Introduction
Acute kidney injury (AKI) is a frequent complication
after cardiac surgery and abdominal aortic aneurysm repair
(1,2). However, ~50% of AKI cases in
hospitalized patients are caused by renal ischemia reperfusion
injury (IRI) (3). The
pathophysiology of AKI is very complex and combines major
ischemia-induced cell stress, a significant burst of free radicals
and pro-inflammatory cytokines [such as interleukin (IL)-1β, IL-6
and tumor necrosis factor (TNF)-α)] evoking a pro-inflammatory
cascade, and subsequent injuries on distant organs, including the
lung, heart, liver and brain (4–6).
This organ crosstalk phenomenon is well-known in critical care
medicine as multiple organ failure due to systemic inflammatory
response syndrome. Acute lung injury (ALI) is the most clinically
relevant remote organ dysfunction associated with AKI (5). The pathological characteristics of
ALI are increased pulmonary vascular permeability, lung edema and
alveolar hemorrhage (7–9). A previous study indicated that when
both AKI and ALI occur, the overall morbidity is as high as 80%
(4). Therefore, improved
understanding of the effects of renal IRI on remote organs,
especially on the lungs, is urgently needed.
Nuclear factor (NF)-κB proteins are a family of
ubiquitously expressed transcription factors. In their inactive
forms, NF-κB proteins are bound by members of the inhibitor of κB
(IκB) family. IκB kinase (IKK)ε is a member of the IKK family,
which influences NF-κB signaling and concomitant gene expression
downstream of IκB (10,11). A previous study indicated that the
NF-κB pathway may aggravate tubular injury and exacerbate a
maladaptive inflammatory response in renal ischemia
reperfusion-induced AKI (12). IKK
family members may be therapeutic targets for lung injury, and the
beneficial effects of IKK proteins may be mediated by inhibition of
the NF-κB pathway (13).
According to Park et al (14) and Bulek et al (15), IKKε may serve an important role in
enhancing lipopolysaccharide-induced and IL-17-mediated
inflammatory responses, including increasing the transcription of
inflammation-related genes in primary airway epithelial cells, and
exacerbating the severity of neutrophilia and pulmonary
inflammation in ALI. However, the molecular mechanism of ALI in the
setting of renal IRI remains unclear. Based on the results of a
previous study, NF-κB-dependent gene expression induced by TNF-α or
IL-1 may be abrogated by IKKε (16); however, to the best of our
knowledge, reports of the role of IKKε in kidney-lung crosstalk in
renal IRI are lacking.
The present study aimed to investigate the possible
molecular mechanisms by which IRI induces ALI, and the effect of
the NF-κB pathway on kidney-lung crosstalk in renal IRI by
primarily focusing on the role of IKKε. The present study may
improve understanding of the pathological mechanisms underlying
IRI-induced ALI and the significance of anti-inflammatory
treatments for patients with IRI.
Materials and methods
Animals
IKKε knockout C57BL/6J mice (IKKε−/−)
(male; age, 6–8-weeks; weight, 25–30 g) were purchased from the
Jackson Laboratory and housed in the Model Animal Research Center
of Nanjing University (Nanjing, China). Wild-type (WT) C57BL/6J
mice (male; age, 6–8-weeks; weight, 25–30 g) were obtained from the
Animal Research Center of Nanjing Medical University (Nanjing,
China). All mice were housed at 2 animals/cage in a
light-controlled environment at 24±1°C, 40–80% humidity, 12-h
light/dark cycles with access to food and water ad libitum
throughout the experimental period. Animal experiments were
performed in compliance with the Institute of Laboratory Animal
Research Guide for the Care and Use of Laboratory Animals of the
NIH and approved by the Institutional Animal Care and Use Committee
of Nanjing Medical University.
Surgical procedures
A total of five experimental groups were evaluated
in this study (n=6 in each group): Two IRI groups (WT and
IKKε−/−), two sham groups (WT and IKKε−/−)
and one control group (WT; no surgery). All procedures were
performed using strict sterile techniques after inducing anesthesia
with an intraperitoneal (IP) injection of pentobarbital (50 mg/kg
body weight). Adequate anesthesia was assessed by pinching the paw
and tail. Animals from each group were placed on a heating blanket
prior to sham and IRI operations. Renal IRI was performed as
previously described (17).
Briefly, the back of the mouse was shaved and a 1.5-cm incision was
made on both sides. Two vascular clamps were applied across both
renal pedicles for 45 min. Occlusion was visually verified by
monitoring the change in the color of the kidney to a paler hue.
After clamp removal, the restoration of blood flow to the kidney
was confirmed by the return of the original color. The incisions on
the back of each mouse were closed in two layers with sutures, and
mice were returned to their cages for 24 or 48 h. The animals were
allowed to recover, and had free access to food and water. Animals
in the sham groups underwent an identical procedure without
vascular clamp placement. Blood and tissue samples were separately
collected at different time points (24 and 48 h) from different
groups.
Plasma parameters
At different time points after operation (24 and 48
h), mice were anesthetized with an IP injection of pentobarbital
(50 mg/kg body weight), and the adequacy of anesthesia was
evaluated by monitoring hind limb reflexes. Blood samples (~0.5 ml)
were obtained from the retroorbital plexus and centrifuged at 1,509
× g for 15 min at 4°C to obtain serum. The samples were stored at
−80°C until further use. Serum creatinine (SCr) and blood urea
nitrogen (BUN) levels were measured as renal function markers using
an Olympus AU2700 automatic biochemistry apparatus (Olympus
Corporation).
Tissue collection and lung wet-to-dry
ratio
Immediately after the blood samples collection
procedure, mice were euthanized with an overdose of pentobarbital
(150 mg/kg body weight). The inferior lobe of the left lung was
harvested to measure the lung wet-to-dry ratio. First, an
arteriovenous (AV) fistula needle was placed in the left atrium.
Saline was slowly forced through another AV fistula needle inserted
into the right ventricle using the injector until the lung changed
color from red to white. The upper lobe of the left lung was
harvested and preserved in liquid nitrogen for western blotting.
The left main bronchus was isolated and cross-clamped. The right
lung was filled with 0.5% low-melting point agarose in 10% formalin
at a constant pressure of 25 cm H2O through a
tracheotomy with an AV fistula needle, allowing the homogenous
expansion of the lung parenchyma. The lung wet-to-dry ratio was
measured by desiccating the lung at 80°C until a constant weight
was obtained. The ratio was calculated as an indicator of lung
edema.
Histological analysis using
hematoxylin and eosin (H&E) staining
For histopathological evaluation of lung injury,
lung tissues were obtained from the mice and subsequently fixed in
10% formaldehyde at room temperature for 24 h and embedded in
paraffin. The 5-µm sections were heated at 60°C for 1 h, before
being dewaxed in xylene and rehydrated using a descending ethanol
series. H&E staining was then performed on sections, with
hematoxylin for 10 min room temperature and eosin for 5 min at room
temperature. Stained sections were visualized using a light
microscope (magnification, ×200) by a pathologist in a blinded
manner.
Western blot analysis
IHC and western blotting were performed as
previously described (12,17). Lung tissue samples were ground in
liquid nitrogen and lysed using RIPA lysis buffer (Beyotime
Institute of Biotechnology) for 30 min. Total protein was
quantified using a bicinchoninic acid assay and 50 µg protein was
separated using 12% SDS-PAGE for 90 min. The separated proteins
were subsequently transferred onto polyvinylidene difluoride
membranes and blocked in TBS with 5% skimmed milk for 2 h at room
temperature. The membranes were incubated with primary antibodies
against GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology,
Inc.), IKKε (1:500; cat. no. ab124766; Abcam), NF-κB phosphorylated
p50 (pi-p50; 1:200; cat. no. sc-271908; Santa Cruz Biotechnology,
Inc.), NF-κB phosphorylated p65 (pi-p65; 1:200; cat. no. sc-166748;
Santa Cruz Biotechnology, Inc.), NF-κB p50 (1:1,000; cat. no.
ab32360; Abcam), NF-κB p65 (1:800; cat. no. ab16502; Abcam)
overnight at 4°C. Following the primary antibody incubation, the
membranes were subsequently incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2370;
1:5,000; Santa Cruz Biotechnology Inc.) for 1 h at 37°C. The
protein-antibody complexes were visualized using Pierce™ Fast
Western Blot Kit, ECL Substrate (cat. no. 35050; Thermo Fisher
Scientific, Inc.) with a chemiluminescence instrument (Tanon
Science and Technology Co., Ltd.). Protein expression was
quantified using ImagePro Plus software (version 6.0; Media
Cybernetics, Inc.).
Immunohistochemical analysis
Immunohistochemistry was performed using
paraffin-embedded tissue sections cut at 4-µm thickness mounted on
glass slides. The slides were then deparaffinized and rehydrated.
Then, the lung sections were incubated with primary antibodies
against TNF-α (1:200; cat. no. ab9739; Abcam), Ki67 (1:2,000; cat.
no. ab15580; Abcam), IL-1β (1:200; cat. no. sc-7884; Santa Cruz
Biotechnology, Inc.), and IL-10 (1:400; cat. no. bs-0698R; BIOSS),
NF-κB phosphorylated p50 (pi-p50; 1:200; cat. no. sc-271908; Santa
Cruz Biotechnology, Inc.), NF-κB phosphorylated p65 (pi-p65; 1:200;
cat. no. sc-166748; Santa Cruz Biotechnology, Inc.) and then with
biotin secondary antibodies (B3640; Sigma-Aldrich; Merck KGaA) at
room temperature for 30 min.
The IHC score was determined using the Fromowitz
standard as previously described (18). The percentage of positive stained
cells was graded as follows, 0–5%, 0; 6–25%, 1; 26–50%, 2; 51–75%,
3; >75%, 4. The intensity of staining was graded as follows:
Absent or faint blush, 0; weak, 1; moderate, 2; strong, 3. Then the
two scores were added.
Statistical analysis
The data are presented as the mean ± standard error
of the mean of at least three independent repeats. The results were
analyzed by one-way ANOVA followed by a Tukey's post hoc test. The
IHC scores were compared by Mann-Whitney U test. SPSS 17 software
(SPPS, Inc.) was used to perform the statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
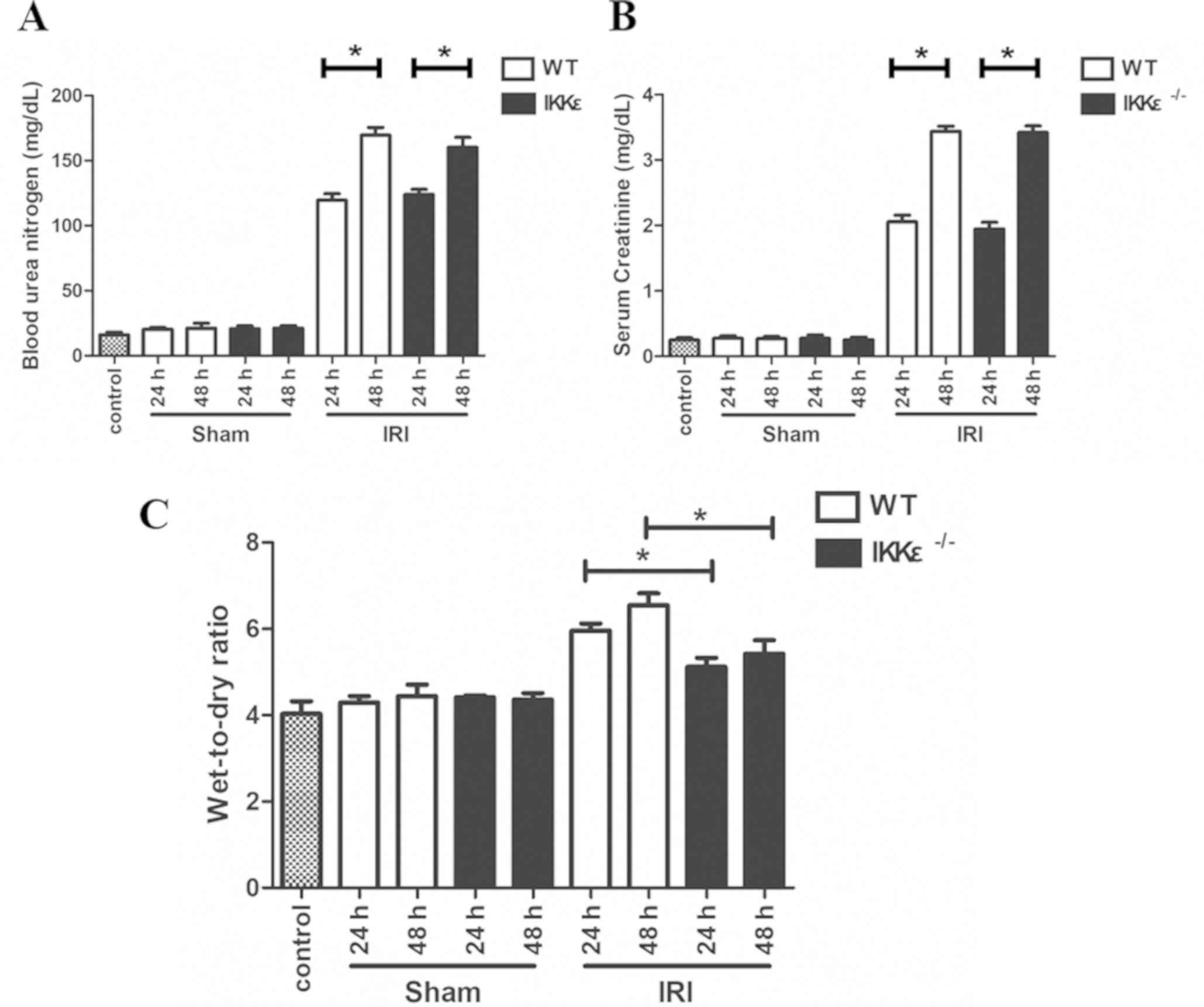
Renal function significantly decreases
following experimental renal IRI
SCr and BUN levels were measured in
IKKε−/− and WT mice at 24 and 48 h after surgical IRI to
confirm the decreased renal function. Compared with the control and
sham groups, the IRI group mice exhibited significant increases in
SCr values and BUN levels at 24 and 48 h (P<0.05; Fig. 1A and B). Additionally, no
significant difference was observed in renal function between the
WT and IKKε−/− groups 24 and 48 h after surgical IRI
(P>0.05; Fig. 1A and B).
IKKε−/− mice exhibit
significantly weaker acute disease and pulmonary edema compared
with that of WT mice
The lung wet-to-dry ratio was detected to evaluate
the degree of pulmonary edema. The results demonstrated a gradually
increasing trend after surgical IRI (Fig. 1C). In addition, the
IKKε−/− mice group exhibited significantly weaker
pulmonary edema compared with that of the WT mice in 24 and 48 h
(P<0.05).
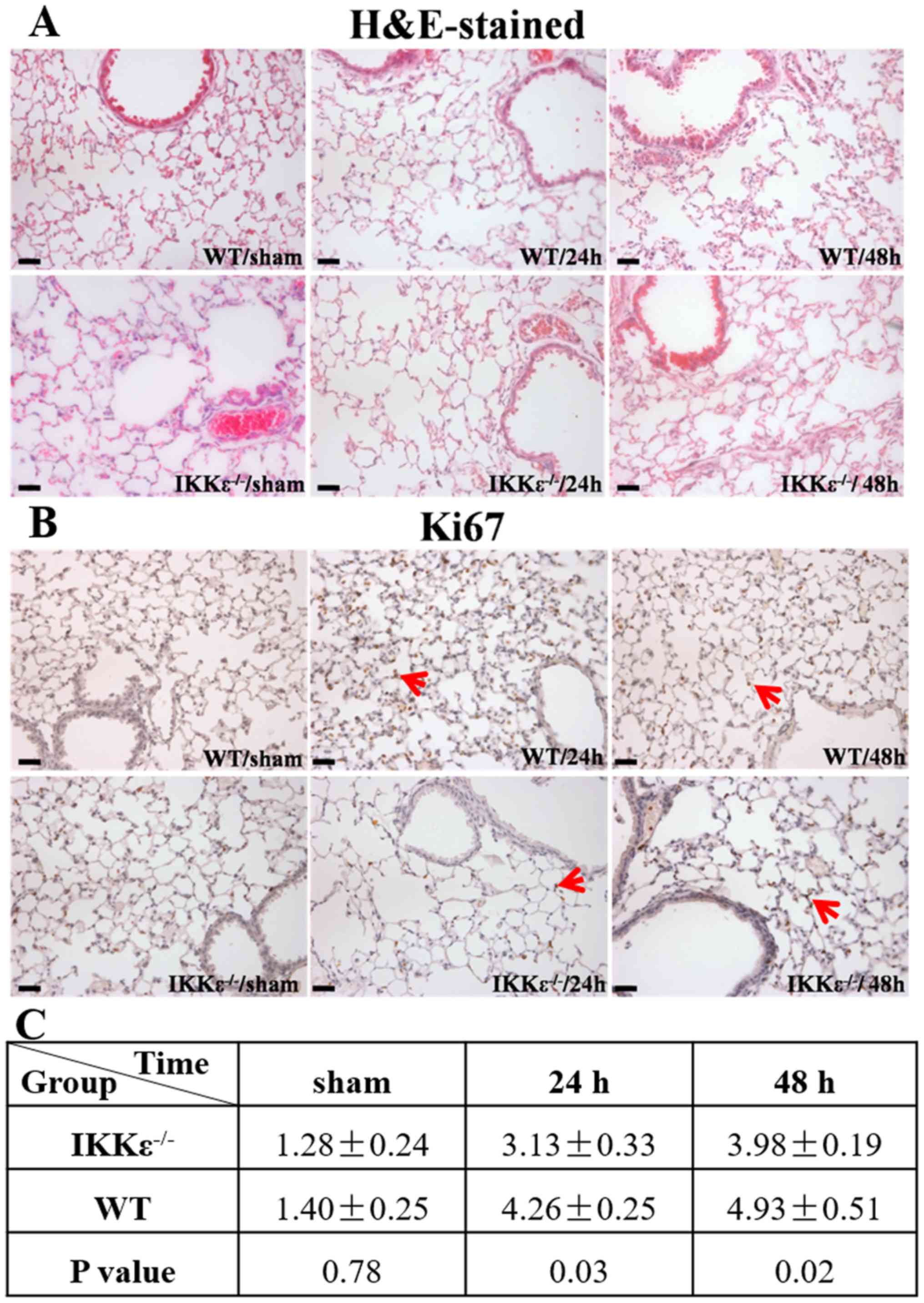
IKKε knockout attenuates experimental
renal IRI-induced lung inflammation
H&E-stained lung sections were examined to
further determine whether IKKε knockout affects experimental renal
IRI-induced ALI. Since the present study indicated that there was
no significant difference in renal function and pulmonary edema
between the control and sham groups at 24 and 48 h, a 48-h
timepoint was used for the sham group in subsequent experiments.
The results demonstrated that in the WT group, renal IRI induced
persistent interstitial edema, focal alveolar hemorrhage, alveolar
wall thickening and inflammatory cell infiltration. By contrast,
lung tissues from the IKKε−/− group exhibited less
damage, which manifested with a disordered and uneven distribution
(Fig. 2A).
Immunohistochemical analysis of the
proliferation-associated antigen Ki67 (Fig. 2B) indicated the absence of mitotic
figures, and very few cells in the sham group were Ki67-positive.
By contrast, compared with those in the IKKε−/− groups,
the number of Ki67-positive cells was significantly increased in
the WT groups at 24 and 48 h (Fig. 2B
and C); thus suggesting cellular proliferation occurred
following kidney IRI in the WT group.
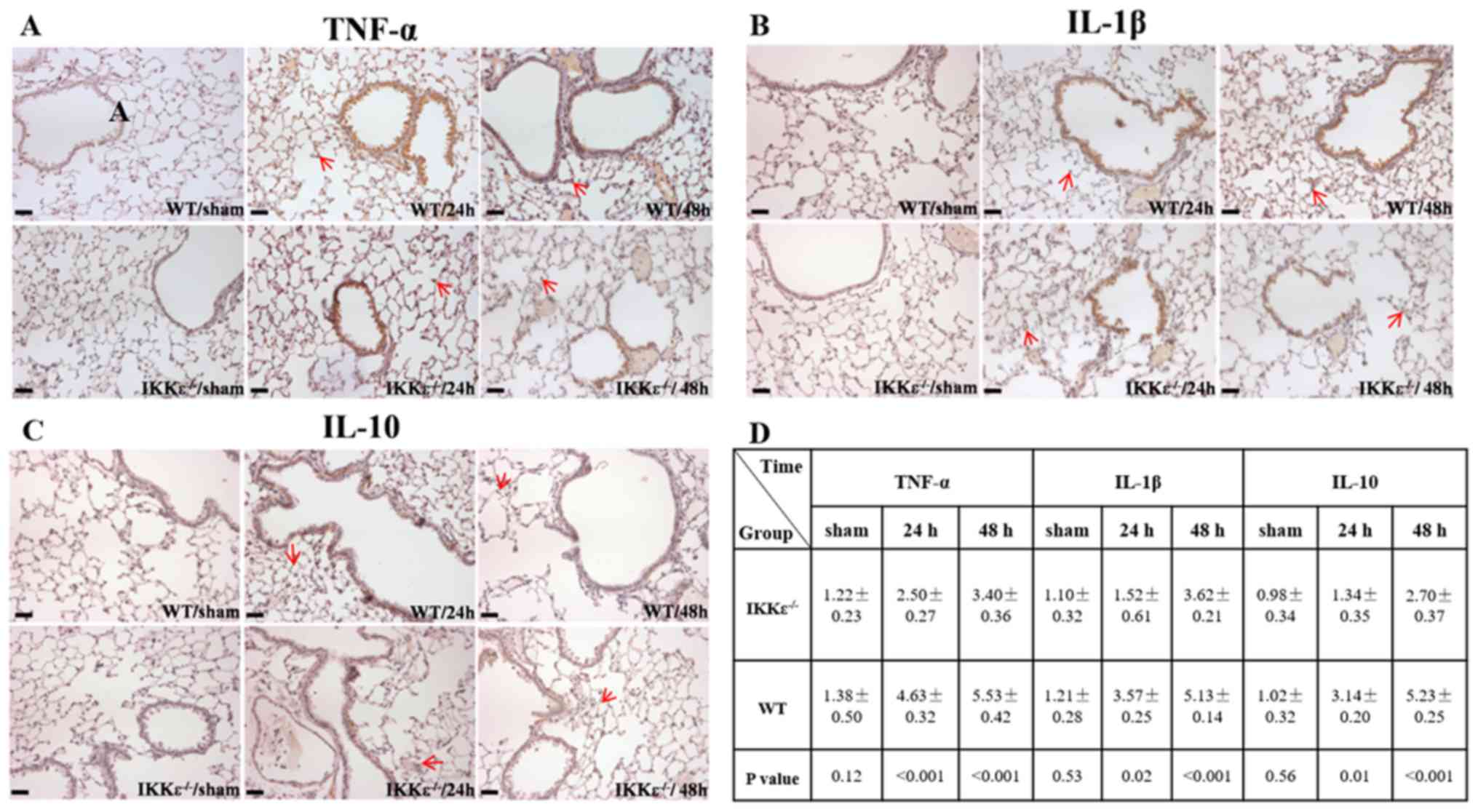
Inflammatory markers, including TNF-α, IL-1β and
IL-10, were detected in lung tissues. The results demonstrated that
the expression levels of TNF-α, IL-1β and IL-10 were significantly
lower in the IKKε−/− groups compared with those in the
WT groups 24 and 48 h post-surgery (P<0.05; Fig. 3A-D). These results suggested that
inflammatory activity was suppressed in the lungs of
IKKε−/− mice.
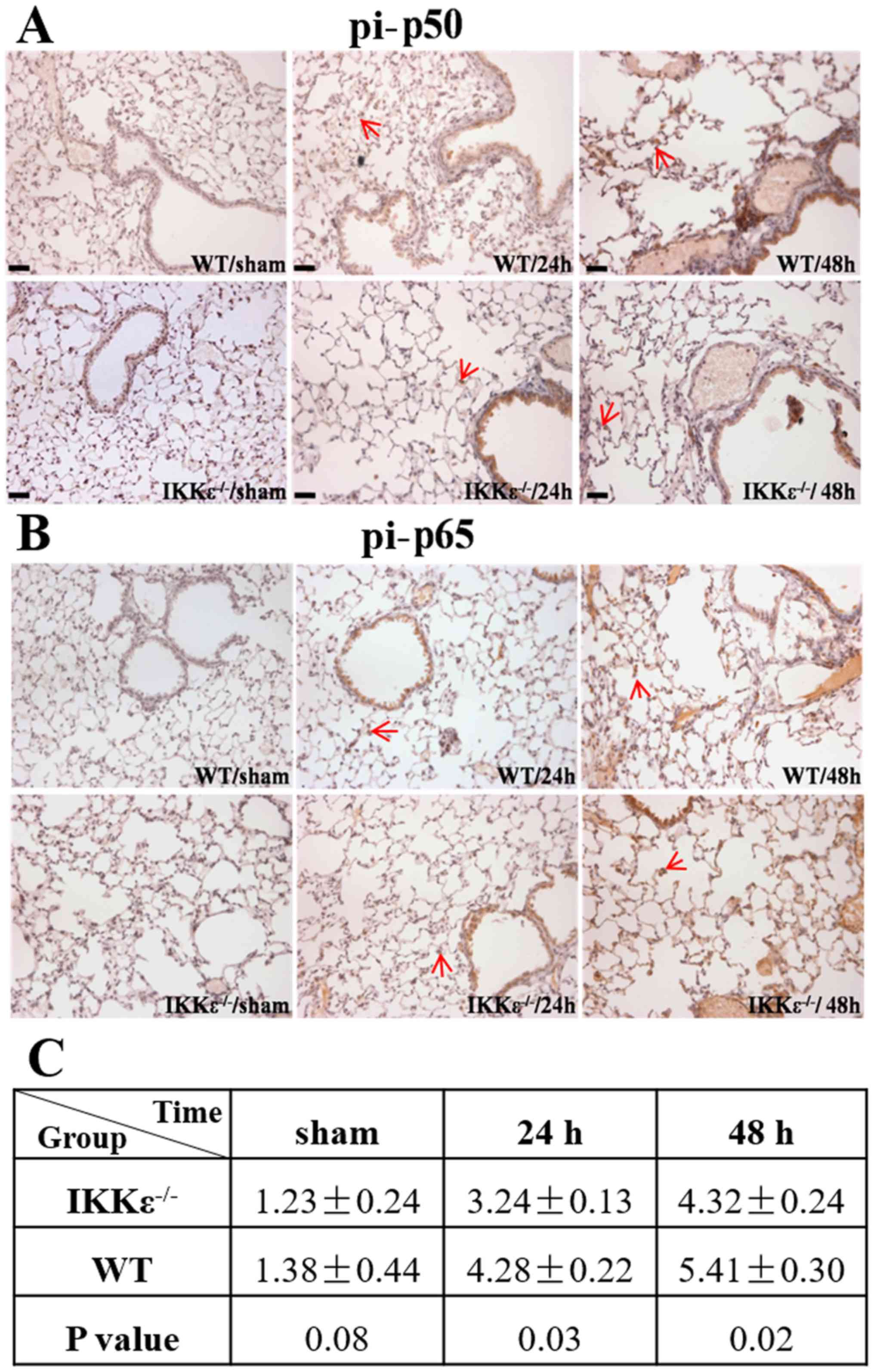
IKKε ablation blocks NF-κB activation
induced by acute ischemic kidney injury
Studies have demonstrated that IKKε participates in
renal IRI (12); however, the
exact mechanism is still unclear. Therefore, the levels of
downstream factors of the NF-κB pathway, such as pi-p50 and pi-p65,
in lung tissues was examined by immunohistochemical staining. The
results demonstrated that the expression levels of pi-p50 and
pi-p65 were significantly lower in bronchial epithelial cells of
the IKKε−/− groups compared with those in the WT groups
(P<0.05; Fig. 4A-C).
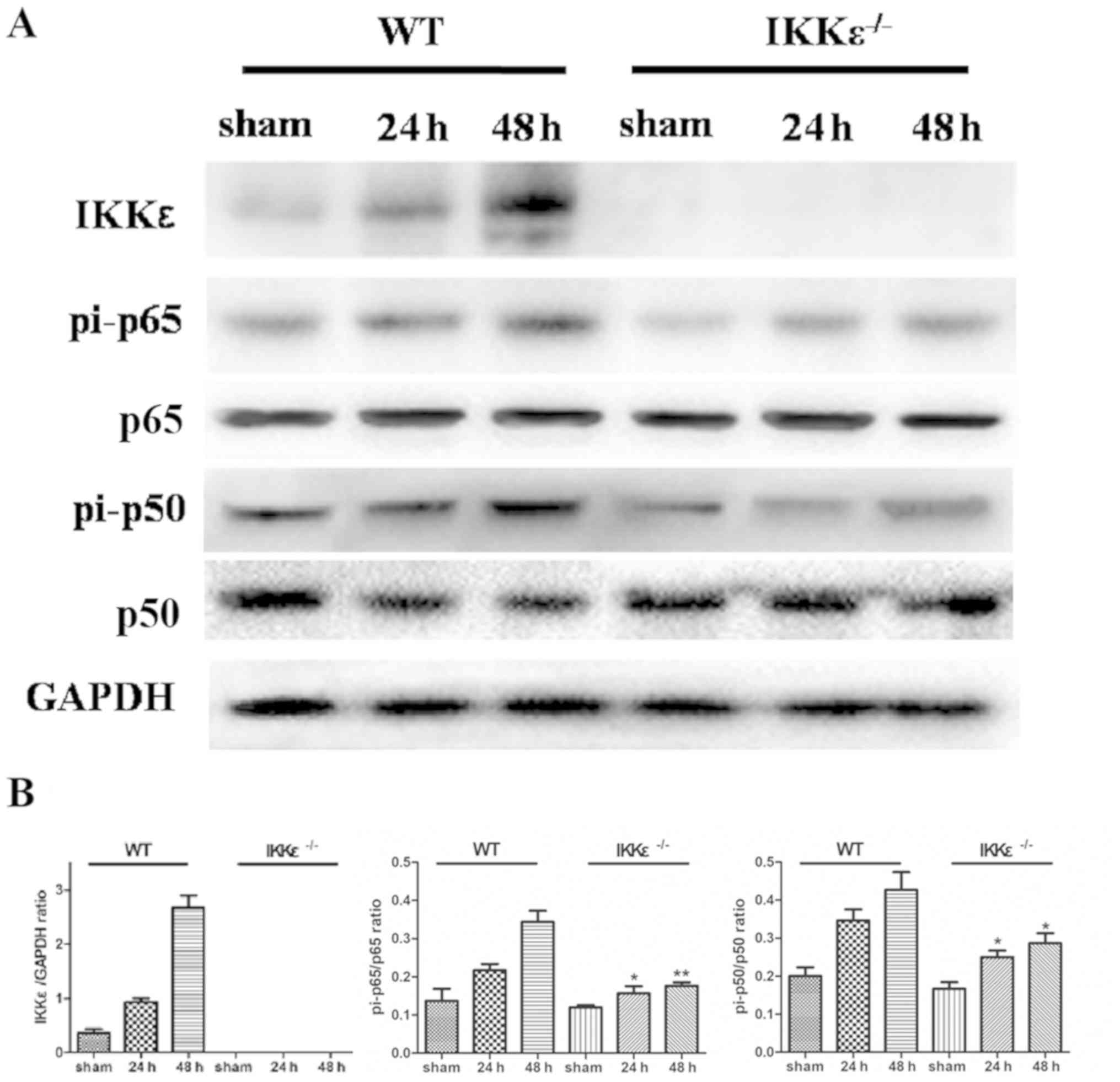
Western blotting was performed to determine the
expression levels of pi-p50 and pi-p65, which are the functionally
active and nuclear forms of NF-κB (11). The results demonstrated that IKKε
expression levels gradually increased in the WT group after acute
ischemic kidney injury (Fig. 5).
In addition, the expression levels of pi-p50 and pi-p65 were
significantly reduced in the IKKε−/− group compared with
those in the WT group (P<0.05), whereas no significant
differences were observed in the total p50 and p65 protein
expression levels (Fig. 5).
Discussion
The pathogenesis of renal IRI is complex and is
still not entirely understood. However, inflammation is currently
accepted as an important pathogenic component (19). Renal IRI has been reported to
result in endothelial and leukocyte activation, reactive oxygen
species production, tubular cell death and the release of
inflammatory mediators, such as cytokines and chemokines (19). AKI has been reported to activate
host innate and adaptive immune responses, and experimental data
have identified both soluble and cellular mediators activated by
the post-ischemic kidney that drive ALI (5,20,21).
Despite the clinical association between renal IRI and ALI, little
is known about the molecular mechanism of kidney-lung crosstalk
following renal IRI. A previous study has observed that increased
expression of TNF-α in the lungs may induce pulmonary inflammatory
damage (22). The present study
used an IKKε−/− mouse model of unilateral IRI and
investigated the possible roles of IKKε−/− and related
inflammatory mediators, such as TNFα, IL-10 and IL-1β. To the best
of our knowledge, the present study is the first to demonstrate
that the IKKε pathway may serve a role in kidney-lung crosstalk
following renal IRI.
The results of the present study demonstrated the
IKKε−/− and WT groups exhibited similar decreased renal
function following experimental AKI; however, the lung wet-to-dry
ratio was significantly decreased in the IKKε−/− group
compared with in the WT group. In addition, this study examined the
morphological and molecular alterations in lung tissues to
investigate the effect of IKKε on ALI following renal IRI. A series
of histopathological changes were demonstrated by H&E staining;
renal IRI induced persistent interstitial edema, focal alveolar
hemorrhage, alveolar wall thickening and inflammatory cell
infiltration in the WT group, whereas lung tissues from the
IKKε−/− group exhibited less damage. The present
findings demonstrated that IKKε knockout may reduce lung edema
after renal IRI. Thus, it was hypothesized that IKKε deficiency may
contribute to the reduction of inflammation in the lungs after
renal IRI. Subsequently, Ki67 levels were measured in lung tissues
by IHC to explore cell proliferation following the pathological
changes. The number of Ki67-positive cells was greater in the lungs
of the WT group compared with that in the IKKε−/− group.
Thus, lung cell proliferation was significantly reduced after
inflammatory injury in the absence of IKKε. Thus, it was
hypothesized that IKKε may be associated with inflammatory cell
infiltration and lung tissue destruction.
In the present study, the expression of inflammatory
markers (TNFα, IL-10, and IL-1β) was detected in lung tissues. The
results demonstrated that TNFα expression levels were elevated
after renal IRI treatment, but were inhibited by IKKε knockout.
Higher TNF-α expression levels were observed in the airway
epithelial cells of the WT group after renal IRI treatment, whereas
lower expression levels were observed in the IKKε−/−
group. Renal IRI has been reported to activate soluble TNFα, and
thus induce TNFα receptor 1-dependent pulmonary cell apoptosis and
microvascular barrier dysfunction (22). Thus, TNF-α may serve a key role in
aggravating the downstream effects of renal IRI. IL-1β is an
important protein participating in NF-κB-induced inflammatory
responses, which has been shown to exhibit a biphasic distribution
in IRI injury models (23,24). Previous studies observed that IL-1β
expression levels were significantly increased and remained high
during the reperfusion period compared with controls (23–25).
In the present study, the expression levels of IL-1β were
investigated during the reperfusion period and the results
indicated that IL-1β expression levels were higher in the WT groups
compared with those in the IKKε−/− groups. These results
suggested that IL-1β may be related to IKKε activation. According
to previous studies, TNF-α, IL-1β, IL-6 and other pro-inflammatory
cytokines inhibited the anti-inflammatory effects mediated by
IL-10, indicating that these factors may perform similar roles in
IRI-related pathways (26,27). Consistent with other studies, the
results of the present study demonstrated that the expression
levels of IL-10 were higher in the WT groups compared with those in
IKKε−/− groups. Thus, it was concluded that the levels
of inflammatory and anti-inflammatory factors were significantly
increased in lung tissues following renal IRI, indicating an
inflammation reaction, and the effects were inhibited by IKKε
knockout. Moreover, in addition to anti-inflammatory effects, IL-10
could also inhibit superoxide production and reduce
metalloproteinase release. The present findings may result from a
compensatory response of IL-10 to TNF-α and other pro-inflammatory
cytokines.
Subsequently, the major components of the NF-κB
signaling pathway were measured. Pi-p65/p50 activate downstream
inflammatory factors of the NF-κB pathway, such as TNFα, IL-10 and
IL-1β (28,29). In the present study, the expression
of the NF-κB cascade factors were investigated. The expression
levels of pi-p65 and pi-p50 were demonstrated to be upregulated in
the lungs of the WT groups following renal IRI compared with those
of the IKKε−/− groups. IKKε phosphorylates NF-κB
(pi-p65), contributing to the NF-κB-dependent expression of target
genes in response to proinflammatory signals (10,30,31).
However, the inhibitory effects were not completely dependent on
IKKε deficiency, indicating other signaling pathways may exist.
Studies have observed that NF-κB activation is separately mediated
by myeloid differentiation primary response 88 via IKKα/β and by
TIR domain-containing adaptor-inducing interferon-β via IKKε
(28,32,33).
Previous studies have reported that myeloperoxidase
activity, neutrophil infiltration, blood oxygen saturation and
pulmonary vascular permeability are important evaluation indicators
of ALI (5,20). Therefore, these factors could be
further explored in future studies.
In conclusion, in the present study, a novel role
for IKKε in regulating renal IRI-induced inflammation in mouse
lungs was identified. In the absence of IKKε, the renal
IRI-associated destruction of mouse lung tissue and inflammatory
responses were substantially prevented, and the expression levels
of components associated with NF-κB signaling were reduced. Based
on these findings and previous studies suggesting a role for IKKε
in NF-κB activation, it was hypothesized that IKKε may serve a
pivotal role in the activity of NF-κB, which in turn could regulate
the expression of genes involved in the inflammatory response in
lungs following renal IRI.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Science and Technology Development Fund of Nanjing Medical
University (grant no. NMUB2019147).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CM designed the study and performed experiments. XM
and AZ designed the study. CZ and HL performed experiments. YQ
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown JR, Cochran RP, Dacey LJ, Ross CS,
Kunzelman KS, Dunton RF, Braxton JH, Charlesworth DC, Clough RA,
Helm RE, et al: Perioperative increases in serum creatinine are
predictive of increased 90-day mortality after coronary artery
bypass graft surgery. Circulation. 114 (1 Suppl):I409–I413. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karkouti K, Wijeysundera DN, Yau TM,
Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B,
Laflamme C, et al: Acute kidney injury after cardiac surgery: Focus
on modifiable risk factors. Circulation. 119:495–502. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Star RA: Treatment of acute renal failure.
Kidney Int. 54:1817–1831. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein CL, Hoke TS, Fang WF, Altmann CJ,
Douglas IS and Faubel S: Interleukin-6 mediates lung injury
following ischemic acute kidney injury or bilateral nephrectomy.
Kidney Int. 74:901–909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Awad AS, Rouse M, Huang L, Vergis AL,
Reutershan J, Cathro HP, Linden J and Okusa MD:
Compartmentalization of neutrophils in the kidney and lung
following acute ischemic kidney injury. Kidney Int. 75:689–698.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinsey GR and Okusa MD: Pathogenesis of
acute kidney injury: Foundation for clinical practice. Am J Kidney
Dis. 58:291–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng J, Hu X, Yuen PS and Star RA:
Alpha-melanocyte-stimulating hormone inhibits lung injury after
renal ischemia/reperfusion. Am J Respir Crit Care Med. 169:749–756.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly KJ: Distant effects of experimental
renal ischemia/reperfusion injury. J Am Soc Nephrol. 14:1549–1558.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nath KA, Grande JP, Croatt AJ, Frank E,
Caplice NM, Hebbel RP and Katusic ZS: Transgenic sickle mice are
markedly sensitive to renal ischemia-reperfusion injury. Am J
Pathol. 166:963–972. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clement JF, Meloche S and Servant MJ: The
IKK-related kinases: From innate immunity to oncogenesis. Cell Res.
18:889–899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marko L, Vigolo E, Hinze C, Park JK, Roel
G, Balogh A, Choi M, Wubken A, Cording J, Blasig IE, et al: Tubular
epithelial NF-KB activity regulates ischemic AKI. J Am Soc Nephrol.
27:2658–2669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shu YS, Tao W, Miao QB, Zhu YB and Yang
YF: Improvement of ventilation-induced lung injury in a rodent
model by inhibition of inhibitory KB kinase. J Trauma Acute Care
Surg. 76:1417–1424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park DW, Jiang S, Liu Y, Siegal GP, Inoki
K, Abraham E and Zmijewski JW: GSK3beta-dependent inhibition of
AMPK potentiates activation of neutrophils and macrophages and
enhances severity of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 307:L735–L745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bulek K, Liu C, Swaidani S, Wang L, Page
RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, et al: The
inducible kinase IKKi is required for IL-17-dependent signaling
associated with neutrophilia and pulmonary inflammation. Nat
Immunol. 12:844–852. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kravchenko VV, Mathison JC, Schwamborn K,
Mercurio F and Ulevitch RJ: IKKi/IKKepsilon plays a key role in
integrating signals induced by pro-inflammatory stimuli. J Biol
Chem. 278:26612–26619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossi M, Delbauve S, Wespes E, Roumeguere
T, Leo O, Flamand V, Le Moine A and Hougardy JM: Dual effect of
hemin on renal ischemia-reperfusion injury. Biochem Biophys Res
Commun. 503:2820–2825. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fromowitz FB, Viola MV, Chao S, Oravez S,
Mishriki Y, Finkel G, Grimson R and Lundy J: ras p21 expression in
the progression of breast cancer. Hum Pathol. 18:1268–1275. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonventre JV and Zuk A: Ischemic acute
renal failure: An inflammatory disease? Kidney Int. 66:480–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tulafu M, Mitaka C, Hnin Si MK, Abe S,
Kitagawa M, Ikeda S, Eishi Y, Kurata S and Tomita M: Atrial
natriuretic peptide attenuates kidney-lung crosstalk in kidney
injury. J Surg Res. 186:217–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
White LE, Cui Y, Shelak CM, Lie ML and
Hassoun HT: Lung endothelial cell apoptosis during ischemic acute
kidney injury. Shock. 38:320–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White LE, Santora RJ, Cui Y, Moore FA and
Hassoun HT: TNFR1-dependent pulmonary apoptosis during ischemic
acute kidney injury. Am J Physiol Lung Cell Mol Physiol.
303:L449–L459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XQ, Lv HW, Tan WF, Fang B, Wang H and
Ma H: Role of the TLR4 pathway in blood-spinal cord barrier
dysfunction during the bimodal stage after ischemia/reperfusion
injury in rats. J Neuroinflammation. 11:622014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stroo I, Stokman G, Teske GJ, Raven A,
Butter LM, Florquin S and Leemans JC: Chemokine expression in renal
ischemia/reperfusion injury is most profound during the reparative
phase. Int Immunol. 22:433–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith PD, Puskas F, Meng X, Lee JH,
Cleveland JC Jr, Weyant MJ, Fullerton DA and Reece TB: The
evolution of chemokine release supports a bimodal mechanism of
spinal cord ischemia and reperfusion injury. Circulation. 126 (11
Suppl 1):S110–S117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal NR, Tsushima K, Eto Y, Tripathi
A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton
MR, et al: Immunological priming requires regulatory T cells and
IL-10-producing macrophages to accelerate resolution from severe
lung inflammation. J Immunol. 192:4453–4464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng J, Wang X, Qian F, Vogel S, Xiao L,
Ranjan R, Park H, Karpurapu M, Ye RD, Park GY, et al: Protective
role of reactive oxygen species in endotoxin-induced lung
inflammation through modulation of IL-10 expression. J Immunol.
188:5734–5740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Khan AM, Maderdrut JL, Simon EE and
Batuman V: The effect of PACAP38 on MyD88-mediated signal
transduction in ischemia-/hypoxia-induced acute kidney injury. Am J
Nephrol. 32:522–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salama M, Farrag SM, Abulasrar SA, Amin
MM, Ali AA, Sheashaa H, Sobh M and Arias-Carrion O: Up-regulation
of TLR-4 in the brain after ischemic kidney-induced encephalopathy
in the rat. CNS Neurol Disord Drug Targets. 12:583–586. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Geng H, Wittwer T, Dittrich-Breiholz O,
Kracht M and Schmitz ML: Phosphorylation of NF-kappaB p65 at Ser468
controls its COMMD1-dependent ubiquitination and target
gene-specific proteasomal elimination. EMBO Rep. 10:381–386. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moreno R, Sobotzik JM, Schultz C and
Schmitz ML: Specification of the NF-kappaB transcriptional response
by p65 phosphorylation and TNF-induced nuclear translocation of IKK
epsilon. Nucleic Acids Res. 38:6029–6044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fitzgerald KA, McWhirter SM, Faia KL, Rowe
DC, Latz E, Golenbock DT, Coyle AJ, Liao SM and Maniatis T:
IKKepsilon and TBK1 are essential components of the IRF3 signaling
pathway. Nat Immunol. 4:491–496. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verstrepen L, Verhelst K, Carpentier I and
Beyaert R: TAX1BP1, a ubiquitin-binding adaptor protein in innate
immunity and beyond. Trends Biochem Sci. 36:347–354.
2011.PubMed/NCBI
|