Introduction
Renal cell carcinoma (RCC) is one of the most
prevalent tumor types in the adult kidney, and the recurrence rate
is high (1). In 2015, a ~66.8%
increase in new RCC cases was reported, with a ~23.4% increase in
mortality within patients with RCC in China (2). While there have been increased
efforts for the development of diagnostic methods, including the
utilization of ultrasound and CT technologies, it remains difficult
to distinguish benign tumors from the malignancy (3). Thus, it is essential to identify
novel non-invasive biomarkers and therapeutic candidates for RCC.
The complex pathogenesis of RCC involves alterations at both the
genetic and epigenetic levels, which can lead to tumorigenesis
(4–8); however, the detailed mechanisms
during the initiation and development of this disease are yet to be
fully elucidated. Numerous pathways including PI3K/Akt/mTOR and
Wnt/β-catenin could be involved in the progression of RCC (4,5,9).
Circular RNAs (circRNAs) are a novel group of
non-coding RNAs that form a continuous loop and are more stable
compared with their linear counterparts (10,11).
Although certain circRNAs, such as CirS-7, have been reported as
putative gene regulators (11),
the detailed functions of most circRNAs are largely unknown. Due to
the absence of free 5′- or 3′-ends, circRNAs are resistant to
exonuclease-induced degradation (11). Previous studies have revealed that
circRNAs could function as microRNA (miRNA/miR) ‘sponges’ that
competitively inhibit the activity of miRNAs (11,12).
In addition, circRNAs are involved in the pathogenesis of numerous
types of diseases, such as nervous system disorders and cancer
(13–15).
In the tumor microenvironment, crosstalk between
malignant cells and adjacent healthy cells is crucial during the
development of a tumor (16).
Exosomes are a novel type of microparticles with a diameter of
40–100 nm, and these are produced by most cell types (17). Cell-secreted exosomes are detected
in numerous types of eukaryotic fluids, such as blood, urine and
cell culture media (18). Exosomes
are crucial during cell-cell signaling. For instance, RNAs are able
to shuttle from one cell to another, known as ‘exosomal shuttle
RNAs’, and can affect protein production in healthy cells (19). Previous studies have also revealed
that circRNAs are abundantly detected in exosomes, and function as
miRNA sponges during gene regulation (20–22).
circRNAs can be transferred into exosomes in colon cancer cells
(23). In addition, mRNAs and
miRNAs can be transferred by exosomes to facilitate genetic
exchange between cells (24).
However, the detailed functions of exosomal circRNAs in cancer
remain largely unknown.
In the present study, the expression patterns of
exosomal circRNAs in patients with RCC were examined, and the
regulatory functions of circRNAs during the progression of RCC were
elucidated. The effects of circ_400068-regulated
miR-210-5p/suppressor of cytokine signaling 1 (SOCS1) signaling on
the development of RCC were elucidated, which could provide novel
insights into the therapeutic strategies of the treatment against
this disease.
Materials and methods
Clinical samples
Human kidney tissue and plasma (10 ml) specimens
were obtained from patients with RCC (n=28, aged 38–72 years old;
16 males and 12 females) at the Department of Urology of The First
Affiliated Hospital of Jinzhou Medical University between May 2014
and April 2017. Para-carcinoma tissues were ≥5-cm from tumor
margin. The expression levels of hsa_circ_400068 were categorized
into low and high groups using the mean value. The biopsies were
examined by two independent pathologists, and the
clinicopathological features of enrolled patients were summarized
in Table I. The study was approved
by the Ethics Committee of The First Affiliated Hospital of Jinzhou
Medical University, Written informed consents were signed by all
patients.
 | Table I.Clinicopathological parameters of
patients with renal cell carcinoma recruited in the current
study. |
Table I.
Clinicopathological parameters of
patients with renal cell carcinoma recruited in the current
study.
|
|
| hsa_circ_400068
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total number of
cases | Low | High | P-value |
|---|
| Sex |
|
|
| 0.392 |
|
Male | 16 | 7 | 9 |
|
|
Female | 12 | 7 | 5 |
|
| Age, years |
|
|
| 0.411 |
|
>60 | 18 | 8 | 10 |
|
|
≤60 | 10 | 6 | 4 |
|
| Tumor size, cm |
|
|
| 0.409 |
|
>5 | 14 | 6 | 8 |
|
| ≤5 | 14 | 8 | 6 |
|
| Histology
gradea |
|
|
| 0.456 |
|
I–II | 17 | 9 | 8 |
|
|
III–IV | 11 | 5 | 6 |
|
Extraction of exosomes in human plasma
and RCC cells culture media
RCC cells were cultured using Dulbecco's modified
Eagle's medium (DMEM) supplemented with streptomycin (100 µg/ml),
penicillin (100 U/ml) and 10% (FBS; HyClone; Cytiva). Exosomes were
isolated using gradient centrifugation as previously described
(22). Circulating blood samples
(~10 ml) were centrifugated at 2,000 × g at 4°C for 10 min and the
plasma was obtained. After centrifugation at 3,000 × g for 30 min,
debris and floating cells were aspirated. Subsequently, the
supernatant was collected and centrifugated at 10,000 × g for 10
min to remove microvesicles, whose sizes are larger compared with
exosomes. The supernatant was spun at 15,000 × g for 60 min. All
centrifugation steps were performed at 4°C. Isolated exosomes
pellets were rinsed using PBS and stored at −80°C until further
use.
Confirmation of exosome using
transmission electron microscope
Isolated exosomes pellets were resuspended in PBS
and fixed using 2.5% of glutaraldehyde (pH 7.2) at 4°C overnight. A
drop of 100 µl the suspension was placed on a parafilm sheet, and a
copper grid coated by carbon was placed onto the drop for 10 sec
and then removed. Uranyl acetate and phosphotungstic acid (0.5 µg,
2%) were added onto the grid at room temperature for 5 sec.
Following the removal of excess liquid using filter paper, the grid
was dried for 10 min at room temperature and the observed using a
transmission electron microscope (H-7600; Hitachi, Ltd.). In total,
10 fields of view were randomly selected for each sample
(magnification, ×200,000).
circRNA microarray
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and a RNeasy
Mini kit (Qiagen GmbH). Samples were then grouped and
Cy3-dUTP-labelled targets were generated using an Arraystar Super
RNA Labeling Kit (Arraystar Inc.) for circRNA array. Human circRNA
array v2 (CapitalBio Technology Co., Ltd.) was designed, ~5,000
genes were mounted onto the chip, the target sequences of these
circRNAs were obtained from circBase (June, 2015 http://www.circbase.org/). Briefly, labeled targets
were hybridized with the samples, which were scanned using Agilent
Microarray scanner (Agilent Technologies, Inc.). Data were
normalized according to the Quantile algorithm (https://www.fon.hum.uva.nl/praat/manual/quantile_algorithm.html).
Arrays were performed using the protocol provided by Agilent
Technologies, Inc. Genes whose fold change was >2 (P<0.05)
were further analyzed. In total, the five most up- or downregulated
circRNAs in plasma exosomes of patients with RCC are listed in
Table II. circ_400068 was the
most upregulated gene in RCC samples and was selected for further
study.
 | Table II.Top five up- and downregulated
circRNAs in the array. |
Table II.
Top five up- and downregulated
circRNAs in the array.
| A, Upregulated |
|---|
|
|---|
| circRNA ID | Fold change | P-value | circRNA type | Genomic
location | Gene symbol |
|---|
|
hsa_circ_0092314 | 4.86 | 0.00772 | Exonic | chr22 | RANBP11 |
|
hsa_circ_0000735 | 3.66 | 0.00664 | Exonic | chr17 | P2RX1 |
|
hsa_circ_0023642 | 2.83 | 0.01453 | Exonic | chr11 | UVRAG |
|
hsa_circ_0068610 | 2.33 | 0.03245 | Exonic | chr3 | TFRC |
|
hsa_circ_0032821 | 2.15 | 0.04664 | Exonic | chr14 | CEP128 |
|
| B,
Downregulated |
|
| circRNA
ID | Fold
change | P-value | circRNA
type | Genomic
location | Gene
symbol |
|
|
hsa_circ_0005730 | 3.61 | 0.04235 | Exonic | chr5 | CDK7 |
|
hsa_circ_0003645 | 3.22 | 0.02664 | Exonic | chr16 | C16orf62 |
|
hsa_circ_0026134 | 2.76 | 0.01664 | Exonic | chr12 | TUBA1C |
|
hsa_circ_0061274 | 2.52 | 0.01863 | Exonic | chr21 | NRIP1 |
|
hsa_circ_0092368 | 2.19 | 0.00791 | Exonic | chr1 | HMGN2 |
Cell culture
The human clear cell RCC cell line Caki-1 and the
papillary RCC cell line Caki-2, as well as a cell line of normal
human kidney cells (HK-2) were obtained from the Shanghai Institute
of Cell Biology, Chinese Academy of Science. Cells were maintained
using DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin (all purchased from Gibco; Thermo Fisher
Scientific, Inc.). Cells were incubated in a humidified incubator
supplied with 5% CO2 at 37°C.
Cell transfection
To generate circ_400068 and SOCS1 knockdown HK-2
cells, SureSilencing short hairpin (sh)RNA plasmids (Qiagen GmbH)
targeting circ_400068 (sh-circ_400068) and the scrambled negative
control (sh-NC; sense,
5′-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3′
and antisense,
5′-AGCTCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3′),
as well as small interfering (si)RNA against SOCS1 (si-SOCS1;
5′-CCUGCACGGAGCAUUAACUTT-3′) and the NC (si-NC;
5′-ACGUGACACGUUCGGAGAATT-3′) were obtained from Shanghai GenePharma
Co., Ltd. Subsequent experiments were performed 48 h
post-transfection.
For miR-210-5p interference, miR-210-5p mimics
(5′-CUGUGCGUGUGACAGCGGCUGA-3′), miR-210-5p inhibitors
(5′-UCAGCCGCUGUCACACGCACAG-3′) and miR-NC (sense,
5′-UUCUUUUCGAACGUGUCACGUTT-3′ and antisense,
ACGUGACACGUUCGGAGAATT-3′) with the non-targeting sequence were
purchased from Shanghai GenePharma Co., Ltd and transfected into
HK-2 cells (ATCC).
To produce HK-2 cells overexpressing circ_400068
(LV-circ_400068) or SOCS1(LV-SOCS1), wild-type (WT) and scrambled
(LV-NC) sequences were integrated into PLCDH-cir vectors (Guangzhou
RiboBio Co., Ltd.).
In total, 25 nM plasmids were used for transfection
together with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Fresh DMEM containing 10% FBS was added
into cells 12 h after transfection. Transfected cells were further
selected using puromycin (0.5 µg/ml; Sigma-Aldrich; Merck KGaA) for
an additional 2 weeks. Non-transfected cells or cells transfected
with sh-circ_400068 and miR-210-5p mimics were further treated
using exosomes (108 particles/ml) isolated from the
culture media of RCC cells (RCC-Exo) 37°C for 24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
miRNA was extracted using a miRNeasy Mini kit
(Qiagen China Co., Ltd.). A TaqMan miRNA assay (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to examine the expression
of miR-210-5p, and the reaction was completed on Applied Biosystem
7500 system (Thermo Fisher Scientific, Inc.). For the detection of
mRNAs and circRNAs, total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. The concentration of extracted RNA
was evaluated using a NanoDrop 1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). Then, cDNA was produced using
PrimeScript™ RT kit (Takara Biotechnology Co., Ltd.), and PCR
program used was: 42°C for 45 min, 99°C for 5 min and 5°C for 5 min
in a PCR cycler. qPCR was conducted using SYBR Green PCR Master mix
(Takara Biotechnology Co., Ltd.). U6 snRNA was used as internal
control for miRNAs. Endogenous GAPDH was used as a reference for
circRNA and mRNA. The forward and reverse primer pairs used were as
follows: circ_400068, forward: 5′-TGATCTCACCCTAAGTTCGC-3′ and
reverse: 5′-CATACCAATCCTCAATCCTC-3′; miR-210-5p, forward:
5′-CCTGCAATATTTGCATGTCG-3′ and reverse:
5′-GTCCCTATTGGCGTTACTATGG-3′; SOCS1, forward:
5′-GCAUCCGCGUGCACUUUCAUU-3′ and reverse:
5′-AAUGAAAGUGCACGCGGAUGC-3′; GAPDH, forward:
5′-ATGTCGTGGAGTCTACTGGC-3′ and reverse: 5′-TGACCTTGCCCACAGCCTTG-3′;
and U6, forward: 5′-CTCGCTTCGGCAGCACA-3′ and reverse:
5′-AACGCTTCACGAATTTGCGT-3′. The thermocycling program included:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec, 60°C for 20 sec and 72°C for 10 sec and final
extension at 72°C for 5 min. Relative gene expression was analyzed
using the 2−∆∆Cq method (25).
Cell proliferation assay
At 24 h post-transfection, cells were harvested and
seeded at 5×103 per well onto a 96-well plate. Cell
proliferation was determined at day 1, 2, 3 and 4. According to the
manufacturer's protocol, 10 µl Cell Counting Kit (CCK)-8 solution
(Beyotime Institute of Biotechnology) was used for each coloring
reaction. After a further incubation at 37°C for 2 h, the
absorbance at 450 nm was quantified using a plate reader (Bio-Rad
Laboratories, Inc.). Each experiment was performed in triplicate
independently.
Cell apoptosis analysis
Cell apoptotic rate was calculated as the percentage
of early apoptotic cells, the percentage of late apoptotic cells,
or the percentage of early + late apoptotic cells. Transfected
cells were placed onto a 6-well plate at 4×104 per well
and then centrifugated at 5,000 × g at room temperature for 5 min.
Cell pellets were washed and re-suspended using PBS. Cells were
stained with PI at room temperature for 5 min. To evaluate cell
apoptotic activity, cell suspension were incubated at 4°C in dark
for 30 min and staining was performed using 5 µl Annexin V-FITC
(Sigma-Aldrich; Merck KGaA). Cell apoptotic rate was determined
using a flow cytometer FACSAria III (BD Biosciences) and the data
were interpreted using FlowJo software (version 7.6; FlowJo
LLC).
Bioinformatics and dual-luciferase
reporter assay
TargetScan (version 6.2; www.targetscan.org/) and miRanda (version 5.0;
www.microrna.org/microrna/) were used to
predict the putative downstream targets of circ_400068 and
miR-210-5p. Wild-type (WT) fragment of the 3′untranslated region
(UTR) on circ_400068 or SOCS1 with potential complementary binding
sites of miR-210-5p were obtained from Shanghai GenePharma Co.,
Ltd. The sequences were integrated onto pmirGLO Dual-Luciferase
miRNA Target Expression vector (Promega Corporation) according to
the manufacturer's protocols. A circ_400068 or SOCS1 3′UTR-mutant
(MUT) vector containing the mutant binding site of miR-210-5p was
produced using a QuikChange Multi Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.). Subsequently, the
plasmids were used to co-transfect 293 cells (ATCC) with miR-210-5p
mimics or mock control (2 µg/µl) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase
activity was examined 48 h after transfection using a Dual
Luciferase Reporter Assay system (Promega Corporation), and firefly
luciferase activity was normalized using Renilla
luciferase.
Western blotting
Total protein was extracted from tissues and cells
using RIPA buffer (Beyotime Institute of Biotechnology). The
concentration of isolated protein was evaluated using BCA assay
(Beyotime Institute of Biotechnology). Equal amount (30 µg) of
protein samples were loaded onto 10% SDS-PAGE and were subsequently
transferred to PVDF membrane (EMD Millipore). Then, the membranes
were blocked in TBS containing 5% non-fat milk at room temperature
for 1 h, followed by incubation using primary antibodies: Ki-67
(1:5,000; cat. no. ab16667; Abcam), caspase-7 (1:500; cat. no.
ab32522; Abcam), cleaved caspase-7 (1:500; cat. no. ab256474;
Abcam), SOCS1 (1:100; cat. no. ab62584; Abcam), E-cadherin (E-cad;
1:5,000; cat. no. ab15148; Abcam), p53 (1:1,000; cat. no. ab131442;
Abcam), GAPDH (1:10,000; cat. no. ab8245; Abcam) and STAT1
(1:5,000; cat. no. ab47425; Abcam) at 4°C overnight. The following
day, membranes were rinsed and incubated in horseradish
peroxidase-conjugated anti-rabbit (1:10,000; cat. no. sc-2357;
Santa Cruz Biotechnology Inc.) or anti-mouse IgG (1:10,000; cat.
no. sc-2371; Santa Cruz Biotechnology Inc.) at room temperature for
1 h. Protein bands were visualized using an ECL detection kit
(Pierce Biotechnology; Thermo Fisher Scientific, Inc.), and blots
were analyzed using ImageJ (version 1.3; National Institutes of
Health). GAPDH was used as internal standard.
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed using JMP 9.0 software (SAS Institute, Inc.). All
the experiments were performed three times. The significance of
differences were interpreted using paired or unpaired Student's
t-test or one-way ANOVA followed by a post hoc Tukey's test. The
association between circ_400068 expression in RCC tissues and
exosomes was evaluated using Pearson's correlation test. P<0.05
was considered to indicate a statistically significant
difference.
Results
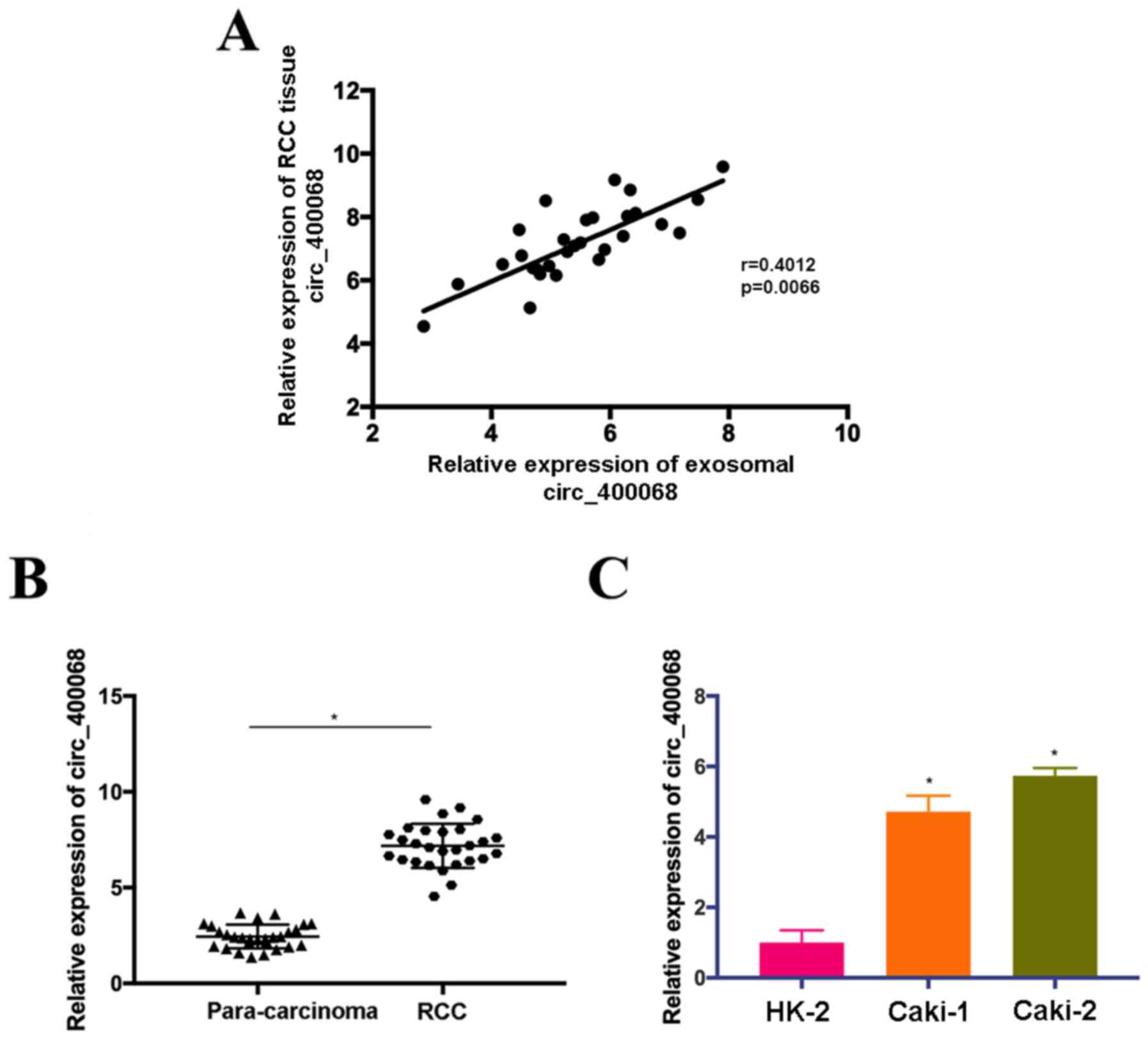
circ_400068 is upregulated in RCC
samples and cells
According to the data from circRNA microarray
(Table II), circ_400068 was
upregulated in plasma exosomes of patients with RCC and selected
for further functional study. The results indicated that the
expression levels of plasma exosomal and tissue circ_400068 in
patients with RCC were moderately positively correlated (Fig. 1A). Therefore, the expression of
circ_400068 in RCC tissues was evaluated. Upregulation of
circ_400068 was observed in RCC tissues compared with adjacent
healthy controls (Fig. 1B).
Additionally, the expression of circ_400068 was elevated in RCC
cells lines in comparison with healthy kidney cells (Fig. 1C). However, no significant
association was observed between circ_400068 in RCC tissues and
clinicopathological characteristics of the patients (Table II). These findings suggested that
the expression of circ_400068 was upregulated in RCC, which could
be associated with the development of this disease.
Treatment with exosomal circ_400068
promotes the proliferation and inhibits the apoptosis of kidney
cells, which were abrogated by sh-circ_400068
In order to elucidate the roles of circ_400068 on
cellular functions, proliferation and apoptosis were examined in
HK-2 cells treated with exosomal circ_400068 extracted from
RCC-Exo. The results demonstrated that the expression of
circ_400068 in HK-2 cells was increased following this treatment
(Fig. 2A). In order to perform
further functional experiments, the cells were also treated with
sh-circ_400068, and the transfection efficiencies were confirmed
using RT-qPCR (Fig. 2B).
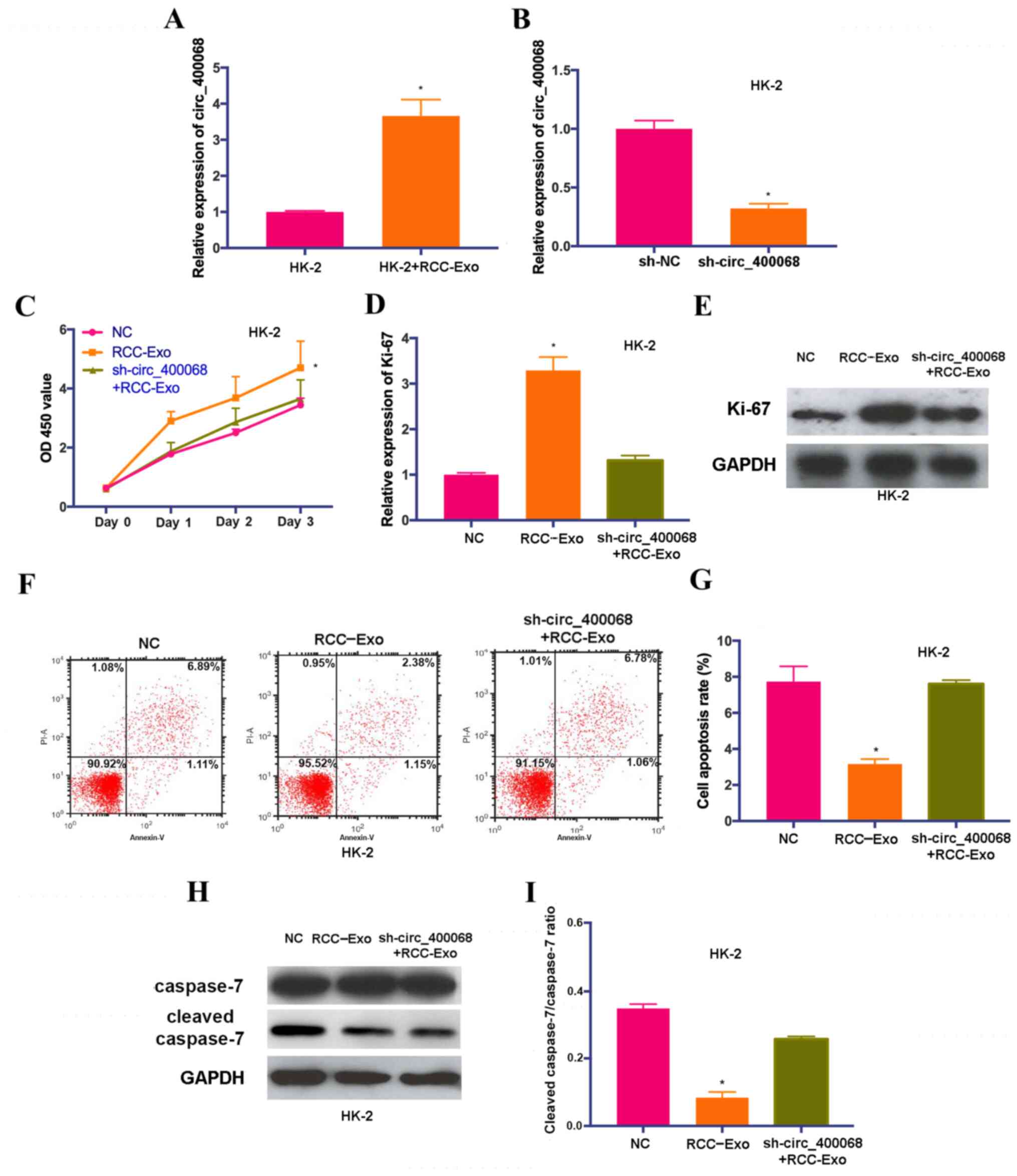 | Figure 2.Exosomal circ_400068 enhances the
proliferation and suppresses the apoptosis of kidney cells, and the
effects are reversed by sh-circ_400068. (A) Expression of
circ_400068 was elevated in HK-2 cells treated with RCC-Exo. (B)
Transfection efficiency of sh-circ_400068 in HK-2 cells was
assessed using reverse transcription quantitative PCR compared with
the sh-NC. (C) Proliferative activity in HK-2 cells were enhanced
following the treatment with RCC-Exo, which was abrogated by
sh-circ_400068. (D) The RNA levels of Ki-67 were increased in cells
treated with RCC-exo and reduced by sh-circ_400068. (E) The protein
levels of Ki-67 were enhanced in cells treated with RCC-exo, which
was reversed by sh-circ_400068. (F) Flow cytometry results
demonstrated that the (G) cell apoptotic rate was significantly
reduced in RCC-Exo-treated HK-2 cells, and the effects were rescued
by the treatment with sh-circ_400068. (H) Western blotting results
indicated that the (I) expression of cleaved caspase-7 was
decreased in HK-2 cells treated with RCC-Exo, which was abrogated
by sh-circ_400068. *P<0.05 vs. NC. NC, negative control; sh-,
short hairpin RNA; RCC-Exo, exosomes isolated from the culture
media of RCC cells; circ, circular RNA; RCC, RCC, renal cell
carcinoma; OD, optical density. |
HK-2 cells were also co-treated with sh-circ_400068
and RCC-Exo. The results of CCK-8 assay suggested that the
proliferation of HK-2 cells was significantly promoted after the
treatment with RCC-Exo, and the effects were abolished by
sh-circ_400068 (Fig. 2C). In line
with these findings, the expression of Ki-67 was upregulated in
HK-2 cells treated with RCC-Exo, which was markedly reversed by the
treatment with sh-circ_400068 (Fig. 2D
and E). Moreover, flow cytometry results indicated that the
apoptotic rate of RCC-Exo-treated cells was significantly decreased
compared with non-treated cells (Fig.
2F and G). The expression of cleaved caspase-7 was
significantly downregulated in HK-2 cells treated with RCC-Exo
compared with the NC, and the effects were reversed by
sh-circ_400068 (Fig. 2H and I).
These data indicated that exosomal circ_400068 produced by RCC
cells was able to enhance cell proliferation and suppress the
apoptosis of healthy kidney cells, which could contribute to the
progression of RCC.
miR-210-5p is a putative downstream
target of circ_400068 in RCC
In order to investigate whether circ_400068 exerts
its regulatory functions by targeting corresponding miRNAs in RCC,
the complementary binding sites of miR-210-5p on circ_400068
transcripts were predicted using miRanda (Fig. 3A). Luciferase reporters carrying WT
(WT-circ_400068) and MUT (MUT-circ_400068) fragments of the
predicted miR-210-5p binding sites were produced. The data
indicated that miR-210-5p mimics significantly abolished the
luciferase activity of vectors containing WT binding sequences, but
not the MUT control (Fig. 3B). In
order to perform further functional experiments, the cells were
also treated with LV-circ_400068, and the transfection efficiencies
were confirmed using RT-qPCR (Fig.
3C).
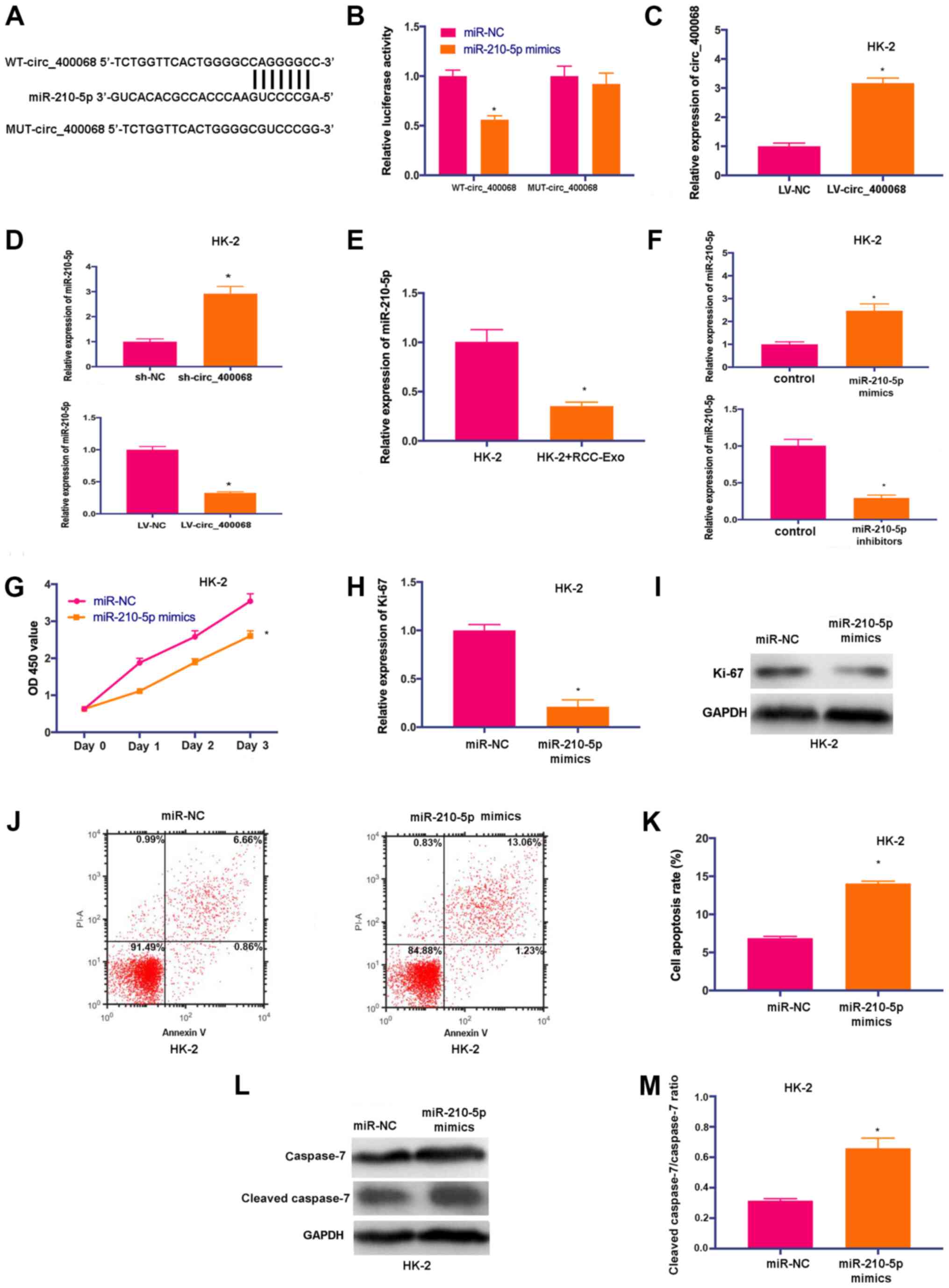 | Figure 3.miR-210-5p is a novel target of
circ_400068 in RCC. (A) Potential binding sites of miR-210-5p on
transcript of circ_400068 were predicted. (B) miR-210-5p mimics
significantly decreased the luciferase activity of WT-circ_400068
compared with miR-NC, but not MUT-circ_400068. (C) Transfection
efficiency of LV-circ_400068 in HK-2 cells. (D) miR-210-5p
expression was determined in HK-2 cells treated with sh-circ_400068
or LV-circ_400068, as well as (E) RCC-Exo using RT-qPCR. (F)
Transfection efficiencies of miR-210-5p mimics or inhibitors in
HK-2 cells were measured via RT-qPCR compared with miR-NC. (G)
Proliferation of HK-2 cells was suppressed following the treatment
with miR-210-5p mimics. (H) Ki-67 expression was decreased by
miR-210-5p mimic in HK-2 cells compared with miR-NC, as determined
by (I) western blotting. (J) Flow cytometry analysis demonstrated
that (K) the apoptotic activity was promoted in HK-2 cells treated
with miR-210-5p mimics compared with miR-NC. (L) Western blotting
results indicated that (M) cleaved caspase-7 expression was
significantly elevated by the transfection with miR-210-5p mimics
in HK-2 cells compared with miR-NC. *P<0.05 vs. respective
control. miR, microRNA; NC, negative control; MUT, mutant; WT,
wild-type; RCC, renal cell carcinoma; RT-qPCR, reverse
transcription quantitative PCR; sh-, short hairpin RNA; RCC-Exo,
exosomes isolated from the culture media of RCC cells; circ,
circular RNA. |
To further study the influences of circ_400068 on
miR-210-5p expression, the expression of miR-210-5p was detected in
HK-2 cells transfected with sh-circ_400068 or LV-circ_400068. The
expression of miR-210-5p was upregulated in cells following the
transfection with sh-circ_400068, but downregulated after
LV-circ_400068 transfection (Fig.
3D). Furthermore, the expression of miR-210-5p was
significantly decreased in RCC-Exo-treated cells (Fig. 3E).
In order to perform further functional studies, HK-2
cells were transfected with miR-210-5p mimics or inhibitors, and
the transfection efficiencies were confirmed via RT-qPCR (Fig. 3F). The proliferative activity of
HK-2 cells was significantly inhibited after the treatment with
miR-210-5p mimics (Fig. 3G). In
addition, the expression of Ki-67 was downregulated in HK-2 cells
transfected with miR-210-5p mimics (Fig. 3H and I). It was identified that
cell apoptosis was enhanced following the treatment with miR-210-5p
mimics (Fig. 3J and K). Similarly,
the expression of cleaved caspase-7 was significantly increased in
HK-2 cells treated with miR-210-5p mimics (Fig. 3L and M). Thus, the results
suggested that miR-210-5p could be a promising target of
circ_400068 in RCC.
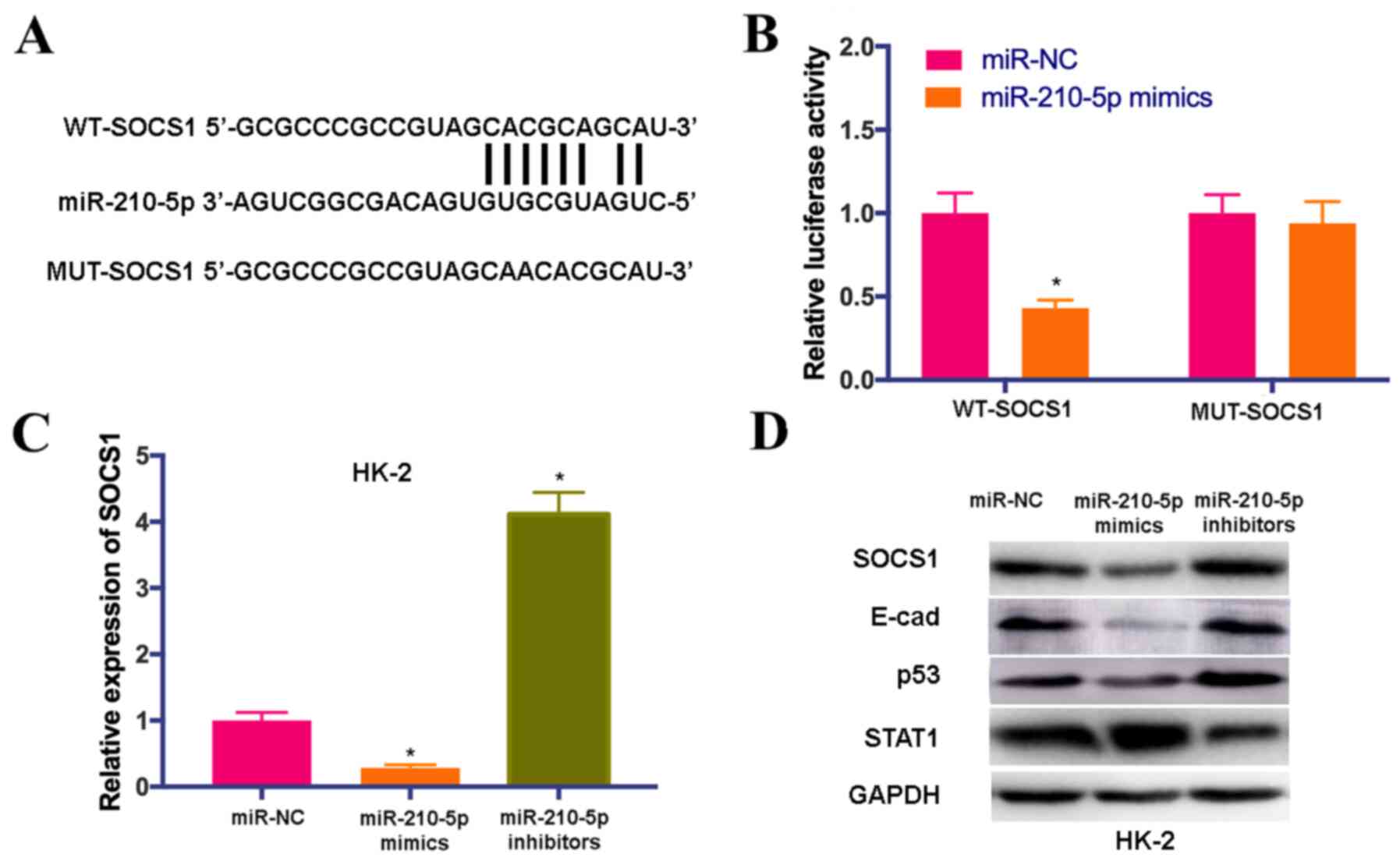
SOCS1 is a novel target of
miR-210-5p
Using the TargetScan database, complementary binding
sequences between SOCS1 and miR-210-5p were predicted (Fig. 4A). In order to elucidate whether
SOCS1 was a putative target of miR-210-5p, WT and MUT sequences of
SOCS1 were inserted after a firefly luciferase coding domain. It
was found that the overexpression of miR-210-5p significantly
decreased the luciferase activity of the SOCS1-WT reporter but not
SOCS1-MUT control (Fig. 4B).
Additional experiments were performed to investigate
whether miR-210-5p affects the expression levels of SOCS1 and its
downstream molecules. RT-qPCR results demonstrated that the
expression of SOCS1 was downregulated by miR-210-5p mimics, but
upregulated by miR-210-5p inhibitors compared with miR-NC (Fig. 4C). IT was found that the protein
expression levels of SOCS1, E-cad and p53 were notably decreased in
HK-2 cells treated with miR-210-5p mimics, while their expression
levels were elevated by treatment with miR-210-5p inhibitors.
However, the expression of STAT1 was significantly upregulated by
miR-210-5p mimics and downregulated by miR-210-5p inhibitors
(Fig. 4D). These findings
indicated that SOCS1 signaling could be downstream target of
miR-210-5p.
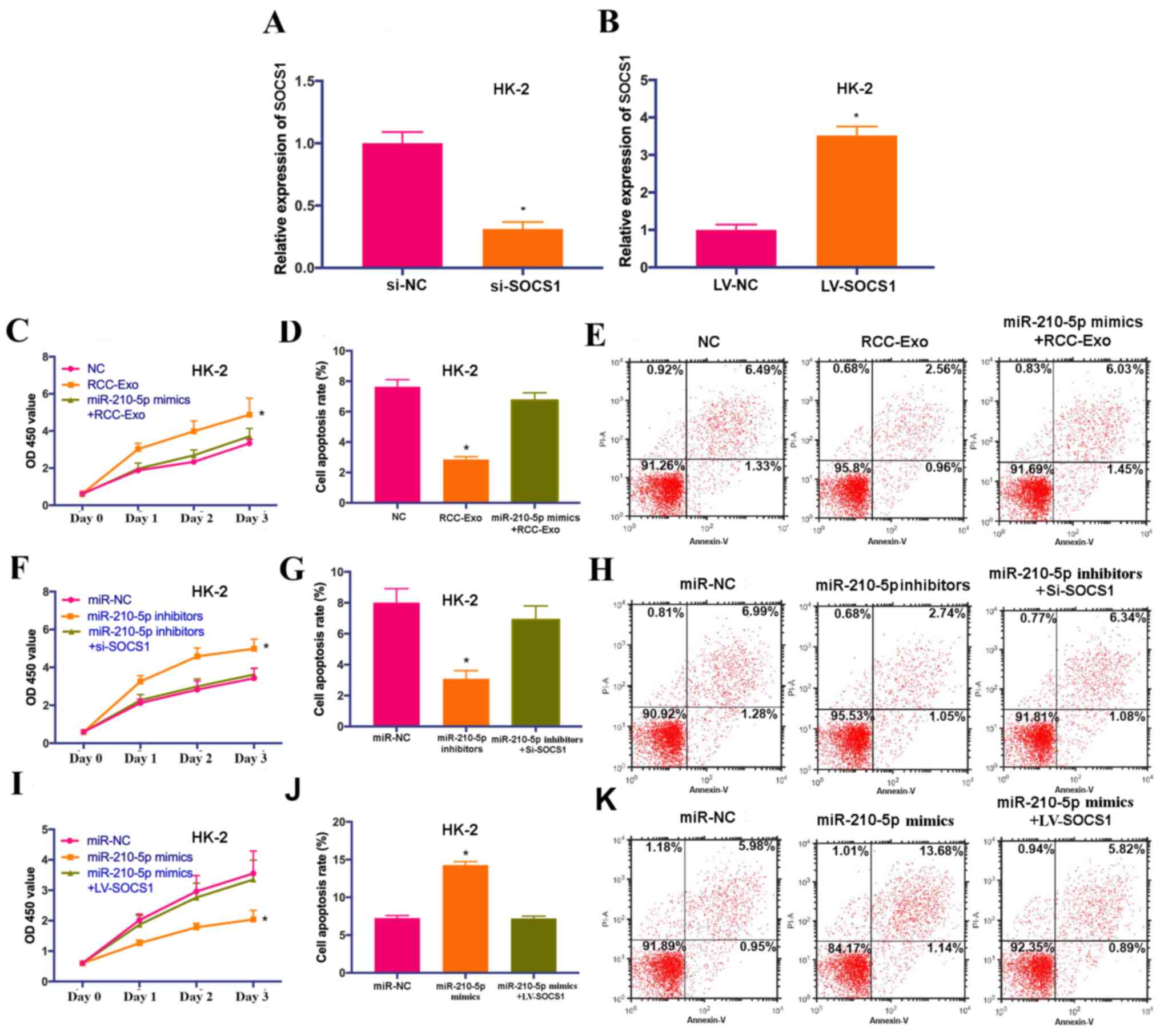
circ_400068 regulates the
proliferation of HK-2 cells by targeting the miR-210-5p/SOCS1
axis
To investigate the involvement of miR-210-5p and
SOCS1 in exosomal circ_400068-modulated biological feature changes
in HK-2 cells, the cells were co-treated with miR-210-5p mimics and
RCC-Exo, miR-210-5p mimics and LV-SOCS1 or miR-210-5p inhibitors
and si-SOCS1. The transfection efficiencies of si-SOCS1 and
LV-SOCS1 were confirmed via RT-qPCR (Fig. 5A and B). The data suggested that
the effects on cell proliferation and apoptosis caused by RCC-Exo
were significantly abolished by miR-210-5p mimics (Fig. 5C-E). Additionally, promoted cell
proliferation caused by miR-210-5p inhibitors was significantly
attenuated by the knockdown of SOCS1, whereas the influences on
cell proliferation and apoptosis triggered by miR-210-5p mimics
were significantly reversed by SOCS1 overexpression (Fig. 5F-K). Collectively, these findings
suggested that circ_400068 may regulate the proliferation of HK-2
cells via the miR-210-5p/SOCS1 signaling pathway. It was also
indicated that exosomal circ_400068 originated from the tumor has
potential as a putative non-invasive biomarker for the prognosis of
RCC, and it may affect the proliferation of healthy kidney cells by
targeting the miR-210/SOCS1 axis.
Discussion
RCC is the most prevalent kidney cancer type in
adults (1). Although the
diagnostic and therapeutic methods have been improved for RCC, it
remains difficult to distinguish benign and malignant tumors
(2). The pathogenesis of RCC is
complex, and the detailed mechanisms are largely unknown.
circRNAs are potential gene regulators, which can
act as miRNA ‘sponges’ (12).
Impaired circRNAs expression levels are associated with the onset
and development of nervous system disorders and cancer (13–15).
During the progression of a tumor, crosstalk between cancer cells
and adjacent healthy cells is crucial, and exosomes produced by
malignant cells serve essential roles during this process (16,19).
Previous studies have reported that exosomal circRNAs are novel
regulators of gene expression (20–22).
For instance, circRNAs are transferred into exosomes and secreted
by colon cancer cells (23).
In the present study, the circRNAs expression array
data suggested that circ_400068 was significantly upregulated in
RCC exosomes. Further functional studies were performed to
investigate the effects of exosomal circ_400068 produced by RCC
cells on the proliferation of healthy kidney cells. Treatment with
RCC-Exo promoted the proliferation of healthy kidney cells, while
cell apoptosis was significantly inhibited. In order to examine the
detailed function of exosomal circ_400068 in tumorigenesis, its
downstream molecules were predicted, and it was identified that a
novel signaling pathway involving a circ_400068/miR-210-5p/SOCS1
axis may regulate the proliferation of kidney cells. miR-210 is
involved in numerous biological processes during the development of
cancer, such as apoptosis, angiogenesis and DNA damage response,
and it is considered as potential biomarker and therapeutic target
in numerous types of tumors, including RCC (26–30).
In line with the present findings, the tumor-promoting role of
SOCS1 and its detailed regulatory functions in colorectal cancer
cells via the downstream molecules E-cad, p53 and STAT1 have been
revealed in a previous report (31).
However, there are limitations to the present study.
For instance, the effects of sh-circ_400068 on the proliferation
and apoptosis of RCC cells in vivo could be evaluated in
future studies. Furthermore, whether circ_400068 regulates SOCS1 in
a miR-210-5p-dependent manner could be investigated.
In conclusion, the present study demonstrated that
exosomal circ_400068 was upregulated in the culture media of RCC
cells, and circ_400068 could be a novel oncogenic factor. The
crosstalk of RCC cells and adjacent healthy kidney cells may be
crucial during tumor progression, and exosomal circ_400048 secreted
by RCC cells could serve essential roles during this process. Thus,
this novel circ_400068/miR-210-5p/SOCS1 axis could be a therapeutic
candidate for the treatment of RCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Fund Program of Liaoning Province (grant no.
20180530058).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS designed this study. HX and JS performed the
experiments and conducted the data analysis. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The First Affiliated Hospital of Jinzhou Medical
University. Written informed consents were signed by all
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dabestani S, Beisland C, Stewart GD,
Bensalah K, Gudmundsson E, Lam TB, Gietzmann W, Zakikhani P,
Marconi L, Fernandéz-Pello S, et al: Intensive Imaging-based
follow-up of surgically treated localised renal cell carcinoma does
not improve Post-recurrence survival: Results from a European
multicentre database (RECUR). Eur Urol. 75:261–264. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheth S, Scatarige JC, Horton KM, Corl FM
and Fishman EK: Current concepts in the diagnosis and management of
renal cell carcinoma: Role of multidetector ct and
Three-dimensional CT. Radiographics. 21 (Suppl):S237–S254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–1365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elfiky AA, Aziz SA, Conrad PJ, Siddiqui S,
Hackl W, Maira M, Robert CL and Kluger HM: Characterization and
targeting of phosphatidylinositol-3 kinase (PI3K) and mammalian
target of rapamycin (mTOR) in renal cell cancer. J Transl Med.
9:1332011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Cristofano C, Minervini A, Menicagli M,
Salinitri G, Bertacca G, Pefanis G, Masieri L, Lessi F, Collecchi
P, Minervini R, et al: Nuclear expression of hypoxia-inducible
factor-1alpha in clear cell renal cell carcinoma is involved in
tumor progression. Am J Surg Pathol. 31:1875–1881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Wang Y, Song Y, Bu R, Yin B, Fei X,
Guo Q and Wu B: Expression profiling and clinicopathological
significance of DNA methyltransferase 1, 3A and 3B in sporadic
human renal cell carcinoma. Int J Clin Exp Pathol. 7:7597–609.
2014.PubMed/NCBI
|
|
8
|
Shang D, Liu Y, Ito N, Kamoto T and Ogawa
O: Defective Jak-Stat activation in renal cell carcinoma is
associated with interferon-alpha resistance. Cancer Sci.
98:1259–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von Schulz-Hausmann S, Schmeel L, Schmeel
F and Schmidt-Wolf I: Targeting the Wnt/beta-catenin pathway in
renal cell carcinoma. Anticancer Res. 34:4101–4108. 2014.PubMed/NCBI
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vicens Q and Westhof E: Biogenesis of
circular RNAs. Cell. 159:13–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conn S, Pillman KA, Toubia J, Conn VM,
Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA and
Goodall GJ: The RNA binding protein quaking regulates formation of
circRNAs. Cell. 160:1125–1134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang
Q, Zhang P, Xiong Z, He C, Huang Z, et al: ZKSCAN1 gene and its
related circular RNA (circZKSCAN1) both inhibit hepatocellular
carcinoma cell growth, migration, and invasion but through
different signaling pathways. Mol Oncol. 11:422–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tlsty T and Coussens L: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–759. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keller S, Sanderson M, Stoeck A and
Altevogt P: Exosomes: From biogenesis and secretion to biological
function. Immunol. Lett. 107:102–108. 2006.
|
|
19
|
Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X,
Shi L, Lu X, Xu W, Lu L, et al: STAT3-regulated exosomal miR-21
promotes angiogenesis and is involved in neoplastic processes of
transformed human bronchial epithelial cells. Cancer Lett.
370:125–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilusz J and Sharp P: Molecular biology. A
circuitous route tononcoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang PF, Wei CY, Huang XY, Peng R, Yang
X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, et al: Circular RNA
circTRIM33-12 acts as the sponge of microRNA-191 to suppress
hepatocellular carcinoma progression. Mol Cancer. 18:1052019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dou Y, Cha DJ, Franklin JL, Higginbotham
JN, Jeppesen DK, Weaver AM, Prasad N, Levy S, Coffey RJ, Patton JG
and Zhang B: Circular RNAs are Down-regulated in KRAS mutant colon
cancer cells and can be transferred to exosomes. Sci Rep.
6:379822016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee J and Lötvall J: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao A, Li G, Péoc'h M, Genin C and
Gigante M: Serum miR-210 as a novel biomarker for molecular
diagnosis of clear cell renal cell carcinoma. Exp Mol Pathol.
94:115–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa
P and Gui Y: Synthetic miRNA-mowers targeting miR-183-96-182
cluster or miR-210 inhibit growth and migration and induce
apoptosis in bladder cancer cells. PLoS One. 7:e522802012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakada C, Tsukamoto Y, Matsuura K, Nguyen
TL, Hijiya N, Uchida T, Sato F, Mimata H, Seto M and Moriyama M:
Overexpression of miR-210, a downstream target of HIF1a, causes
centrosome amplification in renal carcinoma cells. J Pathol.
224:280–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Favaro E, Ramachandran A, McCormick R, Gee
H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, et al:
MicroRNA-210 regulates mitochondrial free radical response to
hypoxia and Krebs cycle in cancer cells by targeting iron sulfur
cluster protein ISCU. PLoS One. 5:e103452010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen WY, Liu WJ, Zhao YP, Zhou L, Zhang
TP, Chen G and Shu H: Induction, modulation and potential targets
of miR-210 in pancreatic cancer cells. Hepatobiliary Pancreat Dis
Int. 11:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tobelaim W, Beaurivage C, Champagne A,
Pomerleau V, Simoneau A, Chababi W, Yeganeh M, Thibault P, Klinck
R, Carrier JC, et al: Tumour-promoting role of SOCS1 in colorectal
cancer cells. Sci Rep. 5:143012015. View Article : Google Scholar : PubMed/NCBI
|