Introduction
Spinal cord injury (SCI) affects millions of
individuals worldwide (1), with
between 16 and 19.4 new cases per million annually in Western
European countries (2), and
characteristically causes life-long neurological consequences
(different degrees of paralysis and sensory impairment of limbs and
trunk) with substantial socioeconomic implications (3). SCI is divided into primary injury and
secondary injury; primary injury refers to the initial physical
damage of the spinal cord caused by an indirect or direct external
force, while the secondary injury is characterized by a series of
physiological and pathological changes to the spinal cord,
including inflammation, oxidative stress, necrosis and neuronal
apoptosis on the basis of the primary damage, further deepening and
expanding the degree and scope of the damage (4–6). The
secondary injury is largely responsible for the neurological
dysfunction associated with SCI. The current therapeutic strategies
for SCI include surgical intervention (grafts and bridges), neural
stem cell transplantation and the administration of high-dose
methylprednisolone (7,8), the neurological recovery remains
limited since there is no consensus about the beneficial effects.
Both molecular therapies (modulation of inflammatory response and
administration of growth-stimulating factors), rehabilitative
training and combinatorial therapies (tissue engineering and
searching for synergistic effects) have raised hopes in developing
novel therapies for attenuating secondary damage (9). Previous studies have demonstrated
that the pathophysiological processes of SCI involved neuronal
inflammation, oxidative stress, neuronal degeneration and
apoptosis, as well as reactive changes in the glia (10–13).
Therefore, further investigations into the pathogenesis may improve
the current understanding of the molecular mechanisms of SCI.
The small-molecule protein sulfiredoxin-1 (SRX1), a
conserved endogenous antioxidative protein, is found widely
distributed in eukaryotes (14).
It was previously reported that SRX1 served an important role in
maintaining the pulmonary antioxidative defense against cigarette
smoke via nuclear factor erythroid-2-related factor 2
(NRF2)-dependent transcriptional regulation (15). SRX1 was also identified to have a
crucial role in cellular damage triggered by oxidative stress
(16,17). Moreover, SRX1 protected cardiac
progenitor cells from oxidative stress and promoted their survival
via ERK/NRF2 signaling (18).
Another study also illustrated the cardioprotective effect of SRX1
via the inhibition of mitochondrial apoptosis through the PI3K/AKT
signaling pathway (19). However,
to the best of our knowledge, the effects of SRX1 on nerve damage
in SCI remain poorly understood. Therefore, the present study aimed
to determine whether the functions of SRX1 in SCI were associated
with oxidative stress and inflammation. In addition, the roles of
NRF2 and the downstream target genes were investigated.
Materials and methods
Establishment of SCI model rats
All experiments were approved by the Institutional
Animal Ethics Committees of University of South China (Hengyang,
China) and were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (NIH Publications no. 8023, revised 1978). In total, 32
male Sprague Dawley rats (6 weeks old, 200–220 g) were purchased
from the Central South University Experiment Center. The animals
were provided with free access to food and water, and maintained in
standardized conditions with a 12-h light/dark cycle, at a
temperature of 22±2°C and humidity of 55±10%. Following one week of
adaptive feeding, all rats received thoracic laminectomies at the
7, 8 and 9th thoracic vertebrae under aseptic conditions; 5%
isoflurane was used to induce anesthesia and rats were maintained
at a surgical plane by continuous inhalation of 2% isoflurane for
the duration of surgery. Subsequently, the rats were randomly
divided into two groups (16 rats per group): Sham and model. The
model group was established by the clip compression technique as
previously described (20,21) and the wound was sutured. The sham
group contained rats who underwent a laminectomy without clip
compression.
During the 8 weeks following surgery, the animal
health and behavior were monitored and recorded once a day
(behavioral assessment data not disclosed). In the sham group, two
rats died within 3 days of the sham operation and one died within 8
to 14 days; no deaths were recorded after 2 weeks. In the model
group, two rats died within 3 days following spinal trauma, none
died from day 4 to day 7, three died on day 8 to day 14 and one
died from day 15 to day 30; no deaths were reported after 31 days
(Table SI). Therefore, there were
10 rats in the model group and 13 rats in the sham group. Autopsy
was performed to identify the cause of death. Subsequently, 8 weeks
after surgery, rats were euthanized through inhalation of 5%
isoflurane until respiration ceased (within 5 min) and decapitation
was used as a secondary method of euthanasia to ensure a humane
death. Spinal cord tissues were then extracted after confirming
cardiac arrest. The following humane endpoints were adhered to
through the study: Emaciation, lethargy, abdominal swelling and
self-mutilation.
Histological analysis
Spinal cord tissues were fixed in 4% formaldehyde at
4°C for 24 h and embedded in paraffin. The paraffin-embedded
tissues were cut into 5-µm thick sections and stained with
hematoxylin for 10 min and eosin for 5 min at room temperature
(H&E).
Spinal cord tissues were fixed in 4% formaldehyde at
4°C for 24 h and embedded in paraffin. A Nissl staining kit
(Beijing Solarbio Science & Technology Co., Ltd.) was used to
stain 8-µm thick spinal cord sections, according to the
manufacturer's instructions. Tissues sections were observed under a
light microscope (magnification, ×200).
Cell culture and treatments
Rat pheochromocytoma PC12 cells (China Center for
Type Culture Collection) were cultured in DMEM (Hyclone; Cytiva),
supplemented with 10% FBS (Hyclone; Cytiva), and maintained at 37°C
under humid conditions with 5% CO2 and 95% air. For
differentiation, PC12 cells were treated with 50 ng/ml nerve growth
factor (Sigma-Aldrich; Merck KGaA) every other day for 6 days at
37°C, which is a widely used method for the in vitro study
of nervous system diseases, including SCI (22–25).
To investigate the function of NRF2 in the
differentiated PC12 cells, the cells were pretreated with the NRF2
activator tert-butylhydroquinone (25 µM, TBHQ; Sigma-Aldrich; Merck
KGaA) or inhibitor ML385 (5 µM; MedChemExpress) for 24 h prior to
the experiments (26–28), then PC12 cells were exposed to 5
µg/ml lipopolysaccharide (LPS; MedChemExpress) for 18 h.
Cell transfection
PC12 cells were seeded into a 6-well plate
(5×105 cells per well). At a confluence of 60%, 8 µg
SRX1 overexpression (Ov) pEX-1 plasmid (Ov-SRX1; Shanghai
GenePharma Co., Ltd.) or an empty control pEX-1 plasmid (Ov-NC) was
transfected into cells using 5 µl Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). PC12 cells were
transfected with Ov-SRX1 or Ov-NC according to the manufacturer's
protocol. After successful transfection, cells were used in
subsequent experiments.
Cell viability assay
The viability of PC12 cells was analyzed using a
Cell Counting Kit-8 (CCK-8) assay (MedChemExpress), according to
the manufacturer's protocols. Briefly, cells at a density of
5×103 cells/ml were plated into a 96-well plate (100 µl
per well) and treated with 0.625, 1.25, 2.5 and 5 µg/ml LPS for 18
h. Subsequently, 10 µl CCK-8 solution was added/well and incubated
for 4 h. Following the incubation, the absorbance was measured at a
wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Western blotting
Total protein was extracted from spinal cord tissues
or PC12 cells using RIPA lysis buffer (Beyotime Institution of
Biotechnology), while nuclear protein was extracted using a Nuclear
and Cytoplasmic Extraction kit (Beyotime Institute of
Biotechnology). Total protein was quantified using a bicinchoninic
acid assay kit (Abcam) and 25 µg protein was separated via 10%
SDS-PAGE. The separated proteins were subsequently transferred onto
PVDF membranes (EMD Millipore) and then membranes were blocked with
5% BSA Blocking Buffer (Beijing Solarbio Science & Technology
Co., Ltd.). The membranes were then incubated with the following
primary antibodies at 4°C overnight: Anti-SRX1 (cat. no. ab203613;
1:1,000; Abcam), anti-peroxiredoxin (PRDX)1 (cat. no. ab15571;
1:1,000; Abcam), anti-PRDX6 (cat. no. ab59543; 1:1,000; Abcam),
anti-thioredoxin reductase (TXNRD) 1 (cat. no. ab16840; 1:1000;
Abcam), anti-superoxide dismutase (SOD)2 (cat. no. ab13534;
1:1,000; Abcam), anti-NRF2 (cat. no. ab89443; 1:1,000; Abcam),
anti-NAD(P)H dehydrogenase quinone (NQO) 1 (cat. no. ab28947;
1:1,000; Abcam), anti-heme oxygenase 1 (cat. no. ab13248; 1:1,000;
HO-1; Abcam), anti-GAPDH (cat. no. ab181603; 1:1,000; Abcam) and
anti-Lamin B1 (cat. no. ab16048; 1:1,000; Abcam). Following the
primary antibody incubation, the membranes were washed with TBS
with Tween-20 (0.05%) and incubated with a goat anti-mouse IgG
HRP-conjugated secondary antibody (cat. no. 31430; 1:10,000; Thermo
Fisher Scientific, Inc.) or a goat anti-rabbit IgG HRP-conjugated
secondary antibody (cat. no. 31460; 1:10,000; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. Protein bands were
visualized using Immobilon Crescendo Western HRP substrate (cat.
no. WBLUR0100; Merck KGaA) and the expression levels were
semi-quantified using ImageJ v1.8.0 software (National Institutes
of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the spinal cord tissues
or PC12 cells using TRI reagent® (Sigma-Aldrich; Merck
KGaA). The reaction templates in PrimeScript™ RT reagent kit
(Takara Bio, Inc.) were added into the tube according to the
manufacturer's protocol, centrifuged for 5 sec (500 × g) at room
temperature and total RNA was reversed transcribed into cDNA at
37°C for 15 min and 85°C for 5 sec. qPCR was subsequently performed
using a SYBR® Premix Ex Taq II kit (Takara Bio, Inc.),
and the following thermocycling conditions were used: One cycle of
95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
The following primers sequences were used for the qPCR: SRX1
forward, 5′-AATCCCCAACCCCTGACTTT-3′ and reverse,
5′-AATCCCCAACCCCTGACTTT-3′; NQO1 forward,
5′-GCGTCTGGAGACTGTCTGGG-3′ and reverse, 5′-CGGCTGGAATGGACTTGC-3′;
HO-1 forward, 5′-GCGAAACAAGCAGAACCCA-3′ and reverse,
5′-GCTCAGGATGAGTACCTCCCA-3′; and GAPDH forward,
5′-TGGCCTCCAAGGAGTAAGAAAC-3′ and reverse,
5′-GGCCTCTCTCTTGCTCTCAGTATC-3′. The expression levels were
calculated using the 2−∆∆Cq method and normalized to the
loading control GAPDH (29).
Determination of malondialdehyde (MDA)
content, and the levels of SOD, reactive oxygen species (ROS) and
inflammatory cytokines
The cell medium of PC12 cells was collected and
centrifugated at 500 × g for 5 min at room temperature. The
concentrations of tumor necrosis factor (TNF)-α (cat. no. PT516;
Beyotime Institute of Biotechnology), interleukin (IL)-1β (cat. no.
PI303; Beyotime Institute of Biotechnology), IL-18 (cat. no.
SEKR-0054; Beijing Solarbio Science & Technology Co., Ltd.) and
IL-10 (cat. no. PI525; Beyotime Institute of Biotechnology) in the
medium supernatants were analyzed using ELISA kits. MDA and SOD in
the cellular supernatants were analyzed using commercial detection
kits (Beyotime Institute of Biotechnology), according to the
manufacturers' protocols. PC12 cells (2×105) were seeded
into a 24-well plate, and loaded with 10 µM
2′,7′-dichlorofluorescin diacetate (DCFH-DA; Invitrogen; Thermo
Fisher Scientific, Inc.) for 20 min in the dark at room
temperature. Intracellular ROS production was observed under a
fluorescence microscope (magnification, ×100; Olympus Corporation)
and analyzed using ImageJ v1.8.0 software (National Institutes of
Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.) and data was presented
as the mean ± SD. Each experiment was repeated three times.
Statistical differences between groups were determined using a
one-way ANOVA, followed by a Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
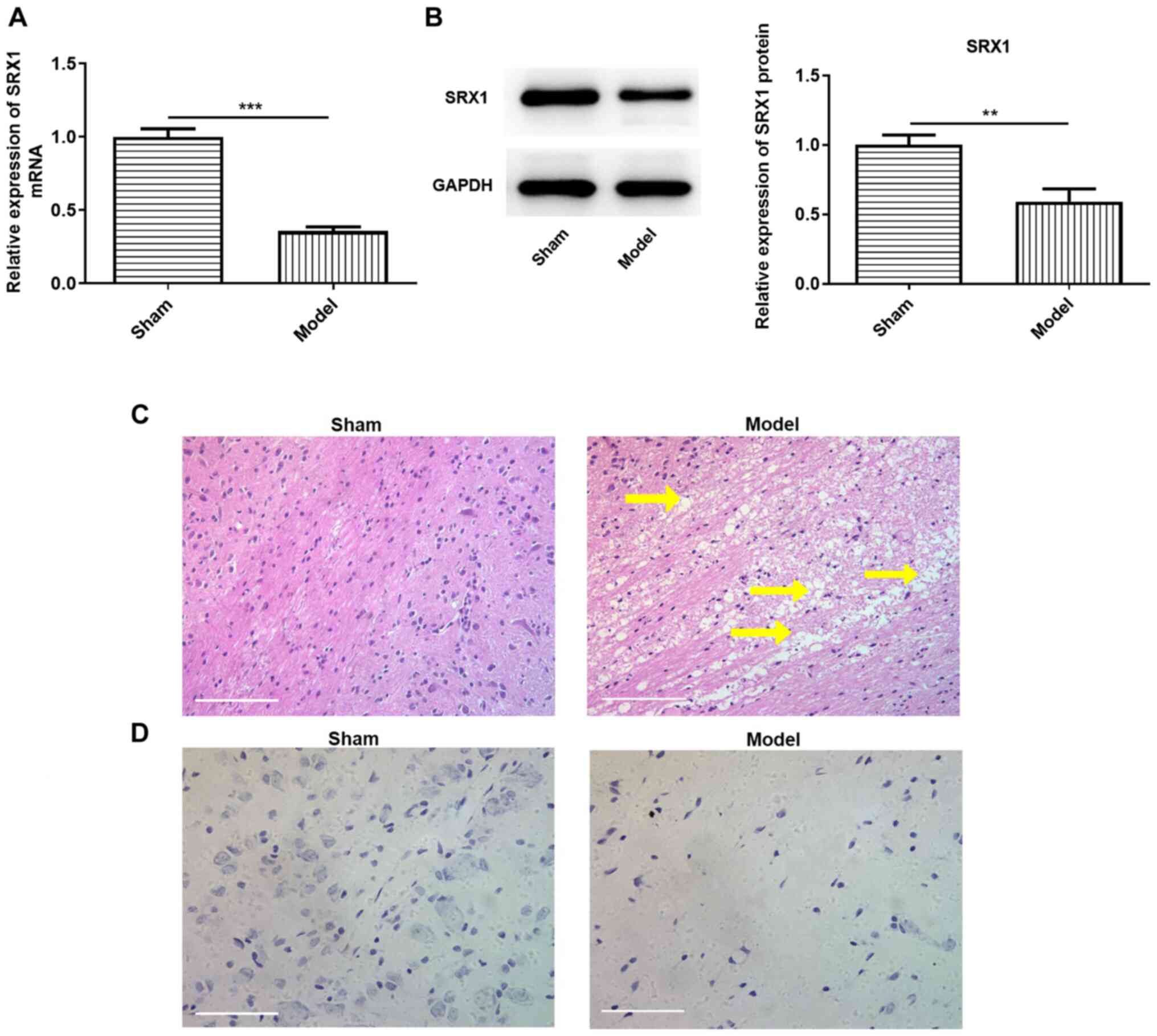
Expression levels of SRX1 are
downregulated in SCI model rats
SCI model rats were established as previously
reported (20,21). The pathological changes in the
spinal cord tissues and the expression levels of SRX1 were
subsequently investigated. The expression levels of SRX1 in the
model group were significantly downregulated at the mRNA and
protein level compared with the sham group (Fig. 1A and B). H&E staining also
revealed numerous irregular cavities in the injured spinal cords of
the model group compared with the sham group (Fig. 1C). In addition, Nissl bodies in the
sham group were larger in shape and more abundant compared with
those in the SCI model group, indicating a weaker protein synthesis
ability of Nissl bodies in the SCI model rats (Fig. 1D). Collectively, these results
suggested that the rat model of SCI was successfully established,
and that the expression levels of SRX1 may be downregulated in
damaged spinal cord tissues.
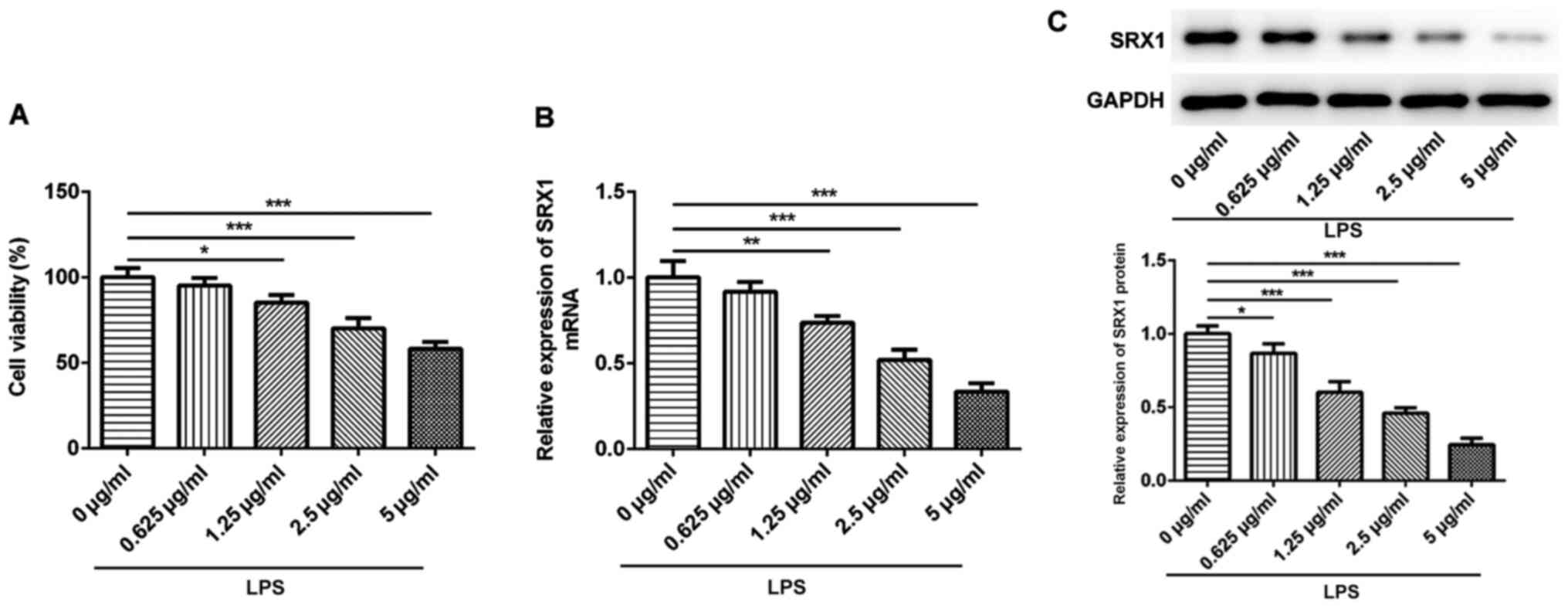
SRX1 expression levels are
downregulated in PC12 cells stimulated with LPS
Subsequently, in vitro experiments were
performed using the PC12 cell line, a common cell model used for
researching neurobiological events, including SCI (22–25).
A range of concentrations of LPS (0.625–5 µg/ml) were used to
stimulate PC12 cells for 18 h as previously described (26) and the viability of PC12 cells was
subsequently determined. The results revealed that 1.25, 2.5 and 5
µg/ml LPS significantly impaired the viability of the PC12 cells
compared with the control cells (Fig.
2A). Subsequently, SRX1 expression levels were analyzed
following the stimulation with LPS; the expression levels of SRX1
were downregulated by LPS in a dose-dependent manner at both the
protein and mRNA level (Fig. 2B and
C). These results suggested that SRX1 expression levels may be
downregulated in PC12 cells challenged with LPS.
Overexpression of SRX1 inhibits the
inflammatory response in PC12 cells
To determine the association between LPS-induced
inflammation and SRX1 in PC12 cells, Ov-SRX1 was constructed and
transfected into PC12 cells; a significant upregulation in the mRNA
expression levels of SRX1 were identified after the cells were
transfected with Ov-SRX1 compared with the control and Ov-NC groups
(Fig. 3A), indicating that the
transfection with the overexpression plasmid was successful. Cell
viability was significantly impaired after stimulation with 5 µg/ml
LPS, so 5 µg/ml LPS was chosen for subsequent experiments. There
was a significant increase in the expression of SRX1 mRNA and
protein after cells were transfected with Ov-SRX1 (Fig. 3B and C). In addition, the results
of the CCK-8 assay revealed that the Ov-SRX1 plasmid significantly
increased the viability of LPS-induced cells compared with cells
treated with LPS only (Fig.
3D).
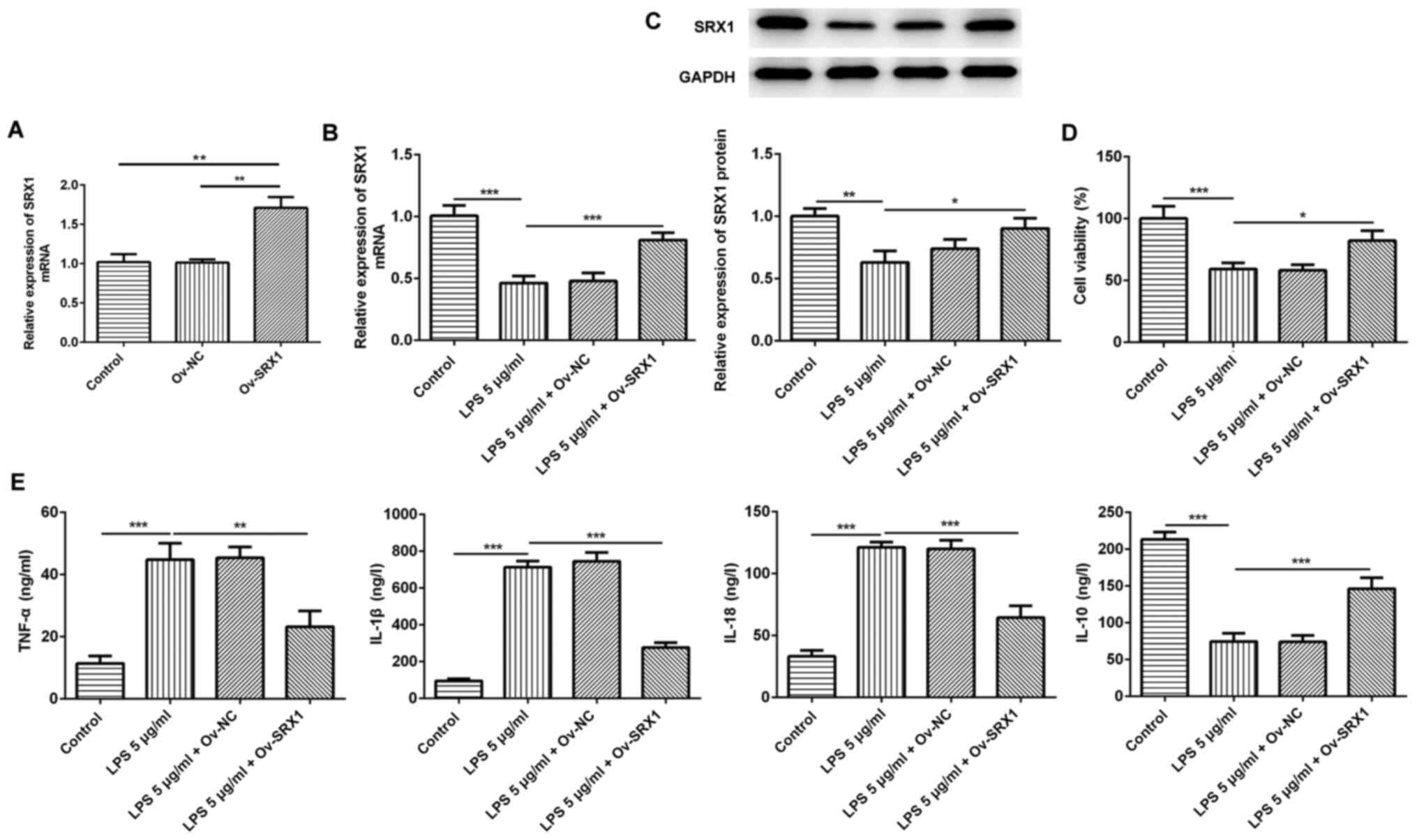 | Figure 3.Overexpression of SRX1 reduces the
inflammatory response in PC12 cells. (A) Transfection efficiency of
Ov-SRX1 in PC12 cells was determined using RT-qPCR. (B) mRNA and
(C) protein expression levels of SRX1 in PC12 cells transfected
with Ov-SRX1 and stimulated with 5 µg/ml LPS were determined using
RT-qPCR and western blotting, respectively. (D) Cell Counting Kit-8
assay was used to determine the cell viability of PC12 cells
transfected with Ov-SRX1 and stimulated with 5 µg/ml LPS. (E)
TNF-α, IL-1β, IL-18 and IL-10 levels in PC12 cells transfected with
Ov-SRX1 and stimulated with 5 µg/ml LPS were detected using ELISA
kits. Data are expressed as the mean ± SD from 3 independent
experiments. *P<0.05, **P<0.01, ***P<0.001. SRX1,
sulfiredoxin-1; LPS, lipopolysaccharide; Ov, overexpression; NC,
negative control; RT-qPCR, reverse transcription-quantitative PCR;
TNF-α, tumor necrosis factor α; IL, interleukin. |
Subsequently, the levels of inflammatory cytokines
secreted by PC12 cells following the overexpression of SRX1 and LPS
stimulation were analyzed. The levels of the proinflammatory
factors, TNF-α, IL-1β and IL-18, were all significantly increased
following the stimulation of LPS compared with the control group,
while the simultaneous transfection of cells with Ov-SRX1 reversed
this effect (Fig. 3E). Conversely,
the secretion of IL-10 was significantly decreased following LPS
treatment compared with the control group, while the simultaneous
transfection with Ov-SRX1 significantly increased the secretion of
IL-10 compared with the LPS treatment group (Fig. 3E). Taken together, these results
indicated that the inflammatory response in PC12 cells may be
attenuated by the overexpression of SRX1.
Overexpression of SRX1 reduces
oxidative stress in PC12 cells
The activation of the inflammatory response results
in the production of ROS, which further exacerbates inflammation
and leads to tissue damage (30).
To investigate the antioxidative role of SRX1 in damaged
neuron-like cells, the intracellular production of ROS was
determined using the fluorescent probe DCFH-DA. Following the
treatment of the cells with 5 µg/ml LPS, the levels of ROS were
significantly increased compared with the control group, whereas
this effect was significantly attenuated following the
overexpression of SRX1 (Fig. 4A).
Furthermore, the MDA content and SOD activity were also analyzed;
LPS treatment significantly increased the generation of MDA
compared with the control group; however, this effect was
significantly impeded by the overexpression of SRX1 (Fig. 4B). The overexpression of SRX1 also
significantly reversed the inhibition over SOD activity induced by
LPS (Fig. 4C). The expression
levels of PRDX1, PRDX6, TXNRD1 and SOD2 were significantly
downregulated in LPS-stimulated cells compared with the control
group; however, a significant upregulation in the expression levels
of these antioxidative proteins was observed in cells transfected
with the Ov-SRX1 plasmid and stimulated with LPS compared with LPS
treatment alone (Fig. 4D).
Altogether, these findings suggested that SRX1 may exert an
antioxidative role in injured PC12 cells.
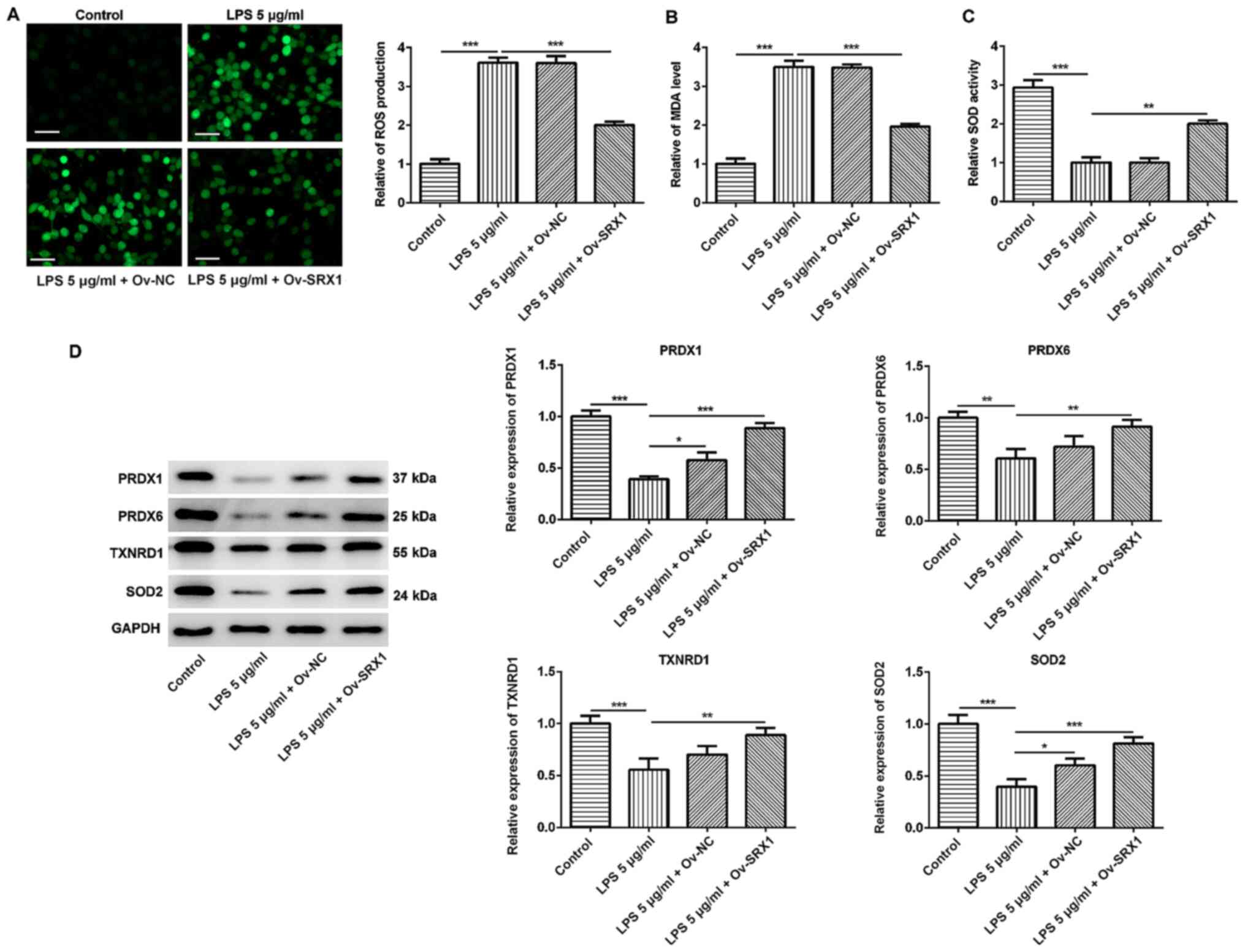 | Figure 4.Overexpression of SRX1 relieves
oxidative stress in PC12 cells. (A) Intracellular ROS in PC12 cells
transfected with Ov-SRX1 and stimulated with 5 µg/ml LPS was
stained using 2′,7′-dichlorofluorescin. Scale bar, 50 µm. (B) MDA
content and (C) SOD activity in PC12 cells transfected with Ov-SRX1
and stimulated with 5 µg/ml LPS were analyzed using commercial
detection kits. (D) Protein expression levels of antioxidative
proteins PRDX1, PRDX6, TXNRD1 and SOD2 in PC12 cells transfected
with Ov-SRX1 and stimulated with 5 µg/ml LPS were analyzed using
western blotting. Data are expressed as the mean ± SD from 3
independent experiments. *P<0.05, **P<0.01, ***P<0.001.
SRX1, sulfiredoxin-1; LPS, lipopolysaccharide; Ov, overexpression;
NC, negative control; ROS, reactive oxygen species; MDA,
malondialdehyde; SOD, superoxide dismutase; PRDX, peroxiredoxin;
TXNRD1, thioredoxin reductase 1. |
NRF2 controls the expression levels of
downstream target genes and SRX1
A previous study indicated that SRX1 exerted an
antioxidative role, which relied on the activation of NRF2. SRX1
was discovered to protect neurons from ischemia/reperfusion-induced
oxidative stress injury, which was regulated by NRF2 (31). The expression levels of NRF2 and
its downstream target proteins NQO1 and HO-1 were all significantly
downregulated in PC12 cells exposed to LPS compared with the
control group (Fig. 5A),
suggesting that NRF2 may participate in the neuronal damage
triggered by LPS.
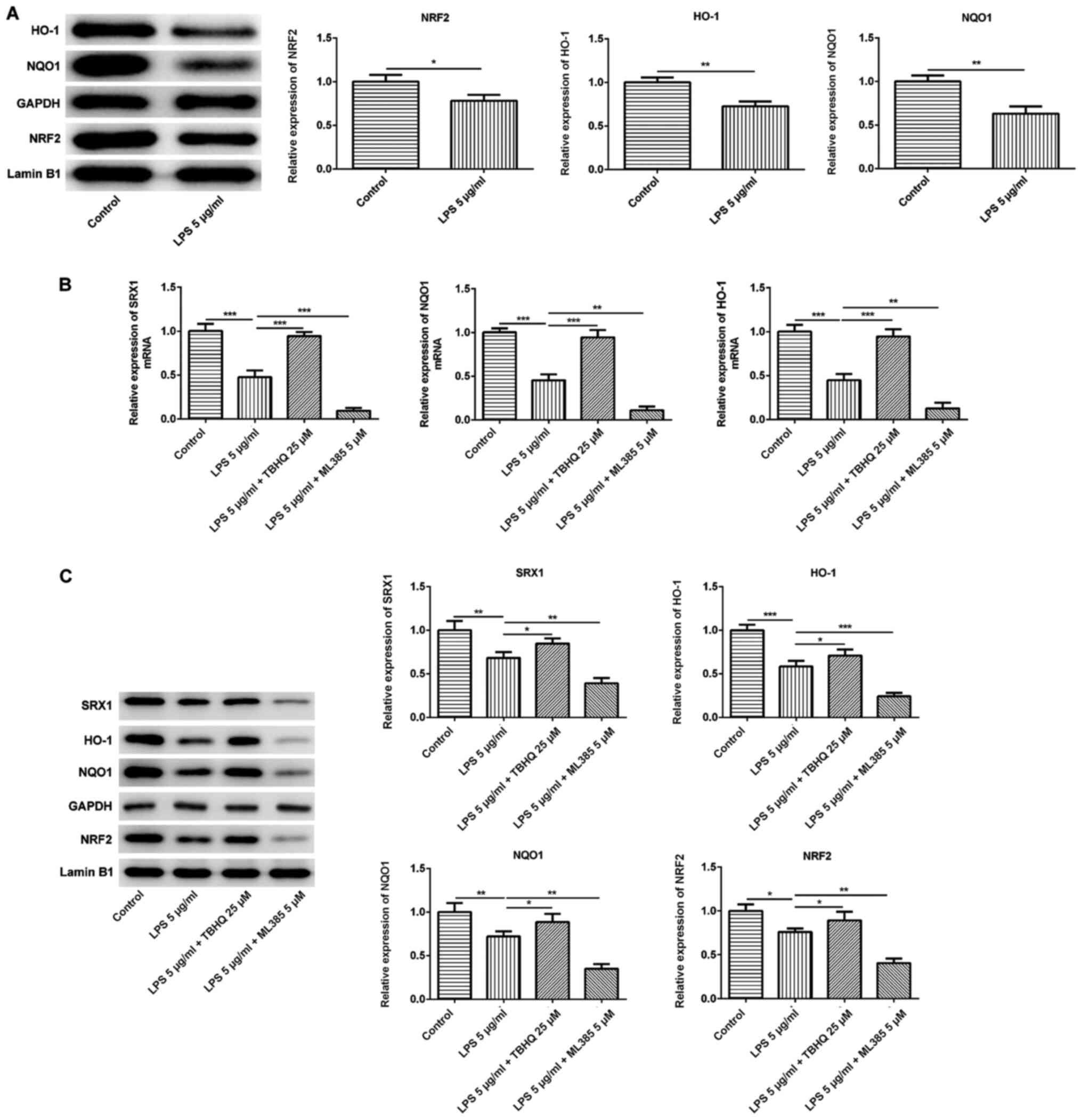 | Figure 5.NRF2 controls the expression levels
of downstream target genes and SRX1. (A) Expression levels of
nuclear NRF2 and downstream target genes NQO1 and HO-1 in PC12
cells stimulated with 5 µg/ml LPS were analyzed using western
blotting. (B) mRNA expression levels of SRX1, NQO1 and HO-1 in PC12
cells stimulated with 5 µg/ml LPS with or without 25 µM TBHQ or 5
µM ML385 treatment were determined using reverse
transcription-quantitative PCR. (C) Western blotting analysis was
performed to analyze the expression levels of nuclear NRF2, NQO1,
HO-1 and SRX1 in PC12 cells stimulated with 5 µg/ml LPS with or
without 25 µM TBHQ or 5 µM ML385 treatment. Data are expressed as
the mean ± SD from 3 independent experiments. *P<0.05,
**P<0.01, ***P<0.001. SRX1, sulfiredoxin-1; LPS,
lipopolysaccharide; Ov, overexpression; NC, negative control; NRF2,
nuclear factor erythroid-2-related factor 2; HO-1, heme oxygenase
1; NQO1, NAD(P)H dehydrogenase quinone 1; TBHQ,
tert-butylhydroquinone. |
Subsequently, the NRF2 inducer, TBHQ, and inhibitor,
ML385, were used to confirm the regulatory effects of NRF2 on SRX1.
The mRNA expression levels of SRX1, NQO1 and HO-1 were then
analyzed using RT-qPCR; TBHQ significantly reversed the suppressive
effects of LPS on the expression levels of SRX1, NQO1 and HO-1,
while ML385 enhanced the inhibitory effects of LPS treatment
(Fig. 5B). In addition, the
protein expression levels of SRX1, NQO1 and HO-1, as well as
nuclear NRF2, were significantly upregulated following the
co-treatment of TBHQ and LPS compared to LPS treatment alone
(Fig. 5C). However, ML385
treatment further downregulated the protein expression levels of
SRX1, NQO1, HO-1 and NRF2 in PC12 cells exposed to LPS compared
with LPS treatment alone. Overall, these findings suggested that
the alleviation of the inflammatory response and oxidative stress
by SRX1 may be modulated by NRF2.
Discussion
SCI is the most serious complication of spinal
injury and often leads to severe dysfunction of the limb below the
injury segment, which brings devastating physical and psychological
harm to patients and imposes huge economic burdens to society
(32). Currently, there are
>1,000,000 patients with SCI in the United States and >12,000
new cases annually (33).
Therefore, it remains an urgent requirement to determine effective
therapeutic strategies for SCI. Inflammation and oxidative stress
in neuron-like cells are commonly considered pathological
alterations of SCI (10,11). However, the precise regulatory
mechanisms of inflammation and oxidative stress in SCI remain
poorly understood.
In the present study, SCI model rats were
established by surgery in an aseptic environment. A large amount of
cavities and decreased Nissl bodies were observed in the injured
spinal cords of the model group compared with the sham group. In
addition, the upregulated expression levels of SRX1 identified in
the spinal cord tissues were of great significance in the present
study. A previous study illustrated that SRX1 protected against
cardiomyocyte injury upon simulated ischemia/reperfusion by
inhibiting mitochondrial apoptosis (19). SRX1 was also discovered to relieve
apoptosis and oxidative stress in primary rat cortical astrocytes
stimulated by oxygen-glucose deprivation or
H2O2, which involved the activation of the
mitochondrial apoptotic pathway (34). However, to the best of our
knowledge, the definite role of SRX1 in SCI has remained
elusive.
LPS, which is composed of lipids and
polysaccharides, is a constituent of the outer cell wall of
Gram-negative bacteria and typically induces oxidative stress and
an inflammatory response in cells (35,36).
In the present study, the mRNA and protein expression levels of
SRX1 were downregulated in PC12 cells exposed to 0.625–5 µg/ml LPS
in a dose-dependent manner. Therefore, to determine the role of
SRX1 in SCI, SRX1 was overexpressed in PC12 cells. The secretion of
the inflammatory cytokines TNF-α, IL-1β and IL-18 was reduced to
differing degrees following the transfection of Ov-SRX1 and LPS
stimulation, while the levels of IL-10 demonstrated the opposite
trend.
In addition, the levels of oxidative stress were
analyzed in PC12 cells transfected with Ov-SRX1 and stimulated with
LPS; ROS production and MDA content were both inhibited following
the overexpression of SRX1 and stimulation of LPS, whereas SOD
activity was increased. ROS scavenging heavily relies on
manganese-dependent SOD, a mitochondrial enzyme encoded by the SOD2
gene (37). PRDX1 and PRDX6 are
members of the PRDX family that reduce the oxidative load and
remove ROS (38,39), thus serving an important role in
the antioxidant processes. In addition, TXNRD1 is an antioxidant
enzyme, which is involved in antioxidant defense and redox
regulation (40). SOD, PRDXs,
glutathione and catalase are major ROS detoxification enzymes
(14). In the current study, the
production of glutathione and catalase was not investigated, thus
future work should aim to investigate this. NRF2 is known to
stimulate anti-inflammatory effects and redox homeostasis,
conferring resistance to oxidative damage induced by exogenous
chemicals and thereby promoting cell survival (41). SRX1 was identified as a pivotal
NRF2-regulated gene responsible for the defense against oxidative
injury in the lung induced by cigarette smoke (15). Another study demonstrated that SRX1
protected human cardiac stem/progenitor cells against oxidative
stress-induced apoptosis through the activation of the ERK/NRF2
signaling pathway (18). NRF2 has
also been reported to activate the antioxidant response
element-dependent gene expression of HO-1, NQO1 and SOD through
evading Kelch-like ECH-associated protein 1 (KEAP1)-mediated
ubiquitination-proteasomal degradation and subsequent nuclear
translocation (42). Thus, the
precise regulatory mechanism between SRX1 and NRF2 in damaged PC12
cells was investigated in the present study. The results indicated
that LPS reduced the expression levels of NRF2 in the nucleus, as
well as the transcription and translation of the downstream target
genes, NQO1 and HO-1. Conversely, the activation of NRF2 with TBHQ
reduced the impact of LPS, while the inhibition of NRF2 with ML385
aggravated the effect caused by LPS, suggesting that the
neuroprotective effect of SRX1 on PC12 cells challenged by LPS may
depend on NRF2.
In conclusion, the findings of the present study
discovered that the expression levels of SRX1 were downregulated in
the injured spinal cord of rats. In addition, the data suggested
that SRX1 may alleviate the inflammatory response and oxidative
stress in neuron-like cells, which was dependent on the nuclear
translocation of NRF2, providing a novel therapeutic target for
nervous system damage. Neuron-like PC12 cells are commonly used to
investigate neuronal damage resulting from spinal cord injury
(22–24,43,44);
however, primary spinal neurons derived from the rat spinal cord
may be more appropriate for the study of SCI and should be used in
further investigations. Moreover, the influence of SRX1-related
drugs on the SCI model rats and the influence of the ubiquitination
of KEAP1 on NRF2 activation should be further studied to determine
the functional recovery of the rats. Further studies addressing
these limitations will help to validate the conclusions of the
present study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZW and ZL made substantial contributions to
conception and design of the study, JO and XS analyzed and
interpreted data, and JL conducted the experiments and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Ethics Committees of University of South China (Hengyang,
China) and were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fakhoury M: Spinal cord injury: Overview
of experimental approaches used to restore locomotor activity. Rev
Neurosci. 26:397–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scivoletto G, Miscusi M, Forcato S,
Ricciardi L, Serrao M, Bellitti R and Raco A: The rehabilitation of
spinal cord injury patients in Europe. Acta Neurochir Suppl.
124:203–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedli L, Rosenzweig ES, Barraud Q,
Schubert M, Dominici N, Awai L, Nielson JL, Musienko P, Nout-Lomas
Y, Zhong H, et al: Pronounced species divergence in corticospinal
tract reorganization and functional recovery after lateralized
spinal cord injury favors primates. Sci Transl Med. 7:302ra1342015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cramer SC, Lastra L, Lacourse MG and Cohen
MJ: Brain motor system function after chronic, complete spinal cord
injury. Brain. 128:2941–2950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26 (Suppl 24):S2–S12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beattie MS: Inflammation and apoptosis:
Linked therapeutic targets in spinal cord injury. Trends Mol Med.
10:580–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin XY, Lai BQ, Zeng X, Che MT, Ling EA,
Wu W and Zeng YS: Cell Transplantation and neuroengineering
approach for spinal cord injury treatment: A summary of current
laboratory findings and review of literature. Cell Transplant.
25:1425–1438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ordikhani F, Sheth S and Zustiak SP:
Polymeric particle-mediated molecular therapies to treat spinal
cord injury. Int J Pharm. 516:71–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva NA, Sousa N, Reis RL and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W,
Lin W, Li Y, Fu H and Li S: Valproic acid attenuates traumatic
spinal cord injury-induced inflammation via STAT1 and NF-κB pathway
dependent of HDAC3. J Neuroinflammation. 15:1502018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin X, Zhu J, Ni H, Rui Q, Sha W, Yang H,
Li D and Chen G: Treatment with 2-BFI attenuated spinal cord injury
by inhibiting oxidative stress and neuronal apoptosis via the Nrf2
signaling pathway. Front Cell Neurosci. 13:5672019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ying X, Tu W, Li S, Wu Q, Chen X, Zhou Y,
Hu J, Yang G and Jiang S: Hyperbaric oxygen therapy reduces
apoptosis and dendritic/synaptic degeneration via the BDNF/TrkB
signaling pathways in SCI rats. Life Sci. 229:187–199. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zanuzzi CN, Nishida F, Sisti MS, Barbeito
CG and Portiansky EL: Reactivity of microglia and astrocytes after
an excitotoxic injury induced by kainic acid in the rat spinal
cord. Tissue Cell. 56:31–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Findlay VJ, Tapiero H and Townsend DM:
Sulfiredoxin: A potential therapeutic agent? Biomed Pharmacother.
59:374–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh A, Ling G, Suhasini AN, Zhang P,
Yamamoto M, Navas-Acien A, Cosgrove G, Tuder RM, Kensler TW, Watson
WH and Biswal S: Nrf2-dependent sulfiredoxin-1 expression protects
against cigarette smoke-induced oxidative stress in lungs. Free
Radic Biol Med. 46:376–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biteau B, Labarre J and Toledano MB:
ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae
sulphiredoxin. Nature. 425:980–984. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Yu S, Wu J, Zou Y and Zhao Y:
Sulfiredoxin-1 protects PC12 cells against oxidative stress induced
by hydrogen peroxide. J Neurosci Res. 91:861–870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, He P, Wang XL, Zhang S, Devejian N,
Bennett E and Cai C: Sulfiredoxin-1 enhances cardiac progenitor
cell survival against oxidative stress via the upregulation of the
ERK/NRF2 signal pathway. Free Radic Biol Med. 123:8–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, He Z, Guo J, Li Z, Wang X, Yang C
and Cui X: Sulfiredoxin-1 protects against simulated
ischaemia/reperfusion injury in cardiomyocyte by inhibiting
PI3K/AKT-regulated mitochondrial apoptotic pathways. Biosci Rep.
36:e003252016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soubeyrand M, Badner A, Vawda R, Chung YS
and Fehlings MG: Very high resolution ultrasound imaging for
real-time quantitative visualization of vascular disruption after
spinal cord injury. J Neurotrauma. 31:1767–1775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Can H, Aydoseli A, Gömleksiz C, Göker B,
Altunrende ME, Dolgun M and Sencer A: Combined and individual use
of pancaspase inhibitor Q-VD-OPh and NMDA receptor antagonist
riluzole in experimental spinal cord injury. Ulus Travma Acil
Cerrahi Derg. 23:452–458. 2017.PubMed/NCBI
|
|
22
|
Goldshmit Y, Tang J, Siegel AL, Nguyen PD,
Kaslin J, Currie PD and Jusuf PR: Different Fgfs have distinct
roles in regulating neurogenesis after spinal cord injury in
zebrafish. Neural Dev. 13:242018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong ZX, Feng SS, Chen SZ, Chen ZM and
Chen XW: Inhibition of MSK1 promotes inflammation and apoptosis and
inhibits functional recovery after spinal cord injury. J Mol
Neurosci. 68:191–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen LM, Song ZW, Hua Y, Chao X and Liu
JB: miR-181d-5p promotes neurite outgrowth in PC12 Cells via
PI3K/Akt pathway. CNS Neurosci Ther. 23:894–906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin TF and Grishanin RN: PC12 cells as
a model for studies of regulated secretion in neuronal and
endocrine cells. Methods Cell Biol. 71:267–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khodagholi F and Tusi SK: Stabilization of
Nrf2 by tBHQ prevents LPS-induced apoptosis in differentiated PC12
cells. Mol Cell Biochem. 354:97–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallorini M, Petzel C, Bolay C, Hiller KA,
Cataldi A, Buchalla W, Krifka S and Schweikl H: Activation of the
Nrf2-regulated antioxidant cell response inhibits HEMA-induced
oxidative stress and supports cell viability. Biomaterials.
56:114–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh A, Venkannagari S, Oh KH, Zhang YQ,
Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S,
et al: Small molecule inhibitor of NRF2 selectively intervenes
therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem
Biol. 11:3214–3225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Chen Y, Yu S, Li L, Zhao X, Li Q,
Zhao J and Zhao Y: Neuroprotective effects of sulfiredoxin-1 during
cerebral ischemia/reperfusion oxidative stress injury in rats.
Brain Res Bull. 132:99–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D,
Luan J, Wang L and Lin D: Coenzyme Q10 regulation of apoptosis and
oxidative stress in H2O2 Induced BMSC death
by modulating the Nrf-2/NQO-1 signaling pathway and its application
in a model of spinal cord injury. Oxid Med Cell Longev.
2019:64930812019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hachem LD, Ahuja CS and Fehlings MG:
Assessment and management of acute spinal cord injury: From point
of injury to rehabilitation. J Spinal Cord Med. 40:665–675. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zhou Y, Yu S, Wu J, Chen Y and
Zhao Y: Sulfiredoxin-1 exerts anti-apoptotic and neuroprotective
effects against oxidative stress-induced injury in rat cortical
astrocytes following exposure to oxygen-glucose deprivation and
hydrogen peroxide. Int J Mol Med. 36:43–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JY, Joo B, Nam JH, Nam HY, Lee W, Nam
Y, Seo Y, Kang HJ, Cho HJ, Jang YP, et al: An aqueous extract of
herbal medicine ALWPs enhances cognitive performance and inhibits
LPS-induced neuroinflammation via FAK/NF-κb signaling pathways.
Front Aging Neurosci. 10:2692018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shah SA, Khan M, Jo MH, Jo MG, Amin FU and
Kim MO: Melatonin stimulates the SIRT1/Nrf2 signaling pathway
counteracting lipopolysaccharide (LPS)-induced oxidative stress to
rescue postnatal rat brain. CNS Neurosci Ther. 23:33–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashtekar A, Huk D, Magner A, La Perle KMD,
Boucai L and Kirschner LS: Alterations in Sod2-induced oxidative
stress affect endocrine cancer progression. J Clin Endocrinol
Metab. 103:4135–4145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kubo E, Chhunchha B, Singh P, Sasaki H and
Singh DP: Sulforaphane reactivates cellular antioxidant defense by
inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress.
Sci Rep. 7:141302017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding C, Fan X and Wu G: Peroxiredoxin 1-an
antioxidant enzyme in cancer. J Cell Mol Med. 21:193–202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tuo L, Xiang J, Pan X, Gao Q, Zhang G,
Yang Y, Liang L, Xia J, Wang K and Tang N: PCK1 downregulation
promotes TXNRD1 expression and hepatoma cell growth via the
Nrf2/Keap1 pathway. Front Oncol. 8:6112018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu S, Lu H and Bai Y: Nrf2 in cancers: A
double-edged sword. Cancer Med. 8:2252–2267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Magesh S, Chen Y and Hu L: Small molecule
modulators of Keap1-Nrf2-are pathway as potential preventive and
therapeutic agents. Med Res Rev. 32:687–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin CW, Chen B, Huang KL, Dai YS and Teng
HL: Inhibition of autophagy by estradiol promotes locomotor
recovery after spinal cord injury in rats. Neurosci Bull.
32:137–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He Z, Zhou Y, Huang Y, Wang Q, Zheng B,
Zhang H, Li J, Liu Y, Wu F, Zhang X, et al: Dl-3-n-butylphthalide
improves functional recovery in rats with spinal cord injury by
inhibiting endoplasmic reticulum stress-induced apoptosis. Am J
Transl Res. 9:1075–1087. 2017.PubMed/NCBI
|