Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide (1). The 5-year
survival rate following diagnosis is estimated to be ~15.6%, which
is lower than the rate observed in colon, breast or prostate cancer
(2). Non-small cell lung cancer
(NSCLC) accounts for 85% of all lung cancer cases and
histologically, it is typically classified in three subtypes:
Large-cell neuroendocrine carcinoma, squamous cell carcinoma and
adenocarcinoma (3). Over the last
several decades, conventional cancer treatments, such as surgery,
chemotherapy and radiotherapy, have been used to treat lung cancer
(4); however, these therapies do
not benefit patients with locally advanced or distant metastatic
disease (stage III/IV) (5).
Therefore, several molecular targeted therapies have been approved
for these patients, including first- and second-generation
epidermal growth factor receptor tyrosine kinase inhibitors
(6). In total, >60% of all
newly diagnosed patients with lung cancer have undergone
radiotherapy alone and in combination with chemotherapy, targeted
therapies and immunotherapy as the first line therapies (7). Thus, there is an urgent requirement
to identify promising therapeutic targets that could inhibit cell
proliferation and induce cell apoptosis to prevent the progression
of lung cancer.
MicroRNAs (miRNAs/miR) are single stranded
non-coding RNAs of ~19–25 nucleotides in length, that are generated
from endogenous hairpin transcripts through a multistep process,
which starts in the nucleus and ends in the cytoplasm (8,9).
miRNAs are partially complementary to one or more mRNA molecules
(10); their main function is to
downregulate gene expression in several ways, including
translational repression, mRNA cleavage and de-adenylation
(11). Accumulating scientific
evidence has demonstrated that miRNAs serve an important role in
cancer progression and treatment. For example, Cazzoli et al
(12) screened 742 miRNAs isolated
from the circulating exosomes of patients with NSCLC and among
them, four miRNAs (miR-378a, miR-379, miR-139-5p and miR-200b-5p)
were identified as screening markers to segregate patients with
lung adenocarcinoma and granuloma. In addition, miR-21, miR-31 and
miR-let7 were discovered to be closely associated with the
diagnostic efficacy and survival rate in lung cancer (13). miR-379 is located on human
chromosome 14q32 within a large miRNA gene cluster (14). Previous evidence indicated that
miR-379-5p exhibited a tumor-suppressive role in several types of
cancer, including osteosarcoma, bladder cancer and melanoma
(15,16). Hao et al (17) also reported that miR-379-5p
expression levels were significantly downregulated in
chemoresistant NSCLC tissues and cells, whereas miR-379-5p
overexpression suppressed eukaryotic translation initiation factor
4 γ 2 (EIF4G2) expression to enhance cisplatin chemosensitivity.
However, the expression profile and the role of miR-379-5p in NSCLC
remains to be fully determined.
β-Arrestins (ARRBs), including ARRB1 and ARRB2, have
been identified as scaffold proteins that mediate the
desensitization and internalization of G protein-coupled receptors
(GPCRs) (18). Furthermore, ARRBs
were discovered to serve as signal transducers, with a previous
study demonstrating that they had important roles in several
physiological processes, such as chemotaxis, the Frank-Starling
force, and pathological conditions including myelofibrosis,
pulmonary fibrosis and asthma (19). Emerging data has also suggested
that the recruitment of ARRBs may represent a major non-G
protein-dependent signaling pathway of GPCRs, such as the
endothelin-1 receptor (ET-1R) in cancer (20). In addition, a study on glioblastoma
multiforme (GBM) revealed that ARRB1 knockdown suppressed GBM cell
proliferation, invasion and glycolysis by inhibiting Src signaling
(21). Regarding lung cancer, a
previous study indicated that nicotine induced the nuclear
translocation of ARRB1, which resulted in the increased expression
of proliferative and survival genes, thereby promoting the growth
and progression of NSCLC (22).
Additionally, Shen et al (23) reported that ARRB1 enhanced the
chemosensitivity of lung cancer through the mediation of DNA
damage.
The present study aimed to investigate the
expression levels of miR-379-5p and ARRB1 in NSCLC. The results
indicated that miR-379-5p overexpression may inhibit cell
proliferation and promote cell apoptosis by targeting ARRB1.
Thereby, this study provided novel insight into lung cancer
treatment.
Materials and methods
Patient studies
The present study was approved by the Ethics
Committee of Weinan Central Hospital and written informed consent
was obtained from each participant prior to the study initiation.
Tumor and para-carcinoma tissues were obtained from 30 patients (20
male and 10 female; 13 aged <50 and 17 aged ≥50) with NSCLC who
underwent surgery at Weinan Central Hospital between January 2017
and March 2018. The patients did not undergo any treatment such as
chemotherapy or radiotherapy before surgery. The tumor and
para-carcinoma tissues were fixed in 10% formalin at room
temperature until use. The clinicopathological features of these
patients are presented in Table
I.
 | Table I.Association between
clinicopathological features of patients with non-small cell lung
cancer and miR-379-5p expression levels. |
Table I.
Association between
clinicopathological features of patients with non-small cell lung
cancer and miR-379-5p expression levels.
|
|
| miR-379-5p
expression levels |
|
|---|
|
|
|
|
|
|---|
| Variable | n | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.8804 |
|
≥50 | 17 | 7 | 10 |
|
|
<50 | 13 | 5 | 8 |
|
| Sex |
|
|
| 0.6023 |
|
Male | 20 | 8 | 12 |
|
|
Female | 10 | 5 | 5 |
|
| Tumor size |
|
|
| 0.0281a |
| <3
cm | 14 | 10 | 4 |
|
| ≥3
cm | 16 | 5 | 11 |
|
| TNM stage |
|
|
| 0.0510 |
|
I–II | 12 | 6 | 6 |
|
|
III–IV | 18 | 3 | 15 |
|
| Metastasis |
|
|
| 0.0371a |
|
Negative | 13 | 7 | 6 |
|
|
Positive | 17 | 3 | 14 |
|
Plasmids and cell culture
The BEAS-2B, A549, PG49 and DMS-114 cell lines were
purchased from the American Type Culture Collection. The cells were
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), and
maintained at 37°C in a humidified incubator containing 5%
CO2 until 90% confluence was reached. The miR-379-5p
mimic (5′-UGGUAGACUAUGGAACGUAGG-3′), miR-negative control (NC)
mimic (5′-GUGGAUUUUCCUCUAUGAUUU-3′), ARRB1 overexpression pcDNA3.1
plasmid (pcDNA3.1-ARRB1), pcDNA3.1 plasmid (negative control for
pcDNA3.1-ARRB1), wild-type (WT) and mutant (MUT) ARRB1 3′
untranslated region (UTR) pGL3 plasmids were obtained from Addgene,
Inc.
Cell transfection
A549 cells (at 1×104 cells/well) were
seeded into 96-well plates and subsequently transfected with miR-NC
mimics, miR-379-5p mimics, pcDNA3.1 and pcDNA3.1-ARRB1.
Untransfected cells were used as the control group. The cells were
incubated at 37°C and when they reached 70–80% confluence, they
were transfected with 500 ng transfectants using 2.5 µl
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following incubation at 37°C for 6 h, the serum-free RPMI-1640
medium was replaced with fresh RPMI-1640 medium containing 10% FBS,
and the cells continued to incubate for 24 h. All experiments were
performed in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
The relative miR-379-5p and mRNA expression levels
were determined using RT-qPCR. Total RNA from tissues and cells was
extracted using the RNeasy Mini kit (Qiagen, Inc.), according to
the manufacturer's protocol, and the concentration was determined
on a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Total RNA was reverse-transcribed into cDNA using M-MLV
Reverse Transcriptase (Promega Corporation). M-MLV RT 5X reaction
buffer (containing Tris-HCl, KCl, MgCl2 and DDT; 5 µl)
and 5 µl dNTP and Oligo DT primer were used for RT. The following
temperature protocol was used for the reverse transcription: 43°C
for 30 min, 97°C for 5 min and 5°C for 5 min. qPCR was subsequently
performed using a PrimeScript™ RT-PCR kit (Qiagen Inc.) following
the manufacturer's protocol. The following thermocycling conditions
were used for the qPCR: Initial denaturation at 95°C for 6 min;
followed by 40 cycles of initiation at 94°C for 30 sec, annellation
at 60°C for 30 sec and elongation at 73°C for 90 sec. The following
primer pairs were used for the qPCR: miR-379-5p forward,
5′-GCGCTGGTAGACTATGGAA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6
forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; ARRB1 forward,
5′-CCTGGATGTCTTGGGTCTG-3′ and reverse, 5′-TGATGGGTTCTCCGTGGTA-3′;
Bcl-2 forward, 5′-CTGCACCTGACGCCCTTCACC-3′ and reverse,
5′-CACATGACCvCCACCGAACTCAAAGA-3′; Bax forward,
5′-GACCAGCATGACAGATTTCTACCA-3′ and reverse,
5′-AACTGAGACTAAGGCAGAAGATG-3′; caspase-3 forward,
5′-CTCGGTCTGGTACAGATGTCGATG-3 ′ and reverse,
5′-GGTTAACCCGGGTAAGAATGTGCA-3′; and GAPDH forward,
5′-ACACCCACTCCTCCACCTTTG-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTAG-3′. All experiments were performed in
triplicate. The mRNA expression levels were quantified using the
2−∆∆Cq method (24).
The endogenous expression levels of U6 and GAPDH were used to
normalize the miR-379-5p and mRNA expression levels,
respectively.
Western blotting
The protein expression levels in tissues and cells
were determined using western blotting. Total and phosphorylated
protein was extracted from the cells using RIPA lysis buffer
(Boster Biological Technology) according to the manufacturer's
protocols. Protein was quantified using a bicinchoninic acid
protein assay kit (Abbkine Scientific Co., Ltd.) and 20 µg
protein/lane was separated via 15% SDS-PAGE. The separated proteins
were subsequently transferred onto PVDF membranes (EMD Millipore)
and blocked with 5% skimmed milk for 2 h at room temperature. The
membranes were incubated with the following primary antibodies for
1 h at room temperature: Anti-ARRB1 (1:1,000; cat. no. ab32099),
anti-PI3K (1:1,000; cat. no. ab191606), anti-phosphorylated
(p)-PI3K (1:1,1000; cat. no. ab182651), anti-AKT (1:500; cat. no.
ab8805), anti-p-AKT (1:1,000; cat. no. ab38449), anti-Bcl-2
(1:1,000; cat. no. ab59348), anti-Bax (1:1,000; cat. no. ab53154),
anti-caspase-3 (1:500; cat. no. ab13847) and anti-GAPDH (1:2,500;
cat. no. ab9485; all from Abcam). Following the primary antibody
incubation, the membranes were incubated with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (1:5,000; cat.
no. ab205718; Abcam) for 45 min at room temperature. The protein
bands were visualized using an ECL Western Blotting kit (Abcam) and
the expression levels were analyzed using ImageJ software (v1.48U;
National Institutes for Health). All experiments were performed in
triplicate. GAPDH protein expression levels were used as an
internal control.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of transfected A549 cells was
assessed using a CCK-8 kit (APeXBIO Technology LLC) according to
the manufacturer's protocols. Briefly, the cells at the density of
1×104 cells/well were seeded into 96-well plates and
following 0, 12, 24 or 48 h, 10 µl CCK-8 reagent was added to each
well and incubated for an additional 24 h in RPMI-1640 medium
supplemented with 10% FBS at 37°C in a humidified incubator
containing 5% CO2. Finally, the optical density of each
well was measured using a microplate reader at 450 nm.
Flow cytometric analysis of
apoptosis
Following transfection, the apoptotic rate of A549
cells was analyzed using flow cytometry. Briefly, 2×105
transfected cells were plated into a 24-well plate and incubated
for 48 h at 37°C with 5% CO2. Subsequently, cells were
incubated with 10 µg/ml propidium iodide (Sigma-Aldrich; Merck
KGaA) and 50 µg/ml Annexin V-FITC (BD Biosciences) at room
temperature for 15 min in the dark. Finally, the stained cells were
analyzed using a FACScan flow cytometer (BD Biosciences) carrying
CellQuest Pro software (version 6.0; BD Biosciences). The apoptotic
cell rate was calculated by the number of (apoptotic cells + death
cells)/all cells.
Bioinformatics analysis and
dual-luciferase reporter assay
To predict the target genes of miR-379-5p,
bioinformatic online tool TargetScan (http://www.targetscan.org) was employed. After
transfection for 24 h, a dual-luciferase reporter assay was
performed to verify the direct interaction between ARRB1 and
miR-379-5p. A549 cells at the density of 2×104
cells/well were seeded into 96-well plates and incubated for 24 h
at 37°C in a humidified atmosphere with 5% CO2. The
cells were subsequently co-transfected with 2.5 µg ARRB1-3′UTR-WT
or ARRB1-3′UTR-MUT pGL3 plasmids, and miR-379-5p mimic or miR-NC
mimic, using 2.5 µl Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and incubated for 24
h following the manufacturer's protocol. The luciferase activity of
the transfected A549 cells was analyzed using a Dual-Luciferase
Reporter Assay kit (BioVision, Inc.), according to the
manufacturer's protocol. The firefly luciferase activity was
normalized to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.01 software (GraphPad Software, Inc.) and all data
are presented as the mean ± SD from three replicates. Statistical
differences between the cancer and normal tissues were performed
using a paired Student's t-test, whereas the association between
the miR-379-5p expression levels and the clinicopathological
features were determined using a χ2-test. A one-way
ANOVA, followed by Tukey's post hoc test for multiple comparisons
was performed to analyze the significant differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-379-5p expression levels are
downregulated and ARRB1 expression levels are upregulated in NSCLC
tissues and cell lines
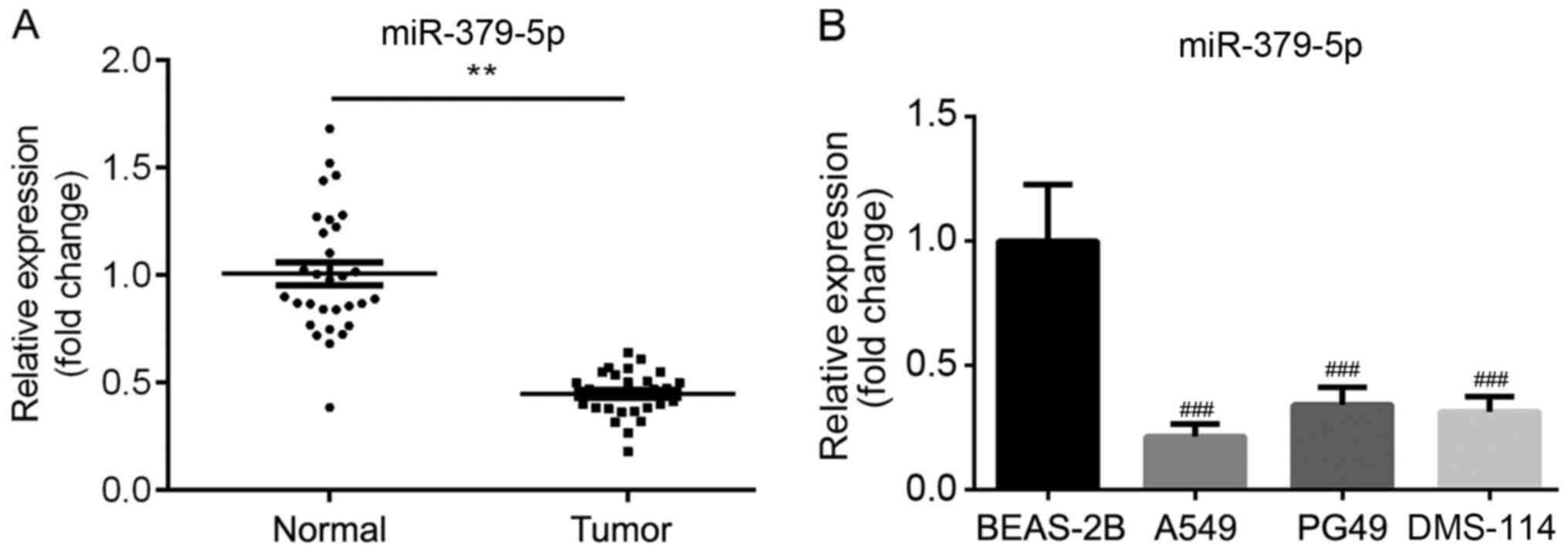
The expression levels of miR-379-5p in normal and
tumor tissues were determined using RT-qPCR. The results revealed
that miR-379-5p expression levels were significantly downregulated
in the tumor tissues compared with the normal tissues (Fig. 1A). Similarly, the expression levels
of miR-379-5p were significantly decreased in NSCLC cells,
including A549, PG49 and DMS-114 compared with normal human
bronchial epithelial cell line (BEAS-2B; Fig. 1B). The high or low expression
levels of miR-379-5p were also discovered to be significantly
associated with the tumor size and metastasis, but not with the
age, sex or TNM stage (Table I).
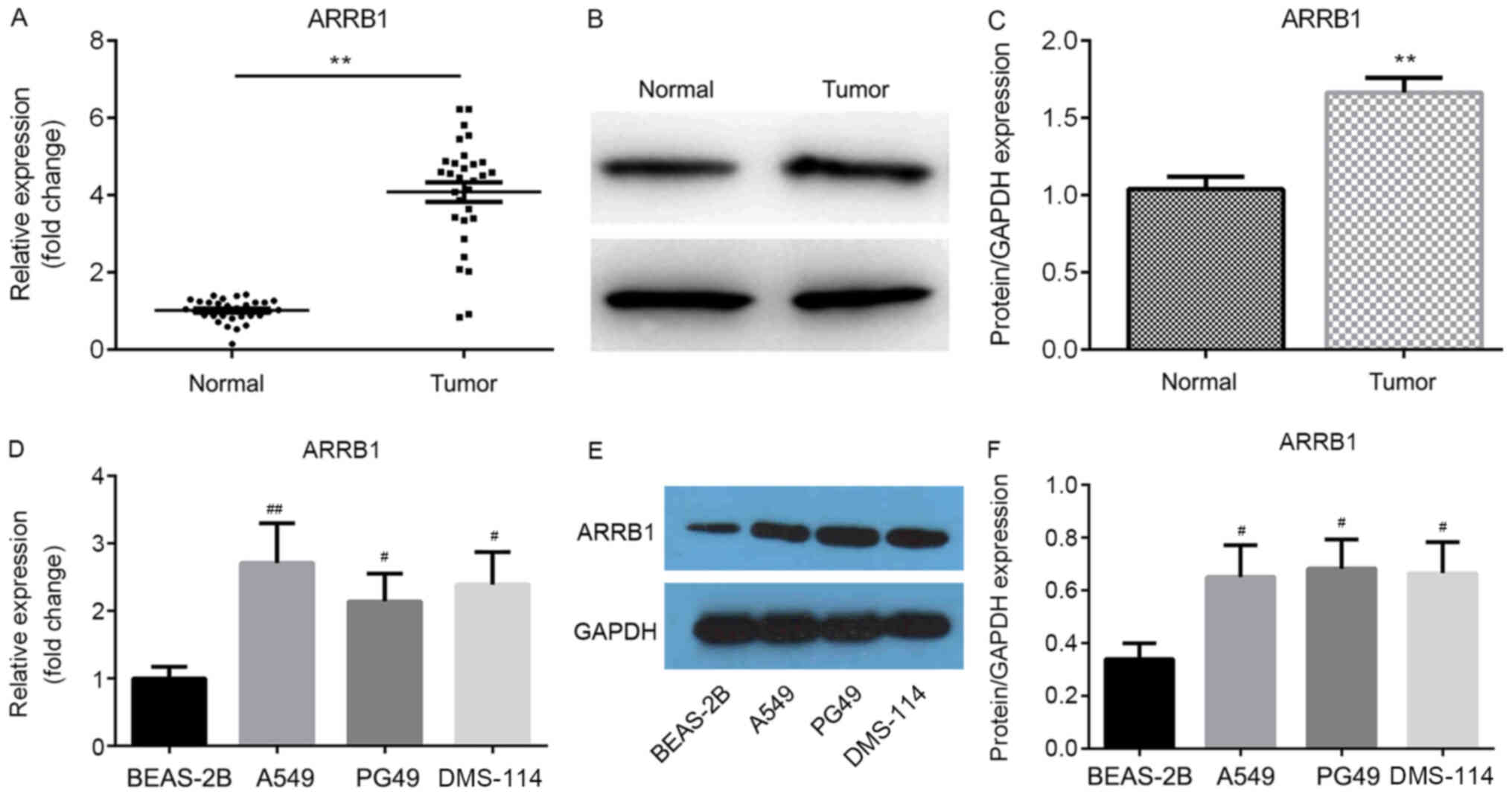
In addition, the mRNA and protein expression levels of ARRB1 in the
tissues and cell lines were determined using RT-qPCR and western
blotting, respectively; both the mRNA (Fig. 2A and D) and protein (Fig. 2B, C, E and F) expression levels of
ARRB1 were significantly upregulated in tumor tissues and cell
lines compared with normal tissues and the BEAS-2B cell line,
respectively.
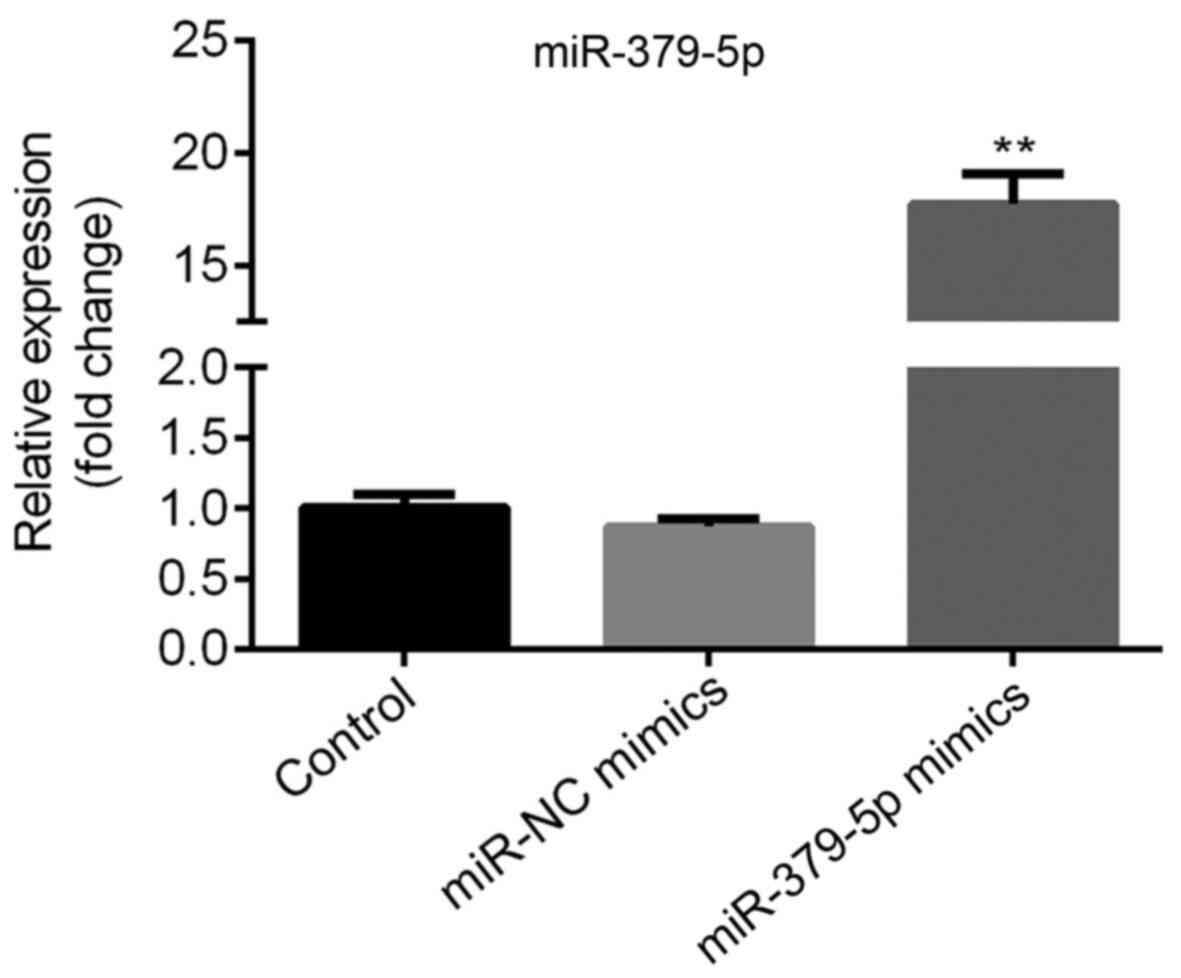
Successful transfection of A549 cells
with miR-379-5p mimics
A549 cells with lower miR-379-5p and higher ARRB1
levels compared with other cells were used to transfected with
miR-379-5p mimics or miR-NC mimics; compared with the miR-NC mimics
group, the expression level of miR-379-5p was significantly
upregulated in the miR-379-5p mimics group (Fig. 3). Therefore, the miR-379-5p mimic
and miR-NC mimic were used for further experimental procedures.
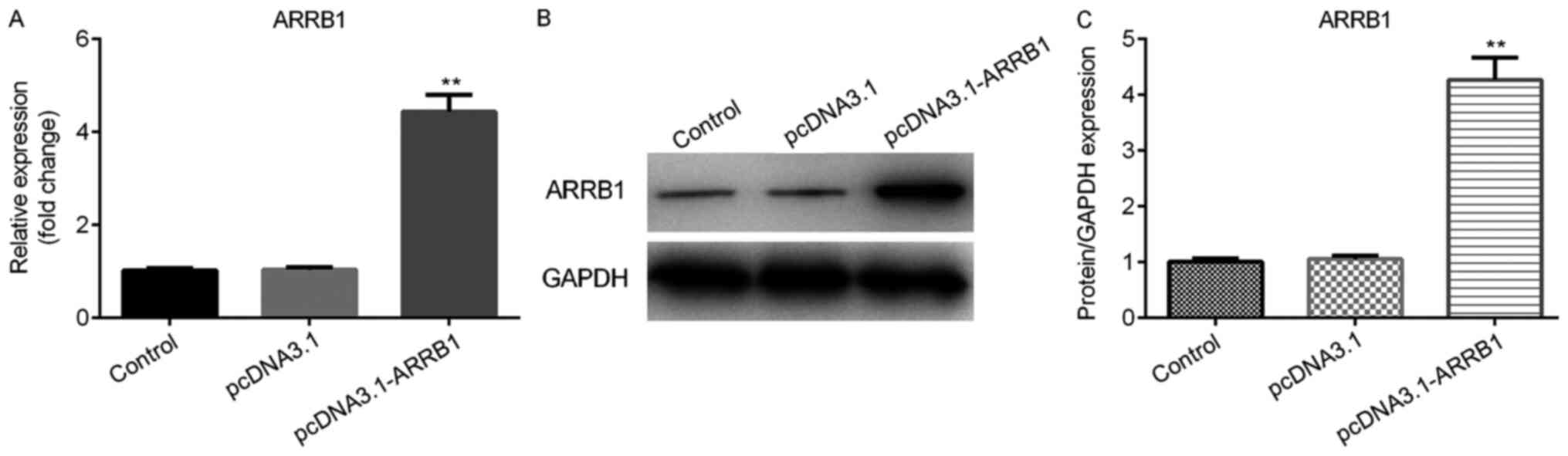
Successful construction of ARRB1
overexpression plasmids
The ARRB1 overexpression plasmids were transfected
into A549 cells in order to verify the successful construction of
overexpression plasmids. Thus, the mRNA and protein expression
levels of ARRB1 were analyzed using RT-qPCR and western blot
analysis, respectively. The results demonstrated that the mRNA
expression level of ARRB1 was significantly upregulated in the
pcDNA3.1-ARRB1 group compared with the pcDNA3.1 group (Fig. 4A). Consistent with the results of
ARRB1 mRNA expression levels, western blotting also revealed that
the protein expression level of ARRB1 was significantly increased
in the pcDNA3.1-ARRB1 group compared with the pcDNA3.1 group
(Fig. 4B and C). Thus, the ARRB1
overexpression plasmids were used for further cell experiments.
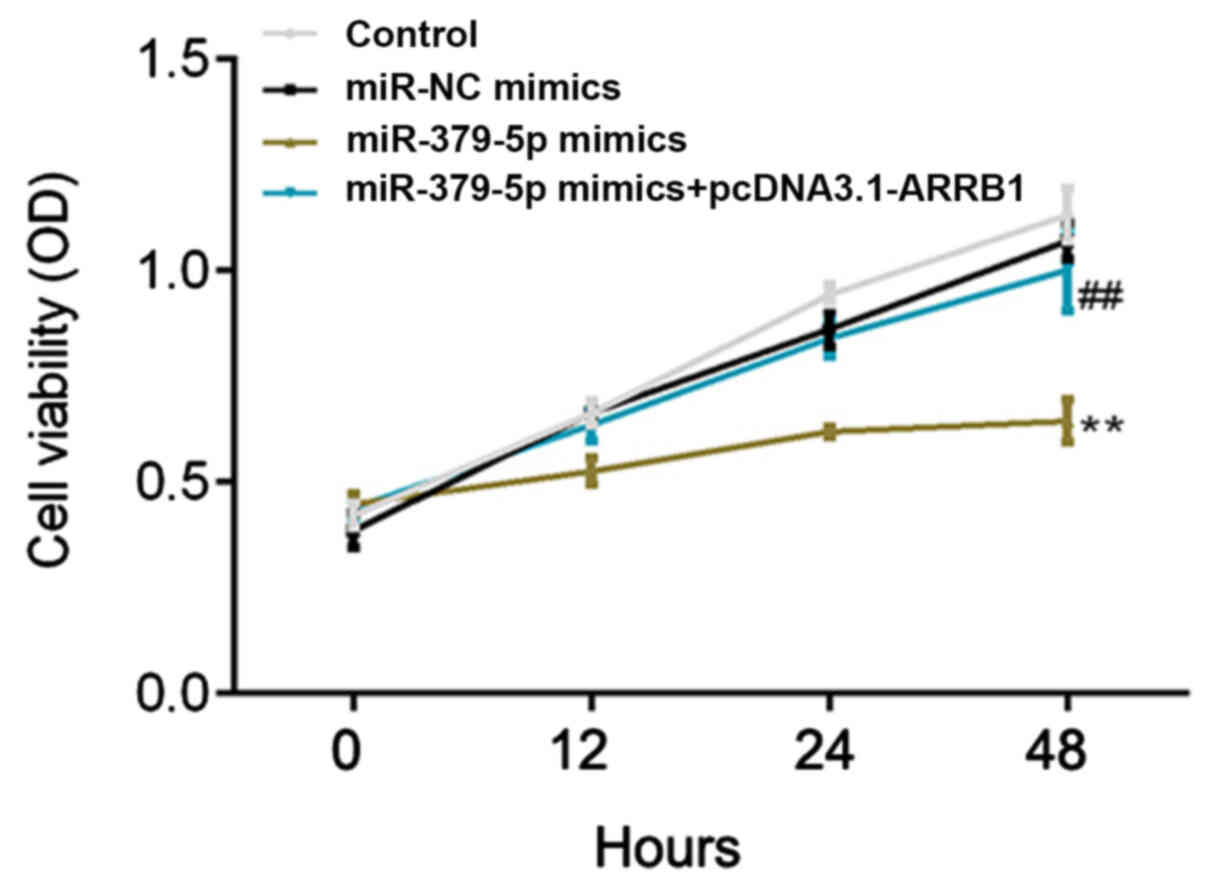
miR-379-5p overexpression inhibits
A549 cell proliferation, while ARRB1 overexpression rescues this
inhibition
Following transfection, the proliferation rate of
A549 cells was detected at 0, 12, 24 and 48 h using a CCK-8 assay.
The proliferation rate was demonstrated to be significantly
inhibited in the miR-379-5p mimics group compared with the miR-NC
mimics group (Fig. 5). Notably, a
significantly increased proliferation rate was observed in the
miR-379-5p mimics + pcDNA3.1-ARRB1 group compared with the
miR-379-5p mimics group (Fig. 5).
These results indicated that miR-379-5p overexpression may
decrease, while ARRB1 overexpression may increase, the cell
proliferation rate.
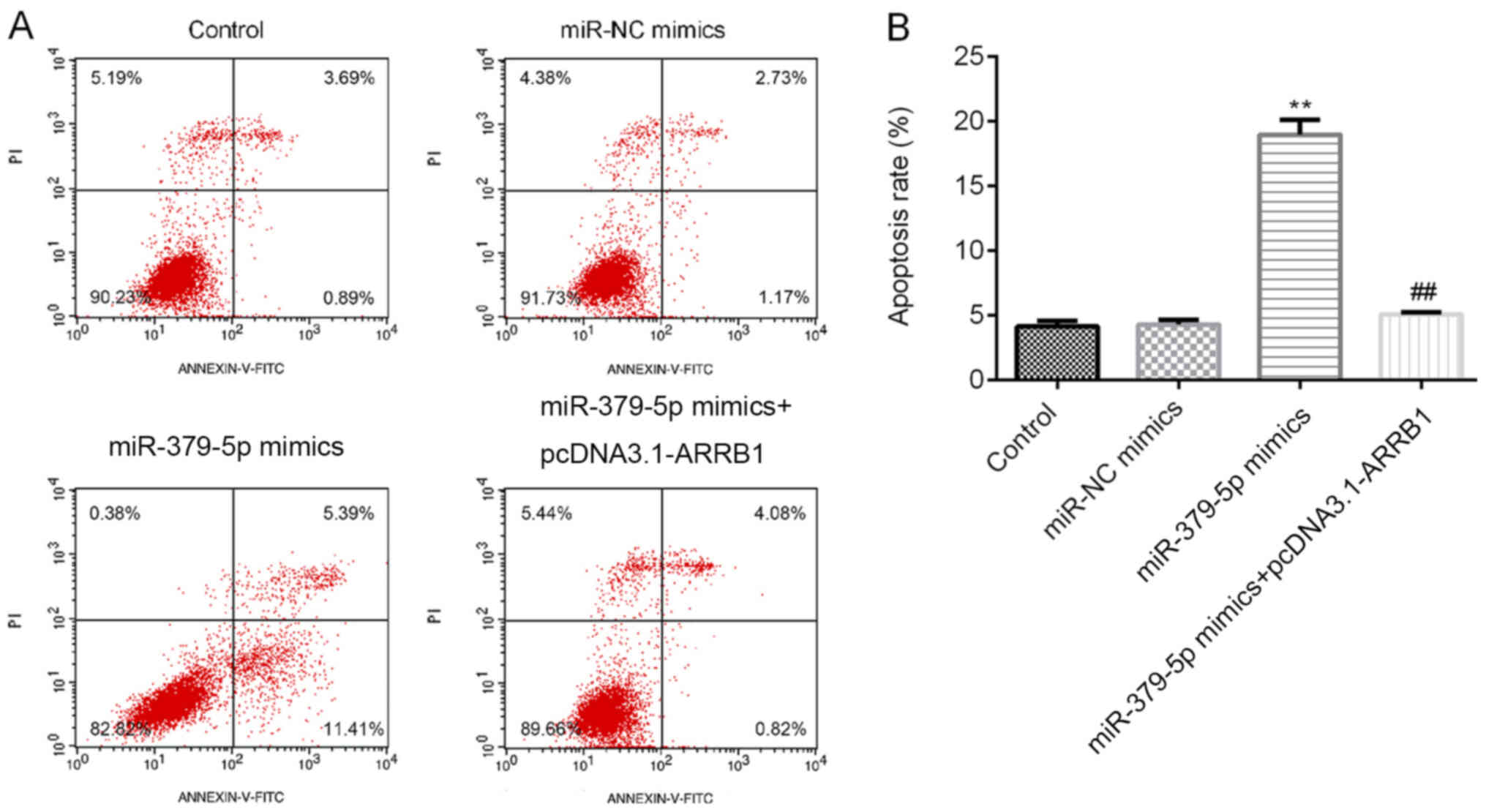
miR-379-5p overexpression promotes
A549 cell apoptosis, while ARRB1 overexpression reverses the
promotion
Following the transfection with the miR-379-5p
mimics, miR-NC mimics or ARRB1 overexpression plasmids, the cells
were incubated for 48 h and the apoptotic cell ratio was determined
using flow cytometric analysis. Among the four groups,
overexpression of miR-379-5p increased the apoptotic rate, while
ARRB1 overexpression decreased the improvement induced by
miR-379-5p (Fig. 6A and B). Thus,
these findings indicated that miR-379-5p overexpression may induce
A549 cell apoptosis, whereas ARRB1 overexpression may reduce cell
apoptosis.
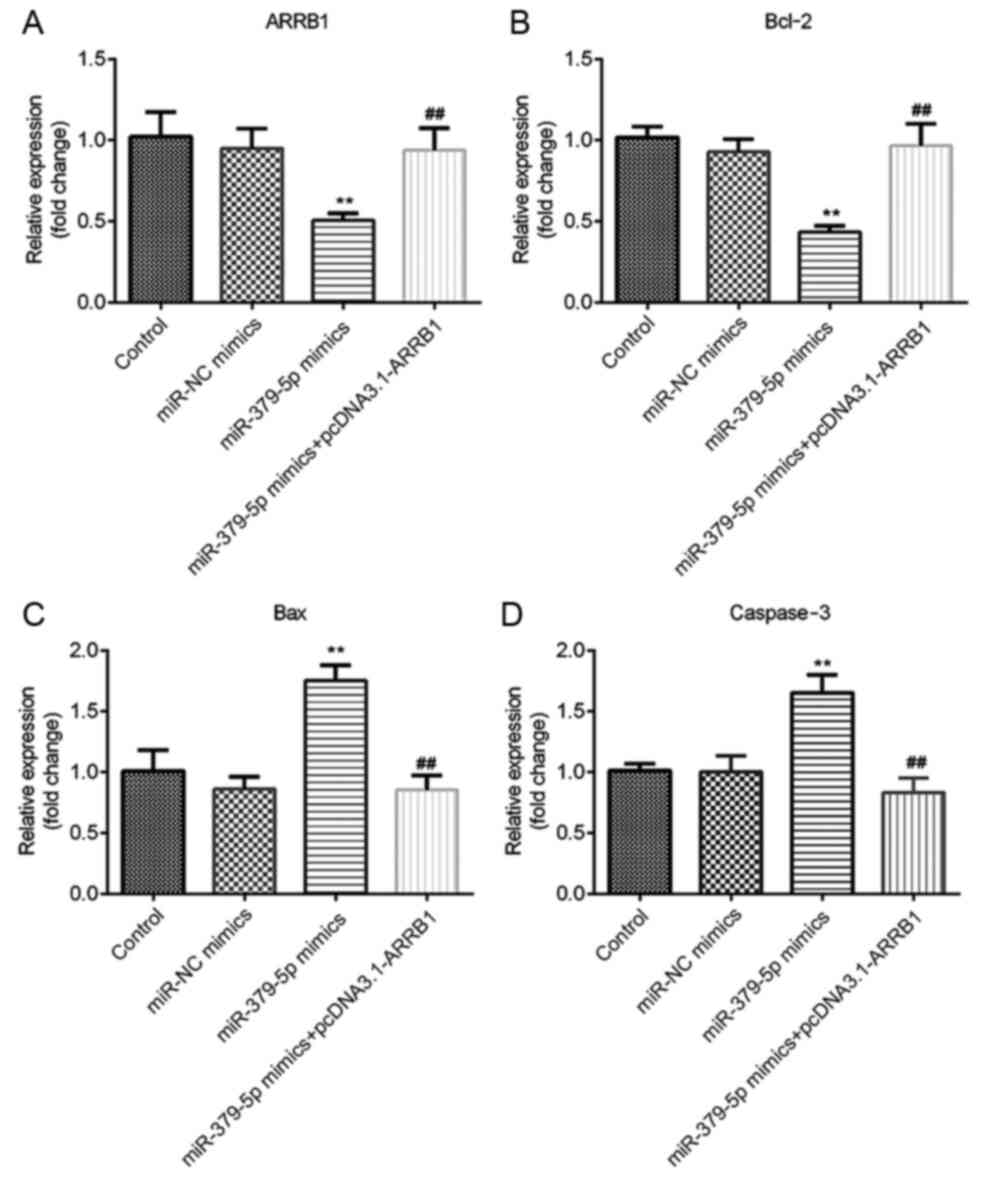
miR-379-5p regulates ARRB1, Bcl-2, Bax
and caspase-3 mRNA expression levels in NSCLC cells by regulating
the expression level of ARRB1
The results of RT-qPCR revealed that ARRB1 and Bcl-2
mRNA expression levels were significantly decreased in the
miR-379-5p mimics group compared with the miR-NC mimics group,
whereas the expression levels were significantly increased in the
miR-379-5p mimics + pcDNA3.1-ARRB1 group compared with the miR-379
mimics group (Fig. 7A and B). In
addition, the mRNA expression levels of Bax and caspase-3 were
significantly upregulated in the miR-379-5p mimics group; there was
no significant difference among the control, miR-NC mimics and
miR-379-5p mimics + pcDNA3.1-ARRB1 groups (Fig. 7C and D). These results indicated
that miR-379-5p overexpression may downregulate ARRB1 and Bcl-2
expression levels, and upregulate Bax and caspase-3 expression
levels, whereas ARRB1 overexpression reversed the downregulation of
Bcl-2 and upregulation of Bax and caspase-3.
miR-379-5p regulates the protein
expression levels of ARRB1, PI3K/p-PI3K, AKT/p-AKT, Bcl-2, Bax and
caspase-3 by regulating the expression level of ARRB1
ARRB1, p-PI3K/PI3K, p-AKT/AKT and Bcl-2 expression
levels were significantly decreased in the miR-379-5p mimics group
compared with the miR-NC mimics group; however, the expression
levels of these proteins in the miR-379-5p mimics group were
significantly reversed following the overexpression of ARRB1
(Fig. 8A and B). Increased
expression levels of Bax and caspase-3 were detected in the
miR-379-5p mimics group; however, these expression levels were
reversed following the overexpression of ARRB1 (Fig. 8A and B). Therefore, these findings
indicated that miR-379-5p may inhibit ARRB1, PI3K/p-PI3K, AKT/p-AKT
and Bcl-2 expression levels, while increasing Bax and caspase-3
protein expression levels. In addition, it was determined that the
overexpression of ARRB1 may reverse these effects.
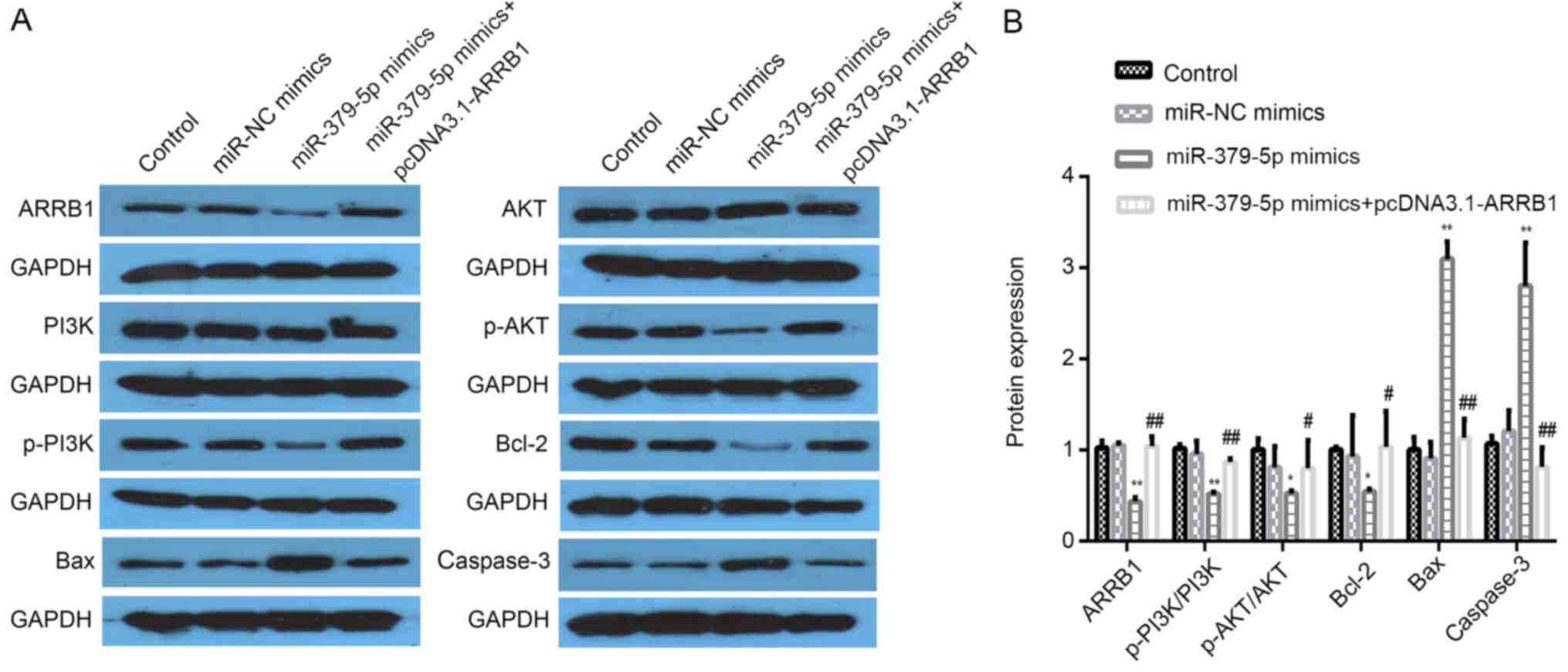 | Figure 8.miR-379-5p mimics downregulate the
expression levels of ARRB1, p-PI3K/PI3K, p-AKT/AKT and Bcl-2 and
upregulate the expression levels of Bax and caspase-3. (A)
Expression levels of ARRB1, p-PI3K/PI3K, p-AKT/AKT, Bcl-2, Bax and
caspase-3 protein were analyzed using western blotting. Each blot
is accompanied by its loading control GAPDH. (B)
Semi-quantification of the expression levels from part A.
*P<0.05 vs. the miR-NC mimics group; **P<0.01 vs. the miR-NC
mimics group; #P<0.05 vs. the miR-379-5p mimics
group; ##P<0.01 vs. the miR-379-5p mimics group. miR,
microRNA; NC, negative control; ARRB1, β-arrestin-1; p-,
phosphorylated. |
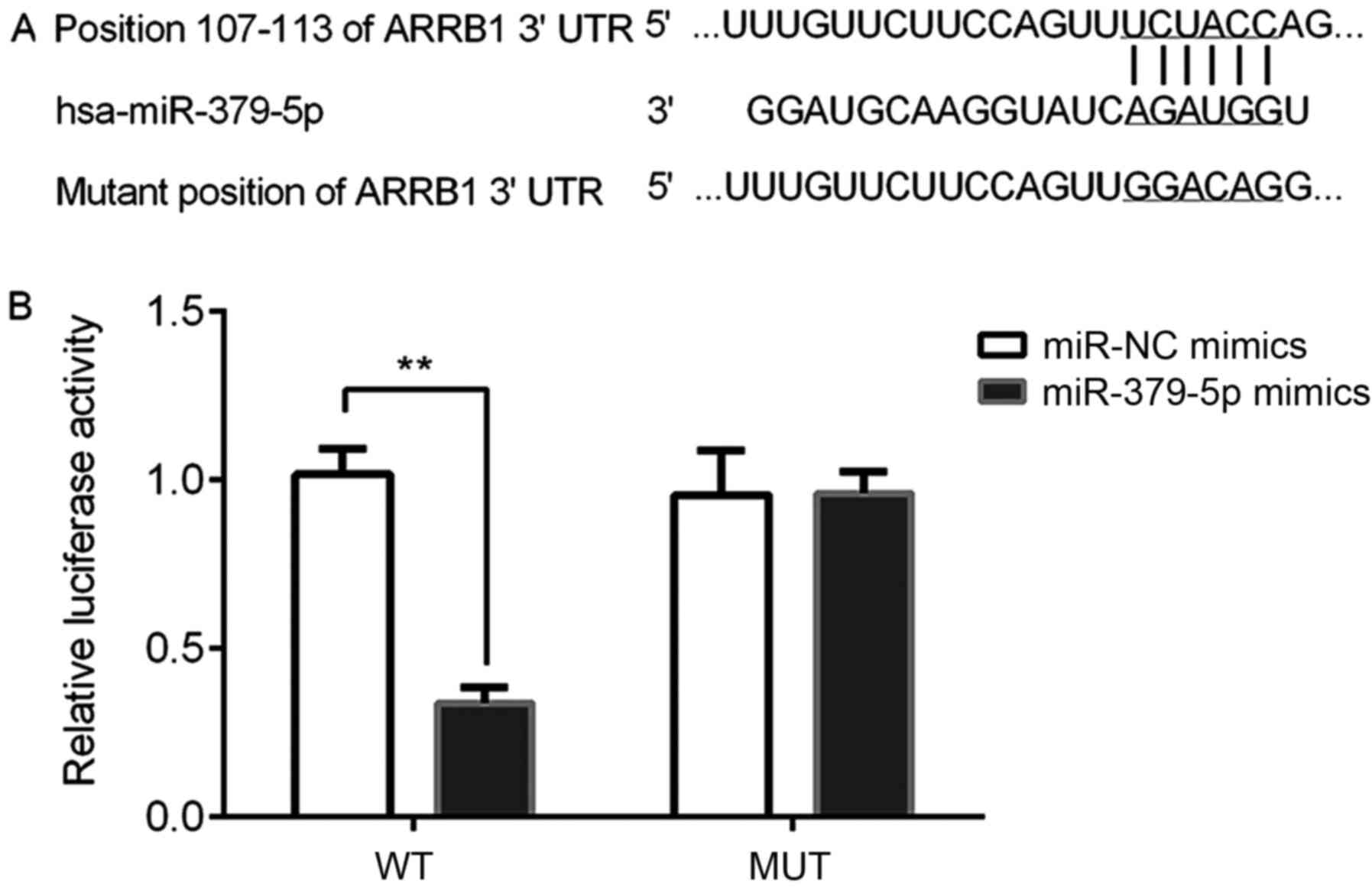
miR-379-5p directly targets ARRB1
A dual-luciferase reporter assay was performed to
determine whether ARRB1 was a direct target gene of miR-379-5p. The
results of the bioinformatics analysis using TargetScan (http://www.targetscan.org) revealed that ARBB1 was a
potential direct target gene of miR-379-5p by binding to the
ARRB1-3′UTR (Fig. 9A).
Subsequently, the dual-luciferase reporter assay demonstrated that
the relative luciferase activity was significantly reduced in the
ARBB1-3′UTR-WT and miR-379-5p mimics group compared with the miR-NC
mimic and ARBB1-3′UTR-WT group (Fig.
9B). Thus, it was verified that miR-379-5p directly targeted
ARRB1.
Discussion
The present study demonstrated that miR-379-5p
expression levels were downregulated in NSCLC and that the
overexpression of miR-379-5p subsequently reduced the expression
levels of ARRB1. Furthermore, miR-379-5p overexpression decreased
the expression levels of p-PI3K/PI3K, p-AKT/AKT and Bcl-2, and
increased the expression levels of Bax and caspase-3 in A549 cells.
These findings resulted in the inhibition of cell proliferation and
induced cell apoptosis. In addition, a dual-luciferase reporter
assay was performed to further verify that miR-379-5p directly
targeted ARRB1. The results of the present study revealed that the
effects of miR-379-5p on cell proliferation and apoptosis were
regulated through the PI3K/AKT signaling pathway by targeting
ARRB1. Therefore, these findings indicated that miR-379-5p may
suppress NSCLC development by directly inhibiting ARRB1 expression
levels, suggesting that it should be further investigated for its
potential application in clinical trials.
miR-379-5p has been identified as a tumor suppressor
in several types of cancer, including bladder cancer, osteosarcoma
and nasopharyngeal carcinoma (15–16,25).
In the present study, it was discovered that the expression levels
of miR-379-5p were downregulated in NSCLC, which was consistent
with a previous study by Hao et al (17). In addition, consistent with the
findings of Dasgupta et al (22), ARRB1 expression levels were also
revealed to be upregulated in NSCLC. ARRBs, multifunctional adapter
proteins, are most commonly known as regulators of GPCR signaling,
and they are often found upregulated in multiple types of human
cancer (26,27). Furthermore, several signaling
pathways have been reported to be regulated by ARRB1, including
AMPK, HIF1A and PI3K/AKT (27,28).
The results of the present study also revealed that ARRB1
downregulation induced by miR-379-5p overexpression inhibited the
expression levels of PI3K/AKT and Bcl-2, whereas Bax and caspase-3
expression levels were increased. These results are consistent with
those reported by Zhang et al (29). Zhan et al (30), suggested that ARRB1 served a vital
role in inhibiting cell apoptosis. This finding is strongly
supported by the results of the present study, as the
miR-379-5p-induced downregulation of ARRB1 resulted in the
inhibition of cell proliferation and increased rates of cell
apoptosis.
A previous study by Rosanò and Bagnato (20) revealed that ET-1R is a regulator of
cell proliferation, survival, motility, cytoskeletal changes,
angiogenesis, metastasis and drug resistance. Additionally, the
study revealed that the functions of ET-1R were strongly regulated
by ARRB1 (20). In addition, the
tumor-related effects of ARRB1 were associated with the capacity of
the cells to migrate, invade, proliferate, undergo apoptosis and to
manage drug resistance (20).
Thus, the effects of ARRB1 in lung cancer should be further
investigated. The present study hypothesized that the miR-379-5p
targeting of ARRB1 may represent a promising therapeutic target in
NSCLC, thus, further studies should focus on the effects of
miR-379-5p on cell migration, invasion and drug resistance by
targeting ARRB1. For example, the targeting of ARRB1 by miR-379-5p
may suppress cancer progression and enhance the efficacy of
chemotherapy, which may subsequently improve NSCLC prognosis.
However, the present study was limited by the fact
that the complete underlying molecular mechanism was not
determined. Thus, the involvement of other signaling pathways or
related factors influenced by miR-379-5p in regulating the
proliferation and apoptosis of lung cancer cells remains to be
further investigated, which is the aim of our future studies.
In conclusion, the present study revealed that
miR-379-5p and ARRB1 expression levels were downregulated and
upregulated in NSCLC, respectively. Furthermore, the overexpression
of miR-379-5p was discovered to downregulate the expression levels
of ARRB1, p-PI3K/PI3K, p-AKT/AKT and Bcl-2, and upregulate the
expression levels of Bax and caspase-3, thus resulting in the
inhibition of cell proliferation and the promotion of cell
apoptosis. Thus, it was hypothesized that miR-379-5p may serve as a
tumor suppressor in NSCLC by directly targeting ARRB1. Overall,
miR-379-5p may be considered as a potential therapeutic target for
patients with NSCLC to prevent the progress of the disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YJ and AW contributed to study design. YJ, PZ and YG
performed all experiments and data analysis. YJ was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weinan Central Hospital and written informed consent
was obtained from each participant prior to the study
initiation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen R, Xia W, Wang S, Xu Y, Ma Z, Xu W,
Zhang E, Wang J, Fang T, Zhang Q, et al: Long noncoding RNA
SBF2-AS1 is critical for tumorigenesis of early-stage lung
adenocarcinoma. Mol Ther Nucleic Acids. 16:543–553. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nanavaty P, Alvarez MS and Alberts WM:
Lung cancer screening: Advantages, controversies, and applications.
Cancer Control. 21:9–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richtmann S, Wilkens D, Warth A,
Lasitschka F, Winter H, Christopoulos P, Herth FJF, Muley T,
Meister M and Schneider MA: FAM83A and FAM83B as prognostic
biomarkers and potential new therapeutic targets in NSCLC. Cancers
(Basel). 11:6522019. View Article : Google Scholar
|
|
4
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung Cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
5
|
Zhang T, Song X, Liao X, Wang X, Zhu G,
Yang C and Xie X: Distinct prognostic values of phospholipase C
beta family members for non-small cell lung carcinoma. Biomed Res
Int. 2019:42565242019.PubMed/NCBI
|
|
6
|
Castellanos-Rizaldos E, Zhang X, Tadigotla
VR, Grimm DG, Karlovich C, Raez LE and Skog JK: Exosome-based
detection of activating and resistance EGFR mutations from plasma
of non-small cell lung cancer patients. Oncotarget. 10:2911–2920.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Hu Q, Xu B, Hu X, Su H, Li Q, Zhang
X, Yue J and Yu J: Peripheral memory and naïve T cells in non-small
cell lung cancer patients with lung metastases undergoing
stereotactic body radiotherapy: Predictors of early tumor response.
Cancer. Cell Int. 19:1212019. View Article : Google Scholar
|
|
8
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cazzoli R, Buttitta F, Nicola MD,
Malatesta S, Marchetti A, Rom WN and Pass HI: Micrornas derived
from circulating exosomes as noninvasive biomarkers for screening
and diagnosing lung cancer. J Thorac Oncol. 8:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Wang Z, Wang Q, Cui Y and Luo S:
Clinical significance of the expression of miRNA-21, miRNA-31 and
miRNA-let7 in patients with lung cancer. Saudi J Biol Sci.
26:777–781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X and Chu J: MicroRNA-379 suppresses
cell proliferation, migration and invasion in nasopharyngeal
carcinoma by targeting tumor protein D52. Exp Ther Med.
16:1232–1240. 2018.PubMed/NCBI
|
|
15
|
Wu D, Niu X, Tao J, Li P, Lu Q, Xu A, Chen
W and Wang Z: MicroRNA-379-5p plays a tumor-suppressive role in
human bladder cancer growth and metastasis by directly targeting
MDM2. Oncol Rep. 37:3502–3508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie X, Li YS, Xiao WF, Deng ZH, He HB, Liu
Q and Luo W: MicroRNA-379 inhibits the proliferation, migration and
invasion of human osteosarcoma cells by targetting EIF4G2. Biosci
Rep. 37:BSR201605422017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao GJ, Hao HJ, Ding YH, Wen H, Li XF,
Wang QR and Zhang BB: Suppression of EIF4G2 by miR-379 potentiates
the cisplatin chemosensitivity in non-small cell lung cancer cells.
FEBS Lett. 591:636–645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JC, Liu B, Zhang RW, Jiao PL, Tan X,
Wang YK and Wang WZ: Overexpression of ß-arrestin1 in the rostral
ventrolateral medulla downregulates angiotensin receptor and lowers
blood pressure in hypertension. Front Physiol. 9:2972018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Grotegut CA, Wisler JW, Li T, Mao
L, Chen M, Chen W, Rosenberg PB, Rockman HA and Lefkowitz RJ:
β-Arrestin 1 regulates β2-adrenergic receptor-mediated skeletal
muscle hypertrophy and contractility. Skelet Muscle. 8:392018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosanò L and Bagnato A: β-arrestin1 at the
cross-road of endothelin-1 signaling in cancer. J Exp Clin Cancer
Res. 35:1212016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lan T, Wang H, Zhang Z, Zhang M, Qu Y,
Zhao Z, Fan X, Zhan Q, Song Y and Yu C: Downregulation of
β-arrestin 1 suppresses glioblastoma cell malignant progression via
inhibition of src signaling. Exp Cell Res. 357:51–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dasgupta P, Rizwani W, Pillai S, Davis R,
Banerjee S, Hug K, Lloyd M, Coppola D, Haura E and Chellappan SP:
ARRB1-mediated regulation of E2F target genes in nicotine-induced
growth of lung tumors. J Natl Cancer Inst. 103:317–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen H, Wang L, Zhang J, Dong W, Zhang T,
Ni Y, Cao H, Wang K, Li Y, Wang Y and Du J: ARRB1 enhances the
chemosensitivity of lung cancer through the mediation of DNA damage
response. Oncol Rep. 37:761–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X and Chu J: MicroRNA-379 suppresses
cell proliferation, migration and invasion in nasopharyngeal
carcinoma by targeting tumor protein D52. Exp Ther Med.
16:1232–1240. 2018.PubMed/NCBI
|
|
26
|
Ma Z, Yu YR, Badea CT, Kovacs JJ, Xiong X,
Comhair S, Piantadosi CA and Rajagopal S: Vascular endothelial
growth factor receptor 3 regulates endothelial function through
β-arrestin. Circulation. 139:1629–1642. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zecchini V, Madhu B, Russell R, Gomes NP,
Warren A, Gaude E, Borlido J, Stark R, Zecchini HI, Rao R, et al:
Nuclear ARRB1 induces pseudohypoxia and cellular metabolism
reprogramming in prostate cancer. EMBO J. 33:1365–1382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Son D, Kim Y, Lim S, Kang HG, Kim DH, Park
JW, Cheong W, Kong HK, Han W, Park WY, et al: MiR-374a-5p promotes
tumor progression by targeting ARRB1 in triple negative breast
cancer. Cancer Lett. 454:224–233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Zhong X, Xiao Y and Chen C:
MicroRNA-296 inhibits colorectal cancer cell growth and enhances
apoptosis by targeting ARRB1-mediated AKT activation. Oncol Rep.
41:619–629. 2019.PubMed/NCBI
|
|
30
|
Zhan Y, Xu C, Liu Z, Yang Y, Tan S, Yang
Y, Jiang J, Liu H, Chen J and Wu B: β-Arrestin1 inhibits
chemotherapy-induced intestinal stem cell apoptosis and mucositis.
Cell Death Dis. 7:e22292016. View Article : Google Scholar : PubMed/NCBI
|