Introduction
Titanium (Ti) is one the most frequently applied
materials for dental implants due to their excellent
osteocompatibility, mechanical property and corrosion resistance
(1–3). The oral cavity is a humid environment
that provides a habitat for acid-producing bacteria, which causes
low pH around the implants (4–6).
This acidity triggers bio-corrosion, which induces Ti ion release
to negatively affect osseointegration (7,8). It
has been previously reported that excessive Ti ions exert
detrimental influences on bone cells, which can in turn negatively
affect peri-implant bone remodeling and osseointegration (9). Following implantation, bone
reconstruction around the implant can be achieved, which is heavily
dependent on the delicate balance between bone resorption and
formation. During this process, osteoclasts and osteoblasts
interact with each other to maintain bone remodeling. As a possible
noxious stimulus on bone cells, Ti ions released during the
corrosion process or Ti nanoparticles escaped during surgery can
disrupt this balance by activating osteoclasts whilst inhibiting
osteoblasts (10–12). Osteoclast-induced bone resorption
is associated with a number of factors, including parathyroid
hormone, interleukin (IL)-17A and semaphorin 3A (13–15).
Excessive titanium ion or nanoparticle accumulation can promote the
expression of TNF-α and IL-6, both of which can provoke
inflammation and osteoclastogenesis (16,17).
In terms of the osteoblastic bone formation, several novel
regulators of osteoblast differentiation have been found, including
caveolin-1 and Doublecortin-like kinase 1 (DCAMKL1, a
serine/threonine kinase) that belongs to the CaM kinase family and
shares homology with the neuronal microtubule binding protein
doublecortin (18,19).
At present, studies reporting the effects of
titanium corrosion products on osteoblasts are insufficient. A
previous study reported that 10 ppm Ti ions, which is the minimum
toxic concentration, was capable of suppressing the growth whilst
promoting the nuclear translocation of Yes-associated protein in
osteoblast-like MC3T3-E1 cells (20). Although the negative effect of Ti
ion exposure has been explored in preliminary studies, the
underlying regulatory mechanism of the effects mediated by
excessive Ti ion accumulation during osteoblast differentiation
remain poorly understood.
Many signaling pathways and key signaling molecules
have been reported to regulate osteogenic differentiation. Bone
morphogenetic protein, Wnt, Hippo and MAPK signaling pathways have
all been previously demonstrated to serve important roles in
regulating osteoblast physiology (21–26).
Among these, the MAPK signaling cascade pathway is particularly
important for processes including cell proliferation, apoptosis and
differentiation by activating nuclear transcription factors
(27). The MAPK family is
comprised of a number of components, including JNK, ERK and p38.
ERK1/2 has been previously associated with cell proliferation
(28) whereas p38 tended to bias
towards cell differentiation (29). JNK signaling has been shown to be
extensively activated by oxidative stress, where it serves as a
regulator of several life-cycle processes, including cell meiosis,
mitosis, differentiation and energy metabolism (30). In particular, JNK activity is
required for the initiation of early osteogenic differentiation in
mesenchymal stem cells (MSCs) (31,32).
By contrast, attenuation of JNK activity using JNK inhibitors was
found to result in increased late stage osteogenic differentiation
(33). These findings suggest that
the MAPK/JNK signaling pathway serves a vital role throughout
different stages of osteogenic differentiation. However, to the
best of our knowledge, there remains an insufficient number of
studies evaluating the relationship between elevated Ti ion levels
and JNK activity, which may prove useful in clarifying the
mechanism behind the effects of Ti ions on osteoblast behaviors.
Consequently, the aim of the present study was to explore the role
of the MAPK/JNK signaling pathway in the molecular mechanism
underlying the effects of excessive Ti ion exposure on the
biological characteristics of MC3T3-E1 osteoblasts.
Materials and methods
Cell culture
MC3T3-E1 cells, an osteoblast-like cell line, were
obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and used as an in vitro model of
osteoblast development for evaluating cellular responses, including
attachment, proliferation and gene expression. MC3T3-E1 cells were
cultured in α-MEM containing 10% FBS and 1% penicillin/streptomycin
(All Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere under 5% CO2. The culture medium was changed
every two days, where cells were digested and passaged every time
80–90% confluence was reached. Titanium atomic absorption standard
solution (1,000 µg/ml) was purchased from Sigma-Aldrich, Merck KGaA
(cat. no. 274933). Cells co-cultured with or without 10 ppm Ti ions
(diluted 100 times from the Titanium atomic absorption standard
solution; 1,000 µg/ml) or inhibitor of the MAPK/JNK pathway (25 µM
SP600125) at 37°C in a humidified atmosphere under 5%
CO2 were denoted as the control, Ti, JNK inhibitor
[cells co-cultured with inhibitor of the MAPK/JNK pathway (25 µM
SP600125) at 37°C in a humidified atmosphere under 5%
CO2] and Ti + JNK inhibitor groups, respectively.
Cell adhesion and spreading assay
The initial cell seeding density into commercially
pure titanium samples (Titanium atomic absorption standard
solution; 1,000 µg/ml) was 5×103 cells/well. Cells were
pretreated with or without the JNK inhibitor SP600125 (cat. no.
8177; Cell Signaling Technologies, Inc.) for 1 h and then
co-cultured with 0 or 10 ppm Ti ions for another 8 h at 37°C in a
humidified atmosphere under 5% CO2. Each sample was
subsequently washed twice with PBS and fixed with 95% alcohol at
4°C overnight. 7 µl/2 ml Rhodamine Phalloidin (Cytoskeleton, Inc.)
was then prepared and added to the cells in the dark for 30 min at
room temperature to achieve cytoskeleton staining. After rinsing
the samples twice with PBS, 1 µl/2 ml DAPI was added (cat. no.
C1002; Beyotime Institute of Biotechnology) for 30 sec for nuclei
staining at room temperature. Each sample was observed at ×200
magnification using a confocal laser scanning microscope (LSM710;
Zeiss AG).
Alkaline phosphatase (ALP) activity
assay
MC3T3-E1 cells (2×105 cells/well) were
initially seeded into a 6-well plate and cultured overnight at 37°C
in a humidified atmosphere under 5% CO2. After
pretreatment with or without SP600125 for 1 h at 37°C, cells were
co-cultured with 0 or 10 ppm Ti ions (without SP600125) for a
further 7 days to investigate the effect of Ti ions on cell
differentiation. Each sample was washed with PBS twice and fixed
with 95% alcohol at 37°C for 30 min. ALP staining was determined
using ALP Stain Kit (cat. no. D001-1-1; Nanjing Jiancheng
Bioengineering Institute) and the duration of this staining
procedure was about 30 min at 37°C. Then the images were then
observed and captured using an Epson Perfection V30 scanner (Seiko
Epson Corporation). ALP activity was analyzed using an ALP Assay
Kit (cat. no. A059-2-2; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocols. Total protein content
was determined using bicinchoninic acid (BCA) protein assay kit
(Nanjing KeyGen Biotech Co., Ltd.). ALP activity relative to that
of control was calculated after normalization to the total protein
content.
Western blotting
The initial cell seeding density of MC3T3-E1 cells
was 2×105 cells/well in a 6-well plate. After 7 days
culture, cell proteins were extracted using RIPA buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology). The quantitation of
total protein was performed using a BCA protein assay kit (Nanjing
KeyGen Biotech Co., Ltd.). Protein lysates (20 µg) were separated
by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF)
films. The membranes were then blocked using 5% non-fat dry milk
for 1 h at room temperature before incubation serially with primary
antibodies overnight at 4°C and secondary antibodies for 2 h at
room temperature. After rinsing three times with PBS to remove the
residual antibodies, the protein bands were visualized using ECL
Western Blot Kit (EMD Millipore). The primary antibodies used in
the present study were as follows: Runt-related transcription
factor 2 (1:1,000, Runx2; cat. no. 12556; Cell Signaling
Technology, Inc.), Osterix (1:1,000, OSX; cat. no. ab22552; Abcam),
JNK (1:1,000, cat. no. 9252; Cell Signaling Technology, Inc.),
phosphorylated (p)-JNK (1:1,000, cat. no. 4668; Cell Signaling
Technology, Inc.) and GAPDH (1:3,000, cat. no. BM1623; Wuhan Boster
Biological Technology, Ltd.). The secondary antibodies used were as
follows: horseradish peroxidase-conjugated goat anti-rabbit IgG
(1:1,000, cat. no. ZB-2301; ZSGB-BIO; OriGene Technologies, Inc.)
and horseradish peroxidase-conjugated goat anti-Mouse IgG (1:1,000,
cat. no. AP124P; EMD Millipore). Protein quantification was
analyzed using ImageJ software (version k1.45; National Institutes
of Health).
Immunofluorescence staining for JNK
nuclear translocation
The initial cell seeding density of MC3T3-E1 cells
was 1×105 cells/well onto 24-well glass coverslips (13
mm diameter) in a 12-well plate. Cells were pretreated with or
without SP600125 for 1 h and then co-cultured with 0 or 10 ppm Ti
ions for another 3 days at 37°C in a humidified atmosphere under 5%
CO2. Afterwards, cells were washed in PBS, fixed with 4%
paraformaldehyde for 15 min at 4°C, treated with 0.5% Triton X-100
for 15 min at room temperature to increase permeability and then
blocked with 10% goat serum (cat. no. 005-000-121; Jackson
ImmunoResearch Laboratories, Inc.) for 1 h at 37°C. The cells were
subsequently incubated with primary antibodies at 4°C overnight,
followed by incubation with FITC-conjugated secondary antibodies
for 1 h at 37°C. All nuclei were stained with 1 µl/2 ml DAPI for 3
min at room temperature before the subcellular localization of
proteins were visualized at ×200 magnification and analyzed using
fluorescence microscopy (Leica DM4000M; Leica Microsystems GmbH).
The primary and secondary antibodies used for immunofluorescence
were as follows: P-JNK (1:100; cat. no. 4668; Cell Signaling
Technology, Inc.), DyLight 549 AffiniPure goat anti-rabbit IgG
(H+L; 1:200; cat no. A23320; Abbkine Scientific Co., Ltd.).
Statistical analysis
Data were analyzed using the SPSS 22.0 software (IBM
Corp.) and presented as the mean ± SD. Group differences were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
*P<0.05 and **P<0.01 were considered to indicate
statistically significant differences.
Results
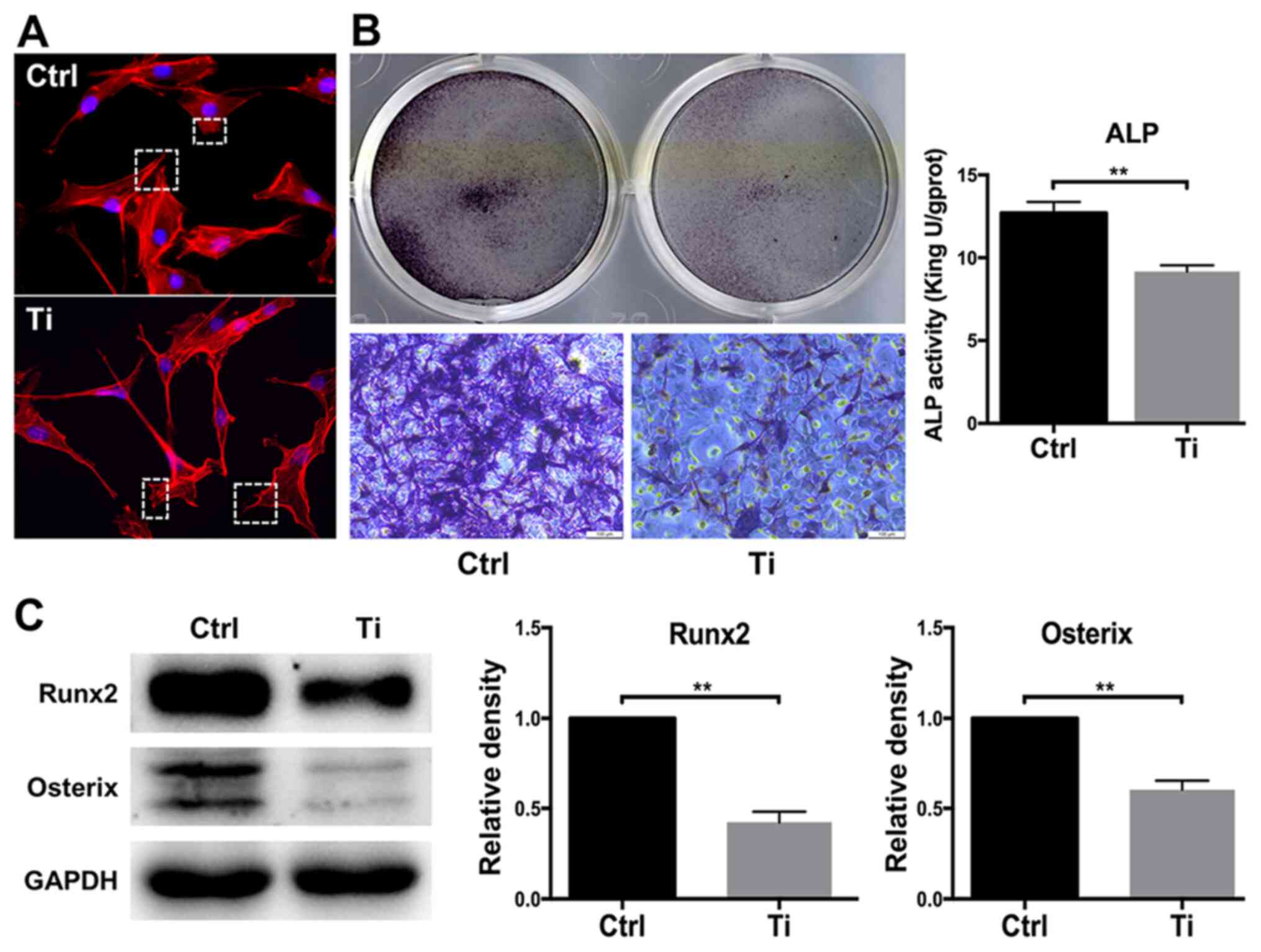
Inhibition of osteoblast behavior
following Ti ion exposure
The morphology of osteoblasts serves a key role in
regulating cell proliferation and differentiation (34). Cell adhesion and spreading were
observed after MC3T3-E1 cells were treated with 0 or 10 ppm Ti ions
for 8 h (Fig. 1A). Compared with
the control group, osteoblasts that were treated with 10 ppm Ti
ions exhibited disordered extension of pseudopodia, disorganized
cell spreading and poor substrate adherence (Fig. 1A), suggesting that 10 ppm Ti ions
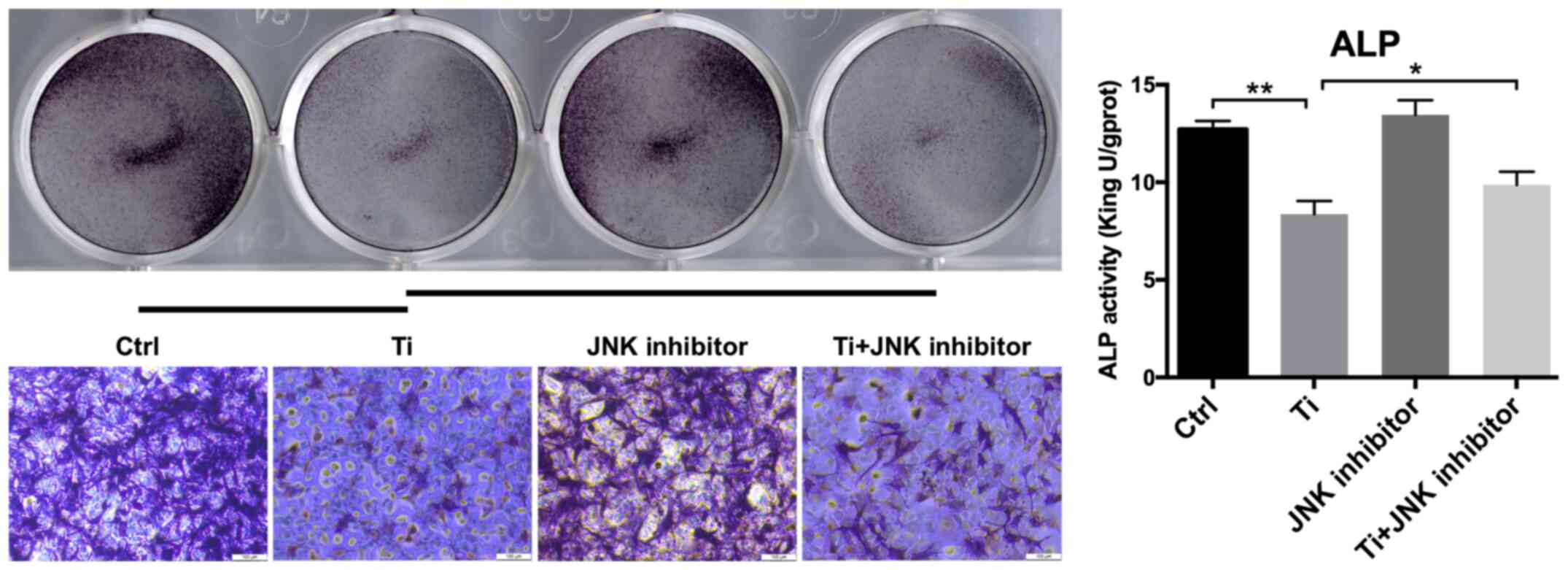
suppressed the first phase of osteoblast differentiation. Since ALP
is widely considered to be a marker of early-stage osteoblast
differentiation (35), ALP
staining and activity was next quantified in MC3T3-E1 cells. ALP
staining in the Ti group was found to be markedly weaker compared
with that in the control group, suggesting that 10 ppm Ti ions
inhibited osteoblast differentiation. A similar finding was
obtained from the levels of ALP activities, which was significantly
lower it the Ti group compared with those in the control group
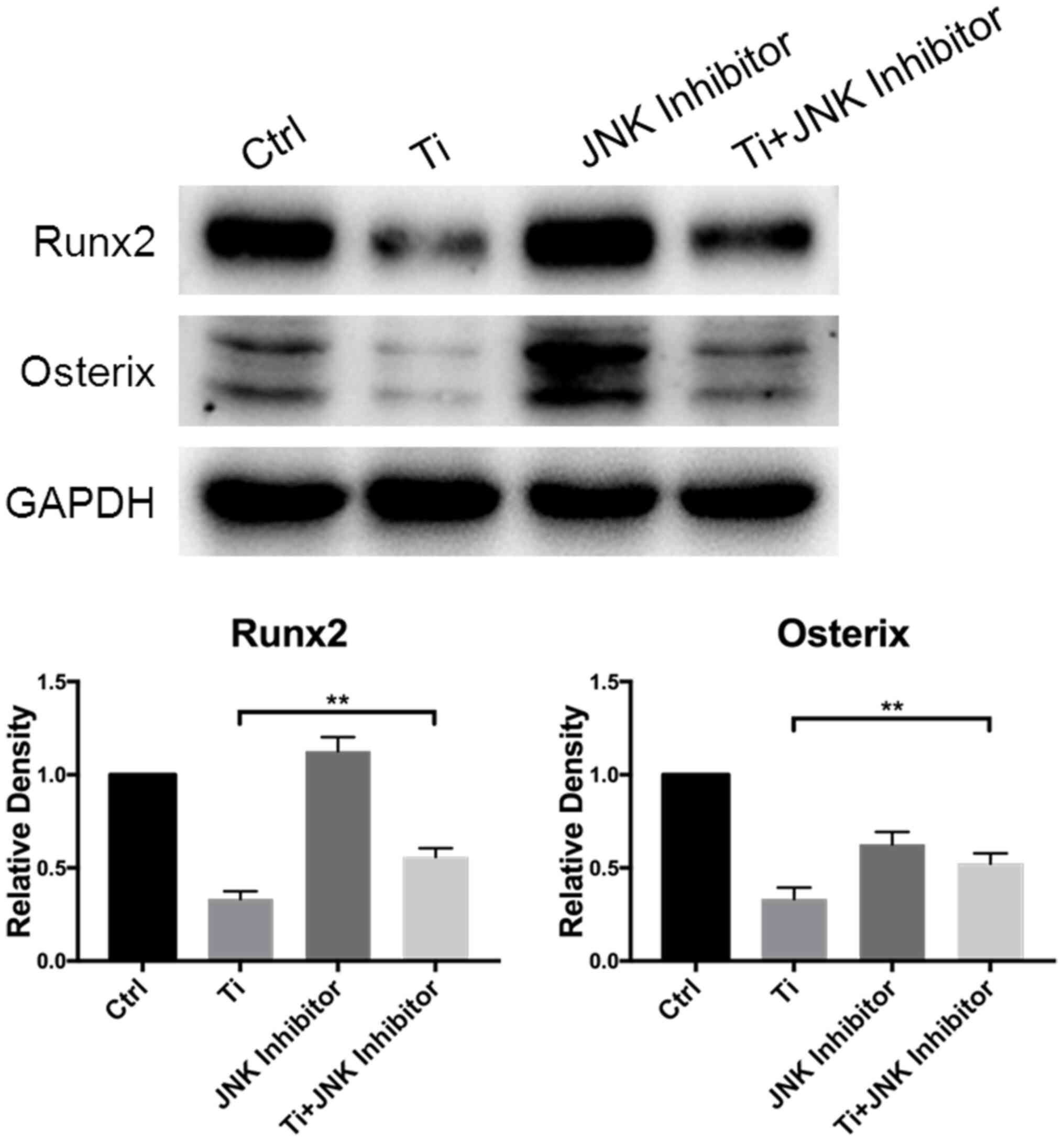
(Fig. 1B). Runx2 and OSX are vital
transcription factors for osteoblast differentiation (36). Therefore, the expression levels of
these two proteins were measured by western blotting to analyze
cell differentiation. After 7 days of culture, Runx2 and OSX
expression was revealed to be downregulated in MC3T3-E1 cells in
the Ti group compared with that in the control group, indicating
that 10 ppm Ti ions can inhibit the early mineralization process of
MC3T3-E1 cells (Fig. 1C).
Therefore, these observations suggest that 10 ppm Ti ions can
disrupt the biological behaviors of osteoblasts, which may be due
to the inhibition of cell adhesion in the early stages of
differentiation.
Activation of the MAPK/JNK signaling
pathway in osteoblasts following Ti ion exposure
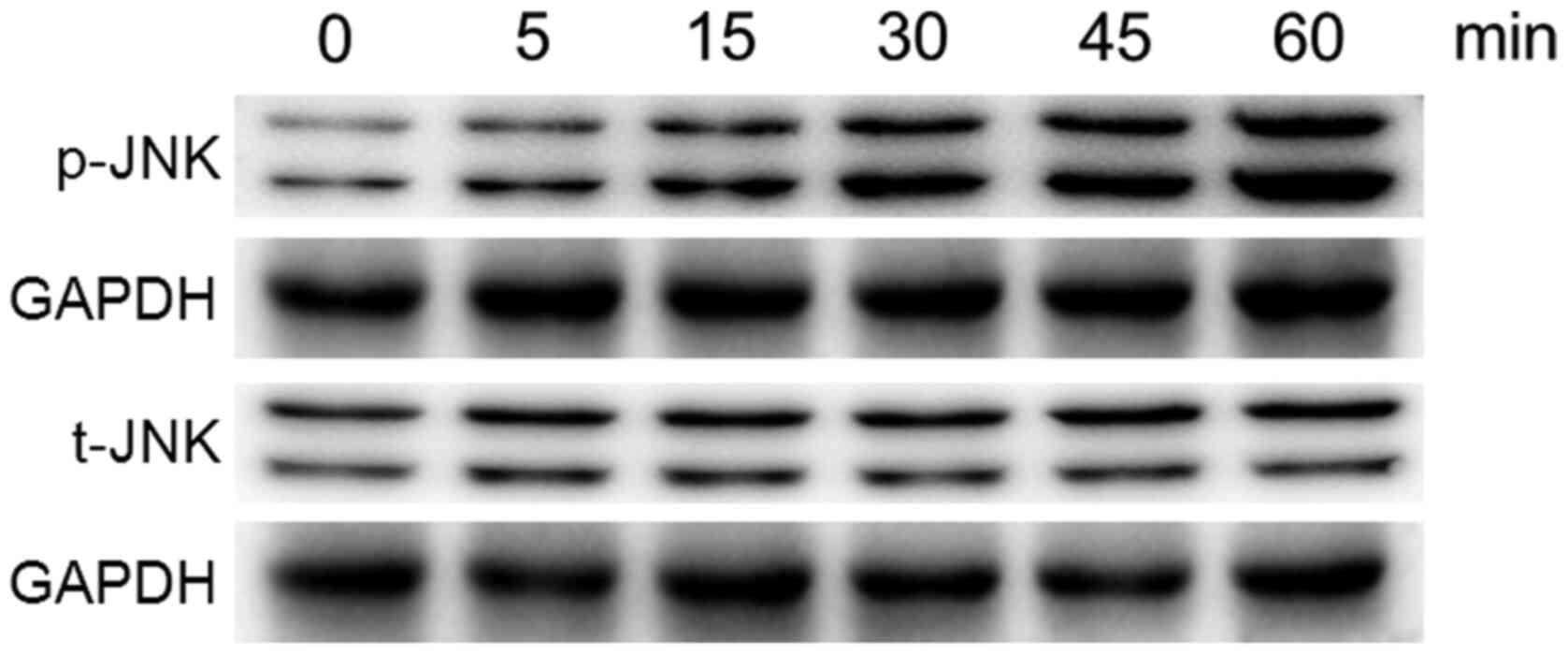
MC3T3-E1 cells were treated with 10 ppm Ti ions at
the early time points up to 60 min, following which the
phosphorylation levels of JNK were analyzed by western blotting,
since the phosphorylation process is rapid (Fig. 2). The levels of p-JNK was found to
be markedly increased following exposure to 10 ppm Ti ions within
60 min. However, the total protein expression of JNK remained
stable. These results suggest that the MAPK/JNK signaling pathway
was activated after treatment with 10 ppm Ti ions.
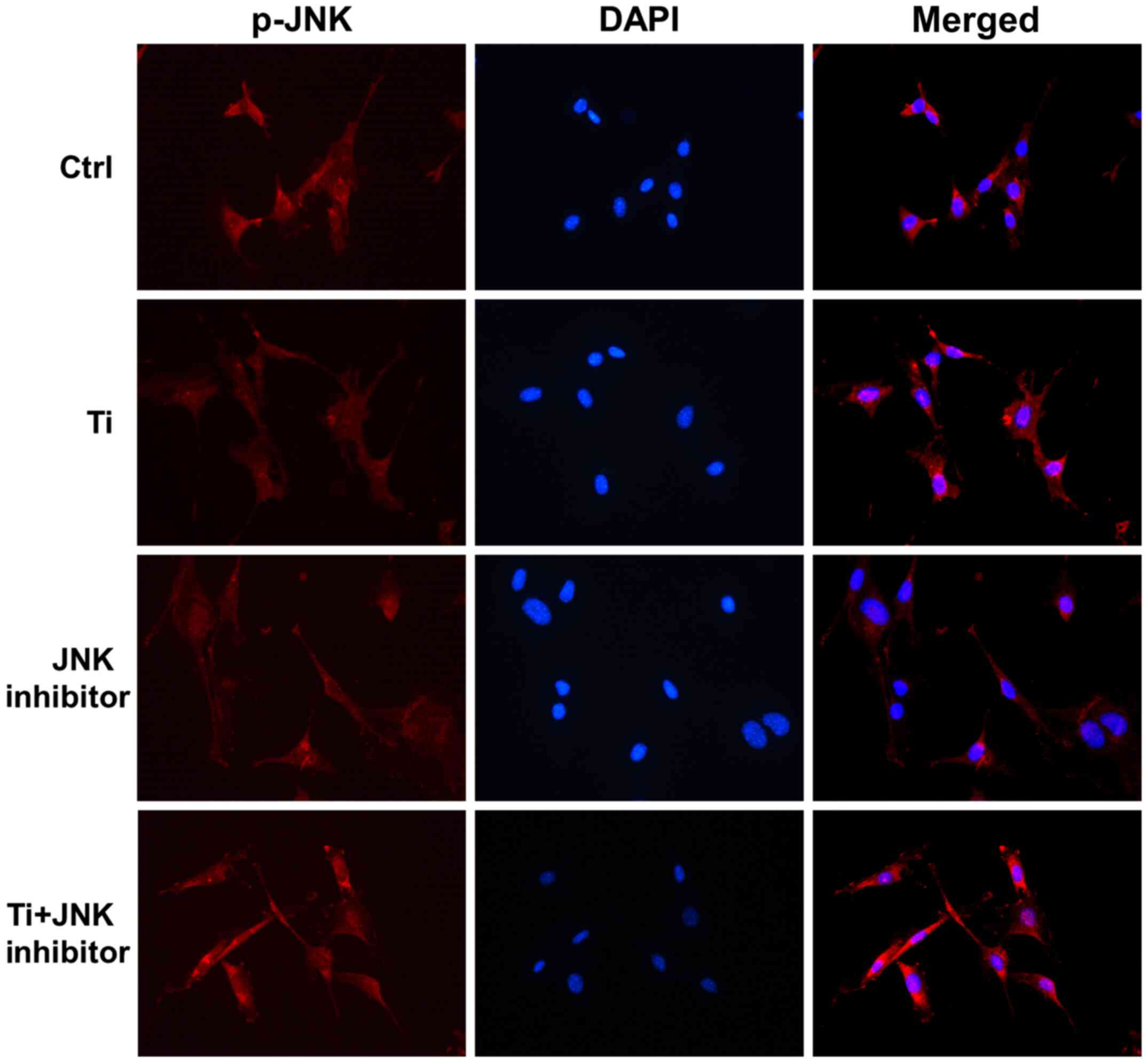
Immunofluorescence assay was used to confirm the
effects of Ti ions on the nuclear translocation of JNK. It was
shown that p-JNK is mainly localized in the cytoplasm (Fig. 3). After being exposed to 10 ppm Ti
ions for 72 h, p-JNK staining became stronger and was more
localized in the nuclei compared with that in the control group,
suggesting that JNK signaling has been activated (Fig. 3). These results suggested that 10
ppm Ti ions can activate the MAPK/JNK signaling pathway by
promoting the phosphorylation and subsequent nuclear translocation
of JNK.
Key role of MAPK/JNK signaling pathway
in the regulation of Ti ion-induced effects in osteoblasts
SP600125, a MAPK/JNK inhibitor, was used to explore
the role of this signaling pathway in the regulation of Ti
ion-induced effects in MC3T3-E1 osteoblasts. The effects of
SP600125 on the nuclear translocation of p-JNK (Fig. 3), cell adhesion (Fig. 4) and cell differentiation (Figs. 5 and 6) were separately investigated in
MC3T3-E1 cells following Ti ion exposure.
As aforementioned, 10 ppm Ti ions were demonstrated
to promote the nuclear localization of p-JNK (Fig. 3). Following pretreatment with
SP600125 for 1 h, the distribution of p-JNK in MC3T3-E1 cells
became more dispersed, where the nuclear staining was less intense
in the JNK inhibitor group compared with that in the control group.
Furthermore, after being treated with Ti ions, nuclear p-JNK
staining in the Ti + JNK inhibitor group was found to be less
overlapped compared with that in the Ti group, that is, following
treatment with Ti ions, there was less nuclear p-JNK red staining
overlapping with blue DAPI in the Ti + JNK inhibitor group compared
with that in the Ti group. These results suggested that 10 ppm Ti
ions activated the MAPK/JNK signaling pathway in MC3T3-E1
cells.
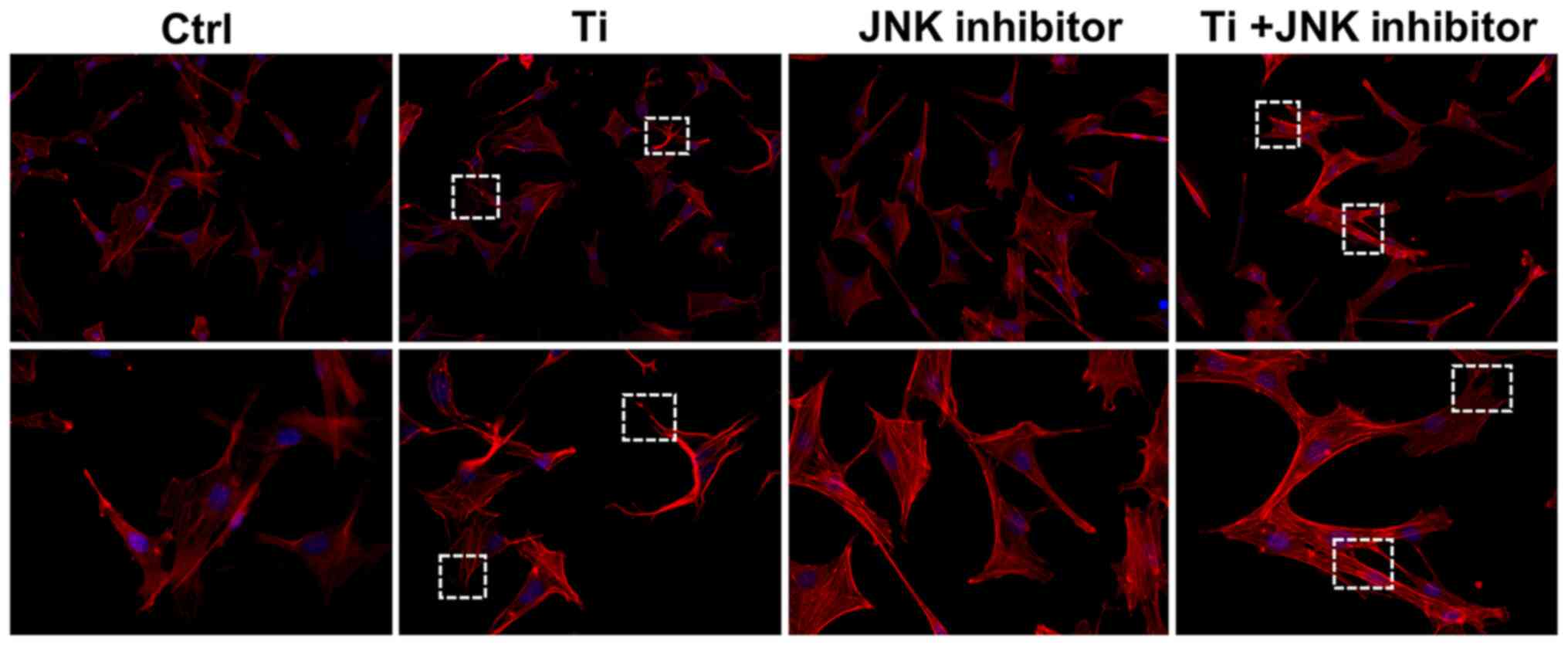
As shown in Fig. 4,
10 ppm Ti ions inhibited the spreading of MC3T3-E1 cells compared
with that in the control group. Following pretreatment with
SP600125, cells in the Ti + JNK inhibitor group were demonstrated
to extended more pseudopodia and spread more evenly (white boxes)
compared with those in the Ti group, suggesting that cell adhesion
and spreading ability were preserved following Ti ion exposure when
the MAPK/JNK pathway was inhibited. As shown in Fig. 5, 10 ppm Ti ions suppressed the ALP
activities in osteoblasts compared with those in the control group.
Following treatment with SP600125, the ALP activities in the Ti +
JNK inhibitor group was found to be significantly improved compared
with those in the Ti group (P=0.0378, *P<0.05). The expression
levels of Runx2 and OSX proteins after Ti ion exposure were also
revealed to be significantly downregulated compared with those in
the control group (Fig. 6). When
the MAPK/JNK pathway was blocked by SP600125, the reduced
expression levels of Runx2 and OSX caused by Ti treatment were
partially reversed, which offset some of the adverse effects
mediated by the Ti ions. Therefore, these findings suggest that 10
ppm Ti ion regulated the osteoblast behaviors through activating
the MAPK/JNK signaling pathway.
Discussion
Although there has been rapid developments in the
dental implantology field over the past decades, attention need to
be paid on the complications associated with dental implants
(37,38). Many concerns have been previously
raised about the response of the oral cavity to corrosive products
generated from titanium-based dental implants (39,40).
Noumbissi et al (41)
previously reported that titanium implant corrosion was a potential
risk factor for the establishment and progression of peri-implant
mucositis and peri-implantitis. These corrosive products, most of
which are comprised of free Ti ions, are typically released from
the damaged passivation film of the titanium (8). Previous evidence has shown that
excessive Ti ions and nanoparticles can hinder cell growth and
division in the tissues surrounding the implants (42). In addition, inflammatory cells or
phagocytic cells around the implants, including osteoclasts,
macrophage and foreign body giant cells, can be activated following
Ti ion exposure. This leads to immune system activation, which may
influence bone formation and increase the risk of implant failure
(43,44). Although some studies have reported
Ti ions-induced cytotoxicity in bone cells, the exact underlying
mechanism remains unclear.
A previous study has demonstrated that 10 ppm Ti
ions was a toxic concentration for osteoblast proliferation
(20). In the present study, the
effects of 10 ppm Ti ions on osteoblast adhesion and early
differentiation characteristics were investigated further. The
results demonstrated that 10 ppm Ti ions could restrict the
extension of osteoblast pseudopodia and inhibit cell spreading.
Osteoblast differentiation covers osteogenic commitment and
osteogenic differentiation (45).
During the osteogenic commitment process, Runx2 is considered to be
one of the core transcription factors (46), whilst ALP and OSX are considered to
be specific markers for the osteogenic differentiation process
(47,48). Analysis of ALP activity and western
blotting showed that the expression of these osteogenic markers was
downregulated following treatment with 10 ppm Ti ions compared with
those in control. These results were consistent a previous finding,
which also showed that 10 ppm Ti ions could inhibit proliferation
of osteoblasts (20).
MAPK signaling pathway has been recognized as an
important regulator of osteoblast differentiation and bone mass
(49,50). The upstream stimulation can
activate the key components of the MAPK signaling pathway by
post-translational modifications, including phosphorylation and
acetylation (51). This then
translates into downstream cascade reactions that can be triggered
to regulate biological processes in the nucleus (52). The role of the MAPK signaling
pathway on osteogenic function is garnering increasing research
attention (53). A previous study
has also demonstrated that JNK and ERK, which are key components of
the MAPK signaling pathway, could serve as regulators of osteoblast
physiology following fluoride exposure (26).
In the present study, the effects of Ti ions on the
MAPK/JNK signaling pathway and changes in the phosphorylation
status of JNK were investigated in MC3T3-E1 osteoblasts. The level
of JNK phosphorylation in MC3T3-E1 osteoblast was shown to be
increased after treatment with 10 ppm Ti ions within 60 min, whilst
the total JNK expression remained unchanged. Nuclear translocation
of p-JNK following exposure to Ti ions was investigated further.
Due to the rapid phosphorylation process, total intracellular
protein expression frequently remains unchanged. The nuclear
translocation status of p-JNK can be applied to independently
measure the activity of JNK (54).
Therefore, p-JNK was focused upon for the immunofluorescence
staining to investigate the nuclear translocation process. As shown
in Fig. 3 merged image, it was
found that the red coverage area in the blue core was greater in
the Ti group compared with the control group, suggesting that the
localization of p-JNK seemed to shift from the cytoplasm into the
nucleus in cells treated with 10 ppm Ti ions compared with that in
the control group. A previous study demonstrated that JNK can be
activated by dual phosphorylation at the threonine (Thr 183) and
tyrosine (Tyr 185) residues (55).
p-JNK can in turn activate the transcription factor c-Jun by
phosphorylation, which is known to form the activator protein-1
transcription complex with c-Jun's principal dimerization partner,
c-Fos (56). This complex finally
binds to specific DNA sequences at target promoters to regulate the
expression of associated genes that participate in the
differentiation function of osteoblasts (57). In the present study, JNK was
activated by Ti ions as the upstream stimulation, where the
activated JNK induced the reactions of nuclear-localized
transcription factors to influence the expressions of osteogenic
markers (Figs. 2 and 3). However, the role of the MAPK/JNK
signaling pathway in the Ti ion-induced osteoblast characteristics
requires further study.
To ascertain the role of JNK in the regulation of
osteoblast behaviors following Ti ion exposure further, subsequent
experiments were performed by inhibiting JNK using the inhibitor
SP600125. SP600125 is a common competitive inhibitor of JNK that
can inhibit the phosphorylation of c-Jun, which have been used in
many in vitro and in vivo studies (58,59).
Unlike small interfering RNAs or short hairpin RNAs products, which
are recognized as powerful tools for targeted gene silencing in
cells, SP600125 can partially reduce JNK expression rather than
completely knocking it out. Therefore, residual amounts of JNK
expression remained a possibility following the use of the JNK
inhibitor in this study. In MC3T3-E1 osteoblasts pretreated with
SP600125, a reduced number of p-JNK molecules went into the nuclei
in the Ti + JNK inhibitor group compared with that in the Ti group.
Importantly, the morphology of osteoblast adhesion was found to be
improved when JNK was blocked in cells exposed to Ti ions,
suggesting the ordered extension of the pseudopodia. In addition,
the levels of osteogenic markers were revealed to be significantly
upregulated when JNK was inhibited in cells exposed to Ti ions,
including the increased activity of ALP, Runx2 and OSX expression.
These results demonstrated that the JNK signaling significantly
regulated osteoblast behaviors under Ti ion exposure, such that JNK
inactivation could attenuate the negative effect of Ti ions on
osteoblast adhesion and osteogenic differentiation.
In conclusion, results from the present study
demonstrated the negative effects of 10 ppm Ti ions on osteoblast
physiology. It was shown that 10 ppm Ti ions can promote JNK
activation by phosphorylation, which could in turn serve a key role
in the regulation of Ti ion-induced cytotoxicity in osteoblasts.
Therapeutic interventions that target JNK signaling may therefore
improve the function of osteoblasts. These findings may serve as a
valuable reference for the further in-depth exploration of the
impact of Ti ions on titanium implant osseointegration and
peri-implant bone loss in vivo, where the mechanism of JNK
signaling involved in regulating bone formation after Ti ion
exposure could be explored further.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81870799), the Key
Research and Development Plan of Jiangsu Province (grant no.
BE2019728), the Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016850), the Southeast University-Nanjing Medical University
Cooperative Research Project (grant no. 2242017K3DN14), the Nanjing
Medical University-SUYAN Group Intelligent Innovation Research and
Development Project (grant no. NMU-SY201806), the Science and
Technology Development Foundation of Nanjing Medical University
(grant no. 2017NJMU217) and the Foundation of Priority Academic
Program Development of Jiangsu Higher Education Institutions (grant
no. 2018-87).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQZ contributed to the design, data acquisition and
analysis and drafted the manuscript. PPM and SMZ contributed to the
experiments performance, data acquisition and analysis. JQ
contributed to the conception, design, data interpretation in the
present study and critically revised the manuscript. All authors
agreed to be accountable for all aspects of the work. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ti
|
titanium
|
|
ALP
|
alkaline phosphatase
|
|
Runx2
|
runt-related transcription factor
2
|
|
OSX
|
Osterix
|
References
|
1
|
Ahn TK, Lee DH, Kim TS, Jang GC, Choi S,
Oh JB, Ye G and Lee S: Modification of titanium implant and
titanium dioxide for bone tissue engineering. Adv Exp Med Biol.
1077:355–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X, Geng H, Gong L, Zhang Q, Li H,
Zhang X, Wang Y and Gao P: Modification of the surface of titanium
with multifunctional chimeric peptides to prevent biofilm formation
via inhibition of initial colonizers. Int J Nanomedicine.
13:5361–5375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu WQ, Shao SY, Xu LN, Chen WQ, Yu XY,
Tang KM, Tang ZH, Zhang FM and Qiu J: Enhanced corrosion resistance
of zinc-containing nanowires-modified titanium surface under
exposure to oxidizing microenvironment. J Nanobiotechnology.
17:552019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Revathi A, Borrás AD, Muñoz AI, Richard C
and Manivasagam G: Degradation mechanisms and future challenges of
titanium and its alloys for dental implant applications in oral
environment. Mater Sci Eng C Mater Biol Appl. 76:1354–1368. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silva TSO, Freitas AR, Pinheiro MLL, do
Nascimento C, Watanabe E and Albuquerque RF: Oral biofilm formation
on different materials for dental implants. J Vis Exp.
24:577562018.
|
|
6
|
Puskar T, Jevremovic D, Williams RJ,
Eggbeer D, Vukelic D and Budak I: A comparative analysis of the
corrosive effect of artificial saliva of variable pH on DMLS and
Cast Co-Cr-Mo dental alloy. Materials (Basel). 7:6486–6501. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noronha Oliveira M, Schunemann WVH, Mathew
MT, Henriques B, Magini RS, Teughels W and Souza JCM: Can
degradation products released from dental implants affect
peri-implant tissues? J Periodontal Res. 53:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Golasik M, Herman M and Piekoszewski W:
Toxicological aspects of soluble titanium-a review of in vitro and
in vivo studies. Metallomics. 8:1227–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wachi T, Shuto T, Shinohara Y, Matono Y
and Makihira S: Release of titanium ions from an implant surface
and their effect on cytokine production related to alveolar bone
resorption. Toxicology. 327:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Deng Z, Gan J, Zhou G, Shi T, Wang
Z, Huang Z, Qian H, Bao N, Guo T, et al:
TiAl6V4 particles promote osteoclast
formation via autophagy-mediated downregulation of interferon-beta
in osteocytes. Acta Biomater. 48:489–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stegen S, van Gastel N, Eelen G,
Ghesquière B, D'Anna F, Thienpont B, Goveia J, Torrekens S, Van
Looveren R, Luyten FP, et al: HIF-1α promotes glutamine-mediated
redox homeostasis and glycogen-dependent bioenergetics to support
postimplantation bone cell survival. Cell Metab. 23:265–279. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Y, Newman H, Shen L, Sharma D, Hu G,
Mirando AJ, Zhang H, Knudsen E, Zhang GF, Hilton MJ and Karner CM:
Glutamine metabolism regulates proliferation and lineage allocation
in skeletal stem cells. Cell Metab. 29:966–978.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Hanai JI, Le PT, Bi R, Maridas D,
DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, et al:
Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell
Metab. 25:661–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li JY, D'Amelio P, Robinson J, Walker LD,
Vaccaro C, Luo T, Tyagi AM, Yu M, Reott M, Sassi F, et al: IL-17A
is increased in humans with primary hyperparathyroidism and
mediates PTH-induced bone loss in mice. Cell Metab. 22:799–810.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayashi M, Nakashima T, Yoshimura N,
Okamoto K, Tanaka S and Takayanagi H: Autoregulation of osteocyte
Sema3A orchestrates estrogen action and counteracts bone aging.
Cell Metab. 29:627–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miron RJ and Bosshardt DD: OsteoMacs: Key
players around bone biomaterials. Biomaterials. 82:1–19. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao S, Sun Y, Li X, Wang J, Yan L, Zhang
Z, Wang D, Dai J, He J and Wang S: Scutellarin inhibits
RANKL-mediated osteoclastogenesis and titanium particle-induced
osteolysis via suppression of NF-κB and MAPK signaling pathway. Int
Immunopharmacol. 40:458–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berger JM, Singh P, Khrimian L, Morgan DA,
Chowdhury S, Arteaga-Solis E, Horvath TL, Domingos AI, Marsland AL,
Yadav VK, et al: Mediation of the acute stress response by the
skeleton. Cell Metab. 30:890–902 e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Li C, Zhang Y, Zhang D, Otterbein
LE and Jin Y: Caveolin-1 selectively regulates microRNA sorting
into microvesicles after noxious stimuli. J Exp Med. 216:2202–2220.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu WQ, Ming PP, Qiu J, Shao SY, Yu YJ,
Chen JX, Yang J, Xu LN, Zhang SM and Tang CB: Effect of titanium
ions on the Hippo/YAP signaling pathway in regulating biological
behaviors of MC3T3-E1 osteoblasts. J Appl Toxicol. 38:824–833.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai S, Wan L, Wang JL, Huang JC and Huang
HX: Gushukang inhibits osteocyte apoptosis and enhances BMP-2/Smads
signaling pathway in ovariectomized rats. Phytomedicine.
64:1530632019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jimi E: The role of BMP signaling and
NF-κB signaling on osteoblastic differentiation, cancer
development, and vascular diseases-is the activation of NF-κB a
friend or foe of BMP function? Vitam Horm. 99:145–170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lerner UH and Ohlsson C: The WNT system:
Background and its role in bone. J Intern Med. 277:630–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang
L and Xu Y: Cross-talking between PPAR and WNT signaling and its
regulation in mesenchymal stem cell differentiation. Curr Stem Cell
Res Ther. 11:247–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia L, Zhang Y, Ji Y, Xiong Y, Zhang W,
Wen Y and Xu X: YAP balances the osteogenic and adipogenic
differentiation of hPDLSCs in vitro partly through the
Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun.
518:154–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu WQ, Yu YJ, Xu LN, Ming PP, Shao SY and
Qiu J: Regulation of osteoblast behaviors via cross-talk between
Hippo/YAP and MAPK signaling pathway under fluoride exposure. J Mol
Med (Berl). 97:1003–1017. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng W, Gu X, Hu D and Hao Y: Co-culture
with synovial tissue in patients with rheumatoid arthritis suppress
cell proliferation by regulating MAPK pathway in osteoblasts. Am J
Transl Res. 11:3317–3327. 2019.PubMed/NCBI
|
|
28
|
Chen J, Cao J and Luo Y: Expression of ERK
and p-ERK proteins of ERK signaling pathway in the kidneys of
fluoride-exposed carp (Cyprinus carpio). Acta Histochem.
116:1337–1341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang D, Li X, Sun L, Huang P, Ying H,
Wang H, Wu J and Song H: Regulation of Hippo signalling by p38
signalling. J Mol Cell Biol. 8:328–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Chen J, Xie L, Wang J, Feng C and Song
J: Protective properties of sesamin against fluoride-induced
oxidative stress and apoptosis in kidney of carp (Cyprinus
carpio) via JNK signaling pathway. Aquat Toxicol. 167:180–190.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizerska-Kowalska M, Slawinska-Brych A,
Kalawaj K, Żurek A, Pawińska B, Rzeski W and Zdzisińska B: Betulin
promotes differentiation of human osteoblasts in vitro and exerts
an osteoinductive effect on the hFOB 1.19 cell line through
activation of JNK, ERK1/2, and mTOR kinases. Molecules.
24:2637–2653. 2019. View Article : Google Scholar
|
|
32
|
Balera Brito VG, Chaves-Neto AH, Landim de
Barros T and Penha Oliveira SH: Soluble yerba mate (Ilex
Paraguariensis) extract enhances in vitro osteoblastic
differentiation of bone marrow-derived mesenchymal stromal cells. J
Ethnopharmacol. 244:1121312019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawabata T, Tokuda H, Fujita K,
Matsushima-Nishiwaki R, Sakai G, Tachi J, Hioki T, Kim W, Iida H,
Otsuka T and Kozawa O: HSP90 inhibitors diminish PDGF-BB-induced
migration of osteoblasts via suppression of p44/p42 MAP kinase.
Biomed Res. 40:169–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayes JS, Khan IM, Archer CW and Richards
RG: The role of surface microtopography in the modulation of
osteoblast differentiation. Eur Cell Mater. 20:98–108. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glynn ER, Londono AS, Zinn SA, Hoagland TA
and Govoni KE: Culture conditions for equine bone marrow
mesenchymal stem cells and expression of key transcription factors
during their differentiation into osteoblasts. J Anim Sci
Biotechnol. 4:402013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ting M, Craig J, Balkin BE and Suzuki JB:
Peri-implantitis: A Comprehensive overview of systematic reviews. J
Oral Implantol. 44:225–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Romanos GE, Delgado-Ruiz R and Sculean A:
Concepts for prevention of complications in implant therapy.
Periodontol 2000. 81:7–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Laura A, Hothi HS, Meswania JM,
Whittaker RK, de Villiers D, Zustin J, Blunn GW, Skinner JA and
Hart AJ: Clinical relevance of corrosion patterns attributed to
inflammatory cell-induced corrosion: A retrieval study. J Biomed
Mater Res B Appl Biomater. 105:155–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu WQ, Qiu J and Zhang FQ: In vitro
corrosion study of different TiO2 nanotube layers on titanium in
solution with serum proteins. Colloids Surf B Biointerfaces.
84:400–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noumbissi S, Scarano A and Gupta S: A
literature review study on atomic ions dissolution of titanium and
its alloys in implant dentistry. Materials (Basel). 12:3682019.
View Article : Google Scholar
|
|
42
|
Mercan S, Bölükbaşı N, Bölükbaşı MK, Yayla
M and Cengiz S: Titanium element level in peri-implant mucosa.
Biotechnol Biotechnol Equip. 27:4002–4005. 2014. View Article : Google Scholar
|
|
43
|
Messer RL, Tackas G, Mickalonis J, Brown
Y, Lewis JB and Wataha JC: Corrosion of machined titanium dental
implants under inflammatory conditions. J Biomed Mater Res B Appl
Biomater. 88:474–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vallés G, González-Melendi P,
González-Carrasco JL, Saldaña L, Sánchez-Sabaté E, Munuera L and
Vilaboa N: Differential inflammatory macrophage response to rutile
and titanium particles. Biomaterials. 27:5199–5211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Costa V, Carina V, Fontana S, De Luca A,
Monteleone F, Pagani S, Sartori M, Setti S, Faldini C, Alessandro
R, et al: Osteogenic commitment and differentiation of human
mesenchymal stem cells by low-intensity pulsed ultrasound
stimulation. J Cell Physiol. 233:1558–1573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zou W, Greenblatt MB, Brady N, Lotinun S,
Zhai B, de Rivera H, Singh A, Sun J, Gygi SP, Baron R, et al: The
microtubule-associated protein DCAMKL1 regulates osteoblast
function via repression of Runx2. J Exp Med. 210:1793–1806. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maehata Y, Takamizawa S, Ozawa S, Kato Y,
Sato S, Kubota E and Hata R: Both direct and collagen-mediated
signals are required for active vitamin D3-elicited differentiation
of human osteoblastic cells: Roles of osterix, an
osteoblast-related transcription factor. Matrix Biol. 25:47–58.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou W, Zhang J, Lin K and Chen F:
Comparison between mandibular and femur derived bone marrow stromal
cells: Osteogenic and angiogenic potentials in vitro and bone
repairing ability in vivo. RSC Adv. 7:56220–56228. 2017. View Article : Google Scholar
|
|
49
|
Zhang DD, Wu YF, Chen WX, Xu Y, Liu SY,
Luo HH, Jiang GM, Wu Y and Hu P: C-type natriuretic peptide
attenuates renal osteodystrophy through inhibition of FGF-23/MAPK
signaling. Exp Mol Med. 51:702019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen M, Chen PM, Dong QR, Huang Q, She C
and Xu W: p38 Signaling in titanium particle-induced MMP-2
secretion and activation in differentiating MC3T3-E1 cells. J
Biomed Mater Res A. 102:2824–2832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li K, Yang F, Zhang G, Song S, Li Y, Ren
D, Miao Y and Song CP: AIK1, a mitogen-activated protein kinase,
modulates abscisic acid responses through the MKK5-MPK6 kinase
cascade. Plant Physiol. 173:1391–1408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang C, Sun H and Zhong Y: Notoginsenoside
R1 promotes MC3T3-E1 differentiation by up-regulating miR-23a via
MAPK and JAK1/STAT3 pathways. Artif Cells Nanomed Biotechnol.
47:603–609. 2019.PubMed/NCBI
|
|
53
|
Ewendt F and Föller M: p38MAPK controls
fibroblast growth factor 23 (FGF23) synthesis in
UMR106-osteoblast-like cells and in IDG-SW3 osteocytes. J
Endocrinol Invest. 42:1477–1483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu S, Parameswaran H, Young SM and
Varisco BM: JNK suppresses pulmonary fibroblast elastogenesis
during alveolar development. Respir Res. 15:342014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fleming Y, Armstrong CG, Morrice N,
Paterson A, Goedert M and Cohen P: Synergistic activation of
stress-activated protein kinase 1/c-Jun N-terminal kinase
(SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4
(MKK4) and MKK7. Biochem J. 145–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen K, Ng PY, Chen R, Hu D, Berry S,
Baron R and Gori F: Sfrp4 repression of the Ror2/Jnk cascade in
osteoclasts protects cortical bone from excessive endosteal
resorption. Proc Natl Acad Sci USA. 116:14138–14143. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ouyang Z, Huang Q, Liu B, Wu H, Liu T and
Liu Y: Rubidium chloride targets Jnk/p38-mediated NF-κB activation
to attenuate osteoclastogenesis and facilitate osteoblastogenesis.
Front Pharmacol. 10:5842019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gao P, Wang H, Liu J, Wu Y, Hei W, He Z,
Cai C, Guo X, Cao G and Li B: miR-128 regulated the proliferation
and autophagy in porcine adipose-derived stem cells through
targeting the JNK signaling pathway. J Recept Signal Transduct Res.
1–6. 2020.(Online ahead of print). View Article : Google Scholar
|
|
59
|
Mulder SE, Dasgupta A, King RJ, Abrego J,
Attri KS, Murthy D, Shukla SK and Singh PK: JNK signaling
contributes to skeletal muscle wasting and protein turnover in
pancreatic cancer cachexia. Cancer Lett. 491:70–77. 2020.(Online
ahead of print). View Article : Google Scholar : PubMed/NCBI
|