Introduction
Angiogenesis, a process of novel blood vessel
formation from the pre-existing vasculature, serves a critical role
in numerous human neovascularization diseases, such as diabetic
retinopathy, ischemia/reperfusion injury and carcinogenesis
(1). Vascular homeostasis is an
intrinsically regulated procedure controlled by the balance of
angiogenic and anti-angiogenic factors during normal physiological
activities (1). Once homeostasis
is interrupted, neovascularization is inevitable, becoming a major
cause of irreversible vision loss in various clinical eye diseases,
including retinopathy of prematurity, diabetic retinopathy and
age-related macular degeneration (AMD) (2–4).
The underlying molecular mechanisms of choroidal
neovascularization (CNV) are multifactorial and complicated.
Previous studies have demonstrated that hypoxia and ischemia serve
important roles in the formation of CNV (5–8).
Multiple hypoxia-induced growth factors and inflammatory cytokines,
including VEGF, hypoxia-inducible factor-1α, TNF-α and IL-1,
stimulated the progression of CNV (5–8).
Among these factors, VEGF is hypothesized to be the most important
inducer of CNV under hypoxic conditions (5–9).
Insulin-like growth factor binding protein-related
protein 1 (IGFBP-rP1) is a soluble-secreted 36-kDa glycoprotein,
also known as IGFBP7, mac25, TAF and angiomodulin (10,11).
IGFBP-rP1 is distinct from other IGFBPs since it exhibits a low
affinity for IGFs and a high affinity for insulin (10,11).
Our previous studies have revealed that IGFBP-rP1 suppressed the
phenotype activation of retinal endothelial cells induced by VEGF
in vitro and inhibited retinal angiogenesis by
downregulating VEGF in vivo (12,13).
Notably, IGFBP-rP1 was demonstrated to be decreased in the aqueous
humor of patients with CNV secondary to AMD compared with control
patients with cataract; however, its specific role remains unknown
(14).
In the present study, IGFBP-rP1 specific small
interfering RNA (siIGFBP-rP1) was transfected into choroidal
endothelial (RF/6A) cells to block the expression of IGFBP-rP1
under hypoxic conditions to investigate the role of
IGFBP-rP1-silencing in the hypoxia-induced angiogenic potential of
choroidal endothelial cells and the underlying mechanisms.
Materials and methods
Media and reagents
Lipofectamine® 3000 transfection reagent
was purchased from Thermo Fisher Scientific, Inc. PrimeScript
reverse transcription (RT) reagent kit and SYBR Premix Ex Taq
Real-Time PCR kit were purchased from Takara Bio, Inc. and were
used in RT-quantitative (q)PCR assays. Recombinant human IGFBP-rP1
and goat anti-human IGFBP-rP1 polyclonal antibody (cat. no. AF1334)
were obtained from R&D Systems, Inc. Rabbit anti-human GAPDH
polyclonal (cat. no. 10494-1-AP) and mouse anti-human β-actin
monoclonal (cat. no. 66009-1-Ig) antibodies were obtained from
ProteinTech Group, Inc. Rabbit anti-human VEGF polyclonal (cat. no.
ab150766) antibodies were purchased from Abcam. The other primary
antibodies, including rabbit anti-human B-RAF monoclonal (cat. no.
14814), rabbit anti-human phosphorylated-MEK (p-MEK) polyclonal
(cat. no. 9121), rabbit anti-human MEK polyclonal (cat. no. 9122),
rabbit anti-human phosphorylated-ERK (p-ERK) polyclonal (cat. no.
9101) and rabbit anti-human ERK polyclonal (cat. no. 9102)
antibodies were purchased from Cell Signaling Technology, Inc.
Horseradish peroxidase (HRP) mouse anti-goat (cat. no. sc2354),
goat anti-rabbit (cat. no. sc-2004) and goat anti-mouse (cat. no.
sc-2005) secondary antibodies were obtained from Santa Cruz
Biotechnology, Inc. The PVDF membranes and Chemiluminescent HRP
Substrate reagent were purchased from EMD Millipore. CellTiter
96® AQueous One Solution Cell Proliferation Assay (MTS
Assay) was purchased from Promega Corporation. Transwell Permeable
Supports with an 8-µm pore polycarbonate filter were purchased from
Corning, Inc. Growth factor-reduced Matrigel matrix was purchased
from BD Biosciences. All other chemicals were of reagent grade and
obtained from Merck KGaA, unless otherwise specified.
Cell line and culture
RF/6A cells, a well-established choroid endothelial
cell line for studying CNV pathogenesis (15–17),
were obtained from the Cell Bank of the Chinese Academy of
Sciences. The cells were cultured in RPMI-1640 medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (GE Healthcare
Life Sciences). The cells were maintained at 37°C in a 5%
CO2-humidified incubator and the medium was changed
every 2 or 3 days. Prior to experimental intervention, the medium
was replaced with serum-free RPMI-1640 for 12 h.
Following siRNA transfection for 24 h, RF/6A cells
were subjected to hypoxic conditions and IGFBP-rP1-restored
conditions for further study. Cobalt chloride (CoCl2;
Merck KGaA) at a final concentration of 200 µmol/l or 1%
O2 in the presence of 5% CO2 and 94%
N2 using a ProOx C21 System (BioSpherix, Ltd.) was used
to mimic hypoxic conditions. To restore IGFBP-rP1 expression,
recombinant human IGFBP-rP1 was added at a final concentration of
200 ng/ml for 24 h in the conditions of the siIGFBP-rP1 duplex 2
group, which has been demonstrated by previous studies (18–20).
All experiments were performed in triplicate.
IGFBP-rP1 gene silencing
siRNAs for IGFBP-rP1 were purchased from Guangzhou
RiboBio Co., Ltd. The sequences of the positive siIGFBP-rP1 duplex
1 and 2 were 5′-TCC-TCC-TCT-TCG-GAC-ACC-T-3′ and
5′-GTC-GCT-ACA-TGC-CCT-GCT-C-3′, respectively. RF/6A cells were
seeded into six-well plates and transfected with 50 nM siRNA using
Lipofectamine® 3000 transfection reagent. Briefly, 50 nM
siRNA in Opti-MEM medium (Thermo Fisher Scientific, Inc.) was mixed
with 4 µl Lipofectamine® 3000 and incubated for 25 min
at room temperature prior to adding the mixture to the cells
cultured in serum-free medium. The cells were incubated at 37°C for
5 h. Following this, the medium was replaced with RPMI-1640
complete medium for 48 h before the level of silencing was
determined by RT-qPCR and western blotting. Scramble control siRNA,
transfection reagent and blank control were used to compare the
effects of siIGFBP-rP1.
RNA extraction and RT-qPCR
Total RNA was extracted from cultured RF/6A cells
using a RNeasy Mini kit (Qiagen, Inc.), according to the
manufacturer's protocol. Total RNA was eluted in 40 µl
nuclease-free water. The concentration and purity of RNA were
measured by spectrophotometry (NanoDrop 8000; Thermo Fisher
Scientific, Inc.) at 15°C within 10 sec. In all RNA preparations,
the ratio of optical density (OD)260 to 280
was 1.9:2.0. Equal amounts of RNA (0.5 µg) were converted into cDNA
using PrimeScript RT reagent kit (Takara Bio, Inc.) and qPCR was
performed using SYBR Premix Ex Taq real-time PCR kit (Takara Bio,
Inc.), according to the manufacturer's protocols. The primers of
IGFBP-rP1 and GAPDH were designed and synthesized by Sangon Biotech
Co., Ltd. GAPDH served as an internal reference for the control.
The primer sequences used were as follows: IGFBP-rP1 forward,
5′-AGC-TGT-GAG-GTC-ATC-GGA-AT-3′ and reverse,
5′-CAG-CAC-CCA-GCC-AGT-TAC-TT-3′; and GAPDH forward,
5′-GAG-TCA-ACG-GAT-TTG-GTC-GT-3′ and reverse,
5′-GAC-AAG-CTT-CCC-GTT-CTC-AG-3′. The One-Step RT-PCR thermocycling
conditions were as follows: 30 min of initial reverse transcription
at 50°C, enzyme heat-activation at 95°C for 15 min, followed by 35
three-step amplification cycles of denaturation at 94°C for 40 sec,
annealing at 60°C for 40 sec and extension at 72°C for 1 min. The
qPCR thermocycling conditions were as follows: enzyme
heat-activation at 95°C for 30 sec, followed by 40 two-step
amplification cycles of denaturation at 95°C for 5 sec, annealing
and extension at 60°C for 35 sec. Relative gene expression was
calculated using a standard curve (the optical density was
determined at 280 nm) and IGFBP-rP1 mRNA levels were normalized to
GAPDH.
Western blotting
The RF/6A cells cultured in the IGFBP-rP1-silencing
group, the hypoxic group and the control group were washed with
phosphate-buffered saline (PBS) twice and lysed in ice-cold RIPA
buffer (1 mmol/l phenylmethyl sulfonylfluoride, 10 µg/ml leupeptin
and 10 µg/ml aprotinin). Following 30 min on ice, lysates were
scraped into microcentrifugation tubes and centrifuged at 14,000 ×
g for 10 min at 4°C. The supernatant was removed and the protein
concentrations were quantified using a Pierce protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cell lysates (30 µg protein/lane) were
separated by 10% SDS-PAGE and transferred onto PVDF membranes.
Following blocking with 5% blocking liquid diluted in Tris-buffered
saline with 0.1% Tween-20 for 1 h at 37°C, membranes were incubated
with different dilutions of primary antibodies overnight at 4°C.
The following day, the membranes were washed three times and
subsequently incubated with HRP-conjugated secondary antibodies for
1 h at room temperature. The primary antibodies used included
IGFBP-rP1 (1:500), B-RAF (1:500), p-MEK (1:1,000), MEK (1:1,000),
p-ERK (1:1,000), ERK (1:1,000), VEGF (1:1,000), GAPDH (1:1,000) and
β-actin (1:1,000) antibodies. The dilution for the secondary
antibodies was 1:5,000. Signals were detected by enhanced
chemiluminescence (Western Lighting Plus-ECL; PerkinElmer, Inc.)
and quantified by densitometry using Gel-Pro Analyzer software
(version no. 4.0; Media Cybernetics, Inc.). GAPDH and β-actin were
used as the internal controls.
Cell viability assay
Cell viability was evaluated by MTS colorimetric
assays, as previously described (12). Briefly, cells were seeded in a
96-well plate (100 µl/well; 5×104 cells/ml) and allowed
to attach overnight in the serum-free medium. Following this, cells
were transfected with siRNA duplex 1 and 2 for 6, 12, 24, 48 and 72
h to plot growth curves. The cells transfected with siRNA duplex 2
for 24 h were then exposed to CoCl2 mimetic and 1%
O2-induced hypoxia for 6, 12, 24, 48 and 72 h at 37°C in
a 5% CO2-humidified incubator to compare differences
between these two types of hypoxic conditions on cell
proliferation. Finally, MTS reagent (20 µl) was added to each well
for 2 h and the absorbance and OD values at 490 nm were recorded
using a Wallac Victor 1420 plate reader (PerkinElmer, Inc.).
Cell motility assay
Wound and Transwell assays were performed to assess
cell motility. Confluent monolayers of the transfected or
untransfected cells (4×105 cells/well) in 6-multiwell
plates were wounded using a pipette tip and washed with PBS three
times. Following this, the plates were incubated in DMEM (1% FBS)
containing 200 µmol/l CoCl2 for 24 h at 37°C in a 5%
CO2 incubator. Images of the wounded area were
immediately recorded at the time of wounding and at 24 h using an
inverted microscope (IX50; Olympus Corporation) at a magnification
of ×400. The relative migration area of cells that had migrated
from the edge of the wound was calculated using ImageJ software
(version 1.50; National Institutes of Health).
The Transwell assays was performed as previously
described (12). Briefly, the
transfected or untransfected cells were placed in the upper chamber
(6×104 cells/chamber) at a final volume of 100 µl of the
serum-free RPMI-1640 medium. Following this, the normal or
CoCl2-induced hypoxic medium was placed in the bottom
chamber at a final volume of 600 µl. Following incubation for 24 h,
the filters were fixed in 10% paraformaldehyde for 20 min at room
temperature and stained with hematoxylin for 15 min at room
temperature. Cells on the lower surface of the filters were counted
in five randomly selected fields/filter under a light microscope
(BX50; Olympus Corporation) at a magnification of ×200.
Tube formation assay
Tube formation assays were performed by placing
ice-cold growth factor-reduced Matrigel matrix in a prechilled
96-well plate (50 µl/well), followed by incubation at 37°C for 30
min to allow polymerization. Next, siIGFBP-rP1 transfected cells
(8×104 cells/well) were seeded on the Matrigel-coated
96-well plate and incubated for 24 h under normal or hypoxic
conditions. Enclosed capillary-like tube structures within the
Matrigel layer from five randomly selected fields/well were
captured under an inverted phase contrast microscope (BX40; Olympus
Corporation) at a magnification of ×50. The tube length was
measured by Image-Pro Plus Analyzer software (version no. 6.0;
Media Cybernetics, Inc.).
Statistical analysis
A one-way ANOVA followed by Dunnett's post-hoc test
was used to evaluate differences between the control and
intervention groups assessed by different experiments. All tests
were performed using SPSS for Windows (version no. 13.0; SPSS
Inc.). Data are presented as the mean + standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
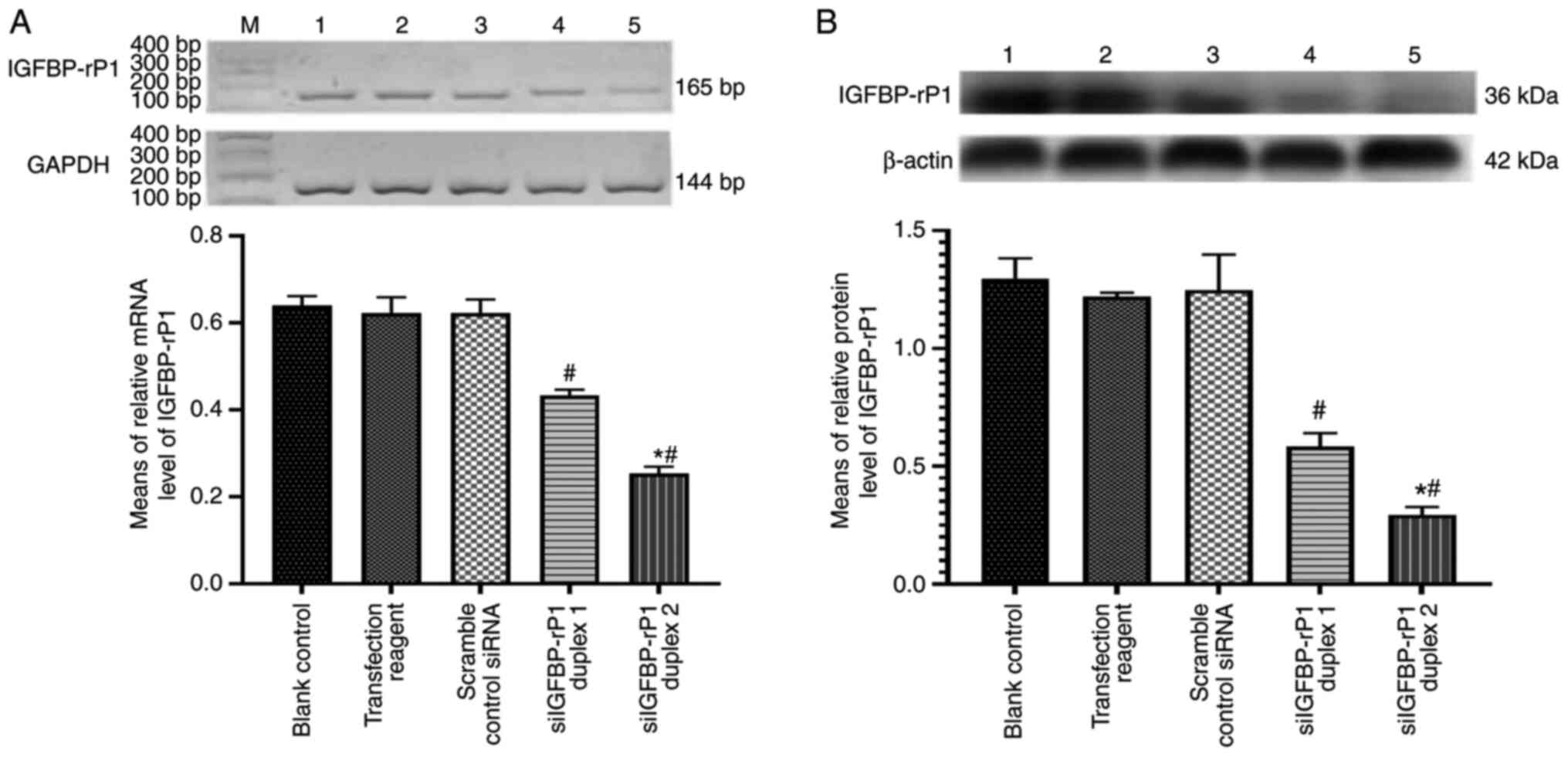
IGFBP-rP1-silencing in RF/6A cells by
siRNA does not exhibit cytotoxicity
Two different siRNA sequences were designed for
transfection to attain a higher knockdown efficiency. Firstly, the
expression of IGFBP-rP1 was confirmed in RF/6A cells without the
specific siRNA transfection. Following this, the effectiveness of
siIGFBP-rP1 transfection was measured by RT-qPCR and western
blotting. IGFBP-rP1 expression exhibited a significant decrease in
siIGFBP-rP1 duplex 1- and 2-transfected cells at the mRNA and
protein levels (P<0.05 vs. the blank control group; Fig. 1). By contrast, there were no
significant differences between the cells cultured in the blank
control, transfection reagent and scramble control siRNA groups
(P>0.05).
Moreover, the cell growth curve demonstrated that
siIGFBP-rP1 duplex 1 and 2 significantly promoted RF/6A cell
proliferation at 12, 24 and 48 h compared with the blank control
group (P<0.01; Fig. 2). No
significant differences in cell viability were observed among the
groups at 6 and 72 h (P>0.05). Furthermore, the siIGFBP-rP1
duplex 2 demonstrated a stronger pro-proliferative effect compared
with the siIGFBP-rP1 duplex 1 at 24 h (P<0.01). Since the
siIGFBP-rP1 duplex 2 exhibited a higher inhibition efficiency and
no cytotoxicity, it was selected for the following experiments.
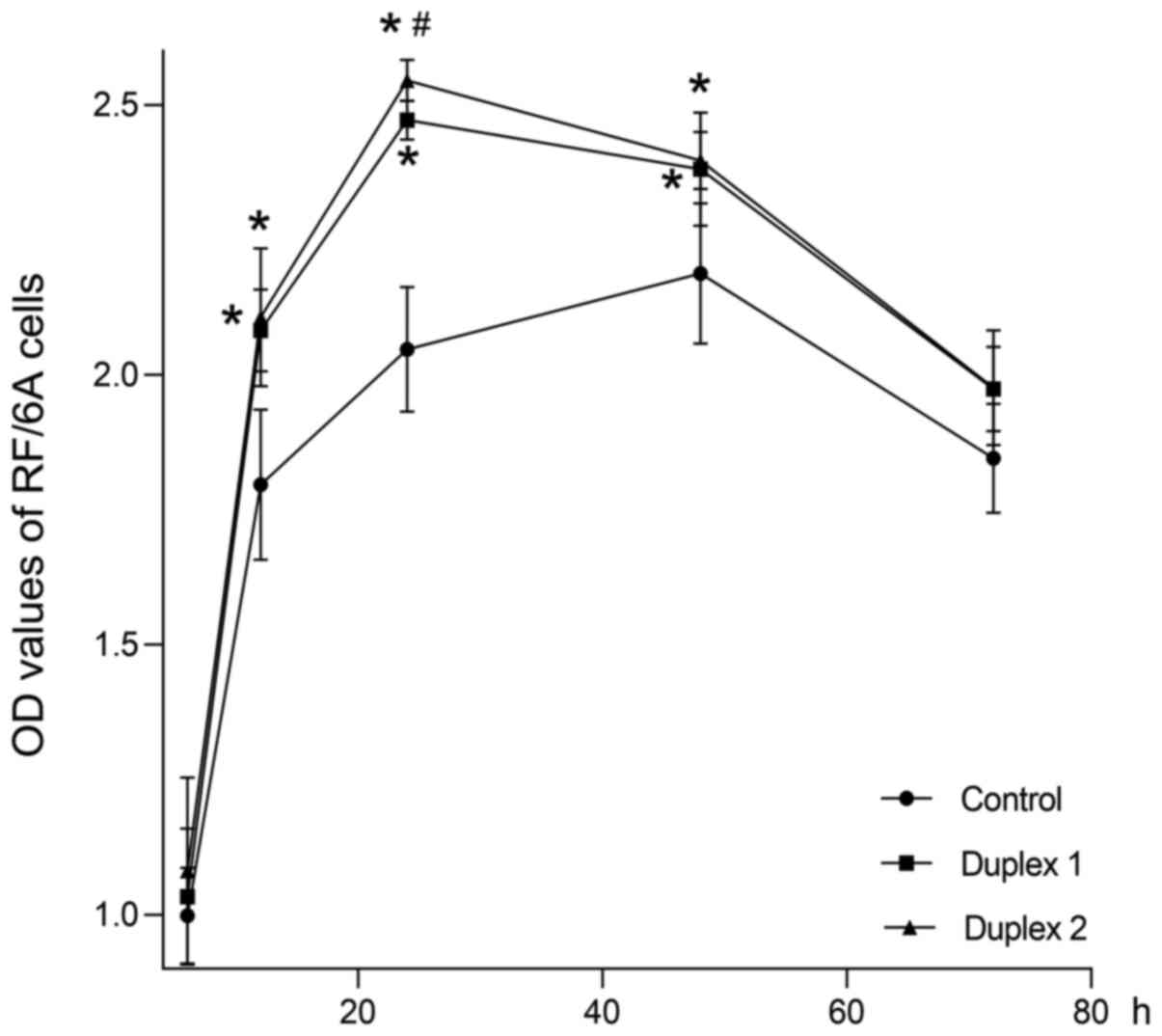
IGFBP-rP1-silencing restores RF/6A
cell viability under hypoxia
As revealed in Fig.
3, the OD values of RF/6A cells detected by MTS colorimetric
assay decreased significantly following 12, 24, 48 and 72 h of
culturing in hypoxic environments, as compared with the control
group, both at 200 µmol/l CoCl2 and at 1% O2
compared with the controls (P<0.01). No significant difference
was observed between the two hypoxic conditions (P>0.05).
Following transfection with siIGFBP-rP1 for 24 h, the OD values
significantly increased following 12, 24 and 48 h of culturing in
normoxic conditions compared controls (P<0.01). Although the OD
values of transfected cells cultured in hypoxic conditions for 12,
24, 48 and 72 h were lower than those of transfected cells cultured
in normoxic conditions, the values were still significantly higher
compared with untransfected cells cultured in hypoxic conditions
(P<0.01). No significant differences were observed among
transfected cells cultured in hypoxic conditions and the control
group at any time-point (P>0.05).
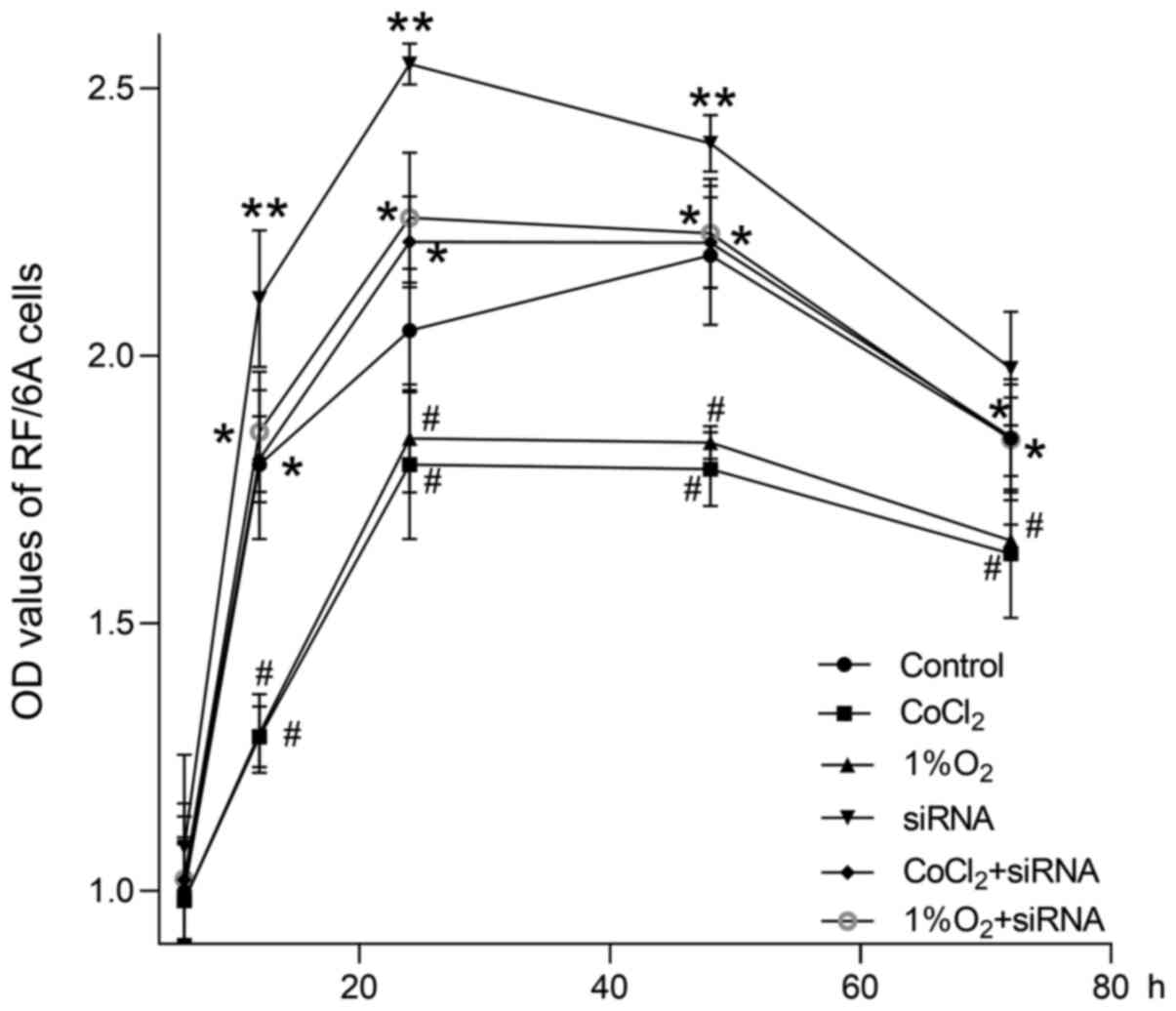 | Figure 3.Cell growth curves of
siIGFBP-rP1-transfected or untransfected cells cultured under
normoxic or hypoxic conditions for 6, 12, 24, 48 and 72 h, as
detected by MTS colorimetric assays. The OD values of hypoxic
groups (CoCl2 and 1% O2) decreased
significantly at 12, 24, 48 and 72 h compared with the control
group (#P<0.01). There was no significant difference
between the hypoxic groups (P>0.05). The OD value of the siRNA
group were significantly increased at 12, 24 and 48 h compared with
controls (**P<0.01). Furthermore, the OD values of the
transfected cells cultured in hypoxic conditions (CoCl2
+ siRNA group and 1% O2 + siRNA group) for 12, 24, 48
and 72 h were significantly lower compared with the siRNA group;
additionally, the values were significantly higher compared with
the hypoxic groups (*P<0.01). No significant differences were
identified between the CoCl2 + siRNA, 1% O2 +
siRNA and control groups (P>0.05). OD values are presented as
the mean ± standard deviation of 4 wells/group and experiments were
performed in triplicate. IGFBP-rP1, insulin-like growth factor
binding protein-related protein 1; siIGFBP-rP1, IGFBP-rP1 specific
siRNA; CoCl2, cobalt chloride; OD, optical density. |
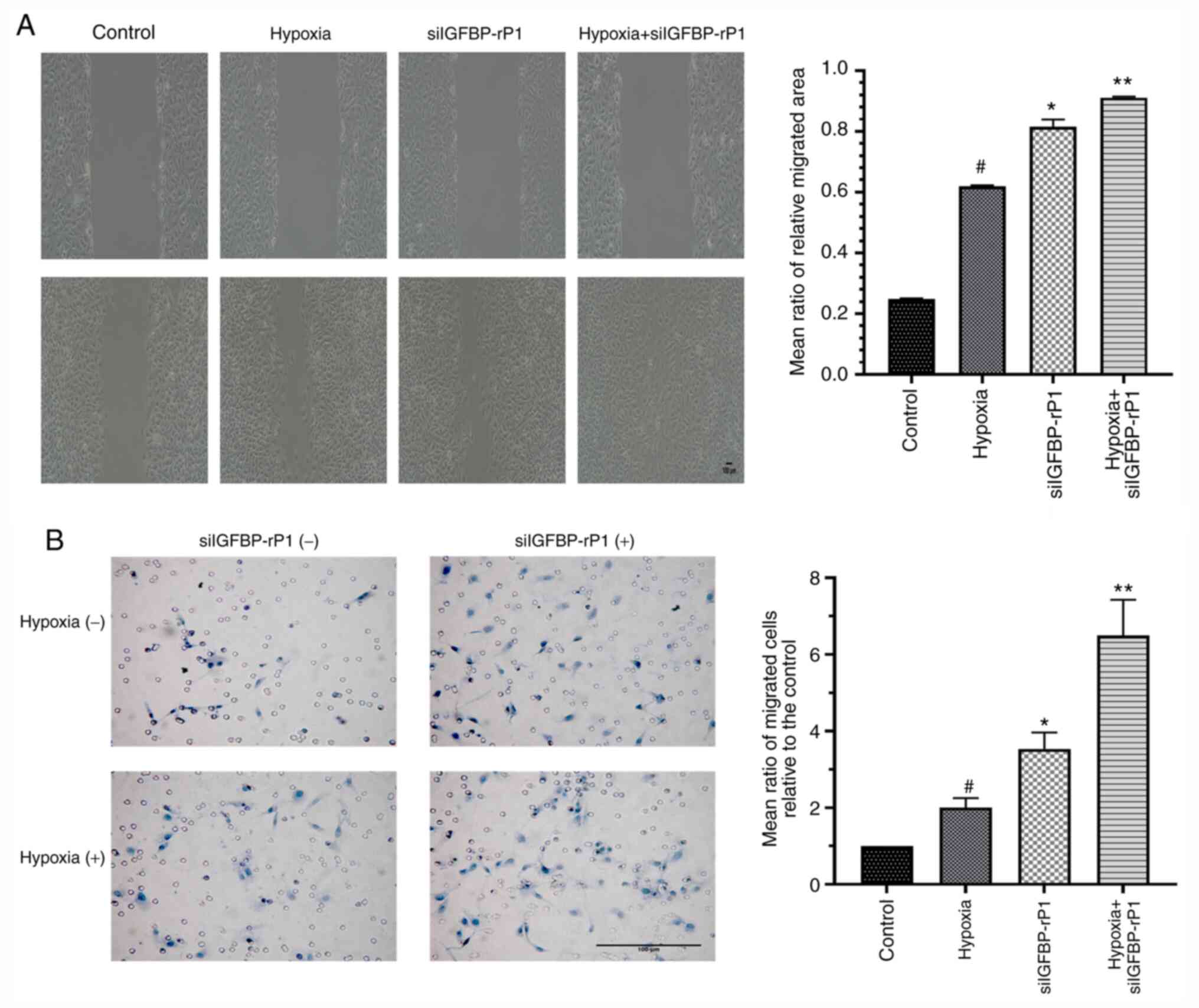
IGFBP-rP1-silencing stimulates
hypoxia-induced migration and tube formation of RF/6A cells
In order to determine the effect of
IGFBP-rP1-silencing on phenotype activation of RF/6A cells under
CoCl2-induced hypoxic conditions, chemotactic motility
and tube formation assays were performed on siIGFBP-rP1 transfected
and untransfected cells under normal and hypoxic conditions. The
scramble control siRNA-transfected cells were used as control
cells.
The relative migration area of cells that had
migrated from the edge of the wound and those that had migrated
across the filter toward the lower surface due to hypoxia were
~2.5× and ~2× that of the control, respectively (Fig. 4). siIGFBP-rP1 transfection
significantly increased cell mobility compared with non-transfected
cells, under both normoxic (P<0.01 vs. the hypoxia group) and
hypoxic conditions (P<0.01 vs. the siIGFBP-rPI group).
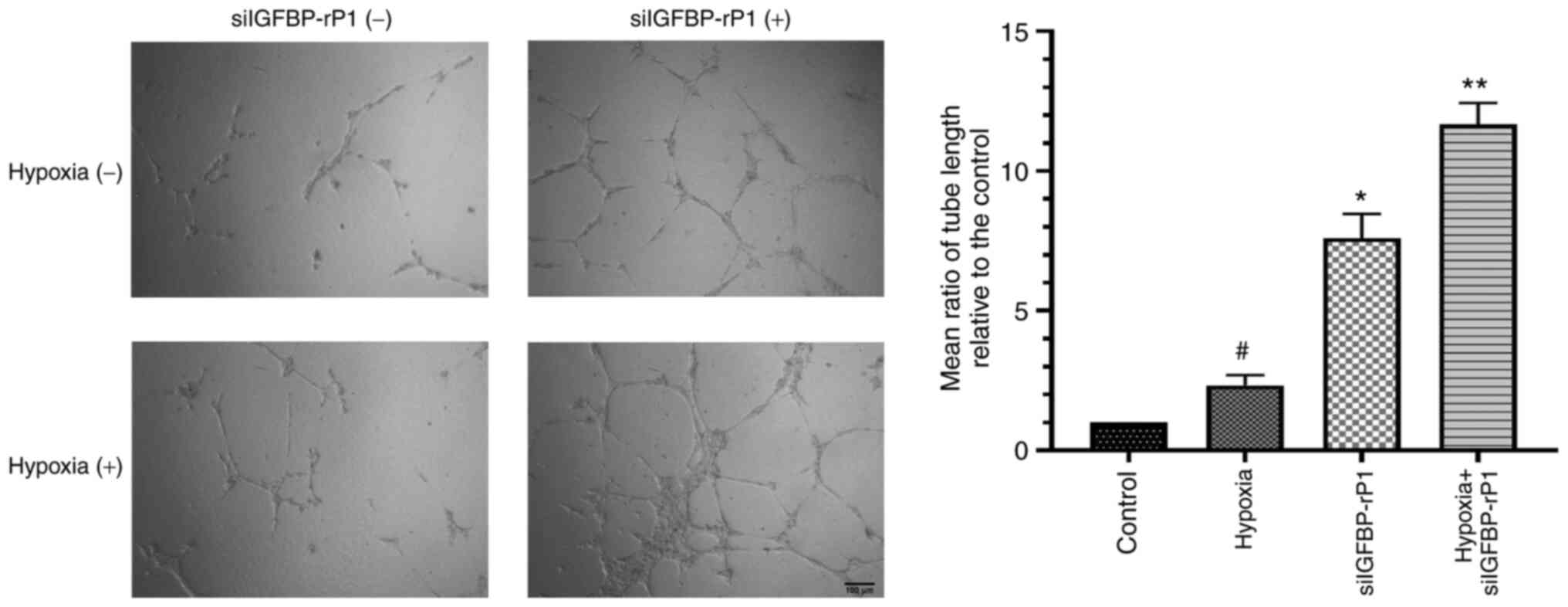
As presented in Fig.
5, extensive and enclosed networks of capillary-like tubes were
observed in the siIGFBP-rP1 and hypoxia + siIGFBP-rP1 groups, while
incomplete and sparse tube networks formed by the untransfected
cells under normal conditions. Tube formation, as determined by the
total tube length, increased ~7- (the siIGFBP-rP1 group vs. the
control group) or ~5-fold (the hypoxia + siIGFBP-rP1 group vs. the
hypoxic group) following siIGFBP-rP1 transfection, as compared with
non-transfection under normal or hypoxic conditions, respectively.
Furthermore, tube length was significantly increased in the hypoxia
group compared with the control group (P<0.01).
IGFBP-rP1-silencing enhances
hypoxia-induced RAF/MEK/ERK signaling pathway activation in RF/6A
cells
To understand the underlying mechanisms of restored
cell viability, enhanced migration and tube formation by
siIGFBP-rP1-transfected cells in hypoxic conditions, RF/6A cells
cultured in normal conditions and CoCl2-induced hypoxic
conditions with or without siIGFBP-rP1 transfection and exogenous
human IGFBP-rP1 were detected to determine the changes of key
molecules in the RAF/MEK/ERK signaling pathway using western
blotting. As revealed in Fig. 6,
under hypoxic conditions, the expression of B-RAF, p-MEK and p-ERK
were significantly increased compared with that under normal
conditions (P<0.05). In addition, following IGFBP-rP1-silencing,
the phosphorylation level of the RAF/MEK/ERK signaling pathway was
significantly increased, both under normal (the siIGFBP-rP1 group
vs. the control group, P<0.05) and hypoxic conditions (the
hypoxia + siIGFBP-rP1 group vs. the hypoxia group, P<0.05).
Furthermore, with the addition of exogenous human IGFBP-rP1, the
upregulation of B-RAF, p-MEK and p-ERK induced by siIGFBP-rP1
transfection significantly decreased (P<0.01). No significant
differences in the expression of MEK and ERK were observed between
the six groups (data not shown).
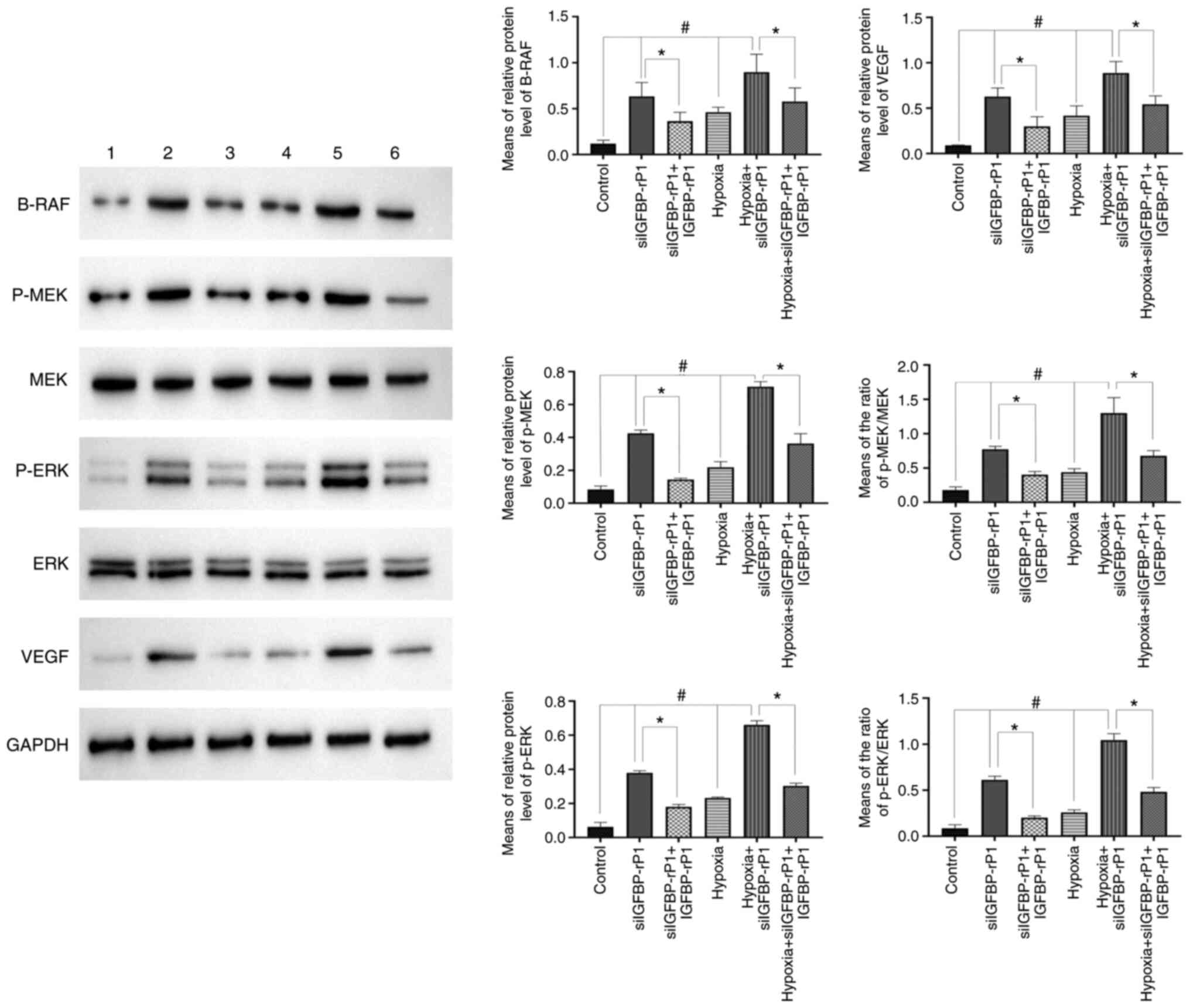 | Figure 6.IGFBP-rP1-silencing upregulates the
hypoxia-induced RAF/MEK/ERK signaling pathway activation and VEGF
expression. Representative images and quantified data demonstrated
that hypoxic stress upregulated B-RAF, p-MEK, p-ERK and VEGF
expression in RF/6A cells compared with controls
(#P<0.05). siIGFBP-rP1 transfection significantly
promoted hypoxia-induced B-RAF, p-MEK, p-ERK and VEGF expression in
RF/6A compared with the hypoxia group (#P<0.05).
IGFBP-rP1 restoration significantly downregulated the expression of
B-RAF, p-MEK, p-ERK and VEGF in siIGFBP-rP1-transfected cells,
under both normoxic and hypoxic conditions, compared with the
siIGFBP-rP1 and hypoxia + siIGFBP-rP1 groups, respectively
(*P<0.01). The membranes were stripped off and probed for the
proteins. Values are presented as the mean ± SD of 3 independent
experiments with similar results and are presented as the
integrated optical density of studied proteins relative to GAPDH.
IGFBP-rP1, insulin-like growth factor binding protein-related
protein 1; siIGFBP-rP1, IGFBP-rP1 specific siRNA. Lane 1, control;
lane 2, siIGFBP-rP1; lane 3, siIGFBP-rP1 + IGFBP-rP1; lane 4,
hypoxia; lane 5, hypoxia + siIGFBP-rP1; lane 6, hypoxia +
siIGFBP-rP1 + IGFBP-rP1. |
IGFBP-rP1-silencing upregulates the
hypoxia-induced VEGF expression in RF/6A cells
Since VEGF expression may be involved in the process
of choroidal angiogenesis mediated by IGFBP-rP1 (12), western blotting was performed to
detect the distinct protein expression of VEGF under different
intervention conditions. As presented in Fig. 6, the expression of VEGF increased
~4- or ~7 fold in the hypoxia group and siIGFBP-rP1 group,
respectively, compared with control. When the transfected cells
were cultured in hypoxic conditions, the expression of VEGF
significantly increased (~8-fold), compared with control.
Additionally, the upregulation of VEGF in siIGFBP-rP1-transfected
cells under hypoxic conditions was markedly increased compared with
transfected cells under normal conditions (P<0.01; data not
shown). Similar to the RAF/MEK/ERK pathway, exogenous human
IGFBP-rP1 significantly downregulated the expression of VEGF in
siIGFBP-rP1-transfected cells, under both normoxic and hypoxic
conditions (P<0.01).
Discussion
The number of patients with AMD, a common
age-associated eye disease, is increasing worldwide due to
exponential population ageing. The projected number of individuals
with AMD for 2020 is 196 million, with an expected increase to 288
million in 2040 (21,22). Since 2010, AMD has become the 3rd
most common cause of blindness and the 4th leading cause of visual
impairment worldwide (23). IGFs,
the family of important proteins that regulate various cellular
processes, including proliferation, differentiation, apoptosis and
neovascularization, may be involved in molecular mechanisms that
contribute to the pathogenesis of AMD, involving oxidative stress,
mitochondrial dysfunction and impaired resistance to molecular
stressors in the choriocapillaris (24–30).
The members of the IGF family, including IGFBP-2, IGFBP-6 and
IGFBP-rP1, were demonstrated to be increased in patients with
exudative AMD (31). However,
further research reported that IGFBP-rP1 was decreased in patients
with neovascular AMD (14). This
difference in IGFBP-rP1 expression highlighted the possibility of
IGFBP-rP1 being an endogenous factor regulating AMD
progression.
The IGFBP-rP1 gene is located on chromosome 4q and
encodes a 256-amino acid protein (10,11).
IGFBP-rP1 is distinct from other IGFBPs, as it can highly bind to
insulin yet lowly to IGFs and IGFBP-rP1 has been demonstrated to
have various functions in different cellular contexts (10,11).
IGFBP-rP1 was described to have both tumor suppressing and
enhancing properties in regard to cell proliferation (32). Additionally, IGFBP-rP1 was
associated with the senescence and apoptosis of different cell
lines, including human breast cancer cell lines and prostate cancer
cell lines (33,34). Previous studies revealed a
stimulatory effect of IGFBP-rP1 in the adhesion, migration and
proliferation of acute myeloid leukemia (35,36).
Accumulating evidence has indicated that IGFBP-rP1 downregulation
may participate in tumorigenesis, acting as a cancer suppressive
factor in various types of cancer, including breast, colorectal,
prostate and hepatocellular cancer (37–40).
Our previous study revealed that IGFBP-rP1 inhibited the
stimulatory effect of VEGF on retinal angiogenesis in vitro
(12). It was also revealed that
IGFBP-rP1 inhibited ocular neovascularization by downregulating
VEGF expression in a mouse model of O2-induced
retinopathy (13). Furthermore,
IGFBP-rP1 was confirmed to be downregulated in the aqueous humor of
patients with wet AMD; however, the downregulation of IGFBP-rP1 in
the pathogenesis of wet AMD remains unknown (14).
In order to examine the effects of IGFBP-rP1 on the
biological behavior of choroidal endothelial cells under a
pathological state, gene silencing was performed to interfere with
the expression of IGFBP-rP1 in choroidal endothelial cells and
hypoxic conditions were used to mimic the internal environment in
patients with AMD. RF/6A cells, a rhesus choroid endothelial cell
line widely used in research to simulate ophthalmic
micro-endothelial cells and explore the influences of external
factors and corresponding mechanisms (15–17,41,42)
were used to observe the influence of IGFBP-rP1-silencing and
hypoxia on CNV formation in vitro. RNA interference, which
allows for the silencing of mammalian genes with great specificity
and potency, has been extensively used in several fields to
investigate gene functions and design novel therapeutic methods
(43–48). Using this technique,
IGFBP-rP1-silencing was successfully accomplished in RF/6A cells.
Cell growth curves confirmed that siIGFBP-rP1 duplex 1 and 2 both
significantly promoted RF/6A cell proliferation at 12, 24 and 48 h,
and siIGFBP-rP1 duplex 2 demonstrated a more significant effect
compared with duplex 1 at 24 h. Furthermore, no significant
differences were observed between the transfected cells and
untransfected cells at 6 and 72 h. These results suggested that
there was no significant impairment in cell viability following the
silencing of IGFBP-rP1 expression at 6–72 h.
Hypoxia is considered to serve an important role in
CNV formation, a critical characteristic of wet AMD and the main
cause of visual impairment in patients with subsequent bleeding or
fibrosis (6–8,49–51).
CoCl2-induced chemical hypoxia, a reliable method of
mimicking hypoxia (52–55), was used to imitate the internal
environment during choroidal angiogenesis. Since CoCl2
can unexpectedly and markedly decrease pyruvate dehydrogenase
phosphorylation and lead to an enhanced glycolytic poise in
mammalian cells (56), the
different effects between CoCl2 mimetic and 1%
O2-induced hypoxia on cell proliferation were compared.
Both hypoxic conditions significantly decreased cell proliferation
and IGFBP-rP1-silencing restored cell viability in these hypoxic
conditions. The difference on cell proliferation was not obvious
between these two types of hypoxic environment under the current
experimental conditions, thus the present study continued to use
CoCl2 mimetic hypoxia in subsequent experiments. The
results demonstrated that hypoxic conditions significantly enhanced
migration and capillary-like tube formation in RF/6A cells. These
results indicated that abnormal environments, including hypoxia,
serve an important role in the development of CNV formation, which
was consistent with previous studies (54,55,57).
The present study further explored the role of
IGFBP-rP1-silencing in the angiogenic potential of RF/6A cells
under hypoxic conditions. The results demonstrated that the
transfected cells under hypoxia were significantly activated to
migrate from the edge of the wound, pass across the filter toward
the lower surface and form extensive and enclosed networks of
capillary-like tubes compared with controls. The migrated area and
number of siIGFBP-rP1 transfected cells in hypoxic conditions, as
well as total tube length, were significantly higher compared with
siIGFBP-rP1 transfected cells in normal conditions. These results
indicated that the downregulation of IGFBP-rP1 significantly
promoted vascular branching and sprouting under hypoxia, which
implied that IGFBP-rP1 may act as an anti-angiogenic regulator of
choroidal angiogenesis. During the process of AMD-related choroidal
vascular homeostasis deterioration and neovascularization, the
downregulation of anti-angiogenic factors has been demonstrated to
be a common pathological manifestation (58–61).
This may explain why the concentration of IGFBP-rP1 in the aqueous
humor of patients with wet AMD was significantly lower compared
with controls (14). Along with
excessively synthesized and released VEGF in the hypoxic eyes of
these patients (9,62), the balance was further disrupted by
the downregulation of IGFBP-rP1, which may significantly accelerate
the occurrence and development of CNV.
The RAF/MEK/ERK signaling pathway serves a critical
role in regulating cell division, proliferation, senescence and
apoptosis during physiological and pathological processes, such as
adipocyte physiology, insulin signaling, oxidative stress and
cancer progression (63–66). Excessive activation of the key
molecules of this signaling pathway in the majority of types of
cancer results in an unbalanced cell proliferation and apoptosis
inhibition or escapement (67,68).
Additionally, it has been confirmed that the activation of the
RAF/MEK/ERK signaling pathway modulated the role of VEGF in tumor
pathogenesis and metastasis (69–72).
The present study demonstrated that the RAF/MEK/ERK signaling
pathway was significantly activated when choroidal endothelial
cells cultured under hypoxic conditions were transfected with
siIGFBP-rP1, while exogenous IGFBP-rP1 downregulated the activation
level of this pathway. IGFBP-rP1 may promote the pro-angiogenic
effect of VEGF, particularly in pathological conditions, including
hypoxia, which was reported by the present results. Furthermore,
the results of the present study revealed that the hypoxia-induced
VEGF expression was significantly upregulated in RF/6A cells by
blocking IGFBP-rP1 expression. The regulation of VEGF expression
demonstrated the same trend as the activation of the RAF/MEK/ERK
signaling pathway. Additionally, previous studies have indicated
that the RAF/MEK/ERK signaling pathway regulated VEGF expression in
diabetic animals (73,74) and hypoxic conditions (75,76).
These results suggested that IGFBP-rP1 may regulate VEGF expression
in choroidal endothelial cells through the RAF/MEK/ERK signaling
pathway under hypoxia. In combination, the inhibition of IGFBP-rP1
further activated the RAF/MEK/ERK signaling pathway and upregulated
VEGF expression, which may concurrently participate in choroidal
endothelial cell phenotype activation, CNV formation and AMD
progression under hypoxia. Since patients with partial wet AMD have
a poor or no response to anti-VEGF treatment, novel drugs that can
resolve this problem are urgently required. IGFBP-rP1 may become a
therapeutic target in choroidal angiogenesis due to its potential
inhibition of ophthalmic neovascularization.
The present study had limitations. Although there
was no significant difference in cell viability between
CoCl2 mimetic and 1% O2-induced hypoxia,
certain effects induced by CoCl2 may differ from those
of actual hypoxia. Therefore, the role of IGFBP-rP1 in RF/6A cells
under physical hypoxia requires further investigation. Furthermore,
despite the extensive application of RF/6A cells as the closest
choroidal endothelial cell line to human species in studies on
choroidal angiogenesis in vitro, the signaling circuitry has
been altered in this immortalized cell line. Therefore, the exact
effect of IGFBP-rP1 on choroidal angiogenesis and the RAF/MEK/ERK
signaling pathway needs to be further explored in human choroidal
endothelial cells and in an in vivo model.
In conclusion, the present study revealed that
IGFBP-rP1-silencing promoted cell proliferation and stimulated
hypoxia-induced migration and tube formation of RF/6A cells.
Additionally, the results confirmed that IGFBP-rP1-silencing
effectively promoted hypoxia-induced angiogenic potential of
choroidal endothelial cells by upregulating VEGF expression,
probably by activating the RAF/MEK/ERK signaling pathway. Further
research should be conducted to determine whether IGFBP-rP1 acts as
an angiogenesis inhibitor in vivo and its effect on other
ocular tissues; the real-world application of IGFBP-rP1 or an
associated drug in clinical practice requires further research in
future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Nature Science Foundation for Young Scholars of China
(grant no. 81200701), the National Key R&D Program of China
(grant nos. 2016YFC0904800 and 2019YFC0840607), the National
Science and Technology Major Project of China (grant no.
2017ZX09304010) and the Bethune·Lumitin Research Funding for Young
or Middle-aged Ophthalmologists (grant no. BJ-LM2019001J).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and HW conducted the siRNA assays, the
restoration assays, data acquisition and were major contributors in
drafting the original manuscript. MM and ZZha performed the cell
viability assays, cell motility assays, tube formation assays,
created graphs and were major contributors in drafting the original
manuscript. ZZhe and HW performed the signaling pathway assays,
analysis and interpretation of data and made major contributions in
editing the original manuscript. XX and TS made substantial
contributions to conception and design, and made critical revising
of the original manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IGFBP-rP1
|
insulin-like growth factor binding
protein-related protein 1
|
|
siIGFBP-rP1
|
IGFBP-rP1 specific siRNA
|
|
AMD
|
age-related macular degeneration
|
|
CNV
|
choroidal neovascularization
|
References
|
1
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daien V, Eldem BM, Talks JS, Korobelnik
JF, Mitchell P, Finger RP, Sakamoto T, Wong TY, Evuarherhe O,
Carter G and Carrasco J: Real-world data in retinal diseases
treated with anti-vascular endothelial growth factor (anti-VEGF)
therapy-a systematic approach to identify and characterize data
sources. BMC Ophthalmol. 19:2062019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campbell M and Doyle SL: Current
perspectives on established and novel therapies for pathological
neovascularization in retinal disease. Biochem Pharmacol.
164:321–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campochiaro PA: Molecular pathogenesis of
retinal and choroidal vascular diseases. Prog Retin Eye Res.
49:67–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Chai Z, Hu D, Ji Q, Xin J, Zhang C
and Zhong J: A global analysis of CNVs in diverse yak populations
using whole-genome resequencing. BMC Genomics. 20:612019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park SM, Lee K, Huh M, Eom S, Park B, Kim
KH, Park DH, Kim DS and Kim HK: Development of an in vitro 3D
choroidal neovascularization model using chemically induced hypoxia
through an ultra-thin, free-standing nanofiber membrane. Mater Sci
Eng C Mater Biol Appl. 104:1099642019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alivand MR, Sabouni F and Soheili ZS:
Probable chemical hypoxia effects on progress of CNV through
induction of promoter CpG demethylation and overexpression of
IL17RC in human RPE cells. Curr Eye Res. 41:1245–1254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andre H, Tunik S, Aronsson M and Kvanta A:
Hypoxia-inducible factor-1α is associated with sprouting
angiogenesis in the murine laser-induced choroidal
neovascularization model. Invest Ophthalmol Vis Sci. 56:6591–6604.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penn JS, Madan A, Caldwell RB, Bartoli M,
Caldwell RW and Hartnett ME: Vascular endothelial growth factor in
eye disease. Prog Retin Eye Res. 27:331–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forbes BE, McCarthy P and Norton RS:
Insulin-like growth factor binding proteins: A structural
perspective. Front Endocrinol (Lausanne). 3:382012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun T, Cao H, Xu L, Zhu B, Gu Q and Xu X:
Insulin-like growth factor binding protein-related protein 1
mediates VEGF-induced proliferation, migration and tube formation
of retinal endothelial cells. Curr Eye Res. 36:341–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang P, Wang H, Cao H, Xu X and Sun T:
Insulin-like growth factor binding protein-related protein 1
inhibit retinal neovascularization in the mouse model of
oxygen-induced retinopathy. J Ocul Pharmacol Ther. 33:459–465.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sung HJ, Han JI, Lee JW, Uhm KB and Heo K:
TCCR/WSX-1 is a novel angiogenic factor in age-related macular
degeneration. Mol Vis. 18:234–240. 2012.PubMed/NCBI
|
|
15
|
Zhu M, Liu X, Wang S, Miao J, Wu L, Yang
X, Wang Y, Kang L, Li W, Cui C, et al: PKR promotes choroidal
neovascularization via upregulating the PI3K/Akt signaling pathway
in VEGF expression. Mol Vis. 22:1361–1374. 2016.PubMed/NCBI
|
|
16
|
Feng Y, Wang J, Yuan Y, Zhang X, Shen M
and Yuan F: miR-539-5p inhibits experimental choroidal
neovascularization by targeting CXCR7. Faseb J. 32:1626–1639. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan C, Hsiao C, Li H, Fang J, Chang D and
Hung C: The inhibitory effects of gold nanoparticles on
VEGF-A-induced cell migration in choroid-retina endothelial cells.
Int J Mol Sci. 21:1092019. View Article : Google Scholar
|
|
18
|
Liu Z, Liu H, Fang W, Yang Y, Wang H and
Peng J: Insulin-like growth factor binding protein 7 modulates
estrogen-induced trophoblast proliferation and invasion in HTR-8
and JEG-3 cells. Cell Biochem Biophys. 63:73–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamura K, Yoshie M, Hashimoto K and
Tachikawa E: Inhibitory effect of insulin-like growth
factor-binding protein-7 (IGFBP7) on in vitro angiogenesis of
vascular endothelial cells in the rat corpus luteum. J Reprod Dev.
60:447–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verhagen HJMP, van Gils N, Martiañez T,
van Rhenen A, Rutten A, Denkers F, de Leeuw DC, Smit MA, Tsui M, de
Vos Klootwijk LLE, et al: IGFBP7 induces differentiation and loss
of survival of human acute myeloid leukemia stem cells without
affecting normal hematopoiesis. Cell Rep. 25:3021–3035. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rozing MP, Durhuus JA, Krogh NM, Subhi Y,
Kirkwood TB, Westendorp RG and Sørensen TL: Age-related macular
degeneration: A two-level model hypothesis. Prog Retin Eye Res.
100825:2019.
|
|
22
|
Wong WL, Su X, Li X, Cheung CM, Klein R,
Cheng CY and Wong TY: Global prevalence of age-related macular
degeneration and disease burden projection for 2020 and 2040: A
systematic review and meta-analysis. Lancet Glob Health.
2:e106–e116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pascolini D and Mariotti SP: Global
estimates of visual impairment: 2010. Br J Ophthalmol. 96:614–618.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipecz A, Miller L, Kovacs I, Czako C,
Csipo T, Baffi J, Csiszar A, Tarantini S, Ungvari Z, Yabluchanskiy
A and Conley S: Microvascular contributions to age-related macular
degeneration (AMD): From mechanisms of choriocapillaris aging to
novel interventions. Geroscience. 41:813–845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lambert NG, ElShelmani H, Singh MK,
Mansergh FC, Wride MA, Padilla M, Keegan D, Hogg RE and Ambati BK:
Risk factors and biomarkers of age-related macular degeneration.
Prog Retin Eye Res. 54:64–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan H and Finkel T: Key proteins and
pathways that regulate lifespan. J Biol Chem. 292:6452–6460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jesko H, Stepien A, Lukiw WJ and
Strosznajder RP: The cross-talk between sphingolipids and
insulin-like growth factor signaling: Significance for aging and
neurodegeneration. Mol Neurobiol. 56:3501–3521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haywood NJ, Slater TA, Matthews CJ and
Wheatcroft SB: The insulin like growth factor and binding protein
family: Novel therapeutic targets in obesity & diabetes. Mol
Metab. 19:86–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slater T, Haywood NJ, Matthews C, Cheema H
and Wheatcroft SB: Insulin-like growth factor binding proteins and
angiogenesis: From cancer to cardiovascular disease. Cytokine
Growth Factor Rev. 46:28–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bach LA: Endothelial cells and the IGF
system. J Mol Endocrinol. 54:R1–R13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cha DM, Woo SJ, Kim HJ, Lee C and Park KH:
Comparative analysis of aqueous humor cytokine levels between
patients with exudative age-related macular degeneration and normal
controls. Invest Ophthalmol Vis Sci. 54:7038–7044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu S, Xu F, Zhang J, Ruan W and Lai M:
Insulin-like growth factor binding protein-related protein 1 and
cancer. Clin Chim Acta. 431:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson HM, Birnbaum RS, Poot M, Quinn LS
and Swisshelm K: Insulin-like growth factor binding protein-related
protein 1 inhibits proliferation of MCF-7 breast cancer cells via a
senescence-like mechanism. Cell Growth Differ. 13:205–213.
2002.PubMed/NCBI
|
|
34
|
Mutaguchi K, Yasumoto H, Mita K, Matsubara
A, Shiina H, Igawa M, Dahiya R and Usui T: Restoration of
insulin-like growth factor binding protein-related protein 1 has a
tumor-suppressive activity through induction of apoptosis in human
prostate cancer. Cancer Res. 63:7717–7723. 2003.PubMed/NCBI
|
|
35
|
Hu S, Chen R, Man X, Feng X, Cen J, Gu W,
He H, Li J, Chai Y and Chen Z: Function and expression of
insulin-like growth factor-binding protein 7 (IGFBP7) gene in
childhood acute myeloid leukemia. Pediatr Hematol Oncol.
28:279–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laranjeira AB, de Vasconcellos JF, Sodek
L, Spago MC, Fornazim MC, Tone LG, Brandalise SR, Nowill AE and
Yunes JA: IGFBP7 participates in the reciprocal interaction between
acute lymphoblastic leukemia and BM stromal cells and in leukemia
resistance to asparaginase. Leukemia. 26:1001–1011. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burger AM, Leyland-Jones B, Banerjee K,
Spyropoulos DD and Seth AK: Essential roles of IGFBP-3 and
IGFBP-rP1 in breast cancer. Eur J Cancer. 41:1515–1527. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu S, Zhang J, Xu F, Xu E, Ruan W, Ma Y,
Huang Q and Lai M: IGFBP-rP1 suppresses epithelial-mesenchymal
transition and metastasis in colorectal cancer. Cell Death Dis.
6:e16952015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seki M, Teishima J, Mochizuki H, Mutaguchi
K, Yasumoto H, Oka K, Nagamatsu H, Shoji K and Matsubara A:
Restoration of IGFBP-rP1 increases radiosensitivity and
chemosensitivity in hormone-refractory human prostate cancer.
Hiroshima J Med Sci. 62:13–19. 2013.PubMed/NCBI
|
|
40
|
Akiel M, Guo C, Li X, Rajasekaran D,
Mendoza RG, Robertson CL, Jariwala N, Yuan F, Subler MA, Windle J,
et al: IGFBP7 deletion promotes hepatocellular carcinoma. Cancer
Res. 77:4014–4025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang H, Wu M, Liu Y, Song L, Li S, Wang
X, Zhang YF, Fang J and Wu S: Serine racemase deficiency attenuates
choroidal neovascularization and reduces nitric oxide and VEGF
levels by retinal pigment epithelial cells. J Neurochem.
143:375–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen S, Zhou Y, Zhou L, Guan Y, Zhang Y
and Han X: Anti-neovascularization effects of DMBT in age-related
macular degeneration by inhibition of VEGF secretion through
ROS-dependent signaling pathway. Mol Cell Biochem. 448:225–235.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Milhavet O, Gary DS and Mattson MP: RNA
interference in biology and medicine. Pharmacol Rev. 55:629–648.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tam C, Wong JH, Cheung R, Zuo T and Ng TB:
Therapeutic potentials of short interfering RNAs. Appl Microbiol
Biotechnol. 101:7091–7111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramachandran PS, Keiser MS and Davidson
BL: Recent advances in RNA interference therapeutics for CNS
diseases. Neurotherapeutics. 10:473–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Corydon TJ: Antiangiogenic eye gene
therapy. Hum Gene Ther. 26:525–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moore NA, Bracha P, Hussain RM, Morral N
and Ciulla TA: Gene therapy for age-related macular degeneration.
Expert Opin Biol Ther. 17:1235–1244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moore SM, Skowronska-Krawczyk D and Chao
DL: Emerging concepts for RNA therapeutics for inherited retinal
disease. Adv Exp Med Biol. 1185:85–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee CS, Choi EY, Lee SC, Koh HJ, Lee JH
and Chung JH: Resveratrol inhibits hypoxia-induced vascular
endothelial growth factor expression and pathological
neovascularization. Yonsei Med J. 56:1678–1685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Biswal MR, Prentice HM, Smith GW, Zhu P,
Tong Y, Dorey CK, Lewin AS and Blanks JC: Cell-specific gene
therapy driven by an optimized hypoxia-regulated vector reduces
choroidal neovascularization. J Mol Med (Berl). 96:1107–1118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang T, Li X, Yu W, Yan Z, Zou H and He
X: Overexpression of thymosin beta-10 inhibits VEGF mRNA
expression, autocrine VEGF protein production, and tube formation
in hypoxia-induced monkey choroid-retinal endothelial cells.
Ophthalmic Res. 41:36–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin J, Yuan F, Shen MQ, Feng YF and He QL:
Vascular endothelial growth factor regulates primate
choroid-retinal endothelial cell proliferation and tube formation
through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem.
381:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li R, Du J and Chang Y: Role of autophagy
in hypoxia-induced angiogenesis of RF/6A cells in vitro. Curr Eye
Res. 41:1566–1570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui K, Zhang S, Liu X, Yan Z, Huang L,
Yang X, Zhu R and Sang A: Inhibition of TBK1 reduces choroidal
neovascularization in vitro and in vivo. Biochem Bioph Res Co.
503:202–208. 2018. View Article : Google Scholar
|
|
56
|
Borcar A, Menze MA, Toner M and Hand SC:
Metabolic preconditioning of mammalian cells: Mimetic agents for
hypoxia lack fidelity in promoting phosphorylation of pyruvate
dehydrogenase. Cell Tissue Res. 351:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cabral T, Mello L, Lima LH, Polido J,
Regatieri CV, Belfort RJ and Mahajan VB: Retinal and choroidal
angiogenesis: A review of new targets. Int J Retina Vitreous.
3:312017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Farnoodian M, Sorenson CM and Sheibani N:
Negative regulators of angiogenesis, ocular vascular homeostasis,
and pathogenesis and treatment of exudative AMD. J Ophthalmic Vis
Res. 13:470–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Farnoodian M, Wang S, Dietz J, Nickells
RW, Sorenson CM and Sheibani N: Negative regulators of
angiogenesis: Important targets for treatment of exudative AMD.
Clin Sci (Lond). 131:1763–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Farnoodian M, Sorenson CM and Sheibani N:
PEDF expression affects the oxidative and inflammatory state of
choroidal endothelial cells. Am J Physiol Cell Physiol.
314:C456–C472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Housset M and Sennlaub F: Thrombospondin-1
and pathogenesis of age-related macular degeneration. J Ocul
Pharmacol Ther. 31:406–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Terao N, Koizumi H, Kojima K, Yamagishi T,
Yamamoto Y, Yoshii K, Kitazawa K, Hiraga A, Toda M, Kinoshita S, et
al: Distinct aqueous humour cytokine profiles of patients with
pachychoroid neovasculopathy and neovascular age-related macular
degeneration. Sci Rep. 8:105202018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gehart H, Kumpf S, Ittner A and Ricci R:
MAPK signalling in cellular metabolism: Stress or wellness? EMBO
Rep. 11:834–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sun Y, Liu W, Liu T, Feng X, Yang N and
Zhou H: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rezatabar S, Karimian A, Rameshknia V,
Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi
M, Monirialamdari M and Yousefi B: RAS/MAPK signaling functions in
oxidative stress, DNA damage response and cancer progression. J
Cell Physiol. 234:14951–14965. 2019. View Article : Google Scholar
|
|
66
|
Marampon F, Ciccarelli C and Zani BM:
Biological rationale for targeting MEK/ERK pathways in anti-cancer
therapy and to potentiate tumour responses to radiation. Int J Mol
Sci. 20:25302019. View Article : Google Scholar
|
|
67
|
Khaliq M and Fallahi-Sichani M: Epigenetic
mechanisms of escape from BRAF oncogene dependency. Cancers
(Basel). 11:14802019. View Article : Google Scholar
|
|
68
|
Degirmenci U, Wang M and Hu J: Targeting
aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells.
9:1982020. View Article : Google Scholar
|
|
69
|
Huang M, Huang B, Li G and Zeng S:
Apatinib affect VEGF-mediated cell proliferation, migration,
invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in
cholangiocarcinoma cell. BMC Gastroenterol. 18:1692018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yamana S, Tokiyama A, Fujita H, Terao Y,
Horibe S, Sasaki N, Satomi-Kobayashi S, Hirata KI and Rikitake Y:
Necl-4 enhances the PLCγ-c-Raf-MEK-ERK pathway without affecting
internalization of VEGFR2. Biochem Biophys Res Commun. 490:169–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gong J, Zhou S and Yang S: Vanillic acid
suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1
and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int J
Mol Sci. 20:e4652019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis-molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang EY, Gao B, Shi HL, Huang LF, Yang L,
Wu XJ and Wang ZT: 20(S)-protopanaxadiol enhances angiogenesis via
HIF-1α-mediated VEGF secretion by activating p70S6 kinase and
benefits wound healing in genetically diabetic mice. Exp Mol Med.
49:e3872017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ye X, Xu G, Chang Q, Fan J, Sun Z, Qin Y
and Jiang AC: ERK1/2 signaling pathways involved in VEGF release in
diabetic rat retina. Invest Ophthalmol Vis Sci. 51:5226–5233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hou SY, Li YP, Wang JH, Yang SL, Wang Y,
Wang Y and Kuang Y: Aquaporin-3 inhibition reduces the growth of
NSCLC cells induced by hypoxia. Cell Physiol Biochem. 38:129–140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y,
Hou M and Wang Z: Notoginsenoside Ft1 promotes angiogenesis via
HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and
Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 84:784–792.
2012. View Article : Google Scholar : PubMed/NCBI
|