Introduction
Sepsis often occurs following infection or injury,
and is one of the primary causes of morbidity and mortality
worldwide (1). It was reported
that sepsis was responsible for 2.8 million deaths in high-income
countries every year (2). The
pathophysiology of sepsis is complex, and there are a number of
risk factors that can contribute to the development of sepsis
(3). Therefore, it is necessary to
understand the mechanistic pathways underlying sepsis in order to
develop an effective treatment.
Long non-coding RNAs (lncRNAs) are a novel family of
regulatory RNA molecules that are >200 nucleotides and have
numerous diverse functions (4).
lncRNAs have roles in the regulation of inflammatory responses in
sepsis (5). For example, lncRNA
Transcript Predicting Survival in AKI was demonstrated to
facilitate HK-2 cell apoptosis and inflammatory responses in
sepsis-induced kidney injury (6).
lncRNA hox transcript antisense RNA could repress proliferation,
and upregulate apoptosis and inflammatory responses in sepsis in
vitro (7). Wang et al
(8) reported that lncRNA nuclear
paraspeckle assembly transcript 1 (NEAT1) knockdown could
ameliorate sepsis-induced myocardial damage in mice, including the
decrease of edema in myocardial tissues in mice, as well as the
inhibition of apoptosis and inflammation. Previous literature has
suggested that lncRNA highly upregulated in liver cancer (HULC) was
involved in the decrease of pre-inflammatory mediators in
lipopolysaccharide (LPS)-induced sepsis in vitro (9). Nevertheless, the mechanism of action
of HULC in LPS-induced sepsis remains to be elucidated.
MicroRNAs (miRNAs/miRs) are a category of important
non-coding RNAs associated with human diseases that participate in
various biological processes (10). At present, an increasing number of
researchers are focusing on miRNAs relevant to sepsis. For
instance, miR-25 was demonstrated to attenuate apoptosis and
increase expression of certain proinflammatory cytokines in
cardiomyocytes treated with LPS (11). Wang et al (12) demonstrated that miR-21-3p affected
sepsis-related cardiac dysfunction by targeting SH3
domain-containing protein 2 (12).
It was revealed that the expression of miR-128-3p was downregulated
in the podocytes of a patient with sepsis (13), which prompted us to investigate the
effect of miR-128-3p on the progression of LPS-induced sepsis in
the present study.
Rac family small GTPase 1 (RAC1) serves as a key
signal transducer that has been demonstrated to control cellular
inflammatory responses (14). It
was reported that RAC1 could modulate the formation of
platelet-derived microparticles and generation of thrombin in
sepsis (15). In the present
study, the role of RAC1 in LPS-induced sepsis was examined.
Materials and methods
Clinical sample collection
A total of 110 patients with sepsis (male/female:
72/38; Age range, 18–38 years; mean age, 56.84±10.17 years) and 100
healthy controls (male/female: 64/36; Age range, 24–81 years; mean
age 59.33±11.24 years) were recruited at the Renmin Hospital of
Wuhan University (Hubei, China) between November 2016 and January
2019. The patients with sepsis, admitted to intensive care units
(ICUs) were diagnosed as septic based on the ‘Definitions for
sepsis and organ failure and guidelines for the use of innovative
therapies in sepsis’ (16). A
total of 110 patients with sepsis, including 36 patients with
sepsis, 47 patients with severe sepsis and 27 patients with septic
shock (17), were involved in the
study. The exclusion criteria were as follows: Patients with
malignancies, those receiving immunosuppressant treatment, pregnant
or lactating women and patients with human immunodeficiency virus.
All participants submitted written informed consent. The current
study was approved by the Ethics Committee of the Renmin Hospital
of Wuhan University.
Samples of blood were taken from patients with
sepsis within 24 h of admission to the ICU. The blood samples of
100 healthy participants were acquired during their physical
examination. Serum was obtained after centrifugation at 400 × g for
15 min at 4°C and stored at −80°C.
Cell culture and LPS treatment
Human dermal microvascular endothelial cells
(HMEC-1; CRL-3243) were commercially procured from American Type
Culture Collection and grown in MCDB 131 Medium, no glutamine
(Gibco; Thermo Fisher Scientific, Inc.) containing 10 ng/ml
epidermal growth factor (EGF; Gibco; Thermo Fisher Scientific,
Inc.), 1 µg/ml hydrocortisone (Sigma-Aldrich; Merck KGaA), 10 mM
glutamine (Gibco; Thermo Fisher Scientific, Inc.) and 10% (V/V)
fetal bovine serum (Thermo Fisher Scientific, Inc.) at 37°C.
To mimic sepsis in vitro, HMEC-1 cells
(5×104/100 µl) maintained in 6-well plates were treated
with 1 µg/ml LPS (Beijing Solarbio Science & Technology Co.,
Ltd.) for 24 h, which could trigger strong immune-inflammatory
responses (18,19), whereas cells treated with dimethyl
sulfoxide (DMSO; Beijing Solarbio Science & Technology Co.,
Ltd.) served as controls.
Cell transfection
Small interfering RNA (siRNA) directed against HULC
(si-HULC, 5′-CCUCCAGAACUGUGAUCCA-3′) and the negative control
(si-NC, 5′-GGACUCUCGGAUUGUAAGAUU-3′) were acquired from Shanghai
GeneChem Co., Ltd. To construct overexpression plasmids, the
sequence of HULC or RAC1 was inserted into a pcDNA3.1 vector
(Hanbio Biotechnology Co., Ltd.), generating pcDNA3.1-HULC (HULC)
or pcDNA3.1-RAC1 (RAC1), with pcDNA3.1 (vector) as the control. In
addition, miR-128-3p mimic (miR-128-3p,
5′-AAAGAGACCGGUUCACUGUGA-3′), miR-128-3p inhibitor
(anti-miR-128-3p, 5′-UUUCUCUGGCCAAGUGACACU-3′) and their
corresponding negative control (miR-NC, 5′-ACGUGACACGUUCGGAGAATT-3′
and anti-miR-NC, 5′-CUAACGCAUGCACAGUCGUACG-3′) were synthesized by
GenePharma Co. Ltd. (Shanghai, China). When cell confluence reached
50%, LPS-treated HMEC-1 cells were transfected with 2 µg plasmids
or 40 nM oligonucleotides using Lipofectamine® 3000
(Beijing Solarbio Science & Technology Co., Ltd.). The
knockdown efficiency was measured in cells transfected without LPS
treatment. After 48 h, cells were subjected to subsequent
investigation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Extraction of total serum RNA and RNA from HMEC-1
cells was performed with TRIzol® LS Reagent (Ambion;
Thermo Fisher Scientific, Inc.). After determination of RNA
concentration and purity using a NanoDrop ND-1000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc.), 1 µg RNA
was utilized to synthesize cDNA (42°C for 60 min and 80°C for 5
min) with the BeyoRT™ First Strand cDNA Synthesis kit (Beyotime
Institute of Biotechnology) for HULC and RAC1, and miScript Reverse
Transcription kit (Qiagen, Inc.) for miR-128-3p. Then, qPCR was
performed with a SYBR-Green mix (Takara Bio, Inc.) with following
thermocycling conditions: Initial denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation at 95°C for 20 sec, annealing
at 60°C for 30 sec and extension at 72°C for 20 sec. The relative
expression of miR-128-3p, HULC, RAC1, IL-6, TNF-α, intercellular
adhesion molecule (ICAM1) and vascular cell adhesion molecule
(VCAM1) was evaluated by the 2−ΔΔCq method (20), miR-128-3p expression was normalized
to U6, whereas HULC, RAC1, IL-6, TNF-α, ICAM1 and VCAM1 expression
was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Primers subjected for amplification were: miR-128-3p, forward (F):
5′-GGTCACAGTGAACCGGTC-3′ and reverse (R): 5′-GTGCAGGGTCCGAGGT-3′;
U6, F: 5′-GCAGGAGGTCTTCACAGAGT-3′ and R:
5′-TCTAGAGGAGAAGCTGGGGT-3′; HULC, F: 5′-TCATGATGGAATTGGAGCCTT-3′
and R: 5′-CTCTTCCTGGCTTGCAGATTG-3′; RAC1, F:
5′-AAGAGAAAATGCCTGCTGTTGTAA-3′ and R: 5′-GCGTACAAAGGTTCCAAGGG-3′;
IL-6, F: 5′-GGTACATCCTCGACGGCATCT-3′ and R:
5′-GTGCCTCTTTGCTGCTTTCAC-3′; TNF-α, F: 5′-CGAGTGACAAGCCTGTAGCC-3′
and R: 5′-GTTGACCTTGGTCTGGTAGG-3′; ICAM1, F:
5′-GGCCTCAGTCAGTGTGA-3′ and R: 5′-AACCCCATTCAGCGTCA-3′; VCAM1, F:
5′-CCGGATTGCTGCTCAGATTGGA-3′ and R: 5′-AGCGTGGAATTGGTCCCCTCA-3′;
GAPDH, F: 5′-GCCAAAAGGGTCATCATCTC-3′ and R:
5′-GGCCATCCACAGTCTTCT-3′.
Cell apoptosis assay
Cell apoptosis was determined using the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (BioVision, Inc.). In brief, HMEC-1 cells were
collected and suspended in 200 µl binding buffer, and then
double-stained with 5 µl Annexin V-FITC and 10 µl PI at room
temperature for 20 min in the dark. Stained apoptotic cells
(Annexin V+ and PI−) were identified by a
flow cytometer (Accuri C6; BD Biosciences) and data were analyzed
utilizing Cell Quest 6.0 software (BD Biosciences).
Western blot analysis
Whole proteins were extracted from HMEC-1 cells with
a protein extraction kit (Beijing Solarbio Science & Technology
Co., Ltd.). After quantification with a bicinchoninic acid assay
kit (Beyotime Institute of Biotechnology), 30 µg protein samples
were separated via SDS-PAGE on a 10% gel, and then subsequently
transferred onto a polyvinylidene fluoride membrane (Thermo Fisher
Scientific, Inc.) using the wet electrophoretic transfer method for
2 h. The membranes were blocked in 5% skimmed milk at room
temperature for 2 h, immersed in diluted primary antibodies at 4°C
overnight, and then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (Goat Anti-Rabbit IgG
H&L; cat. no. ab150077; 1:3,000; Abcam) at room temperature for
2 h. The primary antibodies were purchased from Abcam, including
anti-cleaved-caspase-3 (anti-cleaved-cas3; cat. no. ab32042;
1:1,000), anti-cleaved-cas9 (cat. no. ab2324; 1:1500), anti-IL-6
(cat. no. ab233706; 1:1,500), anti-TNF-α (cat. no. ab183218;
1:1,000), anti-ICAM1 (cat. no. ab109361; 1:1,500), anti-VCAM1 (cat.
no. ab134047; 1:1,500), anti-RAC1 (cat. no. ab155938; 1:1,000) and
anti-β-actin (cat. no. ab115777; 1:2,000). An enhanced
chemiluminescence kit (Amersham; Cytiva) was used to visualize the
protein bands. The intensity of protein bands was determined using
the Quantity One software (4.5.0 basic; Bio-Rad Laboratories,
Inc.).
Dual-luciferase reporter assay
The miRcode (http://www.mircode.org/) was used to search which
miRNAs directly interacted with HULC. The possible target genes of
miR-128-3p were also predicted by the starBase database (http://starbase.sysu.edu.cn/agoClipRNA.php?source=circRNA).
A dual-luciferase reporter assay was performed to confirm the
interaction between miR-128-3p and HULC or RAC1. The segmental
sequence of HULC or 3′untranslated region (3′UTR) of RAC1 at the
predicted binding sites were amplified, followed by insertion into
a pGL3 basic vector (Promega Corporation) to construct HULC
wild-type (WT) or RAC1 WT. The binding sites were mutated by Q5
Site Directed Mutagenesis kit (New England Biolabs, Inc.), and HULC
mutant (MUT) and RAC1 MUT were constructed in a similar manner.
1×105 HMEC-1 cells were co-transfected with 2 µg HULC
WT, HULC MUT, RAC1 WT or RAC1 MUT and 40 nM miR-128-3p or miR-NC
with Lipofectamine 3000 (Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C. After 48 h, the luciferase activity was
measured using the Dual-Lucy Assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). The relative luciferase activity
indicated the ratio of firefly luciferase activity to
Renilla luciferase activity.
RNA binding protein
immunoprecipitation (RIP)
RIP assays were performed using an EZ-Magna RIP kit
(EMD Millipore), following the manufacturer's instructions to
further confirm the target relationship among HULC, miR-128-3p and
RAC1. A total of 1×107 HMEC-1 cells were lysed in RIP
lysis buffer, then the cell extract was incubated with magnetic
beads and an antibody against argonaute 2 (Ago2; cat. no. ab32381;
1:50; Abcam) or immunoglobin G (IgG; cat. no. ab109761; 1:50;
Abcam) at 4°C overnight. After protein digestion,
immunoprecipitated RNA was subjected to RNA isolation and RT-qPCR
for detection of HULC, miR-128-3p and RAC1 expression.
RNA pull-down assays
For the RNA pull-down assay, the Magnetic
RNA-Protein Pull-Down kit (Thermo Fisher Scientific, Inc.) was
used. Biotin-labeled miR-128-3p (Bio-miR-128-3p) and its
corresponding negative control (Bio-miR-NC) were constructed by
Guangzhou RiboBio Co., Ltd. Then, cells were lysed and incubated
with beads binding with Bio-miR-128-3p or Bio-miR-NC. Finally,
beads were washed with wash buffer and subjected for examination of
HULC expression via RT-qPCR.
Statistical analysis
Statistical analyses of data derived from 3 parallel
repeat experiments were performed using SPSS 21.0 software (IBM
Corp.) and GraphPad Prism 7 (GraphPad Software Inc.). Data are
presented as the mean ± standard deviation. Comparisons were
conducted with a Student's t test (for 2 groups) or one-way ANOVA
followed by a Tukey's post hoc test (for ≥3 groups). Pearson
correlation analysis was performed to identify the correlation
between the expression of miR-128-3p and HULC or RAC1 in the serum
of patients with sepsis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dysregulation of HULC and miR-128-3p
in serum of patients with sepsis
Initially, the expression of HULC and miR-128-3p was
evaluated in the serum of patients with sepsis and healthy
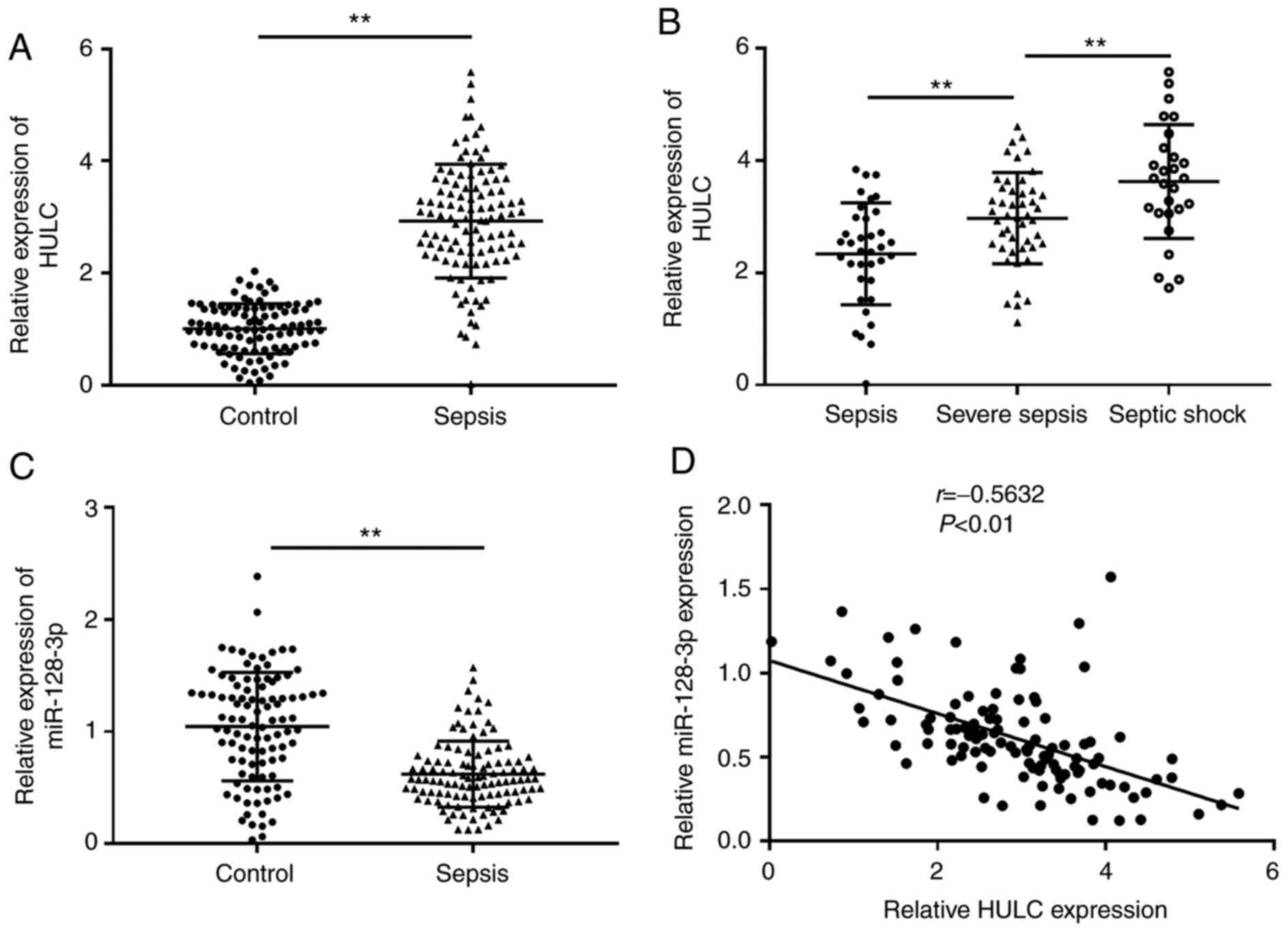
participants (control) via RT-qPCR. As depicted in Fig. 1A, the expression of HULC in the
serum of patients with sepsis was significantly higher than that in
the control. Among the 110 patients with sepsis, the highest level
of HULC was discovered in patients with septic shock (n=27), the
second-highest level of HULC expression was observed in patients
with severe sepsis (n=47) compared with patients with sepsis (n=36)
(Fig. 1B). RT-qPCR analysis
suggested that miR-128-3p expression was significantly
downregulated in the serum of patients with sepsis compared with
those in the control group (Fig.
1C). In addition, there was an inverse correlation between
expression levels of HULC and miR-128-3p in the serum of patients
with sepsis (Fig. 1D). Thus, HULC
and miR-128-3p may be related to the development of sepsis.
Silencing of HULC partially reverses
LPS-induced apoptosis and inflammation in a cell model of
sepsis
To construct a cell model of sepsis, HMEC-1 cells
were treated with LPS or DMSO as a carrier control. Utilizing
RT-qPCR analysis, it was found that LPS treatment significantly
elevated HULC expression when compared with the control (Fig. 2A). In addition, knockdown
efficiency of si-HULC transfection was determined by RT-qPCR
(Fig. 2B). Then, functional
analyses for the role of HULC in LPS-induced HMEC-1 cells were
performed. Flow cytometry indicated that HULC knockdown decreased
the LPS-induced upregulation of apoptosis (Fig. 2C). Subsequent western blotting and
semi-quantitative analysis showed similar results. In Fig. 2D, protein expression levels of
cleaved-cas3 and cleaved-cas9 were significantly upregulated by
LPS, which was reversed in the LPS + si-HULC group. RT-qPCR and
western blot assays were employed to determine the expression
levels of IL-6, TNF-α, ICAM1 and VCAM1, which indicated that LPS
stimulated expression of the four pro-inflammatory factors, and
HULC knockdown largely attenuated the aforementioned promotion
(Fig. 2E and F). Collectively,
silencing of HULC could partially reverse LPS-induced apoptosis and
inflammation in HMEC-1 cells.
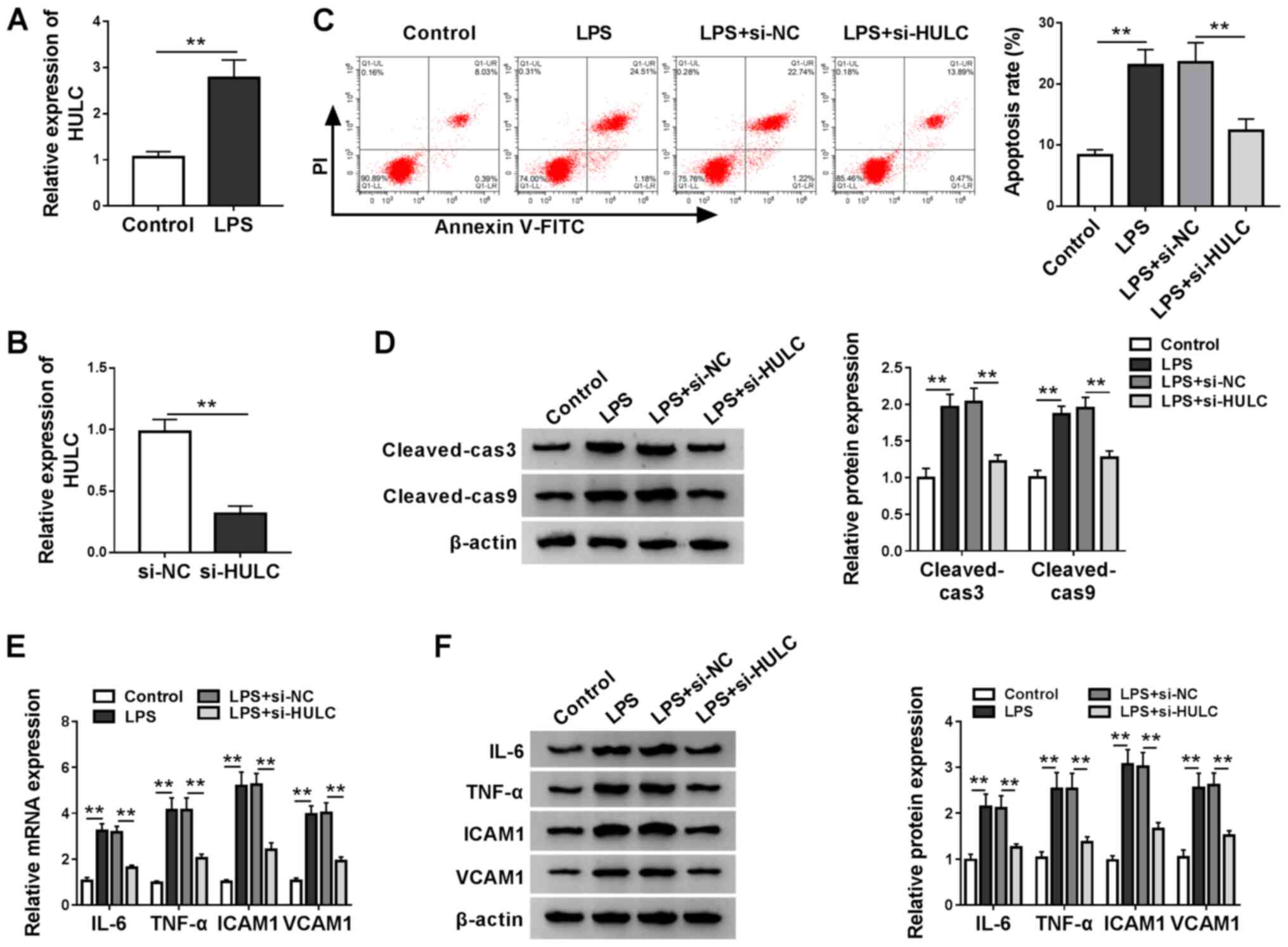 | Figure 2.Silencing of HULC partially reverses
LPS-induced apoptosis and inflammation in a cell model of sepsis.
(A) HULC expression levels in HMEC-1 cells treated with LPS or DMSO
(control). (B) HULC expression in HMEC-1 cells transfected with
si-HULC or si-NC. (C-F) HMEC-1 cells treated with LPS or DMSO
(control) were transfected with si-HULC or si-NC. (C) Apoptotic
rate of transfected HMEC-1 cells. (D) Protein expression levels of
cleaved-cas3 and cleaved-cas9 in transfected HMEC-1 cells. The (E)
mRNA and (F) protein expression levels of IL-6, TNF-α, ICAM1 and
VCAM1 in transfected HMEC-1 cells. **P<0.01. HULC, highly
upregulated in liver cancer; ICAM1, intercellular adhesion
molecule; VCAM1, vascular cell adhesion molecule; siRNA, small
interfering RNA; NC, negative control; LPS, lipopolysaccharide;
HMEC-1, human dermal microvascular endothelial cells; DMSO,
dimethyl sulfoxide; cas, caspase. |
HULC was a sponge of miR-128-3p
Analysis utilizing miRcode revealed that miR-128-3p,
miR-9, miR-150, miR-203, miR-27a-3p and miR-218-5p were predicted
to have possible binding positions for HULC. miR-128-3p (Fig. 3A) was selected for subsequent
investigations as it exhibited the most significant downregulation
in LPS-stimulated HMEC-1 cells transfected with HULC in a
preliminary study among the six miRNAs (data not shown). To
validate this prediction, a dual-luciferase reporter assay was
performed. As shown in Fig. 3B,
co-transfection with miR-128-3p significantly inhibited the
luciferase activity of HULC WT in HMEC-1 cells compared with the
miR-NC group. There was no significant difference in luciferase
activity in HULC MUT. Besides, the RIP assay revealed that both
HULC and miR-128-3p were abundant in Ago2 RIP of HMEC-1 cells
compared with IgG RIP (Fig. 3C). A
large amount of HULC was found to be pulled down by Bio-miR-128-3p
rather than Bio-miR-NC in HMEC-1 cells (Fig. 3D). The aforementioned data
demonstrated that HULC could sponge miR-128-3p. To ascertain the
regulatory effect of HULC on miR-128-3p expression, the present
study upregulated HULC expression levels by transfection, which was
confirmed in HMEC-1 cells (Fig.
3E). Following this, it was found that silencing of HULC
increased miR-128-3p expression, and overexpression of HULC
inhibited miR-128-3p expression (Fig.
3F). Overall, the data showed that HULC targeted miR-128-3p and
negatively modulated its expression.
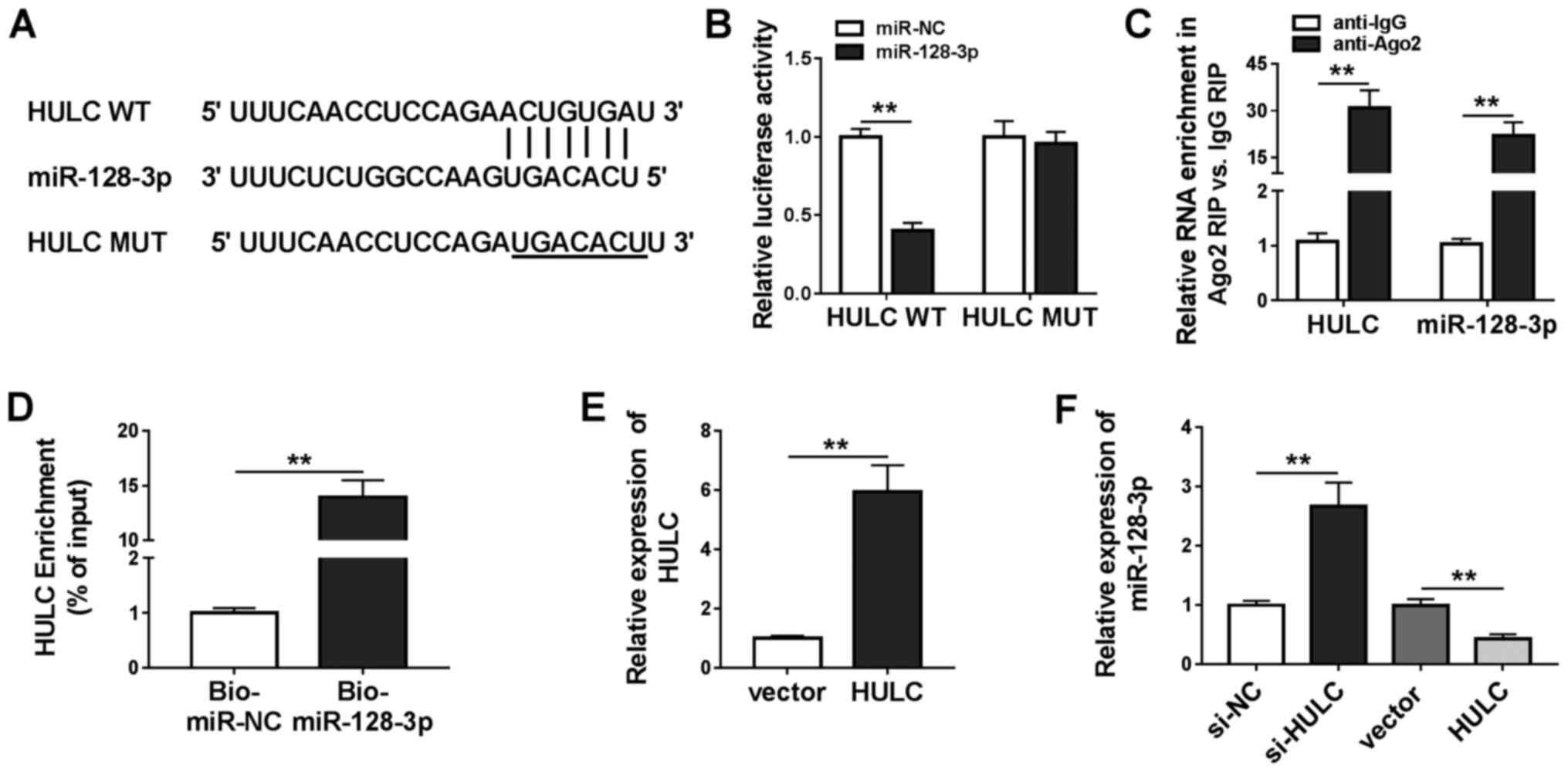 | Figure 3.HULC is a sponge for miR-128-3p. (A)
The putative binding sites between HULC and miR-128-3p, as well as
the MUT. (B) The luciferase activities of HULC WT and HULC MUT in
HMEC-1 cells. (C) The enrichment of HULC and miR-128-3p in the
samples bound to anti-Ago2 or anti-IgG. (D) The enrichment of HULC
pulled down by Bio-miR-128-3p or Bio-miR-NC in HMEC-1 cells. (E)
The expression of HULC in HMEC-1 cells transfected with an empty
vector or HULC-overexpression vector. (F) The expression level of
miR-128-3p in HMEC-1 cells transfected with si-NC, si-HULC, an
empty vector or a HULC-overexpression vector. **P<0.01. WT,
wild-type; MUT, mutant; miR, microRNA; NC, negative control; IgG,
immunoglobin G; HULC, highly upregulated in liver cancer; HMEC-1,
human dermal microvascular endothelial cells; Ago2, argonaute 2;
Bio, biotin; siRNA, small interfering RNA. |
HULC knockdown ameliorates LPS-induced
apoptosis and inflammation in HMEC-1 cells by targeting
miR-128-3p
miR-128-3p expression was measured in LPS-treated
HMEC-1 cells and the control group, RT-qPCR demonstrated that LPS
treatment downregulated miR-128-3p expression (Fig. 4A). Subsequently, the ability of
anti-miR-128-3p to interfere with miR-128-3p expression was
confirmed by RT-qPCR (Fig. 4B). To
clarify the underlying mechanism of HULC in LPS-induced apoptosis
and inflammation of HMEC-1 cells, LPS-treated HMEC-1 cells were
transfected with anti-miR-NC, anti-miR-128-3p, si-HULC +
anti-miR-NC or si-HULC + anti-miR-128-3p. As demonstrated in
Fig. 4C, miR-128-3p interference
promoted the apoptosis of HMEC-1 cells treated with LPS, and also
elevated cell apoptosis of LPS-treated HULC-knockdown HMEC-1 cells.
Western blot analysis indicated that miR-128-3p knockdown elevated
the expression levels of cleaved-cas3 and cleaved-cas9 in
LPS-treated HMEC-1 cells in the untransfected group and the si-HULC
group (Fig. 4D). Furthermore,
silencing of miR-128-3p also significantly promoted the
inflammatory response in LPS-treated HMEC-1 cells in both the
si-HULC group and the untransfected group (Fig. 4E and F). Taken together, silencing
of HULC weakened LPS-induced apoptosis and the inflammatory
response in HMEC-1 cells by targeting miR-128-3p.
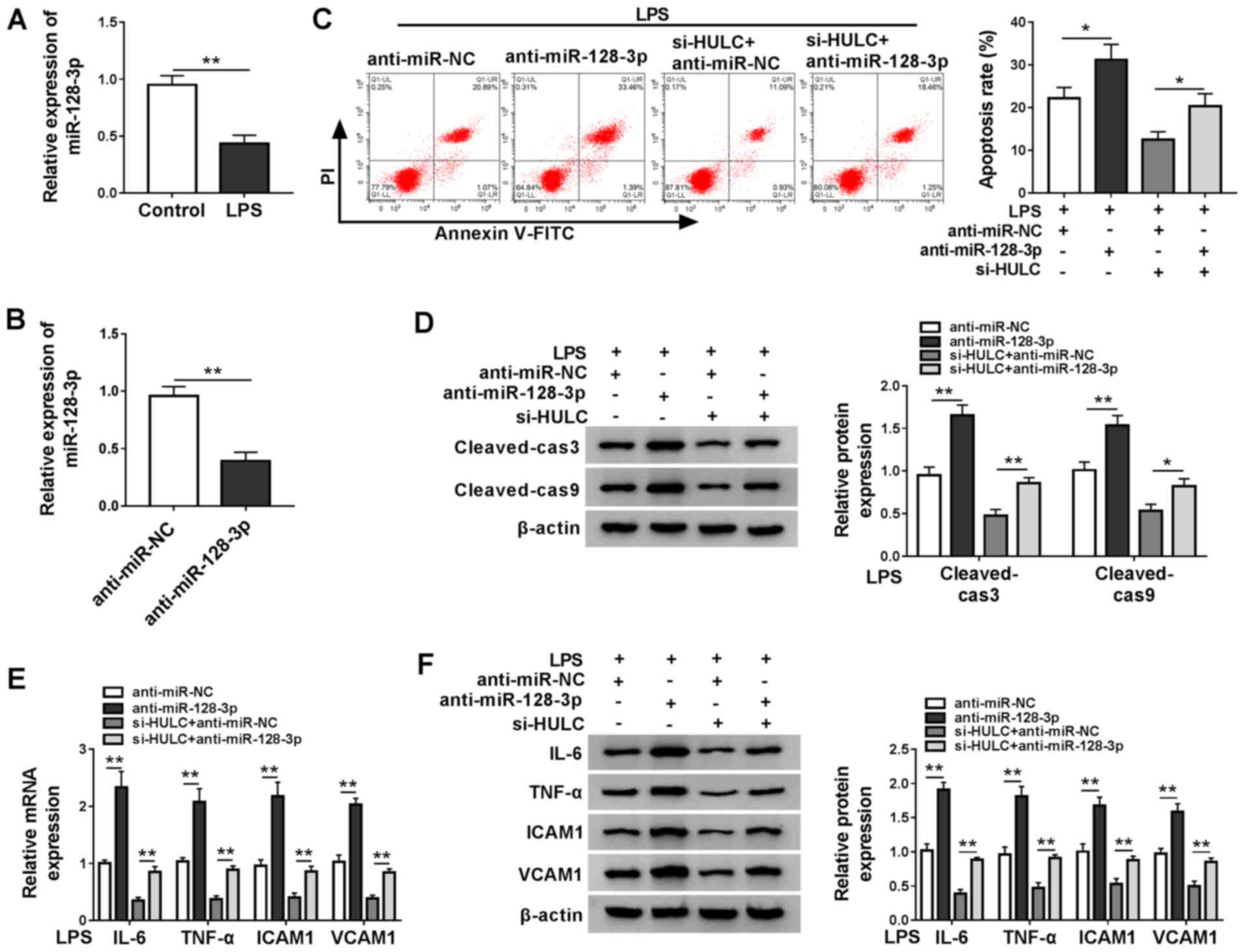 | Figure 4.HULC knockdown ameliorates
LPS-induced apoptosis and inflammation in HMEC-1 cells by targeting
miR-128-3p. (A) miR-128-3p expression in HMEC-1 cells treated with
LPS or DMSO (control). (B) miR-128-3p expression levels in HMEC-1
cells transfected with anti-miR-NC or anti-miR-128-3p. (C-F) HMEC-1
cells treated with LPS were transfected with anti-miR-NC,
anti-miR-128-3p, si-HULC + anti-miR-NC or si-HULC +
anti-miR-128-3p. (C) Apoptotic rate of transfected HMEC-1 cells.
(D) Protein expression levels of cleaved-cas3 and cleaved-cas9 in
transfected HMEC-1 cells. The (E) mRNA and (F) protein expression
levels of IL-6, TNF-α, ICAM1 and VCAM1 in transfected HMEC-1 cells.
*P<0.05. **P<0.01. HULC, highly upregulated in liver cancer;
LPS, lipopolysaccharide; miR, microRNA; NC, negative control;
siRNA, small interfering RNA; ICAM1, intercellular adhesion
molecule; VCAM1, vascular cell adhesion molecule; DMSO, dimethyl
sulfoxide; HMEC-1, human dermal microvascular endothelial cells;
cas, caspase. |
RAC1 is a target of miR-128-3p
The starBase software was applied for searching the
target genes of miR-128-3p, RAC1, kruppel-like factor 4 (KLF4),
disintegrin and metalloproteinase domain-containing protein 10 and
forkhead box protein O4 were identified as candidates. In a
preliminary study, among these four predicted genes, RAC1
expression levels decreased the most in LPS-induced HMEC-1 cells
transfected with miR-128-3p (data not shown). The binding sequence
between miR-128-3p and the 3′UTR of RAC1 is exhibited in Fig. 5A, along with a mutant version.
Dual-luciferase reporter assays and RIP assays demonstrated that
there was a relationship between miR-128-3p and RAC1 (Fig. 5B and C). Furthermore, the present
study found that RAC1 was significantly upregulated in the serum of
patients with sepsis compared with that in the control group
(Fig. 5D). Furthermore, expression
levels of RAC1 mRNA was inversely correlated with miR-128-3p
expression in the serum of patients with sepsis (Fig. 5E). The overexpression efficiency of
miR-128-3p is presented in Fig.
5F. Introduction of miR-128-3p reduced protein expression
levels of RAC1, but transfection with anti-miR-128–3 resulted in
the opposite effect (Fig. 5G).
Additionally, silencing HULC expression led to the reduction of
RAC1 expression, which was reversed by anti-miR-128-3p (Fig. 5H). These results suggested that
RAC1 was a downstream target of miR-128-3p in HMEC-1 cells.
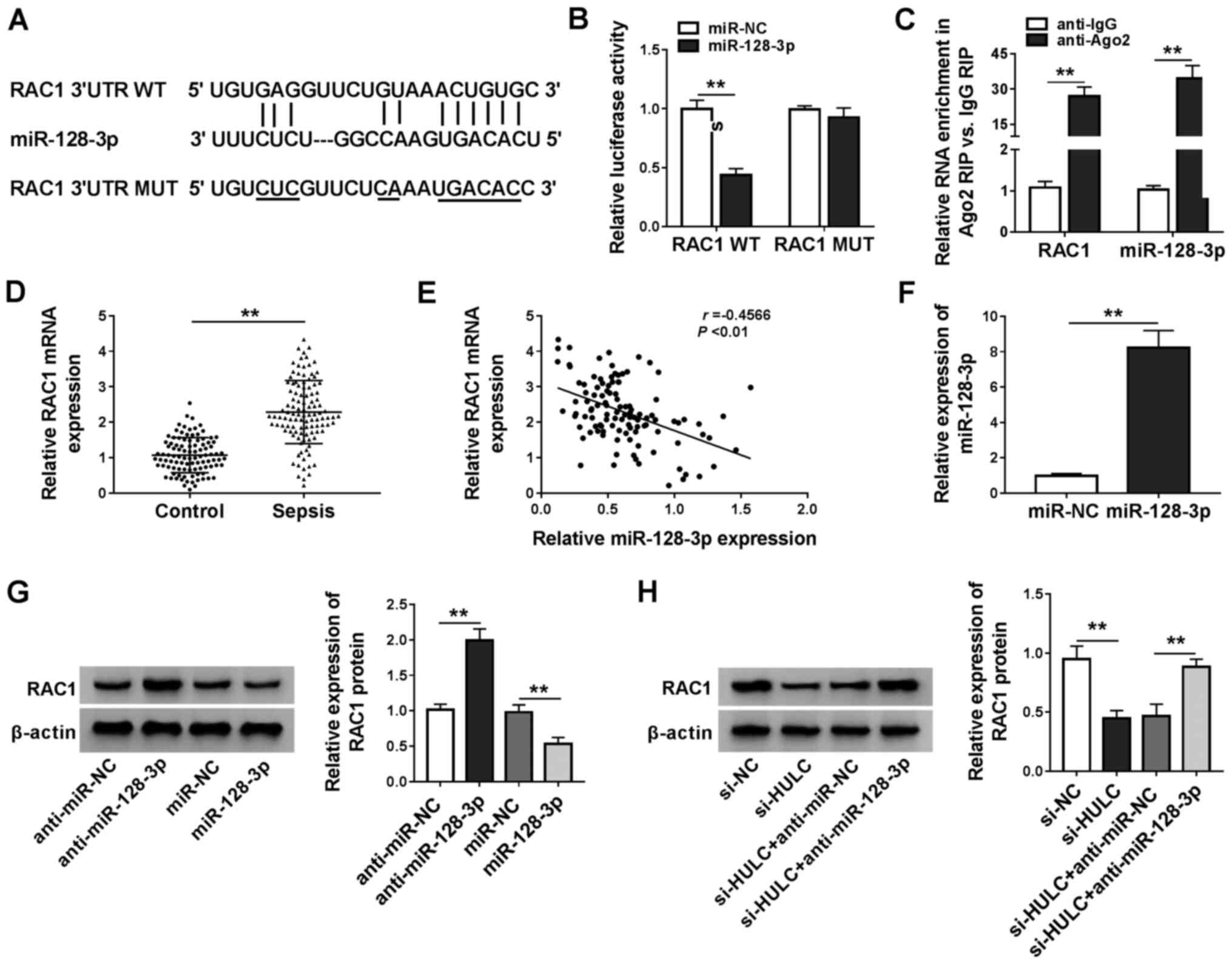 | Figure 5.RAC1 is a target of miR-128-3p. (A)
The predicted binding position between miR-128-3p, the 3′UTR of
RAC1 and the MUT. (B) The luciferase activities of RAC1 WT and RAC1
MUT in HMEC-1 cells. (C) The enrichment of RAC1 and miR-128-3p in
the samples bound to the anti-Ago2 or anti-IgG. (D) The mRNA
expression of RAC1 in the serum of 110 patients with sepsis and 100
healthy participants. (E) Pearson correlation analysis for
expression levels of miR-128-3p and RAC1 mRNA in the serum of 110
patients with sepsis (r=−0.4566, P<0.01). (F) miR-128-3p
expression in HMEC-1 cells transfected with miR-NC or miR-128-3p.
(G) The protein expression of RAC1 in HMEC-1 cells transfected with
anti-miR-NC, anti-miR-128-3p, miR-NC or miR-128-3p. (H) The protein
expression of RAC1 in HMEC-1 cells transfected with si-NC, si-HULC,
si-HULC + anti-miR-NC or si-HULC + anti-miR-128-3p. **P<0.01.
RAC1, Rac family small GTPase 1; miR, microRNA; 3′UTR,
3′untranslated region; WT, wild-type; MUT, mutant; NC, negative
control; IgG, immunoglobin G; Ago2, argonaute 2; HULC, highly
upregulated in liver cancer; siRNA, small interfering RNA; HMEC-1,
human dermal microvascular endothelial cells. |
Upregulation of RAC1 could reverse the
effect of HULC depletion on apoptosis and inflammation in HMEC-1
cells
As indicated in Fig.
6A, LPS treatment also upregulated RAC1 expression levels in
HMEC-1 cells compared with the control group. RAC1 expression
levels were successfully upregulated by transfection with RAC1
(Fig. 6B). Then, to validate the
anti-apoptotic and anti-inflammatory roles of RAC1 in the
HULC-knockdown cells, LPS-induced HMEC-1 cells were transfected
with si-NC, si-HULC, si-HULC + vector or si-HULC + RAC1.
Furthermore, the aforementioned HULC knockdown-induced inhibition
of apoptosis, reduction in cleaved-cas3 and cleaved-cas9 expression
levels and decrease in the inflammatory response were all
attenuated by the co-transfection of RAC1 (Fig. 6C-F). Taken together, HULC knockdown
had anti-apoptotic and anti-inflammatory effects in LPS-induced
HMEC-1 cells via the downregulation of RAC1.
 | Figure 6.Upregulation of RAC1 reverses the
anti-apoptotic and anti-inflammatory effects of HULC knockdown in
HMEC-1 cells. (A) The protein expression of RAC1 in HMEC-1 cells
treated with LPS or DMSO (control). (B) RAC1 protein expression in
HMEC-1 cells transfected with an empty vector or a
RAC1-overexpression vector. (C-F) HMEC-1 cells treated with LPS
were transfected with si-NC, si-HULC, si-HULC + vector or si-HULC +
RAC1. (C) Apoptotic rate of transfected HMEC-1 cells. (D) Protein
expression levels of cleaved-cas3 and cleaved-cas9 in transfected
HMEC-1 cells. The (E) mRNA and (F) protein expression levels of
IL-6, TNF-α, ICAM1 and VCAM1 in transfected HMEC-1 cells.
*P<0.05, **P<0.01. HULC, highly upregulated in liver cancer;
siRNA, small interfering RNA; NC, negative control; LPS,
lipopolysaccharide; RAC1, Rac family small GTPase 1; ICAM1,
intercellular adhesion molecule; VCAM1, vascular cell adhesion
molecule; HMEC-1, human dermal microvascular endothelial cells;
DMSO, dimethyl sulfoxide. |
Discussion
The primary feature of sepsis is the systemic
dysregulation of the inflammatory response after infection
(1), which can result in multiple
organ failure and even death (21). Hence, there is an urgent need to
develop a deeper understanding about sepsis initiation and
progression. The present study identified HULC/miR-128-3p/RAC1 as a
novel potential regulatory axis in a sepsis cell model using
LPS-treated HMEC-1 cells.
LPS is an endotoxin that is able to regulate the
development of myocardial injury caused by sepsis (22), and has been widely used to induce
sepsis models in vitro (23,24).
In the present study, 1 µg/ml LPS was used to treat HMEC-1 cells.
Initially, the effect of LPS on HMEC-1 cells was measured. The data
from RT-qPCR, western blot analysis and flow cytometry suggested
that LPS treatment reinforced apoptosis and inflammatory responses
in treated HMEC-1 cells, which was in line with previous studies
(7,24), thus indicating the successful
establishment of a sepsis model in vitro.
RT-qPCR in the present study revealed that HULC was
highly expressed in the serum of patients with sepsis, especially
the patients with septic shock; as well as in LPS-treated HMEC-1
cells. The HULC gene is 16 kb long and is located at chromosome
6p24.3; it is the first recognized non-coding RNA that is
upregulated in liver cancer tissues, harboring the potential to be
a biomarker of hepatocellular carcinoma (25,26).
Additionally, HULC knockdown has been demonstrated to inhibit
gastric cancer progression via the mediation of the miR-9-5p/myosin
heavy chain 9 axis (27). Chu
et al (28) found that HULC
aggravated ovarian carcinoma progression through the activation of
the PI3K/AKT/mTOR signaling pathway via the downregulation of
miR-125a-3p. The aforementioned reports implied the oncogenic role
of HULC in human cancer types. In the present study, HULC was
knocked down by transfection with specific siRNAs to explore its
role in sepsis in vitro. Functional experiments showed that
HULC knockdown reduced apoptosis and expression levels of
proinflammatory cytokines (IL-6, TNF-α, ICAM1 and VCAM1) in HMEC-1
cells treated with LPS. In other words, silencing of HULC
ameliorated the LPS-mediated injury in HMEC-1 cells, which was
consistent with a previous report (9). Multiple reports have proposed that
lncRNAs play pivotal roles in sepsis by targeting miRNAs (6,7). For
example, NEAT1 upregulated Toll-like receptor 4 to promote
sepsis-induced liver injury by sponging let-7a (29). In the present study, miR-128-3p was
predicted to target HULC, using the online software microRNA.org.
The target relationship between HULC and miR-128-3p was confirmed
by dual-luciferase reporter, RIP and RNA pull-down assays. From the
present data, miR-128-3p was downregulated in the serum of patients
with sepsis and LPS-induced HMEC-1 cells. A significant inverse
correlation between HULC and miR-128-3p was discovered in serum of
patients with sepsis.
miR-128-3p regulates the inflammatory responses
triggered by TNF-α by targeting sirtuin 1 (Sirt1) in bone marrow
mesenchymal stem cells (30).
Selenium could mitigate LPS-induced myocardial inflammation by
modulating the miR-128-3p/p38MAPK-NF-κB pathway (31). miR-128-3p also functions as a tumor
suppressor in human breast cancer (32), glioma (33) and hepatocellular carcinoma
(34). In the present study,
miR-128-3p interference aggravated LPS-mediated damage in HMEC-1
cells, and abolished the HULC knockdown-mediated reduction of
apoptosis and expression levels of proinflammatory cytokines in
LPS-treated HMEC-1 cells. miR-128-3p can modulate inflammatory
responses by binding to the 3′UTR of Sirt1 (30) and KLF4 (35). The present study hypothesized that
miR-128-3p took part in the LPS-induced proinflammatory response by
targeting specific genes. Consequently, the binding position
between miR-128-3p and RAC1 were searched using starBase, following
which they were validated using dual-luciferase reporter and RIP
assays. RT-qPCR analysis demonstrated the high expression of RAC1
in the serum of patients with sepsis and LPS-treated HMEC-1 cells.
In addition, RAC1 mRNA expression was negatively correlated with
miR-128-3p in the serum of patients with sepsis.
RAC1 activity is associated with mitogen-activated
protein kinases involved in proinflammatory activities, therefore
repressing RAC1 activity is hypothesized to be a mechanism to
alleviate the coagulation dysfunction in abdominal sepsis (15). Jiang et al (36) proposed that RAC1 signaling affected
inflammation caused by cigarette smoke in vitro and in
vivo via Erk1/2 MAPK and STAT3 pathways. Inactivation of RAC1
reversed PM2.5-induced inflammation in mouse airways and human
bronchial epithelial cells via the AKT signaling pathway (37). In a preliminary study, miR-128-3p
upregulation suppressed the inflammatory response in LPS-stimulated
HMEC-1 cells, which could be reversed by the introduction of RAC1.
Functional analyses in the current study demonstrated that
accumulation of RAC1 reversed the downregulated apoptosis and
proinflammatory response induced by HULC knockdown in LPS-treated
HMEC-1 cells, which also indicated the participation of RAC1 in the
proinflammatory response induced by LPS.
A few limitations exist in the present study. The
specific signaling pathways involved in the HULC/miR-128-3p/RAC1
axis in the inflammatory response during LPS-induced sepsis in
HMEC-1 cells have not yet been investigated. Furthermore, mouse
models could help to further study the role of HULC in
vivo.
In conclusion, HULC and RAC1 expression were
elevated, and miR-128-3p expression declined in patients with
sepsis and LPS-induced HMEC-1 cells. HULC knockdown could protect
HMEC-1 cells from LPS-triggered injury, which was reversed by
miR-128-3p knockdown or RAC1 overexpression. HULC sponged
miR-128-3p to upregulate RAC1 in LPS-induced HMEC-1 cells,
therefore inhibiting HULC expression could be a potential strategy
in the treatment of sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and YuL conceptualized the study and developed
the methodology. Data analysis and interpretation were performed by
JX, HX and YaL. Validation and investigation were conducted by HX,
YaL and WY. The original draft of the manuscript, along with the
review and editing was conducted by WY, XL and YuL. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All participants submitted written informed consent.
The present study was approved by the ethical review committee of
the Renmin Hospital of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deutschman CS and Tracey KJ: Sepsis:
Current dogma and new perspectives. Immunity. 40:463–475. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adhikari NK, Fowler RA, Bhagwanjee S and
Rubenfeld GD: Critical care and the global burden of critical
illness in adults. Lancet. 376:1339–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cecconi M, Evans L, Levy M and Rhodes A:
Sepsis and septic shock. Lancet. 392:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho J, Chan H, Wong SH, Wang MH, Yu J, Xiao
Z, Liu X, Choi G, Leung CC, Wong WT, et al: The involvement of
regulatory non-coding RNAs in sepsis: A systematic review. Crit
Care. 20:3832016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen J, Liu L, Zhang F, Gu J and Pan G:
LncRNA TapSAKI promotes inflammation injury in HK-2 cells and urine
derived sepsis-induced kidney injury. J Pharm Pharmacol.
71:839–848. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Gu X, Zhou L, Wang S, Zhu L, Huang
Y and Cao F: Long non-coding RNA-HOTAIR promotes the progression of
sepsis by acting as a sponge of miR-211 to induce IL-6R expression.
Exp Ther Med. 18:3959–3967. 2019.PubMed/NCBI
|
|
8
|
Wang SM, Liu GQ, Xian HB, Si JL, Qi SX and
Yu YP: LncRNA NEAT1 alleviates sepsis-induced myocardial injury by
regulating the TLR2/NF-kappaB signaling pathway. Eur Rev Med
Pharmacol Sci. 23:4898–4907. 2019.PubMed/NCBI
|
|
9
|
Chen Y, Fu Y, Song YF and Li N: Increased
expression of lncRNA UCA1 and HULC is required for pro-inflammatory
response during LPS induced sepsis in endothelial cells. Front
Physiol. 10:6082019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rivera-Barahona A, Perez B, Richard E and
Desviat LR: Role of miRNAs in human disease and inborn errors of
metabolism. J Inherit Metab Dis. 40:471–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao Y, Sun F and Lei M: miR-25 inhibits
sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci
Rep. 38:BSR201715112018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Bei Y, Shen S, Huang P, Shi J,
Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al: miR-21-3p controls
sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol
Cell Cardiol. 94:43–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Wang J, Zhang Z and Miao H:
Decreased miR-128 and increased miR-21 synergistically cause
podocyte injury in sepsis. J Nephrol. 30:543–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Luo L, Morgelin M and Thorlacius
H: Rac1 regulates sepsis-induced formation of platelet-derived
microparticles and thrombin generation. Biochem Biophys Res Commun.
487:887–891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM Consensus Conference Committee.
American College of Chest Physicians/Society of Critical Care
Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Shi K, Chen M, Xu L, Hong J, Hu B,
Yang X and Sun R: Elevated miR-155 expression induces
immunosuppression via CD39(+) regulatory T-cells in sepsis patient.
Int J Infect Dis. 40:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Wu J, Li Y and Xing G:
Cytoprotective effect of heat shock protein 27 against
lipopolysaccharide-induced apoptosis of renal epithelial HK-2
cells. Cell Physiol Biochem. 41:2211–2220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang A, Lu H, Wen D, Sun J, Du J, Wang X,
Gu W and Jiang J: The potential roles of long non-coding RNAs in
lipopolysaccharide-induced human peripheral blood mononuclear cells
as determined by microarray analysis. FEBS Open Bio. 9:148–158.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving sepsis campaign: International Guidelines for
Management of Sepsis and Septic Shock: 2016. Crit Care Med.
45:486–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Virzi GM, Clementi A, Brocca A and Ronco
C: Endotoxin effects on cardiac and renal functions and cardiorenal
syndromes. Blood Purif. 44:314–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quoilin C, Mouithys-Mickalad A, Lécart S,
Fontaine-Aupart MP and Hoebeke M: Evidence of oxidative stress and
mitochondrial respiratory chain dysfunction in an in vitro model of
sepsis-induced kidney injury. Biochim Biophys Acta. 1837:1790–1800.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao
S, Lai Z, Wei N, Yang R, Shao Y and He J: miR-23b negatively
regulates sepsis-induced inflammatory responses by targeting ADAM10
in human THP-1 monocytes. Mediators Inflamm. 2019:53065412019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Li Z, Zhang Y, Zhong Q, Chen Q
and Zhang L: Molecular mechanism of HEIH and HULC in the
proliferation and invasion of hepatoma cells. Int J Clin Exp Med.
8:12956–12962. 2015.PubMed/NCBI
|
|
26
|
Panzitt K, Tschernatsch M M, Guelly C,
Moustafa T, Stradner M, Strohmaier HM, Buck CR, Denk H, Schroeder
R, Trauner M and Zatloukal K: Characterization of HULC, a novel
gene with striking up-regulation in hepatocellular carcinoma, as
noncoding RNA. Gastroenterology. 132:330–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Liu Y, Wei C, Yang Z, Chang W and
Zhang X: LncRNA HULC promotes the progression of gastric cancer by
regulating miR-9-5p/MYH9 axis. Biomed Pharmacother. 121:1096072019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu P, Xu L and Su H: HULC functions as an
oncogene in ovarian carcinoma cells by negatively modulating
miR-125a-3p. J Physiol Biochem. 75:163–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang CC and Niu F: LncRNA NEAT1 promotes
inflammatory response in sepsis-induced liver injury via the
Let-7a/TLR4 axis. Int Immunopharmacol. 75:1057312019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu L, Zhang G, Guo C, Zhao X, Shen D and
Yang N: MiR-128-3p mediates TNF-α-induced inflammatory responses by
regulating Sirt1 expression in bone marrow mesenchymal stem cells.
Biochem Biophys Res Commun. 521:98–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Wang S, Zhang Q, Li X and Xu S:
Selenomethionine alleviates LPS-induced chicken myocardial
inflammation by regulating the miR-128-3p-p38 MAPK axis and
oxidative stress. Metallomics. 12:54–64. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao J, Li D and Fang L: MiR-128-3p
suppresses breast cancer cellular progression via targeting LIMK1.
Biomed Pharmacother. 115:1089472019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huo L, Wang B, Zheng M, Zhang Y, Xu J,
Yang G and Guan Q: miR-128-3p inhibits glioma cell proliferation
and differentiation by targeting NPTX1 through IRS-1/PI3K/AKT
signaling pathway. Exp Ther Med. 17:2921–2930. 2019.PubMed/NCBI
|
|
34
|
Huang CY, Huang XP, Zhu JY, Chen ZG, Li
XJ, Zhang XH, Huang S, He JB, Lian F, Zhao YN and Wu GB: miR-128-3p
suppresses hepatocellular carcinoma proliferation by regulating
PIK3R1 and is correlated with the prognosis of HCC patients. Oncol
Rep. 33:2889–2898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu Q, Meng Q, Qi M, Li F and Liu B:
Shear-sensitive lncRNA AF131217.1 inhibits inflammation in HUVECs
via regulation of KLF4. Hypertension. 73:e25–e34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang JX, Zhang SJ, Shen HJ, Guan Y, Liu
Q, Zhao W, Jia YL, Shen J, Yan XF and Xie QM: Rac1 signaling
regulates cigarette smoke-induced inflammation in the lung via the
Erk1/2 MAPK and STAT3 pathways. Biochim Biophys Acta Mol Basis Dis.
1863:1778–1788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Zhang W, Zeng X, Zhao W, Wang Z,
Dong X, Jia Y, Shen J, Chen R and Lin X: Inhibition of Rac1
activity alleviates PM2.5-induced pulmonary inflammation via the
AKT signaling pathway. Toxicol Lett. 310:61–69. 2019. View Article : Google Scholar : PubMed/NCBI
|