Introduction
Acute lung injury (ALI) and its severe form, acute
respiratory distress syndrome, are common inflammatory lung
diseases with high mortality of 30–40% that are triggered by severe
trauma, sepsis and pneumonia (1,2).
Lipopolysaccharide (LPS) derives from the cell wall of
gram-negative bacteria, and it plays a crucial role in inflammatory
responses (3,4). Therefore, finding the molecules
involved in the development of ALI is indispensable for the
treatment of ALI.
Long non-coding RNAs (lncRNAs) are a group of
non-coding RNAs (ncRNAs) with >200 nucleotides. lncRNA cancer
susceptibility candidate 2 (CASC2) has been indicated as an
important molecule involved in the pathogenesis of numerous types
of cancer (5–8). For instance, Wang et al
(9) reported that CASC2 prevented
the epithelial-mesenchymal transition of hepatocellular carcinoma
cells via microRNA (miR/miRNA)-367/F-box/WD repeat-containing
protein 7 signaling. Ba et al (10) demonstrated that CASC2 inhibited the
proliferation and motility of osteosarcoma cells via miR-181a. As
for ALI, Li et al (11)
revealed that CASC2 was downregulated in LPS-treated A549 cells,
and CASC2 accumulation attenuated LPS-induced ALI in vivo
and in vitro. Overall, the present study aimed to
investigate the function of CASC2 in ALI, as well as its functional
mechanism.
miRNAs, as another group of ncRNAs, could regulate
the physiological and pathological processes by regulating the
expression of target genes (12–14).
miRNAs have been reported to participate in the initiation and
progression of lung inflammation (15). Huang et al (16) demonstrated that miR-27b promoted
LPS-induced ALI by regulating nuclear factor erythroid 2-related
factor 2 (Nrf2) and NF-κB signaling. The present study aimed to
elucidate the underlying mechanism of miR-27b in ALI.
TGF-β activated kinase 1 and MAP3K7-binding protein
2 (TAB2) serves as an important upstream adaptor of interleukin-1
(IL-1) signaling to function. TAB2 has been reported to be involved
in the pathogenesis of LPS-induced ALI (17). However, the function of TAB2 in ALI
remains to be revealed.
The present study intended to uncover the role and
the working mechanism of CASC2 in LPS-induced ALI cell model.
Materials and methods
Cell culture
Human alveolar epithelial adenocarcinoma cell line
A549 was acquired from American Type Culture Collection. A549 cells
(2×105 cells/well) in 6-well plates were grown in the
Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin in a humidified atmosphere at 37°C and
5% CO2.
LPS treatment
A549 cells were cultivated in 6-well plates
(2×105 cells/well), and 1 µg/ml LPS (Sigma-Aldrich;
Merck KGaA) or control [dimethyl sulfoxide (DMSO); Sigma-Aldrich;
Merck KGaA] was mixed with the culture medium when the confluence
reached ~80%, and the cells were incubated for 24 h according to a
previous article (18).
Cell transfection
To achieve CASC2 overexpression or knockdown, CASC2
overexpression plasmid (CASC2; 1 µg), pcDNA (1 µg), small
interfering RNA control (si-con; 5′-UUCUCCGAACGUGUCACGUUU-3′; 100
nM) or CASC2 specific siRNA (si-CASC2; 5′-AGACUAUAAUGAUACCUUGGG-3′;
100 nM) were transfected into A549 cells (3×105
cells/well). To achieve miR-27b overexpression or knockdown, miRNA
control (miR-con; 5′-UUCUCCGAACGUGUCACGUUU-3′; 50 nM), miR-27b
mimic (miR-27b; 5′-CCGAAGAUGCUCACCAGCCC-3′; 50 nM), miR-27b
inhibitor (anti-miR-27b; 5′-AUGUUCUUGAAAGCCGAU-3′; 20 nM) or
anti-miR-con (5′-UUGUACUACACAAAAGUACUG-3′; 20 nM) were transfected
into A549 cells (3×105 cells/well). si-TAB2
(5′-ACAACUUCAGGUACUUCAGGG-3′; 100 nM) was transfected into A549
cells (3×105 cells/well) to achieve TAB2 silencing, and
si-con (5′-UUCUCCGAACGUGUCACGUUU-3′; 100 nM) was used as the
control. The aforementioned RNA and overexpression plasmids were
purchased from Shanghai GenePharma Co., Ltd., and the transfection
was carried out with Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After transfection for 24 h,
transfected cells were used for subsequent analysis.
Experimental grouping
In rescue experiments of miR-27b for CASC2, A549
cells were transfected with CASC2 plasmid (1 µg) or pcDNA (1 µg)
alone or together with miR-27b (50 nM) or miR-con (50 nM) for 24 h
followed by 1 µg/ml LPS treatment for 24 h. Thus, A549 cells were
divided into six groups: Control, LPS, LPS + pcDNA, LPS + CASC2,
LPS + CASC2 + miR-con and LPS + CASC2 + miR-27b.
In rescue experiments of TAB2 for miR-27b, A549
cells were transfected with anti-miR-27b (20 nM) or anti-miR-con
(20 nM) alone or together with si-TAB2 (100 nM) or si-con (100 nM)
for 24 h followed by 1 µg/ml LPS treatment for 24 h. Thus, A549
cells were divided into six groups: Control, LPS, LPS +
anti-miR-con, LPS + anti-miR-27b, LPS + anti-miR-27b + si-con and
LPS + anti-miR-27b + si-TAB2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from A549 cells was extracted using
TRIzol® (Beyotime Institute of Biotechnology). The
reverse transcription of CASC2 and the mRNA of TAB2 was performed
using BeyoRT™ First Strand cDNA Synthesis Kit (Beyotime Institute
of Biotechnology) at 42°C for 60 min, whereas the cDNA of miR-27b
was obtained with One Step miRNA RT kit (HaiGene) at 37°C for 60
min followed by 85°C for 5 sec. U6 and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) served as the internal references for
miR-27b, CASC2 and TAB2. The expression of miR-27b, TAB2 and CASC2
was measured using the 2−ΔΔCq method (19). The PCR reaction was conducted using
SYBR-Green [Roche Diagnostics (Shanghai) Co., Ltd.] and an ABI 7500
thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with the following primers: CASC2 forward,
5′-GCACATTGGACGGTGTTTCC-3′ and reverse, 5′-CCCAGTCCTTCACAGGTCAC-3′;
miR-27b forward, 5′-AAAAGTCGACCGAAGATGCTCACCAGCCCTT-3′ and reverse,
5′-AAAAGTCGACGGCAGTGGCCTCTGCCTGGC-3′; TAB2 forward,
5′-AGTACAAGATATCTTTATGG-3′ and reverse,
5′-TGCTGTCTGTGGCTCCTGCT-3′); U6 forward, 5′-ATGGGTCGAAGTCGTAGCC-3′
and reverse, 5′-TTCTCGGCGTCTTCTTTCTCG-3′; and GAPDH forward,
5′-ATGTTCCAGTATGACTCCACTCACG-3′ and reverse,
5′-GAAGACACCAGTAGACTCCACGACA-3′.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
A549 cells were plated in 96-well cell culture
plates at the density of 5×103 cells/well. Following LPS
stimulation and relevant transfection, 20 µl 5 mg/ml MTT reagent
(Sigma-Aldrich; Merck KGaA) was added into the supernatant of A549
cells. A549 cells were incubated with MTT in the incubator at 37°C
with 5% CO2 for 4 h. The culture medium was discarded,
and A549 cells were incubated with DMSO (Sigma-Aldrich; Merck KGaA)
to dissolve formazan crystals. The absorbance of each well was
measured at 490 nm using a microplate reader.
Cell apoptosis analysis
The adherent and non-adherent A549 cells were
harvested and washed using phosphate buffered saline buffer. A549
cells (3×105 cells) were probed with Annexin
V-fluorescein isothiocyanate and propidine iodide using the
Apoptosis Detection Kit (BD Biosciences). A FACS CantoII flow
cytometer (BD Biosciences) was used to identify the apoptotic A549
cells, and the apoptotic rate was analyzed using BD FACSDiva
software (version 8.0.1; BD Biosciences). The apoptotic rate
indicated the percentages of apoptotic A549 cells in the early
stage (Q4) and late stage (Q2).
Enzyme-linked immunosorbent assay
(ELISA)
Human IL-1β (cat. no. DLB50), IL-6 (cat. no. D6050)
and tumor necrosis factor α (TNF-α; cat. no. DTA00D) ELISA kits
(R&D Systems, Inc.) were used to examine the concentrations of
inflammatory cytokines in the supernatant of A549 cells. Briefly,
A549 cells were seeded into 96-well plates (5×103
cells/well), and A549 cells were stimulated with the relevant
treatment the next day. After centrifuging at 2,000 × g for 5 min
at 4°C, cell supernatant was collected to examine the secretion of
inflammatory cytokines according to the instructions of the ELISA
kits.
Bioinformatics analysis
The miRNA targets of CASC2 were predicted using
LncBase software v2.0 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index)
from DIANA Tools, while microT-CDS software v5.0 (http://www.microrna.gr/microT-CDS) from DIANA
Tools was used to predict the mRNA targets of miR-27b.
Dual-luciferase reporter assay
A dual-luciferase reporter assay was performed to
explore the interaction between miR-27b and CASC2 or TAB2 in A549
cells. Transfection was performed using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). CASC2 gene
sequences, including predicted wild-type (WT) or mutant-type (MUT)
binding sites of miR-27b, were inserted into pmirGLO vectors
(Promega Corporation). miR-con, miR-27b, anti-miR-con or
anti-miR-27b and CASC2-WT or CASC2 MUT were co-transfected into
A549 cells (4×104 cells/well). A
Dual-Luciferase® Reporter Assay system (Promega
Corporation) was utilized to measure the luciferase activity after
transfection for 48 h, and the firefly luciferase activity was
normalized to Renilla luminescence. The same protocol was
used for TAB2.
RNA binding protein
immunoprecipitation (RIP) assay
RNA-protein or RNA-RNA complex were isolated using
Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (EMD
Millipore). A549 cells (2×105 cells) were disrupted
using RIP lysis buffer for 5 min on ice. The antibodies, including
anti-Argonaute 2 (anti-Ago2; cat. no. MABE253; EMD Millipore) at
the dilution of 1:50 and anti-Immunoglobulin G (anti-IgG; cat. no.
TS-1L-BK; EMD Millipore) at the dilution of 1:100 were incubated
with protein A/G beads for 1 h at 4°C. Then, the A549 cell lysate
was mixed with the beads to incubate for 4 h at 4°C. Beads were
washed twice using PBS buffer (Sangon Biotech Co., Ltd.), and the
mixture was centrifuged at 21,000 × g for 10 min at 4°C. RNA was
extracted using TRIzol® (Beyotime Institute of
Biotechnology), and the immunoprecipitated RNA was detected by
RT-qPCR.
Western blot assay
Western cell lysis buffer (Beyotime Institute of
Biotechnology) was used to disrupt A549 cells, and the proteins
were quantified using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). The quantified protein samples (25 µg) were
separated on the 5% stacking and 12% separating gels, and
subsequently transferred to a polyvinylidene fluoride membrane. The
membrane was blocked using 5% skimmed milk for 1 h at room
temperature. The membrane was incubated with anti-TAB2 at the
dilution of 1:5,000 (cat. no. ab172412; Abcam) and anti-β-actin at
the dilution of 1:20,000 (cat. no. ab115777; Abcam) at 4°C
overnight, followed by incubation with a horseradish
peroxidase-conjugated secondary antibody (cat. no. ab205718; Abcam)
at the dilution of 1:5,000 for 2 h at room temperature. Several
X-ray films were used to visualize the protein signal through
Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.). Image Lab analysis software (v4.0; Bio-Rad Laboratories,
Inc.) was utilized to evaluate the intensities of protein
bands.
Animal experiment
A total of 12 BALB/c mice (weight, 23–28 g; age, 8
weeks) were obtained from Experimental Animal Center of Huazhong
Agricultural University (Wuhan, China) and maintained in a
specific-pathogen-free environment under standard conditions of
22°C, 60% humidity and a 12 h light/dark cycle. These nude mice
were supplied food and water ad libitum. All experimental
procedures were approved by The Ethical Committee on Animal
Research of Shiyan People's Hospital (Shiyan, China) and were in
accordance with guidelines provided by the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (20). Mice were arbitrarily divided into
four groups (n=3), including the Control group (intraperitoneal
injection of 30 mg/kg normal saline), LPS group (intraperitoneal
injection of 30 mg/kg LPS), LPS + pcDNA group (transfection with
pcDNA empty vector before LPS treatment) and LPS + CASC2 group
(transfection with CASC2 before LPS treatment). Transfection was
conducted using a microinjector to inject the transfection
complexes (50 µl) into the trachea of the mice. After 12 h of
transfection, these nude mice were euthanized using sodium
pentobarbital (100 mg/kg) and the right lung (free of heart and
trachea) were removed. The breathing of the mice was monitored to
verify the death. No mice died during this experiment. Right lung
was rinsed briefly using PBS buffer (Sangon Biotech Co., Ltd.), and
then the right lung was weighed to obtain the wet weight. The right
lung was baked at 56°C for 72 h to acquire the dry weight.
Wet-to-dry (W/D) ratio was obtained by dividing the wet weight of
the right lung by the dry weight. The myeloperoxidase (MPO)
activity of lung tissues was obtained using an MPO determination
kit (Jiancheng Company; http://www.njjcbio.com/products.asp?id=354) at 460 nm.
The expression of CASC2 was detected using RT-qPCR. The levels of
IL-1β, IL-6 and TNF-α were measured using ELISA kits.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 7 software (GraphPad Software, Inc.). Normally distributed
data are presented as the mean ± standard deviation. Comparisons
between groups were analyzed using Student's t-test or one-way
analysis of variance followed by Tukey's post hoc test as
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
CASC2 overexpression ameliorates
LPS-triggered ALI
To investigate the expression pattern and biological
function of CASC2 in ALI, a ALI cell model was established by
treating A549 cells with 1 µg/ml LPS for 24 h. The overexpression
efficiency of the CASC2 plasmid was high in A549 cells (Fig. S1A). As shown in Fig. 1A, the expression of CASC2 was
decreased in the ALI cell model compared with in non-treated A549
cells. Besides, the expression of CASC2 was increased in A549 cells
treated with LPS and the CASC2 overexpression plasmid. LPS
stimulation reduced viability and induced apoptosis of A549 cells
(Fig. 1B and C). In addition, the
overexpression of CASC2 protected A549 cells against LPS-induced
injury. The inflammatory reaction was activated in the ALI model,
and the transfection of CASC2 suppressed LPS-triggered inflammation
(Fig. 1D-F). In summary, these
data suggested that LPS-induced ALI could be attenuated by the
overexpression of CASC2.
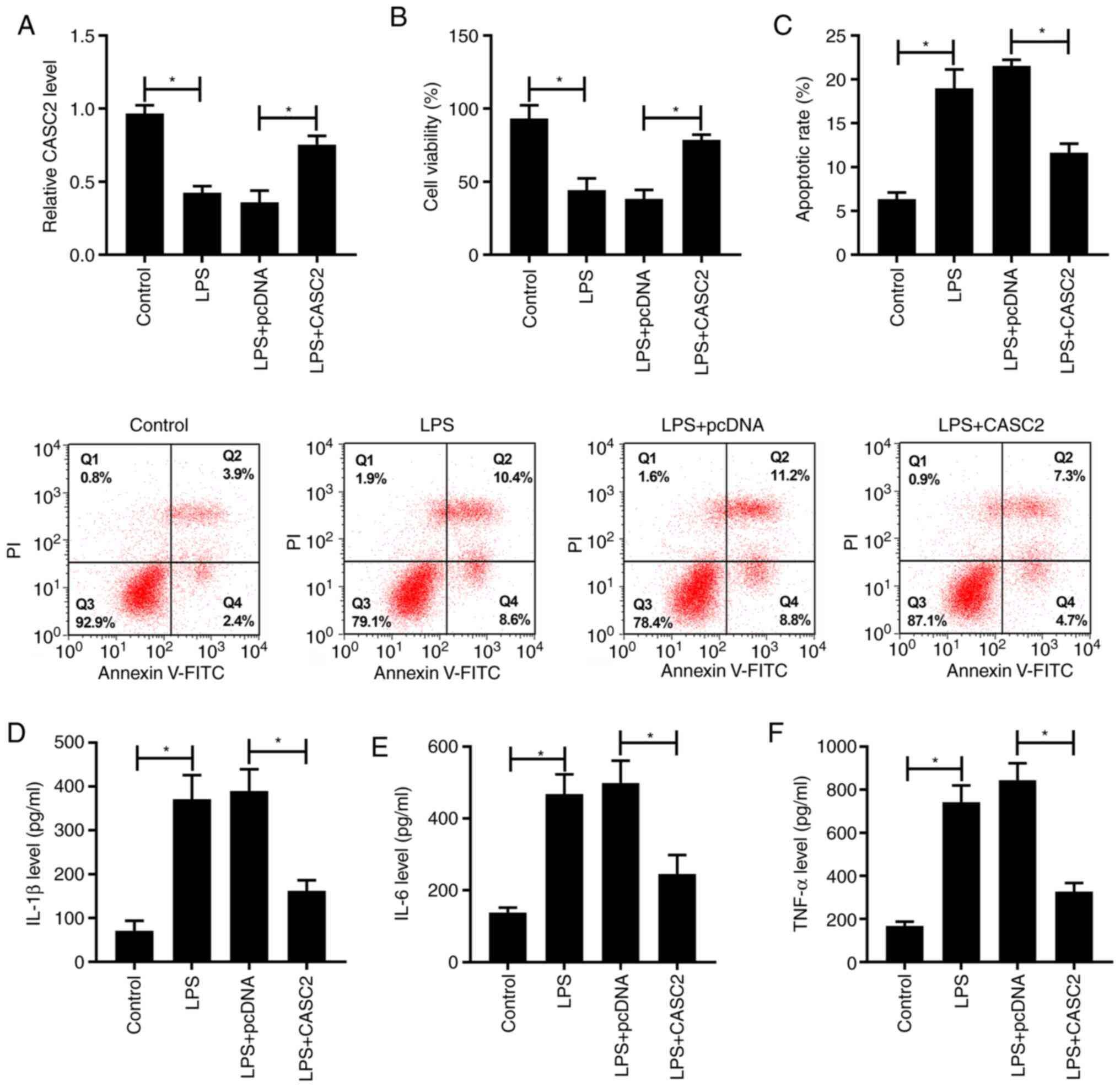 | Figure 1.CASC2 overexpression ameliorates
LPS-induced acute lung injury. A549 cells were stimulated with LPS,
LPS + pcDNA or LPS + CASC2. (A) The expression of CASC2 was
detected in A549 cells via reverse transcription-quantitative PCR.
(B) A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assay was performed to measure the viability of A549 cells. (C) The
apoptotic rate of A549 cells was analyzed by flow cytometry. The
inflammatory factors in A549 cells, including (D) IL-1β, (E) IL-6
and (F) TNF-α, were examined by ELISA. *P<0.05. CASC2, cancer
susceptibility candidate 2; LPS, lipopolysaccharide; IL,
interleukin; TNF-α, tumor necrosis factor α. |
CASC2 could directly bind to miR-27b
to downregulate its expression
miR-27b was predicted to be a potential target of
CASC2 using LncBase database, and the putative binding sites are
listed in Fig. 2A. The
transfection efficiencies of miR-27b and anti-miR-27b were high in
A549 cells, as determined by RT-qPCR (Fig. S1B). The dual-luciferase reporter
assay results showed that transfection of miR-27b or anti-miR-27b
significantly decreased or increased the luciferase activity in the
CASC2-WT group, respectively (Fig.
2B). Besides, the luciferase activity in the CASC2-MUT group
remained unaffected. Furthermore, a RIP assay was conducted to
detect whether CASC2 was present in RNA-induced silencing complex
(RISC), and the results revealed that CASC2 and miR-27b could be
detected in the RISC (Fig. 2C),
suggesting that CASC2 could bind to miR-27b in A549 cells. LPS
exposure could elevate the expression of miR-27b in A549 cells
(Fig. 2D). The transfection of
si-CASC2 significantly reduced CASC2 expression in A549 cells
(Fig. S1A). CASC2 overexpression
significantly downregulated the expression of miR-27b (Fig. 2E), whereas transfection with
si-CASC2 significantly increased the expression of miR-27b in A549
cells, suggesting that miR-27b was inversely regulated by CASC2 in
A549 cells. These data indicated that miR-27b was a target of
CASC2, and CASC2 negatively regulated the expression of miR-27b in
A549 cells.
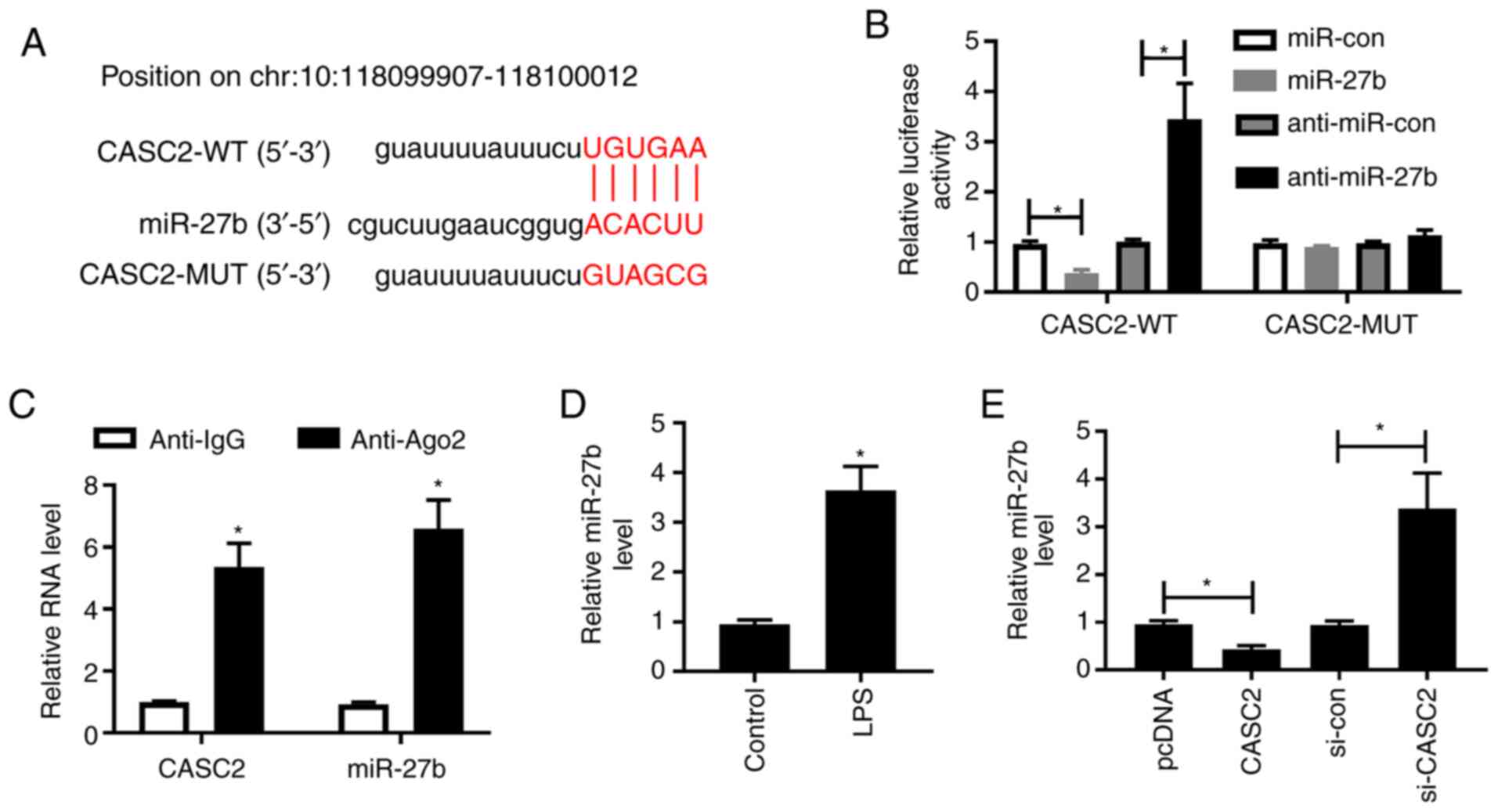 | Figure 2.CASC2 could directly bind to miR-27b
to downregulate its expression. (A) The miR-27b binding sequence in
CASC2 was predicted using LncBase software. (B) A dual-luciferase
reporter assay was performed in A549 cells co-transfected with
CASC2-WT or CASC2-MUT and miR-con, miR-27b, anti-miR-con or
anti-miR-27b. (C) The association between miR-27b and CASC2 was
verified by performing a RNA binding protein immunoprecipitation
assay. (D) RT-qPCR was conducted to measure the expression of
miR-27b in A549 cells treated with LPS. (E) RT-qPCR was performed
to detect the expression of miR-27b in A549 cells transfected with
pcDNA, CASC2, si-con or si-CASC2. *P<0.05 vs. control group.
CASC2, cancer susceptibility candidate 2; LPS, lipopolysaccharide;
miR, microRNA; WT, wild-type; MUT, mutant; con, control; RT-qPCR,
reverse transcription-quantitative PCR; si-, small interfering RNA;
Ago2, Argonaute 2; IgG, Immunoglobulin G. |
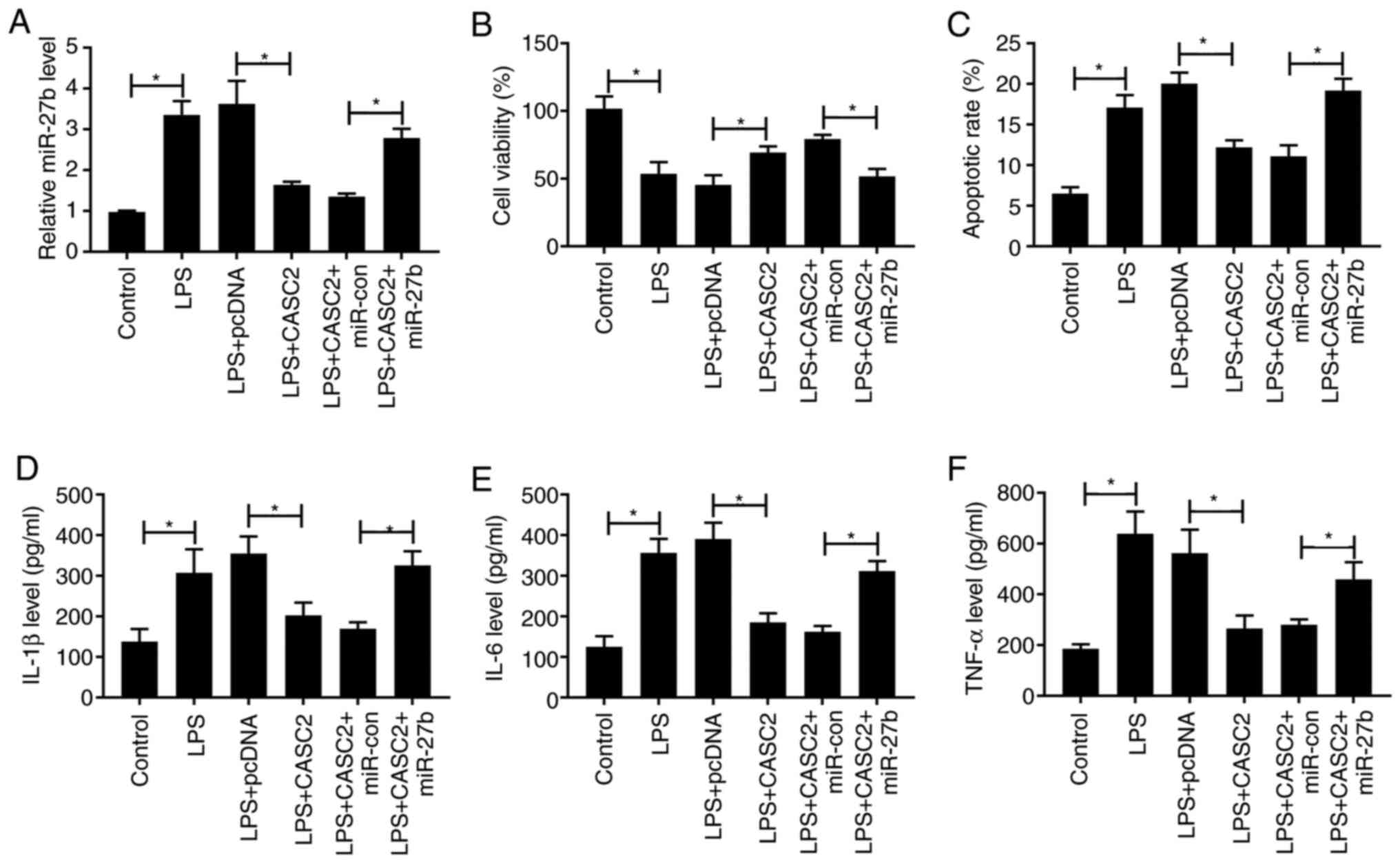
CASC2 protects A549 cells against
LPS-induced inflammatory damage by downregulating miR-27b
To further explore the functions of miR-27b and
CASC2 in LPS-induced ALI, A549 cells were divided into six groups:
Control, LPS, LPS + pcDNA, LPS + CASC2, LPS + CASC2 + miR-con or
LPS + CASC2 + miR-27b. As shown in Fig. 3A, CASC2 overexpression reduced the
expression of miR-27b in the ALI cell model, whereas the expression
of miR-27b was increased in LPS-stimulated cells co-transfected
with CASC2 and miR-27b. The addition of miR-27b reversed the
protective effect of CASC2 transfection on the viability of ALI
model cells (Fig. 3B). Meanwhile,
the apoptosis was significantly increased in the LPS + CASC2 +
miR-27b group compared with that in the LPS + CASC2 + miR-con group
(Fig. 3C). Subsequently, it was
found that the inhibitory effect of CASC2 overexpression on the
inflammatory response was reversed by the overexpression of miR-27b
in ALI model cells (Fig. 3D-F).
Collectively, these data suggested that CASC2 suppressed
LPS-stimulated ALI by downregulating the expression of miR-27b.
TAB2 is a target of miR-27b in A549
cells
microT-CDS software was used to search the target
genes of miR-27b, the putative miR-27b binding sequence in the
3′untranslated region (3′UTR) of TAB2 is shown in Fig. 4A. The luciferase activity was
significantly decreased or increased in A549 cells co-transfected
with TAB2-WT and miR-27b or anti-miR-27b, respectively (Fig. 4B). These results revealed that TAB2
was a direct target of miR-27b in A549 cells. To further validate
these results, a RIP assay was carried out. As shown in Fig. 4C, TAB2 and miR-27b were
co-precipitated with Ago2, demonstrating that TAB2 could bind to
miR-27b in A549 cells. Besides, LPS treatment reduced the protein
expression of TAB2 in A549 cells (Fig.
4D). A549 cells transfected with miR-con, miR-27b, anti-miR-con
or anti-miR-27b were used to explore the modulatory relationship
between miR-27b and TAB2. As exhibited in Fig. 4E, miR-27b transfection reduced the
expression of TAB2, whereas depletion of miR-27b led to the
opposite effect in A549 cells. In summary, TAB2 was shown to be a
target of miR-27b in A549 cells, and miR-27b could negatively
regulate the expression of TAB2.
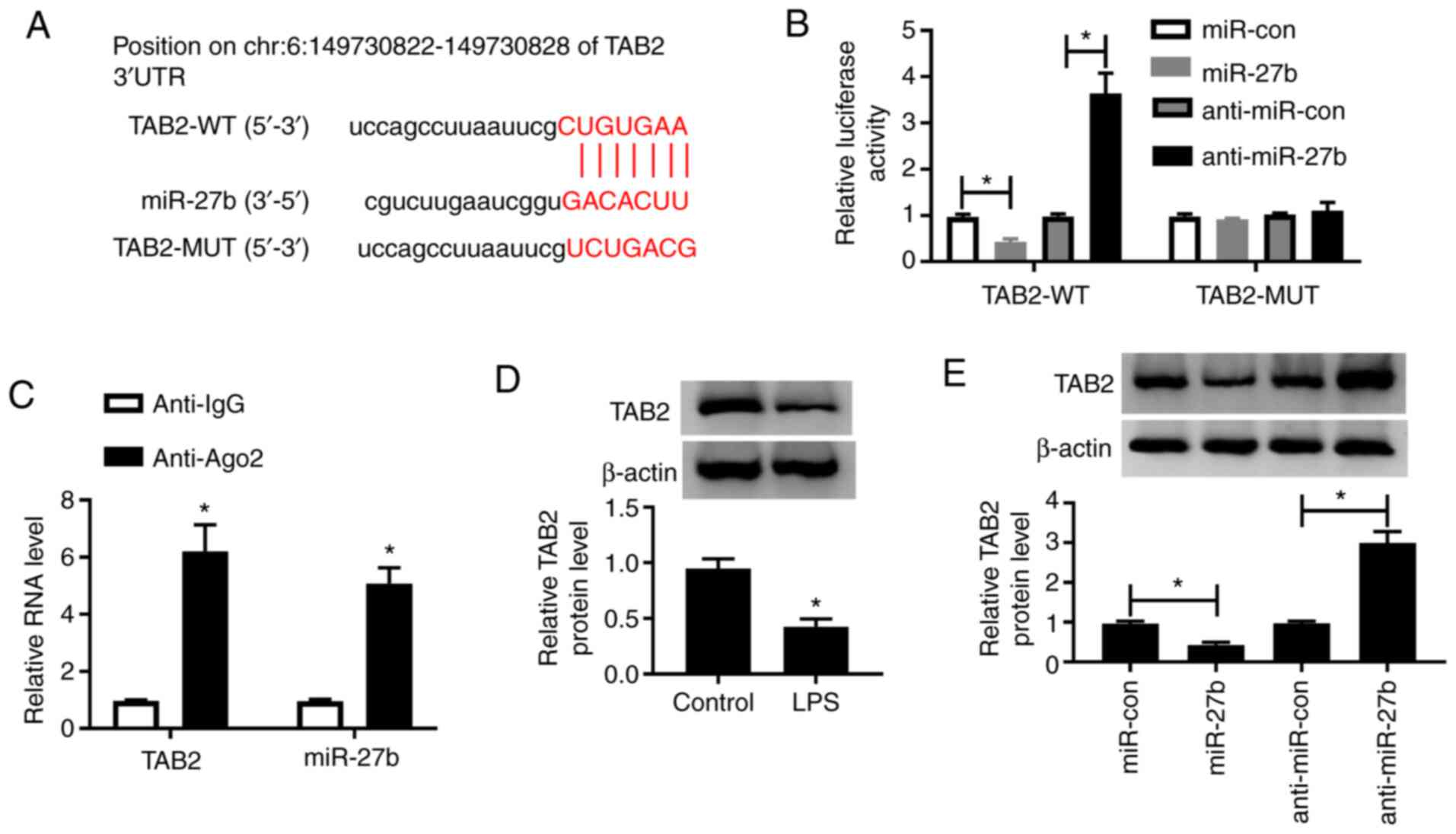 | Figure 4.TAB2 is a target of miR-27b in A549
cells. (A) The binding sites of miR-27b in the 3′UTR of TAB2 were
predicted using microT-CDS software. (B) The target relationship
between miR-27b and TAB2 was confirmed by a dual-luciferase
reporter assay. (C) A RNA binding protein immunoprecipitation assay
was carried out to validate the association between miR-27b and
TAB2 in A549 cells. (D) The expression of TAB2 in LPS-induced A549
cells was examined by western blotting. (E) Western blotting was
conducted to measure the expression of TAB2 in A549 cells
transfected with miR-con, miR-27b, anti-miR-con or anti-miR-27b.
*P<0.05 vs. control group. TAB2, TGF-β activated kinase 1 and
MAP3K7-binding protein 2; LPS, lipopolysaccharide; miR, microRNA;
3′UTR, 3′untranslated region; WT, wild-type; MUT, mutant; con,
control; Ago2, Argonaute 2; IgG, Immunoglobulin G. |
TAB2 depletion attenuates the
protective effect of miR-27b silencing on LPS-induced ALI
To determine whether TAB2 was involved in the
miR-27b-mediated inflammatory injury of ALI model cells,
anti-miR-27b and si-TAB2 were co-transfected into ALI model cells.
The knockdown efficiency of si-TAB2 was high in A549 cells
(Fig. S1C). As indicated in
Fig. 5A, the expression of TAB2
was increased following the depletion of miR-27b in the ALI model,
and this promotor effect was reversed by the silencing of TAB2.
Besides, miR-27b depletion protected A549 cells against the
LPS-induced reduction in viability, and increase in apoptosis and
inflammatory-related cytokine expression. These effects were
reversed by the co-transfection of si-TAB2 and anti-miR-27b
(Fig. 5B-F). Taken together,
miR-27b accelerated LPS-induced ALI by targeting and downregulating
TAB2 expression.
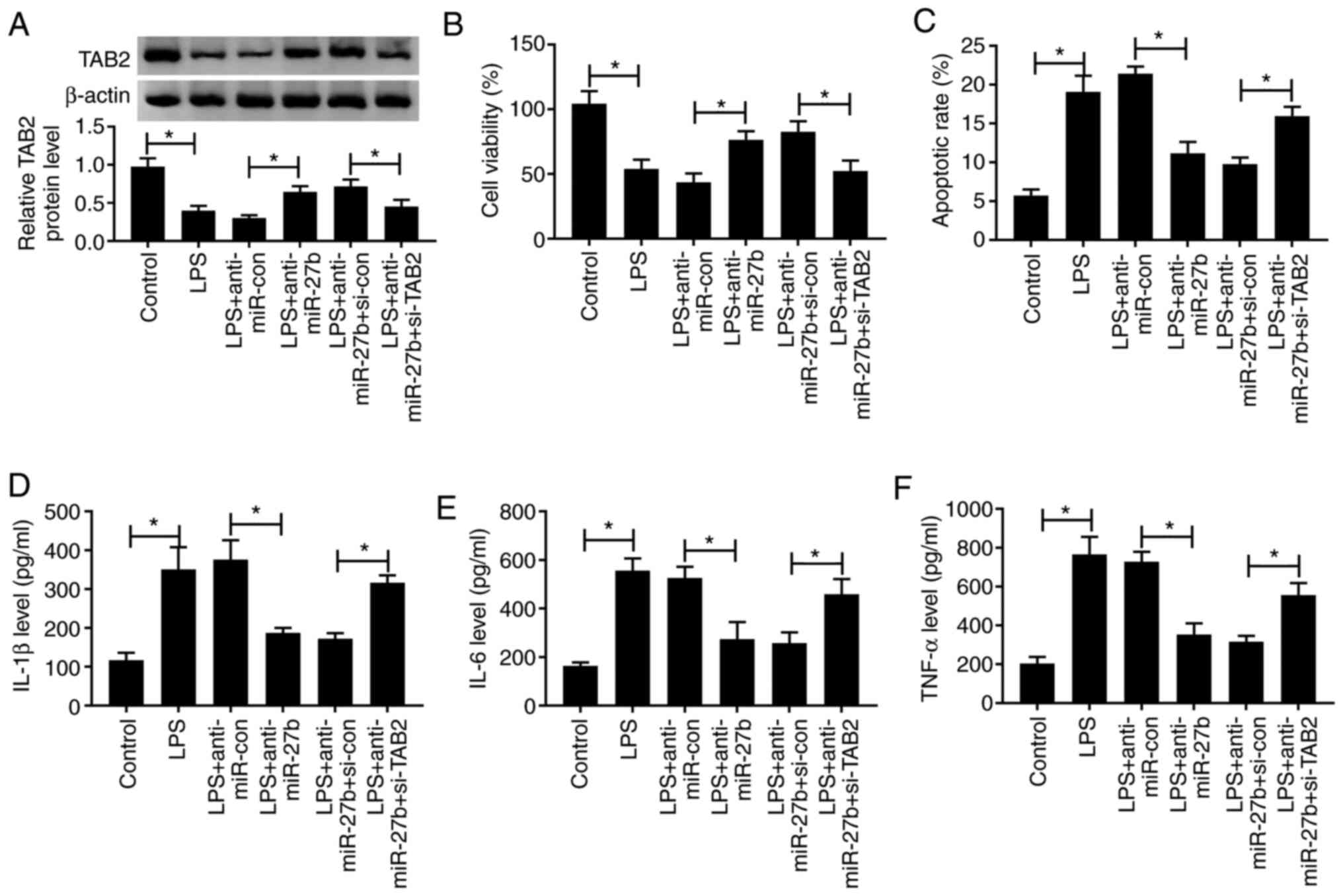 | Figure 5.TAB2 knockdown attenuates the
protective effect of miR-27b silencing on LPS-induced ALI. A549
cells were transfected with anti-miR-27b (20 nM) or anti-miR-con
(20 nM) alone or together with si-TAB2 (100 nM) or si-con (100 nM)
for 24 h followed by 1 µg/ml LPS treatment for 24 h. (A) The
protein expression of TAB2 was determined in A549 cells by western
blotting. (B) A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
was performed to detect the viability of A549 cells. (C) The
apoptotic rate of A549 cells was examined by flow cytometry. (D-F)
Levels of inflammatory-related cytokines in A549 cells were
measured by ELISA. *P<0.05. TAB2, TGF-β activated kinase 1 and
MAP3K7-binding protein 2; LPS, lipopolysaccharide; miR, microRNA;
ALI, acute lung injury; con, control; si-, small interfering RNA;
IL, interleukin; TNF-α, tumor necrosis factor α. |
CASC2 upregulates the expression of
TAB2 by functioning as a competing endogenous (ce)RNA of miR-27b in
A549 cells
The expression of TAB2 was elevated by the
transfection of CASC2, and the addition of miR-27b decreased the
expression of TAB2 in A549 cells (Fig.
6A). Furthermore, CASC2 silencing caused a significant
reduction in the expression of TAB2, whereas the co-transfection of
anti-miR-27b and si-CASC2 increased the expression of TAB2 in A549
cells (Fig. 6B). These data showed
that TAB2 was regulated by the CASC2/miR-27b axis in A549
cells.
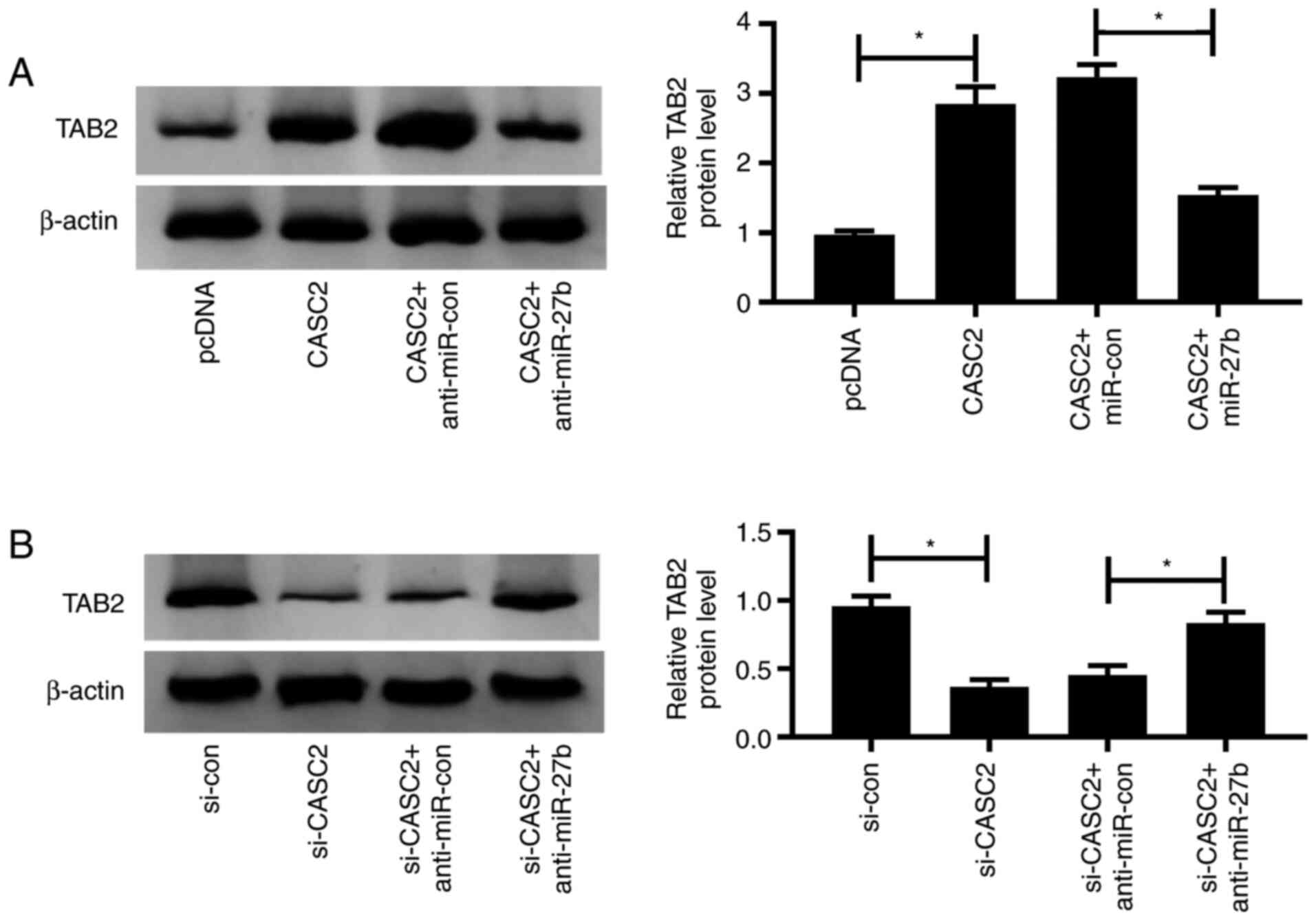 | Figure 6.CASC2 upregulates the expression of
TAB2 by functioning as a competing endogenous RNA of miR-27b in
A549 cells. (A) Western blotting was performed to detect the
expression of TAB2 in A549 cells transfected with pcDNA, CASC2,
CASC2 + miR-con or CASC2 + miR-27b. (B) A549 cells were transfected
with si-con, si-CASC2, si-CASC2 + anti-miR-con or si-CASC2 +
anti-miR-27b, and the expression of TAB2 was examined by western
blotting. *P<0.05. CASC2, cancer susceptibility candidate 2;
TAB2, TGF-β activated kinase 1 and MAP3K7-binding protein 2; miR,
microRNA; con, control; si-, small interfering RNA. |
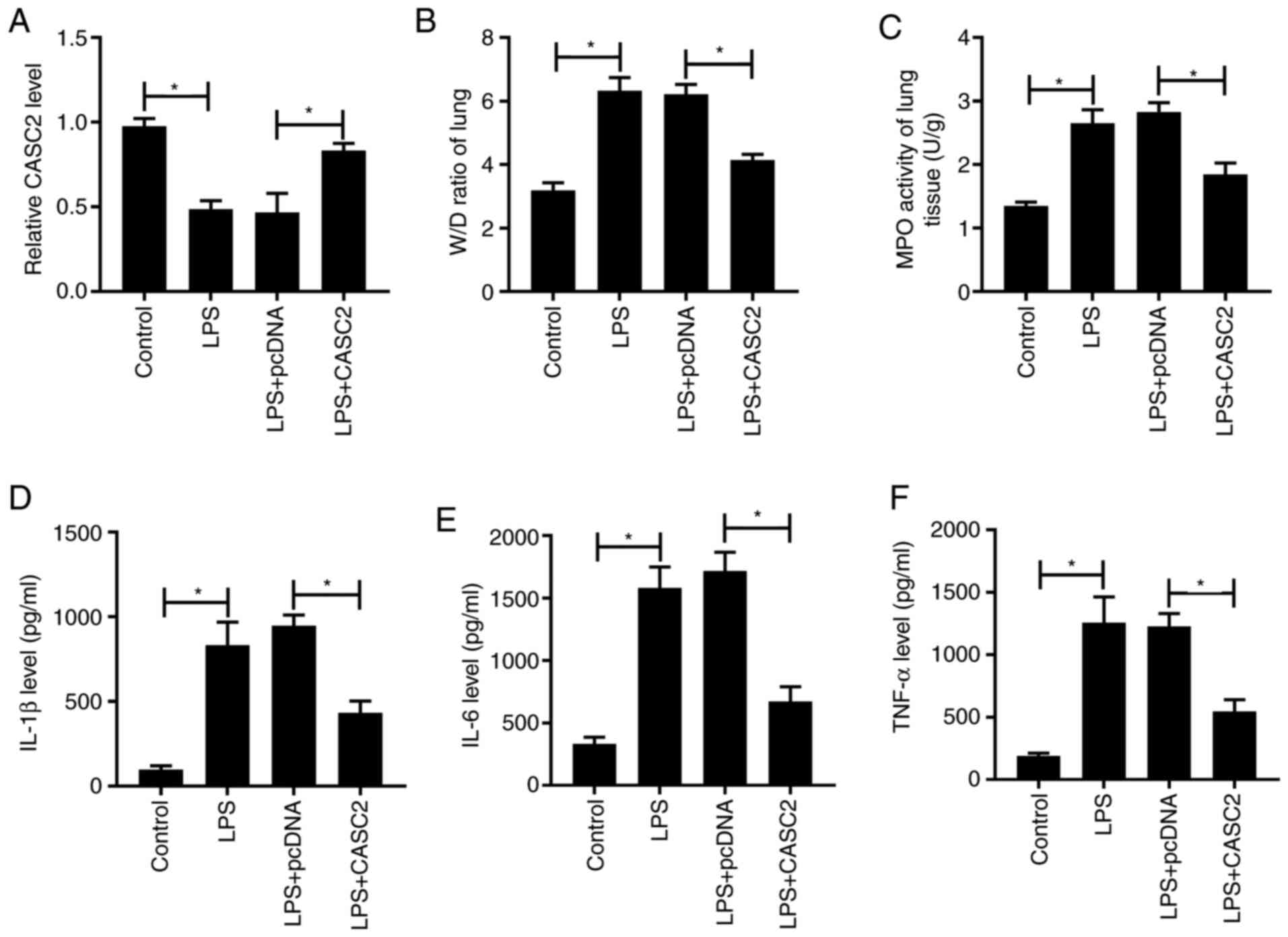
CASC2 overexpression attenuates
LPS-triggered ALI in lung tissues
BALB/c mice were divided into the indicated four
groups. Lung tissue injury was assessed by measuring the W/D ratio,
MPO activity and inflammation. As presented in Fig. 7A, LPS treatment decreased the
expression of CASC2, whereas overexpression of CASC2 upregulated
the expression of CASC2 following LPS treatment. LPS treatment
upregulated the W/D ratio, MPO activity and inflammatory factors in
lung tissues, these effects were partly reversed by the
overexpression of CASC2 (Fig.
7B-F), which suggested that LPS-mediated edema and inflammatory
responses were attenuated by the transfection of CASC2. In
conclusion, LPS-induced ALI in lung tissues was alleviated by the
overexpression of CASC2 in vivo.
Discussion
To elucidate the underlying molecular mechanism of
LPS-induced ALI, A549 cells were treated with 1 µg/ml LPS for 24 h
to construct a cell model of ALI. Cellular inflammatory response
was evaluated by measuring the levels of inflammatory markers
(IL-1β, IL-6 and TNF-α) via ELISA. Cell viability was reduced,
whereas apoptosis and inflammatory responses were increased in the
ALI cell model compared with that in the control group. An
increasing number of studies have demonstrated that lncRNAs exert
important regulatory roles in ALI (21,22).
Dai et al (23) reported
that lncRNA MALAT1 depletion suppressed LPS-induced inflammation
via miR-146a. Li et al (11) found that lncRNA CASC2 inhibited
LPS-induced apoptosis of A549 cells by functioning as a sponge of
miR-144-3p. In the present study, it was found that CASC2
overexpression ameliorated LPS-induced injury of A549 cells.
miR-27b was predicted to directly bind to CASC2
using LncBase database. The binding relationship between miR-27b
and CASC2 was validated by dual-luciferase reporter and RIP assays.
miR-27b was reported to be involved in the occurrence and
development of various types of cancer, including breast cancer and
colorectal cancer (24,25). Feng et al (26) revealed that miR-27b regulated the
metastasis of gastric cancer via nuclear receptor subfamily 2. Wu
et al (27) reported that
lncRNA ZEB2-AS1 accelerated the progression of bladder cancer by
targeting miR-27b. However, there are very few studies focused on
the role of miR-27b in ALI. Huang et al (16) reported that miR-27b facilitated
LPS-induced ALI via Nrf2 protein and the NF-κB signaling pathway.
In the present study, the expression of miR-27b was higher in the
LPS-treated A549 group compared with that in the non-treated group.
Additionally, it was found that miR-27b overexpression reversed the
protective effects of CASC2 expression on LPS-stimulated A549
cells, and the role of miR-27b in ALI was consistent with the
findings of a previous study (16).
miR-27b could modulate the expression of target
genes at the posttranscriptional level (25,28,29).
In the present study, we hypothesized whether miR-27b regulated the
progression of ALI through specific target genes. The complementary
sequence between miR-27b and TAB2 3′UTR was predicted using
microT-CDS software. Subsequently, the association between miR-27b
and TAB2 in A549 cells was validated by conducting dual-luciferase
reporter and RIP assays. TAB2 could be downregulated by LPS
treatment in A549 cells, and it was demonstrated that TAB2 was
negatively regulated by miR-27b in A549 cells. TAB2 is a crucial
upstream adaptor of IL-1 signaling. Yang et al (17) proposed that miR-142a-3p exerted a
protective role in LPS-triggered ALI by downregulating TAB2.
Whereas, the present study found that TAB2 exerted the opposite
effect in LPS-induced A549 cells compared with the previous study
(17). The increased viability and
the downregulation of apoptosis and inflammation caused by miR-27b
silencing were attenuated by the addition of si-TAB2 in LPS-treated
A549 cells. These results demonstrated that miR-27b contributed to
LPS-induced ALI via the downregulation of TAB2. The differences in
the results with the previous study may be due to the differences
in the cells used in the experiment (17). The present study showed that TAB2
was regulated by the CASC2/miR-27b axis, and CASC2 overexpression
alleviated LPS-triggered ALI in vivo.
There were also some limitations of the present
study. Constructing an ALI cell model using two cell lines would
make the results more reliable. Furthermore, there was crosstalk
within the lncRNAs/miRNAs/mRNAs signal pathway, and additional
experiments should be conducted to examine the molecular mechanism
of CASC2 in ALI progression.
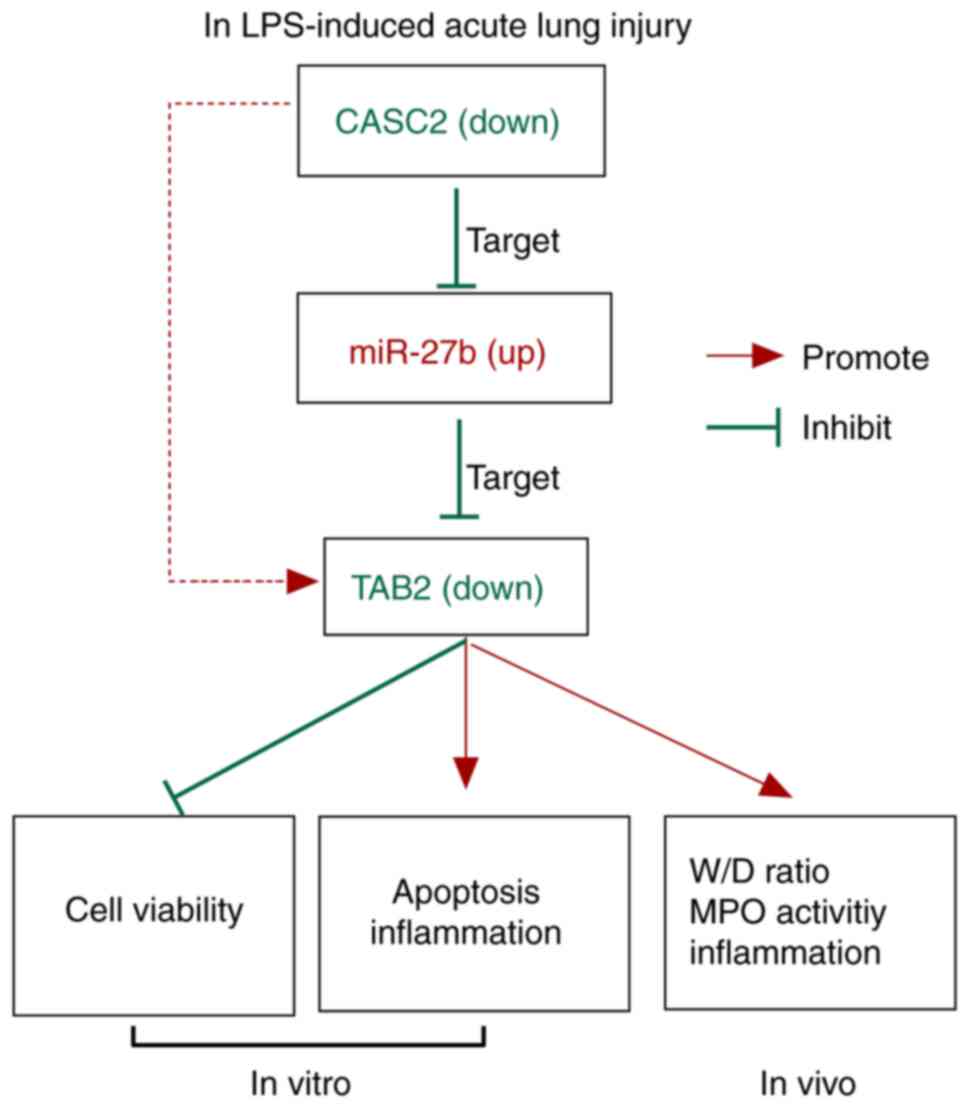
In summary, miR-27b was found to be a direct target
of CASC2, and miR-27b could bind to the 3′UTR of TAB2 in A549
cells. CASC2 protected A549 cells against LPS-induced ALI by
upregulating TAB2 via sponging miR-27b (Fig. 8). These results indicated that
downregulating the expression of miR-27b could be an effective
treatment strategy for patients with ALI.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the study. JM, JL and YC performed the
experiments. JM and YC analyzed the data. XL wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by The
Ethical Committee on Animal Research of Shiyan People's Hospital
(Shiyan, China) and were in accordance with guidelines provided by
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson ER and Matthay MA: Acute lung
injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ragaller M and Richter T: Acute lung
injury and acute respiratory distress syndrome. J Emerg Trauma
Shock. 3:43–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chow JC, Young DW, Golenbock DT, Christ WJ
and Gusovsky F: Toll-like receptor-4 mediates
lipopolysaccharide-induced signal transduction. J Biol Chem.
274:10689–10692. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng Y, Zou W, Hu C, Li G, Zhou S, He Y,
Ma F, Deng C and Sun L: Modulation of CASC2/miR-21/PTEN pathway
sensitizes cervical cancer to cisplatin. Arch Biochem Biophys.
623-624:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao Z, Wang H, Li H, Li M, Wang J, Zhang
W, Liang X, Su D and Tang J: Long non-coding RNA CASC2 inhibits
breast cancer cell growth and metastasis through the regulation of
the miR-96-5p/SYVN1 pathway. Int J Oncol. 53:2081–2090.
2018.PubMed/NCBI
|
|
7
|
Li Y, Lv S, Ning H, Li K, Zhou X, Xv H and
Wen H: Down-regulation of CASC2 contributes to cisplatin resistance
in gastric cancer by sponging miR-19a. Biomed Pharmacother.
108:1775–1782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y,
Wu R, Chen Y, Li W, Zhou H, et al: Down-regulation of lncRNA CASC2
promotes cell proliferation and metastasis of bladder cancer by
activation of the Wnt/β-catenin signaling pathway. Oncotarget.
8:18145–18153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang
C, Dou C, Xu M, Liu Q and Tu K: Long non-coding RNA CASC2
suppresses epithelial-mesenchymal transition of hepatocellular
carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer.
16:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ba Z, Gu L, Hao S, Wang X, Cheng Z and Nie
G: Downregulation of lncRNA CASC2 facilitates osteosarcoma growth
and invasion through miR-181a. Cell Prolif. 51:e124092018.
View Article : Google Scholar
|
|
11
|
Li H, Shi H, Gao M, Ma N and Sun R: Long
non-coding RNA CASC2 improved acute lung injury by regulating
miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell
Biosci. 8:152018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sand M: The pathway of miRNA maturation.
Methods Mol Biol. 1095:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Ma K, Zhang S, Zhang H, Liu J, Wang
X and Li S: Pulmonary microRNA expression profiling in an immature
piglet model of cardiopulmonary bypass-induced acute lung injury.
Artif Organs. 39:327–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Huang L, Zhu G, Pei Z and Zhang
W: Downregulated microRNA-27b attenuates lipopolysaccharide-induced
acute lung injury via activation of NF-E2-related factor 2 and
inhibition of nuclear factor κB signaling pathway. J Cell Physiol.
234:6023–6032. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Yang C, Guo YF, Liu P, Guo S, Yang
J, Zahoor A, Shaukat A and Deng G: MiR-142a-3p alleviates
Escherichia coli derived lipopolysaccharide-induced acute lung
injury by targeting TAB2. Microb Pathog. 136:1037212019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chuang CY, Chen TL, Cherng YG, Tai YT,
Chen TG and Chen RM: Lipopolysaccharide induces apoptotic insults
to human alveolar epithelial A549 cells through reactive oxygen
species-mediated activation of an intrinsic mitochondrion-dependent
pathway. Arch Toxicol. 85:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council Institute for
Laboratory Animal R. Guide for the Care and Use of Laboratory
Animals Washington (DC): National Academies Press (US). Copyright
1996 by the National Academy of Sciences. All rights reserved.
1996
|
|
21
|
Li H, Shi H, Ma N, Zi P, Liu Q and Sun R:
BML-111 alleviates acute lung injury through regulating the
expression of lncRNA MALAT1. Arch Biochem Biophys. 649:15–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu G, Mei H, Chen M, Qin S, Li K, Zhang W
and Chen T: Protective effect of agmatine against hyperoxia-induced
acute lung injury via regulating lncRNA gadd7. Biochem Biophys Res
Commun. 516:68–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen D, Si W, Shen J, Du C, Lou W, Bao C,
Zheng H, Pan J, Zhong G, Xu L, et al: miR-27b-3p inhibits
proliferation and potentially reverses multi-chemoresistance by
targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis.
9:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Y, Yu SY, Chen JJ, Qin J, Qiu YE,
Zhong M and Chen M: MiR-27b directly targets Rab3D to inhibit the
malignant phenotype in colorectal cancer. Oncotarget. 9:3830–3841.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Q, Wu X, Li F, Ning B, Lu X, Zhang Y,
Pan Y and Guan W: miR-27b inhibits gastric cancer metastasis by
targeting NR2F2. Protein Cell. 8:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu X, Yan T, Wang Z, Wu X, Cao G and Zhang
C: LncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and
inhibits apoptosis by regulating miR-27b. Biomed Pharmacother.
96:299–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling YH, Sui MH, Zheng Q, Wang KY, Wu H,
Li WY, Liu Y, Chu MX, Fang FG and Xu LN: miR-27b regulates myogenic
proliferation and differentiation by targeting Pax3 in goat. Sci
Rep. 8:39092018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rong X, Ge D, Shen D, Chen X, Wang X,
Zhang L, Jia C, Zeng J, He Y, Qiu H, et al: miR-27b suppresses
endothelial cell proliferation and migration by targeting Smad7 in
kawasaki disease. Cell Physiol Biochem. 48:1804–1814. 2018.
View Article : Google Scholar : PubMed/NCBI
|