Introduction
Acute myocardial infarction (MI) is a common cause
of death worldwide. The annual mortality and morbidity rates
following MI are 7 and 22%, respectively (1). Reperfusion therapies, including
primary percutaneous coronary intervention and thrombolytic
therapy, are commonly used to attenuate MI damage (2). However, reperfusion therapies may
cause myocardial ischemia reperfusion (I/R) injury when the blood
flow returns to the myocardial tissue (2). The potential molecular mechanisms of
I/R injury include oxidative stress, inflammation, calcium overload
and cytokine release (3). A large
number of reactive oxygen species (ROS) are produced within minutes
of reperfusion, which could oxidize target proteins to trigger
oxidative stress injury, as a physiological second messenger
signaling molecule (4). Abundant
natural biologically active substances from diverse sources (e.g.
luteolin, coptisine, curcumin) can potentially decrease I/R injury
through an antioxidant effect (5–7).
Therefore, natural biologically active substances can be used to
control the development of oxidative stress injury in order to
improve the health of patients with I/R.
Astaxanthin (AST) belongs to a natural xanthophyll
carotenoid that is well-known for its antioxidant,
anti-inflammatory, anti-apoptosis and anticancer abilities
(8–13). Haematococcus pluvialis (H.
pluvialis) is the best natural AST resource as it primarily
contains the 3S, 3′S-AST isomer. The 3S, 3′S-AST molecule is
regarded as the most effective biological antioxidant to eliminate
free radicals (14). AST has an
α-hydroxyketone structure, which is responsible for its strong
antioxidant activity by capturing singlet oxygen and reacting with
free radicals (15). The
antioxidant activity of AST is 500 times and 10 times higher
compared with that in vitamin E and β-carotene, respectively
(14,16). In recent years, AST has been
reported to protect against cardiovascular disease. In rats with
isoproterenol-induced MI, AST treatment decreased the weight of the
heart, inflammatory cell infiltration and myocardial fibrosis by
improving antioxidant enzyme activity (9). Furthermore, AST also attenuated
cardiac dysfunction and fibrosis in a mouse MI model (17). However, the protective roles of 3S,
3′S-AST against ROS-induced damage of cardiomyocytes and the
potential mechanisms involved have not been elucidated.
During I/R injury, cardiac
Na+/K+-ATPase (NKA) function was found to be
altered, and elucidation of the mechanism involved would be
important to develop novel approaches for therapeutic intervention
(18). NKA is a transmembrane
enzyme responsible for transporting Na+ and
K+ ions across the cytomembrane, which establishes and
maintains the ion concentration gradient across the cell plasma
membrane (19). In addition to its
Na pump function, NKA was also discovered to function as a signal
transduction protein. The NKA/Src/Erk1/2 signaling pathway was
found to be used as a feed-forward amplifier for ROS signaling that
aggravates atherosclerosis, dyslipidemia, obesity and diabetes
(20,21). Wang et al (22) demonstrated that ROS could active
the NKA/Src/Erk1/2 signaling pathway to further trigger ROS
production. Emerging studies have demonstrated the NKA/Src/ROS
signal pathway is an underlying target for protecting the heart
from I/R injury (23). Therefore,
the present study was designed to investigate whether 3S, 3′S-AST
exerts protective effects against myocardial oxidative stress
damage by attenuating NKA/Src/Erk1/2 signaling-modulated ROS
amplification.
Materials and methods
Materials
The 3S, 3′S-AST compound was obtained from
Sigma-Aldrich (cat. no. SML0982; Merck KGaA), with ≥97% purity.
Antibodies against Bax (cat. no. 2772S), Bcl-2 (cat. no. 3498S),
caspase-3 (cat. no. 9665S), cleaved-caspase-3 (cat. no. 9664S),
Erk1/2 (cat. no. 4695S) and phosphorylated (p)-Erk1/2
(Thr202/Tyr204; cat. no. 9101S) were purchased from Cell Signaling
Technology, Inc. NKA (cat. no. cs-58629) and anti-c-Src antibodies
(cat. no. cs8056) were obtained from Santa Cruz Biotechnology,
Inc., while anti-p-Src pY418 antibody (cat. no. 44-660G) was
obtained from Thermo Fisher Scientific, Inc. Goat anti-rabbit IgG
(cat. no. 925-68021) and goat anti-mouse IgG (cat. no. 925-68020)
secondary antibodies were purchased from LI-COR Biosciences.
Nitrocellulose (NC) membranes were purchased from EMD Millipore,
while RIPA lysis buffer was purchased from Boster Biological
Technology. FBS was obtained from Biological Industries and the MTT
kit (cat. no. M2128) and DAPI fluorescent dye (cat. no. D9542) were
purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and treatment
H9c2 cells (rat embryonic cardiomyocytes) were
obtained from the Cell Bank of Shanghai Academy of Sciences and
cultured in DMEM (cat. no. C11995500BT; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin-streptomycin at 37°C in a humidified incubator with 5%
CO2. The H9c2 cells were passaged regularly and cultured
to 70–80% confluence prior experimentation. Control group was
treated with DMEM without FBS; DMSO group was treated with DMEM and
DMSO without FBS. To generate a ROS-induced myocardial cell injury
model, H9c2 cells were exposed to 100 µM H2O2
for 1 or 24 h and analyzed for further analysis. A total of 50 mg
3S, 3′S-AST powder was dissolved in 5 ml DMSO and prepared into a
stock solution of 16.8 mM. Cells were pretreated with 3S, 3′S-AST
(5, 10 and 20 µM) for 1 h prior to H2O2
administration.
Cell viability assay
Cell viability was detected using an MTT kit
according to the manufacturer's instructions. H9c2 cells were
plated into 96-well plates (1×103 cells/well), cultured
with DMEM and treated with 3S, 3′S-AST (5, 10, 20 µM) and
H2O2 (50, 100, 200, 400 µM) for 24 h. A total
of 15 µl MTT solution was added into each well and co-incubated for
4 h, at 37°C. After removing the medium, 150 µl DMSO was added to
each well. The absorbance was measured at a wavelength of 490 nm
using a microplate absorbance reader (Multiskan; Thermo Fisher
Scientific, Inc.) and analyzed to assess cell viability.
Cell apoptosis assay
To detect cell apoptosis, H9c2 cells were stained
using a DAPI fluorescent dye to observe nuclear morphology. H9c2
cells were plated into six-well plates (1×105
cells/well), washed with PBS three times and fixed in 4%
paraformaldehyde (Beyotime Institute of Biotechnology) at room
temperature for 10 min. The cells were then washed three times with
PBS and stained using 3 ml DAPI staining solution (1 µg/ml) for 10
min at 37°C. The nuclear morphology was observed using fluorescence
microscopy (Olympus Corporation; magnification, ×200). Healthy
living cells presented with uniform staining of the nuclei.
Apoptotic cells presented with pyknosis and fragmentation of the
nuclei.
Lactate dehydrogenase (LDH) release
analysis
Cell damage was determined using an LDH assay kit
(cat. no. A020; Nanjing Jiancheng Bioengineering Institute) by
detecting LDH release of myocardial cells. H9c2 cells were cultured
at a density of 1×105 cells/well in six-well plates. A
total of 20 µl cell culture medium was added into another 96-well
plate, with 300 µl reaction mixture from the kit and the mixture
was incubated for 30 min at 37°C. LDH activity was measured using a
microplate reader at a wavelength of 450 nm.
Creatine kinase-myocardial band
(CK-MB) activity analysis
CK-MB activity levels were measured using a CK-MB
assay kit (cat. no. E006; Nanjing Jiancheng Bioengineering
Institute) according to manufacturer's protocol. Briefly, H9c2
cells were lysed with PBS buffer, and freeze/thawed three times on
ice for 30 min, followed by centrifugation at 12,000 × g for 15 min
at 4°C to isolate the cell debris. The protein content of samples
was examined using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology). CK-MB activity levels were
detected at a wavelength of 340 nm using a microplate absorbance
reader and expressed as U/g protein.
Malondialdehyde (MDA) and glutathione
peroxidase (GSH-px) level detection
The levels of MDA and GSH-px in H9c2 cells were
detected using MDA (cat. no. A003) and GSH-px assay kits (cat. no.
A005) (both from Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions. Briefly, H9c2 cells
were lysed with PBS buffer and freeze/thawed 3 times on ice for 30
min. The supernatant was isolated by centrifugation at 12,000 × g
for 15 min at 4°C. The MDA content was measured at a wavelength of
532 nm and expressed as nmol/mg protein. The GSH-px activities of
the cell lysate was detected at a wavelength of 412 nm according to
the produced enzyme-catalyzed reaction product.
GSH and glutathione reductase (GR)
level detection
The levels of GSH and GR in H9c2 cells were detected
using GSH (cat. no. A006) and GR assay kits (cat. no. A062) (both
from Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions. Briefly, the H9c2 cells were lysed
with PBS buffer and freeze/thawed three times on ice for 30 min
then. The mixture was then centrifuged at 12,000 × g for 15 min at
4°C to isolate the cell debris. GSH activity levels were detected
at a wavelength of 405 nm and expressed as µmol/g protein, while GR
activity levels were detected at a wavelength of 340 nm using a
spectrophotometer and expressed as U/g protein.
Intracellular ROS measurements
A ROS assay kit (cat. no. S0033; Beyotime Institute
of Biotechnology) was used to detect intracellular ROS levels
according to the manufacturer's instructions.
2′-7′-dichlorofluorescin diacetate (DCFH-DA) was diluted with
serum-free medium (1:1,000). Cells were seeded into a six-well
plate (1×105 cells/well) and treated with
H2O2 and 3S, 3′S-AST for 24 h. The culture
medium was removed and the cells were incubated with DCFH-DA (10
µM) for 20 min at 37°C. Subsequently, the cells were washed with
serum-free medium (three times for 5 min each time) and images were
obtained using a fluorescence microscope (Olympus Corporation;
magnification ×100). ROS fluorescence intensity was analyzed using
Image-Pro Plus (version 5.0; MediaCybernetics, Inc.).
Western blot analysis
Following treatment, H9c2 cells were scraped using a
scraper and the protein lysates were extracted using a
solubilization buffer (containing RIPA and 1% protease inhibitor).
The debris was removed by centrifugation at 12,000 × g for 15 min
at 4°C. The protein concentration was determined using a BCA assay
kit. The protein samples (80 µg) were added to 5X loading buffer
(Beyotime Institute of Biotechnology) and heated at 95°C for 5 min
using metal heating block. Protein samples were separated using 10%
SDS-PAGE and transferred onto a NC membrane, at a constant current
of 300 mA for 1 h under wet transfer conditions. The NC membranes
were blocked with 5% skimmed milk at room temperature, and the
proteins were incubated with the following antibodies overnight at
4°C: NKA (1:200), c-Src (1:200), p-Src (1:1,000), Erk1/2 (1:1,000),
p-Erk1/2 (1:1,000), Bax (1:500), Bcl-2 (1:500), caspase-3 (1:500),
cleaved-caspase-3 (1:500) and GAPDH (1:1,000). The membranes were
then incubated with the following fluorescent secondary antibodies:
Goat anti-rabbit (1:10,000) and goat anti-mouse (1:10,000). The
bands were captured and quantified using an Odyssey Imaging System
(LI-COR Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM Corp.). The results are represented as
the mean ± SEM from at least three independent experiments.
Analysis between two groups were performed using Student's t-test.
Analysis between multiple groups were performed using one-way ANOVA
analysis followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
3S, 3′S-AST attenuates ROS-induced
cell injury in rat cardiomyocytes
To investigate the protective effects of 3S, 3′S-AST
in cardiomyocytes under oxidative stress damage in vitro,
the rat H9c2 embryonic heart-derived cell line was treated with
H2O2 to simulate oxidative stress injury. The
cell viability was evaluated using an MTT assay. As shown in
Fig. 1A and B,
H2O2 significantly decreased cell viability
in H9c2 cells in dose-dependent manner, and pretreatment with 3S,
3′S-AST significantly inhibited the decrease of cell viability
caused by H2O2. Furthermore, with the
increase in the dose of 3S, 3′S-AST, the effect of inhibition was
more significant. Because the 100 µM H2O2
decreased cell viability to ~60%, this concentration was used for
subsequent experiments. LDH and CK-MB are well-known indicators of
cell injury (24); Therefore, they
were measured in the present study. The LDH content in the culture
medium of H2O2-treated cells was
significantly higher compared with the control group, and treatment
with H2O2 significantly increased CK-MB
content in H9c2 cells. However, pretreatment with 3S, 3′S-AST
significantly reduced this increase (Fig. 1C and D). No significant difference
was observed in the 5 µM 3S, 3′S-AST-treated group compared with
the H2O2-treated group.
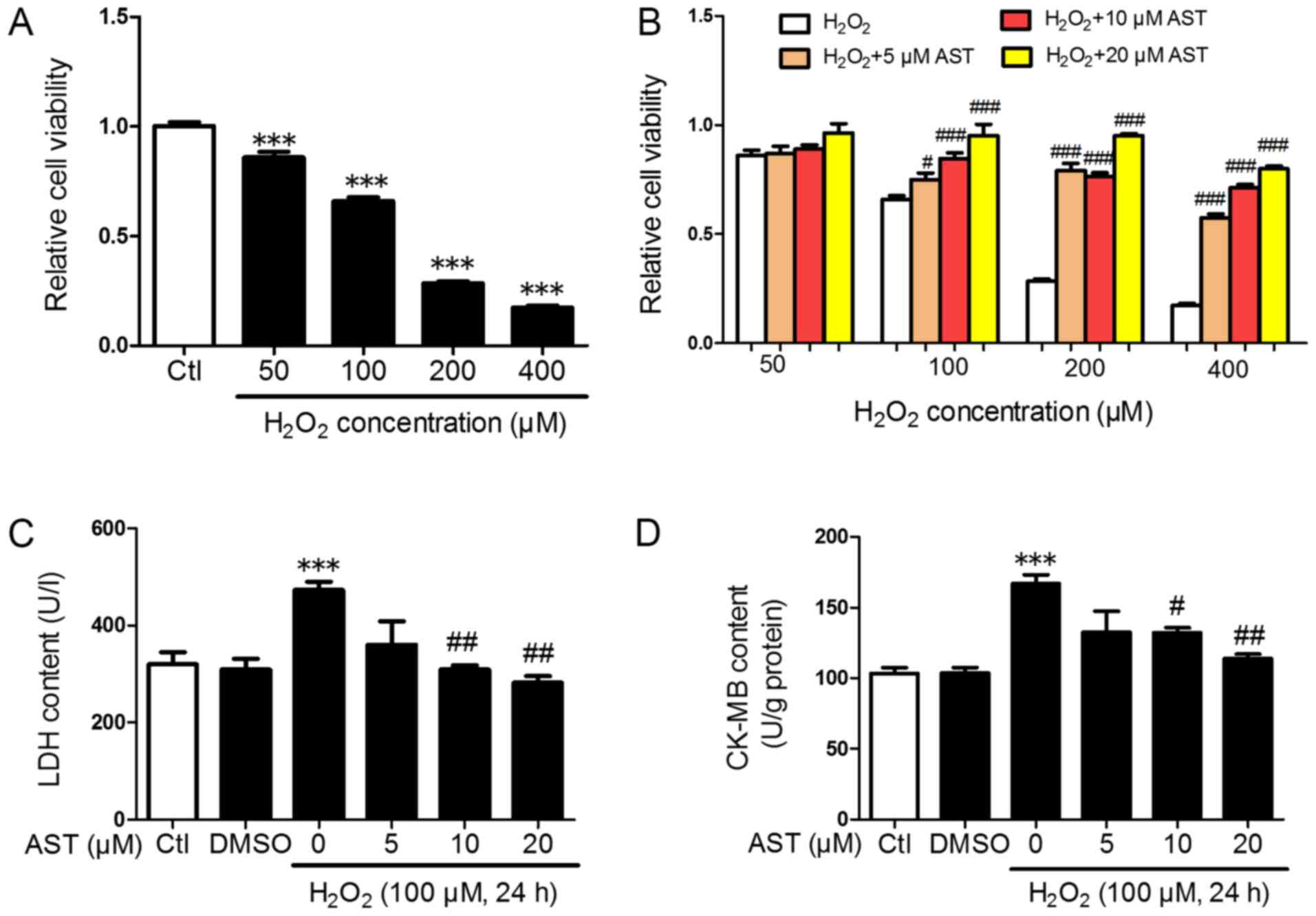 | Figure 1.3S, 3′S-AST attenuates reactive
oxygen species-induced injury in H9c2 cells. (A) Cell viability in
H9c2 cells treated with different concentrations of
H2O2 (50, 100, 200, and 400 µM) for 24 h.
n=8. ***P<0.001 vs. Ctl. (B) Cell viability in cells pretreated
with various concentrations of 3S, 3′S-AST (5, 10 and 20 µM) for 1
h, and then treated with H2O2. n=8.
#P<0.05 and ###P<0.001 vs.
H2O2. (C) H2O2 (100 µM,
24 h) upregulated the LDH content in the culture supernatant of
H9c2 cells, while 3S, 3′S-AST pretreatment decreased LDH content.
n=4. ***P<0.001 vs. Ctl; ##P<0.01 vs.
H2O2. (D) H2O2 (100 µM,
24 h) upregulated CK-MB content in H9c2 cells while 3S, 3′S-AST
pretreatment decreased CK-MB content. n=3. ***P<0.001 vs. Ctl;
#P<0.05 and ##P<0.01 vs,
H2O2. Data are expressed as the mean ±
standard error of the mean. AST, 3S, 3′S-ASTaxanthin; LDH, lactate
dehydrogenase; CK-MB, creatine kinase-myocardial band; Ctl,
control. |
3S, 3′S-AST inhibits ROS-induced
cardiomyocyte apoptosis
DAPI DNA fluorescent staining was utilized to
investigate the effect of 3S, 3′S-AST on cell apoptosis. The
morphology of the nucleus in the H9c2 cells showed uniform staining
in the control and DMSO groups. By contrast,
H2O2-treated cells showed nuclear pyknosis
and fragmentation. Compared with the
H2O2-treated group, the 3S, 3′S-AST-treated
group showed markedly attenuated cell apoptosis, but no significant
difference was observed in the 5 µM 3S, 3′S-AST-treated group
(P>0.05) (Fig. 2A). Western
blot analysis was performed to evaluate the effect of 3S, 3′S-AST
on the expression levels of pro-apoptosis proteins (Bax, caspase-3
and cleaved-caspase-3) and anti-apoptosis protein (Bcl-2) in each
group. The protein expression levels of caspase-3,
cleaved-caspase-3 and Bax were significantly downregulated, while
the expression level of Bcl-2 was significantly upregulated in the
3S, 3′S-AST-treated groups compared with the
H2O2-treated group. These effects were
attenuated treatment with 10 and 20 µM 3S, 3′S-AST (Fig. 2B-F).
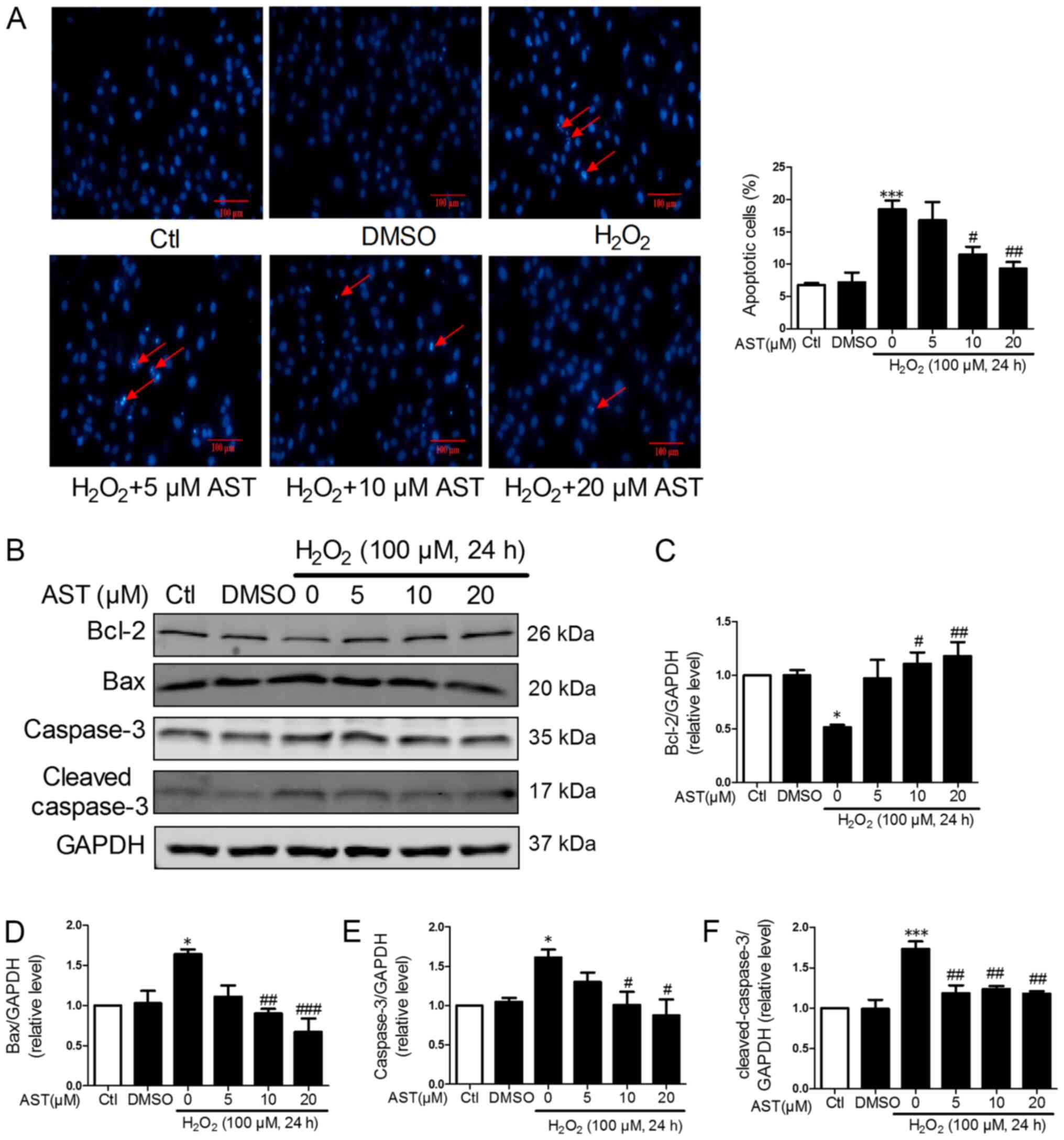 | Figure 2.3S, 3′S-AST attenuates reactive
oxygen species-induced cell apoptosis. (A) Pretreatment with 3S,
3′S-AST decreased the number of apoptotic cells caused by treatment
with H2O2 (100 µM, 24 h). The red arrows
indicate apoptotic cells. Scale bar, 100 µM. (B) Expression levels
of Bcl-2, Bax, caspase-3 and cleaved-caspase-3 were determined by
western blot analysis. Densitometric analysis of (C) Bcl-2, (D)
Bax, (E) caspase-3 and (F) cleaved-caspase-3. Data are expressed as
the mean ± standard error of the mean. n=3. *P<0.05 and
***P<0.001 vs. Ctl; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2. AST, 3S, 3′S-AST. AST, 3S,
3′S-ASTaxanthin; Ctl, control. |
Effects of 3S, 3′S-AST on ROS
accumulation and antioxidant activity
DCFH-DA staining was used to determine ROS
accumulation. Cells treated with 3S, 3′S-AST significantly
attenuated intracellular ROS level induced by
H2O2 (Fig. 3A
and B). It was reported that the NKA/Src signaling pathway,
which regulates ROS amplification, plays a key role in oxidative
stress damage (23). In the
present study, the results indicated that 3S, 3′S-AST may act as an
antioxidant to reduce the ROS level and inhibit the NKA/Src/ROS
amplification loop. Furthermore, the effects of 3S, 3′S-AST on MDA
content and GSH-px, GSH and GR activity levels were also measured.
The results revealed that treatment with H2O2
significantly increased MDA content and significantly decreased
GSH-px, GSH and GR activity levels in H9c2 cells. The effects of 10
and 20 µM 3S, 3′S-AST were more effective compared with cells
treated with 5 µM 3S, 3′S-AST (Fig.
3C-F).
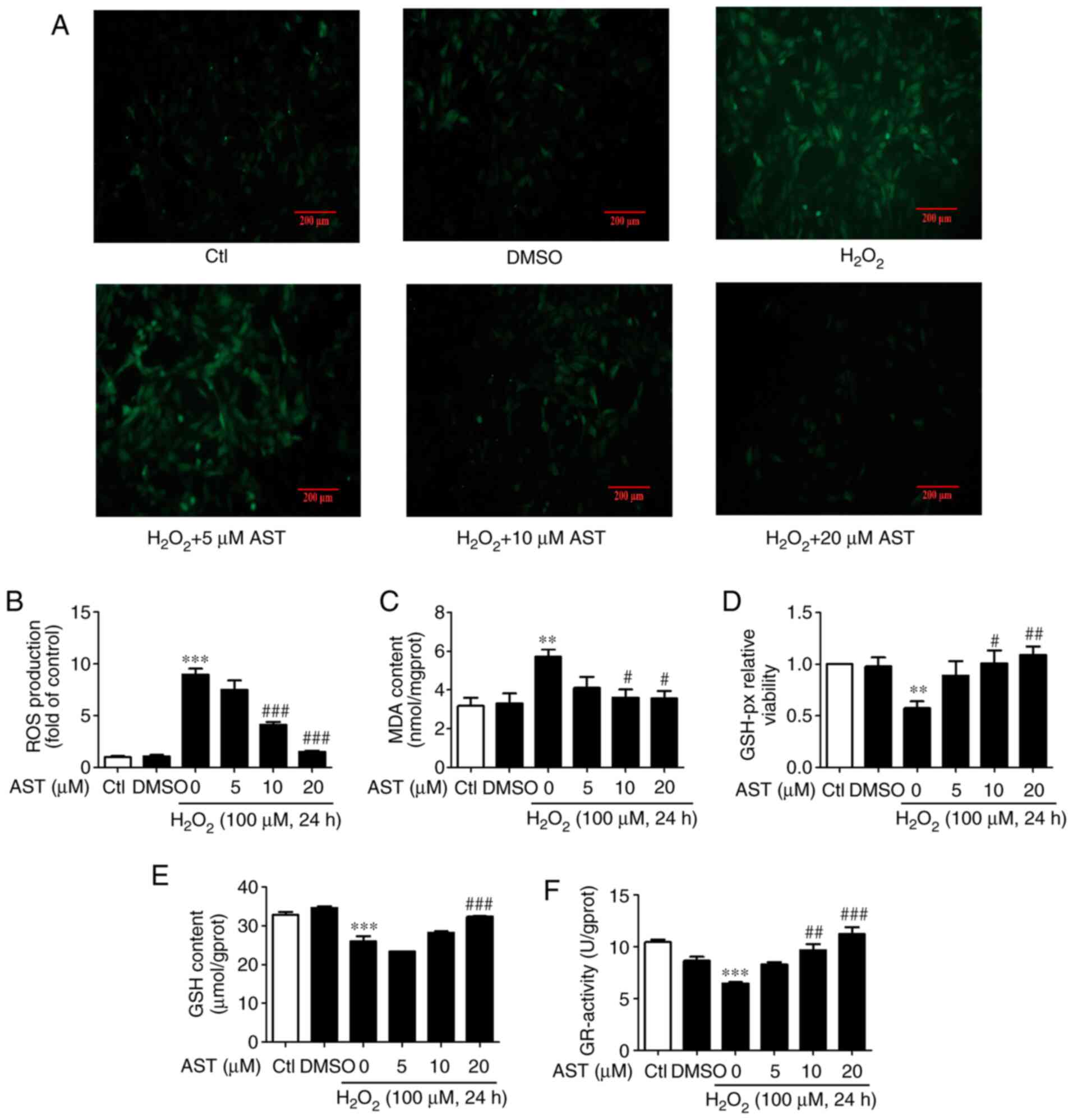 | Figure 3.3S, 3′S-AST inhibits ROS-induced ROS
accumulation and increases antioxidation activity. (A)
Representative ROS staining in H9c2 cells treated with
H2O2 (100 µM, 24 h) and pretreated with 3S,
3′S-AST. Scale bar, 200 µM. (B) Quantitative analysis of ROS
fluorescence intensity. n=3. (C) Pretreatment with 3S, 3′S-AST
decreased MDA content in H9c2 cells treated with
H2O2 (100 µM, 24 h). n=4. (D) Pretreatment
with 3S, 3′S-AST increased GSH-px activity in H9c2 cells treated
with H2O2 (100 µM, 24 h). n=3. (E)
Pretreatment with 3S, 3′S-AST increased GSH content in H9c2 cells
treated with H2O2 (100 µM, 24 h). n=3. (F)
Pretreatment with 3S, 3′S-AST increased GR activity in H9c2 cells
treated with H2O2 (100 µM, 24 h). n=3. Data
are expressed as the mean ± standard error of the mean.
**P<0.01, ***P<0.001 vs. Ctl; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2. AST, 3S, 3′S-ASTaxanthin; Ctl, control;
ROS<, reactive oxygen species; MDA, malondialdehyde; GSH-px,
glutathione peroxidase; GR, glutathione reductase; GSH,
glutathione. |
Effects of 3S, 3′S-AST on the NKA/Src
signaling pathway
ROS is a specific ligand of NKA and could induce
activation of Src and Erk1/2 and activated NKA/Src/Erk1/2 signaling
further promoted ROS production (22). Therefore, the effects of 3S,
3′S-AST on the phosphorylation of Src and its downstream regulator
Erk1/2 in H2O2-treated H9c2 cells was
determined. As shown in Figs. 4
and 5, the phosphorylation of Src
and Erk1/2 was significantly upregulated in
H2O2-treated cells compared with controls.
However, 3S, 3′S-AST inhibited the activation of Src and Erk1/2 and
did not alter the protein expression levels of NKA in cells with
ROS-induced damage. These results indicated that 3S, 3′S-AST
attenuated the activities of the NKA/Src/Erk1/2 signaling pathway,
which acted as one upstream pathway of ROS production and
apoptosis.
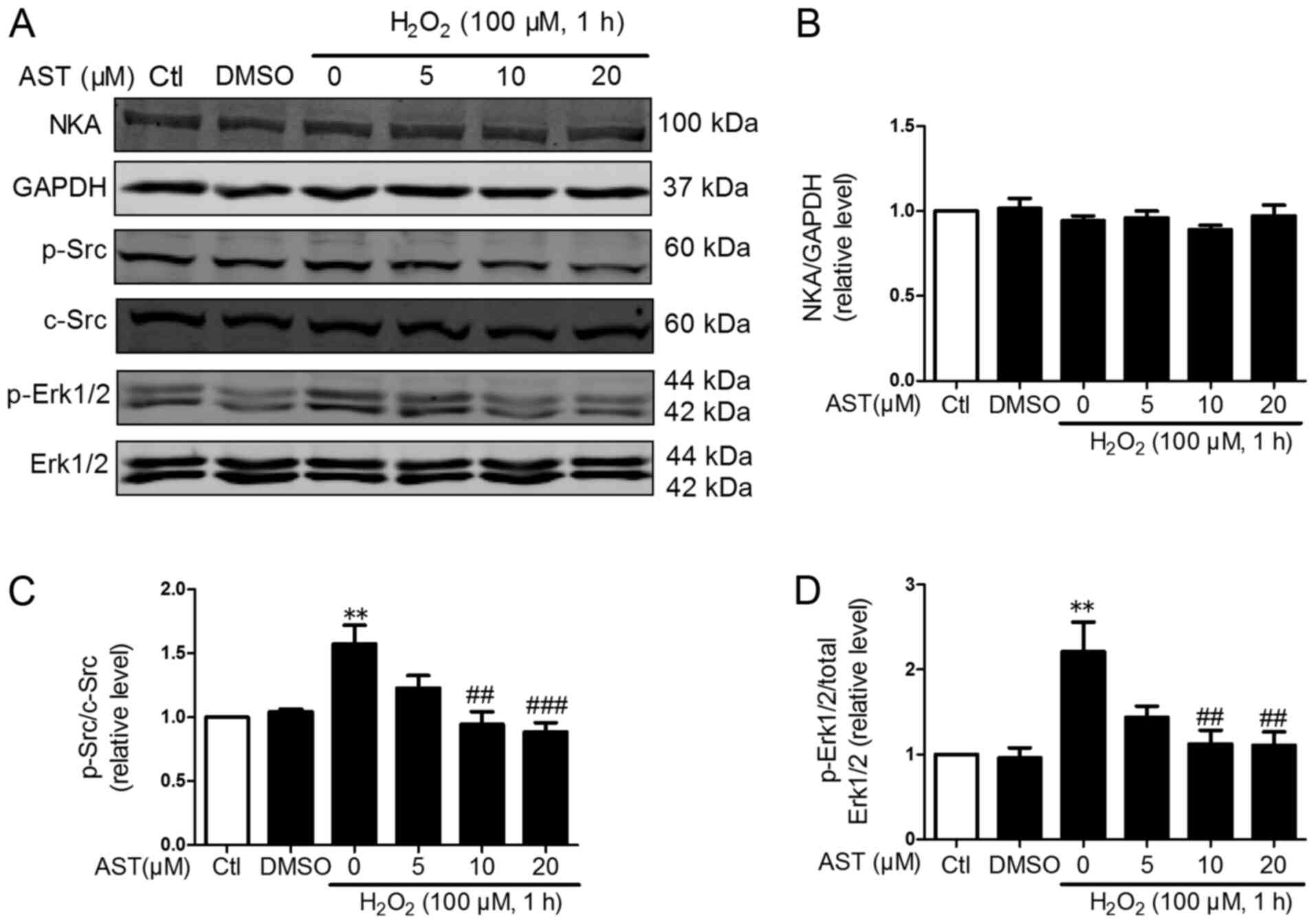 | Figure 4.Effects of 3S, 3′S-AST on the
NKA/Src/Erk1/2 signaling pathway. (A) Levels of NKA, p-Src and
p-Erk1/2 were determined by western blot analysis in H9c2 cells
treated with H2O2 (100 µM, 1 h) and
pretreated with 3S, 3′S-AST. Densitometric analysis of (B) NKA, (C)
p-Src and (D) p-Erk1/2. Data are expressed as the mean ± standard
error of the mean. n=4. **P<0.01 vs. Ctl; ##P<0.01
and ###P<0.001 vs. H2O2. AST,
3S, 3′S-ASTaxanthin; Ctl, control; p, phosphorylated; NKA,
Na+/K+-ATPase. |
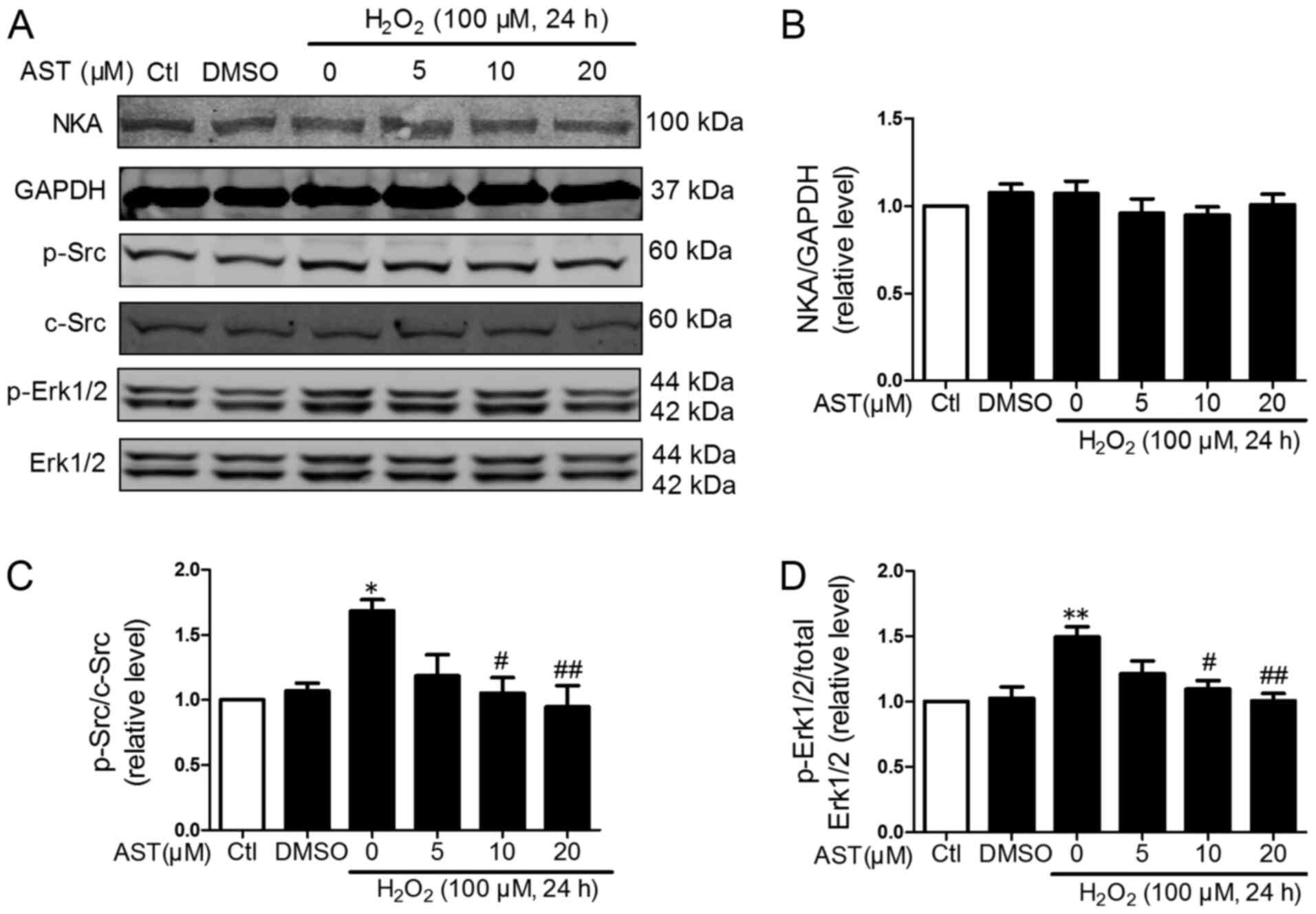 | Figure 5.Effects of 3S, 3′S-AST on the
NKA/Src/Erk1/2 signaling pathway. (A) Levels of NKA, p-Src and
p-Erk1/2 were determined by western blot analysis in H9c2 cells
treated with H2O2 (100 µM, 24 h) and
pretreated with 3S, 3′S-AST. Densitometric analysis of (B) NKA, (C)
p-Src and (D) p-Erk1/2. Data are expressed as the mean ± standard
error of the mean. n=3. *P<0.05 and **P<0.01 vs. Ctl;
#P<0.05 and ##P<0.01 vs.
H2O2. AST, 3S, 3′S-ASTaxanthin; Ctl, control;
p, phosphorylated; NKA, Na+/K+-ATPase. |
Discussion
Restoring blood flow has been confirmed to be an
efficient approach to recover ischemic myocardium (25). However, myocardial reperfusion will
elicit an increase in ROS production in cardiomyocytes (26). Some therapeutic drugs, such as
rosuvastatin (27), have been
available to prevent oxidative stress injury from I/R; however, the
therapeutic effects for I/R injury remain unsatisfactory (28). Therefore, it is essential to
discover safe and effective therapeutic agents. The present study
demonstrated the function of 3S, 3′S-AST in protecting
cardiomyocytes from ROS-induced injury in vitro. The results
indicated that 3S, 3′S-AST treatment inhibited cardiomyocyte
apoptosis and improved antioxidant activity. It was further
indicated that 3S, 3′S-AST treatment prevented intracellular ROS
generation by reducing the activity of the NKA/Src/Erk1/2/ROS
amplification signal transduction pathway.
AST exists in three stereoisomeric forms (3S, 3′S;
3R, 3′R and 3R, 3′S)(29). H.
pluvialis-derived AST primarily contains the 3S, 3′S-form, and
synthetic AST is a mixture of the three stereoisomers [(3S,
3′S):(3R, 3′S):(3R, 3′R), 1:2:1 ratio] (29). It was reported that synthetic AST
could reduce MI size in I/R rabbits (30); however, humans are unable to use
synthetic AST (31), while H.
pluvialis-derived AST is the only AST permitted for human
consumption (31). Synthetic AST
has 20 times lower antioxidant capacity than natural AST and to
date has not been approved for human consumption. The United States
Food and Drug Administration and the European Food Safety Authority
has approved the use of AST from H. pluvialis, as a food
ingredient in the food production process and it can be used as an
additive at dosages below 12–24 mg/day within 30 days (32). Initial preclinical studies also
support the antioxidant ability of AST at a particular dose. For
example, Iwamoto et al (33) found that the resistance of
volunteers to low-density lipoprotein oxidation was enhanced when
AST was administered at doses of 1.8–21.6 mg/day for 14 days.
Yoshida et al (34) showed
that triglyceride and high-density lipoprotein-cholesterol levels
were ameliorated in patients administered with AST at doses of
12–18 mg/day for 12 weeks. Furthermore, in a human clinical study,
the beneficial action of dietary AST was identified at doses of 2
and 8 mg/day for 8 days and was found to regulate the immune
response, oxidative damage and inflammation (35). However, a previous study
investigating the anti-fibrotic effects of different concentrations
of AST (10, 20 and 40 µM) on LX-2 cells revealed that AST could
modulate proliferation and apoptosis of LX-2 cells at the cellular
level (36). H9c2 cells
pre-treated with 0.5–8 µM AST for 6 h and co-incubated with
homocysteine for 72 h revealed that AST alleviated homocysteine
induced cytotoxicity (37). In the
present study, different concentrations of 3S, 3′S-AST (5, 10 and
20 µM) was used to treat H9c2 cells to investigate the effects and
mechanism of 3S, 3′S-AST on oxidative stress. It was found that 10
and 20 µM 3S, 3′S-AST was more effective compared with that for 5
µM, and almost no significant difference was observed in cells
treated with 5 µM 3S, 3′S-AST compared with
H2O2-treated cells.
The bioavailability of AST is affected a number of
factors, including host-related conditions, such as sex, age,
obesity, smoking and alcohol consumption, as well as the structure
of AST or its dispersion medium (14). Some trials have investigated the
bioavailability of AST from H. pluvialis under different
factors. Okada et al (38)
showed that the metabolism of AST in a group of smokers was faster
compared with the non-smokers group, which may be attributed to the
antioxidant effect of AST. A previous study found that dietary oils
may enhance the absorption of AST bioavailability in humans
(39). In addition, Mercke et
al (40) determined the
bioavailability of AST from H. pluvialis dispersed in
synthetic hydrophilic surfactant polysorbate 80 (B), which is a
lipid-based formulation, was 1.7–3.7 times higher compared with the
commercial formulation. Furthermore, studies have shown that the
antioxidant ability of AST from H. pluvialis was improved in
rat plasma and liver tissues after being dissolved in olive oil
(41,42). Satoh et al (43) administered participants with an
oil-based natural AST-rich product for 4 weeks at 20 mg daily, and
there were no safety concerns reported by evaluating toxicity and
efficacy levels. As aforementioned, further research should focus
on improving host-related conditions and identifying new
biomaterials to act as astaxanthin vectors to improve its
bioavailability.
Baburina et al (44) revealed that AST could inhibit
opening of a large-conductance channel (mitochondrial permeability
transition pore) in the rat heart following mitochondrial calcium
overload or oxidative stress by reducing the serine/threonine
protein kinase B/cAMP-responsive element-binding protein signaling
pathways in the mitochondria. Nakao et al (45) found that 0.08% AST had a
significantly positive effect on cardiac function of mice. AST
treatment reversed enhanced lipid peroxidation and reduced
antioxidant enzyme activities in heart tissues caused by
isoproterenol (ISO) administration in rats, which suggested that
AST may protect cardiac tissue in ISO-administered rats by
suppressing oxidative stress and enhancing antioxidant enzyme
functions (9). However, the
effects and mechanism of 3S, 3′S-AST on oxidative stress injury in
myocardial cells caused by I/R was unknown. In the current study,
oxidative stress injury in the H9c2 cells induced by
H2O2 was used to mimic I/R injury. It was
found that 3S, 3′S-AST significantly increased cell viability in
H2O2-treated H9c2 cells. LDH and CK-MB are
known to be markers of cell injury, and release of LDH and content
of CK-MB were significantly reduced by 3S, 3′S-AST.
AST shows effective anti-apoptosis,
anti-inflammatory, anti-cancer, and cardioprotective capacities,
which were associated with its antioxidant activity (14,29).
ROS is a universal inducer of myocardial apoptosis in reperfusion
injury (46). Excessive ROS
production has been considered to be important to induce I/R injury
(46). MDA is the end product of
the lipid peroxidation reaction; therefore, the content of MDA
could reflect the degree of lipid peroxidation (47). To protect molecules from ROS, cells
stimulate antioxidant defense systems, including GSH-px, GSH and
GR, which play important roles in cellular antioxidant activity
(48,49). The present study found that 3S,
3′S-AST could decrease the content of MDA and increase GSH-px, GSH
and GR activity levels in the H9c2 cells. The results indicated
that 10 and 20 µM 3S, 3′S-AST could improve the antioxidant ability
of cardiomyocytes, inhibit excessive ROS accumulation and the
occurrence of lipid peroxidation induced by oxidative damage.
Apoptosis-related proteins, such as Bax, Bcl-2, caspase-3 and p53
play vital roles in cardiomyocyte damage (50,51).
The present study showed that myocardial apoptosis was
significantly inhibited by 3S, 3′S-AST. In addition, the
pro-apoptotic proteins Bax and caspase-3 were significantly
upregulated, while the anti-apoptotic protein Bcl-2 was
significantly decreased in H2O2-treated H9c2
cells. Treatment with 3S, 3′S-AST inhibited the increase of Bax,
caspase-3 and cleaved-caspase-3 and the decrease of Bcl-2 protein
expression.
The NKA was discovered as an ion pump for moving
Na+ and K+ across the cell membrane ~60 years
ago (52). It is a key enzyme in
maintaining the cellular Na+ and K+ ion
gradient in human cardiac myocytes (53). In addition to its ion pumping
function, NKA also serves as a scaffold protein, interacting with
neighboring proteins and facilitating multiple cell signaling
pathways. Wang et al (22)
reported that endogenous cardiotonic steroids could activate the
NKA/Src/Erk1/2 receptor complex to increase ROS levels, and ROS act
as endogenous cardiotonic steroids to stimulate NKA/Src/Erk1/2
activation. Inhibition of the NKA/Src/Erk1/2/ROS amplification loop
exhibited a beneficial effect on I/R injury (23). The present study found that 3S,
3′S-AST could reduce ROS generation induced by
H2O2, and exerted antioxidant effects by
restoring the activation of antioxidant enzymes.
The NKA receptor not only acts as an ion pump,
maintains cell membrane potential and stabilizes cell volume, but
also participates in a variety of protein-protein interactions,
which activates a number of protein cascades and participate in a
variety of signal transductions, including Raf/MEK/ERK,
phospholipase C/protein kinase C, PI3K/Akt, Ca2+ signal
transduction and ROS production (54). However, a number of studies have
shown that these signal transduction pathways can often cross-react
and generate specific cell regulation according to different signal
stimulation. Wu et al (55)
PI3K signaling could be stimulated by NKA with or without the
presence of Src. Src inhibitors can downregulate PI3K signaling in
cells treated with ouabain. Haas et al (56) showed that Src regulate interactions
between NKA and EGFR, resulting in activated Ras/MAPK kinases
cascade reaction. Further study will be performed to investigate
the effects of AST on other signaling pathways, such as Raf/MEK/ERK
or PI3K/Akt.
Decrease of Src and Erk1/2 phosphorylation levels
could inhibit ROS generation, and p-Src and p-Erk1/2 were reduced
by pNaKtide, which showed a cardioprotective effect in I/R injury
(23). In the present study,
treatment with H2O2, for 1 and 24 h
significantly upregulated the levels of p-Src and p-Erk1/2.
Pretreatment with 3S, 3′S-AST attenuated the activation of Src and
Erk1/2 induced by H2O2; however, NKA protein
expression levels were not changed. The present study demonstrated
that 3S, 3′S-AST could inhibit oxidative stress injury of
myocardial cells by reducing the NKA/Src/Erk1/2/ROS amplification
signaling pathway.
In conclusion, 3S, 3′S-AST could inhibit ROS
production and apoptosis by inhibiting the activation of Src and
Erk1/2, and associated oxidant amplification signaling in
myocardial cells following ROS treatment. The current study showed
the therapeutic effects of 3S, 3′S-AST and its potential to be
developed as a clinical approach for oxidative stress-related
myocardial cell injury.
Acknowledgements
Not applicable.
Funding
This study was supported in part by the Nature
Science Foundation of Zhejiang Province (grant no. LQ18H260003),
the Technology Program of Zhejiang Province (grant no. 2017F30001),
Key Subjects of Nutrition of Zhejiang Province (grant no. 16-zc03),
Key Research and Development Program of Zhejiang Province (grant
no. 2019C02028) and Zhejiang Provincial Bureau of Traditional
Chinese Medicine (grant no. 2019ZZ005).
Authors' contributions
XQ and YW designed the project and supervised all
research. XQ wrote the manuscript. ZZ, WH, ML, BZ, LZ, SM, ZH, DL,
ZL and JC performed all experiments and analyzed data. All authors
read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ong SB, Hernández-Reséndiz S,
Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA and
Hausenloy DJ: Inflammation following acute myocardial infarction:
Multiple players, dynamic roles, and novel therapeutic
opportunities. Pharmacol Ther. 186:73–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie B, Liu X, Yang J, Cheng J, Gu J and
Xue S: PIAS1 protects against myocardial ischemia-reperfusion
injury by stimulating PPARγ SUMOylation. BMC Cell Biol. 19:242018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sies H: Hydrogen peroxide as a central
redox signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biol. 11:613–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu D, Li M, Tian Y, Liu J and Shang J:
Luteolin inhibits ROS-activated MAPK pathway in myocardial
ischemia/reperfusion injury. Life Sci. 122:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li
L, Xu XN, Gong LL, Qin HL, Fang LH and Du GH: Coptisine protects
rat heart against myocardial ischemia/reperfusion injury by
suppressing myocardial apoptosis and inflammation. Atherosclerosis.
231:384–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mokhtari-Zaer A, Marefati N, Atkin SL,
Butler AE and Sahebkar A: The protective role of curcumin in
myocardial ischemia-reperfusion injury. J Cell Physiol.
234:214–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faraone I, Sinisgalli C, Ostuni A,
Armentano MF, Carmosino M, Milella L, Russo D, Labanca F and Khan
H: Astaxanthin anticancer effects are mediated through multiple
molecular mechanisms: A systematic review. Pharmacol Res.
155:1046892020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alam MN, Hossain MM, Rahman MM, Subhan N,
Mamun MAA, Ulla A, Reza HM and Alam MA: Astaxanthin prevented
oxidative stress in heart and kidneys of isoproterenol-administered
aged rats. J Diet Suppl. 15:42–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Q, Guo S, Zhou H, Han R, Wu P and Han
C: Astaxanthin protects against early burn-wound progression in
rats by attenuating oxidative stress-induced inflammation and
mitochondria-related apoptosis. Sci Rep. 7:414402017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coombes JS, Sharman JE and Fassett RG:
Astaxanthin has no effect on arterial stiffness, oxidative stress,
or inflammation in renal transplant recipients: A randomized
controlled trial (the XANTHIN trial). Am J Clin Nutr. 103:283–289.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Zhu J, Yin W and Ding X: Astaxanthin
improves cognitive deficits from oxidative stress, nitric oxide
synthase and inflammation through upregulation of PI3K/Akt in
diabetes rat. Int J Clin Exp Pathol. 8:6083–6094. 2015.PubMed/NCBI
|
|
13
|
Kim YJ, Kim YA and Yokozawa T: Protection
against oxidative stress, inflammation, and apoptosis of
high-glucose-exposed proximal tubular epithelial cells by
astaxanthin. J Agric Food Chem. 57:8793–8797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambati RR, Phang SM, Ravi S and
Aswathanarayana RG: Astaxanthin: Sources, extraction, stability,
biological activities and its commercial applications-a review. Mar
Drugs. 12:128–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishimoto Y, Yoshida H and Kondo K:
Potential anti-atherosclerotic properties of astaxanthin. Mar
Drugs. 14:352016. View Article : Google Scholar
|
|
16
|
Zhang ZW, Xu XC, Liu T and Yuan S:
Mitochondrion-permeable antioxidants to treat ROS-burst-mediated
acute diseases. Oxid Med Cell Longev. 2016:68595232016.PubMed/NCBI
|
|
17
|
Shi Y, Lin P, Wang X, Zou G and Li K:
Sphingomyelin phosphodiesterase 1 (SMPD1) mediates the attenuation
of myocardial infarction-induced cardiac fibrosis by astaxanthin.
Biochem Biophys Res Commun. 503:637–643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belliard A, Sottejeau Y, Duan Q, Karabin
JL and Pierre SV: Modulation of cardiac
Na+,K+-ATPase cell surface abundance by
simulated ischemia-reperfusion and ouabain preconditioning. Am J
Physiol Heart Circ Physiol. 304:H94–H103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z and Xie Z: The Na/K-ATPase/Src
complex and cardiotonic steroid-activated protein kinase cascades.
Pflugers Arch. 457:635–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Y, Shapiro AP, Haller S, Katragadda V,
Liu L, Tian J, Basrus V, Malhotra D, Xie Z, Abraham NG, et al:
Involvement of reactive oxygen species in a feed-forward mechanism
of Na/K-ATPase-mediated signaling transduction. J Biol Chem.
288:34249–34258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Tian J, Haas M, Shapiro JI, Askari
A and Xie Z: Ouabain interaction with cardiac
Na+/K+-ATPase initiates signal cascades
independent of changes in intracellular Na+ and
Ca2+ concentrations. J Biol Chem. 275:27838–27844.
2000.PubMed/NCBI
|
|
22
|
Wang Y, Ye Q, Liu C, Xie JX, Yan Y, Lai F,
Duan Q, Li X, Tian J and Xie Z: Involvement of Na/K-ATPase in
hydrogen peroxide-induced activation of the Src/ERK pathway in
LLC-PK1 cells. Free Radic Biol Med. 71:415–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Yin A, Cheng Z, Feng M, Zhang H, Xu
J, Wang F and Qian L: Attenuation of Na/K-ATPase/Src/ROS
amplification signal pathway with pNaktide ameliorates myocardial
ischemia-reperfusion injury. Int J Biol Macromol. 118:1142–1148.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting MicroRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karu I, Tähepõld P, Ruusalepp A and
Starkopf J: Pretreatment by hyperoxia-a tool to reduce
ischaemia-reperfusion injury in the myocardium. Curr Clin
Pharmacol. 5:125–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Lin R, Guo L and Hong M:
Rosuvastatin relieves myocardial ischemia/reperfusion injury by
upregulating PPAR-γ and UCP2. Mol Med Rep. 18:789–798.
2018.PubMed/NCBI
|
|
28
|
Hoffman JJ Jr, Gilbert TB, Poston RS and
Silldorff EP: Myocardial reperfusion injury: Etiology, mechanisms,
and therapies. J Extra Corpor Technol. 36:391–411. 2004.PubMed/NCBI
|
|
29
|
Visioli F and Artaria C: Astaxanthin in
cardiovascular health and disease: Mechanisms of action,
therapeutic merits, and knowledge gaps. Food Funct. 8:39–63. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lauver DA, Lockwood SF and Lucchesi BR:
Disodium Disuccinate Astaxanthin (Cardax) attenuates complement
activation and reduces myocardial injury following
ischemia/reperfusion. J Pharmacol Exp Ther. 314:686–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah MM, Liang Y, Cheng JJ and Daroch M:
Astaxanthin-producing green microalga Haematococcus pluvialis: From
single cell to high value commercial products. Front Plant Sci.
7:5312016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
EFSA Panel on Dietetic Products Nutrition
and Allergies (NDA), . Scientific opinion on the safety of
astaxanthin-rich ingredients (AstaREAL A1010 and AstaREAL L10) as
novel food ingredients. EFSA J. 15–July;2014.(Epub ahead of print).
doi: org/10.2903/j.efsa.2014.3757. PubMed/NCBI
|
|
33
|
Iwamoto T, Hosoda K, Hirano R, Kurata H,
Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S and Kondo K:
Inhibition of low-density lipoprotein oxidation by astaxanthin. J
Atheroscler Thromb. 7:216–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida H, Yanai H, Ito K, Tomono Y,
Koikeda T, Tsukahara H and Tada N: Administration of natural
astaxanthin increases serum HDL-cholesterol and adiponectin in
subjects with mild hyperlipidemia. Atherosclerosis. 209:520–523.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruen-Ngam D, Shotipruk A and Pavasant P:
Comparison of extraction methods for recovery of astaxanthin from
Haematococcus pluvialis. Sep Sci Technol. 46:64–70. 2010.
View Article : Google Scholar
|
|
36
|
Zhu S, Wang T, Luo F, Li H, Jia Q, He T,
Wu H and Zou T: Astaxanthin inhibits proliferation and induces
apoptosis of LX2 cells by regulating the miR29b/Bcl2 pathway. Mol
Med Rep. 19:3537–3547. 2019.PubMed/NCBI
|
|
37
|
Fan CD, Sun JY, Fu XT, Hou YJ, Li Y, Yang
MF, Fu XY and Sun BL: Astaxanthin attenuates homocysteine-induced
cardiotoxicity in vitro and in vivo by inhibiting mitochondrial
dysfunction and oxidative damage. Front Physiol. 8:10412017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okada Y, Ishikura M and Maoka T:
Bioavailability of astaxanthin in Haematococcus algal
extract: The effects of timing of diet and smoking habits. Biosci
Biotechnol Biochem. 73:1928–1932. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiao X, Yang L, Zhang T, Zhou Q, Wang Y,
Xu J and Xue C: Synthesis, stability and bioavailability of
astaxanthin succinate diester. J Sci Food Agric. 98:3182–3189.
2018.PubMed/NCBI
|
|
40
|
Mercke OJ, Lignell A, Pettersson A and
Hoglund P: Oral bioavailability of the antioxidant astaxanthin in
humans is enhanced by incorporation of lipid based formulations.
Eur J Pharm Sci. 19:299–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barros MP, Marin DP, Bolin AP, de Cássia
Santos Macedo R, Campoio TR, Fineto C Jr, Guerra BA, Polotow TG,
Vardaris C, Mattei R and Otton R: Combined astaxanthin and fish oil
supplementation improves glutathione-based redox balance in rat
plasma and neutrophils. Chem-Biol Interact. 197:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rao AR, Reddy RLR, Baskaran V, Sarada R
and Ravishankar GA: Characterization of microalgal carotenoids by
mass spectrometry and their bioavailability and antioxidant
properties elucidated in rat model. J Agr Food Chem. 58:8553–8559.
2010. View Article : Google Scholar
|
|
43
|
Satoh A, Tsuji S, Okada Y, Murakami N,
Urami M, Nakagawa K, Ishikura M, Katagiri M, Koga Y and Shirasawa
T: Preliminary clinical evaluation of toxicity and efficacy of a
new astaxanthin-rich Haematococcus pluvialis extract. J Clin
Biochem Nutr. 44:280–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baburina Y, Krestinin R, Odinokova I,
Sotnikova L, Kruglov A and Krestinina O: Astaxanthin inhibits
mitochondrial permeability transition pore opening in rat heart
mitochondria. Antioxidants (Basel). 8:5762019. View Article : Google Scholar
|
|
45
|
Nakao R, Nelson OL, Park JS, Mathison BD,
Thompson PA and Chew BP: Effect of astaxanthin supplementation on
inflammation and cardiac function in BALB/c mice. Anticancer Res.
30:2721–2725. 2010.PubMed/NCBI
|
|
46
|
Kalogeris T, Bao Y and Korthuis RJ:
Mitochondrial reactive oxygen species: A double edged sword in
ischemia/reperfusion vs preconditioning. Redox Biol. 2:702–714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma JJ, Yu YG, Yin SW, Tang CH and Yang XQ:
Cellular uptake and intracellular antioxidant activity of
Zein/Chitosan nanoparticles incorporated with quercetin. J Agric
Food Chem. 66:12783–12793. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goc Z, Szaroma W, Kapusta E and Dziubek K:
Protective effects of melatonin on the activity of SOD, CAT, GSH-Px
and GSH content in organs of mice after administration of SNP. Chin
J Physiol. 60:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Couto N, Wood J and Barber J: The role of
glutathione reductase and related enzymes on cellular redox
homoeostasis network. Free Radic Biol Med. 95:27–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lopez-Neblina F, Toledo AH and
Toledo-Pereyra LH: Molecular biology of apoptosis in ischemia and
reperfusion. J Invest Surg. 18:335–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips SJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Skou JC: The influence of some cations on
an adenosine triphosphatase from peripheral nerves. Biochim Biophys
Acta. 23:394–401. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pierre SV and Xie Z: The Na,K-ATPase
receptor complex: Its organization and membership. Cell Biochem
Biophys. 46:303–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui X and Xie Z: Protein interaction and
Na/K-ATPase-mediated signal transduction. Molecules. 22:9902017.
View Article : Google Scholar
|
|
55
|
Wu J, Akkuratov EE, Bai Y, Gaskill CM,
Askari A and Liu L: Cell signaling associated with
Na(+)/K(+)-ATPase: Activation of phosphatidylinositide 3-kinase
IA/Akt by ouabain is independent of Src. Biochemistry.
52:9059–9067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Haas M, Wang H, Tian J and Xie Z:
Src-mediated inter-receptor cross-talk between the
Na+/K+-ATPase and the epidermal growth factor
receptor relays the signal from ouabain to mitogen-activated
protein kinases. J Biol Chem. 277:18694–18702. 2002. View Article : Google Scholar : PubMed/NCBI
|



















