Introduction
Inflammation occurs in response to a variety of
stimuli, such as tissue damage, infection, or cancer (1–3).
While acute inflammation is of short duration and represents an
early body reaction that resolves quickly, chronic inflammation is
defined as a prolonged process whereby tissue destruction and
inflammation occur simultaneously (4). In the early stages of inflammation,
the vascular endothelium is activated by cytokines, leading to
adhesion and transmigration of leukocytes into the site of
inflammation. Some of the pro-inflammatory processes acquired at
the endothelium and leukocytes are mediated by the transcription
factor NF-κb (5). Failure to treat
inflammation can lead to various diseases associated with chronic
inflammation, including arthritis, atherosclerosis, and even cancer
(6). For most of these conditions,
no satisfactory treatment has been established.
NF-κB is a transcription factor that plays an
important role in cellular stress responses, including the DNA
damage response (7). NF-κB
activation induces the expression of >200 genes that regulate
inflammation, as well as cell death/apoptosis (8). The DNA damage response is known to
play a pivotal role in ageing and carcinogenesis. It consists of a
signal transduction cascade that is initiated by DNA double-strand
breaks and leads to DNA repair, cell cycle arrest or programmed
cell death (9). Evidence strongly
suggests that inflammation is linked to multiple pathologies, such
as cancer and obesity (10), by
inducing cellular and molecular damage through the activation of
several signaling pathways, including the NF-κB pathway.
The aim of the present study was to identify natural
products with anti-inflammatory effects on NF-κB activity and
further characterize active compounds from plant products for drug
discovery. By screening 112 natural products for their
anti-inflammatory properties, it was identified that Sohakuhi
(Morus alba Linn. bark) extract markedly suppressed
NF-κB-dependent luciferase reporter activity in murine 4T1 cells
without affecting cell viability. The anti-inflammatory effect of
Sohakuhi on tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL)-induced cellular damage was evaluated in human HaCaT
keratinocytes. TRAIL triggered the phosphorylation of p65, a
subunit of NF-κB, leading to cellular damage in HaCaT cells.
However, treatment with Sohakuhi extract protected HaCaT cells
against TRAIL-induced damage. Moreover, Sohakuhi also upregulated
the expression of the anti-apoptotic proteins Bcl-xL and Bcl-2.
Importantly, through chemical fractionation of Sohakuhi extract,
moracin O and P were determined to mediate its anti-inflammatory
effect.
Materials and methods
Cells and reagents
The murine B16F10 and 4T1 cell lines were obtained
from the American Type Culture Collection and maintained at 37°C in
Eagle's minimal essential medium or RPMI-1640 medium (Nissui
Pharmaceutical Co., Ltd.) containing 10% FBS (Nichirei Biosciences,
Inc.), respectively. Human HaCaT keratinocytes (provided by Dr
Takeda, Juntendo University, Tokyo, Japan) were maintained at 37°C
in culture medium consisting of DMEM (Nissui Pharmaceutical Co.,
Ltd.) supplemented with 10% FBS, 100 U/l penicillin G and 100 mg/l
streptomycin at 37°C with 5% CO2. Human recombinant
TRAIL (rTRAIL) was purchased from PeproTech, Inc.
In vitro NF-κB luciferase reporter
assay
B16F10NFκB and 4T1NFκB cells expressing firefly
luciferase under the control of an NF-κB response element were
established as previously described (11,12)
and maintained at 37°C in RPMI-1640 medium containing 10% FBS.
Briefly, B16F10NFκB and 4T1NFκB cells were generated by
transfecting the B16F10 and 4T1 cell lines with
pGL4.32-luc2P/NF-κB-RE/Hygro vector (Promega Corporation)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were selected on hygromycin B and
cloned by limiting dilution.
B16F10NFκB cells and 4T1NFκB cells in the
exponential growth phase were seeded at a final concentration of
4×104 cells/well in a 96-well plate. After 3-h
incubation, the cells were co-cultured with 50 µg/ml extract from
112 natural products (Table SI)
for 24 h. At the end of the assay, 900 µg/ml D-luciferin was added,
and the plates were incubated for another 30 min. Luciferase
activity was measured by the GloMax®-Multi Detection
System (Promega Corporation).
Cell viability assay
Cell viability was quantified using the WST-1 cell
proliferation reagent (Dojindo Molecular Technologies, Inc.).
B16F10NFκB or 4T1NFκB cells were seeded on a 96-well plate and
co-cultured with extracts from 112 natural products (Table SI) at 50 µg/ml for 24 h. In a
separate experiment, HaCaT cells were seeded on a 96-well plate at
a density of 104 cells/well and pre-treated with 20
ng/ml rTRAIL for 1 h. The cells were then cultured with or without
Sohakuhi extract (6.25 to 50 µg/ml) for 24 h. After incubation,
WST-1 solution was added and used according to the manufacturer's
instructions, and absorbance was measured at 450 nm using a
microplate reader. Cell viability was calculated as a percentage of
the control.
Caspase-3 and −7 activity assay
For measurement of the activities of caspase-3 and
−7, the Caspase-Glo® 3/7 assay system (Promega
Corporation) was used according to the manufacturer's instructions.
Briefly, HaCaT cells (5×103 cells/well in a 96-well
plate) were pre-treated with 20 ng/ml rTRAIL for 1 h. The cells
were then cultured with Sohakuhi extract for 24 h, and the
Caspase-Glo® 3/7 reagent was then added. After 30-min
incubation, caspase-3 and −7 activities were measured using the
GloMax®-Multi Detection System (Promega
Corporation).
Identification of the active
components of Sohakuhi extract
To identify the bioactive components of Sohakuhi
extract, 200 g root bark of Morus alba Linn. was decocted in
1 l water for 50 min. The water extract was fractionated by
water-methanol gradient reverse-phase medium-pressure liquid
chromatography (RP-MPLC) fractionation. Mass spectrometry and
1H-nuclear magnetic resonance (NMR) analysis were used
to identify compounds in the isolated fractions. Extraction and
isolation of Sohakuhi was conducted using a water-methanol gradient
MPLC fractionation system and high-performance liquid
chromatography (Accela™ HPLC system; Thermo Fisher Scientific,
Inc.) profiles of water extracts of Sohakuhi were determined at
254-nm ultraviolet wavelength. A Capcell Pak C18 MG III S-5
(4.5×250 mm, 5 µm; Shiseido Co., Ltd.) column was used for HPLC
analysis at a flow rate of 1 ml/min at 40°C. A gradient elution
system composed of 5% CH3CN (v/v) (A) and H2O
was used as follows: 0–4 min, 5% A; 4–8 min, 5–10% B; 8–12 min,
10–15% B; 12–15 min, 15–20% B; 15–18 min, 20–25% B; 18–21 min,
25–30% B; 21–25 min, 30–35% B; 25 min, 40% B. The active fraction
was further analyzed using a CHCl3-MeOH gradient RP-MPLC
fractionation system with mass spectrometry and 1H-NMR
analysis to identify the active compounds of Sohakuhi. The MS
conditions were set as follows: Negative ESI mode, spray voltage
4.5 kV, capillary voltage 40.0 kV, tube lens 150 V, capillary
temperature 270°C, sheath gas flow rate 50 units, aux gas flow rate
10 units, and scan range m/z 50–2,000. A polytyrosine solution was
used for instrument calibration before each experiment.
Western blot analysis
HaCaT cells were seeded at a density of
5×105 cells/well and cultured for 24 h in a 6-well
plate. After treatment, the cells were washed in cold PBS, scraped
and lysed in whole-cell lysis buffer (25 mmol/l HEPES, pH 7.7; 300
mmol/l NaCl; 1.5 mmol/l MgCl2; 0.2 mmol/l EDTA; 0.1%
Triton X-100; 20 mmol/l β-glycerophosphate; 1 mmol/l
Na3VO4; 1 mmol/l
phenylmethylsulfonylfluoride; 1 mmol/l dithiothreitol; 10 mg/ml
aprotinin; 10 mg/ml leupeptin). Cell lysates (10 µl/lane) were
resolved by SDS-PAGE on 7.5–15% gels, then transferred to an
Immobilon-P nylon membrane (EMD Millipore). The membranes were
treated with Block Ace (Dainippon Sumimoto Pharma Co., Ltd.) for at
least 2 h at room temperature, then probed with primary antibodies
at 4°C overnight, followed by horseradish peroxidase-conjugated
secondary antibodies (1:2,000; cat. nos. P0448 and P0260; Dako;
Agilent Technologies, Inc.). Bands were visualized using ECL
reagents (Amersham; Cytiva). The primary antibodies used were
specific for Bcl-2 (clone no. D55G8; cat. no. 4223), Bcl-xL (clone
no. 54H6; cat. no. 2764), p65 (clone no. L8F6; cat. no. 6956),
phosphorylated-p65, (clone no. 93H1; cat. no. 3033; all Cell
Signaling Technology, Inc.) and β-actin (clone no. C4; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.). All primary antibodies
were used at 1:1,000 dilution.
Statistical analysis
All data are presented as the mean ± SEM of three
independent experiments. SPSS versions 23 and 25 software (IBM
Corp.) were used to analyze data. Statistical analysis was carried
out using one-way ANOVA followed by Bonferroni correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sohakuhi suppresses NF-κB activity in
murine cancer cell lines
To identify novel anti-inflammatory agents in plant
products, 112 medicinal plant extracts (Table SI) were screened for their effect
on NF-κB activity using cell lines that express luciferase under
the control of an NF-κB response element. Among the tested
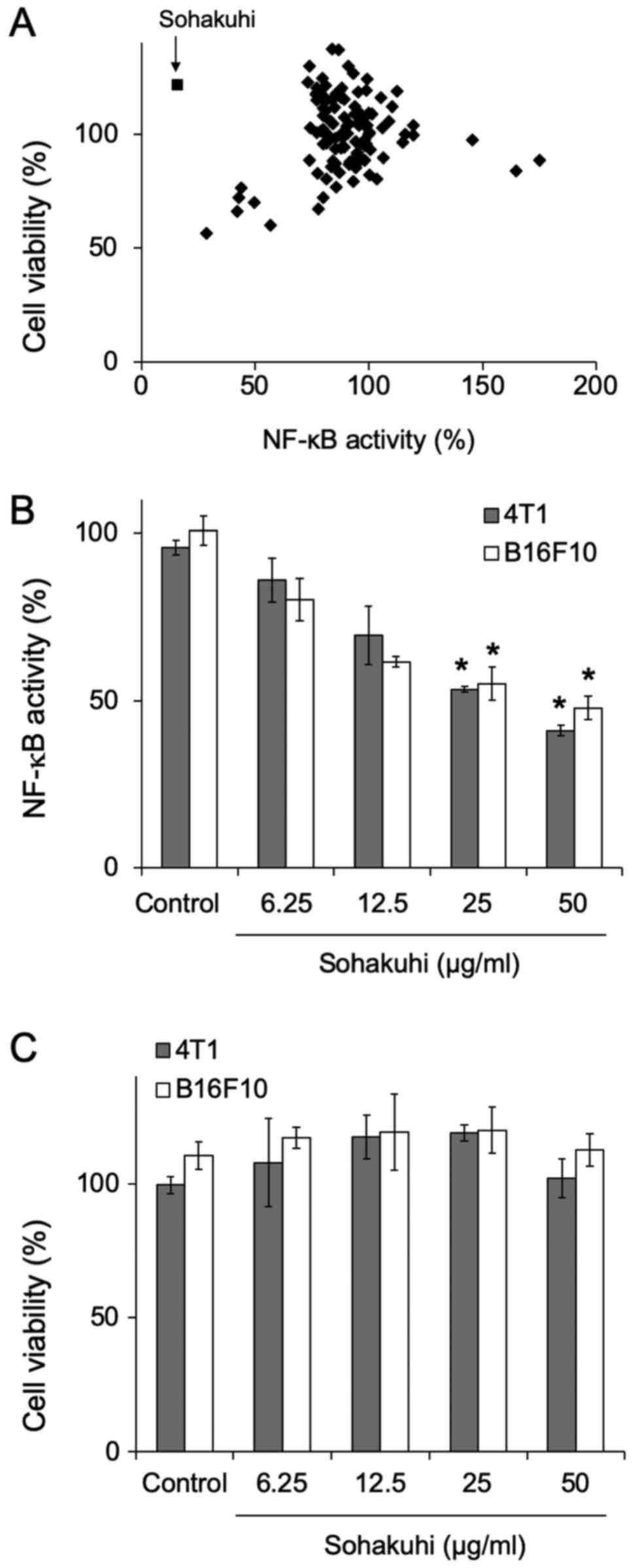
extracts, Sohakuhi markedly suppressed NF-κB activity without
affecting the viability of 4T1 cells (Fig. 1A). Furthermore, NF-κB activity was
suppressed by Sohakuhi extract in 4T1 cells and B16F10 cells in a
dose-dependent manner (Fig. 1B).
However, Sohakuhi extract did not display any cytotoxic effect,
even at doses reaching 50 µg/ml (Fig.
1C).
Cytoprotective effect of Sohakuhi
extract on TRAIL-induced cellular damage in human
keratinocytes
To examine the biological utility of Sohakuhi
extract, human HaCaT keratinocytes were incubated with rTRAIL as an
inflammatory stimulus to induce NF-κB activation and cytotoxicity.
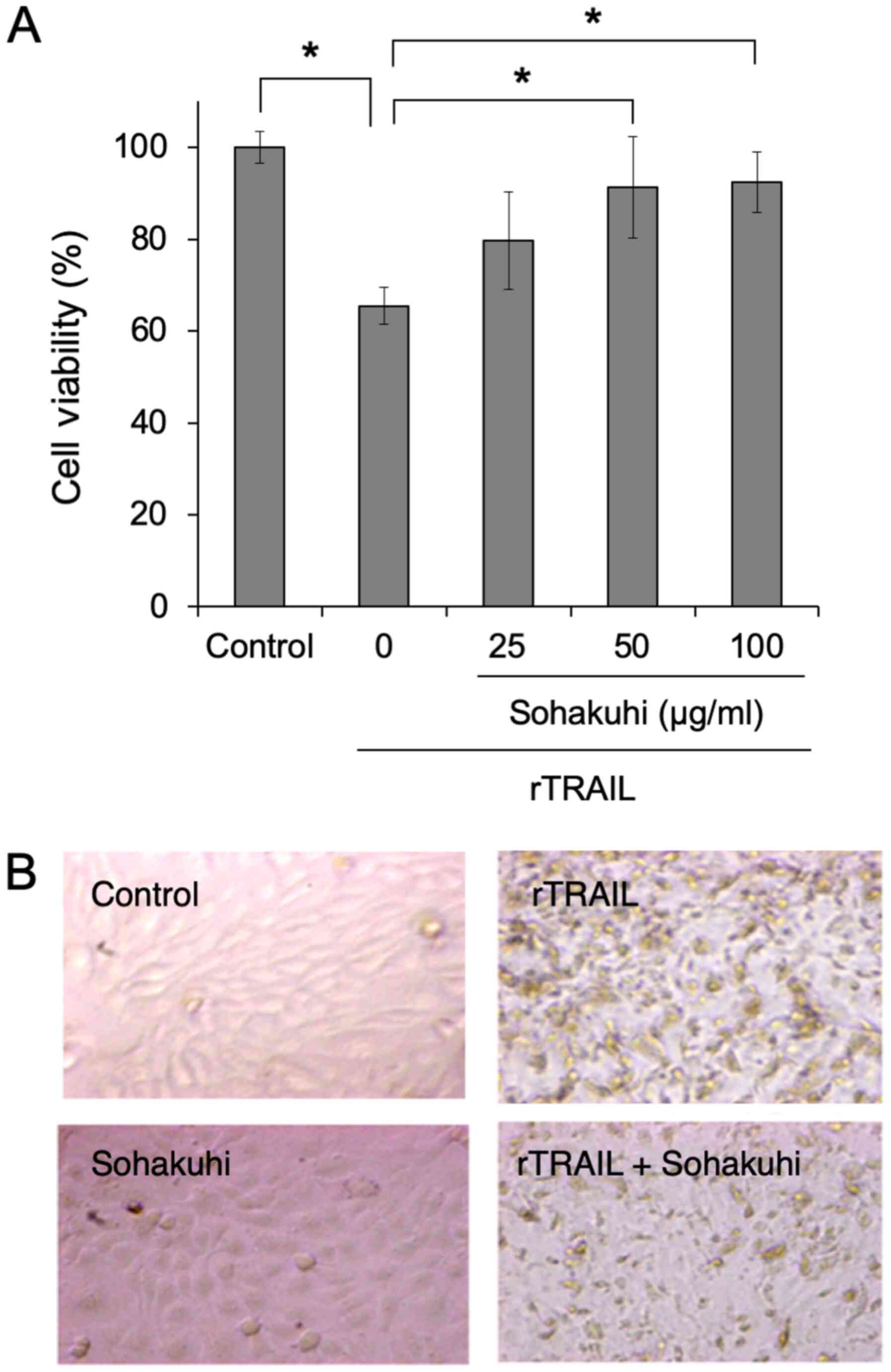
Compared with the control, rTRAIL treatment induced significant
cytotoxicity in HaCaT cells. However, pre-treatment with Sohakuhi
extract significantly protected HaCaT cells from TRAIL-induced
cytotoxicity in a dose-dependent manner (Fig. 2A). The cytoprotective effect of
Sohakuhi extract was evident from microscopic observation of cell
morphology (Fig. 2B). These
results indicated the cytoprotective effect of Sohakuhi extract
against TRAIL-induced cellular damage in human keratinocytes.
Anti-apoptotic effect of Sohakuhi
extract on HaCaT cells
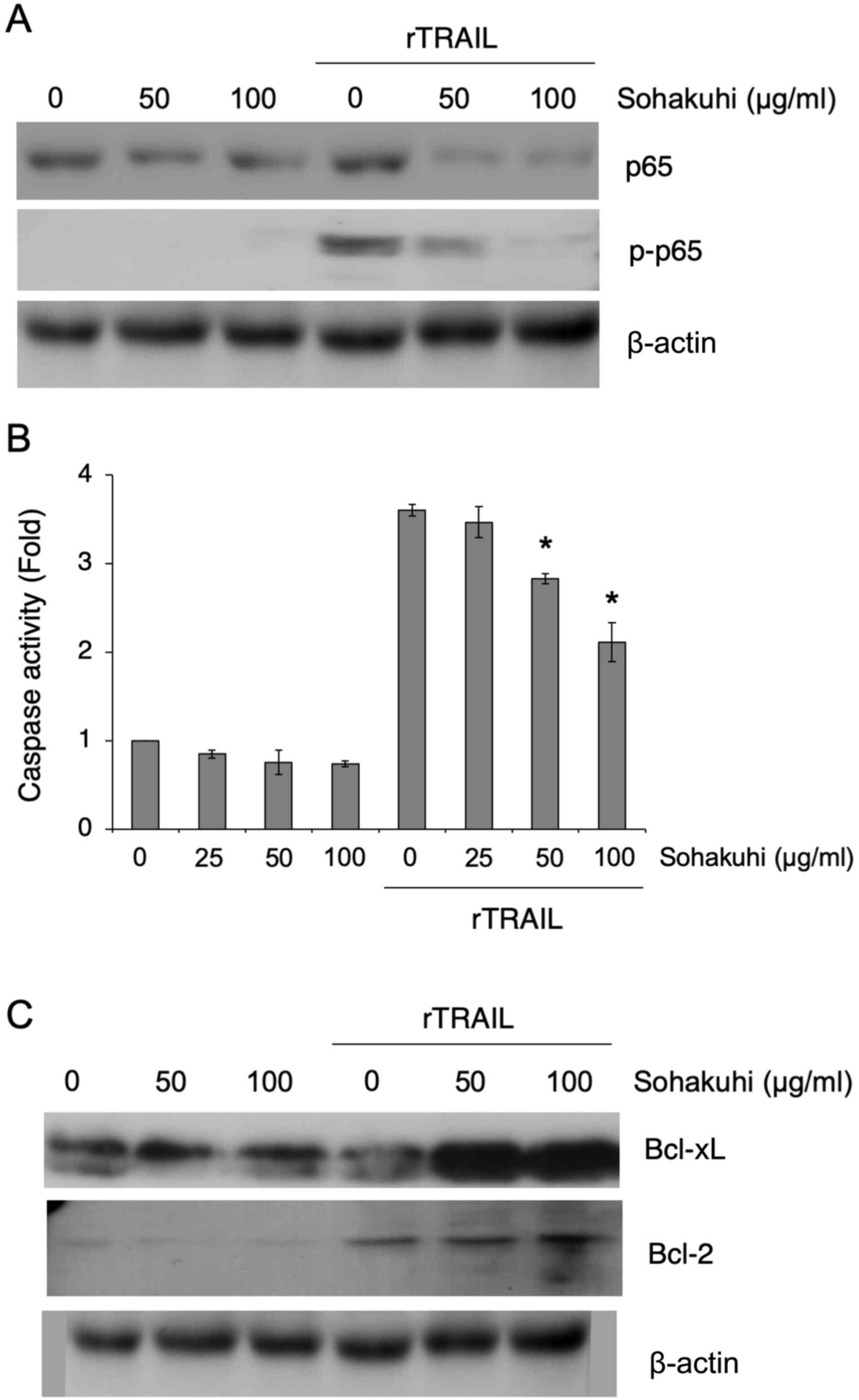
To confirm whether Sohakuhi extract affects NF-κB
activation in HaCaT cells, the expression of the p65 subunit of
NF-κB and its phosphorylation status following rTRAIL stimulation
was assessed in the presence or absence of Sohakuhi extract
(Fig. 3A). Phosphorylation of p65
was detectable in HaCaT cells following rTRAIL stimulation, but
suppressed in the presence of Sohakuhi extract. While Sohakuhi
extract treatment reduced total p65 expression in rTRAIL-stimulated
HaCaT cells, there was almost no effect on the basal expression of
p65 in unstimulated HaCaT cells, suggesting that the effect of
Sohakuhi extract might be specific to TRAIL-induced NF-κB activity.
Treatment with the Sohakuhi extract also showed an inhibitory
effect on TRAIL-induced apoptosis, as seen in the suppression of
caspase-3/7 activation (Fig. 3B).
Importantly, the anti-apoptotic proteins Bcl-xL and Bcl-2 were
upregulated in Sohakuhi extract-treated HaCaT cells following TRAIL
stimulation (Fig. 3C). These
results indicated that the cytoprotective effect of Sohakuhi
extract might result from inhibition of TRAIL-induced apoptosis and
regulation of anti-apoptotic proteins.
Identification of the active component
of Sohakuhi extract to inhibit NF-κB activation
As Sohakuhi extract significantly inhibited NF-κB
activity and displayed a cytoprotective effect against
inflammation-associated cellular damage of human keratinocytes, the
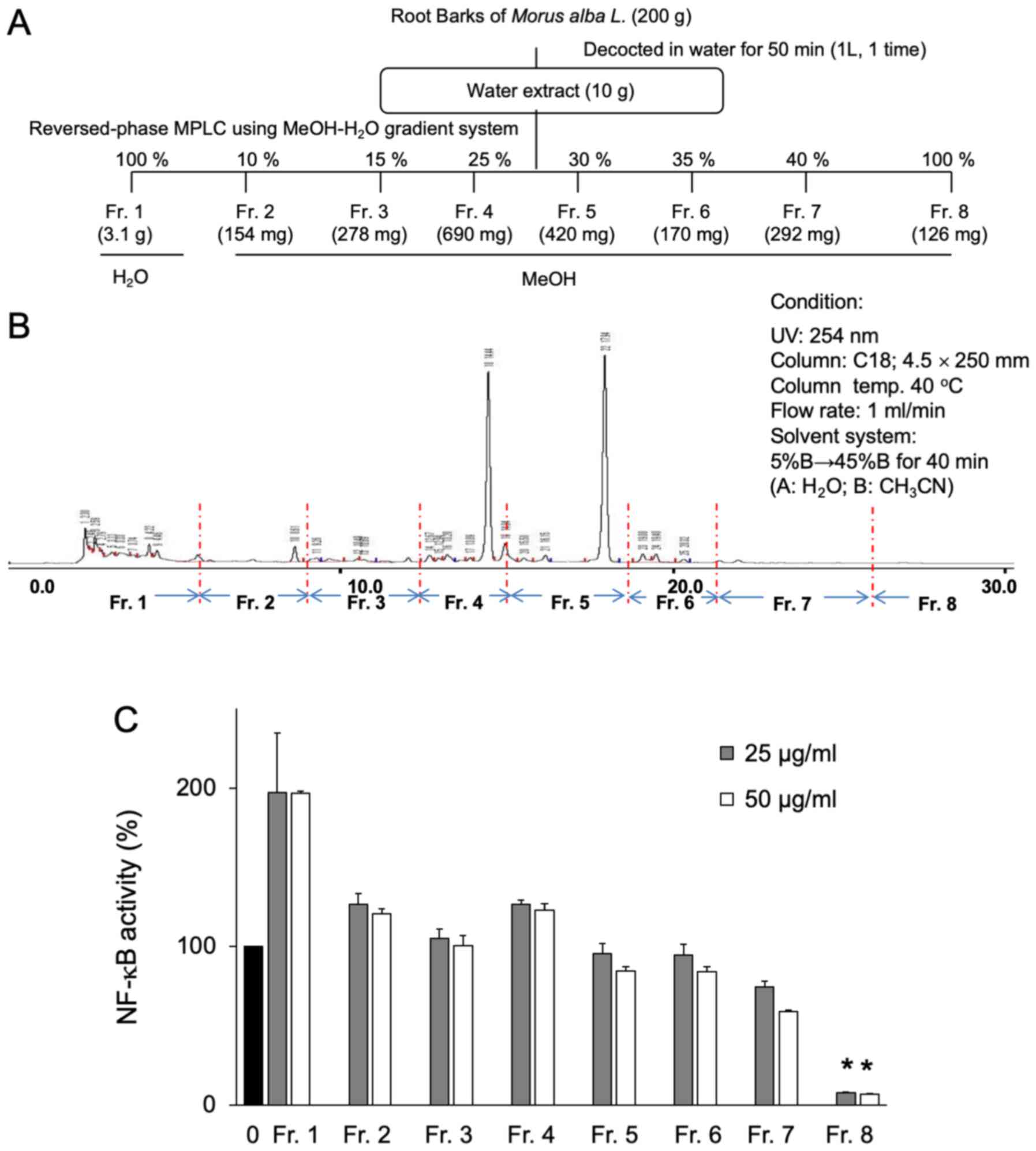
bioactive components of Sohakuhi extract were then analyzed. A
total of 8 yield fractions were isolated from a Sohakuhi water
extract using RP-MPLC with methanol-water gradient (Fig. 4A). The preparative HPLC data for
these 8 yield fractions are presented in Fig. 4B. Amongst those fractions, fraction
8 effectively and exclusively inhibited NF-κB activity (Fig. 4C). Considering that fraction 8 was
eluted with 100% methanol in the water-methanol gradient, the
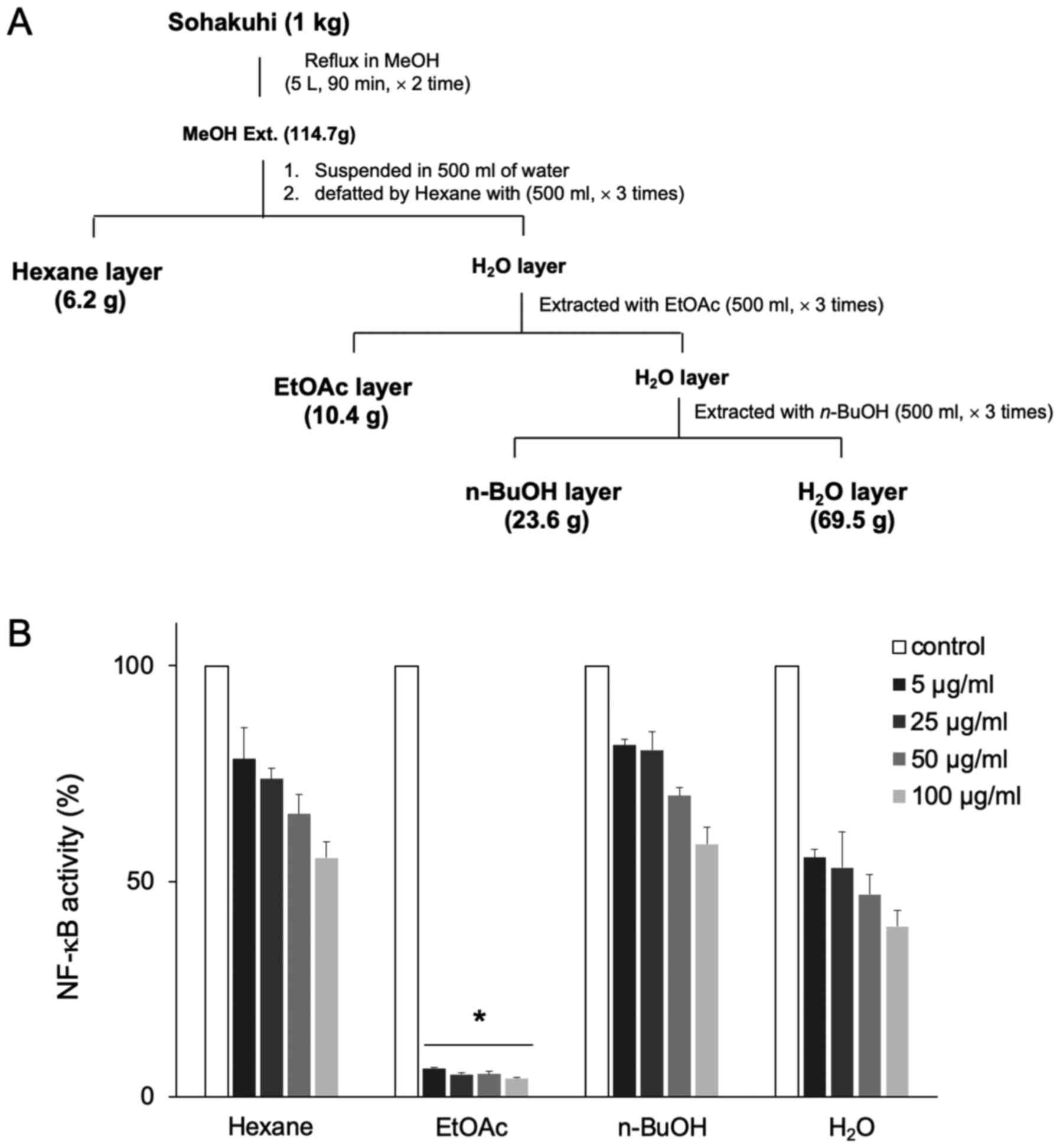
methanol extract of Sohakuhi was then fractionated into hexane,
ethyl acetate, n-butanol and a residual water layer (Fig. 5A) to test the activity of each
layer with respect to NF-κB inhibition. Amongst those layers, the
ethyl acetate layer displayed very potent inhibition of NF-κB
activity in 4T1 cells, even at the lowest dose tested, 5 µg/ml
(Fig. 5B). These results indicated
that the active component of Sohakuhi was fractionated into an
ethyl acetate layer from the methanol extract.
Isolation of moracin O and P as active
compounds of Sohakuhi
Using a CHCl3-MeOH gradient RP-MPLC
fractionation system with mass spectrometry and 1H-NMR
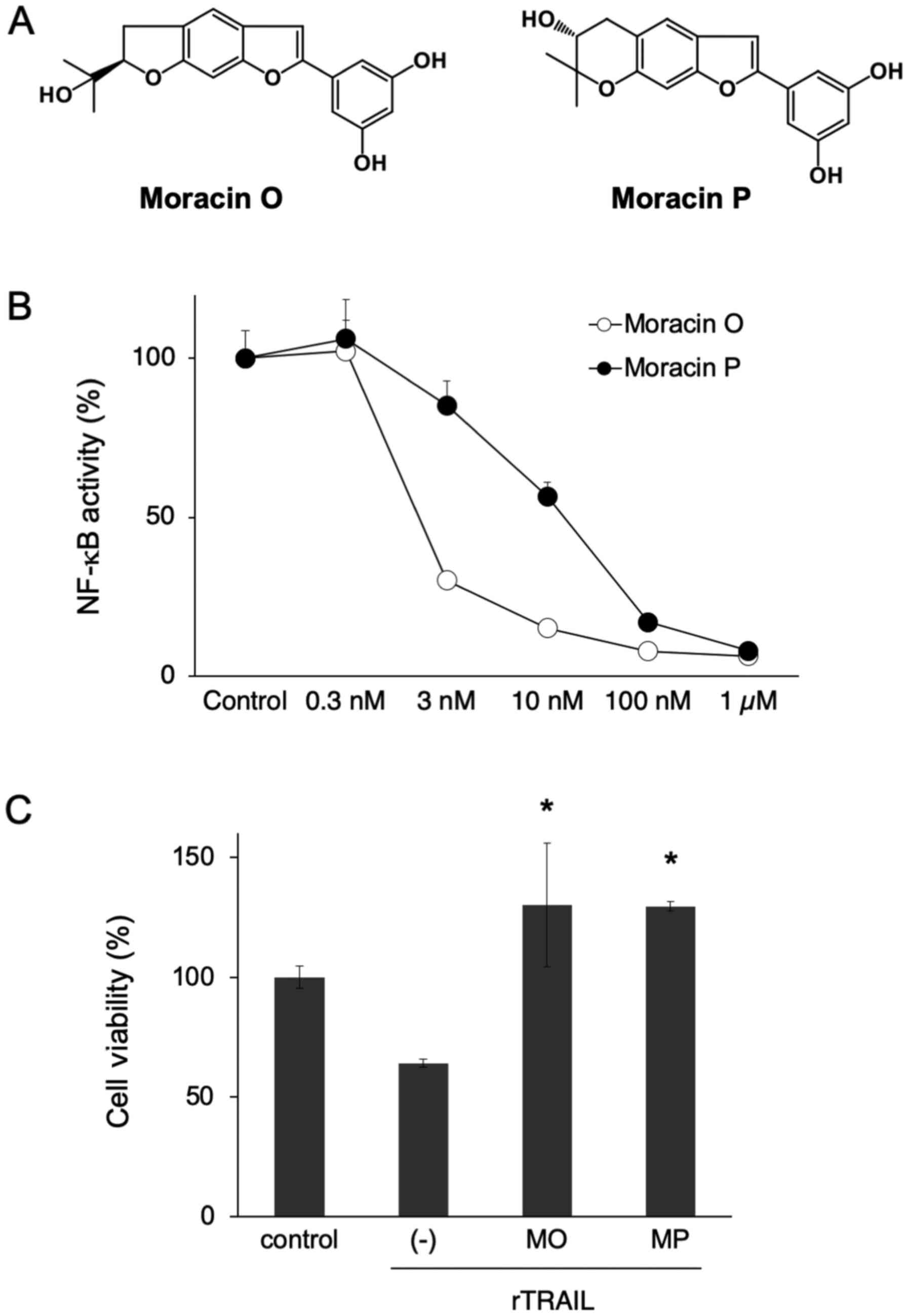
analysis, two major compounds, moracin O and P, were identified in
the ethyl acetate layer of Sohakuhi methanol extract (Fig. 6A). Importantly, both moracin O and
P significantly inhibited NF-κB activity in 4T1 cells, starting at
a dose of 3 nM (Fig. 6B).
Additionally, both moracin O and P showed significant
cytoprotective effects against TRAIL-induced cellular damage in
HaCaT cells (Fig. 6C). Thus,
moracin O and P were identified as active compounds of Sohakuhi
that suppressed NF-κB activity and exerted a cytoprotective effect
against TRAIL-induced damage in HaCaT cells.
Discussion
The aim of the present study was to identify a novel
anti-inflammatory drug candidate that can target NF-κB activation.
A total of 112 plant extracts were screened, identifying Sohakuhi
as a promising anti-inflammatory compound. The white mulberry tree
(Morus alba Linn.) is a deciduous tree originating from
Asia, especially China, but currently cultivated in subtropical,
tropical and mild environmental conditions (13,14).
Morus alba Linn. tree bark, fruits and leaves contain
proteins, carbohydrates, calcium, iron, ascorbic acid, thiamine,
folic acid and vitamin D (15). It
has been used in conventional and natural medicine to treat
diabetes, atherosclerosis, hyperlipidemia, hypertension,
neurodegenerative disease and cancer (13). Phytochemical studies have reported
that alkaloids, flavonoids, flavones, flavanones, stilbenes,
benzophenones, coumarin derivatives, terpenoid stilbenes,
oxyresveratrol and resveratrol were present in Morus species
(16–18). Moreover, oxyresveratrol can
suppress inflammation by inhibiting nitric oxide (NO) production,
inducible NO synthase expression, prostaglandin E2 production and
NF-κB activation in macrophages (19). Moracin R, C, O, P and D belong to
the 2-arylbenzofuran group, and display inhibitory activity against
the differentiation of 3T3-L1 adipocytes and NO production in
RAW264.7 cells (20). The
2-arylbenzofurans of Morus species, moracin O and P,
markedly suppress hypoxia-inducible factor-1 (HIF-1) activity in
the human Hep3B hepatocellular carcinoma cell line (21–23).
Moracin-M-3′-O-β-D-glucopyranoside has been reported to suppress
12-O-tetradecanoylphorbol-13-acetate-induced tumor progression in
mouse skin, and the underlying mechanism may involve the inhibition
of leukocyte infiltration, epidermal hyper-proliferation, oxidative
stress, and the endogenous tumor promoter TNF (24).
NF-κB plays a central role in inflammation, immunity
and several other cellular responses (25–27).
A variety of ligands and receptors can activate the NF-κB signaling
pathway, including TNF and TNF receptor superfamily molecules that
are key to inflammatory responses (24,28).
Amongst these TNF superfamily members, TRAIL is known to be a
potent inducer of apoptosis through activation of inflammatory
signaling pathways (29,30). In the present study, TRAIL induced
cellular damage of HaCaT keratinocytes through NF-κB activation.
Moreover, both moracin O and P significantly protected HaCaT cells
against TRAIL-induced cytotoxicity, possibly through the inhibition
of apoptosis. Considering that NF-κB-mediated inflammation has been
implicated in the regulation of cellular damage by balancing anti-
and pro-apoptotic protein expression (31–34),
moracin O and P may protect against HaCaT cell damage through their
anti-inflammatory activity by targeting the NF-κB pathway. In the
present study, moracin O and P as novel anti-inflammatory compounds
inhibiting cellular damage induced by NF-κB activation.
The aforementioned effects of Sohakuhi extract and
its active components, moracin O and P, suggested that these
compounds may prove beneficial for the treatment of inflammatory
diseases. Although the exact mechanism of action remains to be
determined, the present study demonstrated that both moracin O and
P represent promising phytochemicals that may act as novel
anti-inflammatory or cytoprotective agents through the suppression
of the NF-κB pathway.
Supplementary Material
Supporting Data
Acknowledgements
The authors are grateful to Dr Kei Takahashi
(University of Tokyo) for her technical support.
Funding
The present study was partly supported by The
Ronpaku Program of Japan Science and Promotion of Science (grant
no. R11815), The Research Grant from The Research Foundation of
First Bank of Toyama, The Grant-in-Aid for Scientific Research on
Innovative Areas (grant no. 17H06398), The Ministry of Education,
Culture, Sports, Science and Technology of Japan, and The
Cooperative Research Project from The Institute of Natural
Medicine, University of Toyama.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BH, LU and FL performed the experiments and analyzed
the data. SY and YH analyzed and interpreted the data. BH and YH
wrote the manuscript and YH designed the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviation
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
References
|
1
|
Fachrudin N, Waltenberger B, Cabaracdic M,
Atanasov AG, Malainer C, Schachner D, Heiss EH, Liu R, Noha SM,
Grzywacz AM, et al: Identification of plumericin as apotent new
inhibitor of the NF-kB pathway with anti-inflammatory activity in
vitro and vivo. Br J Pharmacol. 171:1676–1686. 2014.PubMed/NCBI
|
|
2
|
Weis U: Inflammation. Nature.
454:4272008.PubMed/NCBI
|
|
3
|
Tabas I and Glass CK: Anti-Inflammatory
therapy in chronic disease: Challenenges and opportunities.
Science. 339:166–172. 2013.PubMed/NCBI
|
|
4
|
Patel M, Murugananthan and Gowda KPS: In
vivo animal models in preclinical evaluation of anti inflammatory
activity - A review. Int J Pharm Res Allied Sci. 1:01–05. 2012.
|
|
5
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009.PubMed/NCBI
|
|
6
|
Yuan Q, Zhang X, Liu Z, Song S, Xue P,
Wang J and Ruan J: Ethanol extract of adiantum capillus-veneris L.
Suppress the production of inflammatory mediator by inhibiting
NF-kB activation. J Ethnopharmacol. 147:603–611. 2013.PubMed/NCBI
|
|
7
|
Giuliani C, Bucci I and Napolitano G: The
role of the transcription factor nuclear factor-kappa B in thyroid
autoimmunity and cancer. Front Endocrinol (Lausanne).
9:4712018.PubMed/NCBI
|
|
8
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006.PubMed/NCBI
|
|
9
|
De Bont R and van Larebeke N: Endogenous
DNA damage in humans: A review of quantitative data. Mutagenesis.
19:169–185. 2004.PubMed/NCBI
|
|
10
|
Deng T, Lyon CJ, Bergin S, Caligiuri MA
and Hsueh WA: Obesity, inflammation, and cancer. Annu Rev Pathol.
11:421–449. 2016.PubMed/NCBI
|
|
11
|
Takahashi K, Takeda K, Saiki I, Irimura T
and Hayakawa Y: Functional roles of tumor necrosis factor-related
apoptosis-inducing ligand-DR5 interaction in B16F10 cells by
activating the nuclear factor-κB pathway to induce metastatic
potential. Cancer Sci. 104:558–562. 2013.PubMed/NCBI
|
|
12
|
Takhashi K, Nagai N, Ogura K, Tsuneyama K,
Saiki I, Irimura T and Hayakawa Y: Mammary tissue microenvironment
determines T cell-dependent breast cancer-associated inflammation.
Cancer Sci. 106:867–874. 2015.PubMed/NCBI
|
|
13
|
Flaczyk E, Kobus-Cisowska J, Przeor M,
Korczak J, Remiszewski M, Korbas E and Buchowski M: Chemical
characterization and antioxidative properties of polish variety of
Morus alba L, leaf aqueous extracts from the laboratory and
pilot-scale processes. Agriculture Sci. 4:141–147. 2013.
|
|
14
|
Amarowicz R, Pegg RB and Bautista DA:
Antibacterial activity of green tea polyphenols against escherichia
coli K 12. Nahrung. 44:60–62. 2000.PubMed/NCBI
|
|
15
|
Butt MS, Nazir A, Sultan MT and Schoen K:
Morus alba L.Nature's functioning tonic. Trends Food SciTechnol.
19:505–512. 2008.
|
|
16
|
Fukai T, Hano Y, Hirakura K, Nomura T,
Uzawa J and Fukushima K: Structures of two natural hypotensive
Diels-Alder type adducts, mulberrofuran F and G, from the
cultivated mulberry tree (Morus lhou KOIDZ). Chem Pharm Bull
(Tokyo). 33:3195–3204. 1985.PubMed/NCBI
|
|
17
|
Ueda S, Matsumoto J and Nomura T: Four new
natural diels-alder type adducts, Mulberrofuran E, Kuwanon Q, R,
and V from callus culture of Morus Alba, L. Chem Pharm Bull.
32:350–353. 1984.
|
|
18
|
Fukai T, Hano Y, Hirakura K, Nomura T and
Uzawa J: Structure of mulberrofuran H, A Novel 2-arylbenzofuran
derivative from the cultivated mulberry tree (Morus lhou (Ser.)
Koidz). Chem Pharm Bull. 32:808–811. 1984.
|
|
19
|
Chung KO, Kim BY, Lee MH, Kim YR, Chung
HY, Park JH and Moon JO: In-vitro and in-vivo anti-inflammatory
effect of oxyresveratrol from morus alba L. J Pharm Pharmacol.
55:1695–1700. 2003.PubMed/NCBI
|
|
20
|
Yang ZG, Matsuzaki K, Takamatsu S and
Kitanaka S: Inhibitory effects of constituents from Morus
alba var. multicaulis on differentiation of 3T3-L1 cells
and nitric oxide production in RAW264.7 cells. Molecules.
16:6010–6022. 2011.PubMed/NCBI
|
|
21
|
Kaur N, Xia Y, Jin Y, Dat NT, Gajulapati
K, Choi Y, Hong YS, Lee JJ and Lee K: The first total synthesis of
moracin O and moracin P, and establishment of the absolute
configuration of moracin O. Chem Commun (Camb). 14:1879–1881.
2009.
|
|
22
|
Dat NT, Jin X, Lee K, Hong YS, Kim YH and
Lee JJ: Hypoxia-inducible factor-1 inhibitory benzofurans and
chalcone-derived diels-alder adducts from Morus species. J Nat
Prod. 72:39–43. 2009.PubMed/NCBI
|
|
23
|
Xia Y, Jin Y, Kaur N, Choi Y and Lee K:
HIF-1α inhib-itors: Synthesis and biological evaluation of novel
moracin O and P analogs. Eur J Med Chem. 46:2386–2396.
2011.PubMed/NCBI
|
|
24
|
Khyade VB, Khyade VV, Khyade SV and Moser
MB: Influence of Moracin on DMBA-TPA induced skin tumerigenesis in
the mouse. Int J Bioassays. 3:3509–3516. 2014.
|
|
25
|
Baldwing AS Jr: The NF-kappaB and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996.PubMed/NCBI
|
|
26
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866.
1999.PubMed/NCBI
|
|
27
|
Harper N, Farrow SN, Kaptein A, Cohen GM
and MacFarlane M: Modulation of tumor necrosis factor
apoptosis-inducing ligand-induced NF-kappaB activation by
inhibition of apical caspases. J Biol Chem. 276:34743–34752.
2001.PubMed/NCBI
|
|
28
|
Hu WH, Johnson H and Shu HB: Tumor
necrosis factor-related apoptosis inducing ligand receptors signal
NF-kappaB and JNK activation and apoptosis through distinct
pathways. J Biol Chem. 274:30603–30610. 1999.PubMed/NCBI
|
|
29
|
Jeremias I and Debatin KM: TRAIL induces
apoptosis and activation of NF-kappaB. Eur Cytokine Netw.
9:687–688. 1998.PubMed/NCBI
|
|
30
|
Keane MM, Rubinstein Y, Cuello M,
Ettenberg SA, Banerjee P, Nau MM and Lipkowitz S: Inhibition of
NF-kappaB activity enhances TRAIL mediated apoptosis in breast
cancer cell lines. Breast Cancer Res Treat. 64:211–219.
2000.PubMed/NCBI
|
|
31
|
Kim YS, Schwabe RF, Qian T, Lemasters JJ
and Brenner DA: TRAIL mediated apoptosis requires NF-kappaB
inhibition and the mitochondrial permeability transition in human
hepatoma cells. Hepatology. 36:1498–1508. 2002.PubMed/NCBI
|
|
32
|
Oya M, Ohtsubo M, Takayanagi A, Tachibana
M, Shimizu N and Murai M: Constitutive activation of nuclear
factor-kappaB prevents TRAIL-induced apoptosis in renal cancer
cells. Oncogene. 20:3888–3896. 2001.PubMed/NCBI
|
|
33
|
Southall MD, Isenberg JS, Nakshatri H, Yi
Q, Pei Y, Spandau DF and Travers JB: The platelet-activating factor
receptor protects epidermal cells from tumor necrosis factor (TNF)
alpha and TNF-related apoptosis-inducing ligand-induced apoptosis
through an NF-kappa B-dependent process. J Biol Chem.
276:45548–45554. 2001.PubMed/NCBI
|
|
34
|
Wang P, Du B, Yin W, Wang X and Zhu W:
Resveratrol attenuates CoCl2-induced cochlear hair cell
damage through upregulation of sirtuin1 and NF-κB deacetylation.
PLoS One. 8:e808542013.PubMed/NCBI
|