Introduction
Periodontitis (PD) is a chronic inflammatory disease
that leads to progressive destruction of the periodontal ligament
and alveolar bone and even causes the teeth to become loose and
fall out (1). Currently, the
incidence of chronic periodontitis is >90% in China, posing a
serious threat to human oral health (2). Soft-tissue and bone-tissue
destruction in PD caused by prolonged inflammation is initiated by
bacterial colonization and invasion around teeth near the bottom of
the periodontal pocket. It is well known that dental bacterial
biofilms are the initiating factor of PD (3). Among these pathogenic bacteria,
Porphyromonas gingivalis (P. gingivalis) and
Prevotella intermedia (P. intermedia) have been
indicated to have a strong relationship with PD initiation and
progression (4,5). P. gingivalis is a key
pathogenic factor in PD that accounts for a majority of periodontal
tissue damage (6,7), and P. intermedia is frequently
isolated from dental plaques of patients with periodontal diseases.
P. intermedia is also associated with other oral infections,
including pregnancy gingivitis (8,9).
Therefore, PD is associated with P. intermedia and P.
gingivalis. These species are considered periodontal pathogens
that invade periodontal pockets and are frequently associated with
periodontal breakdown (10–12).
Typical initial therapy for PD involves mechanical and
nanotechnological methods and near-infrared photodynamic therapy to
clean bacterial plaques (13,14).
However, complete elimination of pathogenic bacteria by mechanical
cleaning is impossible, as certain pathogens can may be embedded in
soft tissue (15).
Nanotechnological methods may cause damage to the body; however,
this remains uncertain at present. Hence, drug application is an
important adjuvant therapy for PD. There are multiple
anti-microbial options, such as metronidazole, chlorhexidine,
minocycline, doxycycline and tetracycline, but these may result in
drug resistance and oral dysbacteriosis (16).
Numerous natural products from Traditional Chinese
Medicine have been indicated to be suitable for the treatment of PD
due to their anti-bacterial effects, including herbal compounds
(17), Morus alba leaves
(18), psoralen and angelicin
(19). Diosgenin (Dios) is a
naturally occurring steroidal sapogenin and is one of the major
bioactive compounds in dietary fenugreek seeds (Fig. 1). Dioscin, as a derivative of Dios,
has anti-Candida efficiency (20). Dios has a unique structural
similarity to estrogen. In addition to being a lactation aid, Dios
has been indicated to have hypocholesterolemic (21), gastro- and hepatoprotective
(22), anti-oxidative (23), anti-inflammatory, anti-diabetic and
anti-tumorigenic effects (24).
These notable biological properties of Dios (e.g., anti-oxidative,
anti-inflammatory, anti-diabetic and anti-osteoclastogenic) make it
suitable for the treatment of PD. However, whether Dios has
efficacy against PD-associated pathogenic bacteria (e.g., P.
intermedia and P. gingivalis) has remained to be
examined. As a potential novel clinical therapeutic application for
the prevention and treatment PD, it is worthwhile to study the
anti-bacterial activity of Dios, e.g. by investigating its
anti-bacterial effects on PD-associated bacteria.
In the present study, the anti-bacterial effects of
Dios on P. gingivalis and P. intermedia were
evaluated by a direct contact test (DCT), the Cell Counting Kit
(CCK)-8 assay and counting of colony-forming units (CFU) in
vitro. In addition, the anti-bacterial biofilm effects of Dios
on P. gingivalis and P. intermedia were determined by
live/dead cell staining in vitro.
Materials and methods
Bacterial preparation
The anti-bacterial properties of Dios (cat. no.
CSN12576; CSNpharm) were evaluated using P. gingivalis [no.
American Type Culture Collection (ATCC)33227] and P.
intermedia (no. ATTC 25671; both from ATCC) as model
gram-negative bacteria. Glycerol stock solutions were used to
inoculate defined overnight cultures in tryptic soy broth (TSB;
Biti Medical Device Co., Ltd.) medium under anaerobic conditions
(80% N2, 10% H2 and 10% CO2) at
37°C. One milliliter of each cell suspension was subcultured and
harvested during the exponential growth phase. Subsequently, 100 µl
of the P. gingivalis and P. intermedia solutions in a
96-well plate were monitored in a microplate spectrophotometer
(Power Wave XS2; BioTek Instruments, Inc.) at 600 nm and samples
with an optical density (OD) of ~0.12 were used in the following
experiments. A 0.2% chlorhexidine (CHX) solution was used to
establish a positive control group (25,26).
DCT
The test compounds were prepared at a concentration
of 25 µM in anhydrous ethanol. A total of 90 μl TSB with
different dilutions of Dios was added to a 96-well microplate and
10 µl bacterial suspension (prepared OD=0.12) was added.
Subsequently, the plate with final concentrations of Dios of 1–100
µmol/l was measured in a microplate spectrophotometer. Wells
containing media inoculated with bacteria but without compound were
used as a control group. Each sample contained 0.1% (v/v) anhydrous
ethanol. The 96-well microplates were incubated at 37°C in an
anaerobic incubator (80% N2, 10% H2 and 10%
CO2) and the absorbance of the 96-well microplate was
read every 30 min in a microplate spectrophotometer to determine
the absorbance value at 600 nm. Bacteria were treated with
different concentrations of Dios for 2 h. Each group contained 5
replicate wells and the experiment was repeated three times.
CCK-8 assay
A CCK-8 assay was used to detect the viability of
the bacteria in the present study. A total of 10 μl of P.
gingivalis or P. intermedia bacteria (prepared OD=0.12)
was cultured with 90 µl fresh TSB medium containing different
concentrations of Dios. According to the bacterial dynamics, after
culturing for 120 min in an anaerobic incubator at 37°C, CCK-8
solution (10 µl/well) was added to the 96-well plate. After
co-incubating for 30 min at a normal temperature in the dark, the
96-well plate was placed in a microplate spectrophotometer to
determine the absorption value at 450 nm, which reflected the
number of live cells in each well. Each group contained 5 replicate
wells. Cell viability was expressed as the mean ± standard
deviation (SD) of the absorbance for five wells for each group. The
experiment was repeated three times.
CFU assay
The anti-bacterial activity of Dios against P.
gingivalis and P. intermedia was determined by
spread-plate CFU counting. The resulting colonies were counted to
determine the CFUs and the growth inhibitory activity of the drug.
The bacterial suspensions was incubated with Dios at different
concentrations. Subsequently, 10 µl of 10-fold serial dilutions of
the bacteria at different concentrations were plated onto brain
heart infusion (BHI; Difco) agar plates and the plates were further
incubated for 24 h at 37°C under anaerobic conditions, with 3
replicate plates in each group. The colonies were counted after
incubation at 37°C for 24 h. Representative images of the BHI agar
plates were acquired with an iPhone 7 Plus (Apple Inc.). Data from
three replicate plates were acquired and the CFU count log
reduction was calculated using GraphPad Prism 7 (GraphPad Software,
Inc.). All experiments were performed under anaerobic
conditions.
Biofilm viability
Cell slides were placed at the bottom of a 24-well
plate. Subsequently, 300 µl of a P. gingivalis or P.
intermedia suspension was cultured on each cell slide. After
static growth for 1 h at 37°C under anaerobic conditions, 2 ml
fresh TSB culture solution was added to the 24-well plate and the
cells were further cultured in an anaerobic incubator for 24 h to
form bacterial biofilms. After washing with PBS 3 times, 2 ml of
fresh medium containing 25 or 50 µM Dios was added for
cocultivation for 1 h. Experiments with TSB and bacteria but no
compound and with 0.2% CHX and an equal amount of bacterial
suspension were also set up. After gently washing with PBS 3 times,
200 µl of working solution from the LIVE/DEAD BacLight Bacterial
Viability Kit (Shanghai Yeasen Biotechnology Co., Ltd.) was added
into the 24-well plate, followed by incubation in the dark at 37°C
for 15 min. After washing with PBS three times, the biofilm was
imaged using confocal laser scanning microscopy (CLSM; Nikon AI
Plus; Nikon Corp.) at excitation wavelengths of 488 nm (calcein-AM)
and 561 nm (propidium iodide), with dead bacteria stained red and
live bacteria stained green. The data were plotted to analyze the
percent distribution of live and dead bacteria according to green
and red fluorescence intensities. Images were obtained with a 20×
objective and at least three images of randomly selected fields
were collected for each sample.
Statistical analysis
All data were obtained from at least three
independent experiments. Values are expressed as the mean ± SD.
Analysis was performed using one-way ANOVA followed Dunnett's
multiple-comparisons test with GraphPad Prism 7 (GraphPad Software,
Inc.) and Microsoft Excel (Office 365; Microsoft Corp.). P<0.05
was considered to indicate statistical significance.
Results
Effects of Dios on planktonic P.
gingivalis and P. intermedia
The anti-bacterial activity of Dios against P.
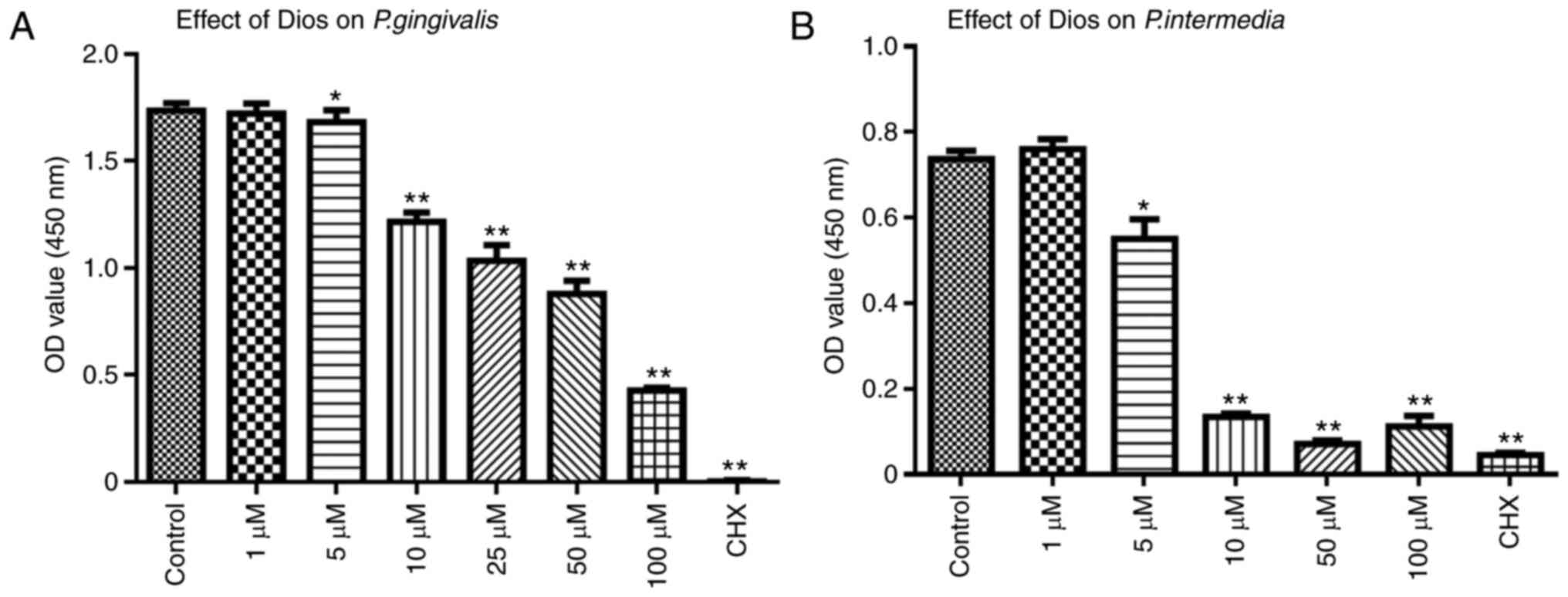
gingivalis and P. intermedia was assessed by a DCT. As
presented in Fig. 2, it was
demonstrated that the growth of P. gingivalis or P.
intermedia was inhibited after incubation with Dios for 60, 90
and 120 min. Furthermore, increasing concentrations of Dios led to
increasingly obvious growth inhibition of P. gingivalis and
P. intermedia (5–100 µM, P<0.05). However, there was no
significant difference between the control group and the 1 µM group
(P>0.05). In summary, the growth of P. gingivalis or
P. intermedia was dose-dependently inhibited by Dios.
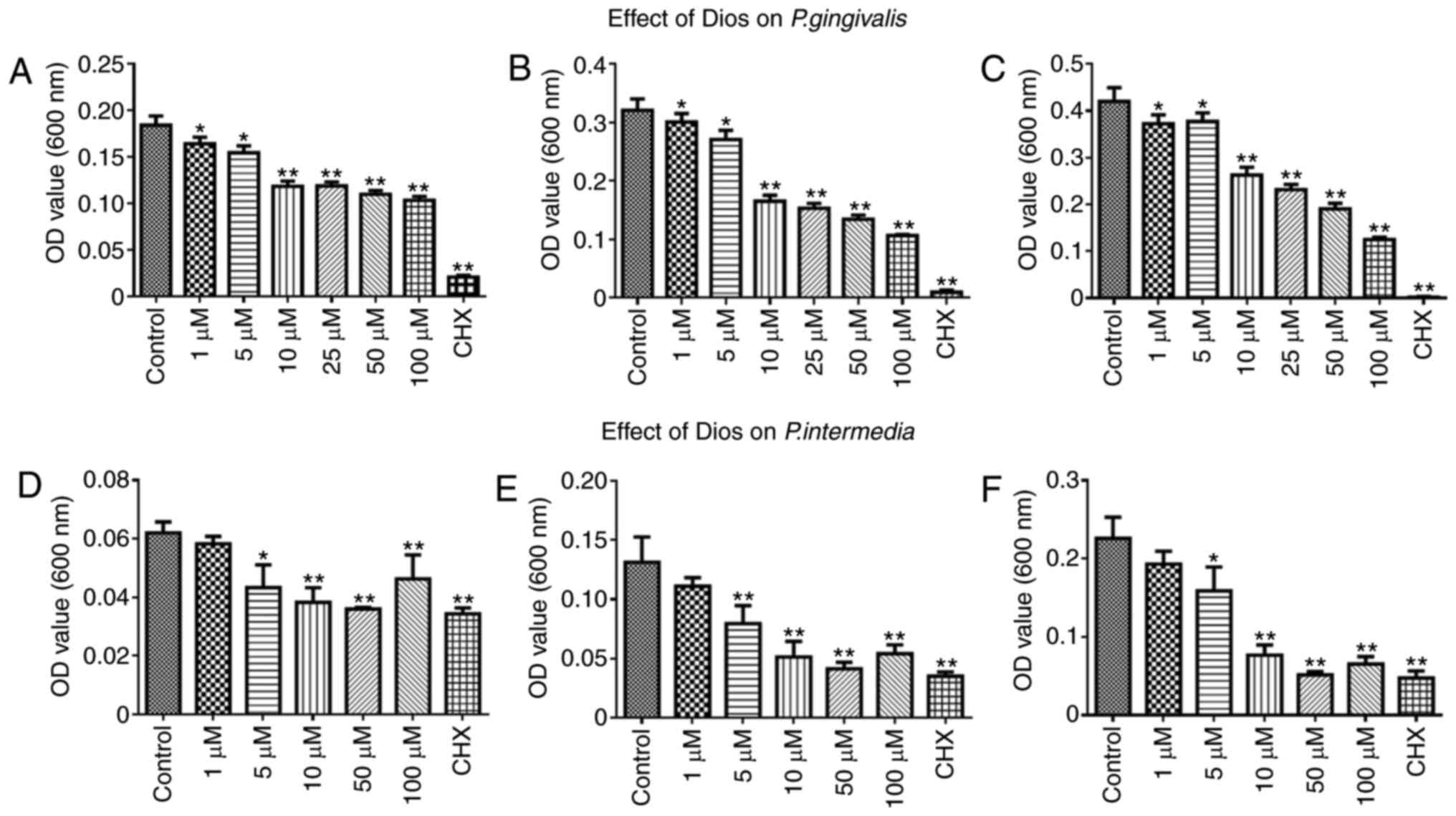 | Figure 2.Effect of Dios on P.
gingivalis and P. intermedia determined by a direct
contact test. Effect of Dios against P. gingivalis over
three periods: (A) 60 min, (B) 90 min, (C) 120 min; effect of Dios
against P. intermedia over three periods: (D) 60 min, (E) 90
min, (F) 120 min. Bacterial suspensions in tryptic soy broth
without Dios were used as a control. Data are presented as the mean
± SD (n=5). *P<0.05, **P<0.01 vs. control group. Dios,
diosgenin; OD, optical density; CHX, chlorhexidine; P.
gingivalis, Porphyromonas gingivalis; P. intermedia,
Prevotella intermedia. |
Effects of Dios on planktonic P.
gingivalis and P. intermedia
The anti-bacterial activity of Dios was further
confirmed by the CCK-8 assay. As presented in Fig. 3, the bacterial activity decreased
after treatment with Dios and was negatively correlated with the
dose of Dios. Consistent with the previous results, 1 µM Dios did
not have any marked effects on bacterial activity; furthermore, the
growth of P. gingivalis or P. intermedia was
dose-dependently inhibited by Dios.
Effects of Dios on planktonic P.
gingivalis and P. intermedia
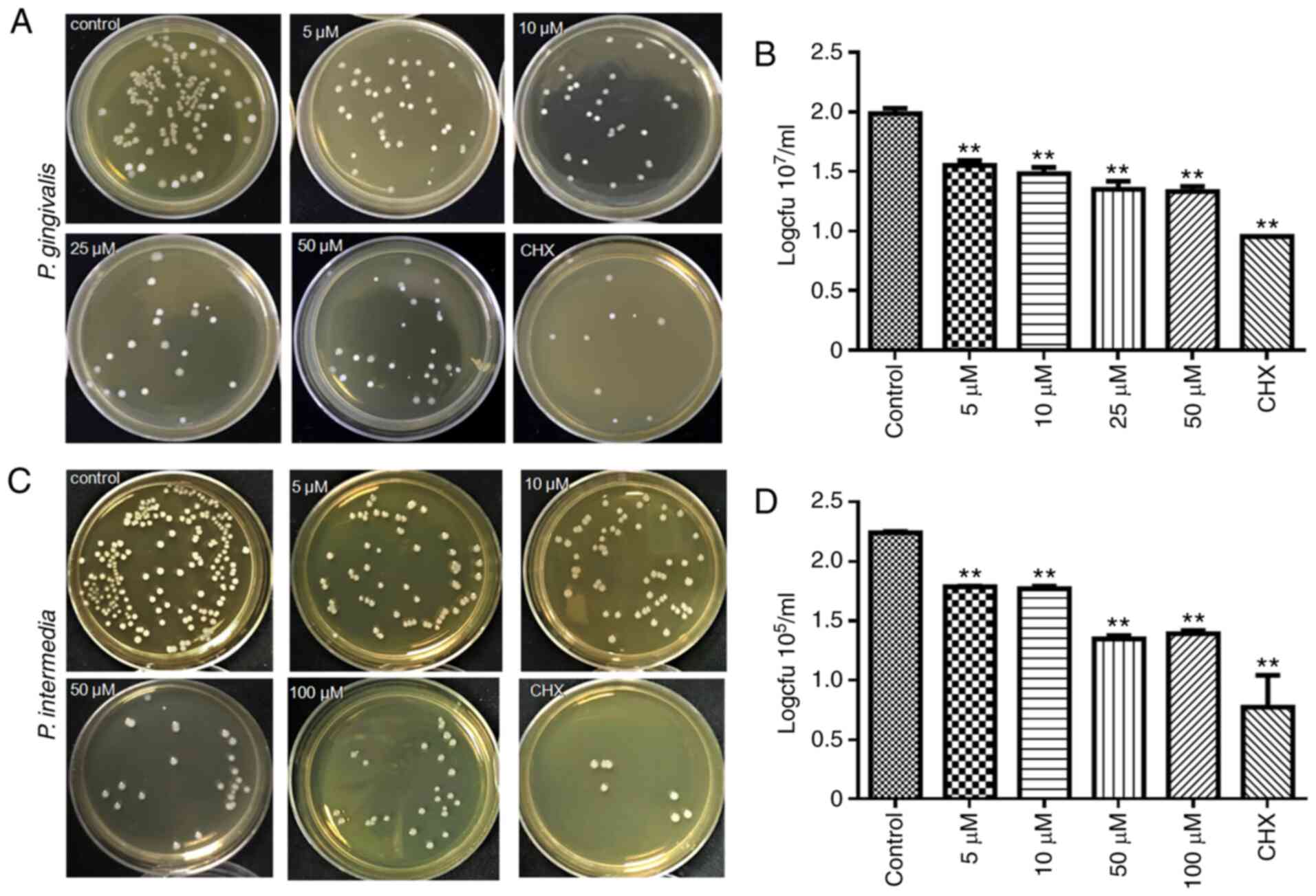
To investigate the anti-bacterial activity of Dios
against P. gingivalis or P. intermedia, the CFU
counting method was used (Fig. 4).
After coculture with Dios for 24 h, the number of bacterial CFU was
markedly decreased. As the concentration of Dios increased to 5,
10, 50 and 100 µM, the number of bacterial CFU decreased
correspondingly (Fig. 4A and C).
The log statistics of the clone count indicated a significant
anti-bacterial effect (Fig. 4B and
D). The levels of both P. gingivalis and P.
intermedia in the 5, 10, 50, 25 and 100 µM groups were lower
than those in the control group by one order of magnitude
(P<0.05). The results obtained confirmed the dose-dependent
anti-microbial activity of Dios.
Anti-biofilm effects of Dios
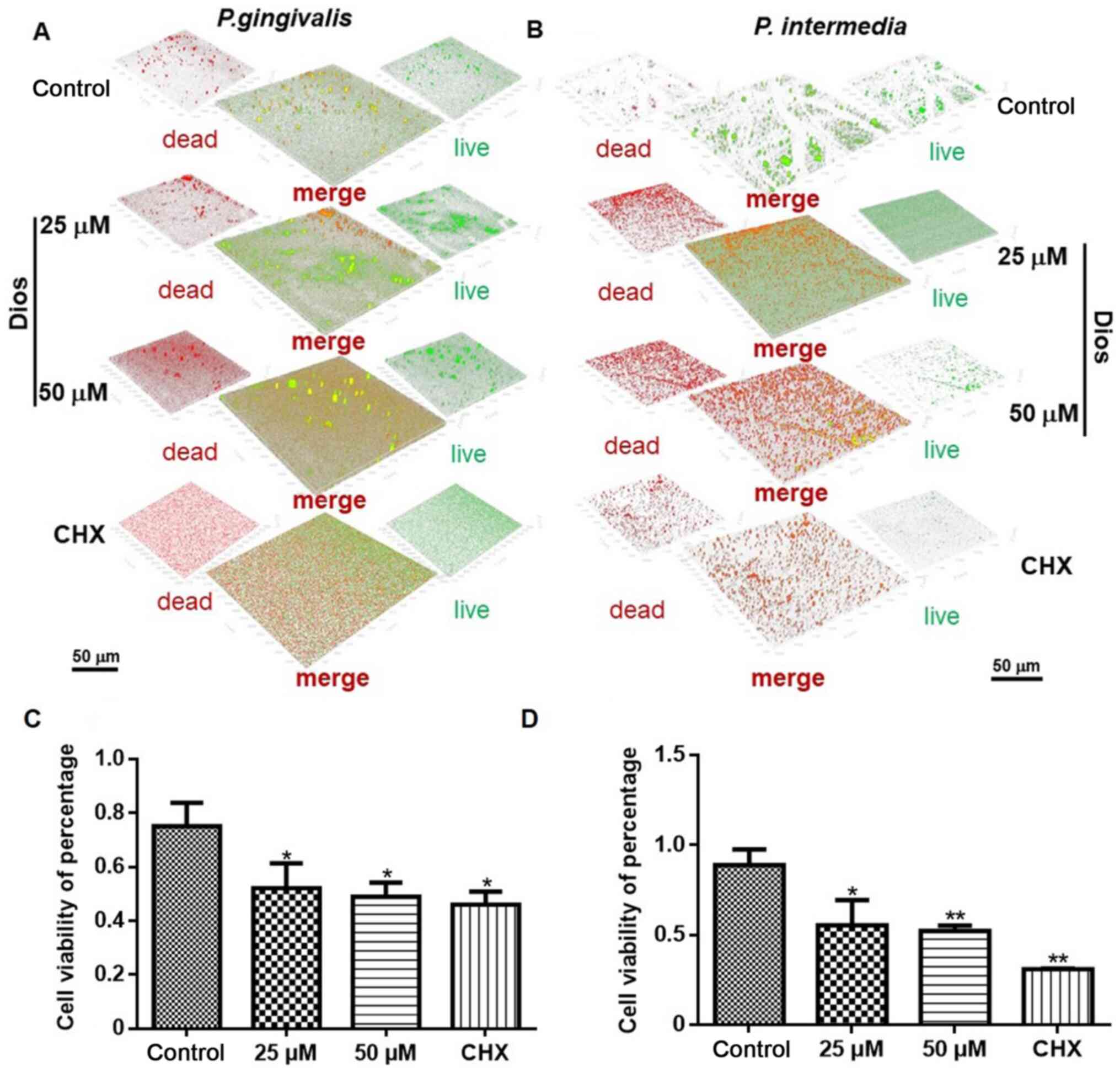
The viability of mature biofilms was investigated by
CLSM. The biofilm viability in the 25 and 50 µM treatment groups
was markedly lower than that in the control group (P<0.05). The
CLSM images displayed the distributions of live (green) and dead
(red) bacteria within the biovolume of interest in the biofilms.
After the bacterial biofilms were treated for 24 h, the control
group displayed mostly green fluorescence (Fig. 5A and B), with cell viabilities of
88.73 and 74.12% for the P. intermedia and P.
gingivalis biofilms, respectively. Approximately 37.84 and
47.68% of the P. intermedia cells had died in the 25 and 50
µM groups, respectively, and 47.73 and 52.91% of the P.
gingivalis cells had died in the 25 and 50 µM groups,
respectively. The proportions of dead P. intermedia and
P. gingivalis cells after incubation with Dios were lower
than those after incubation with 0.2% CHX (68.83 and 56.68%,
respectively), but were obviously higher than those in the control
groups (Fig. 5C and D).
According to the above results, the Dios treatment
groups exhibited superior anti-bacterial effects against P.
intermedia and P. gingivalis compared with those in the
control group, demonstrating the anti-microbial activity of
Dios.
Discussion
PD, induced by oral pathogenic bacteria, is a
chronic inflammatory disease that leads to periodontal bone
destruction and periodontal soft tissue loss (27). Dios is a natural steroid sapogenin
obtained from Dioscorea and potato plants. Glycoside ligands, which
are involved in the synthesis of steroids, have pharmacological
effects, including anti-inflammatory, anti-tumor and anti-oxidant
effects (28). In the present
study, the effects of Dios on two key periodontal pathogens, P.
gingivalis and P. intermedia were examined. The results
suggested that Dios not only inhibited the planktonic growth of
P. gingivalis and P. intermedia but also impaired
P. gingivalis and P. intermedia biofilm viability,
which suggested that Dios may be a novel effective agent for PD
therapy in the future.
Dios, a well-known steroid sapogenin derived from
plants, has been used as a starting material for the production of
steroidal hormones. Dios exhibits a vast range of pharmacological
activities, including cardioprotective, anti-diabetic,
neuroprotective, immunomodulatory, estrogenic and skin protective
effects (29), mainly by
decreasing oxidative stress, preventing inflammatory events
(30), promoting cellular
differentiation/proliferation (31), and regulating the T-cell immune
response (32). Dios inhibits the
production of proinflammatory cytokines (33), enzymes and adhesion molecules
(34). Furthermore, Dios drives
cellular growth/differentiation through the estrogen receptor
cascade and transcriptional factor peroxisome
proliferator-activated receptor γ (35). Dios is also able to reduce
ovariectomy-induced bone loss by enhancing osteoblast genesis and
inhibiting osteoclastogenesis (36) by downregulating Akt signaling
cascades (37). More importantly,
Dios is a naturally occurring steroidal sapogenin that easily
combines with cholesterol in the cell membrane, resulting in
destruction of cell membrane structure and function and promoting
cell dissolution (38). In
addition, this sapogenin is able to effectively prevent DNA
replication and promote phagocytic clearance (39,40),
change voltage-dependent ion channels and destroy the mitochondrial
structure (41). All of these
pharmacological roles are linked to the anti-bacterial effects of
Dios.
Chronic inflammation in PD is difficult to control,
as it is difficult to eliminate oral pathogenic bacteria. In the
present study, it was hypothesized that Dios may be a potential
anti-bacterial medicine for the treatment of PD. Considering the
advantages of high efficiency, low toxicity and lack of microbial
resistance (42), research has
increasingly focused on Traditional Chinese Medicines as
periodontal medications. Furthermore, several plant extracts used
in Traditional Chinese Medicine, such as those of Platycodi Radix,
Paeoniae Radix (43) and burdock
roots (44), were recently
demonstrated to have strong anti-bacterial effects. However, the
impact of Dios on oral pathogens, particularly periodontal
pathogens, has remained elusive. In the present study, the effects
of Dios on two key periodontal pathogens, namely P.
gingivalis and P. intermedia, were examined. The results
of the DCT and CCK-8 assays demonstrated the anti-bacterial effects
of Dios on P. gingivalis and P. intermedia at
concentrations ranging from 5 to 100 µM in vitro. The CFU
counting results further indicated the anti-bacterial effects of
Dios against P. gingivalis and P. intermedia in
vitro. Relative to planktonic bacteria, it is well known that
bacterial biofilms are more challenging to eradicate. In the
present study, bacterial biofilm models of P. gingivalis and
P. intermedia were constructed in vitro. The CLSM
results suggested that bacterial biofilm viability was much lower
after treatment with an appropriate concentration of Dios. All of
these results proved the anti-bacterial effects of Dios on P.
gingivalis and P. intermedia. However, the mechanism
underlying the anti-bacterial effects remains to be determined.
Considering the complexity of bacterial biofilms in PD, further
studies should be performed to investigate the influence of Dios on
dental plaque biofilms, which contain numerous other
microorganisms.
In conclusion, Dios inhibited the growth of P.
gingivalis and P. intermedia as planktonic bacteria and
in biofilms in vitro, which suggested that this compound may
have potential applications in PD therapy. However, the
anti-bacterial mechanisms and biocompatibility require further
research prior to the clinical application of Dios in PD
treatment.
Acknowledgements
The authors thank Professor Lijun Luo from the
Department of Periodontology, Affiliated Hospital of Stomatology,
Tongji University (Shanghai, P.R. China) for providing P.
gingivalis and Professor Xiujun Zhang from Oral Medicine
Research Center Affiliated Shandong University (Jinan, P.R. China)
for providing P. intermedia.
Funding
This study was supported by grants from the Science
Foundation of Shanghai Health and Family Planning Commission (grant
no. 20184Y0110) the Science Foundation of Shanghai Health and
Family Planning Commission (grant no. 19ZR1439600) and the Shanghai
Jiading District Health and Family Planning Commission (grant no.
2019-KY-ZYY-08 XX).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQ and QT designed the experiments. SC performed the
experiments and conducted the statistical analysis of the data. SQ,
SC and XZ drafted the manuscript. XZ, TS and QP helped with the
bacterial preparation, collected the data and performed statistical
analyses. SQ, SC and YX acquired funding. YX analyzed and
interpreted the data, and drafted and edited the manuscript. All
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Page RC and Kornman KS: The pathogenesis
of human periodontitis: An introduction. Periodontol 2000. 14:9–11.
1997.PubMed/NCBI
|
|
2
|
Ravald N and Johansson CS: Tooth loss in
periodontally treated patients: A long-term study of periodontal
disease and root caries. J Clin Periodontol. 39:73–79.
2012.PubMed/NCBI
|
|
3
|
Minty M, Canceil T, Serino M, Burcelin R,
Terce F and Blasco-Baque V: Oral microbiota-induced periodontitis:
A new risk factor of metabolic diseases. Rev Endocr Metab Disord.
20:449–459. 2019.PubMed/NCBI
|
|
4
|
How KY, Song KP and Chan KG:
Porphyromonas gingivalis: An overview of periodontopathic
pathogen below the gum line. Front Microbiol. 7:532016.PubMed/NCBI
|
|
5
|
Reddy PRT, Vandana KV and Prakash S:
Antibacterial and anti-inflammatory properties of plantago ovata
forssk. Leaves and seeds against periodontal pathogens: An in vitro
study. Ayu. 39:226–229. 2018.PubMed/NCBI
|
|
6
|
Hajishengallis G, Chavakis T,
Hajishengallis E and Lambris JD: Neutrophil homeostasis and
inflammation: Novel paradigms from studying periodontitis. J Leukoc
Biol. 98:539–548. 2015.PubMed/NCBI
|
|
7
|
Van der Velden U, Abbas F, Armand S, Loos
BG, Timmerman MF, Van der Weijden GA, Van Winkelhoff AJ and Winkel
EG: Java project on periodontal diseases. The natural development
of periodontitis: Risk factors, risk predictors and risk
determinants. J Clin Periodontol. 33:540–548. 2006.PubMed/NCBI
|
|
8
|
Raber-Durlacher JE, van Steenbergen TJ,
Van der Velden U, de Graaff J and Abraham-Inpijn L: Experimental
gingivitis during pregnancy and post-partum: Clinical,
endocrinological, and microbiological aspects. J Clin Periodontol.
21:549–558. 1994.PubMed/NCBI
|
|
9
|
Kornman KS and Loesche WJ: The subgingival
microbial flora during pregnancy. J Periodontal Res. 15:111–122.
1980.PubMed/NCBI
|
|
10
|
Kolenbrander PE, Palmer RJ Jr, Periasamy S
and Jakubovics NS: Oral multispecies biofilm development and the
key role of cell-cell distance. Nat Rev Microbiol. 8:471–480.
2010.PubMed/NCBI
|
|
11
|
Periasamy S and Kolenbrander PE:
Mutualistic biofilm communities develop with Porphyromonas
gingivalis and initial, early, and late colonizers of enamel. J
Bacteriol. 191:6804–6811. 2009.PubMed/NCBI
|
|
12
|
Socransky SS, Haffajee AD, Cugini MA,
Smith C and Kent RL Jr: Microbial complexes in subgingival plaque.
J Clin Periodontol. 25:134–144. 1998.PubMed/NCBI
|
|
13
|
Graziani F, Karapetsa D, Alonso B and
Herrera D: Nonsurgical and surgical treatment of periodontitis: How
many options for one disease? Periodontol 2000. 75:152–188.
2017.PubMed/NCBI
|
|
14
|
Qi M, Li X, Sun X, Li C, Tay FR, Weir MD,
Dong B, Zhou Y, Wang L and Xu HHK: Novel nanotechnology and
near-infrared photodynamic therapy to kill periodontitis-related
biofilm pathogens and protect the periodontium. Dent Mater.
35:1665–1681. 2019.PubMed/NCBI
|
|
15
|
Slots J: Periodontitis: Facts, fallacies
and the future. Periodontol 2000. 75:7–23. 2017.PubMed/NCBI
|
|
16
|
Pretzl B, Sälzer S, Ehmke B, Schlagenhauf
U, Dannewitz B, Dommisch H, Eickholz P and Jockel-Schneider Y:
Administration of systemic antibiotics during non-surgical
periodontal therapy-a consensus report. Clin Oral Investig.
23:3073–3085. 2019.PubMed/NCBI
|
|
17
|
Moro MG, Silveira Souto ML, Franco GCN,
Holzhausen M and Pannuti CM: Efficacy of local phytotherapy in the
nonsurgical treatment of periodontal disease: A systematic review.
J Periodontal Res. 53:288–297. 2018.PubMed/NCBI
|
|
18
|
Gunjal S, Ankola AV and Bhat K: In vitro
antibacterial activity of ethanolic extract of Morus alba
leaf against periodontal pathogens. Indian J Dent Res. 26:533–536.
2015.PubMed/NCBI
|
|
19
|
Li X, Yu C, Hu Y, Xia X, Liao Y, Zhang J,
Chen H, Lu W, Zhou W and Song Z: New application of psoralen and
angelicin on periodontitis with anti-bacterial, anti-inflammatory,
and osteogenesis effects. Front Cell Infect Microbiol.
8:1782018.PubMed/NCBI
|
|
20
|
Yang L, Liu X, Zhong L, Sui Y, Quan G,
Huang Y, Wang F and Ma T: Dioscin inhibits virulence factors of
Candida albicans. Biomed Res Int.
2018:46517262018.PubMed/NCBI
|
|
21
|
Tikhonova MA, Yu CH, Kolosova NG,
Gerlinskaya LA, Maslennikova SO, Yudina AV, Amstislavskaya TG and
Ho YJ: Comparison of behavioral and biochemical deficits in rats
with hereditary defined or D-galactose-induced accelerated
senescence: Evaluating the protective effects of diosgenin.
Pharmacol Biochem Behav. 120:7–16. 2014.PubMed/NCBI
|
|
22
|
Chen Z, Xu J, Wu Y, Lei S, Liu H, Meng Q
and Xia Z: Diosgenin inhibited the expression of TAZ in
hepatocellular carcinoma. Biochem Biophys Res Commun.
503:1181–1185. 2018.PubMed/NCBI
|
|
23
|
Sethi G, Shanmugam MK, Warrier S, Merarchi
M, Arfuso F, Kumar AP and Bishayee A: Pro-apoptotic and anti-cancer
properties of diosgenin: A comprehensive and critical review.
Nutrients. 10:6452018.
|
|
24
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. 137:259–269. 2018.PubMed/NCBI
|
|
25
|
Zand F, Zahed L, Mansouri P, Dehghanrad F,
Bahrani M and Ghorbani M: The effects of oral rinse with 0.2 and 2%
chlorhexidine on oropharyngeal colonization and ventilator
associated pneumonia in adults' intensive care units. J Crit Care.
40:318–322. 2017.PubMed/NCBI
|
|
26
|
Haydari M, Bardakci AG, Koldsland OC, Aass
AM, Sandvik L and Preus HR: Comparing the effect of 0.06 -, 0.12
and 0.2% Chlorhexidine on plaque, bleeding and side effects in an
experimental gingivitis model: A parallel group, double masked
randomized clinical trial. BMC Oral Health. 17:1182017.PubMed/NCBI
|
|
27
|
Kinane DF, Stathopoulou PG and Papapanou
PN: Periodontal diseases. Nat Rev Dis Primers.
3:170382017.PubMed/NCBI
|
|
28
|
Selim S and Al Jaouni S:
Anti-inflammatory, antioxidant and antiangiogenic activities of
diosgenin isolated from traditional medicinal plant, Costus
speciosus (Koen ex.Retz.) Sm. Nat Prod Res. 30:1830–1833.
2016.PubMed/NCBI
|
|
29
|
Chen Y, Tang YM, Yu SL, Han YW, Kou JP,
Liu BL and Yu BY: Advances in the pharmacological activities and
mechanisms of diosgenin. Chin J Nat Med. 13:578–587.
2015.PubMed/NCBI
|
|
30
|
Kiasalari Z, Rahmani T, Mahmoudi N,
Baluchnejadmojarad T and Roghani M: Diosgenin ameliorates
development of neuropathic pain in diabetic rats: Involvement of
oxidative stress and inflammation. Biomed Pharmacother. 86:654–661.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu L, Dong H, Zhao J, Wang Y, Yang Q, Jia
C and Ma J: Diosgenin stimulates rat TM4 cell proliferation through
activating plasma membrane translocation and transcriptional
activity of estrogen receptors. Biol Reprod. 92:242015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang CH, Liu DZ and Jan TR: Diosgenin, a
plant-derived sapogenin, enhances regulatory T-cell immunity in the
intestine of mice with food allergy. J Nat Prod. 73:1033–1037.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai B, Seong KJ, Bae SW, Chun C, Kim WJ
and Jung JY: A synthetic diosgenin primary amine derivative
attenuates LPS-stimulated inflammation via inhibition of NF-κB and
JNK MAPK signaling in microglial BV2 cells. Int Immunopharmacol.
61:204–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi KW, Park HJ, Jung DH, Kim TW, Park
YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, et al: Inhibition of
TNF-α-induced adhesion molecule expression by diosgenin in mouse
vascular smooth muscle cells via downregulation of the MAPK, Akt
and NF-κB signaling pathways. Vascul Pharmacol. 53:273–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tharaheswari M, Jayachandra Reddy N, Kumar
R, Varshney KC, Kannan M and Sudha Rani S: Trigonelline and
diosgenin attenuate ER stress, oxidative stress-mediated damage in
pancreas and enhance adipose tissue PPARγ activity in type 2
diabetic rats. Mol Cell Biochem. 396:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tao X, Qi Y, Xu L, Yin L, Han X, Xu Y,
Wang C, Sun H and Peng J: Dioscin reduces ovariectomy-induced bone
loss by enhancing osteoblastogenesis and inhibiting
osteoclastogenesis. Pharmacol Res. 108:90–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Srinivasan S, Koduru S, Kumar R,
Venguswamy G, Kyprianou N and Damodaran C: Diosgenin targets
Akt-mediated prosurvival signaling in human breast cancer cells.
Int J Cancer. 125:961–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo N, Tong T, Ren N, Tu Y and Li B:
Saponins from seeds of genus Camellia: Phytochemistry and
bioactivity. Phytochemistry. 149:42–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu K, Berenjian S, Larsson R, Gullbo J,
Nygren P, Lövgren T and Morein B: Nanoparticulate Quillaja
saponin induces apoptosis in human leukemia cell lines with a high
therapeutic index. Int J Nanomedicine. 5:51–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cai BX, Jin SL, Luo D, Lin XF and Gao J:
Ginsenoside Rb1 suppresses ultraviolet radiation-induced apoptosis
by inducing DNA repair. Biol Pharm Bull. 32:837–841. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balestrazzi A, Agoni V, Tava A, Avato P,
Biazzi E, Raimondi E, Macovei A and Carbonera D: Cell death
induction and nitric oxide biosynthesis in white poplar (Populus
alba) suspension cultures exposed to alfalfa saponins. Physiol
Plant. 141:227–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L and Wei W: Anti-inflammatory and
immunoregulatory effects of paeoniflorin and total glucosides of
paeony. Pharmacol Ther. 207:1074522020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Minami M, Takase H, Taira M and Makino T:
In Vitro Effect of the Traditional Medicine Hainosan (Painongsan)
on Porphyromonas gingivalis. Medicines (Basel). 6:582019.
View Article : Google Scholar
|
|
44
|
Gentil M, Pereira JV, Sousa YT, Pietro R,
Neto MD, Vansan LP and de Castro França S: In vitro evaluation of
the antibacterial activity of Arctium lappa as a phytotherapeutic
agent used in intracanal dressings. Phytother Res. 20:184–186.
2006. View Article : Google Scholar : PubMed/NCBI
|