Introduction
As a part of the immune system, the complement
system is initially involved in host defense against pathogens.
Notably, recent research has indicated that the complement system
may also have certain non-immune functions and serve roles in
physiological and pathological processes (1,2); for
example, the complement system was discovered to be involved in the
control of tissue morphogenesis, wound healing and synaptic pruning
(1).
Human C3a anaphylatoxin (C3a), a C3 split fragment
produced during the activation of the complement system, is one of
the complement molecules that have been reported to serve
non-immune roles. C3a was initially reported to be involved in the
chemotaxis, recruitment and activation of inflammatory cells by
binding to the C3a receptor (C3aR) on immune cells (3–5).
However, C3aR has also been demonstrated to be expressed by certain
non-immune cells, including in liver, kidney and nerve cells.
Additionally, C3aR signaling has been revealed to participate in
various physiological and pathological processes, including in the
regulation of metabolism (6,7),
histogenesis (8,9) and tissue repair (10). In the nervous system, C3aR was
reported to be expressed by gliocytes, neural stem cells, neural
progenitors and mature neurons (11–13),
and was found to be involved in the regulation of neurogenesis and
neural regeneration (14).
Notably, C3aR signaling was demonstrated to be directly associated
with the structure of neural development processes. Furthermore,
C3aR has been implicated in axonal sprouting, neurite extension,
synapsis formation and the recovery of injured neural structure and
function (14,15).
Podocytes exhibit an octopus-like dendritic
morphology consisting of a cell body, major processes and foot
processes, similar to neural cells. Foot processes from neighboring
podocytes interdigitate with each other, cover the outer side of
the glomerular basement membrane and constitute one of the most
important components of the glomerular filtration barrier. This
unique architecture is the basis for podocyte function (16). Damage to podocyte structure,
including foot process effacement, which is the retraction and
flattening of foot processes, was confirmed to be directly
associated with proteinuria in kidney diseases and the recovery of
podocyte structure lead to remission (17,18).
Despite the importance of the cellular structure in podocyte
function, the mechanism underlying the management and regulation of
podocyte architecture remains unclear.
C3aR expression in glomerular podocytes has been
reported by several studies (19,20).
However, data concerning the role of C3aR in podocytes is limited.
Considering the morphological similarity between nerve cells and
podocytes, and the role of C3aR in the neurogenesis, particularly
in the regulation of neural morphology, we hypothesized that C3aR
may serve a role in the development and management of podocyte
architecture.
Derived from primarily cultured human glomerular
podocytes, the conditionally immortal human podocyte line (HPC)
mimics the process of podocyte architecture development. During the
proliferation condition, the cells exhibit a typical cobblestone
morphology. In the maturation condition, the cells undergo
proliferation arrest, enlargement of the cell bodies and the
development of the arborized form, with the formation of processes
and a slit diaphragm complex, and the expression of
podocyte-specific markers (21).
To investigate the possible role of C3aR signaling
in the regulation of podocyte architecture development, the current
study examined the effect of C3aR activation on the development of
podocyte morphology by the overexpression of C3a in HPC cells and
discussed the underlying molecular mechanism.
Materials and methods
Cell culture and stable overexpression
of C3a in HPC cells
HPC was provided by Professor M.A. Saleem
(Children's Renal Unit and Academic Renal Unit, University of
Bristol) and cultured as previously described (21). Briefly, the cells were cultured in
RPMI-1640 medium (Dalian Meilun Biology Technology Co., Ltd.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1X Insulin-Transferrin-Selenium (Gibco; Thermo Fisher
Scientific, Inc.) at 33°C for propagation (the proliferation
condition) and at 37°C for differentiation (the maturation
condition).
To obtain HPC cells stably overexpressing secretory
C3a, the cells cultured in the proliferation condition were
transfected with a recombinant lentivirus Lenti-C3a. The lentivirus
was constructed based on a previous study (22), in which mouse secretory C3a was
expressed by inserting the IL-6 signal peptide sequence prior to
the C3a coding sequence. Similarly, a recombinant human C3a gene
IL6 C3a containing the IL-6 signal peptide sequence (117–203 of
NM_000600.3) and C3a coding sequence (2076–2306 of NM_000064.2) was
synthesized. Following this, the expression vector
pLenti6.3-IL6C3a-IRES2-EGFP was constructed by inserting the IL6C3a
gene into the BamHI/AscI site of the
pLenti6.3-MCS-IRES2-EGFP vector (Invitrogen; Thermo Fisher
Scientific, Inc.). Finally, lentivirus Lenti-C3a was produced in
293T cells using the expression vector pLenti6.3-IL6C3a-IRES2-EGFP.
Infection of HPC cells was performed when the cells cultured in the
proliferation condition reached ~50% confluence.
At 2 days post-infection, the transfected cells were
examined under a fluorescence microscope and >80% of the cells
were found to have been transfected successfully. The cells were
transferred to the selection medium containing 10 µg/ml
blasticidin. Two weeks later, stably transfected HPC cells
(HPC-C3a) were obtained. Vector pLenti6.3-MCS-IRES2-EGFP was
directly used to construct the negative control (NC) lentivirus
(Lenti-NC). Infection with Lenti-NC produced the HPC-NC cells.
HPC-C3a cells cultured in medium containing 1 µM SB290157 (Merck
KGaA) were used as the C3aR blocking control.
In the C3a stimulation tests, purified human C3a
(Merck KGaA) was directly added to the cell culture medium once
every two days at 37°C for a total of 6 days at a final
concentration of 100 nM.
Cell morphology observation
To examine the morphological changes in the process
of maturation, HPC cells (including untransfected HPC, HPC-NC,
HPC-C3a and SB290157-treated HPC-C3a cells) were examined under
phase contrast (Leica Microsystems GmbH; magnification, ×200) and
fluorescence microscopes (Zeiss GmbH; magnification, ×400)
following 0, 2, 7 and 14 days of being in the maturation condition.
Given that normal HPC cells do not exhibit fluorescence, only the
morphology of HPC-NC, HPC-C3a and HPC-C3a cells treated with
SB290157 were visualized using fluorescence microscopy.
Cell adhesion assay
Cell adhesion assays were performed in rat tail
collagen I-coated 24-well plates. Following treatment with various
conditions, the cells (HPC, HPC-NC and HPC-C3a) were detached using
Accutase solution (Sigma-Aldrich; Merck KGaA) and 2×105
cells were allowed to attach to the collagen I-coated plates in 0.5
ml RPMI-1640 medium supplemented with 0.1% FBS. Time course
adhesion assays were performed at time points 30 min, 1 h and 3 h
at 37°C in a 5% CO2 incubator. Following this, the
non-adherent cells were collected, and the adherent cells were
detached with Accutase solution. The numbers of both adherent and
non-adherent cells were counted using a cell counter. The cell
adhesion ability was represented by the ratio of adherent cells to
non-adherent cells and was further calibrated to that of
non-treated HPC cells (the adhesion ability of non-treated HPC
cells was defined as 1.0). To compare the adhesive ability between
HPC, HPC-NC, HPC-C3a and SB290157-treated HPC-C3a cells, the cells
were cultured in the maturation condition for 6 days before the
assay began.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from the
cultured HPC, HPC-NC or HPC-C3a cells. cDNA was generated using a
PrimeScriptTM RT Master Mix kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol, and analyzed by RT-qPCR
using 1 µl of cDNA, 250 nM primers and UltraSYBR mixture (CoWin
Biosciences). 18S RNA was used as the endogenous control. The
sequences of the primers are presented in Table I. The following thermocycling
conditions were used to detect the expression levels of nephrin:
Initial denaturation at 95°C for 5 min; followed by 40 cycles of
95°C for 20 sec, 58°C for 10 sec and 72°C for 45 sec; and a final
extension at 72°C for 5 min. The following thermocycling conditions
were used for the rest of the genes: Initial denaturation at 95°C
for 5 min; followed by 40 cycles of 95°C for 20 sec, 60°C for 20
sec and 72°C for 30 sec; and a final extension at 72°C for 5 min.
The expression levels were quantified using the 2−∆∆Cq
method (23) and finally
normalized to the expression levels of in the HPC control cells
(which was set to 1.0).
 | Table I.Primers used for reverse
transcription-PCR. |
Table I.
Primers used for reverse
transcription-PCR.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| COL1A1 |
atcaaccggaggaatttccgt |
caccaggacgaccaggttttc |
| COL4A1 |
cggatcacattgacatgaaacc |
accagtccatgctcgcaaag |
| COL4A2 |
tggacctgatggaaagcgag |
tgctcctttcagcccaaaca |
| COL4A3 |
ggtggcctgagagcctga |
tcacagaagcactggccttt |
| COL4A4 |
ccagcccgcctccaac |
gtgaggttctgtgttctgggt |
| FN1 |
gagaataagctgtaccatcgcaa |
cgaccacataggaagtcccag |
| LAMA1 |
ttagccaccgggaacctaaag |
gccatagcagatacacatgcct |
| LAMB1 |
tgactttcaagacattccgtcc |
aggcgaagtatctatacacaccc |
| LAMC1 |
actgccactgacatcagagta |
gcttgcgtgtccattacatttac |
| LAMA2 |
gccaacgctgagaaacttgag |
gagctccacaaaaccaggct |
| LAMB2 |
agcaatgggcagagttggaa |
ggctcaaggcagacaggatt |
| LAMC2 |
ggagctggagtttgacacgaa |
agcttctgctccagtaagacc |
| LAMA3 |
gggtgctgctcaaagcaaag |
gctgcagtacagaggcttca |
| LAMB3 |
cagtcacagagcaggaggtg |
tctccaggtctctcggaagg |
| LAMC3 |
tacggcaaacagaacccctc |
gatcagccagcagagtcctg |
| ITGA1 |
gtgcttattggttctccgttagt |
cacaagccagaaatcctccat |
| ITGA2 |
tccagagtaacctccagggg |
gccagggttttccagactga |
| ITGA3 |
cccagaggaccaaggaaacc |
attgttcaggtctgccaggg |
| ITGA5 |
ggagaggagcctgtggagta |
cctccttggcagtaaccctg |
| ITGB1 |
gtaaccaaccgtagcaaagga |
tcccctgatcttaatcgcaaaac |
| ITGB3 |
gaagcagagtgtgtcacgga |
acatgacactgcccgtcatt |
| DAG1 |
ggaaggaggctttgccatct |
gaacacactggaggtctggg |
| PTK2 |
catgccctcaaccagggatt |
cacgctgtccgaagtacagt |
| ILK |
gcagcccgagtcccgaggata |
gcgccgagtcccctggattg |
| NRP1 |
gcggacttttccagctctct |
cagttggcctggtcgtcat |
| TLN1 |
agaacggaaacctgccagag |
tgaattgcctggtttgcacg |
| TLN2 |
tgacctaaattggctggatcac |
aacttccgtctaagcagcaac |
| PXN |
catggacgacctcgacgc |
gaggctgctggtggatgaat |
| FERMT2 |
gatgctgccctttcagacct |
ctggtgtgccactggattct |
| VCL |
cgtccgggttggaaaagaga |
gacctcagcctcatcgaagg |
| ACTA2 |
gggactaagacgggaatcct |
gtcacccacgtagctgtctt |
| WT1 |
cccattgccatttggtgtgg |
ccgatgccttgctctctgat |
| TGFB1 |
atggagagaggactgcggat |
tagtgttccccactggtccc |
| 18S RNA |
tttctcgattccgtgggtgg |
agcatgccagagtctcgttc |
| C3aR |
tgaagccttcagctactgtctcag |
ggacaatgatggaggggatgag |
| C3a |
aagtcggcaagtaccccaag |
agttgcagcagtccaggaag |
| CPD |
cccgaccagtttagcaccg |
actgagccaccatgcagattt |
| CPM |
tgtgggttcttgttgtgggg |
agggtctttgccatcactgg |
| CPN1 |
ggtgattacttccggctgct |
cactttggctctgactccgt |
| CPN2 |
acaccagactcctctctgct |
gagcagaacacctcctggac |
| CPZ |
gctccttacagtcctggtcg |
aagtgctctgacagtgtggg |
| Nephrin |
tggcgattcctgcctccgtt |
ttctgctgggagccctcgtt |
Western blotting
Total protein was extracted from the cultured HPC,
HPC-NC or HPC-C3a cells using RIPA lysis buffer (Beyotime Institute
of Biotechnology) containing a Protease Inhibitor Cocktail (Roche
Diagnostics) at 4°C. Total protein was quantified using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology) and 10 µg
protein/lane was separated via 8% SDS-PAGE, then transferred to a
PVDF membrane (Merck KGaA). Following blocking with 5% skim milk
powder at room temperature for 4 h, the membrane was probed with
primary antibodies, including rabbit anti-human C3aR polyclonal
(cat. no. sc-20138; Santa Cruz Biotechnology, Inc.; dilution
1:1,000), rabbit anti-human integrin β1 polyclonal (ITGB1; cat. no.
9699S; Cell Signaling Technology, Inc.; dilution 1:1,500), rabbit
anti-human vinculin (VCL; cat. no. 26520-1-AP; ProteinTech Group,
Inc.; dilution 1:1,000), rabbit anti-human protein tyrosine kinase
2 (PTK2; cat. no. 71433; Cell Signaling Technology, Inc.; dilution
1:1,000) or mouse anti-human GAPDH monoclonal (cat. no. MB001;
Bioworld Technology, Inc; dilution 1:10,000) antibodies at 4°C
overnight. The membranes were then incubated with horseradish
peroxidase (HRP)-conjugated secondary goat anti-rabbit IgG (cat.
no. HS101-01; TransGen Biotech Co., Ltd; dilution 1:2,000) or goat
anti-mouse IgG (cat. no. BS12478; Bioworld Technology, Inc.;
dilution 1:10,000) antibodies for 1 h at room temperature. The
immunolabeled proteins were detected by chemiluminescence using the
Chemiluminescent HRP substrate (Merck KGaA). Densitometric analysis
was performed using ImageJ version 1.52a software (National
Institutes of Health).
Cyto-immunofluorescence
The HPC, HPC-NC or HPC-C3a cells growing on glass
coverslips were washed with PBS, fixed in 4% paraformaldehyde at
4°C for 30 min and permeabilized in 0.5% Triton X-100 at room
temperature for 10 min. Following blocking with 3% BSA (Beyotime
Institute of Biotechnology) for 30 min, the cells were incubated
with rabbit anti-human C3aR (dilution 1:50) or rabbit anti-human
VCL (dilution 1:100) antibodies at room temperature for 2 h. After
washing with PBS three times, the cells were incubated with
Cy3-labled goat anti-rabbit IgG antibodies (cat. no. 33108ES60;
Shanghai Yeasen Biotechnology Co., Ltd.; dilution 1:200) at room
temperature for 30 min. The cells were washed again with PBS and
exposed to DAPI solution (Beyotime Institute of Biotechnology) at
room temperature for 5 min. Following washing with PBS, the cells
were mounted, and fluorescent images were captured with a confocal
microscope (LSM-800; Zeiss GmbH; magnification, ×400). Homologous
serum was used instead of the primary antibody for the control.
Measurement of C3a levels
C3a levels was measured using a human C3a ELISA kit
(BD Biosciences), according to the manufacturer's protocol.
Statistical analyses
Data are presented as the mean ± SEM. SPSS software
(version no. 19.0; SPSS, Inc.) was used for data analysis. Multiple
comparisons were performed using one-way ANOVA and Tukey's post hoc
test was used in the comparison between two groups. All statistical
tests were two-tailed. P<0.05 was considered to indicate a
statistically significant difference.
Results
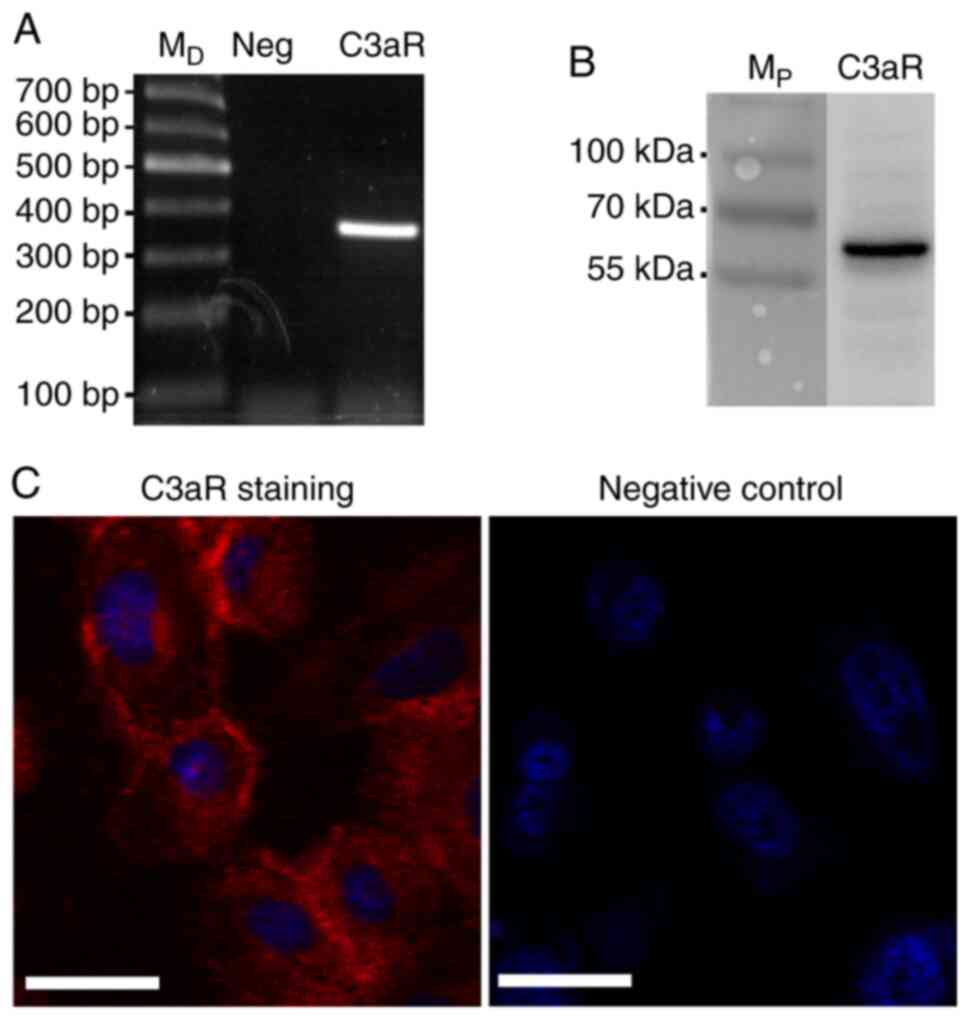
C3aR is expressed by HPC cells
To investigate the effect of C3aR activation in HPC
cells, the expression of C3aR was examined. The results of RT-qPCR,
western blotting and immunofluorescence demonstrated that C3aR was
expressed by HPC cells (Fig. 1).
The expression of C3aR was observed on the cell surface and in the
cytoplasm.
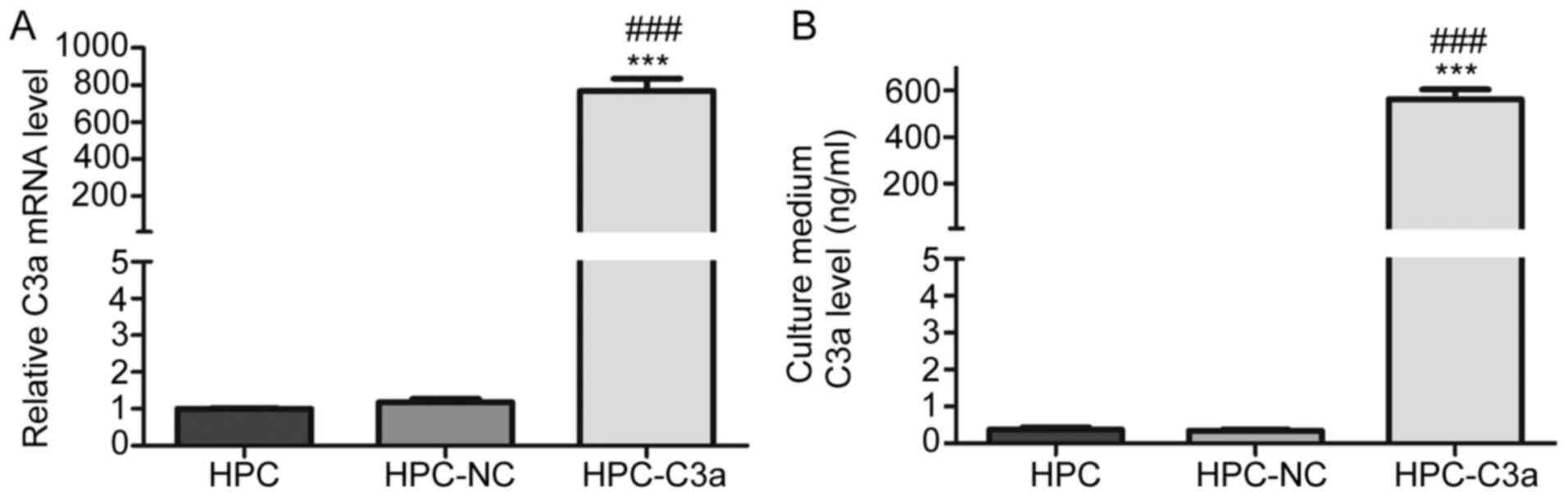
Secretory C3a is overexpressed in
HPC-C3a cells
To determine whether C3a is overexpressed by HPC-C3a
cells, C3a mRNA and protein levels were measured by RT-qPCR and
ELISA, respectively. RT-qPCR results demonstrated that the
expression levels of C3a mRNA was significantly increased in the
HPC-C3a cells compared with HPC-NC and untransfected HPC cells
(768.18±116.11 vs. 1.17±0.16 and 1.00±0.02, respectively; Fig. 2A). Furthermore, the ELISA analysis
reported that the expression of C3a protein was significantly
increased in the cultured medium of HPC-C3a cells compared with the
HPC-NC and untransfected HPC cells (563.63±69.86 vs. 0.34±0.06 and
0.37±0.11 ng/ml, respectively; Fig.
2B).
Influence of overexpression of C3a in
the phenotype of HPC cells during their maturation
As shown in Fig.
3A, at the proliferation condition, HPC cells grew with typical
cobblestone morphology. Shifting the cells to the maturation
condition resulted in the arrest of cell proliferation and
differentiation. The cell bodies enlarged in a time-dependent
manner and various cellular projections developed. Within two
weeks, the cells converted from individual cobblestone morphology
into an arborized appearance, which resembled the morphology of
mature glomerular podocytes in vivo (21). Similar to the untransfected HPC
cells, HPC-NC cells exhibited typical cobblestone morphology in the
proliferation condition and ceased proliferation in the maturation
condition. Additionally, HPC-NC cells became enlarged and developed
arborized morphology within two weeks. No obvious morphological
change was observed in HPC-C3a cells cultured in the proliferation
condition. However, these cells failed to undergo cell body
expansion, which the HPC-NC and untransfected HPC cells underwent
in the maturation condition. HPC-C3a cells cultured in the
maturation condition appeared to exhibit decreased adhesive
capacity and became extremely sensitive to the regular change of
the medium. Increased contracted cells, which could be easily
detached from the surface of the culture plate with gentle shaking,
were observed in the HPC-C3a group starting on the 5th day
following transference to the maturation condition and the regular
change of medium would induce contraction of the cells immediately
(within <30 min). The cell numbers of HPC-C3a decreased markedly
since about the 6th day. Within 2 weeks, most of the HPC-C3a cells
were lost and the cells still remained in culture plate failed to
develop into the arborized appearance as the untreated HPC and
HPC-NC cells did. Addition of SB290157 (SB) blocked the phenotypic
alterations caused by Lenti-C3a infection. The morphological
differences in the formation of the arborized morphology, which was
observed in HPC-NC and SB290157-treated HPC-C3a cells, but not in
HPC-C3a cells, were more clearly pronounced under the fluorescence
microscope since HPC-NC and HPC-C3a cells have fluorescence due to
the expression of EGFP (Fig. 3B).
However, as the untransfected HPC cells have no fluorescence, these
cells were not observed under the fluorescence microscope.
Furthermore, HPC-C3a cells exhibited decreased adhesion capacities
compared with the C3a group, as confirmed by the adhesion assays
(Fig. 3C).
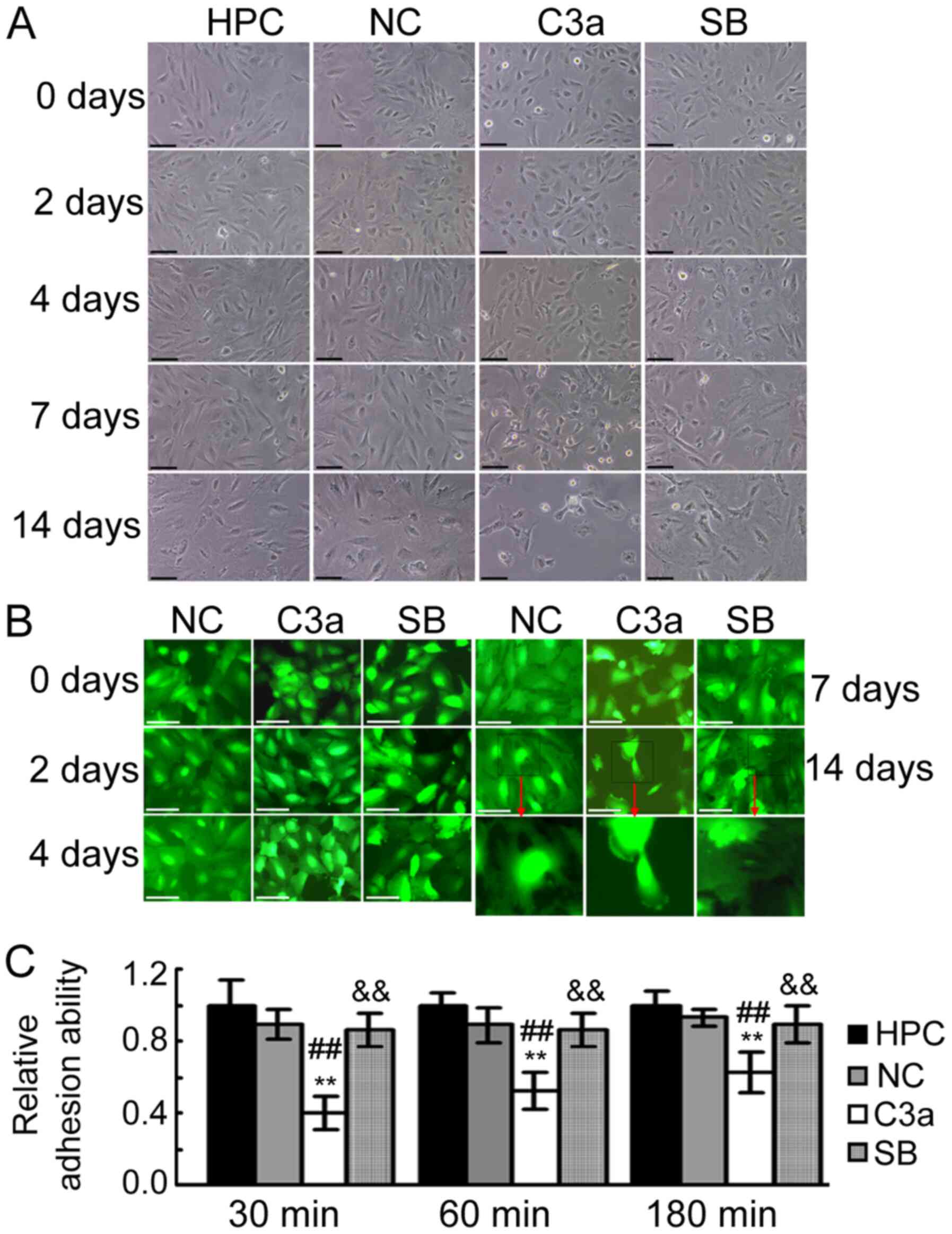 | Figure 3.Influence of C3A anaphylatoxin
overexpression on the morphology and adhesion ability of HPC cells
during maturation. (A) Representative images taken under a phase
contrast microscope demonstrating the morphology of HPC, NC, C3a
cells and C3a cells treated with 1 µM of SB290157 cultured during
the maturation condition for 0, 2, 4, 7 and 14 days. Scale bar, 100
µM. (B) Representative images taken with a fluorescence microscope
revealing the morphology of NC, C3a and SB cells cultured during
the maturation condition for 0, 2, 4, 7 and 14 days. As normal HPC
cells do not exhibit fluorescence, the untransfected HPC cells were
not included in the cell morphology observational experiments under
fluorescence microscopy. Scale bar, 50 µM. (C) Results of adhesion
analysis. The adhesion ability of HPC, NC, C3a and SB cells was
analyzed following culturing in the maturation condition for 6
days. **P<0.01 vs. the HPC group; ##P<0.01 vs. the
NC group; &&P<0.01 vs. the C3a group. C3a,
HPC cells overexpressing human C3A anaphylatoxin; HPC, human
podocyte cell; NC, HPC cells transfected with negative control
vector; SB, C3a cells treated with SB290157. |
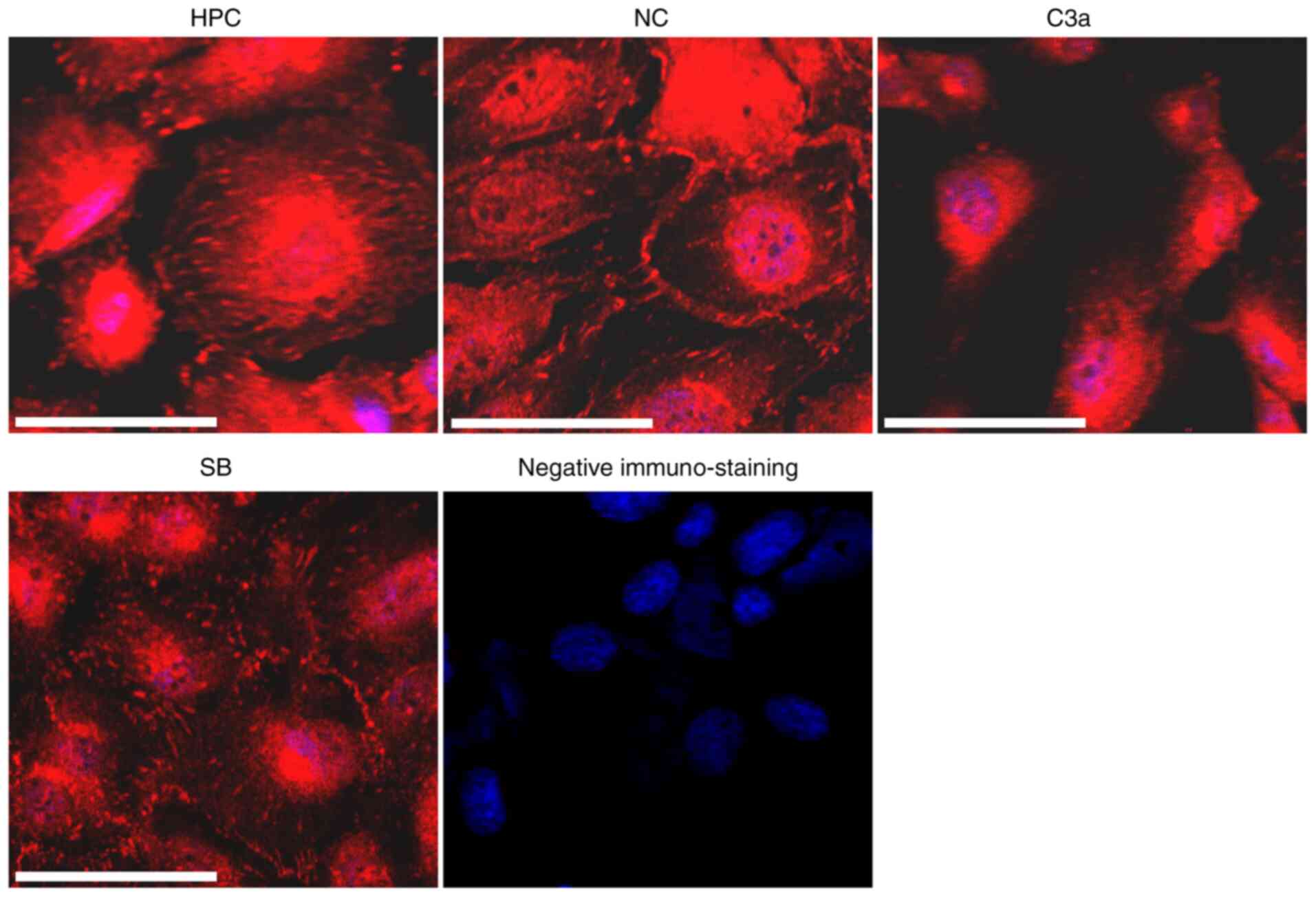
Effect of C3a overexpression on the
structure of focal adhesion (FA)
FAs are macromolecular complexes located at the
sites of cell adhesion to extracellular matrices (ECMs) and serve a
key role in cell adhesion to the ECM (24). To determine whether the decreased
adhesion capacity of HPC-C3a cells is associated with the
alteration of FAs, the cells were stained with antibodies against
VCL, a core component of FAs. Different immunostaining patterns for
VCL were observed in the HPC-C3a cells compared with the
untransfected HPC and HPC-NC cells (Fig. 4). Immunostaining was observed
mainly in the center of HPC-C3a cells and fewer and smaller
VCL-positive plaques were observed in the peripheral area of the
cells. SB290157 treatment (SB) blocked the VCL-induced alterations
in the HPC-C3a cells.
Effect of C3a overexpression on the
expression of cell adhesion-associated genes
To explore the underlying mechanism of decreased
adhesion capacity in HPC-C3a cells, the expression changes of ECM
cell adhesion-associated genes were observed. A total of 33 genes
that have been reported to be associated with podocyte adhesion
were examined. These included 15 genes [collagen I α1 (COL1A1),
collagen IV α1 (COL4A1), collagen IV α2 (COL4A2), collagen IV α3
(COL4A3), collagen IV α4 (COL4A4), fibronectin-1 (FN1), laminin β1
(LAMB1), laminin α1 (LAMA1), laminin γ1 (LAMC1), laminin β1
(LAMB1), laminin β2 (LAMB2), laminin γ2 (LAMC2), laminin α3
(LAMA3), laminin β3 (LAMB3) and laminin γ3 (LAMC3)] encoding ECM
components, 6 genes [integrin α1 (ITGA1), integrin α2 (ITGA2),
integrin α3 (ITGA3), integrin α5 (ITGA5), ITGB1 and integrin β3
(ITGB3)] encoding integrins and 9 genes [dystroglycan 1 (DAG1),
PTK2, integrin linked kinase (ILK), neuropilin 1 (NRP1), talin 1
(TLN1), talin 2 (TLN2), paxillin (PXN), fermitin family member 2
(FERMT2) and VCL] encoding cytoplasmic molecules associated
directly with FAs. Additionally, the gene encoding Wilms' tumor 1
(WT1), the molecular marker for podocytes, α smooth muscle actin
(ACTA2), the molecular marker for mesenchymal cells and for
transforming growth factor β1 (TGFB1), an important molecule in
regulation of cell proliferation, differentiation, growth,
survival, apoptosis, adhesion and migration, were examined. The
RT-qPCR results revealed that the expression levels of COL1A1, FN1,
LAMA1, LAMB1, LAMC1, ITGA1, ITGA2, ITGB1, PTK2, NRP1, TLN2, FERMT2
and VCL mRNA in HPC-C3a cells were significantly decreased compared
with these levels in the untransfected HPC and HPC-NC cells.
However, the addition of SB290157 prevented or reduced the decrease
in the expression of COL1A1, FN1, LAMA1, LAMB1, LAMC1, ITGA1,
ITGA2, ITGB1, PTK2, NRP1, TLN2, FERMT2 and VCL gene (Table II). No significant changes in the
expression of COL4A1, COL4A2, COL4A3, COL4A4, LAMA2, LAMB2, LAMC2,
LAMA3, LAMB3, LAMC3, ITGA3, ITGA5, ITGB3, DAG1, ILK, PXN, TLN1,
PXN, ACTA2, WT1 and TGFB1 were observed between groups.
 | Table II.Expression levels of cell
adhesion-associated genes. |
Table II.
Expression levels of cell
adhesion-associated genes.
|
| Relative mRNA
level |
|---|
|
|
|
|---|
| Gene | HPC | HPC-NC | HPC-C3a | HPC-C3a+SB |
|---|
| COL1A1 | 1±0.06 | 1.19±0.34 |
0.28±0.04b,c |
0.75±0.11d |
| COL4A1 | 1±0.05 | 1.08±0.31 | 1.17±0.17 | 1.14±0.16 |
| COL4A2 | 1±0.06 | 0.95±0.09 | 1.04±0.11 | 1.00±0.12 |
| COL4A3 | 1±0.10 | 1.36±0.39 | 1.34±0.43 | 1.15±0.15 |
| COL4A4 | 1±0.06 | 1.08±0.07 | 1.02±0.22 | 0.97±0.04 |
| FN1 | 1±0.13 | 1.02±0.28 |
0.19±0.05b,c |
0.62±0.09a,d |
| LAMA1 | 1±0.16 | 0.98±0.16 |
0.19±0.03b,c |
0.80±0.12e |
| LAMB1 | 1±0.07 | 1.01±0.08 |
0.21±0.07b |
0.79±0.04b,e |
| LAMC1 | 1±0.16 | 1.07±0.19 |
0.18±0.03b,c |
0.75±0.04a,e |
| LAMA2 | 1±0.24 | 0.99±0.12 | 1.32±0.40 | 0.94±0.19 |
| LAMB2 | 1±0.03 | 0.97±0.13 | 1.00±0.17 | 0.96±0.22 |
| LAMC2 | 1±0.12 | 0.93±0.16 | 1.00±0.18 | 0.92±0.05 |
| LAMA3 | 1±0.05 | 1.05±0.20 | 1.12±0.15 | 0.92±0.05 |
| LAMB3 | 1±0.07 | 1.04±0.19 | 1.11±0.25 | 1.00±0.02 |
| LAMC3 | 1±0.05 | 0.94±0.33 | 1.22±0.29 | 1.05±0.12 |
| ITGA1 | 1±0.22 | 0.94±0.15 |
0.40±0.04b,c |
0.92±0.03e |
| ITGA2 | 1±0.14 | 0.86±0.29 |
0.26±0.08b,c |
0.90±0.14e |
| ITGA3 | 1±0.18 | 0.92±0.09 | 0.98±0.09 | 0.92±0.13 |
| ITGA5 | 1±0.13 | 1.07±0.24 | 0.98±0.40 | 1.06±0.22 |
| ITGB1 | 1±0.14 | 0.86±0.29 |
0.27±0.07b,c |
0.80±0.15e |
| ITGB3 | 1±0.18 | 1.05±0.18 | 1.11±0.15 | 1.09±0.10 |
| DAG1 | 1±0.15 | 1.05±0.11 | 1.08±0.10 | 1.04±0.16 |
| PTK2 | 1±0.26 | 0.85±0.10 |
0.38±0.08b,c |
0.80±0.09e |
| ILK | 1±0.04 | 0.98±0.13 | 1.01±0.11 | 0.96±0.07 |
| NRP1 | 1±0.08 | 1.06±0.14 |
0.45±0.12b,c |
0.90±0.19e |
| TLN1 | 1±0.08 | 0.93±0.11 | 0.98±0.16 | 0.92±0.15 |
| TLN2 | 1±0.12 | 0.92±0.15 |
0.48±0.08b,c |
0.86±0.08e |
| PXN | 1±0.14 | 1.14±0.25 | 1.24±0.05 | 0.93±0.21 |
| FERMT2 | 1±0.15 | 0.94±0.11 |
0.55±0.15b,c |
0.90±0.09d |
| VCL | 1±0.11 | 1.00±0.13 |
0.60±0.02b,c |
090±0.19d |
| ACTA2 | 1±0.10 | 0.96±0.19 | 0.99±0.12 | 0.93±0.18 |
| WT1 | 1±0.11 | 0.90±0.07 | 0.97±0.13 | 0.91±0.15 |
| TGFB1 | 1±0.11 | 0.94±0.22 | 0.90±0.13 | 0.97±0.19 |
Additionally, the decreased expression of ITGB1,
PTK2 and VCL in HPC-C3a cells was confirmed by western blotting
compared with HPC and HPC-NC cells; this decrease was blocked by
addition of 1 µM SB290157 (Fig.
5).
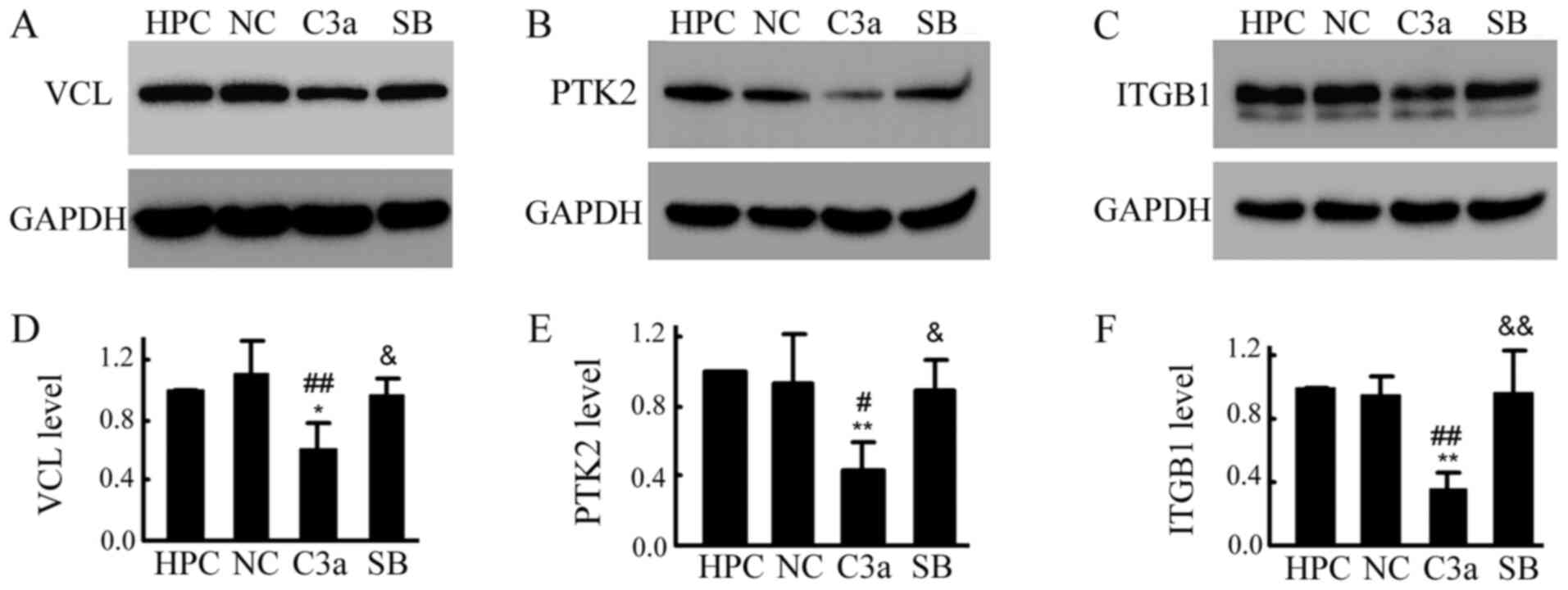 | Figure 5.Overexpression of C3a significantly
decreased the levels of VCL, PTK2 and ITGB1 in HPC cells. All cells
(HPC, NC, C3a and SB) were cultured in the maturation condition for
6 days. Total proteins were extracted and (A-C) western blotting
and (D-F) statistical analysis for VCL, PTK2 and ITGB1 was
performed. *P<0.05, **P<0.01 vs. the HPC group;
#P<0.05, ##P<0.01 vs. HPC-NC group;
&P<0.05, &&P<0.01 vs. the
C3a group. C3a, human C3A anaphylatoxin; PTK2, protein tyrosine
kinase 2; ITGB1, integrin β1; HPC, human podocyte cell line; NC,
negative control; SB, SB290157; VCL, vinculin. |
Given the importance of nephrin in the maintenance
of the structure and function of podocytes, the expression of the
nephrin gene was examined by RT-qPCR. However, no significant
differences were observed between groups (data not shown).
Addition of C3a to the medium only
partially mimicked the effect of overexpression of C3a in HPC
cells
To further examine the effect of C3aR activation on
the maturation of HPC cells, the cells were treated with purified
human C3a. Similar to the effect of C3a overexpression, the
expression of FN1, LAMB1, FERMT2, LAMA1 and LAMC1 in HPC cells
treated with C3a for 6 days (adding purified C3a every two days)
was significantly decreased compared with the untreated HPC group.
The addition of SB290157 prevented the decrease in the expression
of FN1, LAMB1, FERMT2, LAMA1 and LAMC1 in HPC cells. However, C3a
had no significant effect on the expression of COL1A1, ITGA1,
ITGA2, ITGB1, PTK2, NRP1, TLN2 or VCL. Additionally, C3a failed to
induce obvious change in the distribution of VCL-positive FAs,
adhesive capacity or the detachment of cells (Fig. 6).
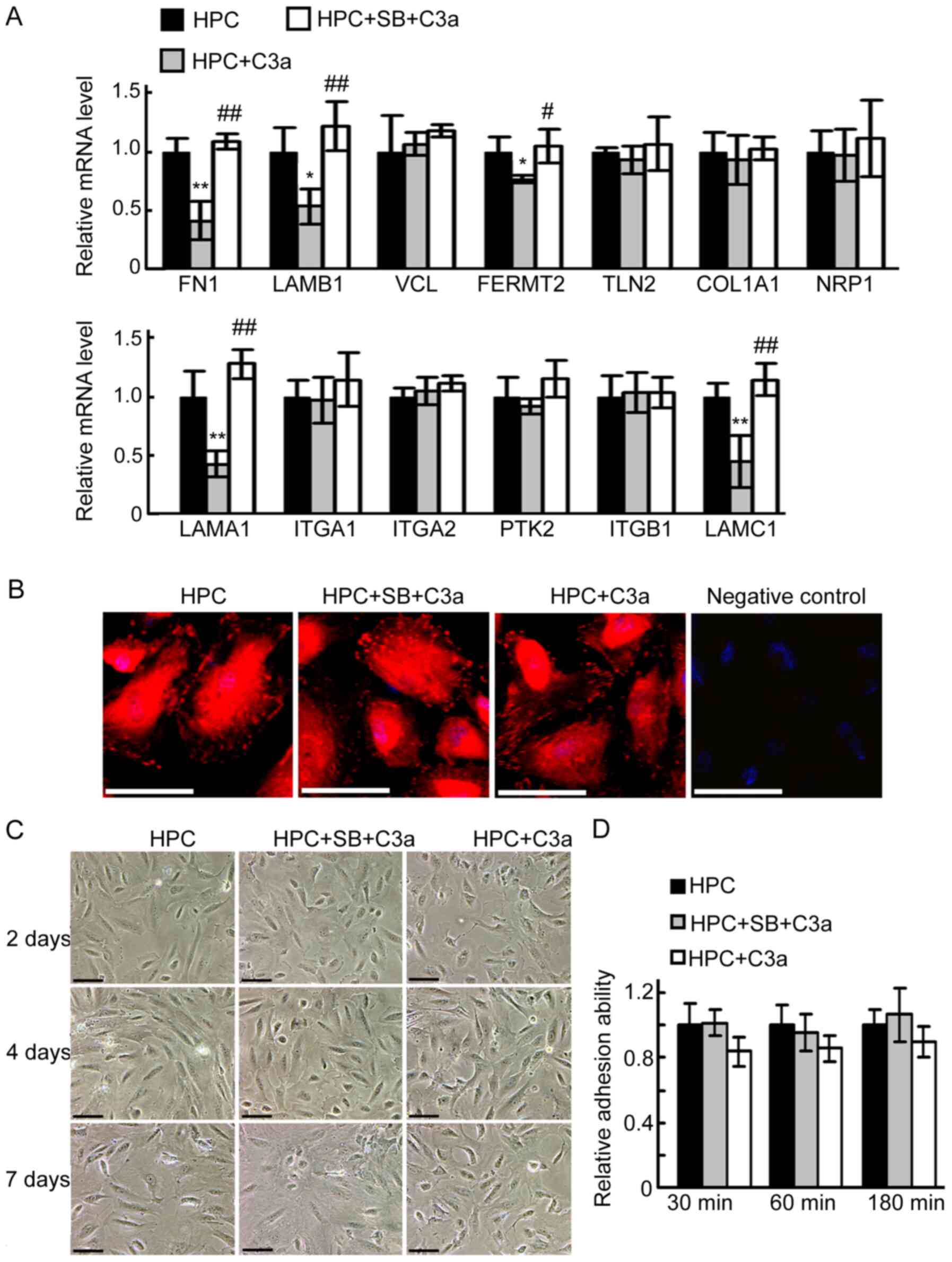 | Figure 6.Addition of C3a to the medium only
partially mimics the effect of overexpression of C3a in HPC cells.
HPC cells were divided into three groups: HPC, HPC+SB+C3a and
HPC+C3a. Morphological changes were observed under a phase contrast
microscope. (A) Reverse transcription-PCR analysis of the
expression of focal adhesion-associated genes. *P<0.05,
**P<0.01 vs HPC group; #P<0.05,
##P<0.01 vs HPC+C3a group. (B) Immunofluorescence
staining for VCL. Scale bar, 50 µM. (C) Representative images taken
under phase contrast microscopy demonstrated cell morphology. Scale
bar, 100 µM. (D) Results of adhesion analysis. COL1A1, collagen I
α1; FN1, fibronectin 1; LAMA1, laminin α1; LAMB1, laminin β1;
LAMC1, laminin γ1; ITGA1, integrin α1; ITGA2, integrin α2; ITGB1,
integrin β1; PTK2, protein tyrosine kinase 2; NRP1, neuropilin 1;
TLN2, talin 2; FERMT2, fermitin family member 2; VCL, vinculin;
C3a, human C3a anaphylatoxin; HPC, human podocyte cell; SB,
SB290157. |
HPC cells express
carboxypeptidases
Given that C3a is easily inactivated by
carboxypeptidases by cleaving the C-terminal arginine in
vivo (25–27), we hypothesized that inactivation of
C3a may be one of the causes for the different effects observed
between C3a overexpression and addition of purified C3a. To
investigate this hypothesis, the expression of carboxypeptidases in
HPC cells was examined. As presented in Fig. 7, the results of RT-PCR demonstrated
that although carboxypeptidases N1 (CPN1) and N2 (CPN2) were not
expressed in HPC cells, carboxypeptidases Z (CPZ), M (CPM) and D
(CPD) were indeed expressed in the cells, supporting our hypothesis
that the C3a added to the HPC cultured medium may be degraded by
the carboxypeptidases produced by the cells.
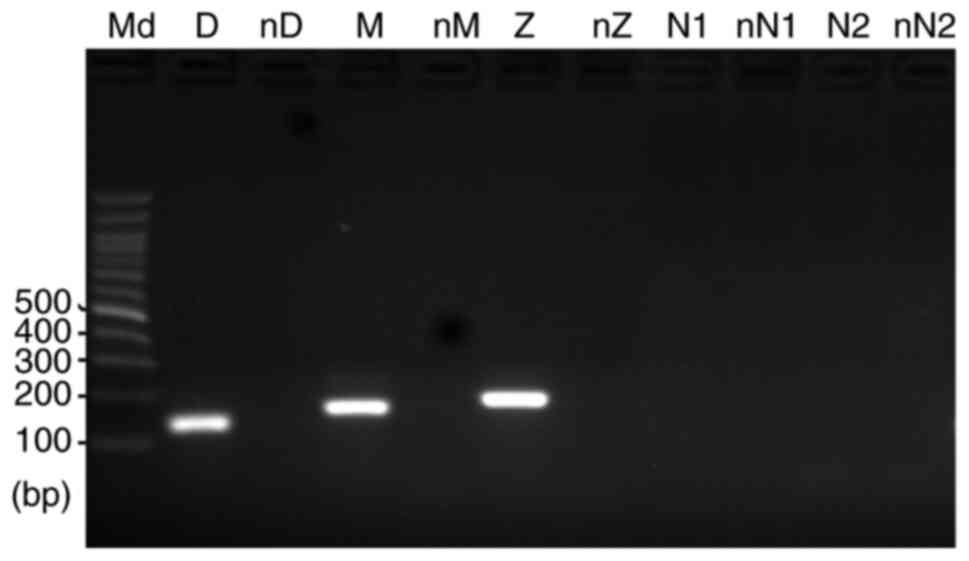 | Figure 7.Results of reverse transcription-PCR
demonstrated that human podocyte cells express genes for
carboxypeptidases Z, M and D. However, cells did not express genes
for carboxypeptidases N1 or N2. Md, DNA molecular marker; D,
carboxypeptidase D; M, carboxypeptidase M; Z, carboxypeptidase Z;
N1, carboxypeptidase N1; N2, carboxypeptidase N2; nD, nM, nZ, nN1
and nN2, negative controls for carboxypeptidases D, M, Z, N1 and
N2, respectively. |
Discussion
To the best of our knowledge, the present study is
the first to investigate the effect of the C3a anaphylatoxin
receptor (C3aR) activation on the maturation of podocytes by using
human podocyte (HPC) cells. Similar to a previous report (21), HPC cells proliferated and exhibited
typical cobblestone morphology when cultured in the proliferation
condition. Proliferation ceased and the cells gradually
differentiated into mature podocytes with enlarged cell bodies and
arborized appearance in the maturation condition. Infection of HPC
cells with control lentivirus did not induce an obvious change in
the cells. However, infection of HPC cells with Lenti-C3a, which
increased the levels of secretory C3a in the cells, induced marked
phenotypic changes, including significantly decreased adhesion
capacity, increased tendency to contract and to be detached from
the surface of tissue culture plates and failure to expand cell
bodies and develop arborized appearance.
Proper attachment of cells to the extracellular
matrix (ECM) is a basic requirement to build a multicellular
organism. It is the basis for adherent cells to survive and
function normally. Given the key role of cell adhesion to the ECM
in the behavior of cells, including cellular differentiation,
expansion, survival, apoptosis and the maintenance of cell
morphology (24,28), it was hypothesized that the
markedly decreased adhesion capacity of the cells may be the
fundamental cause for the different phenotypes observed in the
HPC-C3a cells. In accordance with this hypothesis, addition of the
C3aR-specific antagonist SB290157 prevented the decrease in cell
adhesion capacity, along with the recovery of the ability for the
cells to expand their cell bodies and develop into the arborized
appearance.
Cells, including podocytes, attach to ECM mainly
through the formation of FAs. FAs are large macromolecular
assemblies consisting of ECM components, ECM receptors (integrins)
and certain cytoplasmic proteins, and serve as the linkages between
the ECM and cellular skeleton (29,30).
In addition to anchoring cells, FAs function as signal sensors,
which inform the cells about the condition of the ECM to influence
the cells to alter their behaviors in response to survival,
apoptosis, spreading, differentiation and migration (24). To determine whether the decreased
adhesion capacity observed in the HPC-C3a cells was associated with
the impairment of FAs, the changes in the structure of FAs was
examined by immunostaining for VCL. As a core component of FAs, VCL
comprises an N-terminal head domain, a proline-rich linker and a
C-terminal tail domain. By binding to talin by its head domain
(31) and to paxillin, F-actin and
phosphatidylinositol- 4,5-bisphosphate by its tail domain (32,33),
VCL serves a pivotal role in stabilizing FAs and controls the
potency of cell adhesion binding to ECM (34,35).
As expected, C3a overexpression markedly altered the distribution
of VCL in HPC cells. Additionally, C3aR inhibition with SB290157
blocked this alteration.
To further explore the cause of the decreased
adhesion ability in the HPC-C3a cells, the changes in the
expression of FA structure- and function-associated genes was
examined. Overexpression of C3a significantly reduced the
expression level of FAs-associated genes, including the genes
encoding for LAMA1, LAMB1, LAMC1, FN1, COL1A1, ITGA1, ITGB1, ITGA2,
VCL, TLN2, PTK2, NRP1 and FERMT2. LAMA1, LAMB1 and LAMC1 are the
three chains of laminin 1. Laminin, collagen and fibronectin are
well-known components of the ECM. By interacting with their
receptors, ECM components provide mechanical support for cells and
have an important role in regulating cell shape and function. For
instance, laminin 1 has been reported to serve an important role in
orchestrating the development and differentiation of neural cells
including extension, migration, survival and neurite outgrowth
(36–38). Additionally, similar roles have
been demonstrated in other ECM components (39). Laminin 1, collagen 1 and
fibronectin 1 exert their cellular regulatory roles by interacting
with integrins, including α1β1, α2β1 and α3β1. By binding to their
ECM ligands by their extracellular ‘lobster claws’ domain and
interacting with the cytoskeleton system via their cytoplasmic
parts, integrins bridge the ECM and cytoskeleton, and are therefore
the structural center of the FAs (40,41).
Additionally, integrins serve a key role in signal transduction
associated with cell growth, division, survival, differentiation
and apoptosis (40). Integrins are
obligate heterodimers and each integrin has two subunits: α and β.
Mammals exhibit 24 α and 9 β integrins. Among them, integrins α1,
α2 and β1 have been reported to be expressed by podocytes and α1β1,
α2β1 and α3β1 have been well documented to serve important role in
the normal attachment of podocytes to the glomerular basement
membrane (42). Genetic deficit or
decreased expression of these integrins have been demonstrated to
be implicated in abnormal renal development (43), decreased adhesion capacity of
glomerular podocytes, podocyte damage (44,45)
and renal dysfunction (46,47).
VCL, TLN2, PTK2, NRP1 and FERMT2 are the major cytoplasmic
components associated with FAs. Either by connecting integrins to
cytoskeleton or by mediating signal transduction, these proteins
exert their roles in cell adhesion and adhesion-associated cellular
function (48–51). Thus, the significantly decreased
expression of FA-associated genes explained the decreased adhesion
ability of HPC-C3a cells observed in the current study.
As an important component of the slit diaphragm,
nephrin serves a key role in podocyte-associated nephropathy.
However, the current study did not observe significant change in
the expression of nephrin by C3a overexpression in an RT-qPCR
assay.
The present study observed that the effect of C3a
overexpression on the phenotype of HPC cells was not completely
mimicked by direct addition of C3a to the cell culture medium.
In vivo, C3a is easily inactivated by carboxypeptidases,
which catalyze the removal of the C-terminal arginine of C3a
(25). There are various
carboxypeptidases. Among them, CPN is produced mainly by the liver,
circulates in the plasma and contributes to the inactivation of
anaphylatoxins including C3a, C4a and C5a (26). Furthermore, CPM, CPD and CPZ have
been reported to be able to remove the C-terminal arginine of
peptides (27). Moreover, they
were hypothesized to function outside the cell and function
normally at neutral pH (27). To
investigate the cause for the different effects between the
overexpression of C3a and the direct addition of C3a on HPC cells,
the expression of CPN (encoded by the genes CPN1 and CPN2), CPM,
CPD and CPZ genes in HPC cells were examined. HPC cells expressed
CPM, CPD and CPZ genes; however, the cells did not express CPN.
Therefore, it seems reasonable to hypothesize that directly added
C3a may be inactivated by HPC cell derived carboxypeptidases,
including CPM, CPD and CPZ, and thus can only activate C3aR in HPC
cells temporarily. This is quite different compared with C3a
overexpression in the cells, in which C3a is produced continuously
and, therefore, may induce sustained receptor activation.
The present studies had certain limitations.
Firstly, as the current study was an in vitro study, in
vivo investigations are required. Secondly, while the present
study only observed the influence of C3a overexpression in the
morphological maturation of podocytes, the pathways through which
the overexpressed C3a may exert its role remain to be clarified.
Lastly, as all the commercial kits detected both C3a and C3a desArg
(the inactivated form of C3a), it could not be confirmed whether
the added C3a was directly inactivated by podocyte-derived
carboxypeptidases.
In summary, through the overexpression of the
secretory C3a in HPC cells, the current study investigated the
effect of sustained activation of C3aR on the maturation of HPC
cells. The results demonstrated that sustained activation of C3aR
impaired the morphological maturation of HPC cells. This effect may
be associated with the decreased adhesion ability of the cells to
the ECM and caused by the decreased expression of FA-associated
genes. These results revealed novel function of C3aR signaling in
podocytes and provided novel insight into the mechanism underlying
the regulation of podocyte morphology development. Presently, it is
not clear whether the overactivation of C3aR induces impairment of
podocyte maturation in vivo. However, chronically increased
complement activation has been reported in renal diseases (52–56),
which may indicate increased C3a and the chronic activation of C3aR
in renal cells, including podocytes and renal progenitor cells [for
instance, parietal epithelial cells (57–61)]. The possible pathological role of
excessive C3aR signaling in the dysregulation of podocyte
architecture and podocyte regeneration requires further
research.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Science
Foundation of Taizhou City (grant no. 1801ky02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JMZ designed the current research, performed
experiments and drafted and revised the manuscript. SSW and XT
performed the experiments, analyzed data and drafted and revised
the manuscript. DJC participated in data analysis and revised the
manuscript. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hawksworth OA, Coulthard LG, Mantovani S
and Woodruff TM: Complement in stem cells and development. Semin
Immunol. 37:74–84. 2018.PubMed/NCBI
|
|
2
|
Hawksworth OA, Coulthard LG and Woodruff
TM: Complement in the fundamental processes of the cell. Mol
Immunol. 84:17–25. 2017.PubMed/NCBI
|
|
3
|
Kwan WH, van der Touw W, Paz-Artal E, Li
MO and Heeger PS: Signaling through C5a receptor and C3a receptor
diminishes function of murine natural regulatory T cells. J Exp
Med. 210:257–268. 2013.PubMed/NCBI
|
|
4
|
Peng Q, Li K, Sacks SH and Zhou W: The
role of anaphylatoxins C3a and C5a in regulating innate and
adaptive immune responses. Inflamm Allergy Drug Targets. 8:236–246.
2009.PubMed/NCBI
|
|
5
|
Sacks SH: Complement fragments C3a and
C5a: The salt and pepper of the immune response. Eur J Immunol.
40:668–670. 2010.PubMed/NCBI
|
|
6
|
Lim J, Iyer A, Suen JY, Seow V, Reid RC,
Brown L and Fairlie DP: C5aR and C3aR antagonists each inhibit
diet-induced obesity, metabolic dysfunction, and adipocyte and
macrophage signaling. FASEB J. 27:822–831. 2013.PubMed/NCBI
|
|
7
|
Ohinata K and Yoshikawa M: Food intake
regulation by central complement system. Adv Exp Med Biol.
632:35–46. 2008.PubMed/NCBI
|
|
8
|
Bénard M, Raoult E, Vaudry D, Leprince J,
Falluel-Morel A, Gonzalez BJ, Galas L, Vaudry H and Fontaine M:
Role of complement anaphylatoxin receptors (C3aR, C5aR) in the
development of the rat cerebellum. Mol Immunol. 45:3767–3774.
2008.PubMed/NCBI
|
|
9
|
Broders-Bondon F, Paul-Gilloteaux P,
Gazquez E, Heysch J, Piel M, Mayor R, Lambris JD and Dufour S:
Control of the collective migration of enteric neural crest cells
by the Complement anaphylatoxin C3a and N-cadherin. Dev Biol.
414:85–99. 2016.PubMed/NCBI
|
|
10
|
Xu XH, Peng HS, Sun MQ, Hu M, Zhang R,
Wang WH, He XY and Xiao XR: C-terminal peptide of anaphylatoxin C3a
enhances hepatic function after steatotic liver transplantation: A
study in a rat model. Transplant Proc. 42:737–740. 2010.PubMed/NCBI
|
|
11
|
Davoust N, Jones J, Stahel PF, Ames RS and
Barnum SR: Receptor for the C3a anaphylatoxin is expressed by
neurons and glial cells. Glia. 26:201–211. 1999.PubMed/NCBI
|
|
12
|
Rahpeymai Y, Hietala MA, Wilhelmsson U,
Fotheringham A, Davies I, Nilsson AK, Zwirner J, Wetsel RA, Gerard
C, Pekny M, et al: Complement: A novel factor in basal and
ischemia-induced neurogenesis. EMBO J. 25:1364–1374.
2006.PubMed/NCBI
|
|
13
|
Shinjyo N, de Pablo Y, Pekny M and Pekna
M: Complement Peptide C3a Promotes Astrocyte Survival in Response
to Ischemic Stress. Mol Neurobiol. 53:3076–3087. 2016.PubMed/NCBI
|
|
14
|
Stokowska A, Atkins AL, Morán J, Pekny T,
Bulmer L, Pascoe MC, Barnum SR, Wetsel RA, Nilsson JA, Dragunow M,
et al: Complement peptide C3a stimulates neural plasticity after
experimental brain ischaemia. Brain. 140:353–369. 2017.PubMed/NCBI
|
|
15
|
Shinjyo N, Ståhlberg A, Dragunow M, Pekny
M and Pekna M: Complement-derived anaphylatoxin C3a regulates in
vitro differentiation and migration of neural progenitor cells.
Stem Cells. 27:2824–2832. 2009.PubMed/NCBI
|
|
16
|
Schlondorff J: How many Achilles' heels
does a podocyte have? An update on podocyte biology. Nephrol Dial
Transplant. 30:1091–1097. 2015.PubMed/NCBI
|
|
17
|
Nagata M: Podocyte injury and its
consequences. Kidney Int. 89:1221–1230. 2016.PubMed/NCBI
|
|
18
|
Reiser J and Sever S: Podocyte biology and
pathogenesis of kidney disease. Annu Rev Med. 64:357–366.
2013.PubMed/NCBI
|
|
19
|
Li X, Ding F, Zhang X, Li B and Ding J:
The Expression Profile of Complement Components in Podocytes. Int J
Mol Sci. 17:4712016.PubMed/NCBI
|
|
20
|
Locatelli M, Buelli S, Pezzotta A, Corna
D, Perico L, Tomasoni S, Rottoli D, Rizzo P, Conti D, Thurman JM,
et al: Shiga toxin promotes podocyte injury in experimental
hemolytic uremic syndrome via activation of the alternative pathway
of complement. J Am Soc Nephrol. 25:1786–1798. 2014.PubMed/NCBI
|
|
21
|
Saleem MA, O'Hare MJ, Reiser J, Coward RJ,
Inward CD, Farren T, Xing CY, Ni L, Mathieson PW and Mundel P: A
conditionally immortalized human podocyte cell line demonstrating
nephrin and podocin expression. J Am Soc Nephrol. 13:630–638.
2002.PubMed/NCBI
|
|
22
|
Boos L, Campbell IL, Ames R, Wetsel RA and
Barnum SR: Deletion of the complement anaphylatoxin C3a receptor
attenuates, whereas ectopic expression of C3a in the brain
exacerbates, experimental autoimmune encephalomyelitis. J Immunol.
173:4708–4714. 2004.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI
|
|
24
|
Riveline D, Zamir E, Balaban NQ, Schwarz
US, Ishizaki T, Narumiya S, Kam Z, Geiger B and Bershadsky AD:
Focal contacts as mechanosensors: Externally applied local
mechanical force induces growth of focal contacts by an
mDia1-dependent and ROCK-independent mechanism. J Cell Biol.
153:1175–1186. 2001.PubMed/NCBI
|
|
25
|
Yadav P, Goyal VD, Gaur NK, Kumar A,
Gokhale SM, Jamdar SN and Makde RD: Carboxypeptidase in prolyl
oligopeptidase family: Unique enzyme activation and
substrate-screening mechanisms. J Biol Chem. 294:89–100.
2019.PubMed/NCBI
|
|
26
|
Matthews KW, Mueller-Ortiz SL and Wetsel
RA: Carboxypeptidase N: A pleiotropic regulator of inflammation.
Mol Immunol. 40:785–793. 2004.PubMed/NCBI
|
|
27
|
Reznik SE and Fricker LD:
Carboxypeptidases from A to Z: Implications in embryonic
development and Wnt binding. Cell Mol Life Sci. 58:1790–1804.
2001.PubMed/NCBI
|
|
28
|
Horton ER, Byron A, Askari JA, Ng DHJ,
Millon-Frémillon A, Robertson J, Koper EJ, Paul NR, Warwood S,
Knight D, et al: Definition of a consensus integrin adhesome and
its dynamics during adhesion complex assembly and disassembly. Nat
Cell Biol. 17:1577–1587. 2015.PubMed/NCBI
|
|
29
|
Zaidel-Bar R, Cohen M, Addadi L and Geiger
B: Hierarchical assembly of cell-matrix adhesion complexes. Biochem
Soc Trans. 32:416–420. 2004.PubMed/NCBI
|
|
30
|
Zamir E and Geiger B: Molecular complexity
and dynamics of cell-matrix adhesions. J Cell Sci. 114:3583–3590.
2001.PubMed/NCBI
|
|
31
|
Peng X, Maiers JL, Choudhury D, Craig SW
and DeMali KA: α-catenin uses a novel mechanism to activate
vinculin. J Biol Chem. 287:7728–7737. 2012.PubMed/NCBI
|
|
32
|
Grashoff C, Hoffman BD, Brenner MD, Zhou
R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al:
Measuring mechanical tension across vinculin reveals regulation of
focal adhesion dynamics. Nature. 466:263–266. 2010.PubMed/NCBI
|
|
33
|
Hemmings L, Rees DJ, Ohanian V, Bolton SJ,
Gilmore AP, Patel B, Priddle H, Trevithick JE, Hynes RO and
Critchley DR: Talin contains three actin-binding sites each of
which is adjacent to a vinculin-binding site. J Cell Sci.
109:2715–2726. 1996.PubMed/NCBI
|
|
34
|
Goldmann WH: Role of vinculin in cellular
mechanotransduction. Cell Biol Int. 40:241–256. 2016.PubMed/NCBI
|
|
35
|
Izard T and Brown DT: Mechanisms and
functions of vinculin interactions with phospholipids at cell
adhesion sites. J Biol Chem. 291:2548–2555. 2016.PubMed/NCBI
|
|
36
|
Desban N, Lissitzky JC, Rousselle P and
Duband JL: alpha1beta1-integrin engagement to distinct laminin-1
domains orchestrates spreading, migration and survival of neural
crest cells through independent signaling pathways. J Cell Sci.
119:3206–3218. 2006.PubMed/NCBI
|
|
37
|
Mruthyunjaya S, Parveen D, Shah RD,
Manchanda R, Godbole R, Vasudevan M and Shastry P: Gene expression
analysis of laminin-1-induced neurite outgrowth in human
mesenchymal stem cells derived from bone marrow. J Biomed Mater Res
A. 103:746–761. 2015.PubMed/NCBI
|
|
38
|
Yamada M and Sekiguchi K: Molecular Basis
of Laminin-Integrin Interactions. Curr Top Membr. 76:197–229.
2015.PubMed/NCBI
|
|
39
|
Bachman H, Nicosia J, Dysart M and Barker
TH: Utilizing Fibronectin Integrin-Binding Specificity to Control
Cellular Responses. Adv Wound Care (New Rochelle). 4:501–511.
2015.PubMed/NCBI
|
|
40
|
Iwamoto DV and Calderwood DA: Regulation
of integrin-mediated adhesions. Curr Opin Cell Biol. 36:41–47.
2015.PubMed/NCBI
|
|
41
|
Li Z, Lee H and Zhu C: Molecular
mechanisms of mechanotransduction in integrin-mediated cell-matrix
adhesion. Exp Cell Res. 349:85–94. 2016.PubMed/NCBI
|
|
42
|
Sachs N and Sonnenberg A: Cell-matrix
adhesion of podocytes in physiology and disease. Nat Rev Nephrol.
9:200–210. 2013.PubMed/NCBI
|
|
43
|
Kanasaki K, Kanda Y, Palmsten K, Tanjore
H, Lee SB, Lebleu VS, Gattone VH Jr and Kalluri R: Integrin
beta1-mediated matrix assembly and signaling are critical for the
normal development and function of the kidney glomerulus. Dev Biol.
313:584–593. 2008.PubMed/NCBI
|
|
44
|
Dessapt C, Baradez MO, Hayward A, Dei Cas
A, Thomas SM, Viberti G and Gnudi L: Mechanical forces and TGFbeta1
reduce podocyte adhesion through alpha3beta1 integrin
downregulation. Nephrol Dial Transplant. 24:2645–2655.
2009.PubMed/NCBI
|
|
45
|
He P, Liu D, Zhang B, Zhou G, Su X, Wang
Y, Li D, Yang X, Hepatitis B and Virus X: Hepatitis B Virus X
Protein Reduces Podocyte Adhesion via Downregulation of α3β1
Integrin. Cell Physiol Biochem. 41:689–700. 2017.PubMed/NCBI
|
|
46
|
Borza CM, Su Y, Chen X, Yu L, Mont S,
Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, et al:
Inhibition of integrin α2β1 ameliorates glomerular injury. J Am Soc
Nephrol. 23:1027–1038. 2012.PubMed/NCBI
|
|
47
|
Pozzi A, Jarad G, Moeckel GW, Coffa S,
Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, et al:
Beta1 integrin expression by podocytes is required to maintain
glomerular structural integrity. Dev Biol. 316:288–301.
2008.PubMed/NCBI
|
|
48
|
Chen C, Li R, Ross RS and Manso AM:
Integrins and integrin-related proteins in cardiac fibrosis. J Mol
Cell Cardiol. 93:162–174. 2016.PubMed/NCBI
|
|
49
|
Li SY, Mruk DD and Cheng CY: Focal
adhesion kinase is a regulator of F-actin dynamics: New insights
from studies in the testis. Spermatogenesis.
3:e253852013.PubMed/NCBI
|
|
50
|
Mierke CT: The role of focal adhesion
kinase in the regulation of cellular mechanical properties. Phys
Biol. 10:0650052013.PubMed/NCBI
|
|
51
|
Yasuda-Yamahara M, Rogg M, Frimmel J,
Trachte P, Helmstaedter M, Schroder P, Schiffer M, Schell C and
Huber TB: FERMT2 links cortical actin structures, plasma membrane
tension and focal adhesion function to stabilize podocyte
morphology. Matrix Biol. 68-69:263–279. 2018.PubMed/NCBI
|
|
52
|
Heeringa SF and Cohen CD: Kidney diseases
caused by complement dysregulation: Acquired, inherited, and still
more to come. Clin Dev Immunol. 2012:6951312012.PubMed/NCBI
|
|
53
|
Jalal D, Renner B, Laskowski J, Stites E,
Cooper J, Valente K, You Z, Perrenoud L, Le Quintrec M, Muhamed I,
et al: Endothelial microparticles and systemic complement
activation in patients with chronic kidney disease. J Am Heart
Assoc. 7:e0078182018.PubMed/NCBI
|
|
54
|
Lesher AM and Song WC: Review: Complement
and its regulatory proteins in kidney diseases. Nephrology
(Carlton). 15:663–675. 2010.PubMed/NCBI
|
|
55
|
Mizuno M, Suzuki Y and Ito Y: Complement
regulation and kidney diseases: Recent knowledge of the
double-edged roles of complement activation in nephrology. Clin Exp
Nephrol. 22:3–14. 2018.PubMed/NCBI
|
|
56
|
Salant DJ: Introduction:
Complement-mediated kidney diseases. Semin Nephrol. 33:477–478.
2013.PubMed/NCBI
|
|
57
|
Eng DG, Sunseri MW, Kaverina NV, Roeder
SS, Pippin JW and Shankland SJ: Glomerular parietal epithelial
cells contribute to adult podocyte regeneration in experimental
focal segmental glomerulosclerosis. Kidney Int. 88:999–1012.
2015.PubMed/NCBI
|
|
58
|
Shankland SJ, Freedman BS and Pippin JW:
Can podocytes be regenerated in adults? Curr Opin Nephrol
Hypertens. 26:154–164. 2017.PubMed/NCBI
|
|
59
|
Bao L, Osawe I, Haas M and Quigg RJ:
Signaling through up-regulated C3a receptor is key to the
development of experimental lupus nephritis. J Immunol.
175:1947–1955. 2005.PubMed/NCBI
|
|
60
|
Braun MC, Reins RY, Li TB, Hollmann TJ,
Dutta R, Rick WA, Teng BB and Ke B: Renal expression of the C3a
receptor and functional responses of primary human proximal tubular
epithelial cells. J Immunol. 173:4190–4196. 2004.PubMed/NCBI
|
|
61
|
Horton ER, Byron A, Askari JA, Ng DHJ,
Millon-Frémillon A, Robertson J, Koper EJ, Paul NR, Warwood S,
Knight D, et al: Definition of a consensus integrin adhesome and
its dynamics during adhesion complex assembly and disassembly. Nat
Cell Biol. 17:1577–1587. 2015.PubMed/NCBI
|