Introduction
Breast cancer is the most common malignancy and the
leading cause of cancer-associated death in women worldwide
(1). Among patients with breast
cancer, ~60–70% are hormone-responsive, thus endocrine therapy has
become the standard treatment strategy for disease management in
these patients (2). Tamoxifen, a
selective estrogen receptor (ER) modulator that blocks the binding
between estrogen and its cognate receptor, is most commonly used to
treat ER-positive breast cancer, especially in premenopausal
patients (3). A 5-year course of
adjuvant therapy with tamoxifen significantly reduces the 15-year
risk of recurrence and death in patients with breast cancer
(4). Although the initial response
to tamoxifen is satisfactory, the majority of patients develop
resistance and tumor progression (5). Moreover, tamoxifen-resistant (TAMR)
breast cancer is more prone to invasion and metastasis (6), thus the 5-year survival rate of
patients with breast cancer drops from 80 to 27% with the
occurrence of distant metastasis (7).
Several mechanisms influencing resistance to
endocrine therapy have been identified. The identified mechanisms
are complex and primarily focused on the epigenetic regulation,
mutation, truncation and fusion events of the ER1 gene (8,9). Long
non-coding RNAs (lncRNAs) have been shown to function as master
regulators for gene expression and play a critical role in various
biological functions and disease processes including cancer
(10). As such, accumulating
evidence has demonstrated that lncRNA H19 serves an important role
in the proliferation, metastasis, chemoresistance and endocrine
resistance of breast cancer (11,12).
Recent research has reported that H19 is substantially upregulated
in TAMR breast cancer cell lines and tumor tissues, and is
associated with tamoxifen resistance (13). Several other studies have
demonstrated that H19 dysregulation influences tumor metastasis by
regulating the epithelial-mesenchymal transition (EMT) of tumor
cells (14,15). Zhou et al (16) demonstrated that H19 could promote
EMT in breast cancer cells. However, to the best of our knowledge,
the role of H19 in TAMR breast cancer has not been previously
reported.
Curcumin, a natural compound derived from turmeric,
has been reported to possess antitumor effects, including the
prevention of metastasis and progression in multiple cancer types,
including pancreatic cancer, breast cancer and chronic myeloid
leukemia (17). Curcumin exerts its
anticancer effects by targeting multiple intracellular signaling
pathways. In vitro investigation has demonstrated that
curcumin can inhibit EMT in pancreatic cancer cells by blocking the
hedgehog signaling pathway, and the same inhibitory effect has been
identified in breast cancer cells (18,19).
In addition, curcumin was reported to enhance the radio-sensitivity
of renal cancer cells and increase the response of pancreatic
cancer cells to gemcitabine (20).
However, the effect of curcumin on EMT in TAMR breast cancer cells
is not completely understood. Therefore, the present study aimed to
determine whether H19 induced EMT in TAMR breast cancer, and to
investigate whether curcumin blocked H19-mediated effects in TAMR
breast cancer cells.
Materials and methods
Cell culture, TAMR-MCF-7 cell
establishment and curcumin treatment
The MCF-7 human breast cancer cell line was obtained
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. MCF-7/TAMR cells were established by treating
MCF-7 cells at 37°C with 1 µM 4-hydroxytamoxifen (Sigma-Aldrich;
Merck KGaA) for 3 weeks and then 100 nM 4-hydroxytamoxifen for 6
months, as previously described (21). Cells were cultured in RPMI-1640
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C with 5% CO2. Curcumin
(Sigma-Aldrich; Merck KGaA) was dissolved in DMSO (Sigma-Aldrich;
Merck KGaA) to prepare a 10 mM stock solution and aliquots were
stored at −20°C. Curcumin stock solution was diluted in culture
medium so that the final DMSO content was <0.1%. Cells were
treated with curcumin at a dose of 5, 10, 20, 30 or 40 µM for 48 h
at 37°C with 5% CO2. Untreated cells were used as a
control.
Cell viability
MCF-7/TAMR cells were seeded (5×103
cells/well) into 96-well plates and incubated at 37°C with 5%
CO2 for 24 h. Cells were treated with different
concentrations of curcumin (0, 5, 10, 20, 30 and 40 µM) for 48 h at
37°C with 5% CO2. Subsequently, 10 µl Cell Counting
Kit-8 solution (Sigma-Aldrich; Merck KGaA) was added to each well
and incubated at 37°C for 1 h. DMSO (0.1%) was added to the control
wells. The optical density was determined at a wavelength of 450 nm
using the iMark™ Microplate Absorbance Reader (Bio-Rad
Laboratories, Inc.).
H19 knockdown and overexpression in
MCF-7/TAMR cells
H19 pcDNA3.1 expression vector (H19-epv), empty
vector negative control (H19-epv-NC), scrambled small interfering
(si)RNA negative control (siNC cat. no. siN0000001-1-5) and H19
siRNA (5′-CCTGTAACCAAAAGTGACCG-3′) were obtained from Guangzhou
RiboBio Co., Ltd. Briely, 2×104 MCF-7/TAMR cells were
plated in phenol red-free medium containing 10% FBS in 6-well
plates, then transfected with 100 nM siRNA (siRNAH19 or siNC) or 1
µg of H19 expression plasmid (H19-epv or empty vector) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as previously described (20) when cells reached 70% confluence.
Following 24-h incubation at 37°C with 5% CO2, cells
were treated with curcumin for subsequent experiments as
aforementioned.
Wound healing assay
MCF7/TAMR cells were seeded (5×105
cells/well) into 6-well plates. Following transfection, cells were
treated with curcumin for 48 h at 37°C. At 90–100% confluence, a
sterile pipette tip was used to form a single scratch across the
cell monolayer. After washing with PBS, cells were incubated in
RPMI-1640 supplemented with 1% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C with 5% CO2. The width of the
wound in each group was examined at 0, 24 and 48 h using a light
microscope (magnification, ×40; Olympus Corporation), then analyzed
using Image J software (version 1.8.0; National Institutes of
Health). The migration rate was calculated as: Migration rate =
(Width of the wound at 0 h - Width of the wound at 24 or 48 h) /
Width of the wound at 0 h.
Transwell invasion assay
Following transfection and curcumin treatment, cell
invasion was assessed using modified Boyden chambers (pore size,
8.0 µm; Costar; Corning, Inc.). The Transwell inserts were
pre-coated with Matrigel overnight at 37°C with 5% CO2.
A total of 2×104 transfected cells were resuspended in
200 µl serum-free medium and plated into the upper chambers of the
Transwell inserts in a 24-well plate. Subsequently, 600 µl
RPMI-1640 supplemented with 20% FBS was added to the lower
chambers. Following incubation at 37°C for 24 h, non-invading cells
were removed with cotton swabs and the filters were rinsed with
PBS. Invading cells were fixed with methanol for 20 min at room
temperature, stained with 1% crystal violet for 30 min at room
temperature. Stained cells were counted in eight random microscopic
fields using a light microscope (magnification, ×200; Olympus
Corporation).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA (1 µg)
was reverse transcribed into cDNA using the PrimeScript RT reagent
kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Subsequently, qPCR was performed using iQ™
SYBR® Green Supermix (Bio-Rad Laboratories, Inc.) and
the iQ™5 real-time detection system (Bio-Rad Laboratories, Inc.).
The following primers were used for qPCR: H19 forward,
5′-GTCCGGCCTTCCTGAACACCTT-3′ and reverse,
5′-GCTTCACCTTCCAGAGCCGAT-3′; E-cadherin forward,
5′-CAACAAAGACAAAGAAGGCAAGG-3′ and reverse,
5′-TGAGAGAAGAGAGTGTATGTGGC-3′; vimentin forward,
5′-GGAGGAGATGCTTCAGAGAGAG-3′ and reverse,
5′-GGATTTCCTCTTCGTGGAGTTTC-3′; Snail forward,
5′-AGGACCACAGTGGCTCAGAAAGGAPDH-3′ and reverse,
5′-TGATGACCCTTTTGGCTCCC-3′; and GAPDH forward,
5′-GGAAGCTTGTCATCAATGGAAATC-3′ and reverse,
5′-TGATGACCCTTTTGGCTCCC-3′. The following thermocycling conditions
were used for qPCR: 95°C for 5 min; followed by 40 cycles at 95°C
for 15 sec, 60°C for 30 sec and 72°C for 30 sec. mRNA expression
levels were quantified using the 2−ΔΔCq method (22) and normalized to the internal
reference gene GAPDH.
Western blotting
Total protein was extracted from cells using RIPA
buffer (Sigma-Aldrich; Merck KGaA) containing 1% PMSF, 0.3%
protease inhibitor and 0.1% phosphorylated proteinase inhibitor.
Total protein was quantified using the BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg) were
separated via 12% SDS-PAGE and transferred to nitrocellulose
membranes, which were blocked in blocking buffer [5% non-fat dry
milk in TBS with 0.5% Tween, (TBS-T)] at room temperature for 2 h.
After washing with TBS-T, the membranes were incubated overnight at
4°C with primary antibodies targeted against: E-cadherin (1:300;
Abcam; cat. no. ab40772), N-cadherin (1:300; Abcam; cat. no.
ab76011), vimentin (1:300; Abcam; cat. no. ab16700), Snail (1:300;
Abcam; cat. no. ab216347) and GAPDH (1:1,000; cat. no. G9545;
Sigma-Aldrich; Merck KGaA). Following primary incubation, the
membranes were incubated for 2 h at room temperature with a
HRP-conjugated Affinipure goat anti-rabbit secondary antibody
(1:500; Abcam; cat. no. ab6721). Protein bands were visualized
using Immobilon Western Chemilum HRP Substrate (cat. no. WBKLS0100;
EMD Millipore) and the expression levels of each protein were
analyzed using Image Lab software version 4.1 (Bio-Rad
Laboratories, Inc.).
Immunofluorescence assay
Cells were plated onto confocal laser small dishes
at a density of 5×104, and treated with curcumin as
aforementioned. The cells on chamber slides were washed with PBS
for 15 min, fixed in 4% paraformaldehyde for 30 min at room
temperature and permeabilized with 0.1% Triton X-100 for 5 min at
room temperature. After three washes with PBS (5 min each),
non-specific binding was blocked with 3% BSA (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Subsequently, cells
were incubated with primary antibodies targeted against E-cadherin
(1:100 in PBS with 1% BSA) and N-cadherin (1:100 in PBS with 1%
BSA) for 2 h at room temperature. Following washing with PBS, cells
were incubated with Alexa Fluor 488 goat anti-rabbbit IgG
(Proteintech, cat. no. SA00006-2) for E-cadherin and Alex Fluor 594
goat anti-rabbbit IgG (Proteintech; cat. no. SA00006-4) secondary
antibody for 1 h at room temperature. After three 5-min washes with
PBS, cell nuclei were stained with 10 µg/ml Hoechst 33258 for 10
min at room temperature. Following washing with PBS, cells were
visualized using a fluorescence microscope (magnification,
×200).
Statistical analysis
Data are presented as the mean ± SEM. Comparisons
among multiple groups were analyzed using one-way ANOVA following
by the SNK or Tukey's post hoc test. Comparisons between two groups
were analyzed using an unpaired Student's t-test. Statistical
analyses were performed using SPSS software (version 21.0; IBM
Corp.). Each experiment was performed at least three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
H19 induces EMT in MCF-7/TAMR
cells
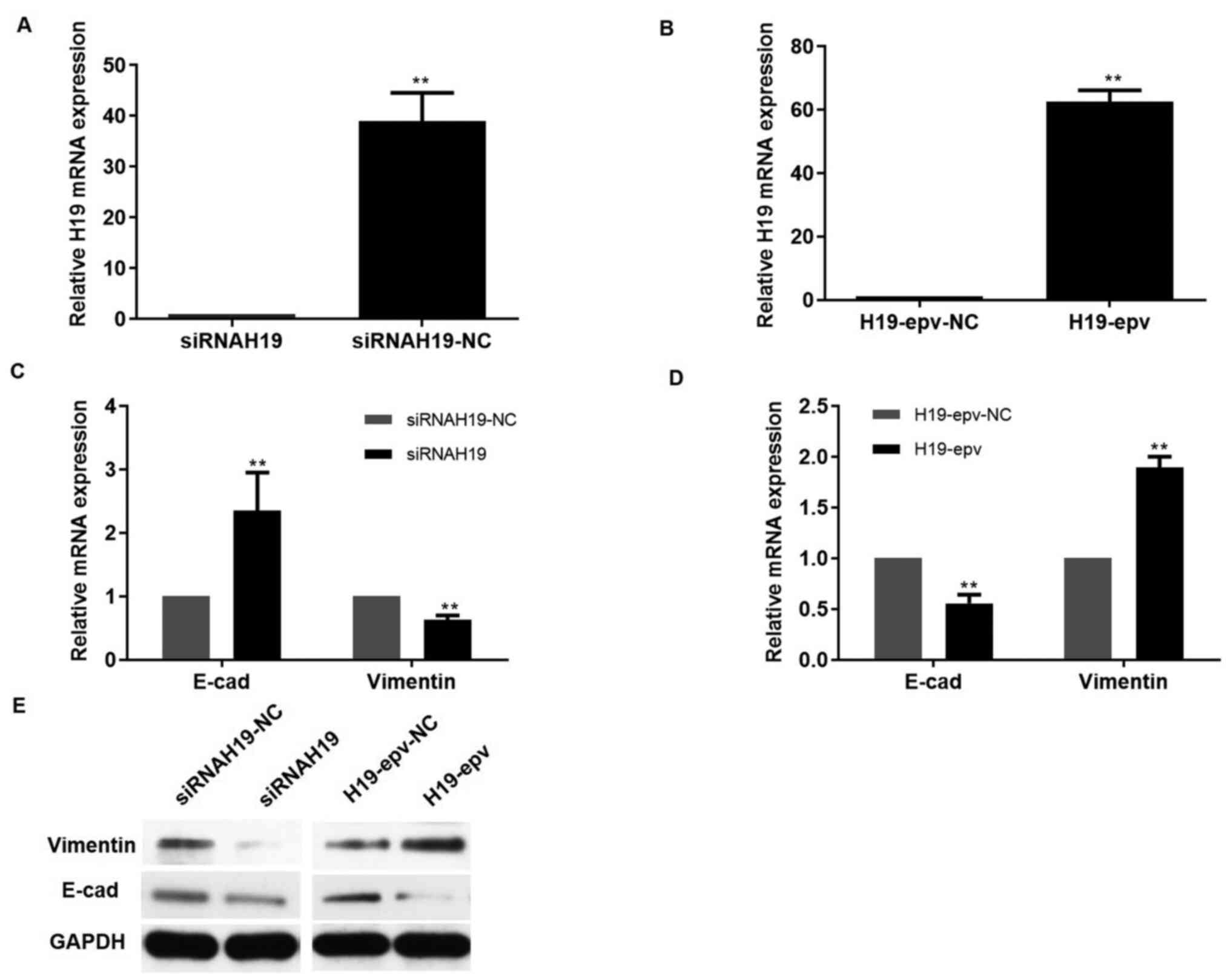
To determine the effects of H19 on MCF-7/TAMR cell
EMT, H19 was overexpressed and knocked down using HPV-epv and
siRNAH19, respectively. The results indicated that following
transfection with siRNAH19 for 24 h, H19 expression was
significantly decreased by 40-fold, vimentin expression was notably
downregulated and E-cadherin expression was markedly upregulated
compared with the siRNAH19-NC group (Fig. 1A, C and E). To further clarify the
role of H19 in EMT, H19 was overexpressed in MCF-7/TAMR cells. At
24 h post-transfection, a 60-fold significant increase in H19
expression levels was observed in H19-epv-transfected MCF-7/TAMR
cells compared with H19-epv-NC-transfected cells (Fig. 1B). Moreover, H19 overexpression
notably increased vimentin expression levels and markedly decreased
E-cadherin expression levels compared with the H19-epv-NC group
(Fig. 1D and E). Collectively, the
aforementioned results suggested that H19 promoted EMT in TAMR
breast cancer cells.
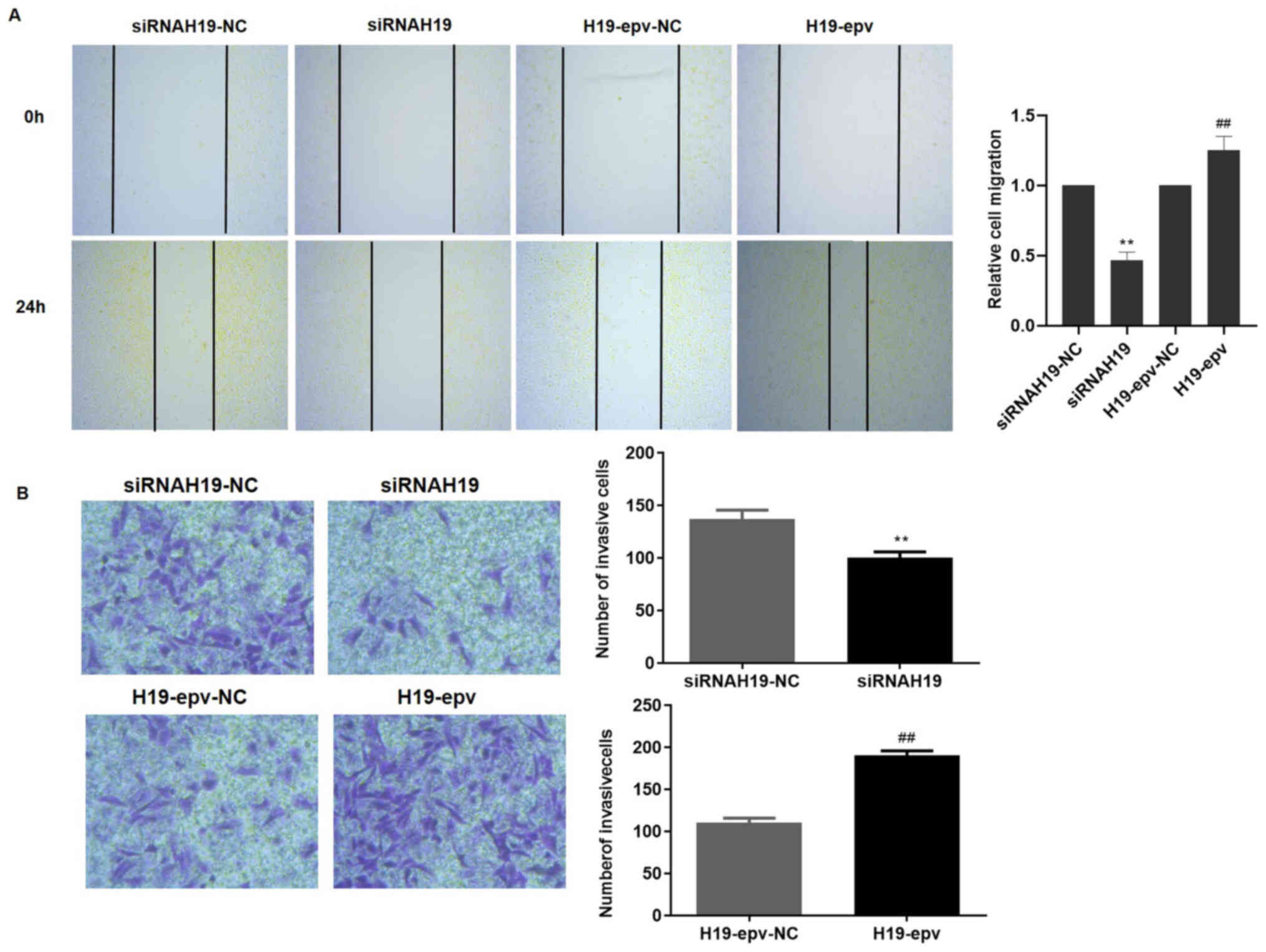
H19 promotes MCF-7/TAMR cell migration
and invasion
Wound healing and Transwell assays were performed to
determine the influence of H19 expression on MCF-7/TAMR cell
migration and invasion. Following transfection with H19-epv or
siRNAH19 for 24 h, the results demonstrated that H19 overexpression
promoted wound closure, whereas H19 knockdown inhibited wound
closure compared with the H19-epv-NC and siRNAH19-NC groups,
respectively (Fig. 2A).
Furthermore, the Transwell invasion assay results suggested that
H19 overexpression significantly promoted MCF-7/TAMR cell invasion
compared with the H19-epv-NC group, whereas H19 knockdown
significantly decreased cell invasion compared with the siRNAH19-NC
group (Fig. 2B). The results
indicated that H19 induced TAMR breast cancer cell migration and
invasion.
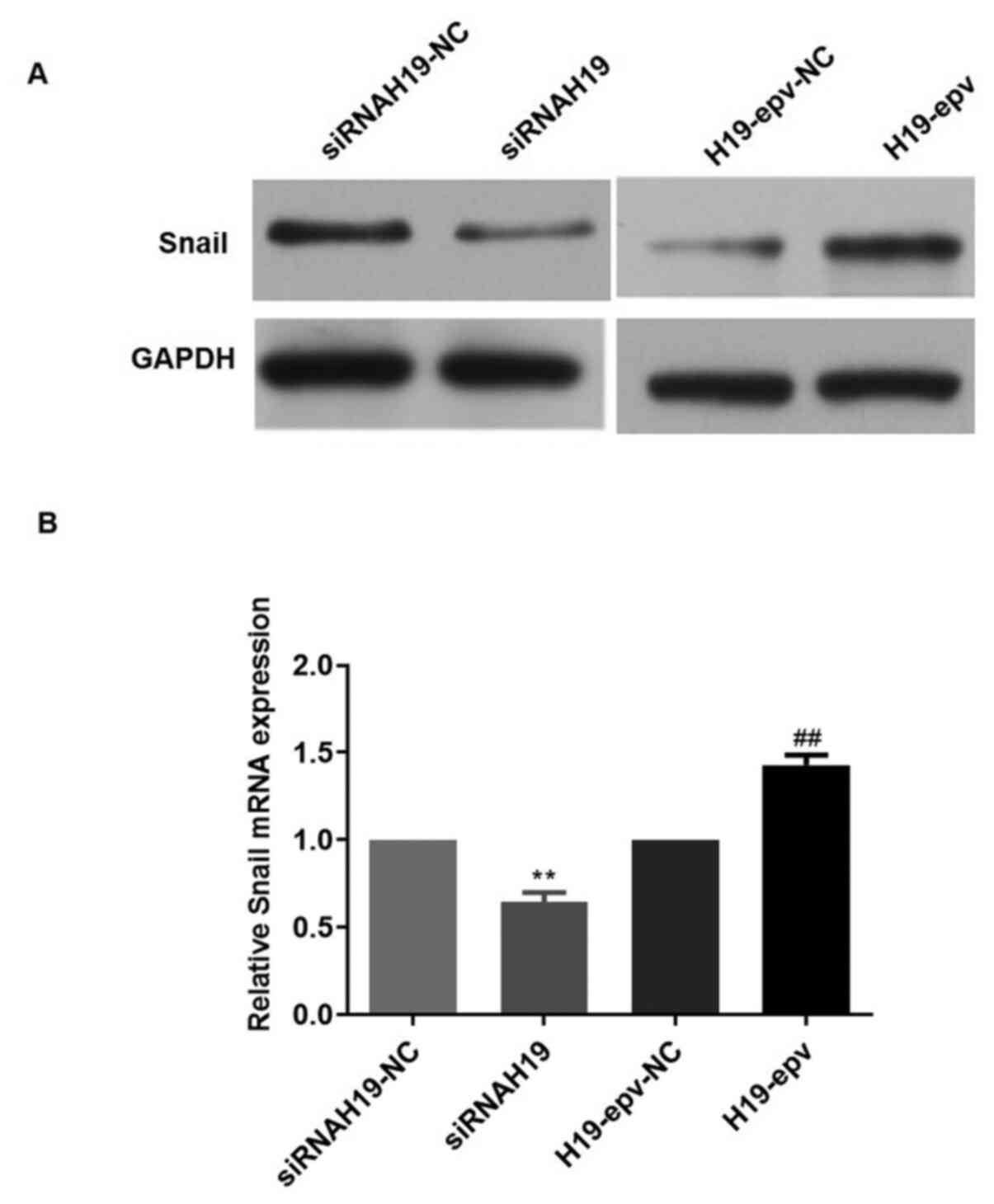
H19 upregulates Snail, promoting
MCF-7/TAMR cell EMT
Snail is a key regulator of the EMT process
(23). Therefore, to investigate
the mechanisms underlying H19-induced EMT, the mRNA and protein
expression levels of Snail in MCF-7/TAMR cells following
transfection with H19-epv or siRNAH19 were determined (Fig. 3). H19 overexpression notably
increased Snail mRNA and protein expression levels compared with
the H19-epv-NC group. However, H19 knockdown markedly decreased the
mRNA and protein expression levels of Snail compared with
siRNAH19-NC. The results suggested that H19 promoted EMT in TAMR
breast cancer cells via Snail upregulation.
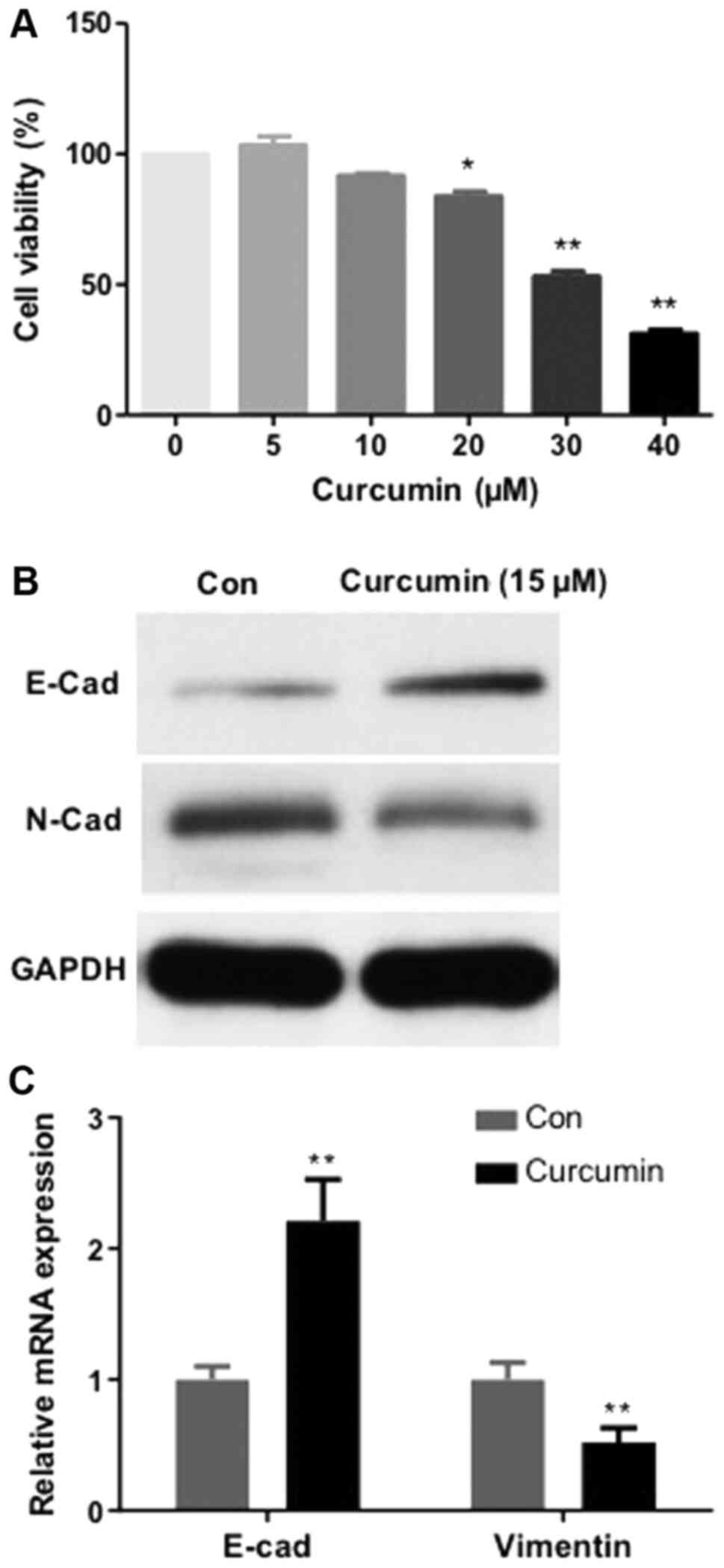
Curcumin inhibits H19-induced EMT in
MCF-7/TAMR cells
The impact of curcumin on MCF-7/TAMR cells
proliferation was investigated. As demonstrated in Fig. 4A, administration of MCF-7/TAMR cells
with curcumin for 48 h inhibited proliferation in a dose-dependent
manner, with an IC50 value of 31.7 µM. No significant differences
in MCF-7/TAMR cell viability were observed at curcumin
concentrations up to 20 µM. The expression levels of EMT biomarkers
(E-cadherin and N-cadherin) were subsequently assessed via RT-qPCR
and western blotting. Compared with the control group, following
treatment with curcumin for 48 h, the expression levels of the
mesenchymal marker N-cadherin were notably reduced, whereas the
expression levels of the epithelial marker E-cadherin were markedly
increased (Fig. 4B and C). The
results indicated that curcumin inhibited MCF-7/TAMR cell EMT.
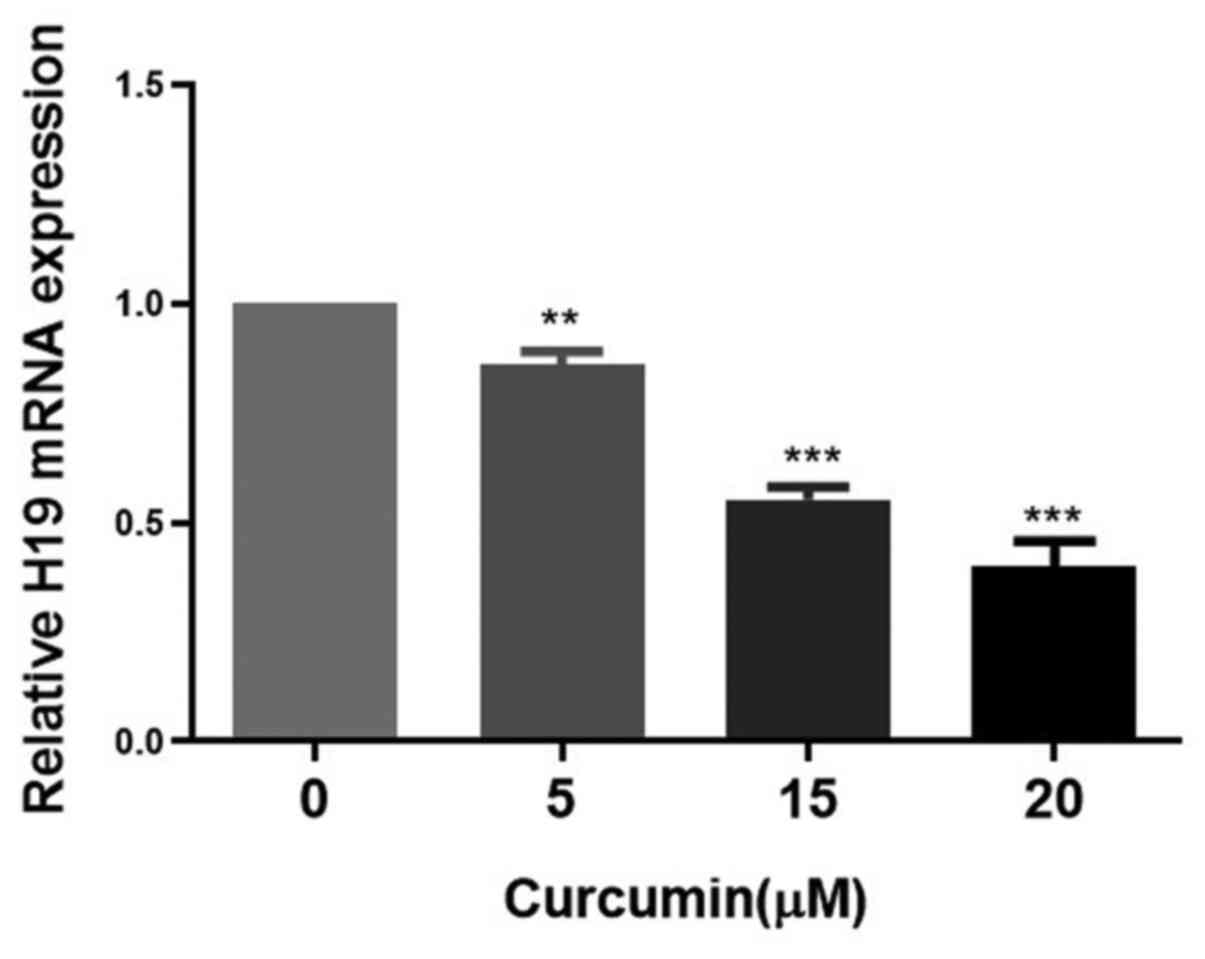
Subsequently, the effects of different
concentrations of curcumin on the expression of H19 in MCF-7/TAMR
cells were assessed. H19 expression was significantly decreased in
a dose-dependent manner by concentrations of curcumin between 5 and
20 µM for 48 h (Fig. 5). Therefore,
15 µM curcumin was selected for use in subsequent experiments. To
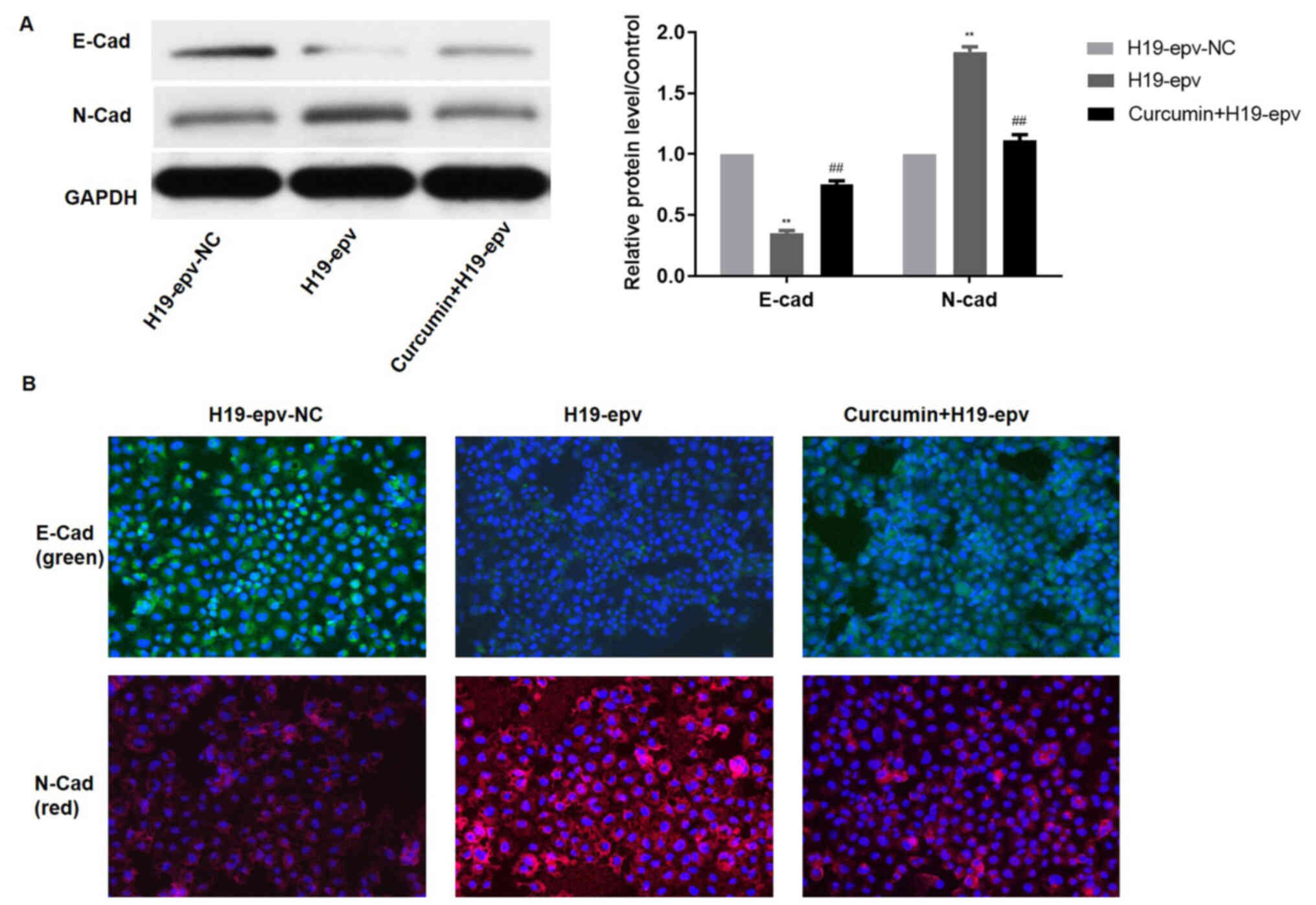
verify the influence of curcumin on H19-induced EMT, the expression
levels of E-cadherin and N-cadherin were evaluated in MCF-7/TAMR
cells following H19 overexpression in the presence or absence of
curcumin. Compared with the H19-epv group, the protein expression
levels of N-cadherin were notably decreased and E-cadherin
expression levels were significantly increased in
H19-epv-transfected cells treated with curcumin for 48 h (Fig. 6A). Immunofluorescence analysis
indicated that H19-overexpression cells displayed notably lower
levels of E-cadherin expression and considerably higher levels of
N-cadherin expression compared with the H19-epv-NC group. However,
following treatment with curcumin for 48 h, the expression levels
of E-cadherin and N-cadherin in H19-overexpression cells were
recovered (Fig. 6B). The results
suggested that curcumin reversed H19-induced effects on EMT.
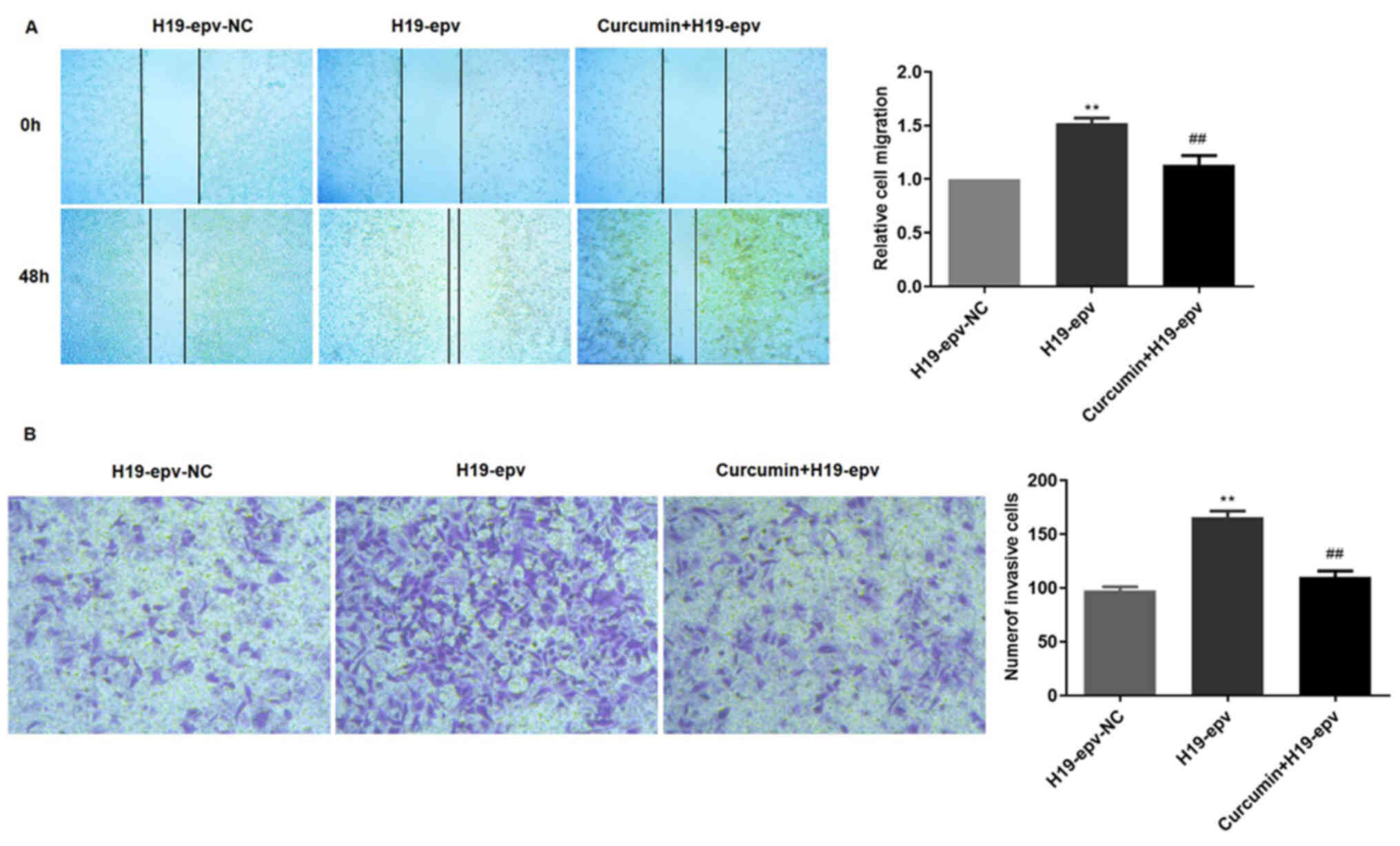
Curcumin inhibits H19-induced
MCF-7/TAMR cell migration and invasion
To verify the influence of curcumin on H19-induced
migration and invasion, MCF-7/TAMR cells were transfected with
H19-epv for 24 h, and then incubated in the presence or absence of
curcumin for 48 h. The results demonstrated that cell migration in
the H19-epv group was significantly increased compared with
H19-epv-NC. However, treatment with curcumin for 48 h significantly
decreased wound closure in H19-overexpression cells (Fig. 7A). The Transwell invasion assay
results also indicated that the number of invading cells was higher
in the H19-overexpression group compared with the NC group, but
curcumin treatment significantly decreased H19
overexpression-induced cell invasion (Fig. 7B). Collectively, the results
indicated that curcumin inhibited MCF-7/TAMR cell migration and
invasion resulting from H19-induced EMT.
Discussion
Despite its wide use for the treatment of breast
cancer, a third of patients with breast cancer develop resistance
to tamoxifen, which results in tumor progression (24). Additionally, TAMR breast cancer is
more prone to invasion and metastasis (6), thus identifying the mechanisms
underlying drug-resistant tumor metastasis, as well as novel
therapeutic strategies is critical to improve patient survival. H19
was previously reported to contribute to tamoxifen resistance in
breast cancer cells via various molecular mechanisms, including the
regulation of autophagy, and Notch and hepatocyte growth factor
signaling (13).
Previous studies have indicated that H19 is
upregulated in a variety of cancer cell types, including breast
cancer, gastric cancer and glioma cells, and that H19 knockdown
inhibits tumor growth, migration and invasion (25,26).
EMT is important for tumor cell migration and invasion (27), and E-cadherin and vimentin are
important markers of EMT. During EMT, E-cadherin and vimentin
expression levels are downregulated and upregulated, respectively
(28). H19 also mediates migration
and EMT-related activity in lung cancer and cisplatin-resistance
ovarian cancer cells (24,26). Collectively, the aforementioned
studies suggested that H19 upregulation is associated with
invasion, metastasis and EMT in different types of cancer cells. In
the present study, the influence of H19 on migration, invasion and
EMT was investigated by performing H19 overexpression and knockdown
in TAMR breast cancer cells. Compared with the H19-epv-NC group,
H19 overexpression promoted migration and invasion, increased
vimentin expression and decreased E-cadherin expression. H19
knockdown displayed the opposite effect, inhibiting migration and
invasion, increasing E-cadherin expression levels and decreasing
vimentin expression levels compared with the siRNAH19-NC group. The
results suggested that H19 promoted EMT in MCF-7/TAMR cells via
regulating the expression of vimentin and E-cadherin. As a zinc
finger transcriptional repressor, Snail is a key regulator of EMT
that downregulates and upregulates the expression of epithelial and
mesenchymal genes, respectively (29). The results of the present study
suggested that H19, as an upstream factor, upregulated the
expression levels of Snail, which decreased E-cadherin expression,
thus triggering EMT in MCF-7/TAMR cells.
Accumulating evidence has demonstrated that curcumin
exerts anticancer effects via multiple signaling pathways. Hu et
al (30) demonstrated that
curcumin significantly inhibited breast cancer cell proliferation
by inhibiting the phosphorylation of AKT/mTOR signaling
pathway-related proteins. Curcumin was also reported to induce
autophagy and activate lysosomal functioning by inhibiting the
AKT/mTOR signaling pathway and activating transcription factor EB
(30). In hepatoma cells, curcumin
inhibits TGF-β1-induced EMT by suppressing Smad2 signaling pathway
activation (31). A recent study
also indicated that curcumin inhibits superoxide dismutase-induced
migration, invasion and EMT-related gene expression in pancreatic
cancer (32). In the present study,
curcumin exerted an inhibitory effect on viability and EMT in
MCF-7/TAMR cells and significantly attenuated H19-induced
migration, invasion and EMT. The present study provided several
insights into the mechanism underlying curcumin-mediated regulation
of H19-induced EMT and migration. Firstly, compared with the
siRNAH19-NC group, H19 overexpression notably upregulated vimentin
expression and markedly downregulated E-cadherin expression, which
indicated that H19 promoted EMT in TAMR breast cancer cells.
Furthermore, the wound healing and Transwell invasion assays
demonstrated that H19 overexpression promoted migration and
invasion in MCF-7/TAMR cells compared with the H19-epv-NC group.
The present study had several limitations. The role of curcumin on
H19-induced EMT requires further investigation. For instance, an
animal model should be applied to validate the molecular mechanism
related to the H19/Snail/E-cadherin axis in regulation of
MCF-7/TAMR EMT and demonstrate the therapeutic potential of
curcumin. Therefore, the mechanism of the present study requires
further investigation using in vivo models of TAMR breast
cancer.
In conclusion, the present study suggested that H19
promoted EMT, migration and invasion in MCF-7/TAMR cells, whereas
curcumin inhibited H19-induced effects. Therefore, the use of
curcumin to inhibit the H19/Snail/E-cadherin axis may serve as a
promising therapeutic option for patients with TAMR breast
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fujian
Science and Technology Innovation Joint Fund Project (grant no.
2017Y9067), the Medical Science Research Project (grant no.
BJBQEKYJJ-B19001CS), the Young and Middle-Aged Backbone Talents
Project (grant no. 2019-ZQN-35), the Science and Technology
Department of Fujian Province (grant no. 2017Y9098), the High-Level
Hospital Foster Grants from Fujian Provincial Hospital (grant no.
2019HSJJ06), the Fujian Natural Science Foundation Project (grant
no. 2019J01177) and the Startup Fund for Scientific Research,
Fujian Medical University (grant nos. 2016QH106 and
2017XQ1024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and JZ conceptualized the study. JC, BZ and HS
designed the study. JC, HS, MX and GZ carried out the experiments
and curated the data. JC, CX and XH analyzed the data. JC and HS
wrote the manuscript. JZ reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poggio F, Ceppi M, Lambertini M, Bruzzi P,
Ugolini D, Bighin C, Levaggi A, Giraudi S, D'Alonzo A, Vaglica M,
et al: Concurrent versus sequential adjuvant chemo-endocrine
therapy in hormone-receptor positive early stage breast cancer
patients: A systematic review and meta-analysis. Breast.
33:104–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y and Liu Y: Endocrine therapy for
breast cancer: Past and present. Zhonghua Yi Shi Za Zhi. 45:28–32.
2015.(In Chinese). PubMed/NCBI
|
|
4
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), ; Davies C, Godwin J, Gray R, Clarke
M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, et al: Relevance
of breast cancer hormone receptors and other factors to the
efficacy of adjuvant tamoxifen: patient-level metaanalysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burstein HJ, Temin S, Anderson H, Buchholz
TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky
AJ, et al: Adjuvant endocrine therapy for women with hormone
receptor-positive breast cancer: American society of clinical
oncology clinical practice guideline focused update. J Clin Oncol.
32:2255–2269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang M: Tamoxifen resistance in breast
cancer. Biomol Ther (Seoul). 20:256–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park M, Kim J, Phuong NTT, Park JG, Park
JH, Kim YC, Baek MC, Lim SC and Kang KW: Involvement of the P2X7
receptor in the migration and metastasis of tamoxifen-resistant
breast cancer: Effects on small extracellular vesicles production.
Sci Rep. 9:115872019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Marchi T, Liu NQ, Stingl C, Timmermans
MA, Smid M, Look MP, Tjoa M, Braakman RB, Opdam M, Linn SC, et al:
4-protein signature predicting tamoxifen treatment outcome in
recurrent breast cancer. Mol Oncol. 10:24–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Zhou C, Jiang H, Liang L, Shi W,
Zhang Q, Sun P, Xiang R, Wang Y and Yang S: ZEB1 induces ER-α
promoter hypermethylation and confers antiestrogen resistance in
breast cancer. Cell Death Dis. 8:e27322017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng F, Li TT, Wang KL, Xiao GQ, Wang JH,
Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M, et al: H19/let-7/LIN28
reciprocal negative regulatory circuit promotes breast cancer stem
cell maintenance. Cell Death Dis. 8:e25692017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu QN, Wang G, Guo Y, Peng Y, Zhang R,
Deng JL, Li ZX and Zhu YS: LncRNA H19 is a major mediator of
doxorubicin chemoresistance in breast cancer cells through a
cullin4A-MDR1 pathway. Oncotarget. 8:91990–92003. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basak P, Chatterjee S, Bhat V, Su A, Jin
H, Lee-Wing V, Liu Q, Hu P, Murphy LC and Raouf A: Long non-coding
RNA H19 acts as an estrogen receptor modulator that is required for
endocrine therapy resistance in ER+ breast cancer cells. Cell
Physiol Biochem. 51:1518–1532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Zhou Y, He J, Sun H and Jin Z: Long
non-coding RNA H19 mediates ovarian cancer cell
cisplatin-resistance and migration during EMT. Int J Clin Exp
Pathol. 12:2506–2515. 2019.PubMed/NCBI
|
|
15
|
Ye Y, Guo J, Xiao P, Ning J, Zhang R, Liu
P, Yu W, Xu L, Zhao Y and Yu J: Macrophages-induced long noncoding
RNA H19 up-regulation triggers and activates the miR-193b/MAPK1
axis and promotes cell aggressiveness in hepatocellular carcinoma.
Cancer Lett. 469:310–322. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W, Ye XL, Xu J, Cao MG, Fang ZY, Li
LY, Guan GH, Liu Q, Qian YH and Xie D: The lncRNA H19 mediates
breast cancer cell plasticity during EMT and MET plasticity by
differentially sponging miR-200b/c and let-7b. Sci Signal.
10:eaak95572017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L, Xiao X, Lei J, Duan W, Ma Q and Li
W: Curcumin inhibits hypoxia-induced epithelial mesenchymal
transition in pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:3728–3734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gallardo M and Calaf GM: Curcumin inhibits
invasive capabilities through epithelial mesenchymal transition in
breast cancer cell lines. Int J Oncol. 49:1019–1027. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li G, Wang Z, Chong T, Yang J, Li H and
Chen H: Curcumin enhances the radiosensitivity of renal cancer
cells by suppressing NF-κB signaling pathway. Biomed Pharmacother.
94:974–981. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Xie S, Yang J, Xiong H, Jia Y,
Zhou Y, Chen Y, Ying X, Chen C, Ye C, et al: The long noncoding RNA
H19 promotes tamoxifen resistance in breast cancer via autophagy. J
Hematol Oncol. 12:812019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mills JN, Rutkovsky AC and Giordano A:
Mechanisms of resistance in estrogen receptor positive breast
cancer: Overcoming resistance to tamoxifen/aromatase inhibitors.
Curr Opin Pharmacol. 41:59–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Si H, Chen P, Li H and Wang X: Long
non-coding RNA H19 regulates cell growth and metastasis via miR-138
in breast cancer. Am J Transl Res. 11:3213–3225. 2019.PubMed/NCBI
|
|
26
|
Liao S, Yu C, Liu H, Zhang C, Li Y and
Zhong X: Long non-coding RNA H19 promotes the proliferation and
invasion of lung cancer cells and regulates the expression of
E-cadherin, N-cadherin, and vimentin. OncoTargets Ther.
12:4099–4107. 2019. View Article : Google Scholar
|
|
27
|
Jiang GM, Wang HS, Zhang F, Zhang KS, Liu
ZC, Fang R, Wang H, Cai SH and Du J: Histone deacetylase inhibitor
induction of epithelial-mesenchymal transitions via up-regulation
of Snail facilitates cancer progression. Biochim Biophys Acta.
1833:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer - observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al Khatib AM, Stepan AE, Margaritescu C,
Simionescu C and Ciurea RN: E-cadherin and snail immunoexpression
in colorectal adenocarcinomas. Curr Health Sci J. 45:204–209.
2019.PubMed/NCBI
|
|
30
|
Hu S, Xu Y, Meng L, Huang L and Sun H:
Curcumin inhibits proliferation and promotes apoptosis of breast
cancer cells. Exp Ther Med. 16:1266–1272. 2018.PubMed/NCBI
|
|
31
|
Cao MT, Liu HF, Liu ZG, Xiao P, Chen JJ,
Tan Y, Jiang XX, Jiang ZC, Qiu Y, Huang HJ, et al: Curcumin
downregulates the expression of Snail via suppressing Smad2 pathway
to inhibit TGF-β1-induced epithelial-mesenchymal transitions in
hepatoma cells. Oncotarget. 8:108498–108508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Jiang Z, Xiao X, Wang Z, Wu Z, Ma Q
and Cao L: Curcumin inhibits superoxide dismutase-induced
epithelial-to-mesenchymal transition via the PI3K/Akt/NF-κB pathway
in pancreatic cancer cells. Int J Oncol. 52:1593–1602.
2018.PubMed/NCBI
|