Introduction
Endometrial cancer (EC) is a common malignant
gynecological tumor arising from the endometrium and is the fourth
most common cause of cancer among females (1–3). It
currently accounts for ~50% of newly diagnosed gynecological
malignancies (4). The morbidity and
mortality of EC are increasing year by year, which negatively
affects the health of females worldwide (5,6).
Although significant advances in therapeutic strategies have been
achieved, including hormonal agents, cytotoxic chemotherapy and
surgery combined with adjuvant chemotherapy, which contribute
towards improvements in the survival rates of patients with EC, the
prognosis of patients with EC remains poor (7,8).
Therefore, understanding the pathogenesis of EC is important to
identify preventative and therapeutic strategies for EC.
Cullin 4A (CUL4A), a member of the cullin family,
acts as a scaffold protein to bind DNA damage-binding protein 1 and
subsequently form a ubiquitin ligase E3 complex to mediate the
degradation of numerous substrates (9,10).
Therefore, CUL4A mediates multiple cellular processes, including
proliferation, DNA replication, apoptosis and hematopoiesis
(11,12). Emerging evidence supports that CUL4A
is responsible for the regulation of certain tumor suppressor
genes, including p53 and p27 (13,14).
Increasing evidence suggests that CUL4A expression was highly
upregulated in a variety of different types of human cancer,
including non-small cell lung, colorectal, ovarian and breast
cancer, which promoted tumor cell proliferation, invasion and
migration (15–18). However, the biological functions of
CUL4A in EC are not completely understood.
In the present study, the expression of CUL4A in
several EC cell lines and human endometrial epithelial cells
(hEECs), as well as the potential underlying mechanisms, were
examined. The results indicated that CUL4A regulated EC cell
proliferation, invasion and migration by interacting with COP9
signalosome subunit 6 (CSN6), which provided a theoretical basis
and potential therapeutic strategy for EC.
Materials and methods
The Cancer Genome Atlas (TCGA)
database analysis
Human RNA-sequencing data from uterine corpus
endometrial carcinoma (UCEC) projects, which included 546 patients
with EC and 35 normal tissues were obtained from TCGA analysis in
UALCAN database (ualcan.path.uab.edu/). Student's t-test was used to
determine statistical significance of CUL4A expression between
normal and tumor samples.
Cell culture
Several human endometrial cancer cell lines
(Ishikawa, KLE, RL95-2 and AN3CA) and a normal human endometrial
epithelial cell line (hEECs) were obtained from Shanghai Institute
for Biological Sciences (Shanghai, China. Cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2.
Cell transfection
The short hairpin (sh) RNAs targeted against CUL4A
(shRNA-CUL4A-1 and shRNA-CUL4A-2) and a negative control (NC)
scrambled shRNA (shRNA-NC) were provided by Shanghai GenePharma
Co., Ltd.). In addition, a CSN6-overexpression plasmid (pcDNA-CSN6)
and its empty vector (pcDNA-NC) were purchased from of Shanghai
Integrated Biotech Solutions Co., Ltd. Cells were plated into
6-well plates (1×106 cells/well) overnight.
Subsequently, cells were transfected using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 24 h post-transfection, transfection
efficiency was assessed via western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Cell Counting kit-8 (CCK-8) assay
Cell proliferation was assessed using the CCK-8 kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), according to the
manufacturer's protocol. In brief, transfected RL95-2 cells were
cultured on a 96-well plate and incubated for 24, 48 or 72 h at
37°C. Subsequently, 10 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc., Rockville, MD, USA) was added to each well.
Following incubation for 2 h at 37°C, the optical density at a
wavelength of 450 nm was detected using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Transwell invasion assay
A Transwell invasion assay was conducted using 8-µm
Transwell chambers (Corning Inc.) coated with diluted BD Matrigel
(BD Biosciences) overnight at 37°C. In brief, 2×105
cells were cultured in the upper chamber with serum-free DMEM, and
medium containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
was plated in the lower chamber. Following incubation for 24 h at
37°C, cells on the lower surface of the membrane were fixed with
methanol for 20 min at 37°C and stained with crystal violet for 30
min at 37°C. The number of invaded cells was counted and images
were captured using an inverted light microscope (magnification,
×200).
Wound healing assay
Cell migration was assessed by conducting a wound
healing assay. Cells were plated onto 6-well plates and cultured
overnight at 37°C in a humidified atmosphere. At 80% confluence, a
linear wound was gently created in the cell monolayer using a
pipette tip and the cells were washed three times with
phosphate-buffered saline (PBS). Cells were incubated in FBS-free
DMEM for 48 h at 37°C. Migratory cells were observed using an IX711
phase contrast microscope (Olympus Corporation) at 0 and 48 h
(magnification, ×200).
Co-immunoprecipitation (IP) assay
For co-IP assays, RL95-2 cells were washed in 2 ml
PBS (Beyotime Institute of Biotechnology) and centrifuged at 850 ×
g at room temperature for 5 min to collect the cells. Then, cells
were lysed in lysis buffer for IP (Beyotime Institute of
Biotechnology). Lysates were incubated with 1 µg antibodies against
CUL4A (1:1,000; cat. no. 2699T; Cell Signaling Technology, Inc.),
CSN6 (1:1,000; cat. no. sc-393023; Santa Cruz Biotechnology, Inc.)
and control lgG (1:1,000; cat. no. 3900S; Cell Signaling
Technology, Inc) plus Protein A/G beads (Santa Cruz Biotechnology,
Inc.) at 4°C for 2 h. After washing the beads with PBS (Beyotime
Institute of Biotechnology) three times, immunoprecipitates were
analyzed via western blotting.
RT-qPCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using a Reverse
Transcription kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Subsequently, qPCR was performed using
SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) and an ABI 7500
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: Pre denaturation at
95°C for 10 min, then 40 cycles of denaturation at 95°C for 15 sec
and annealing at 60°C for 1 min. The following primer sequences
were used: CUL4A, forward, 5′-AAGAGCAGGCAACAAAGAAGCCAC-3′ and
reverse 5′-TTGGCCAGTAGCCCATTGTGAGTA-3′; CSN6, forward,
5′-TCATCGAGAGCCCCCTCTTT-3′ and reverse, 5′-CCAATGCGTTCCGCTTCCT-3′;
and GAPDH, forward, 5′-GAGTCAACGGATTTGGTCG-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′. Relative expression was quantified
using the 2−ΔΔCq method and normalized to the internal
reference gene GAPDH (19).
Western blotting
Total protein was extracted using RIPA buffer
(Beyotime Institute of Biotechnology). Total protein was quantified
using a BCA kit. Subsequently, equal amounts of protein (40
µg/lane) were separated via 10% SDS-PAGE and transferred onto
polyvinylidene membranes (EMD Millipore; Merck KGaA), which were
blocked with 5% skimmed fat milk overnight at 4°C. The membranes
were incubated with primary antibodies. Following primary
incubation, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:3,000; cat. no. 7074S;
Cell Signaling Technology, Inc.) at room temperature for 1.5 h.
Proteins were visualized using Image Quant LAS 4000 (Cytiva) and
scanned using ImageJ software (National Institutes of Health) with
GAPDH as the loading control. Anti-CUL4A (cat. no. 2699T),
anti-matrix metallopeptidase (MMP)2 (cat. no. 40994S), anti-MMP9
(cat. no. 13667T), anti-p53 (cat. no. 2527T) and anti-GAPDH (cat.
no. 5174T) antibodies (all 1:1,000) were obtained from Cell
Signaling Technology, Inc.
Statistical analysis
All experiments were repeated three times
independently. Data are presented as the mean ± standard deviation.
Statistical analyses were conducted using SPSS (version 19.0; IBM
Corp.) and GraphPad Prism (version 6; GraphPad Software, Inc.)
software. Comparisons between two groups were analyzed using the
unpaired Student's t-test. Comparisons among multiple groups were
analyzed using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CUL4A is highly expressed in EC
tissues and cells
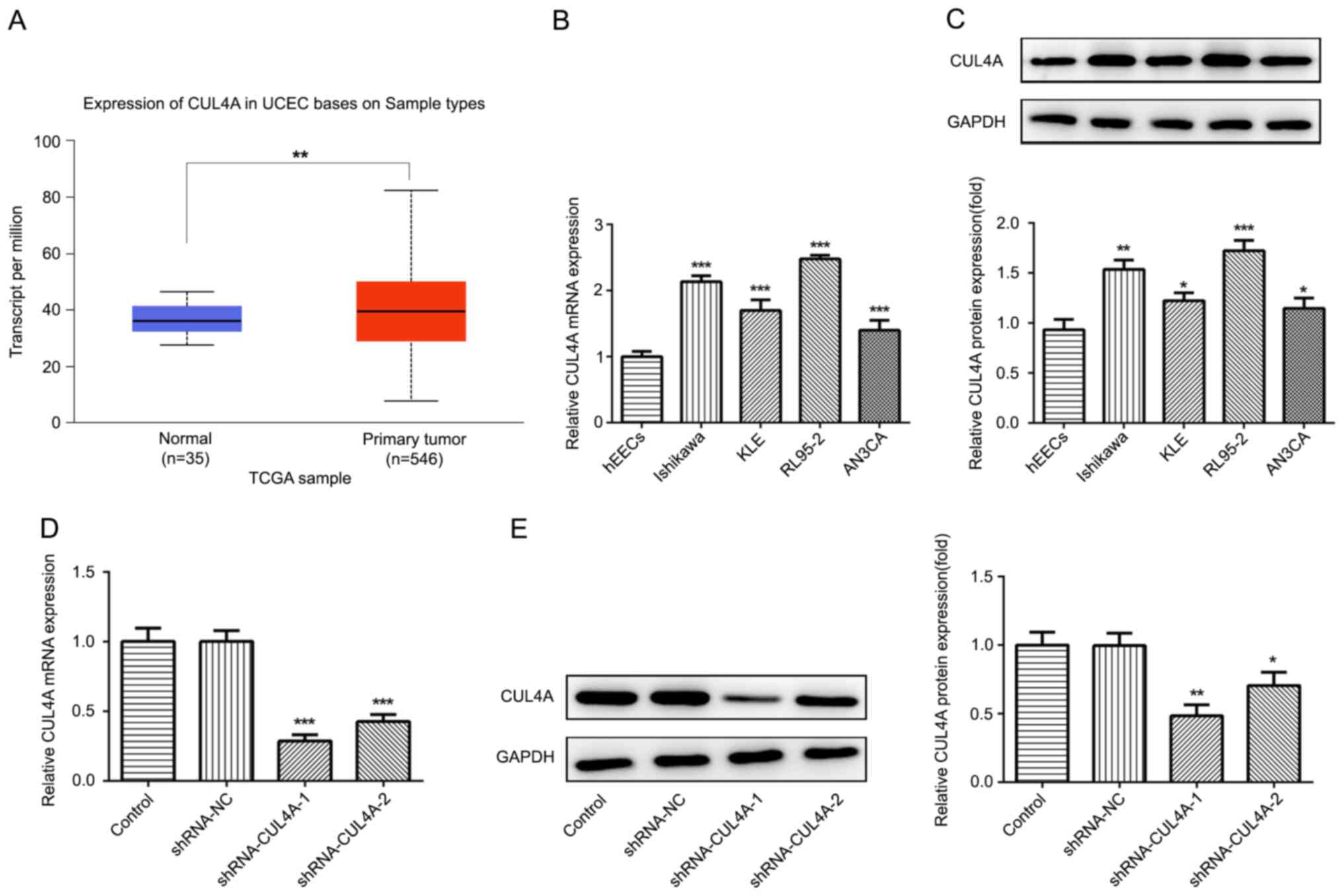
To investigate the functions of CUL4A in EC, TCGA
database was used to analyze the level of CUL4A in EC tumor tissues
(n=546) and adjacent non-tumor tissues (n=35). As presented in
Fig. 1A, the expression level of
CUL4A was increased in EC tissues compared with that in adjacent
non-tumor tissues. Next, Ishikawa, KLE, RL95-2 and AN3CA EC cell
lines, as well as one normal human endometrial epithelial cell line
(hEECs) were employed to determine the level of CUL4A via RT-qPCR
and western blotting. The mRNA and protein expression of CUL4A was
markedly upregulated in EC cell lines, compared with hEECs
(Fig. 1B and C). The expression of
CUL4A was highest in RL95-2 cells; therefore, RL95-2 cells were
selected for subsequent experiments.
 | Figure 1.CUL4A is highly expressed in EC
tissues and cell lines. (A) TCGA database was used to analyze the
mRNA expression level of PITPNA-AS1 in NSCLC tumor tissues and
adjacent non-tumor tissues. **P<0.01 vs. normal. CUL4A
expression in EC cell lines (Ishikawa, KLE, RL95-2 and AN3CA) and
normal human endometrial epithelial cells (hEEC) was examined via
(B) RT-qPCR and (C) western blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. hEEC. CUL4A expression following transfection
with shRNA-CUL4A-1 or shRNA-CUL4A-2 was detected via (D) RT-qPCR
and (E) western blotting. *P<0.05, **P<0.01 and ***P<0.001
vs. shRNA-NC. CUL4A, cullin 4A; EC, endometrial cancer; TCGA, The
Cancer Genome Atlas; NSCLC, non-small cell lung cancer; RT-qPCR,
reverse transcription-quantitative PCR; shRNA, short hairpin RNA;
NC, negative control. |
CUL4A-knockdown inhibits EC
proliferation, invasion and migration
The effects of CUL4A on the functions of EC cells
were investigated in the present study. To begin with, RL95-2 cells
were transfected with shRNA-CUL4A-1 or shRNA-CUL4A-2. The levels of
CUL4A were significantly decreased following transfection with
shRNA-CUL4A, compared with shRNA-NC (Fig. 1D and E). shRNA-CUL4A-1 induced lower
expression levels of CUL4A, compared with shRNA-CUL4A-2, and was
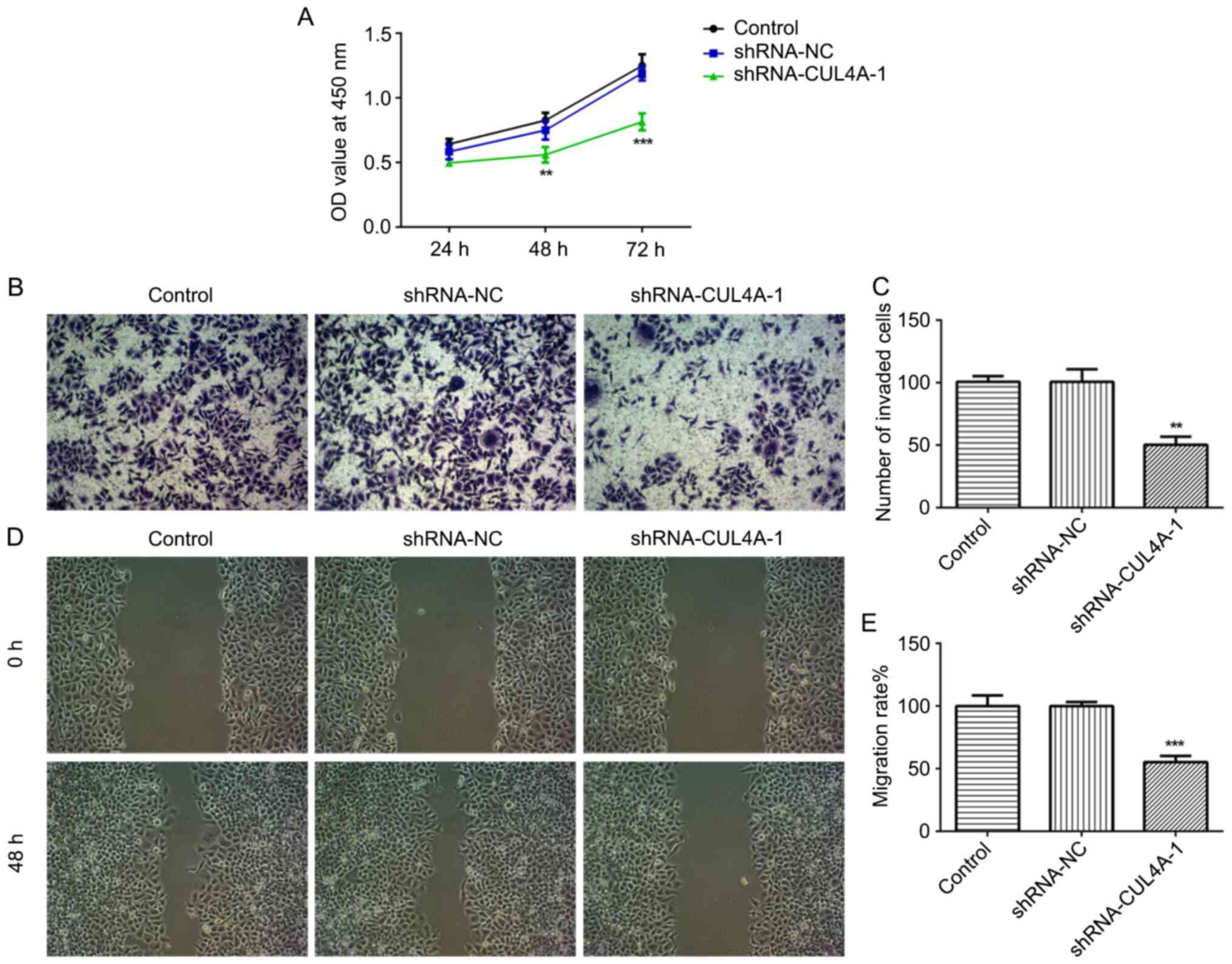
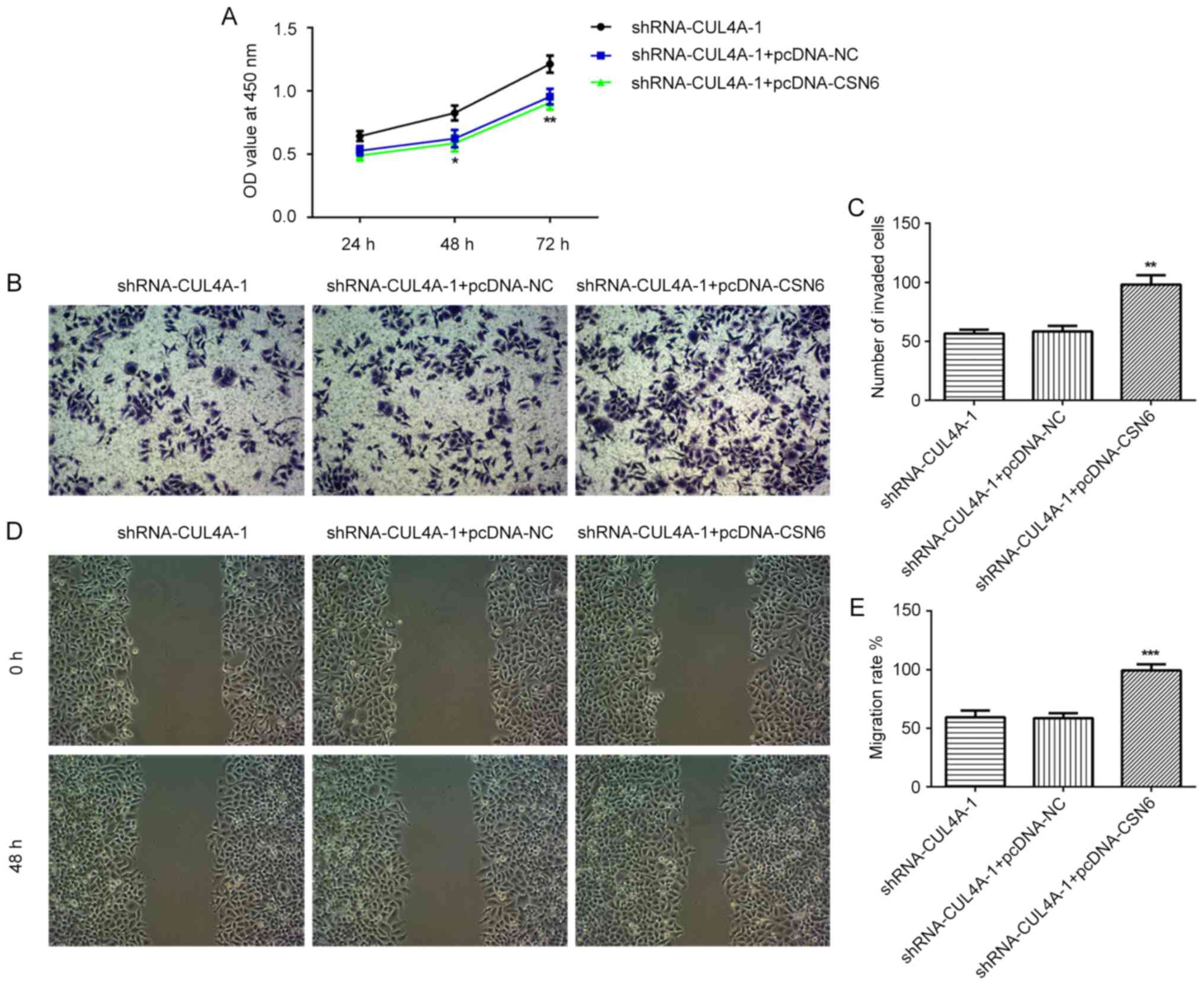
used for subsequent experiments. The CCK-8 assay indicated that
CUL4A-knockdown inhibited RL95-2 cell proliferation, compared with
shRNA-NC (Fig. 2A). In addition,
RL95-2 cell invasion and migration were markedly suppressed by
shRNA-CUL4A-1, compared with shRNA-NC (Fig. 2B-E). Furthermore, western blotting
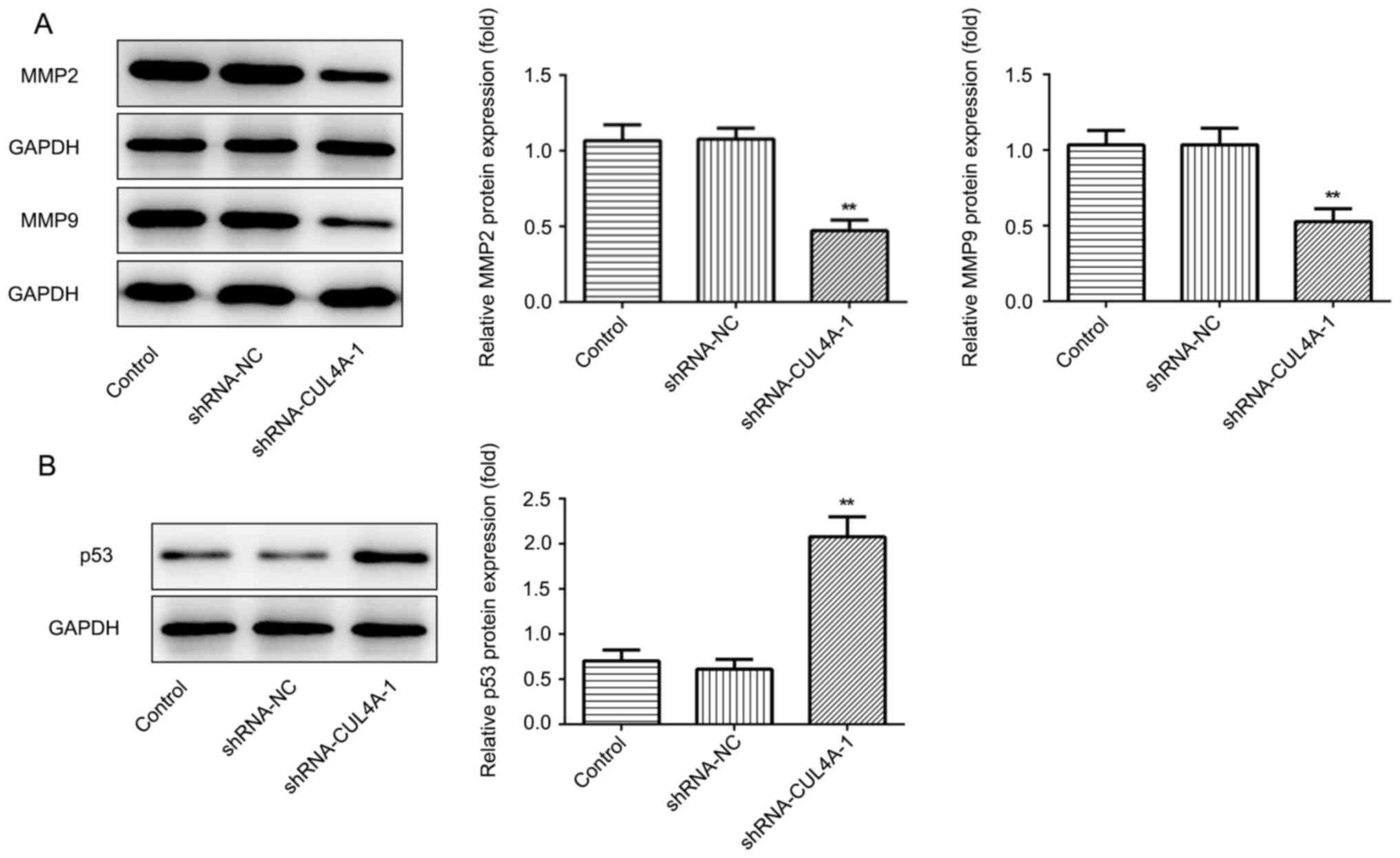
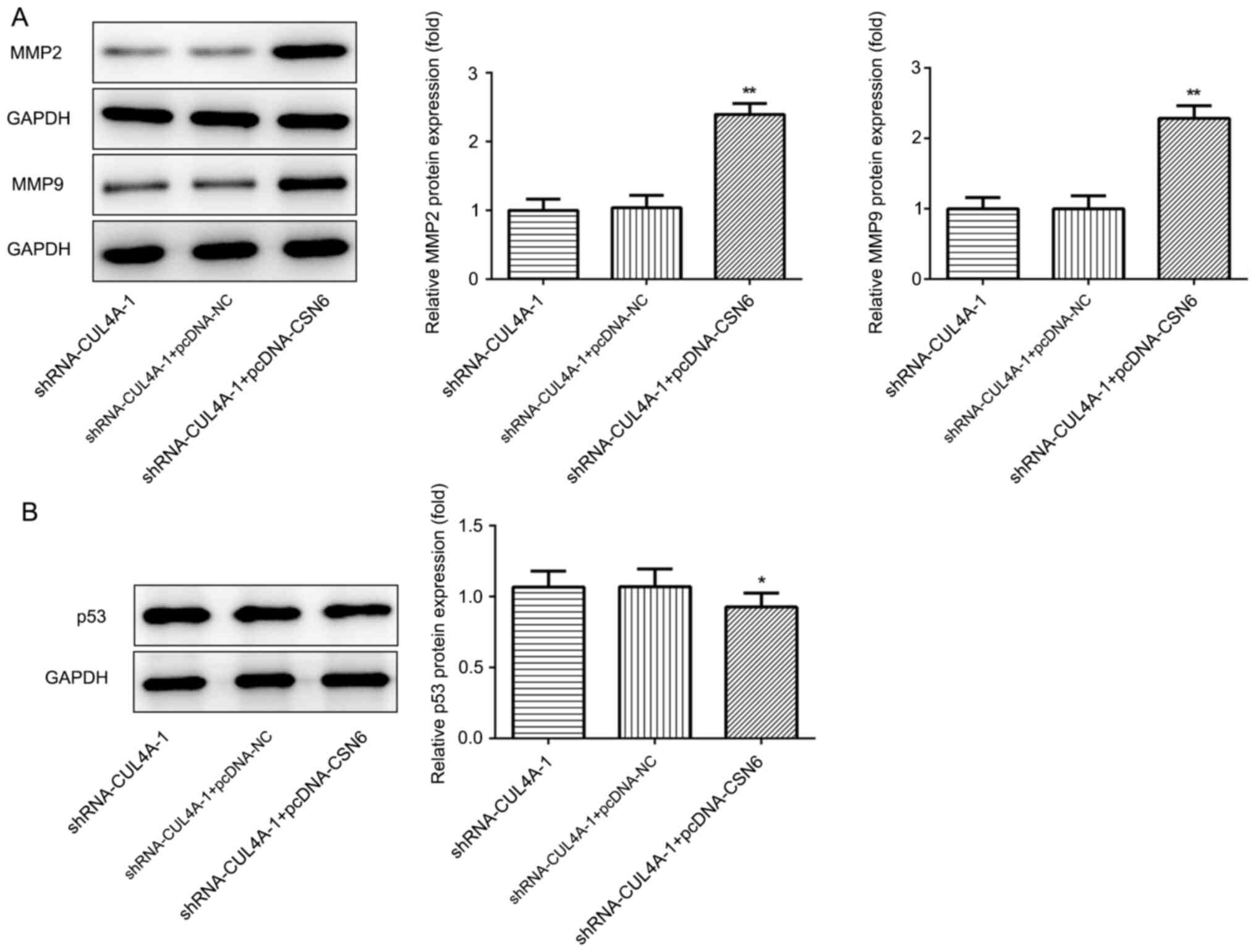
was performed to detect the expression of migration-related
proteins MMP2 and MMP9. CUL4A-knockdown decreased the expression of
MMP2 and MMP9, compared with shRNA-NC (Fig. 3A). The results demonstrated that
CUL4A-knockdown inhibited EC cell proliferation, invasion and
migration.
CUL4A-knockdown promotes the
expression of p53
Western blotting was performed to assess the effect
of CUL4A-knockdown on the expression of p53, a tumor suppressor
gene regulated by CUL4A. shRNA-CUL4A transfection notably
upregulated p53 protein expression, compared with shRNA-NC
(Fig. 3B). The results indicated
that CUL4A-knockdown increased p53 expression.
CSN6 interacted with CUL4A
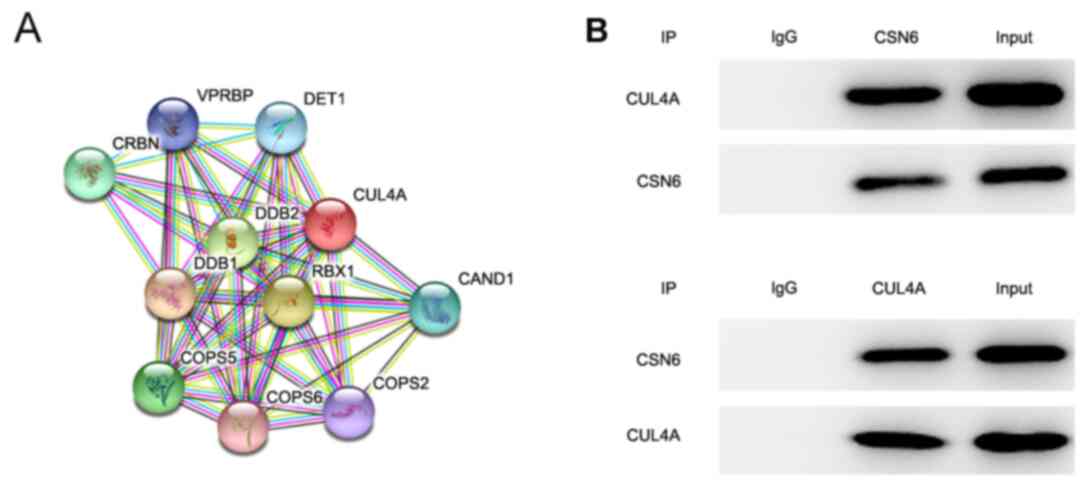
To further investigate the molecular mechanisms
underlying CUL4A-mediated EC cell proliferation, invasion and
migration, the STRING database was applied to detect the potential
proteins interacting with CULA4. The results indicated that CSN6
interacted with CULA4 (Fig. 4A).
CSN6 is a pivotal subunit of the constitutive photomorphogenesis 9
(COP9) signalosome (CSN). To further verify the interaction between
CUL4A and CSN6, a Co-IP assay was performed. The results indicated
that there was a strong interaction between CUL4A and CSN6
(Fig. 4B). Taken together, these
results indicated that CSN6 interacted with CUL4A in RL95-2
cells.
CSN6-overexpression attenuates the
inhibitory effects of CUL4A-knockdown on EC cell proliferation,
invasion and migration
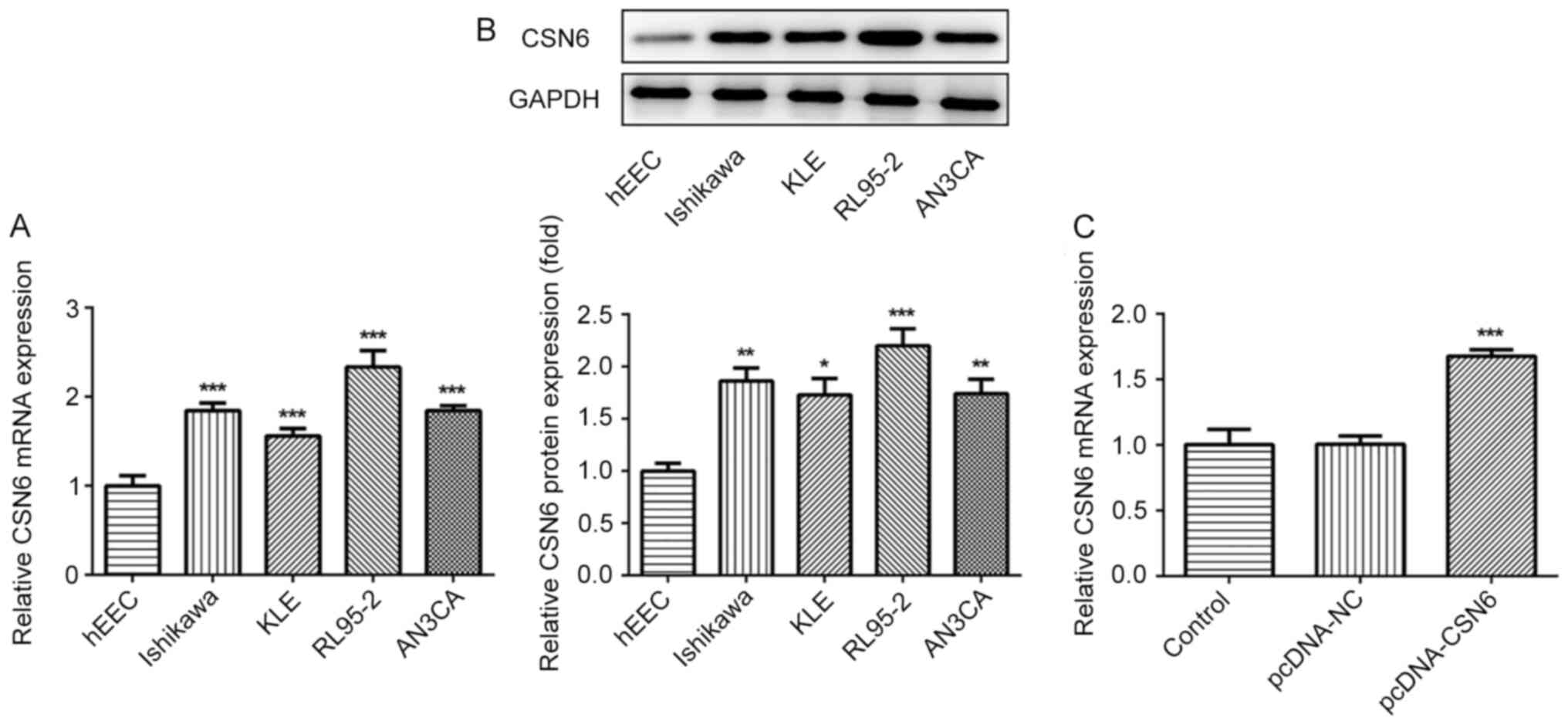
The expression of CSN6 in EC cell lines was
evaluated via RT-qPCR and western blotting. The level of CSN6 was
markedly increased in EC cell lines, compared with hEECs (Fig. 5A and B). Subsequently, a
CSN6-overexpression plasmid was transfected into cells, which
notably increased CSN6 expression, compared with pcDNA-NC (Fig. 5C). Subsequently, the effects of
CSN6-overexpression on CUL4A-knockdown RL95-2 cell proliferation,
invasion and migration were detected. CSN6-overexpression relieved
the inhibitory effects of CUL4A-knockdown on RL95-2 cell
proliferation (Fig. 6A).
Furthermore, CSN6-overexpression increased cell invasion (Fig. 6B and C) and migration (Fig. 6D and E) in CUL4A-knockdown RL95-2
cells. The expression levels of MMP2 and MMP9 presented the same
trend as aforementioned (Fig. 7A).
Taken together, these results indicated that CSN6-overexpression
attenuated the inhibitory effects of CUL4A knockdown on EC cell
proliferation, invasion and migration.
CSN6-overexpression downregulates the
expression of p53, compared with CUL4A-knockdown alone
To further clarify the regulatory mechanisms
underlying CUL4A in EC, the effects of CSN6-overexpression on p53
expression were examined via RT-qPCR and western blotting. As shown
in Fig. 7B, CSN6-overexpression
decreased the expression levels of p53 protein in CUL4A-knockdown
RL95-2 cells. The results indicated that CUL4A regulated the
expression of p53 by interacting with CSN6.
Discussion
As one of the most common gynecological malignancies
worldwide, the morbidity and mortality of EC are increasing.
Although a number of studies have suggested that diverse aberrantly
expressed genes in EC may aggravate malignant behavior (20,21),
the mechanism underlying the progression and metastasis of EC
requires further investigation. To the best of our knowledge, the
present study was the first to investigate the expression status,
functions and molecular mechanisms underlying CUL4A in EC. The
results indicated that CUL4A regulated EC cell proliferation,
invasion and migration by interacting with CSN6, which provided a
theoretical basis and potential therapeutic target for the
treatment of EC.
Increasing evidence has indicated that the
expression of CUL4A is markedly increased in a variety of types of
human cancer, and may serve as a potential oncogene (17,22).
For example, CUL4A was overexpressed in the tissues of patients
with non-small cell lung cancer, and CUL4A-knockdown suppressed
lung cancer cell invasion and metastasis (23). CUL4A inhibition also restrained the
progression of breast cancer (24).
Additionally, CUL4A is highly expressed in ovarian cancer and
functions as an oncogene (25).
However, the functions of procollagen-lysine, 2-oxoglutarate
5-dioxygenase 3 in EC have not been reported and require further
investigation. Increasing evidence has suggested that uncontrolled
proliferation, invasion and migration are dominant features of
cancer cells, which exert crucial effects in the development
process of human cancer (26,27).
In the present study, notably upregulated CUL4A expression was
observed in EC cells, and CUL4A-knockdown refrained EC cell
proliferation, invasion and migration, which was consistent with
previous studies (28,29). Taken together, these results
indicated that CUL4A-knockdown serves protective roles in EC.
CUL4A participates in the proteolysis of p53, and
CUL4A depletion results from an accumulation of p53 (13). p53 is a tumor suppressor gene, which
is involved in the growth and metastasis of various types of cancer
(30–32). CSN6, a pivotal subunit of the
constitutive COP9 CSN, is notably upregulated in a number of
different types of human cancer (33,34).
Previous studies have highlighted the importance of CSN6 as a
regulator of the degradation of cancer-related protein p53
(35,36). In the present study, the STRING
database identified that CSN6 interacted with CULA4, and the Co-IP
assay verified this interaction. The expression of CSN6 was
significantly enhanced in EC cells, and CSN6 overexpression
attenuated the inhibitory effects of CUL4A-knockdown on EC cell
proliferation, invasion and migration. Furthermore, CSN6
overexpression markedly reduced p53 expression in CUL4A-knockdown
EC cells. The results suggested that CUL4A interacted with CSN6 and
further modulated p53 expression.
In conclusion, to the best of our knowledge, the
present study was the first to investigate the pivotal roles of
CUL4A in the functions of EC cells. CUL4A regulated EC cell
proliferation, invasion and migration by interacting with CSN6 and
further modulating p53 expression. The results also suggested that
CUL4A may serve as a potential biomarker and therapeutic target for
the treatment of EC, which provides an innovative perspective for
the clinical therapy of EC. However, the usage of only one EC cell
line, lack of pull down experiment using the purified CUL4 and CSN6
proteins and the addition of EtBr to the co-IP are limitations of
the present study, a comprehensive analysis resolving these issues
is required in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XW searched the literature, designed the
experiments, analyzed the data and wrote the manuscript. XW and TC
performed the experiments. TC revised the manuscript. All authors
read and approval the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Tucci C, Capone C, Galati G, Iacobelli
V, Schiavi MC, Di Donato V, Muzii L and Panici PB: Immunotherapy in
endometrial cancer: New scenarios on the horizon. J Gynecol Oncol.
30:e462019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shu S, Liu X, Xu M, Gao X, Fan J, Liu H
and Li R: MicroRNA-424 regulates epithelial-mesenchymal transition
of endometrial carcinoma by directly targeting insulin-like growth
factor 1 receptor. J Cell Biochem. 2018.(Epub ahead of print).
|
|
3
|
Sun P, Mao X, Gao M, Huang MM, Chen LL,
Ruan GY, Huang WY, Braicu EI and Sehouli J: Novel endocrine
therapeutic strategy in endometrial carcinoma targeting
estrogen-related receptor b by XCT790 and siRNA. Cancer Manag Res.
10:2521–2535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bendifallah S, Ballester M and Darai E:
Endometrial cancer: Predictive models and clinical impact. Bull
Cancer. 104:1022–1031. 2017.(in French). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tran AQ and Gehrig P: Recent advances in
endometrial cancer. F1000Res. 6:812017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wright JD, Burke WM, Wilde ET, Lewin SN,
Charles AS, Kim JH, Goldman N, Neugut AI, Herzog TJ and Hershman
DL: Comparative effectiveness of robotic versus laparoscopic
hysterectomy for endometrial cancer. J Clin Oncol. 30:783–791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong W, Feng H, Santiago FE and Kipreos
ET: CUL-4 ubiquitin ligase maintains genome stability by
restraining DNA-replication licensing. Nature. 423:885–889. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higa LA, Wu M, Ye T, Kobayashi R, Sun H
and Zhang H: CUL4-DDB1 ubiquitin ligase interacts with multiple
WD40-repeat proteins and regulates histone methylation. Nat Cell
Biol. 8:1277–1283. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma P and Nag A: CUL4A ubiquitin
ligase: A promising drug target for cancer and other human
diseases. Open Biol. 4:1302172014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin X, Ma YC, Zhu WY and Fan L: CUL4A
expression is associated with tumor stage and prognosis in
nasopharyngeal carcinoma. Medicine (Baltimore). 98:e180362019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nag A, Bagchi S and Raychaudhuri P: Cul4A
physically associates with MDM2 and participates in the proteolysis
of p53. Cancer Res. 64:8152–8155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Jia N, Kapur R and Chun KT: Cul4A
targets p27 for degradation and regulates proliferation, cell cycle
exit, and differentiation during erythropoiesis. Blood.
107:4291–4299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bian WG, Zhou XN, Song S, Chen HT, Shen Y
and Chen P: Reduced miR-363-3p expression in non-small cell lung
cancer is associated with gemcitabine resistance via targeting of
CUL4A. Eur Rev Med Pharmacol Sci. 23:649–659. 2019.PubMed/NCBI
|
|
16
|
Sui XM, Zhou H, Zhu L, Wang DQ, Fan SM and
Zhao W: CUL4A promotes proliferation and metastasis of colorectal
cancer cells by regulating H3K4 trimethylation in
epithelial-mesenchymal transition. OncoTargets Ther. 10:735–743.
2017. View Article : Google Scholar
|
|
17
|
Han XN, Fang ZL, Wang H, Jiao RF, Zhou J
and Fang N: CUL4A functions as an oncogene in ovarian cancer and is
directly regulated by miR-494. Biochem Biophys Res Commun.
480:675–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YS, Liu XY, Zheng H, Wang Q, An L and
Wei GW: Suppression of CUL4A attenuates TGF-β 1-induced
epithelial-to-mesenchymal transition in breast cancer cells. Int J
Mol Med. 40:1114–1124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai L, Wang H and Yang Q: CRKL
overexpression promotes cell proliferation and inhibits apoptosis
in endometrial carcinoma. Oncol Lett. 13:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin QY, Chen H, Zhang MF, Xiong HZ and
Jiang QP: Knocking down FAM83B inhibits endometrial cancer cell
proliferation and metastasis by silencing the PI3K/AKT/mTOR
pathway. Biomed Pharmacother. 115:1089392019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang TJ, Xue D, Zhang CD, Zhang ZD, Liu
QR and Wang JQ: Cullin 4A is associated with epithelial to
mesenchymal transition and poor prognosis in perihilar
cholangiocarcinoma. World J Gastroenterol. 23:2318–2329. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hung MS, Chen YC, Lin PY, Li YC, Hsu CC,
Lung JH, You L, Xu Z, Mao JH, Jablons DM and Yang CT: Cul4A
modulates invasion and metastasis of lung cancer through regulation
of ANXA10. Cancers (Basel). 11:6182019. View Article : Google Scholar
|
|
24
|
Wang YS, Liu XY, Zheng H, Wang Q, An L and
Wei GW: [Corrigendum] Suppression of CUL4A attenuates TGF-β
1-induced epithelial-to-mesenchymal transition in breast cancer
cells. Int J Mol Med. 40:16112017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu RF, Cai LM, Chi YG, Ding XC and Wu XQ:
miR-377 targets CUL4A and regulates metastatic capability in
ovarian cancer. Int J Mol Med. 41:3147–3156. 2018.PubMed/NCBI
|
|
26
|
Liu Z, He W, Gao J, Luo J, Huang X and Gao
C: Computational prediction and experimental validation of a novel
synthesized pan-PIM inhibitor PI003 and its apoptosis-inducing
mechanisms in cervical cancer. Oncotarget. 6:8019–8035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie PM, Wang XL, Kong M, Bai XY and Jiang
T: TRAF4 promotes endometrial cancer cell growth and migration by
activation of PI3K/AKT/Oct4 signaling. Exp Mol Pathol. 108:9–16.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong Y, Xiang XJ, Feng M, Chen J, Fang ZL
and Xiong JP: CUL4A promotes cell invasion in gastric cancer by
activating the NF-κB signaling pathway. Biologics. 11:45–53.
2017.PubMed/NCBI
|
|
29
|
Xu YY, Wang YS, Ma GX, Wang Q and Wei GW:
CUL4A is overexpressed in human pituitary adenomas and regulates
pituitary tumor cell proliferation. J Neurooncol. 116:625–632.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao ZH, Wang WW, Niu LH, Zhang JY and
Jiang GZ: p53-induced long non-coding RNA PGM5-AS1 inhibits the
progression of esophageal squamous cell carcinoma through
regulating miR-466/PTEN axis. IUBMB Life. 71:1492–1502. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Chen HQ, Yang HF, Li Y, Chen DJ,
Huang YJ, He LX, Zheng CF, Wang LQ, Wang J, et al: Epigenetic
silencing of ALX4 regulates microcystin-LR induced hepatocellular
carcinoma through the P53 pathway. Sci Total Environ. 683:317–330.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De U, Son JY, Sachan R, Park YJ, Kang D,
Yoon K, Lee BM, Kim IS, Moon HR and Kim HS: A new synthetic histone
deacetylase inhibitor, MHY2256, induces apoptosis and autophagy
cell death in endometrial cancer cells via p53 acetylation. Int J
Mol Sci. 19:27432018. View Article : Google Scholar
|
|
33
|
Jhan JH, Lee YC, Li WM, Chang LL, Hsu WC,
Lin HH, Liang PI, Hsu YL, Wu WJ, Lee HY, et al: The prognostic
value of CSN6 expression in upper tract urothelial carcinomas.
Kaohsiung J Med Sci. 35:559–565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du WQ, Liu ZX, Zhu WT, Li TT, Zhu Z, Wei
L, Song J and Pei DS: CSN6 promotes tumorigenesis of gastric cancer
by ubiquitin-independent proteasomal degradation of
p16INK4a. Cancer Biol Med. 16:514–529. 2019.PubMed/NCBI
|
|
35
|
Hou J, Deng Q, Zhou J, Zou J, Zhang Y, Tan
P, Zhang W and Cui H: CSN6 controls the proliferation and
metastasis of glioblastoma by CHIP-mediated degradation of EGFR.
Oncogene. 36:1134–1144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang WQ, Tang M, Zhang L, Xu X, Qi X, Yang
Y, Jin F and Chen B: Clinical implications of CSN6 protein
expression and correlation with mutant-type P53 protein in breast
cancer. Jpn J Clin Oncol. 43:1170–1176. 2013. View Article : Google Scholar : PubMed/NCBI
|