Introduction
Pulmonary arterial hypertension (PAH) is a
relatively rare, debilitating and deadly lung disease diagnosed as
a hemodynamic disease through a cardiac catheter (1). There are a number of causes of
pulmonary hypertension, such as collagen disease, human
immunodeficiency virus infection and anorexia (2). PAH is characterized by continuous
vasoconstriction, rapid remodeling of small blood vessels, vascular
proliferation, and the aberrant growth of pulmonary arterial smooth
muscle cells (PASMCs), leading to a gradual increase in pulmonary
vascular resistance, and ultimately to right ventricular failure
and death (3). The migration and
proliferation of PASMCs are the main pathological bases of
pulmonary arterial remodeling and pulmonary hypertension (3,4).
Inhibition of abnormal proliferation and migration of PASMCs can
effectively reverse pulmonary artery remodeling, thereby reducing
pulmonary circulation resistance. Therefore, elucidating the
underlying mechanism of PASMC proliferation is expected to provide
novel targets for PAH treatment (5). Activated platelet-derived growth
factor (PDGF) is composed of polypeptides (A and B chains), forming
homo- or heterodimers and stimulating the surface receptors of A
and B cells (6). Abnormal PDGF
signaling may lead to atherosclerosis and aberrant remodeling of
peripheral blood vessel-related vessels (7). According to the literature, the
expression of PDGF and its receptor is increased in PAH PASMCs
(8,9). PDGF has been demonstrated to be a
powerful mitogen that stimulates excessive cell proliferation, and
has the ability to stimulate the excessive division and
proliferation of PASMCs both in vitro and in vivo
(10).
Nuclear factor of activated T cells (NFATs) were
first discovered in T cells as their main activator. The primary
function of NFATs is to increase the transcription of inflammatory
mediators and activate both T and B cells (11). Although inflammation is known to
play a role in PAH, it has not yet been adequately studied.
According to related articles, an increase in inflammatory
mediators has been found in the serum of patients with PAH and in
remodeled PA, and there is a clinical link between PAH and
autoimmune diseases such as scleroderma (12,13).
NFAT expression or function has been described in several types of
non-lymphocytes, including mast, endothelial and neuronal cells
(14). The PAH animal model also
showed NFAT expression in PASMCs (15). Bonnet et al (16) suggested that the
Ca2+-NFAT signaling pathway in patients with idiopathic
pulmonary hypertension is activated, which manifests as an increase
in Ca2+ and activation of calcineurin (CaN), thereby
promoting the phosphorylation of NFAT and its transposition. Once
inside the nucleus, NFAT combines with DNA sequences to promote
transcription and cell proliferation. Pulmonary arterioles in
normal subjects and those with secondary pulmonary hypertension
have low expression of NFAT1, whereas those with idiopathic
pulmonary hypertension have a high expression of NFAT1 (17). PAH is associated with abnormal
expression and activity of NFAT (17). Studies have demonstrated that the
expression of NFAT3 protein in the pulmonary arteries of PAH animal
models is increased, and serotonin 5-HT2A receptor antagonists can
downregulate the expression of NFAT protein, indicating that 5-HT2A
receptors may be involved in the process of NFAT3 upregulation
during PAH (18–20). A possible explanation is that
activation of the 5-HT2A receptors leads to an increase in
intracellular Ca2+, which promotes NFAT activation and
translocation into the nucleus to combine with gene sequences that
promote proliferation and inhibit apoptosis, resulting in cell
proliferation and eventually pulmonary artery refactoring.
PDGF reportedly regulates NFAT (21). However, how PDGF and NFAT are
related to the pathogenesis of PAH remains unclear. Therefore, the
present study aimed to explore the interaction between PDGF and
NAFT in the pathogenesis of PAH.
Materials and methods
Subculture and identification of
PASMCs
PASMCs were cultured following type II collagenase
digestion (22). A Sprague-Dawley
rat (aged 3 months and weighing 250 g) was anesthetized with
intraperitoneal sodium pentobarbital injection. The anesthetized
rat was fixed on the operating table, the chest cavity was exposed,
and the distal pulmonary artery was harvested under a
stereomicroscope with microsurgical scissors. After removing the
endothelial tissues, the blood vessels were cut into small pieces
with scissors and placed in type II collagenase to digest
flocculent material. The digestion was terminated with 20% DMEM
(Gibco; Thermo Fisher Scientific, Inc.) in high-sugar culture
solution and then centrifuged at 200 × g for 5 min at 4°C. The
lower layer of the precipitate was removed, resuspended in the DMEM
solution, and cultured in a flask. The fluid was changed the next
day, and identification was performed 1 week later. Primary
cultured cells were subcultured in flasks. PASMCs with good growth
from the second to sixth passages were used for subsequent
experiments. Anti-α-actin immunohistochemical staining was used to
identify PASMCs. PASMCs treated with PDGF were the PDGF group;
PASMCs treated with PBS were the Vehicle group; PASMCs without any
treatment were the Blank group. Cyclosporin A (CsA, C-3662) was
purchased from Sigma-Aldrich (Merck KGaA). The PASMCs were treated
with 0.5 µg/ml CsA for 48 h in the Colony formation and Transwell
experiments. The present study was approved by the Ethics Committee
of The Fourth Affiliated Hospital of Kunming Medical University,
The Second People's Hospital of Yunnan Province (Kunming,
China).
Cell viability and proliferation
A Cell Counting Kit-8 (CCK-8) assay was used to
detect PASMC viability. PASMCs were treated with different
concentrations (0, 10, 20, 50, 80, 100 and 200 ng/ml) of PDGF
(PeproTech Inc.) from day 1 to day 3 of the cell culture at 37°C
with 5% CO2, cells treated with 0 ng/ml PDGF served as
Control. Cells were seeded onto 96-well plates in the logarithmic
growth phase at a density of 5×104/well and cultured
overnight. After rewarming, 10 µl CCK-8 (Abcam) was added to each
cell group and cells were incubated for 1 h. The absorbance of
cells at 450 nm was measured by a microplate micrograph.
Colony formation assay
Cells in the logarithmic growth phase of each group
were digested with EDTA + 0.25% trypsin and blown into single
cells, and suspended in DMEM culture solution of 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). A total of ~200
cells were inoculated into a 10 cm cell culture dish containing 10
ml DMEM + 10% FBS, and the cells were evenly dispersed by gentle
rotation. The cells were placed in an incubator at 37°C, 5%
CO2 and saturated humidity for 10–14 days. The cell
growth was observed in time during the culture, and the culture was
stopped when the macroscopic clones appeared in the culture dish.
The supernatant was discarded and carefully washed twice with PBS.
The cells were fixed with 5 ml of methanol for 15 min at room
temperature and then stained using Giemsa solution for 15–30 min at
room temperature. Each treatment run triplicate, and the colonies
were counted with an optical microscope (Olympus Corporation) at
×10 magnification.
Cell cycle detection using flow
cytometry
PASMCs cultured in each group were digested with
0.25% trypsin (without EDTA), and the cells were digested to obtain
a single-cell suspension. The cells were collected by
centrifugation (at 200 × g) for 5 min at 4°C, and washed twice in
pre-chilled PBS, and then fixed overnight at 4°C with 70% ethanol.
After the fixation, the cells were treated with 500 µl PBS
containing 50 µg/ml propidium iodide (PI), 100 µg/ml RNase A, 0.2%
Triton X-100, and incubated for 30 min in the dark at 4°C. The
processed samples were detected by flow cytometry (CytoFLEX LX;
Beckman Coulter, Inc.). The data analysis was calculated using
FlowJo software (FlowJo LLC).
Western blotting
The PASMCs in each group were cultured overnight and
then lysed using RIPA buffer. The total protein was extracted in
RIPA buffer, separated on polyacrylamide gels (30 µg per well), and
immobilized on polyvinylidene fluoride (PVDF) membrane. After
blocking with 5% non-fat milk at room temperature for 1.5 h, the
membranes were incubated at 4°C overnight with primary antibodies;
when PVDF membranes were used, they were washed three times with
PBST (0.5% Tween-20 in PBS). Following washing in PBST, the
membranes were treated with horseradish peroxide (HRP)-conjugated
goat anti-rabbit IgG; (1:5,000; cat. no. ab205718) or goat
anti-mouse IgG H&L (1:5,000; cat. no. ab205719; Abcam)
secondary antibodies at room temperature for 2 h. Finally, an
enhanced chemiluminescence kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to treat the membrane for
visualization. The following primary antibodies were used in the
study (all from Abcam): Anti-cyclin D1 (1:2,000; cat. no.
ab134175), anti-CDK4 (1:1,000; cat. no. ab108357), anti-PCNA
(1:1,000; cat. no. ab29), anti-NFATc2 (1:1,000; cat. no. ab169140),
anti-NFATc4 (1:1,000; cat. no. ab3447), anti-β-actin (1:1,000; cat.
no. ab8227) and anti-laminin B1 (1:5,000; cat. no. ab256380).
The isolation of nuclear and cytoplasmic fractions
was carried out according to the REAP method (23). Briefly, cells were washed twice with
ice-cold PBS, and the cell pellet was lysed in 0.1% NP-40 PBS lysis
buffer. The nuclei were then isolated by differential
centrifugation at 10,000 × g for 10 sec at 4°C, and the supernatant
was retained as the cytoplasmic fraction. For western blotting, the
nuclei were sonicated in 0.1% NP-40 PBS lysis buffer for further
analysis. The samples were kept on ice during sonication, and the
frequency was 20 kHz, 2 pulses, 8 sec.
Wound healing assay
Horizontal lines were drawn across the well with a
marker pen and ruler on the back of the 6-well plate. Cell
suspensions at a concentration of 1×106/ml were
prepared, and 1×105/ml cells were added to each well.
Vertical lines were scratched with a smaller pipette tip and ruler.
Then, the cells were washed three times in PBS after which the
floating cells were removed and serum-free medium was added. These
cells were cultured at 37°C with 5% CO2 for 24 h and
images were obtained thereafter (24), using an optical microscope (Olympus
Corporation) at ×40 magnification. The quantification was
calculated with ImageJ software (v 1.80, National Institutes of
Health).
Reverse transcription-quantitative
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The purity
and concertation of extracted RNA were determined by the NanoDrop™
ND-1000 (Thermo Fisher Scientific, Inc.). PrimeScript™ RT reagent
Kit (Takara Bio, Inc.) was used to prepare cDNA according to the
manufacturer's instructions. RT-qPCR was carried out using the SYBR
Green PCR Master Mix (Takara Bio, Inc.), under the following
conditions: 94°C for 5 min, followed by 32 cycles of 94°C for 40
sec, 57°C for 40 sec and 72°C for 1 min, with a final elongation
step at 72°C for 5 min. RT-qPCR was performed in triplicate, and
the expression of RNA was calculated using the 2−ΔΔCq
method (25). mRNA expression was
normalized against GAPDH, allowing comparison of mRNA levels. The
primers used in this study are listed in Table I.
 | Table I.List of primers used in reverse
transcription-quantitative PCR. |
Table I.
List of primers used in reverse
transcription-quantitative PCR.
| Gene name | Primer sequences
(5′→3′) |
|---|
| NFATc1 | F:
CACTCAGAAAATCCCAACTTACC |
|
| R:
CAGGTTTGGGGTCATTCTGC |
| NFATc2 | F:
CAGGCAGGTTCACAGGTGTG |
|
| R:
CTGAGATGTCCTGCAACAACC |
| NFATc3 | F:
CTGCTGCTGCTGGTGAGATC |
|
| R:
GGTAAAGAAGGCCCCTACCATC |
| NFATc4 | F:
GGCTACAATGAGAAGCCATTG |
|
| R:
CTTCAGGATTCCAGCACAGTC |
| NFAT5 | F:
CAAACCAGAACCCGATGGC |
|
| R:
GTGACCCTTGAACCAACTGG |
Transwell migration assay
Cell migration and invasion were measured using
Transwell migration assay as previously described (26). Briefly, 400 ml culture medium (DMEM
+ 1% FBS; Gibco; Thermo Fisher Scientific, Inc.) and 600 ml
complete medium (DMEM + 10% FBS; Gibco; Thermo Fisher Scientific,
Inc.) were separately added to the upper and lower chambers,
respectively; 5×104 cells/ml were plated in the upper
well. After incubation for 36 h at 37°C, a cotton-tipped swab was
used to remove the non-invading cells away from the upper surface
of the membrane. Following which, the invading cells were fixed
with methanol for 10 min and stained with 0.1% crystal violet
hydrate for 5 min at room temperature. The stained cells were
counted as cells per field using an optical microscope (Olympus
Corporation) at ×10 magnification, at least three fields were
observed for each experiment.
Small interfering (si)RNA
PASMCs were transfected with non-targeting control
siRNA or NFATc2 siRNA using Lipofectamine® 2000 reagent
(Thermo Fisher Scientific, Inc.). The following primer sequences
were used: NFATc2 siRNA sense, 5′-UCAGUAAACACAACUUUGGACCC-3′ and
antisense, 5′-CCAAAGUUGUGUUUACUGAGAUU-3′; and negative control
siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Non-targeting control siRNA and NFATc2
siRNA sequences were purchased from Ambion (Thermo Fisher
Scientific, Inc.). The concentration of control siRNA and NFATc2
siRNA was 60–80 nM. Following transfection for ~24 h, the PASMCs
were used in RT-qPCR, Transwell, colony formation and western
blotting assays.
Statistical analysis
SPSS version 26.0 (IBM Corp.) and GraphPad Prism 8
(GraphPad Software, Inc.) statistical software packages were used
for data analysis. ImageJ (version 1.80; National Institutes of
Health) was used to performed semi-quantitative analysis. All
experiments were conducted at least three times. The results are
presented as the mean ± standard deviation. Student's t-test or
one-way analysis of variance followed by a Tukey's post hoc test
were used to calculate the statistical differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
PDGF enhances PASMC viability and
proliferation
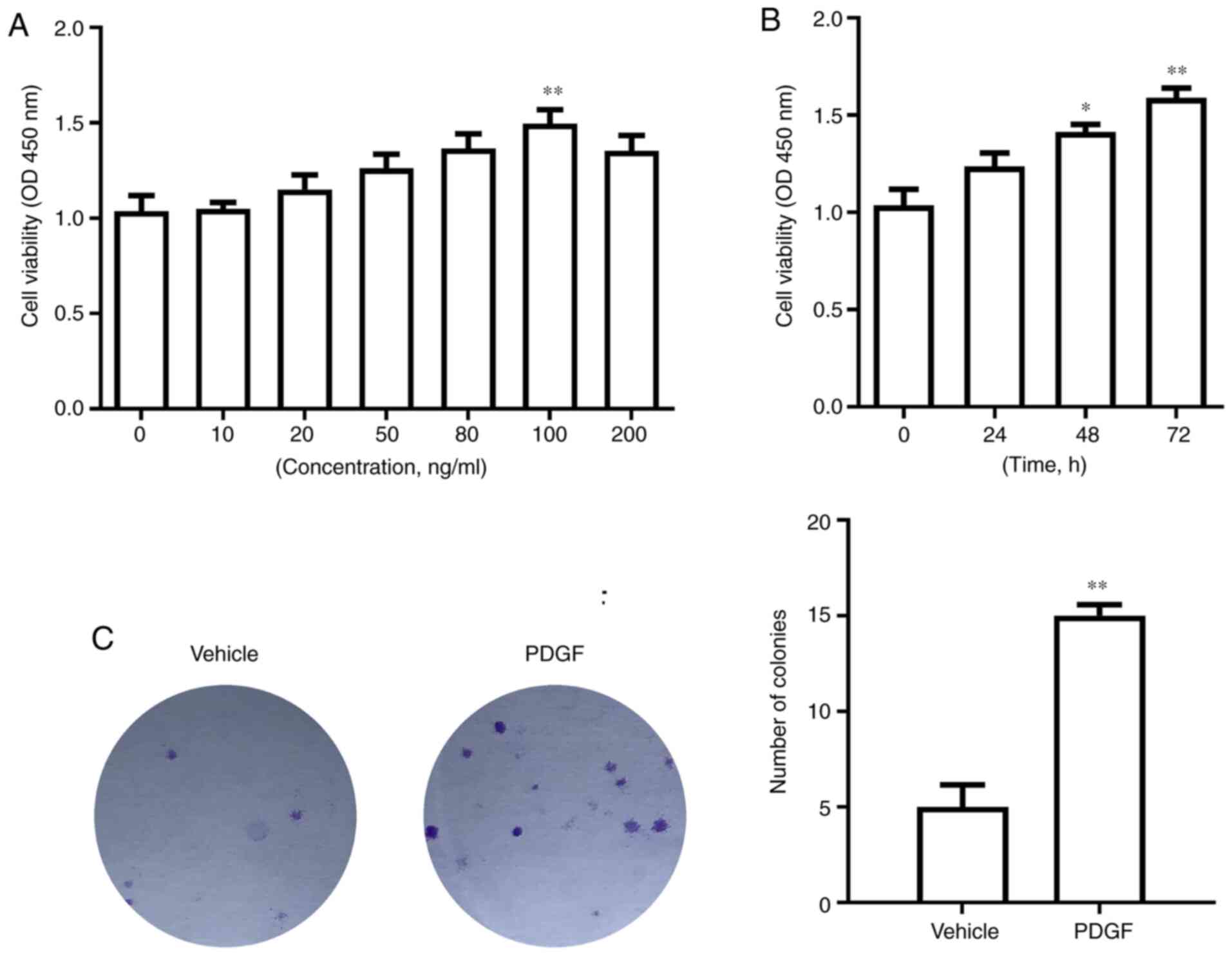
PASMCs were treated with different concentrations of
PDGF for 72 h. The CCK-8 experiments showed that as PDGF
concentration increased, PASMC viability increased. When the
concentration of PDGF reached 100 ng/ml, PASMC viability reached
its peak compared with 0 ng/ml (P<0.01; Fig. 1A). At the same time, following
treatment with 100 ng/ml PDGF for 72 h, PASMC viability was
significantly higher compared with that of the 0 h treatment group
(P<0.01; Fig. 1B). After
treatment with 100 ng/ml PDGF, cell proliferation increased, and
the number of colonies increased significantly compared with the
vehicle group (P<0.01; Fig.
1C).
PDGF promotes division of PASMCs
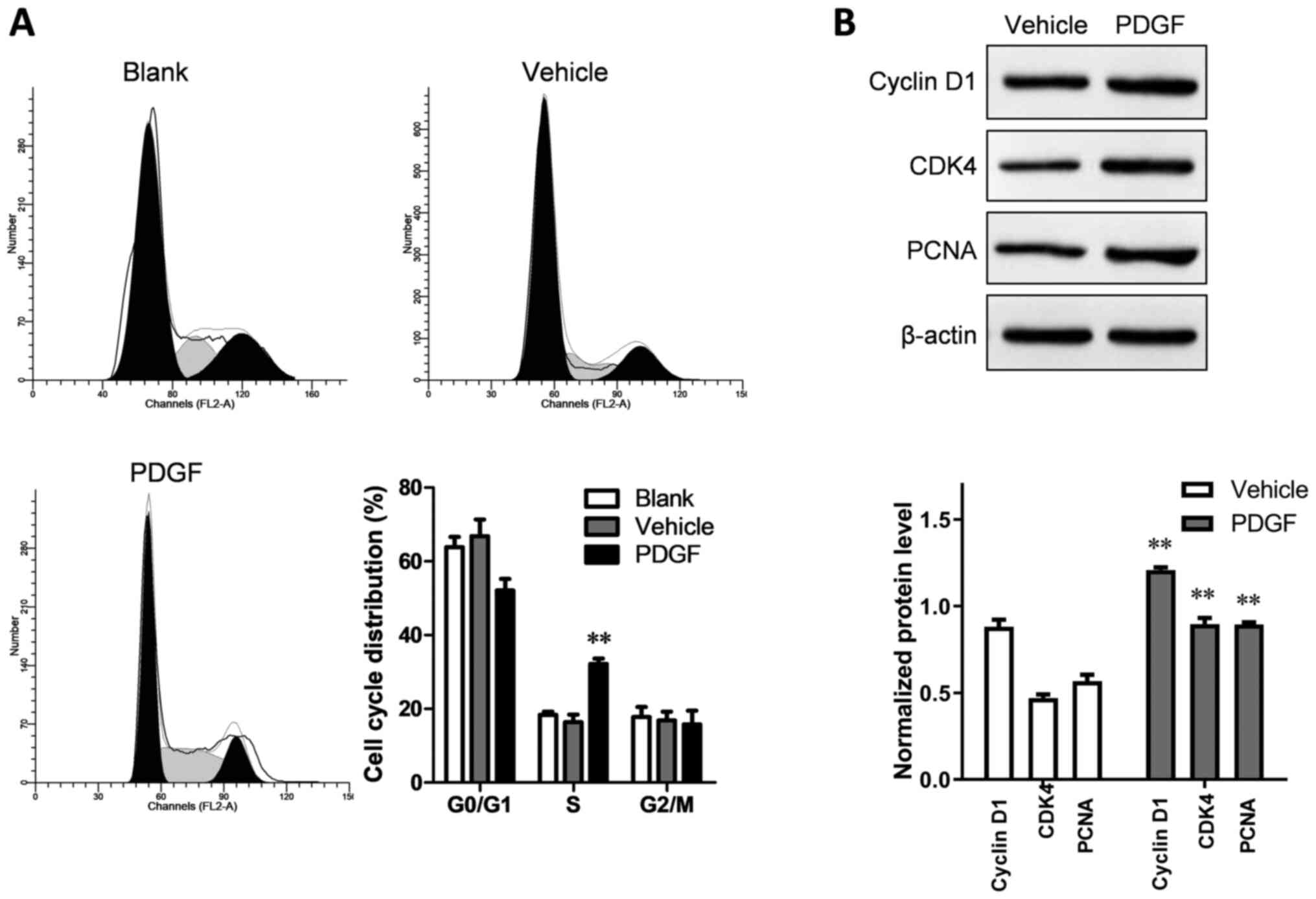
Compared with the blank and vehicle groups, the
PDGF-treated group showed a significantly increased proportion of
PASMCs in the synthesis (S) phase (P<0.01; Fig. 2A). Western blotting was used to
detecT cell cycle-related proteins. The relative expression levels
of cyclin D1, CDK4 and PCNA in the PDGF-treated group were
significantly higher than in the vehicle group (P<0.01; Fig. 2B).
PDGF enhances PASMC migration
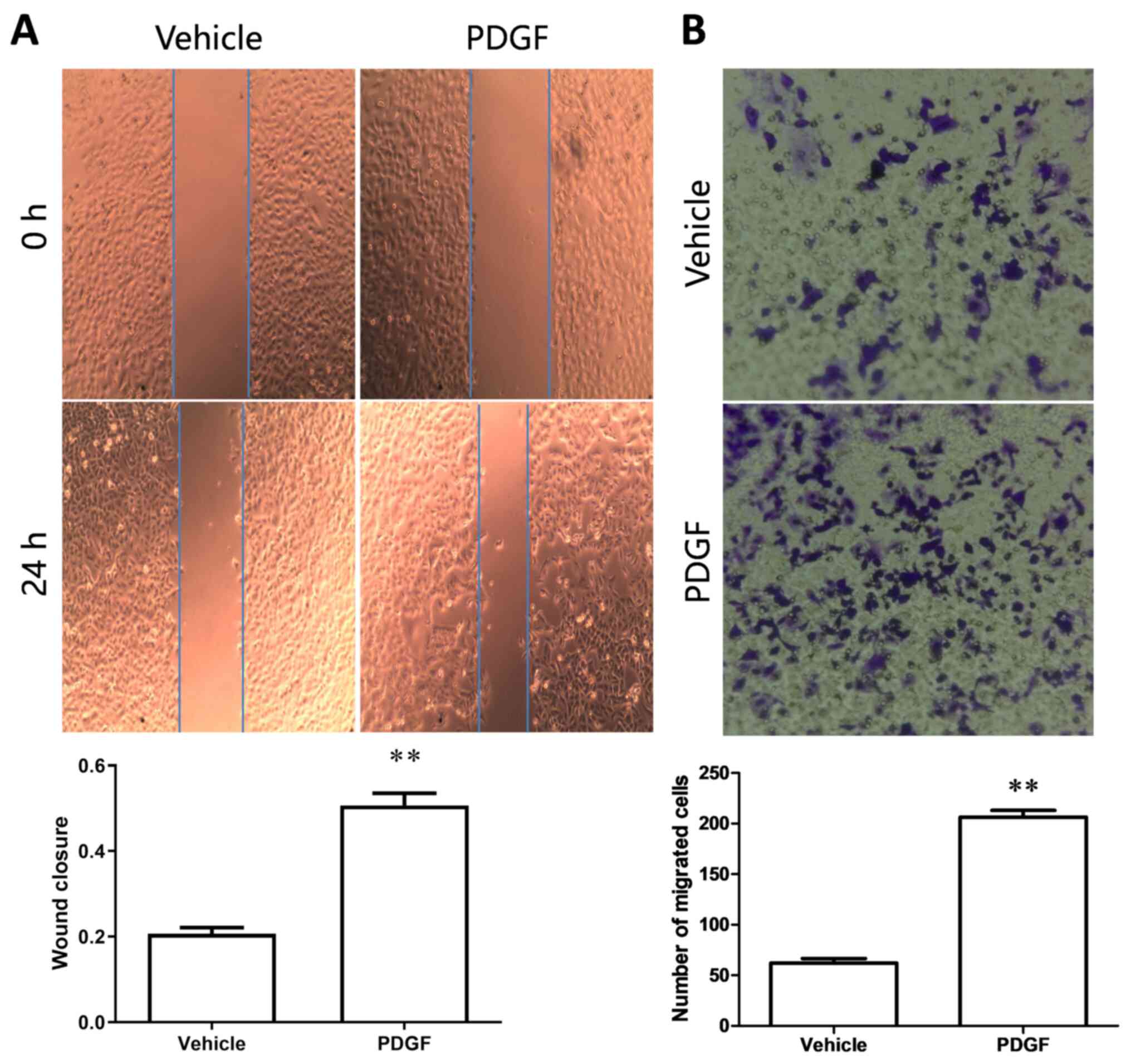
Wound healing and Transwell migration assays were
used to detect cell migration following treatment with PDGF. After
24 h of PDGF treatment, the wound closure rate was significantly
higher than in the vehicle group (Fig.
3A), and the results of the Transwell migration assay showed
that the number of migrated cells in the PDGF group was
significantly higher than that in the vehicle group (Fig. 3B). The aforementioned results
indicated that PDGF treatment increased PASMC migration.
Effects of PDGF on the expression of
NFATc1-5 in PASMCs
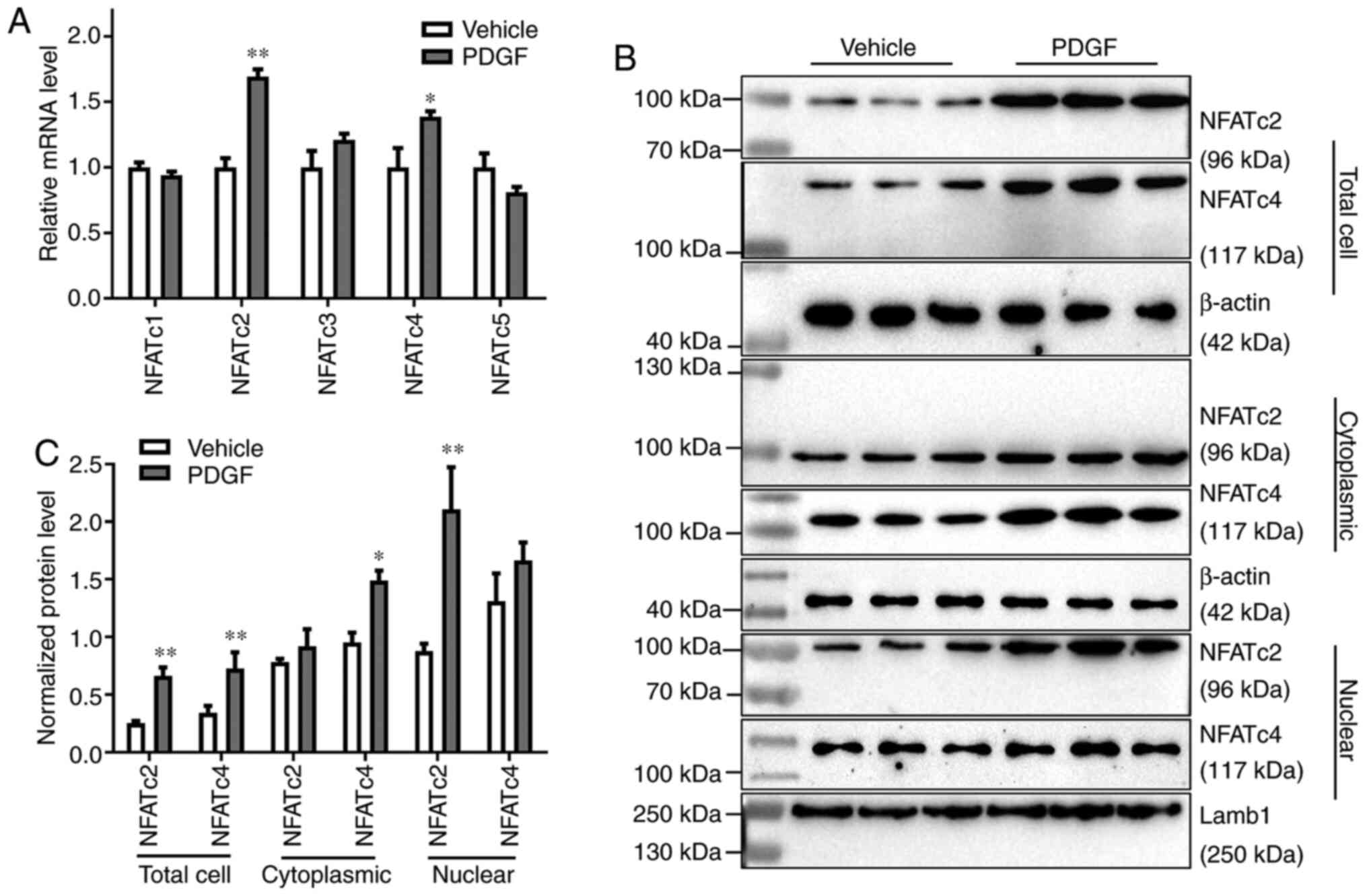
The NFAT transcription factor family consists of
five members, namely NFATc1, NFATc2, NFATc3, NFATc4 and NFATc5
(27). The mRNA expression levels
of all five members in PDGF-treated PASMCs were detected by
RT-qPCR. The relative mRNA expression levels of NFATc2 and NFATc4
were significantly increased after PDGF treatment (P<0.05;
Fig. 4A). To compare the protein
expression levels of NFATc2 and NFATc4 in different parts of the
cell, western blotting was used to detect the expression of NFATc2
and NFATc4 in the total cell, cytoplasm and nucleus following PDGF
treatment. Compared with the vehicle group, the expression of
NFATc2 in the nucleus and total cell increased significantly
(P<0.01); although an increase was also detected in cytoplasmic
levels, this was not significant. NFATc4 expression was
significantly increased in total cell and cytoplasm (P<0.05),
but the expression did not show a significant change in the nucleus
(Fig. 4B and C).
Roles of NFATc2 on PDGF-induced
proliferation and migration of PASMCs
To evaluate the role of NFATc2 in PDGF-induced cell
proliferation and migration, cells pretreated with NFAT pathway
inhibitors, CsA or siNFATc2, were treated with PDGF, and then
western blotting, RT-qPCR, colony formation and Transwell assays
were performed to evaluate changes in cell proliferation and
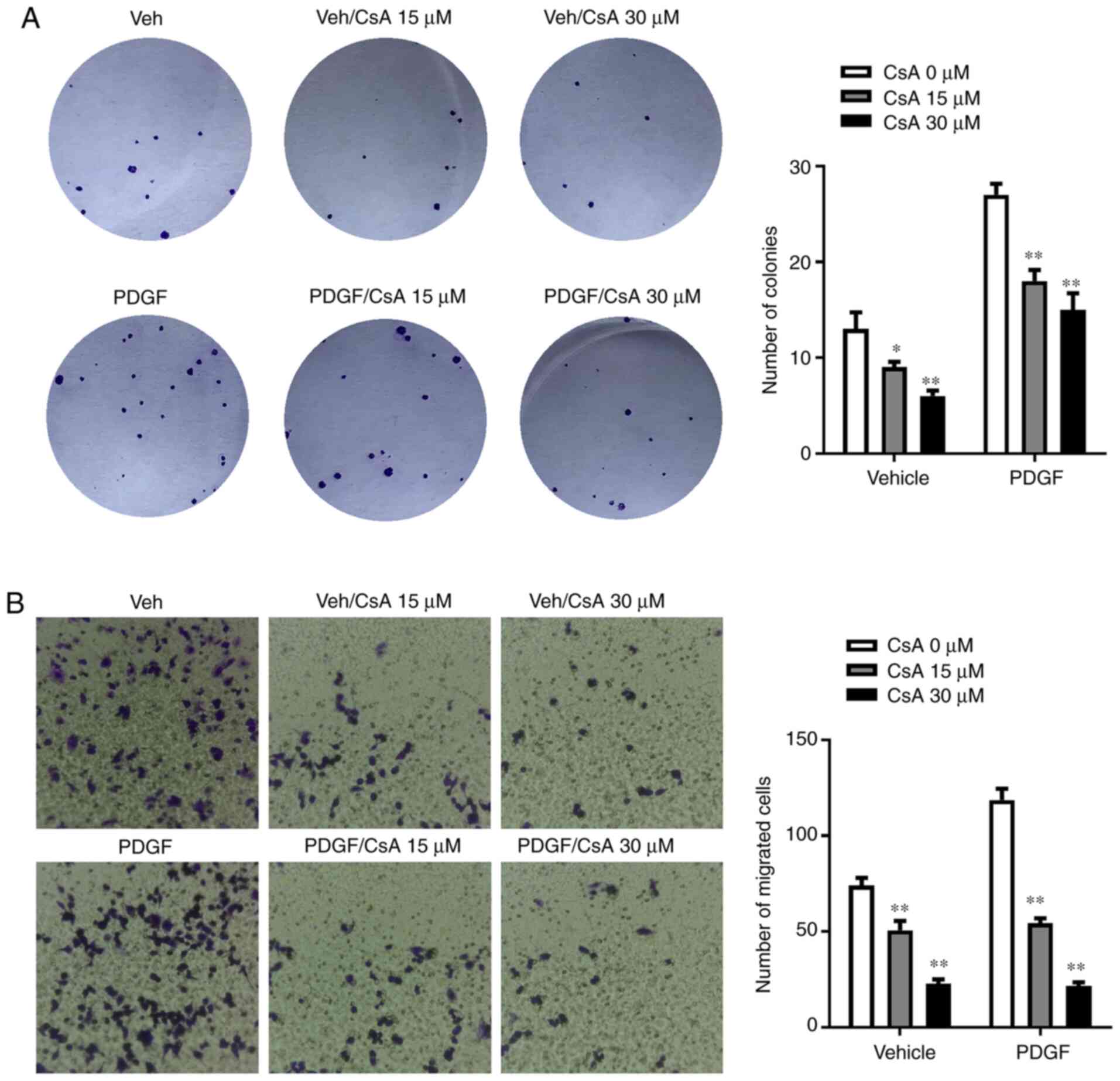
migration. As shown in Fig. 5A, the
number of colonies following 30 µM CsA treatment was significantly
lower than after 0 µM CsA treatment (P<0.01). Furthermore, the
number of colonies in the PDGF-treated group also significantly
decreased after the addition of 30 µM CsA (P<0.01). The results
of the cell migration experiments were similar to those of the
colony formation assay. Following 30 µM CsA treatment, cell
migration in the PDGF and vehicle group decreased significantly
(P<0.01; Fig. 5B).
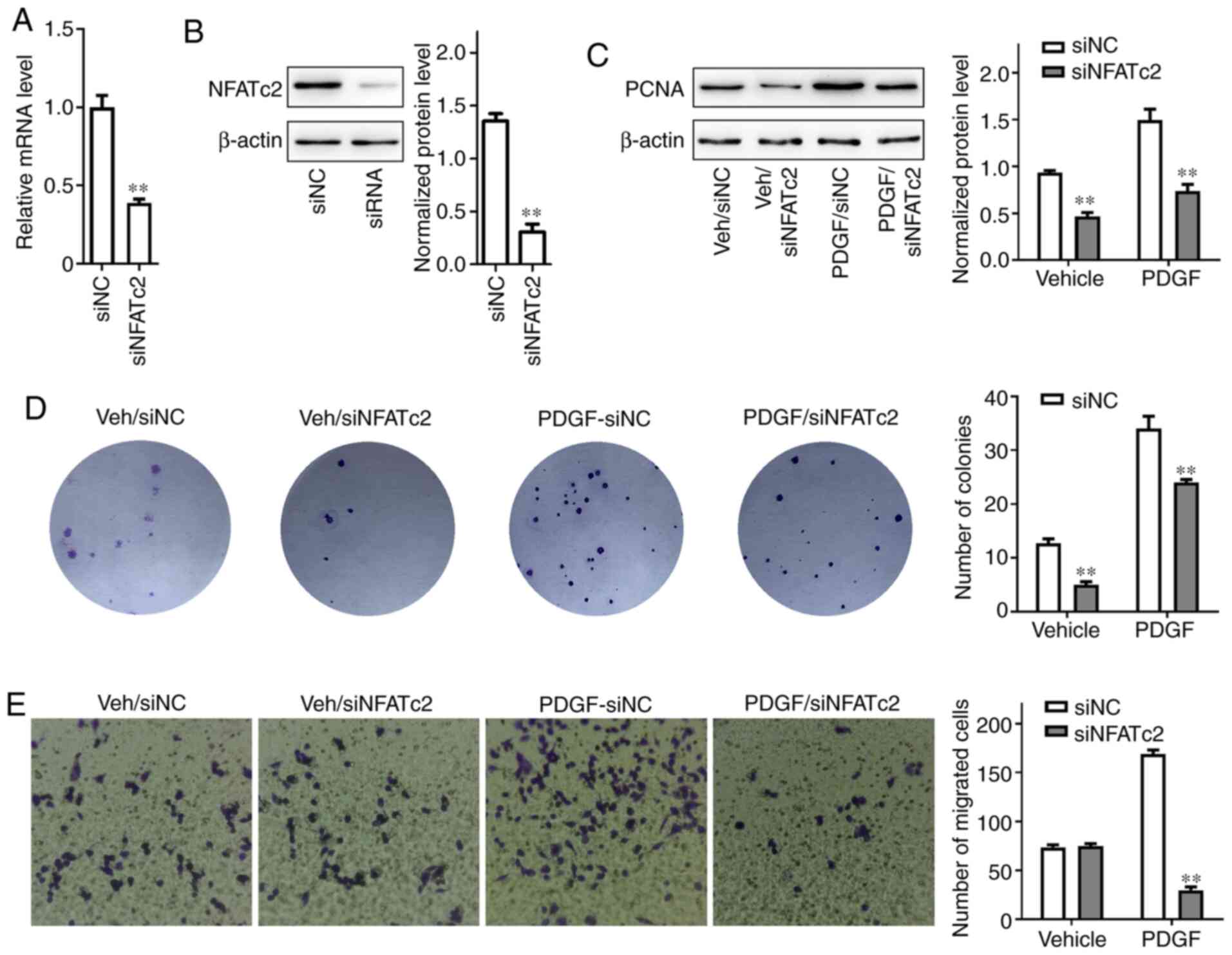
After the cells were pretreated with the NFAT
pathway inhibitor siNFATc2, RT-qPCR was used to detect the relative
expression of NFATc2 mRNA. The relative expression of mRNA in the
siNFATc2 group decreased significantly (P<0.01; Fig. 6A). Additionally, western blotting
demonstrated that following transfection with siNFATc2, the
expression of NFATc2 significantly decreased in PASMCs (Fig. 6B). The normalized protein level of
PCNA in cells pretreated with siNFATc2 in the vehicle and PDGF
groups was significantly lower than that in the siNC group
(P<0.01; Fig. 6C). Colony
formation experiments were performed to further examine the effect
of siNFATc2 on the proliferation of PASMCs. In both the vehicle and
PDGF groups, the number of colonies following siNFATc2 treatment
was significantly reduced (P<0.01; Fig. 6D). A Transwell assay was used to
detecT cell migration. Transfection with siNFATc2 significantly
reduced the number of migrated cells in the PDGF group (P<0.01),
but had no significant effect on the vehicle group (Fig. 6E).
Discussion
PAH is a serious condition characterized by
increased pulmonary circulatory resistance and pulmonary artery
remodeling, which can eventually lead to right ventricular failure
and death (28). The increased
pulmonary vascular resistance in PAH is partly due to increased
proliferation of PASMCs (29).
PDGF, as an important mitogenic promoter, has been demonstrated to
promote PASMC proliferation and migration (30), which is consistent with the present
results. The results of the current study showed thaT cell
viability was highest when cells were treated with 100 ng/ml PDGF.
PDGF-treated PASMCs were analyzed using flow cytometry. The number
of cells in the S phase was significantly higher than that in the
vehicle and blank groups. To verify this result, western blotting
was performed to determine the expression of cell cycle-related
proteins. The results verified that PDGF increased PASMC division.
Furthermore, to investigate the effect of PDGF on PASMC migration,
wound healing and Transwell migration assays were conducted after
PDGF treatment. Migration of PASMCs after PDGF treatment was
significantly increased. Several growth factors, such as PDGF,
fibroblast growth factor 2 and epidermal growth factor, are
implicated in abnormal PASMC proliferation and migration, which
contributes to pulmonary vascular remodeling in PAH (31). This finding was consistent with the
present results of PDGF.
It is commonly known that intrinsic changes in
Ca2+ homeostasis in PASMCs plays an important role in
pulmonary vasoconstriction and vascular reconstruction in PAH
(32) Of note, it was previously
discovered that store-operated Ca2+ entry (SOCE) is the
main channel that regulates Ca2+ influx in PASMCs and
regulates PASMC proliferation, apoptosis and migration in PAH
(33,34). The CaN/NFAT pathway is the most
important downstream signaling pathway of SOCE and is involved in
numerous physiological and pathological processes (32). An increase in cytosolic free
Ca2+ concentration [(Ca2+)cyt] in PASMCs is a
major trigger for pulmonary vasoconstriction and an important
underlying mechanism of pulmonary vascular remodeling via
stimulation of PASMC proliferation and inhibition of PASMC
apoptosis (35).
Based on the aforementioned reports, in the present
study it was speculated that PDGF may regulate NFAT by increasing
the Ca2+ concentration, which would eventually lead to
the occurrence of PAH. RT-qPCR was performed to detect the mRNA
expression levels of NFATc1, NFATc2, NFATc3, NFATc4 and NFAT5 in
PASMCs treated with PDGF. Following PDGF treatment, the relative
expression levels of NFATc2 and NFATc4 mRNA were significantly
higher than those in the vehicle group. As a nuclear factor, NFAT
can only play a role once it is transferred into the nucleus
(36). When the relative expression
of NFATc2 and NFATc4 proteins were compared in different parts of
the cell it was revealed that the expression of NFATc2 in total
cell lysates and the nucleus was significantly increased. Although
NFATc4 was notably increased in the nucleus, there was no
significant change in the relative expression; hence, NFATc2 was
chosen as the research target and investigated in the following
assays.
Colony formation and cell migration assays were
performed to verify the role of NFATc2 in PDGF-induced PASMC
proliferation and migration. Additionally, Ca2+-NFAT
signaling inhibitor CsA was used to treat PASMCs in each group. The
results showed that the number of colonies was significantly
reduced following the addition of 30 µM CsA, compared with the
vehicle group, indicating that CsA could reduce the proliferation
of PASMCs. To verify whether CsA treatment significantly reduced
PASMC migration, PASMCs were transfected with siNFATc2. The results
showed that, compared with siNC, the proliferative and migratory
abilities of PASMCs were significantly reduced following
transfection with siNFATc2. However, the findings of the colony
formation assays showed inconsistent effects of NFATc2 inhibition,
as PDGF is supposed to reduce number of colonies in the presence of
CsA or siNFATc2. This difference was primarily due to the fact that
PGDF can be influenced by other signaling pathways, and PGDF can
regulate cell adherence via the signaling pathway independent of
NFATc2, such as the Akt/mTOR signaling pathway (37). According to a previous study by
Ogawa et al (38), PDGF
upregulates stromal interaction molecule 1 (STIM1)/calcium
release-activated calcium channel protein 1 (Orai1) via the
Akt/mTOR signaling pathway in PASMCs, thereby enhancing
Ca2+ entry.
At present, drugs that target endothelin are the
first choice in the treatment of PAH (39). During development, endothelial cells
secrete the polypeptide platelet-derived growth factor (PDGF). As
endothelial cells differentiate and undergo tubulogenesis, secreted
PDGF is thought to form a concentration gradient, which is sensed
by surrounding smooth muscle precursors via the tyrosine kinase
receptor PDGFR-β to promote their migration and proliferation,
resulting in recruitment and assembly of the vessel wall.
The present study showed that in PDGF-treated rat
PASMCs, the relative expression of NFATc2 in the nucleus changed
significantly compared with vehicle cells, and the proliferative
and migratory abilities of rat PASMCs was significantly reduced
following treatment with NFATc2 inhibitors. In conclusion, this
study indicated that PDGF mediated PASMC proliferation and
migration by regulating NFATc2, which could be used as a potential
target for PAH treatment.
Acknowledgements
Not applicable.
Funding
The present research was funded by grants from the
‘Special and Joint Program’ of Yunnan Provincial Science and
Technology Department and Kunming Medical University [grant nos.
2018FE001(−082), 2018FE001(−206)], the Yunnan Health Training
Project of High Level Talents (grant nos. H-2019026 and H-2018095),
and the Young Academic and Technical Leaders of Yunnan Province
(grant no. 2017HB053).
Availability of data and materials
All data generated or analyzed during this study are
included in this manuscript.
Authors' contributions
FYZ and SLX performed the experiments, interpreted
the data, and were major contributors in writing the manuscript.
CFZ, JL and YZ were responsible for data analysis and
visualization. JY and XQX significantly contributed to the design
and conception of the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Fourth Affiliated Hospital of Kunming Medical
University, The Second People's Hospital of Yunnan Province
(Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PASMCs
|
pulmonary arterial smooth muscle
cells
|
|
PAH
|
pulmonary arterial hypertension
|
|
PDGF
|
platelet-derived growth factor
|
|
NFAT
|
nuclear factor of activated T
cells
|
|
CsA
|
cyclosporin A
|
References
|
1
|
Rubin LJ, Badesch DB, Barst RJ, Galie N,
Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al:
Bosentan therapy for pulmonary arterial hypertension. N Engl J Med.
346:896–903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humbert M, Deng Z, Simonneau G, Barst RJ,
Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA and
Morse JH: BMPR2 germline mutations in pulmonary hypertension
associated with fenfluramine derivatives. Eur Respir J. 20:518–523.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker J, Undem C, Yun X, Lade J, Jiang H
and Shimoda LA: Role of Rho kinase and Na+/H+
exchange in hypoxia-induced pulmonary arterial smooth muscle cell
proliferation and migration. Physiol Rep. 4:e127022016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou X, Chen J, Luo Y, Liu F, Xu G and Gao
Y: Silencing of STIM1 attenuates hypoxia-induced PASMCs
proliferation via inhibition of the SOC/Ca2+/NFAT
pathway. Respir Res. 14:22013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cimminiello C, Arpaia G, Aloisio M, Uberti
T, Rossi F, Pozzi F and Bonfardeci G: Platelet-derived growth
factor (PDGF) in patients with different degrees of chronic
arterial obstructive disease. Angiology. 45:289–293. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perros F, Montani D, Dorfmüller P,
Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E,
Mussot S, Mercier O, et al: Platelet-derived growth factor
expression and function in idiopathic pulmonary arterial
hypertension. Am J Respir Crit Care Med. 178:81–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antoniu SA: Targeting PDGF pathway in
pulmonary arterial hypertension. Expert Opin Ther Targets.
16:1055–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schermuly RT, Dony E, Ghofrani HA,
Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N,
Seeger W and Grimminger F: Reversal of experimental pulmonary
hypertension by PDGF inhibition. J Clin Invest. 115:2811–2821.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Macian F: NFAT proteins: Key regulators of
T-cell development and function. Nat Rev Immunol. 5:472–484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorfmüller P, Perros F, Balabanian K and
Humbert M: Inflammation in pulmonary arterial hypertension. Eur
Respir J. 22:358–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voelkel NF, Cool C, Lee SD, Wright L,
Geraci MW and Tuder RM: Primary pulmonary hypertension between
inflammation and cancer. Chest. 114 (Suppl 3):225S–230S. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vihma H, Pruunsild P and Timmusk T:
Alternative splicing and expression of human and mouse NFAT genes.
Genomics. 92:279–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senavirathna LK, Huang C, Yang X, Munteanu
MC, Sathiaseelan R, Xu D, Henke CA and Liu L: Hypoxia induces
pulmonary fibroblast proliferation through NFAT signaling. Sci Rep.
8:27092018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonnet S, Rochefort G, Sutendra G, Archer
SL, Haromy A, Webster L, Hashimoto K, Bonnet SN and Michelakis ED:
The nuclear factor of activated T cells in pulmonary arterial
hypertension can be therapeutically targeted. Proc Natl Acad Sci
USA. 104:11418–11423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen R, Yan J, Liu P, Wang Z, Wang C,
Zhong W and Xu L: The role of nuclear factor of activated T cells
in pulmonary arterial hypertension. Cell Cycle. 16:508–514. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacLean MMR: The serotonin hypothesis in
pulmonary hypertension revisited: Targets for novel therapies (2017
Grover conference series). Pulm Circ. 8:20458940187591252018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maroteaux L, Ayme-Dietrich E,
Aubertin-Kirch G, Banas S, Quentin E, Lawson R and Monassier L: New
therapeutic opportunities for 5-HT2 receptor ligands.
Pharmacol Ther. 170:14–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han J, Tian H, Liu Y and Fan F:
Sarpogrelate attenuates pulmonary arterial hypertension via
calcium/calcineurin axis. Front Biosci (Landmark Ed). 24:607–615.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hassoun PM, Mouthon L, Barberà JA,
Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML,
Michelakis ED, Morrell NW, et al: Inflammation, growth factors, and
pulmonary vascular remodeling. J Am Coll Cardiol. 54 (Suppl
1):S10–S19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao C, Yu J, Taylor L, Polgar P, McComb ME
and Costello CE: Protein expression by human pulmonary artery
smooth muscle cells containing a BMPR2 mutation and the action of
ET-1 as determined by proteomic mass spectrometry. Int J Mass
Spectrom. 378:347–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nabbi A and Riabowol K: Rapid isolation of
nuclei from cells in vitro. Cold Spring Harb Protoc. 2015:769–772.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong L, Zhu L, Wang S and Zhang Z:
Transthyretin regulates the migration and invasion of JEG-3 cells.
Oncol Lett. 13:1242–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vihma H, Luhakooder M, Pruunsild P and
Timmusk T: Regulation of different human NFAT isoforms by neuronal
activity. J Neurochem. 137:394–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukumoto Y, Tawara S and Shimokawa H:
Recent progress in the treatment of pulmonary arterial
hypertension: Expectation for rho-kinase inhibitors. Tohoku J Exp
Med. 211:309–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nie X, Chen Y, Tan J, Dai Y, Mao W, Qin G,
Ye S, Sun J, Yang Z and Chen J: MicroRNA-221-3p promotes pulmonary
artery smooth muscle cells proliferation by targeting AXIN2 during
pulmonary arterial hypertension. Vascul Pharmacol. 116:24–35. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sysol JR, Natarajan V and Machado RF: PDGF
induces SphK1 expression via Egr-1 to promote pulmonary artery
smooth muscle cell proliferation. Am J Physiol Cell Physiol.
310:C983–C992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pullamsetti SS, Savai R, Seeger W and
Goncharova EA: Translational advances in the field of pulmonary
hypertension. From cancer biology to new pulmonary arterial
hypertension therapeutics. Targeting cell growth and proliferation
signaling hubs. Am J Respir Crit Care Med. 195:425–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He RL, Wu ZJ, Liu XR, Gui LX, Wang RX and
Lin MJ: Calcineurin/NFAT signaling modulates pulmonary artery
smooth muscle cell proliferation, migration and apoptosis in
monocrotaline-induced pulmonary arterial hypertension rats. Cell
Physiol Biochem. 49:172–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Potier M, Gonzalez JC, Motiani RK,
Abdullaev IF, Bisaillon JM, Singer HA and Trebak M: Evidence for
STIM1- and Orai1-dependent store-operated calcium influx through
ICRAC in vascular smooth muscle cells: Role in proliferation and
migration. FASEB J. 23:2425–2437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Earley S and Brayden JE: Transient
receptor potential channels in the vasculature. Physiol Rev.
95:645–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song S, Carr SG, McDermott KM, Rodriguez
M, Babicheva A, Balistrieri A, Ayon RJ, Wang J, Makino A and Yuan
JX: STIM2 (stromal interaction molecule 2)-mediated increase in
resting cytosolic free Ca2+ concentration stimulates
PASMC proliferation in pulmonary arterial hypertension.
Hypertension. 71:518–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao SC, Wu H, Xie J, Chang CP, Ranish JA,
Graef IA and Crabtree GR: Calcineurin/NFAT signaling is required
for neuregulin-regulated Schwann cell differentiation. Science.
323:651–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Razmara M, Heldin CH and Lennartsson J:
Platelet-derived growth factor-induced Akt phosphorylation requires
mTOR/Rictor and phospholipase C-γ1, whereas S6 phosphorylation
depends on mTOR/Raptor and phospholipase D. Cell Commun Signal.
11:32013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogawa A, Firth AL, Smith KA, Maliakal MV
and Yuan JX: PDGF enhances store-operated Ca2+ entry by
upregulating STIM1/Orai1 via activation of Akt/mTOR in human
pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol.
302:C405–C411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O'Callaghan DS, Savale L, Montani D, Jaïs
X, Sitbon O, Simonneau G and Humbert M: Treatment of pulmonary
arterial hypertension with targeted therapies. Nat Rev Cardiol.
8:526–538. 2011. View Article : Google Scholar : PubMed/NCBI
|