Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for
80–90% of pancreatic tumors and is the 4th most common cause of
cancer-related mortality in the USA. Moreover, its 5-year survival
rate is <5%, with a median survival time of <6 months.
Surgical resection and chemotherapy are the main approaches to the
treatment of pancreatic cancer; however, <20% of patients are
eligible for surgery at diagnosis (1). For patients with pancreatic cancer
with locally advanced or metastatic tumors, chemotherapy is the
standard treatment. Gemcitabine (Gem) has been used as a first-line
treatment for patients with pancreatic cancer since the late 1990s
(2). However, the sensitivity of
pancreatic cancer to Gem is poor. Therefore, understanding the
mechanisms of Gem resistance is important for the effective
treatment of pancreatic cancer.
Among several proposed mechanisms that mediate
acquired Gem resistance in patients with pancreatic cancer, one
possible mechanism is drug efflux via ATP-binding cassette
(ABC)-transporters (3–5). ABC-transporter superfamily proteins
contribute to multidrug resistance (MDR) by transporting the
substrates of anticancer drugs out of cancer cells, ultimately
decreasing their intracellular accumulation (6). MDR in cancer is a condition in which
cancer cells become resistant to structurally-unrelated anticancer
drugs (7). Among ABC transporters,
ABC subfamily B member 1 (ABCB1), C member 1 (ABCC1) and G member 2
(ABCG2), are strongly associated with MDR (8,9). It
has been reported that the increase in ABCG2 protein expression is
essential for Gem resistance in pancreatic cancer cells (10), but the underlying mechanisms that
regulate ABCG2 expression are yet to be elucidated.
Previous studies have suggested that the
Wnt/β-catenin signaling pathway may be an upstream molecular
mechanism regulating the expression of ABCG2 (11–14).
In lung cancer cell lines, cisplatin can indirectly affect the
expression of ABCG2 by changing the expression of β-catenin
(11). microRNAs are reported to
enhance cisplatin resistance in colon cancer cells by increasing
the expression of ABCG2 through Wnt proteins (12). Furthermore, cisplatin, methotrexate
and doxorubicin can upregulate the expression of ABCG2, and also
activate the Wnt/β-catenin pathway, thus inducing MDR in
osteosarcoma during chemotherapy (13). In acute and chronic liver diseases,
the Wnt pathway also upregulates the expression of ABCG2 in liver
tissue (14).
Among a large class of secreted Wnt proteins, Wnt5a
has been identified as a biomarker of poor clinical outcome in
patients with ovarian cancer, as well as a mediator of
chemoresistance (15). Upregulated
expression levels of cellular Wnt5a and secreted Wnt5a are observed
in multidrug-resistant KB-V1, NCI/ADR-RES and SW620-MDR1 cells of
the cervix, ovaries and colon, respectively (16). Elevated expression of Wnt5a
regulates the Wnt signaling pathway to support cancer cell
resistant to chemotherapy (16).
Moreover, in in vitro experiments using the bladder cancer
cell lines UM-UC-3 and SW780, the drug resistance of UM-UC-3a cells
to Gem increased after the overexpression of Wnt5a protein, while
the drug resistance of SW780 cells to Gem decreased wwith Wnt5a
downregulation (17). In addition,
inhibition of Wnt5a leads to significant increases in the
drug-induced apoptosis of pancreatic cancer cells, both in
vitro and in vivo (18).
The aim of the current study was to investigate the
upstream regulators of ABCG2 expression and their roles in Gem
resistance in pancreatic cell lines. It was identified that ABCG2
was upregulated by Wnt5a through the upregulation of Frizzled class
receptor 7 (FZD7), a receptor involved in Wnt signaling.
Importantly, FZD7 was required for Wnt5a-induced resistance of
pancreatic cancer cells to Gem treatment. Thus, the present
findings may provide insight into the mechanisms of drug-resistance
in pancreatic cancer.
Materials and methods
Human pancreatic cancer specimens
A total of 24 patient pancreatic cancer specimens
and adjacent para-carcinoma tissue specimens were obtained from the
Department of Pancreatic and Biliary Surgery, China Medical
University. All patients provided written consent to undergo
curative surgical resection and 30-months follow-up and to the use
of their samples for research. The present study was approved by
the Ethics Committee of The First Hospital of China Medical
University. All patients with pancreatic ductal adenocarcinoma
(PDAC) were diagnosed by a certified pathologist according to the
WHO guidelines. The pathological TNM stage was assessed according
to the criteria of the 6th edition of the TNM classification of the
American Joint Committee on Cancer (AJCC). All 24 patients were in
stage Ia-IIb, according to the AJCC criteria, and underwent radical
excision operation. ABCG2 expression did not correlate with sex,
age or TNM stage, but correlated with histological grade (Table I).
 | Table I.Relationship between ABCG2 expression
and clinicopathological characteristics of patients with pancreatic
ductal adenocarcinoma. |
Table I.
Relationship between ABCG2 expression
and clinicopathological characteristics of patients with pancreatic
ductal adenocarcinoma.
|
|
| ABCG2 expression n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Total cases, n | Negative | Positive |
P-valuea |
|---|
| Sex |
|
|
| 0.431 |
|
Male | 13 | 4 (16.7) | 9 (37.5) |
|
|
Female | 11 | 7 (29.2) | 4 (16.7) |
|
| Median age |
|
|
| 0.341 |
|
≤55 | 9 | 3 (12.5) | 6 (25.0) |
|
|
>55 | 15 | 8 (33.3) | 7 (29.2) |
|
| Histological
grade |
|
|
| 0.027 |
|
Good | 7 | 6 (25.0) | 1 (4.2) |
|
|
Moderate | 7 | 3 (12.5) | 4 (16.7) |
|
|
Poor | 10 | 2 (8.3) | 8 (33.3) |
|
| AJCC TNM stage |
|
|
| 0.248 |
| IA | 5 | 3 (12.5) | 0 |
|
| IB | 4 | 2 (8.3) | 4 (16.7) |
|
|
IIA | 9 | 4 (16.7) | 6 (25.0) |
|
|
IIB | 6 | 2 (8.3) | 3 (12.5) |
|
| pT status |
|
|
| 0.389 |
| T1 | 6 | 4 (16.7) | 2 (8.3) |
|
| T2 | 7 | 2 (8.3) | 5 (20.8) |
|
| T3 | 11 | 5 (20.8) | 6 (25.0) |
|
| pN status |
|
|
| 0.813 |
| N0 | 18 | 8 (33.3) | 10 (41.7) |
|
| N1 | 6 | 3 (12.5) | 3 (12.5) |
|
Immunohistochemistry (IHC)
The PDAC tissue was immediately cut into small
pieces and placed in 4% paraformaldehyde for fixation at room
temperature for 12 h, and then paraffin embedded. IHC analysis was
performed on 4–6 µm paraffin sections of human PDAC tissues. Xylene
and gradient alcohols were used to deparaffinize and hydrate,
respectively. Then, 3% H2O2 was added to the
sections to remove endogenous peroxidase. Sections were incubated
with citrate buffer to repair antigen, and blocked with BSA (5%;
Sangon Tech Co., Ltd.; cat. no. A600332) at 4°C for 30 mins.
Primary antibodies were used as follows: ABCG2 (1:400; Cell
Signaling Technology, Inc.; cat. no. 42078) and Wnt5a (1:300; Cell
Signaling Technology, Inc.; cat. no. 2530). After incubation with
primary antibodies overnight at 4°C in a wet box, incubated with
horseradish peroxidase-conjugated secondary antibody (1:2,000;
Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. 31460) at room
temperature for 30 min. Diaminobenzidine (Boster Biological
Technology) was used at room temperature for 10–20 min to stain the
sections dissolved in Tris-HCl and H2O2.
Then, sections were re-stained in hematoxylin at room temperature
for 5–15 sec, dehydrated with gradient alcohol and xylene and
sealed with cover slides. The images were captured under a light
microscope at ×40 magnification.
The percentage of positive cells was divided into
five grades (percentage scores): i) 0, <10%; ii) 1, 10–25%; iii)
2, 25–50%; iv) 3, 50–75%; and v) 4, >75%. In addition, the
intensity of staining was divided into four grades (intensity
scores): i) 0, No staining; ii) 1, light brown; iii) 2, brown; and
iv) 3, dark brown. ABCG2/Wnt5a staining positivity was evaluated
using IHC scores, which were calculated as: IHC score=percentage
score × intensity score. Thus, based on the percentage and
intensity scores, target protein staining was classified into four
groups: i) IHC score ≤3, negative; ii) IHC score >3 and ≤6,
weak; iii) IHC>6 and ≤9, moderate; and iii) IHC>9, strong
(19).
Survival analysis was performed by comparing the
postoperative survival time of all ABCG2 positive specimens (IHC
scores >3 and ≤12, weak to strong positives) and negative
specimens (IHC scores ≤3, negative) using GraphPad Prism version
7.0. Spearman's correlation analysis was conducted between Wnt5a
and FZD7 IHC scores in 24 specimens. In addition, specimens of
deceased patients were scored according to postoperative survival
time: 0–2 months were set as 1, 3–5 months as 2, 6–8 months as 3,
and so on. Then, a Spearman's correlation analysis was conducted
between this survival scores and ABCG2 IHC scores. Spearman's
correlation analysis was conducted using SPSS 22.0 software (IBM
Corp.). P<0.05 was considered to be statistically significant
difference.
Cell culture
Human pancreatic carcinoma cell lines, Capan-2,
Panc-1, SW1990 and AsPC-1, were purchased from American Type
Culture Collection. All cell lines were cultured and maintained in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (cat. no. CCS30009.02; MRC Biotechnology Co., Ltd.; http://www.mrcing.com/pd.jsp?id=7), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were incubated at 37°C
in a humidified atmosphere containing 5% CO2.
Recombinant human Wnt5a (rhWnt5a) was purchased from R&D
Systems, Inc. (cat. no. 645-WN-010).
Transient transfection
The FZD7-expressing plasmid and small interfering
(si)RNA targeting FZD7, as well as the control vector and control
siRNA, were purchased from Jikai Gene Technology Co., Ltd (cat. no.
GIEE0156818). The targeting sequences of FZD7 siRNAs were as
follows: i) siFZD7-1; 5′-AGTACCTGATGACCATGAT-3′; ii) siFZD7-2,
5′-AGCCGTACCACGGAGAGAA-3′; and iii) siFZD7-3,
5′-GTTCGTCTACCTCTTCATA-3′. Transfection was performed using
Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Capan-2 cells were
seeded at 1×106 cells/well into 6-well plates 12–18 h
prior to transfection. According protocol, when cells were 70–80%
confluent, a mixture of 2,500 ng siRNA and 6 µl Lipofectamine™ 3000
regent was added per well. A total of 6 h after transfection, the
medium was replaced with complete medium and intervention reagents
were added to culture medium, as indicated. Cells were collected
after 48 h of additional culture.
Western blot analysis
Capan-2 cells were lysed in cold RIPA lysis buffer
(Beyotime Institute of Biotechnology) and quantified using the BCA
method. Subsequently, the proteins (25 µg of protein loaded per
lane) were separated by SDS-PAGE using 8% gels, then transferred to
a PVDF membrane. The membranes were blocked in 5% non-fat dried
milk in TBS-Tween 20 for 2 h at room temperature and then incubated
overnight with specific primary antibodies against ABCG2 (1:1,000;
Cell Signaling Technology, Inc.; cat. no. 42078), Wnt5a (1:1,000;
Cell Signaling Technology, Inc.; cat. no. 2530) and FZD7 (1:1,000;
Abcam; cat. no. ab64636) at 4°C. GAPDH (1:10,000; ProteinTech
Group, Inc.; cat. no. HRP-60004) was used as a control. After
incubation with horseradish peroxidase-conjugated secondary
antibody (1:10,000; Invitrogen; Thermo Fisher Scientific, Inc.;
cat. no. 31460) for 2 h at room temperature. SuperSignal
Chemiluminescent Substrates (Thermo Fisher Scientific, Inc.) and
imaging systems were used to collect the results. ImageJ (ImageJ
v1.8.0; National Institutes of Health) was used for
densitometry.
MTT assay
Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2 as aforementioned, and
plated at a density of 20,000 cells/well in 96-well plates, then
divided into several groups: Capan-2 vs. Capan-2 treated with
rhWnt5a; conCapan-2 treated with rhWnt5a vs. siFZD7-Capan-2 treated
with rhWnt5a; Capan-2 vs. Capan-2 treated with rhWnt5a and
recombinant human Frizzled-7 Fc Chimera (rhFc, a recombinant human
immunoglobulin that can bind to the Fzd7 molecule and blocks its
biological activity, so that it loses its normal function; R&D
Systems, Inc.; cat. no. 6178-FZ-050) together. Capan-2 cells were
cultured with rhWnt5a for 12 h prior Gem (Eli Lilly and Company)
treatment. Concentrations of Gem range from 0–10 µM. Drug
resistance was examined 48 h after Gem treatment using an MTT
assay. A total of 4 h after the addition of MTT to the culture
medium, formazan was dissolved using DMSO, fluorescence was
measured using a microplate reader at a wavelength of 570 nm.
Flow cytometry assay for cell
apoptosis analysis
Cells were detached and labeled using an Annexin
V-PE/7-AAD apoptosis detection kit (cat. no. KGA1015-1018; Nanjing
KeyGen Biotech Co., Ltd.), according to manufacturer's
instructions. Apoptotic and necrotic cells were quantified using a
flow cytometer (BD Accuri™ C6; BD Biosciences) and the BD Accuri C6
Plus 1.0.23.1 software (BD Biosciences) was used for analysis. A
total of 20,000 cells were analyzed for each sample. Cells negative
for Annexin V-PE and 7-AAD were considered viable. Cells with
Annexin V-PE+ and 7-AAD− staining were
indicative of early apoptosis, while Annexin V-PE+ and
7-AAD+ cells were considered as late apoptotic and
necrotic cells.
Establishment of Gem-resistant cell
lines
PDAC cells were continuously treated with a low
concentration of Gem to obtain drug-resistant cell line (20), such as the AsPC-1/Gem, Capan-2/Gem
and Panc-1/Gem Gem-resistant cell lines. Cells were incubated at
37°C in a humidified atmosphere containing 5% CO2 as
aforementioned. First, the IC50 of Gem was measured for
the parental cells using MTT assay, then 1/8 of the IC50
for parental cells was designated as the initial induction
concentration. After 24 h, the culture medium was changed, and
culture was continued until the cells reached 70–80% confluent.
Then, adherent cells were digested and passaged. Cells were
cultured in a medium containing a higher concentration of Gem (1/4
IC50) after subculture. This was repeated several times,
as Gem concentration reached above IC50. The cells were
maintained in normal culture and frozen. After 3 days, cells were
thawed and cultured with medium containing Gem at IC50.
Cells were observed under a light microscope, if cells grew well,
the drug-resistant cell lines were considered established.
Statistical analysis
All data presented as the mean ± SD of three
independent experiments. The association between FZD7 expression
and clinicopathological characteristics was analyzed using a
χ2 test. Statistical evaluation of continuous data was
performed using one-way ANOVA followed by Tukey's post hoc test for
multigroup comparisons, or a paired t-tests for comparison
of differences between two groups. Wilcoxon's signed rank test was
used to analyze the IHC data using SPSS 22.0 software (IBM Corp.),
the Spearman's correlation analysis was conducted between Wnt5a and
FZD7 IHC scores using SPSS 22.0 software (IBM Corp.). Survival
analysis was carried out using GraphPad Prism version 7.0 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Bioinformatics analysis
Correlation analysis of gene expression was
performed using ‘R2: Genomics Analysis and Visualization Platform’
(https://hgserver1.amc.nl/) (21). The correlation analysis of Wnt5a vs.
ABCG2 was performed in data set of ‘Tumor Colon CRC cell
pop.-Calon-24-MAS5.0-u133p2’, ‘Tumor Lymphoma (T-cell
Angioimmunoblastic)-Teh-20-MAS5.0-u133p2’, ‘Exp Glioblastoma
5aza-dC drug-Mueller-12-MAS5.0-u133a’, ‘Tumor Wilms
VLRWT-Perlman-39-MAS5.0-u133a’ and ‘Mixed
Pancreatic-Kanai-22-MAS5.0-u133p2’. The correlation analysis of
Wnt5a vs. FZD7 and FZD7 vs. ABCG2 were performed in data set of
‘Tumor Pancreatic adenocarcinoma-TCGA-178-rsem-tcgars’, ‘Tumor
Pancreatic ductal adenocarcinoma-Yeh-132-custom-4hm44k’ and ‘Tumor
Pancreatic ductal adenocarcinoma
(ICGC)-Perez-91-complex-ilmnht12v4’.
Results
ABCG2 is expressed in human pancreatic
cancer tissues
To determine the protein expression levels of ABCG2
and Wnt5a in human pancreatic cancer tissue, IHC was performed on
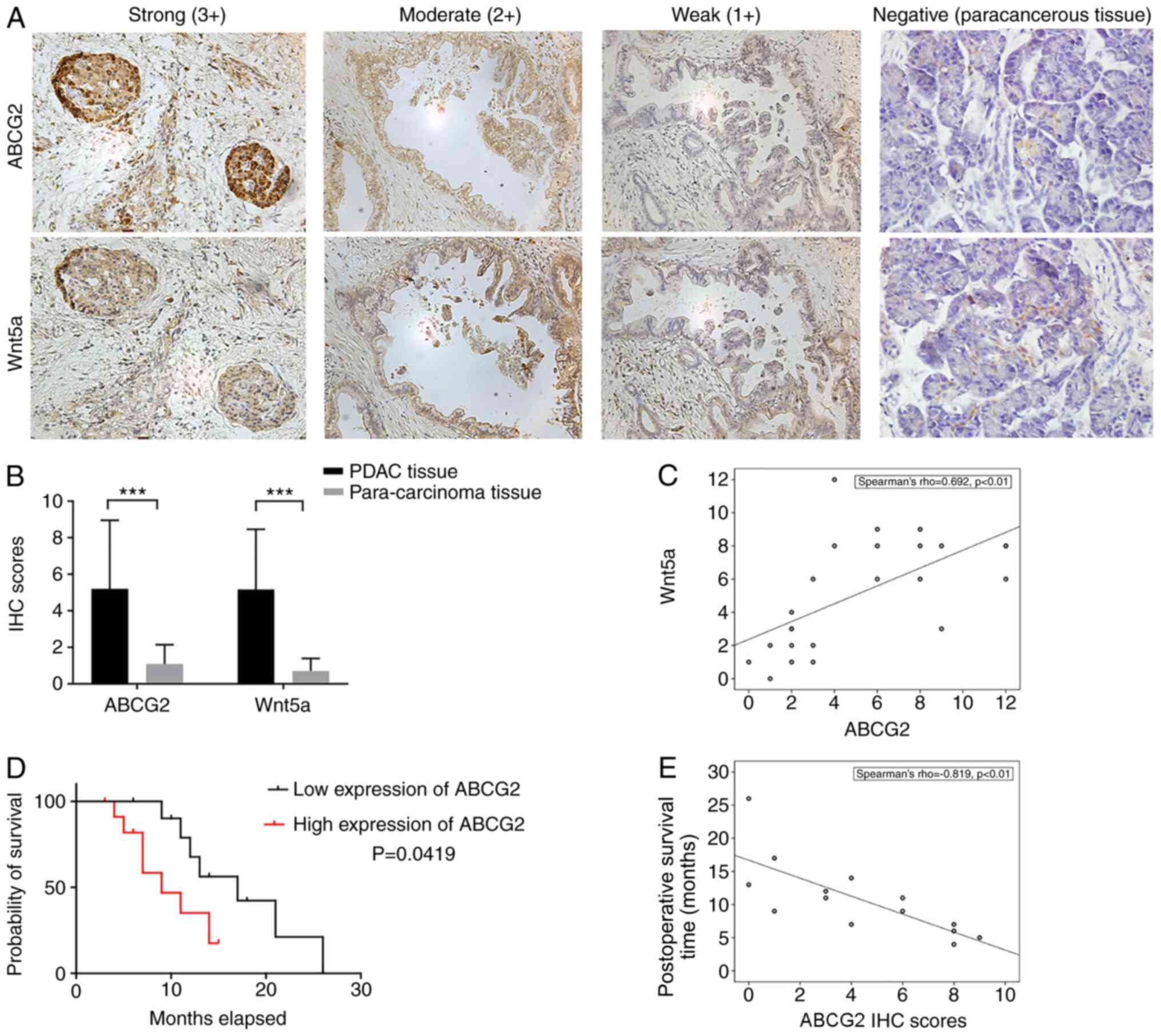
24 PDAC and matched para-carcinoma tissue specimens. IHC results
confirmed that Wnt5a and ABCG2 were expressed in different degrees
in pancreatic cancer tissues (Fig.
1A) of the 24 PDAC samples, IHC scores for ABCG2 ranged between
4–12 in 13 cases, giving a positive rate of 54.2%. The IHC scores
for Wnt5a ranged between 4–12 in 14 cases, giving a positive rate
of 58.3%. The expression levels of ABCG2 and Wnt5a genes were
significantly higher in the PDAC tissues compared with adjacent
tissues (Fig. 1B). The Spearman's
correlation coefficient between ABCG2 and Wnt5a expression levels
in the 24 PDAC specimens reached 0.692, suggesting a positive
correlation between Wnt5a and ABCG2 expression (Fig. 1C).
Survival analysis demonstrated the high ABCG2
expression group displayed significantly lower probability of
survival, compared with the low-expression group (Fig. 1D). While the postoperative survival
time of the 24 cases ranged between 3–26 months, the median
survival time of ABCG2− group (11 cases) and
ABCG2+ group (13 cases), were 17 months and 9 months,
respectively (Table I). Moreover,
the ABCG2 IHC scores in deceased patients negatively correlated
with the postoperative survival time, with a Spearman's coefficient
of −0.819 (Fig. 1E).
Wnt5a protein expression is positively
correlates with ABCG2
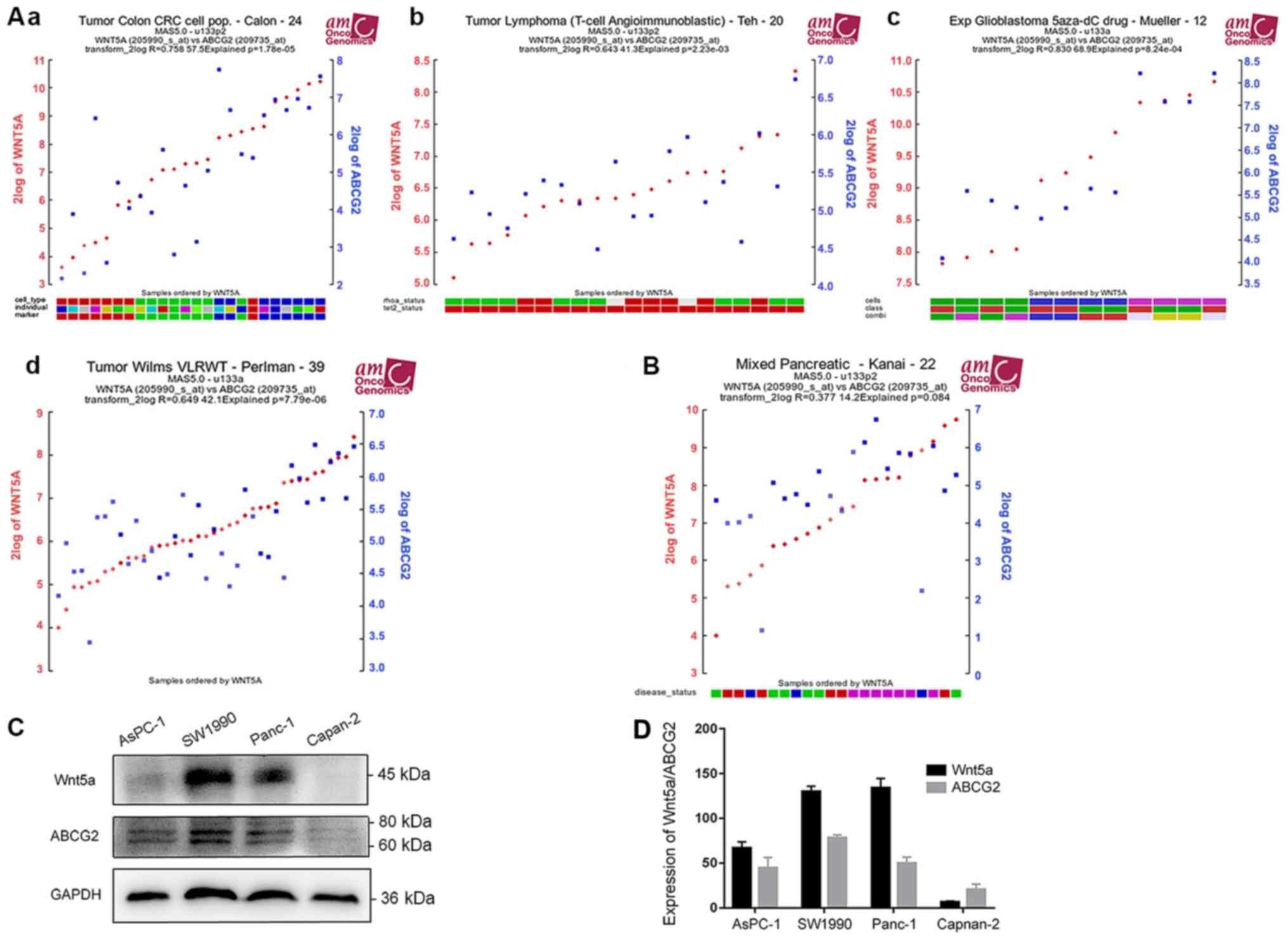
To further determine the association between Wnt5a
and ABCG2 expression levels, The correlation analysis of Wnt5a and
ABCG2 was performed using the gene correlation module of the R2
microarray analysis and visualization platform, which identified
that the expression levels of Wnt5a and ABCG2 were positively
correlated in colon cancer (data set of ‘Tumor Colon CRC cell
pop.-Calon-24-MAS5.0-u133p2’), lymphoma [data set of ‘Tumor
Lymphoma (T-cell Angioimmunoblastic)-Teh-20-MAS5.0-u133p2’], glioma
(data set of ‘Exp Glioblastoma 5aza-dC
drug-Mueller-12-MAS5.0-u133a’) and nephroblastoma (data set of
‘Tumor Wilms VLRWT-Perlman-39-MAS5.0-u133a’), with a Pearson
correlation coefficient ranging between 0.643–0.83 (Fig. 2A). The Pearson coefficient was 0.377
in mixed pancreatic diseases (data set of ‘Mixed
Pancreatic-Kanai-22-MAS5.0-u133p2’) (Fig. 2B), suggesting a moderate correlation
between the expression levels of Wnt5a and ABCG2 in pancreatic
diseases.
To examine the expression of Wnt5a and ABCG2 in
human pancreatic cancer, the protein expression levels for Wnt5a
and ABCG2 in four different types of pancreatic cancer cell lines
were measured using western blotting. SW1990 and PANC-1, which had
higher expression levels of WNT5a, also expressed ABCG2 at
relatively high level (Fig. 2C).
Since the protein levels of both Wnt5a and ABCG2 in Capan-2 cells
were lowest among the tested cell lines, these cells were selected
for subsequent experiments.
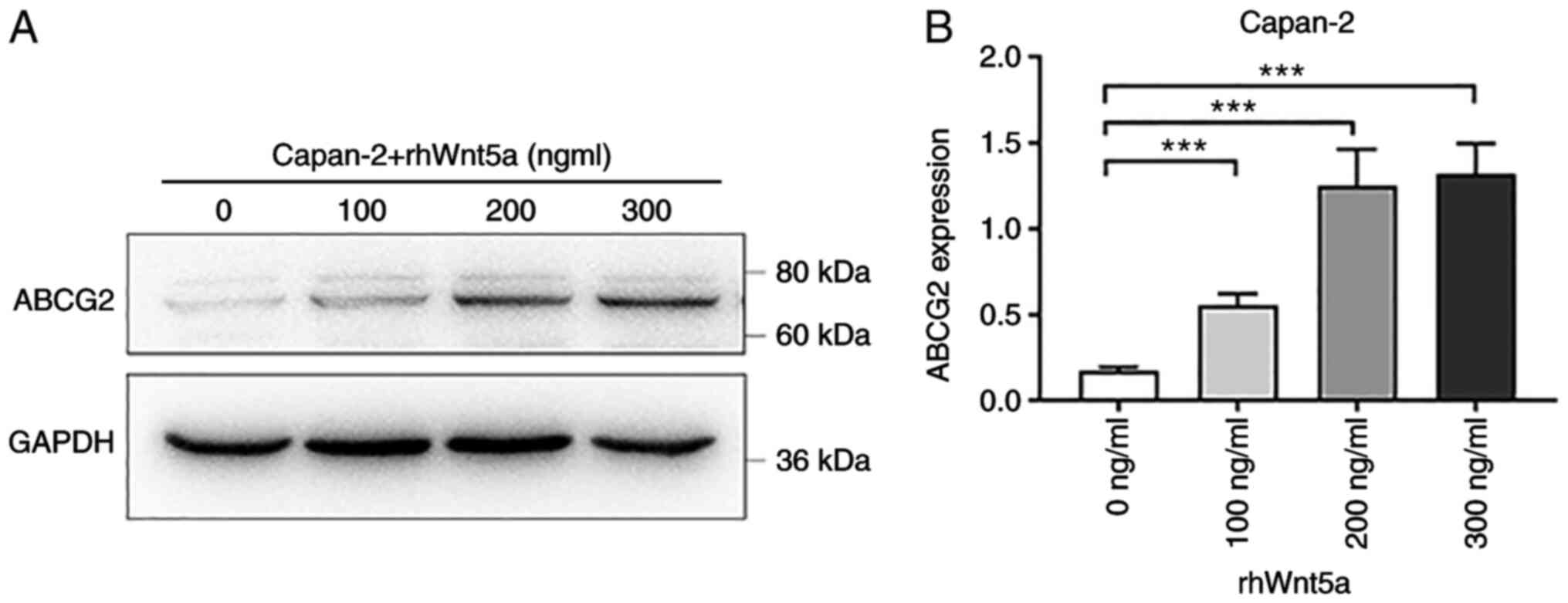
Recombinant human Wnt5a protein
upregulates the expression of ABCG2 in Capan-2 cells
Based on the positive correlation between Wnt5a and
ABCG2 expression in colon cancer, lymphoma, glioblastoma, Wilms'
tumor (Fig. 2A) and pancreatic
cancer cell lines, including Capan-2, it was hypothesized that
ABCG2 expression may be regulated by Wnt5a. Thus, to evaluate
whether Wnt5a was an upstream regulator of ABCG2, different
concentrations of rhWnt5a protein were added to the culture medium
of Capan-2 cells, and changes in ABCG2 protein expression were
assessed following treatment. After 48 h of administration of 100,
200 or 300 ng/ml rhWnt5a, cells were collected and subjected to
western blot analysis. The protein expression levels of ABCG2
increased following rhWnt5a treatment in a dose-dependent manner
(Fig. 3), suggesting that the
expression of ABCG2 was regulated by Wnt5a.
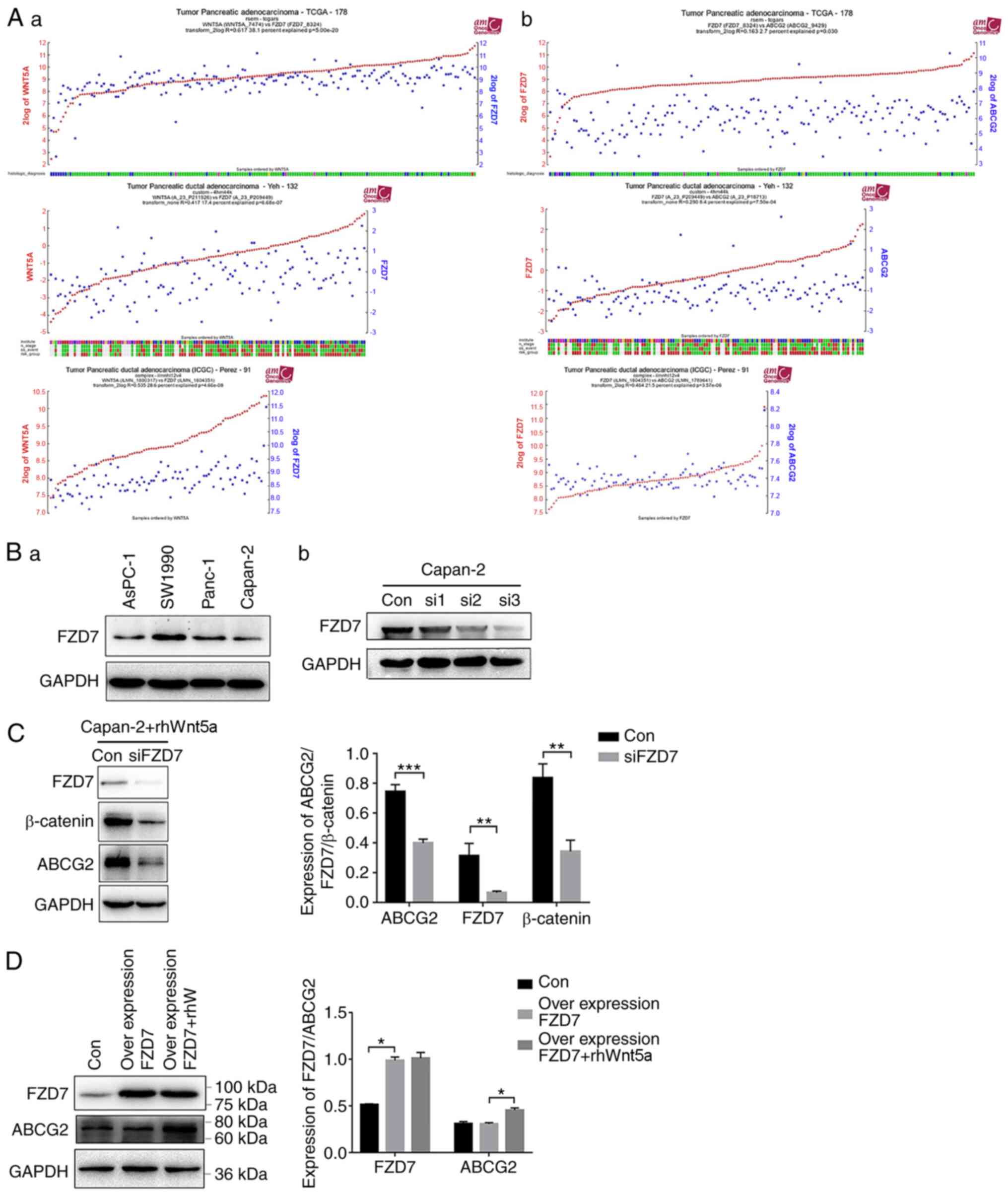
Wnt5a regulates the expression of
ABCG2 gene through FZD7
FZD7 is a receptor proteins in the Wnt pathway.
Mining of the ‘R2: Genomics Analysis and Visualization Platform’
(https://hgserver1.amc.nl/) database
identified a positive correlation between Wnt5a expression and FZD7
in pancreatic carcinoma. For the three different datasets [data set
of ‘Tumor Pancreatic adenocarcinoma-TCGA-178-rsem-tcgars’, ‘Tumor
Pancreatic ductal adenocarcinoma-Yeh-132-custom-4hm44k’ and ‘Tumor
Pancreatic ductal adenocarcinoma
(ICGC)-Perez-91-complex-ilmnht12v4’], Pearson coefficients between
Wnt5a and FZD7 were 0.617 (P=5.0×10−20), 0.417
(P=6.68×10−7) and 0.535 (P=4.66×10−8),
respectively (Fig. 4A-a).
Furthermore, R2 database demonstrated that FZD7 was positively
correlated with ABCG2 expression, with Pearson coefficients between
FZD7 and ABCG2 expression levels of 0.163 (P=0.030), 0.290
(P=7.5×10−4) and 0.464 (P=3.57×10−6),
respectively, for the same three aforementioned datasets (Fig. 4A-b). These findings indicated that
FZD7 may serve a role in regulating the expression of Wnt5a and/or
ABCG2 in pancreatic cancer.
Western blot analysis identified that FZD7 was
expressed in four different types of pancreatic cancer cell lines
(Fig. 4B-a). Moreover, it was found
that the siRNA for FZD7 successfully depleted FZD7 expression in
Capan-2 cells (Fig. 4B-b). Next,
siFZD7 cells were used to examine the effect of FZD7 on
Wnt5a-induced upregulation of ABCG2. rhWnt5a (200 ng/ml) was added
to the siFZD7 Capan-2 cells. The cells were collected and western
blot analysis performed 48 h after the treatment. The expression
levels of β-catenin, which are intracellular signal transducers in
the Wnt pathway (22), were
significantly lower in the siFZD7 cells compared with control siRNA
cells, in the presence of rhWnt5a (Fig.
4C), suggesting that physiological expression of FZD7 is
required for the upregulation of ABCG2 by Wnt5a. However, the
overexpression of FZD7 alone did not increase the ABCG2 protein
expression (Fig. 4D). These results
indicated that FZD7 is necessary, but not sufficient for
Wnt5a-induced upregulation of ABCG2 in Capan-2 cells.
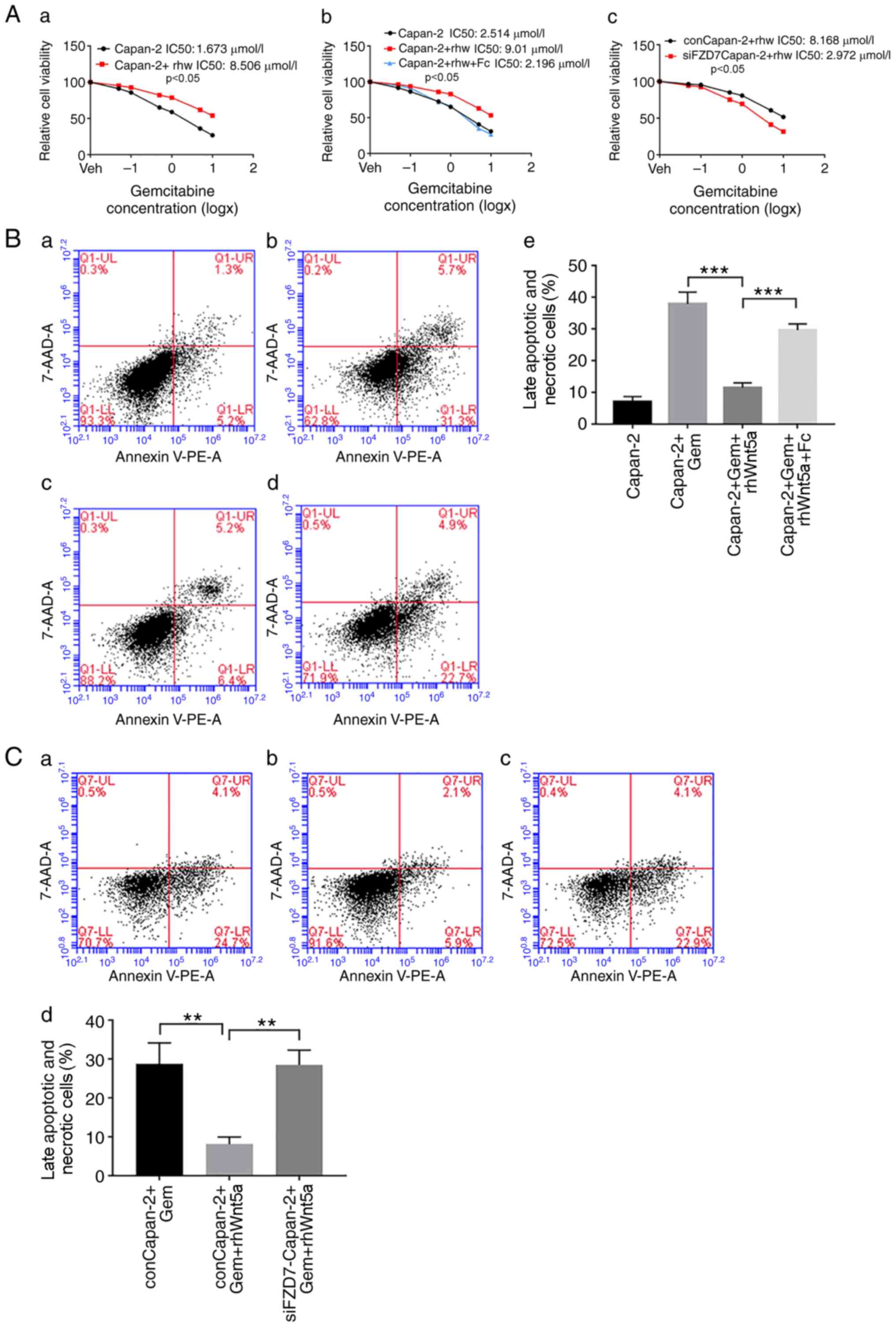
Role of FZD7 in Wnt5a-induced Gem
resistance in Capan-2 cells
Gem is used to treat a number of cancer types,
including pancreatic cancer. MTT assays demonstrated that the
IC50 of Gem in Capan-2 cells was increased following the
addition of rhWnt5a (Fig. 5A-a),
indicating that Wnt5a could directly induce resistance of Capan-2
cells to Gem. To investigate the role of FZD7 in this process, FZD7
expression was knocked down using siRNA, or its function was
inhibited using the FZD7 inhibitor rhFc (300 ng/ml). While rhWnt5a
increased the IC50 value of Gem, the addition of rhFc
attenuated this effect (Fig. 5A-b).
In addition, in siFZD7 Capan-2 cells, Gem has a lower
IC50 compared with control cells (Fig. 5A-c).
Moreover, the frequency of late apoptotic (lower
right quadrant) and necrotic cells (upper right quadrant) induced
by Gem (Fig. 5B-a and b) decreased
following rhWnt5a treatment (Fig.
5B-c), compared with untreated cells. However, treatment with
rhFc reduced the frequency of apoptotic and necrotic cells,
compared with cells treated with rhWnt5a alone (Fig. 5B-d and e). Moreover, while rhWnt5a
decreased the number of late apoptotic and dead cells induced by
Gem in control siRNA cells (Fig. 5C-a
and b), no such effects were observed in the FZD7 siRNA cells
(Fig. 5C-c and d). Collectively,
these data suggested that FZD7 may be a key factor for the
induction of Gem resistance via Wnt5a in pancreatic cancer
cells.
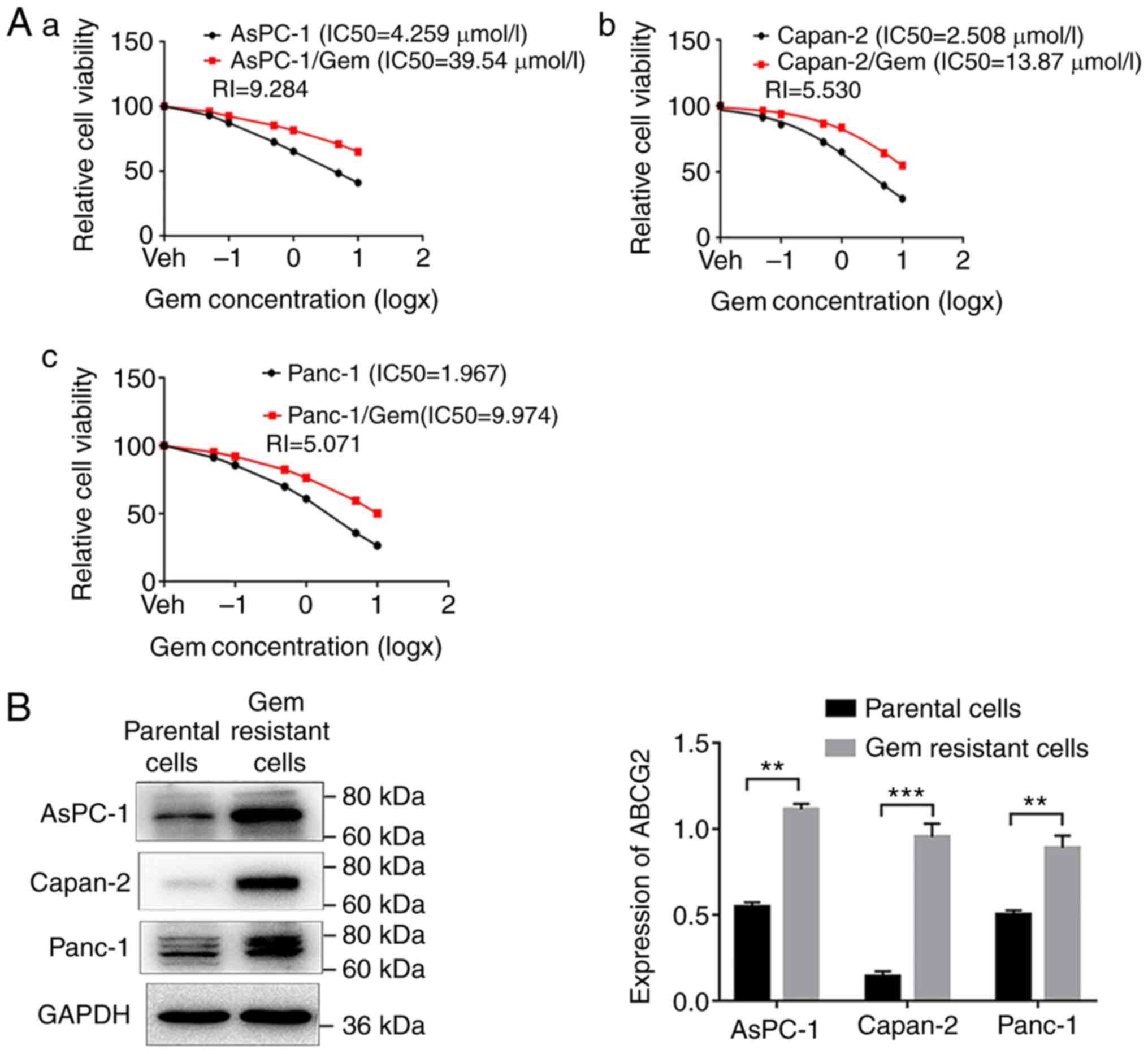
Gem-resistant cells express higher
levels of ABCG2 compared with parental cells
To determine whether high ABCG2 expression was
associated with Gem resistance in different cell lines, pancreatic
carcinoma cell lines resistant to Gem (AsPC-1/Gem, Capan-2/Gem and
Panc-1/Gem) were established using a low concentration of Gem
continuously. Next, the IC50 of Gem was measured using
an MTT assay in these cell lines and compared with the
IC50 in the parental cells (Fig. 6A). The IC50 of Gem was
increased from 4.259, 2.508 and 1.967 µM to 39.54, 13.87 and 9.974
µM, respectively, in AsPC-1/Gem, Capan-2/Gem and Panc-1/Gem cells
as compared with their parent cells (Fig. 6A), indicating successful
establishment of Gem-resistant pancreatic cancer cell lines.
Moreover, the drug-resistant cells expressed significantly higher
levels of ABCG2 compared with the parental cells (Fig. 6B), suggesting that upregulation of
ABCG2 may contribute to Gem resistance of pancreatic cancer
cells.
Discussion
Pancreatic cancer is associated with poor prognosis,
and the lack of effective chemotherapeutic drugs has been a
challenge for the treatment of pancreatic cancer. For instance, one
such issue for chemotherapy is drug resistance. Gem is one of few
drugs used for first-line chemotherapy for pancreatic cancer. But
it is effective in only 23.8% of PDAC cases (22), and development of Gem
chemoresistance serves an important role in this ineffectiveness
(23). The present study aimed to
elucidate the molecular mechanisms underlying Gem resistance in
order to provide a novel therapy strategy for PDAC. It has been
reported that the ABC-transporter superfamily proteins serve
important roles in MDR (6). And the
increase in the protein expression of ABCG2 has been shown to be
required for Gem resistance in pancreatic cancer cells (10). This finding is consistent with the
present results, which indicated that the protein expression of
ABCG2 was significantly higher in three different Gem-resistant
pancreatic cancer cell lines, compared with parental control cells.
Moreover, the IHC staining of 24 PDAC and adjacent tissue samples
demonstrated that high expression of ABCG2 correlated with poor
prognosis. Therefore, it would be beneficial to examine whether the
poor prognosis resulting from the high ABCG2 expression was
associated with increased drug-resistance in vivo.
To identify the upstream regulators of ABCG2
expression, the present study focused on Wnt signaling proteins, as
previous studies have reported the possible function of the Wnt
signaling pathway in the regulation of ABCG2 expression; however,
most studies were performed in cancer types other than pancreatic
cancer (11–14). Among the numerous Wnt proteins, the
current study focused on Wnt5a, as it is considered a mediator of
chemoresistance (15). To the best
of the authors' knowledge, no other Wnt family members have been
reported to regulate ABCG2. Furthermore, the inhibition of Wnt5a
increases the sensitivity of pancreatic cancer cells to drug
treatment in vitro and in vivo (18). In the current study, a positive
correlation between Wnt5a and ABCG2 gene expression levels was
identified using the R2 database. In addition, Wnt5a expression was
positively correlated with ABCG2 expression in the 24 PDAC samples
and in four different PDAC cell lines. As Wnt5a is a secreted
protein that affects cells in an autocrine or paracrine manner, and
most PDAC cell lines have the capacity to respond to exogenous Wnt
stimulation (24), rhWnt5a was used
to treat Capan-2 cells in order to assess its effect on ABCG2
expression. An increase in ABCG2 protein expression in Capan-2
cells was observed after Wnt5a treatment, supporting the hypothesis
that Wnt5a may be an upstream regulator of ABCG2 in pancreatic
cancer cells.
Wnt proteins are series of secretory proteins that
bind to Frizzle receptors on cell membranes in an autocrine or
paracrine manner and activate intracellular molecular cascade
(classical pathway or non-classical pathway) (24). For instance, once a classical
pathway is active, there is an accumulation of un-phosphorylated
β-catenin in the cytosol, which can translocate to the nucleus,
interacting with T cell-specific factor or lymphoid
enhancer-binding factor to activate the Wnt target genes, such as
c-Myc and cyclin D1 (25). The Wnt
protein act as ligands, whether from paracrine or autocrine of
peripheral cells, to effective bind to Frizzle receptors, and is
key for the activation and biological function of the Wnt signaling
pathway. Therefore, the present study further investigated which
type of Frizzle receptor could be involved in the regulation of
ABCG2 expression. The data extracted from the R2 database suggested
that Wnt5a protein expression correlated with the Wnt receptor
FZD7. The expression of ABCG2 was induced by Wnt5a in a
dose-dependent manner. The Wnt5a-mediated upregulation of ABCG5 was
inhibited by the Fzd7 knockdown, suggesting that Wnt5a regulatesd
the expression of ABCG2 through Fzd7. It has been previously
reported that Wnt5a and FZD7 are highly expressed in melanoma, and
that Wnt5a enhances the proliferation and viability of melanoma
cells through FZD7 (26). Thus, the
current study used a FDZ7 inhibitor and FDZ7 siRNA to inhibit the
function or knockdown the expression of FDZ7. Subsequently, the
effect of these treatments on the expression of ABCG2 was evaluated
in pancreatic cells. Inhibition of FDZ7 function and FDZ7 knockdown
both attenuated Wnt5a-induced upregulation of ABCG2, as well as the
resistance of pancreatic cells to Gem, indicating a critical role
for FZD7 in the regulation of ABCG2 and drug resistance in
pancreatic cancer cells. Interestingly, FZD7 alone was not able to
induce Gem resistance, suggesting that FZD7 was necessary but not
sufficient for induction of drug resistance in pancreatic cancer
cells.
A subpopulation of tumor cells with self-renewal and
multi-lineage differentiation capacity, defined as cancer stem
cells (CSCs), has been implicated in tumor resistance to therapy,
as well as recurrence, invasiveness, metastasis and reduced patient
survival time (27–29). Cancer cells with a stem cell
phenotype are more resistant to anti-cancer drugs and express
higher levels of ABCG2 compared with those without stem cell
phenotype (29–31). The activation of ABCG2 (32) or ABCB1 (31) is considered to be one of the
mechanisms for drug resistance of CSCs. For instance, the presence
of ABCG2 in certain populations of CSCs increases the likelihood of
resistance to various anticancer drugs (33,34).
Based on the fact that ABCG2 is highly expressed in certain CSC
cells (29), ABCG2 was proposed as
one of the phenotypes of CSCs (35). Recently, Sasaki et al
(36) isolated ABCG2 positive
pancreatic cancer cells in 3D culture conditions that had stemness
characteristics and anti-cancer drug resistance. Therefore, it
would be interesting to determine whether ABCG2 is required for
maintaining stemness of pancreatic CSCs and whether this accounts
for the resistance of pancreatic cancer cells to anticancer
drugs.
In conclusion, proteins in the Wnt pathway, such as
Wnt5a and FZD7, serve an important role in regulating the
expression of ABCG2, as well as in the anticancer drug resistance
of pancreatic cancer cells.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81572360).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The bioinformatics datasets generated and/or analyzed
during the current study are available in the ‘R2: Genomics
Analysis and Visualization Platform’ repository (https://hgserver1.amc.nl/).
Authors' contributions
ZBZ performed the majority of experiments and
drafted the manuscript. SG performed some of the experiments, as
well as conducted data statistical analysis and English translation
modification. YHX and CHZ designed the project, performed the
interpretation of patient data regarding the PDAC and gave final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethical Committee of
The First Hospital of China Medical University [approval no.
(2015)100]. Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matano E, Tagliaferri P, Libroia A,
Damiano V, Fabbrocini A, De Lorenzo S and Bianco AR: Gemcitabine
combined with continuous infusion 5-fluorouracil in advanced and
symptomatic pancreatic cancer: A clinical benefit-oriented phase II
study. Br J Cancer. 82:1772–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu M, Li L, Liu Z, Jiao Z, Xu P, Kong X,
Huang H and Zhang Y: ABCB2 (TAP1) as the downstream target of SHH
signaling enhances pancreatic ductal adenocarcinoma drug
resistance. Cancer Lett. 333:152–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Xue X, Wang F, An Y, Tang D, Xu Y,
Wang H, Yuan Z, Gao W, Wei J, Zhang J and Miao Y: Expression and
promoter methylation analysis of ATP-binding cassette genes in
pancreatic cancer. Oncol Rep. 27:265–269. 2012.PubMed/NCBI
|
|
5
|
Hopper-Borge E, Xu X, Shen T, Shi Z, Chen
ZS and Kruh GD: Human multidrug resistance protein 7 (ABCC10) is a
resistance factor for nucleoside analogues and epothilone B. Cancer
Res. 69:178–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Binkhathlan Z and Lavasanifar A:
P-glycoprotein inhibition as a therapeutic approach for overcoming
multidrug resistance in cancer: Current status and future
perspectives. Curr Cancer Drug Targets. 13:326–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choudhuri S and Klaassen CD: Structure,
function, expression, genomic organization, and single nucleotide
polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP)
efflux transporters. Int J Toxicol. 25:231–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gatti L, Beretta GL, Cossa G, Zunino F and
Perego P: ABC transporters as potential targets for modulation of
drug resistance. Mini Rev Med Chem. 9:1102–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu MC, Pan MR, Chu PY, Tsai YL, Tsai CH,
Shan YS, Chen LT and Hung WC: Protein arginine methyltransferase 3
enhances chemoresistance in pancreatic cancer by methylating
hnRNPA1 to increase ABCG2 Expression. Cancers (Basel). 11:82018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vesel M, Rapp J, Feller D, Kiss E, Jaromi
L, Meggyes M, Miskei G, Duga B, Smuk G, Laszlo T and Karner I:
ABCB1 and ABCG2 drug transporters are differentially expressed in
non-small cell lung cancers (NSCLC) and expression is modified by
cisplatin treatment via altered Wnt signaling. Respir Res.
18:522017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen B, Zhang D, Kuai J, Cheng M, Fang X
and Li G: Upregulation of miR-199a/b contributes to cisplatin
resistance via Wnt/β-catenin-ABCG2 signaling pathway in
ALDHA1+colorectal cancer stem cells. Tumor Biol.
39:1–14. 2017. View Article : Google Scholar
|
|
13
|
Martins-Neves SR, Paiva-Oliveira DI,
Wijers-Koster PM, Abrunhosa AJ, Fontes-Ribeiro C, Bovée JV,
Cleton-Jansen AM and Gomes CM: Chemotherapy induces stemness in
osteosarcoma cells through activation of Wnt/β-catenin signaling.
Cancer Lett. 370:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spee B, Carpino G, Schotanus BA,
Katoonizadeh A, Vander Borght S, Gaudio E and Roskams T:
Characterisation of the liver progenitor cell niche in liver
diseases: Potential involvement of Wnt and Notch signaling. Gut.
59:247–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng C, Zhang X, Yu H, Wu D and Zheng J:
Wnt5a as a predictor in poor clinical outcome of patients and a
mediator in chemoresistance of ovarian cancer. Int J Gynecol
Cancer. 21:280–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hung TH, Hsu SC, Cheng CY, Choo KB, Tseng
CP, Chen TC, Lan YW, Huang TT, Lai HC, Chen CM and Chong KY: Wnt5a
regulates ABCB1 expression in multidrug-resistant cancer cells
through activation of the non-canonical PKA/β-catenin pathway.
Oncotarget. 5:12273–12290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao J and Wang Q, Wu G, Li S and Wang Q:
miR-129-5p inhibits Gemcitabine resistance and promotes cell
apoptosis of bladder cancer cells by targeting Wnt5a. Int Urol
Nephrol. 50:1811–1819. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong
SC, Chan AT, Sung JJ and Tao Q: WNT5A exhibits tumor-suppressive
activity through antagonizing the Wnt/beta-catenin signaling, and
is frequently methylated in colorectal cancer. Clin Cancer Res.
14:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Zhou C, Cheng L, Yan B, Chen K,
Chen X, Zong L, Lei J, Duan W, Xu Q, et al: Inhibiting YAP
expression suppresses pancreatic cancer progression by disrupting
tumor-stromal interactions. J Exp Clin Cancer Res. 37:692018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDermott M, Eustace AJ, Busschots S,
Breen L, Crown J, Clynes M, O'Donovan N and Stordal B: In vitro
development of chemotherapy and targeted therapy drug-resistant
cancer cell lines: A Practical Guide with Case Studies. Front
Oncol. 6:402014.PubMed/NCBI
|
|
21
|
Qiu X, Jiao J, Li Y and Tian T:
Overexpression of FZD7 promotes glioma cell proliferation by
upregulating TAZ. Oncotarget. 7:85987–85999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang C, Shi S, Meng Q, Liang D, Ji S,
Zhang B, Qin Y, Xu J, Ni Q and Yu X: Complex roles of the stroma in
the intrinsic resistance to gemcitabine in pancreatic cancer: Where
we are and where we are going. Exp Mol Med. 49:e4062017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amrutkar M and Gladhaug IP: Pancreatic
Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 9:1572017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arensman MD, Kovochich AN, Kulikauskas RM,
Lay AR, Yang PT, Li X, Donahue T, Major MB, Moon RT, Chien AJ and
Dawson DW: WNT7B mediates autocrine Wnt/β-catenin signaling and
anchorage-independent growth in pancreatic adenocarcinoma.
Oncogene. 33:899–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anastas JN, Kulikauskas RM, Tamir T, Rizos
H, Long GV, von Euw EM, Yang PT, Chen HW, Haydu L, Toroni RA, et
al: WNT5A enhances resistance of melanoma cells to targeted BRAF
inhibitors. J Clin Invest. 124:2877–2890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frank NY, Schatton T and Frank MH: The
therapeutic promise of the cancer stem cell concept. J Clin Invest.
120:41–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: Promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crea F, Danesi R and Farrar WL: Cancer
stem cell epigenetics and chemoresistance. Epiqenomics. 1:63–79.
2009. View Article : Google Scholar
|
|
30
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RT and Fan ST: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song KH, Choi CH, Lee HJ, Oh SJ, Woo SR,
Hong SO, Noh KH, Cho H, Chung EJ, Kim JH, et al: HDAC1 upregulation
by NANOG promotes multidrug resistance and a stem-like phenotype in
immune edited tumor cells. Cancer Res. 77:5039–5053. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang H, Xiong W, Chen L, Lv Z, Yang C and
Li Y: Knockdown of the long noncoding RNA HOTTIP inhibits cell
proliferation and enhances cell sensitivity to cisplatin by
suppressing the Wnt/β-catenin pathway in prostate cancer. J Cell
Biochem. 120:8965–8974. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding XW, Wu JH and Jiang CP: ABCG2: A
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasaki N, Ishiwata T, Hasegawa F,
Michishita M, Kawai H, Matsuda Y, Arai T, Ishikawa N, Aida J,
Takubo K and Toyoda M: Stemness and anti-cancer drug resistance in
ATP-binding cassette subfamily G member 2 highly expressed
pancreatic cancer is induced in 3D culture conditions. Cancer Sci.
109:1135–1146. 2018. View Article : Google Scholar : PubMed/NCBI
|