Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE)
refers to the clinical symptoms of hypoxic-ischemic damage to the
brain caused by asphyxia during the perinatal period, which is
associated with a high morbidity worldwide of ~737 per 100,000 in
2015 (1–3). Ischemia/reperfusion (I/R)-induced
brain injury is one of the most common causes of neonatal brain
injury (4). It arises from the
temporary interruption of blood supply followed by the recovery of
perfusion and concomitant reoxygenation, which exacerbates the
dysfunction and leads to damage to the brain parenchyma (5). This disease is life threatening to the
newborn and one of the most common causes of neonatal disability
affects ~5,000–20,000 infants per year in Europe (1–4/1,000 live
births in high-income countries) and ~1,000,000 infants per year
worldwide (6,7). Although several efforts have been made
to determine the underlying mechanisms of cerebral I/R injury, the
precise molecular mechanisms remain largely unknown. Thus, a better
understanding of the disease is imperative in order to aid the
identification of novel targets for the development of effective
therapies.
A number of studies have demonstrated that rapid
reperfusion possesses the ability to rescue dying cells in ischemic
areas; however, it also results in the excessive release of
inflammatory mediators and the formation of oxidative stress, which
together, contribute to the exacerbation of brain injury (8,9).
Increasing evidence suggests that both inflammation and oxidative
stress accelerate neuronal apoptosis in the development of cerebral
I/R injury (10,11).
S100a8 (also known as calgranulin A or migration
inhibitory factor-related protein 8) is a member of the S100
protein family of calcium-modulated proteins (12). It is well known that S100a8 is
involved in the pathobiology of inflammatory disorders (13). S100a8 is highly expressed in the
brains of mice following focal cerebral I/R injury (14). Increasing evidence suggests that
S100a8/a9 genes are significantly upregulated during the early
stages of myocardial I/R injury (15,16).
Notably, S100a8/a9 plays a vital role in regulating
macrophage-mediated renal repair following I/R (17). The Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) http://string-db.org/cgi/input.pldatabase revealed
that Grb2-associated binder 1 (GAB1), an intracellular scaffolding
adaptor (18), may interact with
S100a8 (15). In addition,
increasing evidence suggests that GAB1 protects against myocardial
I/R injury and oxidative stress in cardiomyocytes (19,20).
Activation of the GAB1/Src/β-catenin signaling pathway may protect
the integrity of the blood-brain barrier following cerebral
hemorrhage (21). Thus, the
function of S100a8 in cerebral I/R injury, and whether it can
protect against this disease by regulating GAB1 expression, has
attracted research interest.
In the present study, oxygen-glucose deprivation and
reoxygenation (OGD/R)-induced BV2 cell injury were used to mimic a
model of cerebral I/R injury in vitro. The effects of S100a8
on inflammation, oxidative stress and the apoptosis of BV2 cells,
and its potential molecular mechanisms were investigated. Taken
together, the results of the present study may provide a novel
therapeutic target for cerebral I/R injury.
Materials and methods
Cells and cell culture
The murine microglia cell line, BV2, was provided by
the China Center for Type Culture Collection and maintained in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). BV2 cells were cultured in a 95% air/5%
CO2 incubator at 37°C, at saturated humidity.
Prediction of target genes
Search tool for recurring instances of neighboring
genes (STRING) database (https://string-db.org/cgi/input.pl; version 11.0) was
used. STRING is an open-source platform for studying the
miRNA-ncRNA, miRNA-mRNA, ncRNA-RNA, RNA-RNA, RBP-ncRNA and RBP-mRNA
interactions from CLIP-seq, degradome-seq and RNA-RNA interactome
data.
Cell transfection
Short hairpin (sh)RNAs targeting S100a8
(shRNA-S100a8-1 and shRNA-S100a8-2; 500 ng/µl; 50 nmol), scrambled
negative control (NC) shRNA, small interfering (si)RNA against GAB1
and scrambled siRNA-NC were purchased from Shanghai GenePharma Co.,
Ltd. BV2 cells were seeded into 6-well plates (with DMEM) at a
density of 1×106 cells/well 24 h prior to transfection
at 37°C for 6 h. Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
transfection. Transfection efficiency was assessed using reverse
transcription-quantitative (RT-q) PCR and western blot analyses 24
h post-transfection.
OGD/R treatment
OGD/R was performed to generate I/R models in
vitro. BV2 cells were seeded into 95-cm cell culture dishes at
a density of 1×106 cells/well and incubated at 37°C for
6 h. To mimic OGD/R injury, cells in the logarithmic growth phase
were cultured in glucose-free DMEM and placed in a hypoxic chamber
(Thermo Fisher Scientific, Inc.) supplemented with a gas mixture of
1% O2, 94% N2 and 5% CO2 at 37°C
for 6 h. OGD was terminated by restoration with high-glucose DMEM
and incubation under normoxic conditions (95% air and 5%
CO2) at 37°C for 12 h. BV2 cells without any treatment
were used as a control.
Flow cytometric analysis of
apoptosis
Apoptosis was detected using an apoptosis detection
kit (cat. no. KGA102, Nanjing KeyGen Biotech Co., Ltd.). Following
transfection for 24 h, following the manufacturer's manual, BV2
cells were stained with Annexin V-fluorescein isothiocyanate and
propidium iodide at room temperature in the dark for 15 min.
Apoptosis cells were subsequently analyzed using a flow cytometer
(BD Biosciences) and FlowJo software version 7.6.5 (FlowJo
LLC).
Immunoprecipitation (IP) assay
BV2 cells (1×107) were washed in 2 ml PBS
(Beyotime Institute of Biotechnology) and centrifuged at 850 × g at
room temperature for 5 min to collect the cells. OGD/R-exposed BV2
cells were lysed using lysis buffer for IP (Beyotime Institute of
Biotechnology). Lysates were incubated with the indicated
antibodies S100a8 (cat. no. 73425; 1:1,000 dilution), GAB-1 (cat.
no. 12747; 1:1,000 dilution) and control lgG for 1 µg plus Protein
A/G beads (Santa Cruz Biotechnology, Inc.) at 4°C for 2 h. The
beads were washed with PBS (Beyotime Institute of Biotechnology)
three times and the immunoprecipitants were assessed via western
blot analysis.
Concentrations of inflammatory
cytokines
Culture medium (the supernatant) was collected at
the end of the reoxygenation stage. The levels of inflammatory
cytokines, including tumor necrosis factor-α (TNF-α; cat. no.
F11632), interleukin (IL)-1β (cat. no. F10770) and IL-6 (cat. no.
F10830) were assessed in the culture supernatant using enzyme
linked immunosorbent assay kits (Shanghai Westang Bio-Tech Co.,
Ltd).
Detection of oxidative stress-related
markers
After 12 h of reoxygenation, the culture supernatant
was harvested. The concentration of malondialdehyde (MDA; cat. no.
A003-4-1), as well as the activities of superoxide dismutase (SOD;
cat. no. A001-3-2) and glutathione peroxidase (GSH-Px; cat. no.
A005-1-2) were determined using chemical colorimetric detection
kits (Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's instructions.
RT-qPCR
Following the different treatments, total RNA was
extracted from BV2 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed into cDNA using PrimeScript RT reagent (Thermo Fisher
Scientific, Inc.). Following the manufacturer's protocols, Using
SuperScript™ III Platinum™ One-Step qRT-PCR kit (Thermo Fisher
Scientific, Inc.; cat. no. 11732020) amplification reactions were
initially incubated at 94°C for 5 min, followed by 35 cycles of
94°C/30 sec, 54°C/30 sec, and 72°C/20 sec with a final extension at
72°C for 2 min. Thermocycling conditions of the qPCR were: 5 min at
95°C, with 40 cycles for 30 sec at 95°C and 45 sec at 65°C. Gene
expression was quantified using the ABI 7500 PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following primer
sequences were used for qPCR: S100A8 forward,
5′-AAATCACCATGCCCTCTACAAG-3′ and reverse,
5′-CCCACTTTTATCACCATCGCAA-3′; GAB1 forward,
5′-GTTGATGCTGGGTTGACATTCA-3′ and reverse,
5′-GAAAATCCGGTCGATGGTGTT-3′; cyclooxygenase-2 (COX-2) forward,
5′-ACGGTCCTGAACGCATTTATG-3′ and reverse,
5′-TTGGCCCCATTTAGCAATCTG-3′; induced nitric oxide synthase (iNOS)
forward, 5′-CTCTTCGACGACCCAGAAAAC-3′ and reverse,
5′-CAAGGCCATGAAGTGAGGCTT-3′; and GAPDH forward,
5′-TTGTCATGGGAGTGAACGAGA-3′ and reverse,
5′-CAGGCAGTTGGTGGTACAGG-3′. Relative expression levels were
measured using the 2−ΔΔCq method (22) and normalized to the internal
reference gene GAPDH.
Western blotting
Following the different treatments, BV2 cells were
collected and total protein was extracted using RIPA buffer
(Applygen Technologies, Inc.). Total protein was quantified using
the bicinchoninic acid kit (Beyotime Institute of Biotechnology)
and 50 µg protein/lane was separated via SDS-PAGE and proteins (30
µg/lane) were separated via SDS-PAGE (15%). The separated proteins
were subsequently transferred onto nitrocellulose membranes (EMD
Millipore), blocked with 5% non-fat milk at room temperature for 2
h and incubated with primary antibodies at 4°C overnight against:
S100A8 (cat. no. 47310T), GAB1 (cat. no. 3232T), COX-2 (cat. no.
12282T), iNOS (cat. no. 13120S), Src (cat. no. 109381), β-catenin
(cat. no. 32572), Bax (cat. no. 14796S), Bcl-2 (cat. no. 3498T),
cleaved caspase-3 (cat. no. 14220T) and GAPDH (cat. no. 5174T), all
at 1:1,000 and purchased from Cell Signaling Technology, Inc.
Following the primary incubation, the membranes were washed with
TBS containing Tween-20 (0.1%) and incubated at room temperature
for 1.5 h with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody 1:5,000 (cat. no. SA00001-9, ProteinTech
Group, Inc.) or goat anti-mouse IgG secondary antibody 1:5,000
(cat. no. SA00001-8, ProteinTech Group, Inc.). Protein bands were
visualized using the Odyssey Western Blot Analysis system (LI-COR
Biosciences) and quantified using ImageJ software version 7.6.5
(National Institutes of Health).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. Statistical
analysis was performed using GraphPad Prism 5 software (GraphPad
Software, Inc.) and all data are presented as the mean ± SEM.
Unpaired two-tailed Student's t-test was used to compare
differences between two groups, while one-way analysis of variance
followed by Tukey's post hoc test was performed to compare
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
S100a8 is highly expressed in
OGD/R-exposed BV2 cells
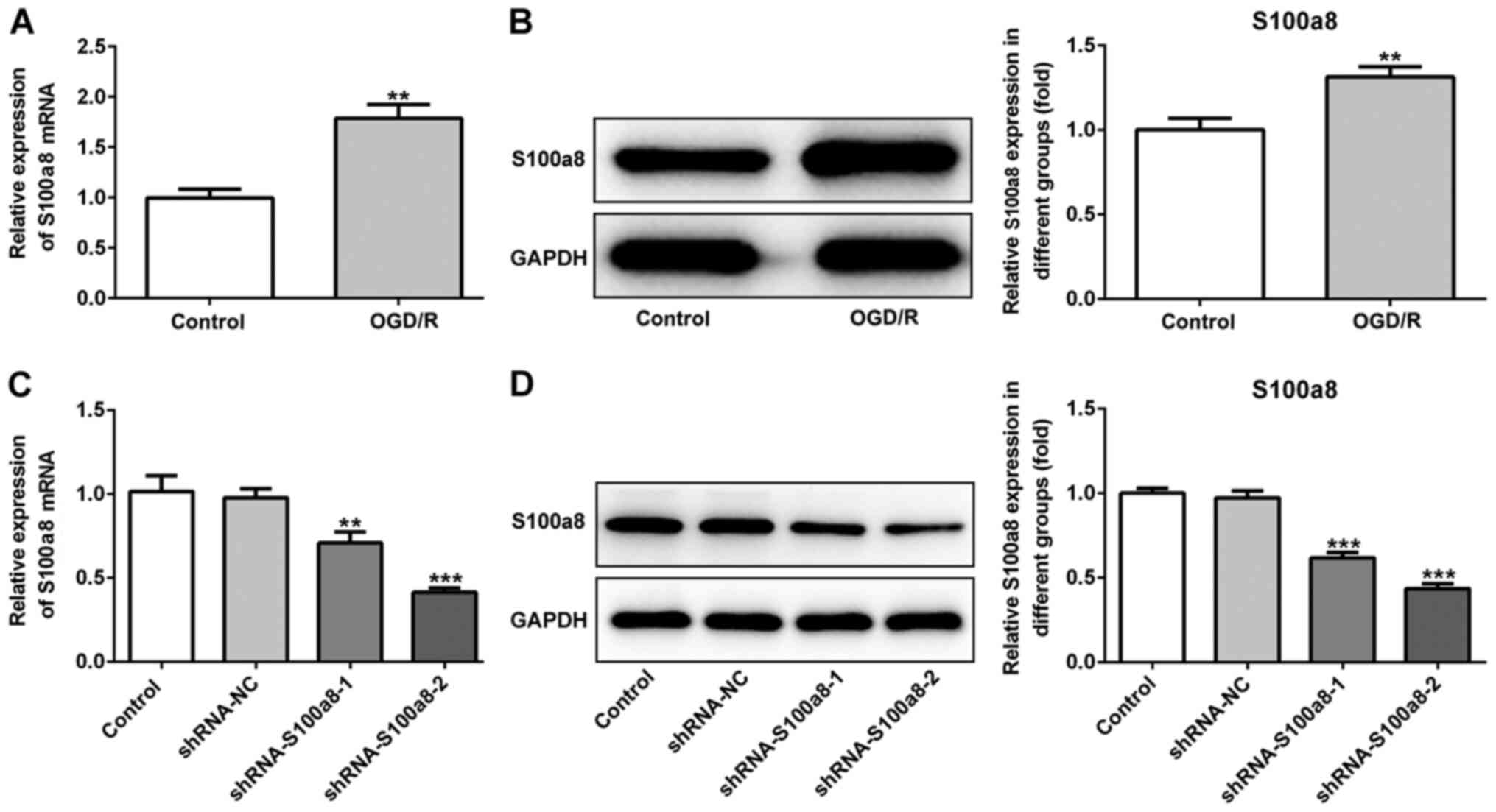
To determine the role of S100a8 in BV2 cells
subjected to OGD/R, RT-qPCR and western blot analyses were
performed to detect S100a8 expression. The results demonstrated
that S100a8 expression was significantly increased at the
transcriptional and post-transcription level following exposure to
OGD/R (Fig. 1A and B).
Subsequently, shRNA-S100a8-1 or shRNA-S100a8-2 were transfected
into BV2 cells, and transfection efficiency was assessed via
RT-qPCR and western blot analyses. The results indicated that
transfection with shRNA-S100a8-2 decreased S100a8 expression to a
greater degree than shRNA-S100a8-1 (Fig. 1C and D). Thus, BV2 cells transfected
with shRNA-S100a8-2 were used for subsequent experimentation.
S100a8 silencing inhibits inflammation
and oxidative stress in OGD/R-exposed BV2 cells
As presented in Fig.
2A-C, exposure to OGD/R significantly increased the contents of
inflammatory factors, including TNF-α, IL-1β and IL-6, whereas
S100a8 silencing inhibited the secretion of the aforementioned
factors compared with the NC group. In addition, commercial kits
were used to determine the levels of oxidative stress-related
markers. The results demonstrated that the concentration of MDA was
notably enhanced, accompanied by the decreased activities of SOD
and GSH-Px in BV2 cells following exposure to OGD/R; however, these
effects were reversed following transfection with siRNA-S100a8-2
(Fig. 2D-F). To determine the
potential underlying molecular mechanisms, the expression levels of
COX-2 and iNOS were assessed via western blot and RT-qPCR analyses,
respectively. The results demonstrated that COX-2 and iNOS
expression were markedly upregulated in the OGD/R group compared
with the control group, whereas S100a8 silencing downregulated
COX-2 and iNOS expression (Fig. 2G and
H). Taken together, these results suggest that S100a8 silencing
suppresses inflammation and oxidative stress in OGD/R-exposed BV2
cells.
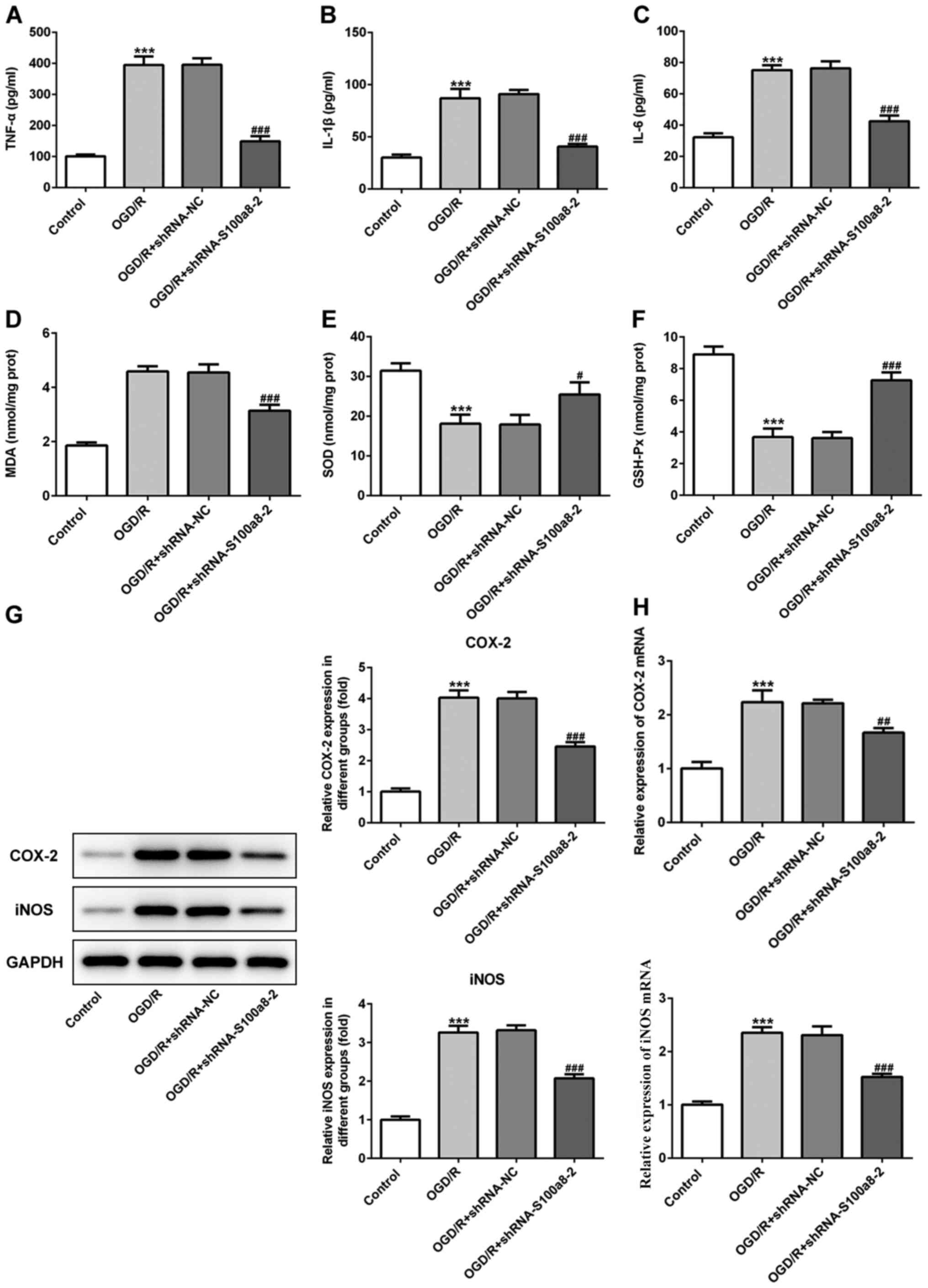 | Figure 2.S100a8 silencing suppresses
inflammation and oxidative stress in OGD/R-exposed BV2 cells. The
expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were
assessed using ELISA kits. The concentration of (D) MDA, as well as
the activities of (E) SOD and (F) GSH-Px were determined using
commercial kits. (G) Western blot and (H) reverse
transcription-quantitative PCR analyses were performed to determine
the expression of COX-2 and iNOS following transfection with
shRNA-S100a8-2. ***P<0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. OGD/R +
shRNA-NC. OGD/R, oxygen-glucose deprivation and reoxygenation;
TNF-α, tumor necrosis factor α; IL, interleukin; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; COX-2, cyclooxygenase-2; iNOS, induced nitric oxide
synthase; sh, short hairpin; NC, negative control. |
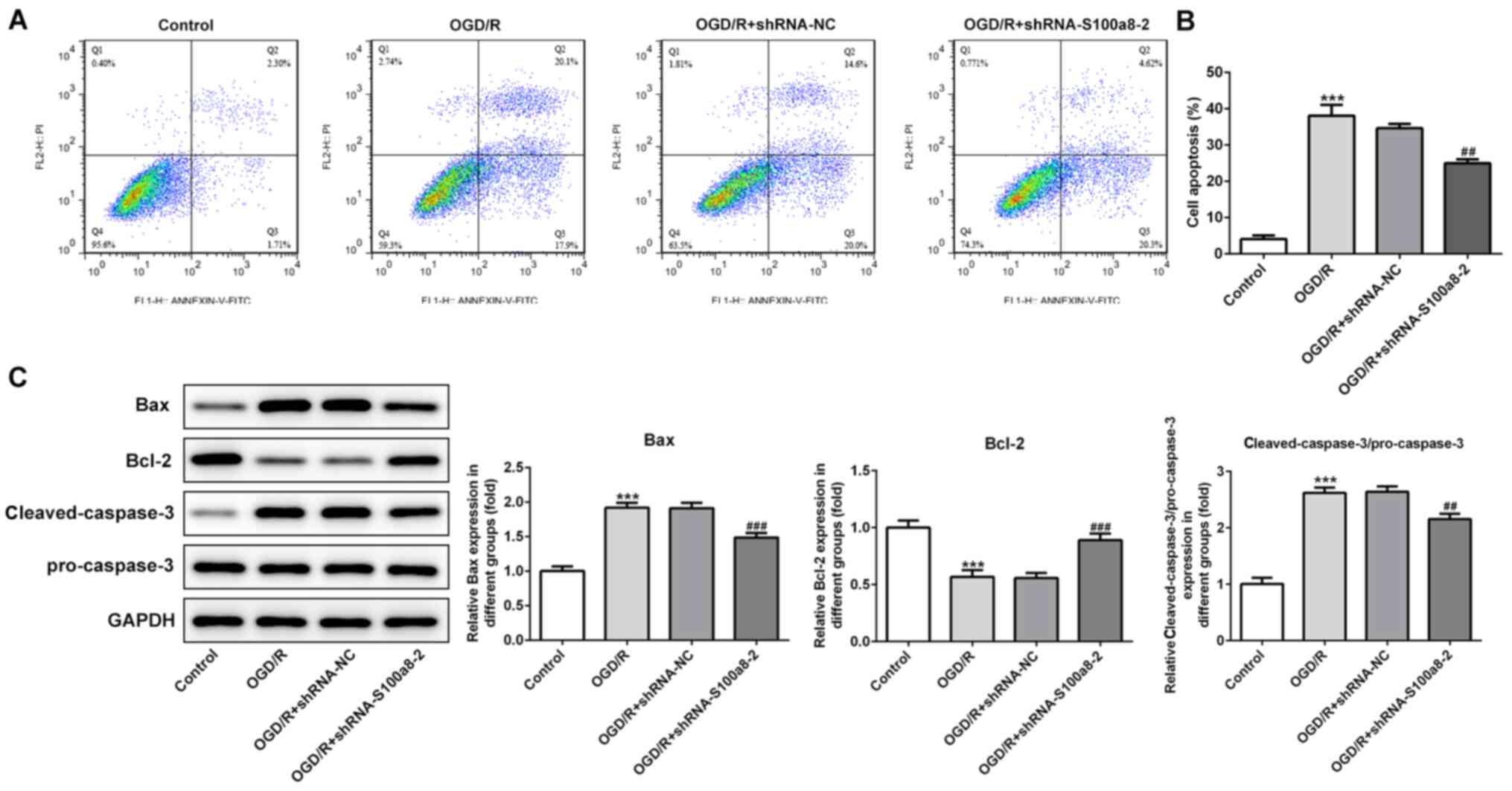
S100a8 silencing restrains apoptosis
in OGD/R-induced BV2 cells
To investigate the function of S100a8 silencing on
the apoptosis of OGD/R-exposed BV2 cells, flow cytometric analysis
was performed to detect the apoptotic rate. The results
demonstrated that exposure to OGD/R markedly enhanced the apoptotic
rate of BV2 cells relative to the control group, whereas S100a8
silencing decreased cell apoptosis (Fig. 3A and B). Consistently, the
expression levels of the pro-apoptotic proteins, Bax and
cleaved-caspase-3/pro-caspase-3, were notably upregulated
accompanied by downregulated expression of the anti-apoptotic
protein, Bcl-2, following OGD/R stimulation; however, these effects
were reversed following transfection with shRNA-S100a8-2 (Fig. 3C). Collectively, these results
suggest that S100a8 silencing inhibits the apoptosis of BV2 cells
subjected to OGD/R.
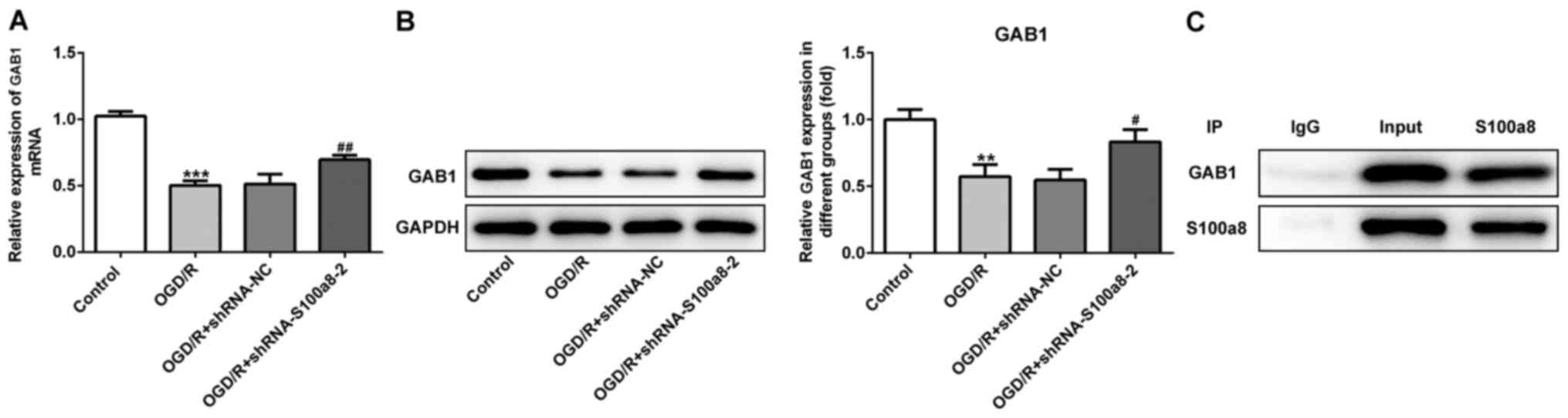
GAB1 directly interacts with
S100a8
To further determine the molecular mechanisms
underlying S100a8-mediated inflammation, oxidative stress and the
apoptosis of OGD/R-exposed BV2 cells, the STRING database was used
to predict the potential proteins interacting with S100a8. The
results indicated that GAB1 directly interacts with S100a8. As
presented in Fig. 4A and B, GAB1
expression markedly decreased in the OGD/R-exposed group compared
with the NC group, while S100a8 silencing evidently increased GAB1
expression compared with the OGD/R-exposed group. The IP assay
confirmed a strong association between GAB1 and S100a8 (Fig. 4C). Taken together, these results
suggest that GAB1 can directly interact with S100a8 in
OGD/R-exposed BV2 cells.
GAB1 silencing alleviates the
inhibitory effects of S100a8 silencing on inflammation and
oxidative stress in OGD/R-exposed BV2 cells
siRNA-GAB1-1 or siRNA-GAB1-2 were transfected into
BV2 cells and GAB1 expression was determined (Fig. 5A and B). The results demonstrated
that GAB1 expression was significantly downregulated following
transfection, and that transfection with siRNA-GAB1-1 decreased
GAB1 expression compared with the control group. Thus, BV2 cells
transfected with siRNA-GAB1-1 were used for subsequent
experimentation. The expression of downstream targets of GAB1,
including Src and β-catenin was assessed via western blot analysis.
Exposure to OGD/R significantly downregulated the expression of
both Src and β-catenin compared with the control, while S100a8
silencing evidently increased their expression compared with the NC
group (Fig. 5C). However, GAB1
silencing restored the inhibitory effects of S100a8 silencing on
the expression levels of Src and β-catenin (Fig. 5C). In addition, as presented in
Fig. 5D-F, GAB1 silencing enhanced
the expression levels of TNF-α, IL-1β and IL-6 relative to the
siRNA-NC group in the OGD/R-exposed BV2 cells, along with S100a8
silencing. Simultaneously, GAB1 silencing attenuated the effects of
S100a8 silencing on the level of MDA and the activities of SOD and
GSH-Px (Fig. 5G-I). As expected,
the expression levels of COX-2 and iNOS markedly increased
following silencing of GAB1 and S100a8 compared with S100a8
silencing alone (Fig. 5J and K).
Collectively, these results indicate that GAB1 silencing abrogates
the inhibitory effects of S100a8 silencing on inflammation and
oxidative stress in OGD/R-exposed BV2 cells.
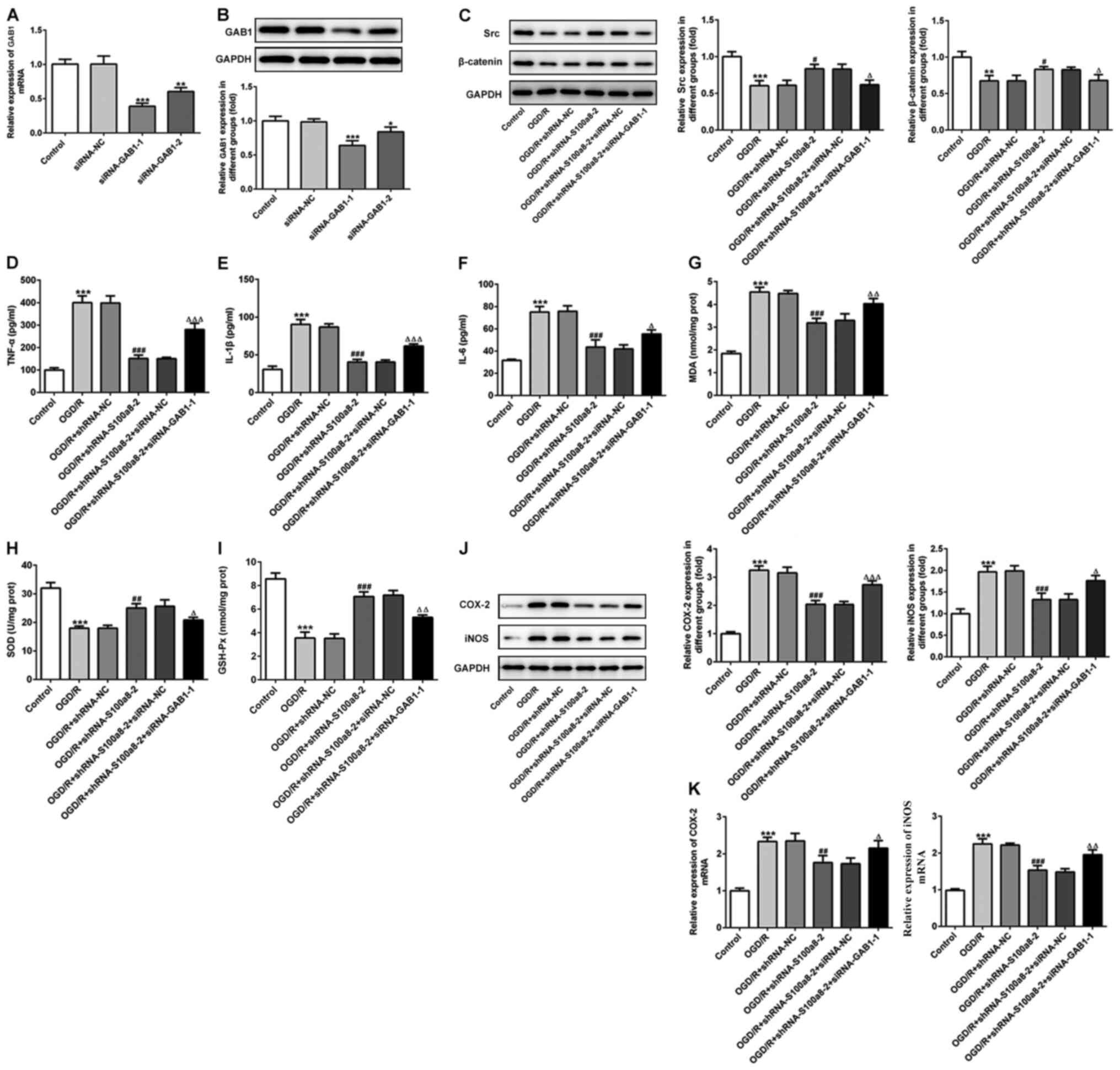 | Figure 5.GAB1 silencing alleviates the
inhibitory effects of S100a8 silencing on inflammation and
oxidative stress in OGD/R-exposed BV2 cells. (A) RT-qPCR and (B)
western blot analyses were performed to determine GAB1 expression
following transfection with siRNA-GAB1-1 or siRNA-GAB1-2. (C)
Expression of downstream targets of GAB1, including Src and
β-catenin was assessed via western blot analysis. Expression levels
of (D) TNF-α, (E) IL-1β and (F) IL-6 were determined using ELISA
kits. The concentration of (G) MDA, as well as the activities of
(H) SOD and (I) GSH-Px were determined using commercial kits. (J)
Western blot and (K) RT-qPCR analyses were performed to determine
the expression levels of COX-2 and iNOS. *P<0.05, **P<0.01,
***P<0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. OGD/R +
shRNA-NC; ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001 vs. OGD/R + shRNA-S100a8-2 + siRNA-NC.
GAB1, Grb2-associated binder 1; OGD/R, oxygen-glucose deprivation
and reoxygenation; RT-qPCR, reverse transcription-quantitative PCR;
si, small interfering; TNF-α, tumor necrosis factor α; IL,
interleukin; MDA, malondialdehyde; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; COX-2, cyclooxygenase-2; iNOS,
induced nitric oxide synthase; sh, short hairpin; NC, negative
control. |
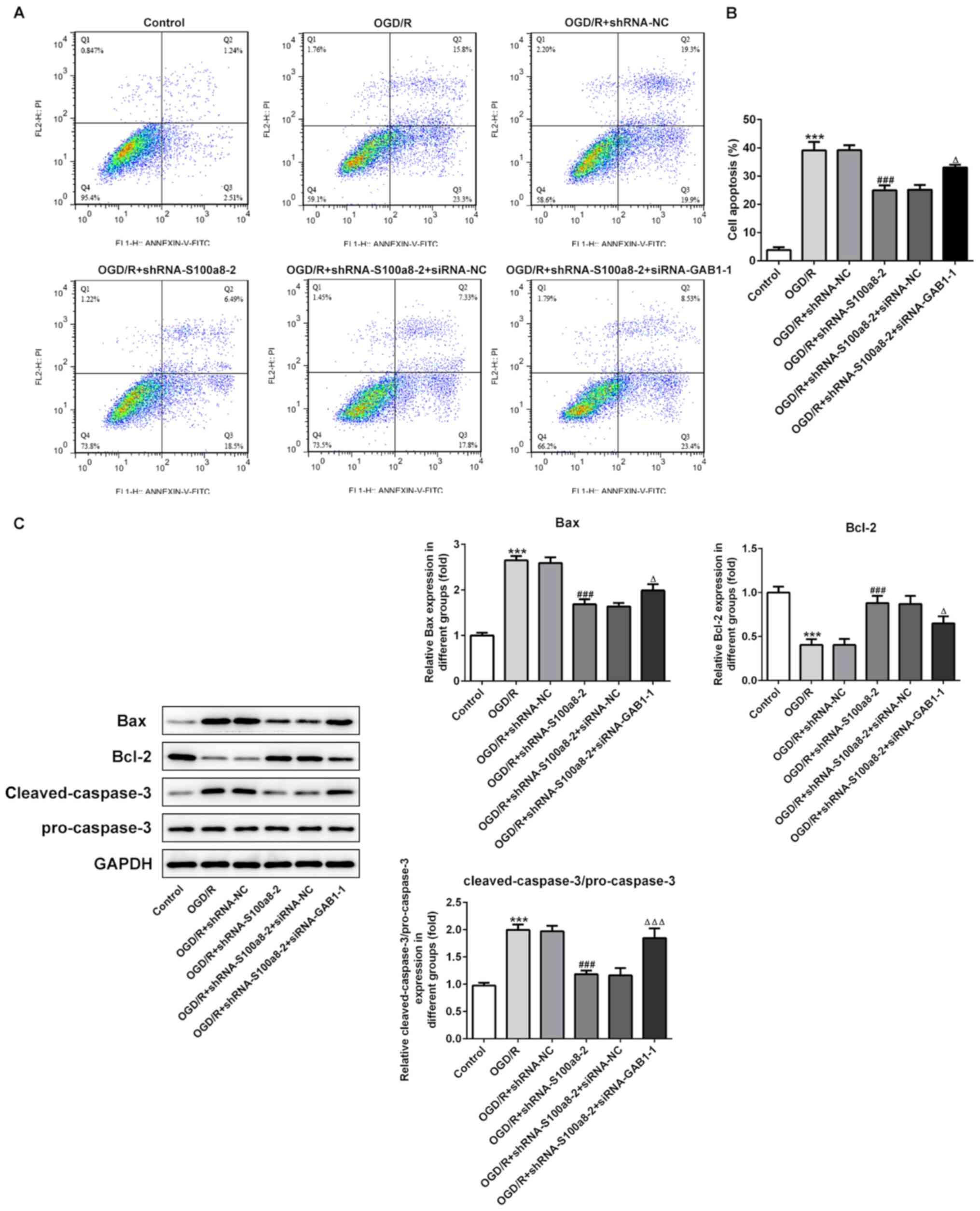
GAB1 silencing blocks the inhibitory
effects of S100a8 silencing on the apoptosis of OGD/R-exposed BV2
cells
The results demonstrated that silencing of GAB1 and
S100a8 promoted the apoptosis of BV2 cells induced by OGD/R
compared with S100a8 silencing alone (Fig. 6A and B). In addition, the expression
levels of Bax and cleaved-caspase-3/pro-caspase-3 decreased,
coupled with an evident increase in Bcl-2 expression following
S100a8 silencing, which was abrogated following transfection with
shRNA-S100a8-2 and siRNA-GAB1-1 (Fig.
6C). Taken together, these results suggest that GAB1 silencing
blocks the inhibitory effects of S100a8 silencing on the apoptosis
of OGD/R-exposed BV2 cells.
Discussion
Cerebral I/R injury is one of the most common causes
of neonatal brain injury about 1 million or 23% of newborn deaths
worldwide every year (23,24). It arises from the temporary
interruption of blood supply followed by the recovery of perfusion
and concomitant reoxygenation, whereby reperfusion induces neuronal
injury in the brain (25).
Neuroinflammation and neuronal apoptosis have been reported to be
notably increased in the processes of I/R, both of which have been
confirmed to play crucial roles in I/R-induced neuronal damage
(26) The results of the present
study indicated that S100a8 was highly expressed in OGD/R-exposed
BV2 cells. Furthermore, S100a8 silencing attenuated OGD/R-induced
inflammation, oxidative stress and the apoptosis of BV2 cells by
upregulating GAB1 expression.
It is well-known that inflammation and oxidative
stress are closely associated with the progression of I/R-induced
multiple organ injury (27–29). Increasing evidence suggests that
inflammation is largely caused by microglia that release
cytotoxicity or pre-inflammatory markers, including TNF-a, IL-1β,
IL-6, COX-2 and iNOS (30–32). The excessive production of reactive
oxygen species (ROS) results in the imbalance of oxidation and
antioxidants in organisms, which plays a catalytic role in the
progression of cerebral I/R injury (33). MDA, the end product of lipid
peroxidation induced by ROS during oxidative stress, causes
oxidative damage by destroying multiple biomacromolecules in
biological membranes or organelles, such as lipids, enzymes and
nucleic acids (34,35). In addition, SOD and GSH-Px,
important antioxidant enzymes, can suppress and attenuate brain
tissue injury induced by ROS cytotoxicity during I/R (36). Previous studies have highlighted the
importance of S100a8 in the pathobiology of inflammatory disorders
(11,37,38).
Treatment with S100a8/a9 has been demonstrated to markedly increase
the secretion of the aforementioned pro-inflammatory cytokines in
cultured BV2 microglial cells (14). Notably, S100A8 induces cytokine
expression via the ROS-dependent activation of NF-κB (39). A previous study reported that S100a8
is highly expressed in the brains of mice following focal cerebral
I/R (25). The results of the
present study demonstrated that S100a8 was highly expressed in
OGD/R-exposed BV2 cells. In addition, S100a8 silencing notably
decreased the expression levels of inflammatory markers, decreased
the content of MDA and increased the activities of SOD and GSH-Px
in OGD/R-stimulated BV2 cells. Collectively, the results of the
present study demonstrated that S100a8-knockdown can effectively
attenuate OGD/R-induced BV2 cell injury by suppressing inflammation
and oxidative stress.
Apoptosis is one of the main forms of cell death
following cerebral I/R (40).
Increasing evidence indicates that inflammation and oxidative
stress contribute to the apoptosis of microglia in the development
of brain injury induced by I/R (41,42).
Caspase-3, a member of the caspase family, is one of the key
proteins that dominates the apoptotic pathway (43). The activated expression of
caspase-3, considered the ‘golden’ index for the detection of
apoptosis, can result in a cascade of apoptotic reactions (44). Bcl-2 family members also play an
important role in the regulation of apoptosis (45). The Bcl-2 family is composed of
antiapoptotic and proapoptotic proteins, and Bcl-2 and Bax are the
two crucial proteins during apoptosis (46). Increasing evidence suggests that
S100a8 can induce apoptosis in several cells of different origins
such as skin and cancer cells (47–49).
Consistent with previous findings (50,51),
the results of the present study indicated markedly downregulated
expression of the proapoptotic proteins, Bax and
cleaved-caspase-3/pro-caspase-3, and upregulated expression of the
antiapoptotic protein, Bcl-2, following S100a8 silencing and OGD/R
exposure in BV2 cells, respectively. Taken together, these results
suggest that S100a8 silencing suppresses the apoptosis of
OGD/R-exposed BV2 cells.
The STRING database revealed that GAB1 interacts
with S100a8. Increasing evidence indicates that GAB1 protects
cardiomyocytes against myocardial I/R injury and oxidative stress
(15). The absence of GAB1 in
endothelial cells can accelerate angiotensin II-dependent vascular
inflammation and aortic atherosclerosis (52). Additionally, activation of the
GAB1/Src/β-catenin signaling pathway can protect the integrity of
the blood-brain barrier following cerebral hemorrhage (21). The results of the present study
demonstrated that S100a8 silencing increased GAB1 expression in BV2
cells exposed to OGD/R. In addition, the IP assay confirmed that
there was a strong association between GAB1 and S100a8. Notably,
GAB1-knockdown reversed the effects of S100a8 silencing on
inflammation, oxidative stress and apoptosis. Collectively, these
results suggest that S100a8 silencing can attenuate OGD/R-induced
BV2 cell injury by regulating GAB1 expression.
In conclusion, the results of the present study
demonstrate that S100a8 silencing alleviates inflammation,
oxidative stress and the apoptosis of BV2 cells induced by OGD/R by
upregulating GAB1 expression. These findings potentially provide
insight into the pathogenesis of cerebral I/R injury and may also
provide a novel direction for the development of therapeutic
strategies for this condition. However, there are limitations to
the present study. First, it was an in vitro study and no
in vivo experiments were performed and second, the molecular
mechanisms underlying the effects of S100a8 inhibition on BV2 cells
function were not fully investigated. These issues require further
in-depth investigations and will be addressed in future
studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH designed the study and wrote the manuscript; CL
performed the experiments, collected and analyzed the data. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayakawa K, Tanda K, Koshino S, Nishimura
A, Kizaki Z and Ohno K: Pontine and cerebellar injury in neonatal
hypoxic-ischemic encephalopathy: MRI features and clinical
outcomes. Acta Radiol. Jan 24–2020.(Epub ahead of print). doi:
10.1177/0284185119900442. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Douglas-Escobar M and Weiss MD:
Hypoxic-ischemic encephalopathy: A review for the clinician. JAMA
Pediatr. 169:397–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dixon BJ, Reis C, Ho WM, Tang J and Zhang
JH: Neuroprotective strategies after neonatal hypoxic ischemic
encephalopathy. Int J Mol Sci. 16:22368–22401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howlett JA, Northington FJ, Gilmore MM,
Tekes A, Huisman TA, Parkinson C, Chung SE, Jennings JM,
Jamrogowicz JJ, Larson AC, et al: Cerebrovascular autoregulation
and neurologic injury in neonatal hypoxic-ischemic encephalopathy.
Pediatr Res. 74:525–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Busl KM and Greer DM: Hypoxic-ischemic
brain injury: Pathophysiology, neuropathology and mechanisms.
NeuroRehabilitation. 26:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snyder EJ, Perin J, Chavez-Valdez R,
Northington FJ, Lee JK and Tekes A: Head Ultrasound resistive
indices are associated with brain injury on diffusion tensor
imaging magnetic resonance imaging in neonates with
hypoxic-ischemic encephalopathy. J Comput Assist Tomogr.
44:687–691. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solevag AL, Schmolzer GM and Cheung PY:
Novel interventions to reduce oxidative-stress related brain injury
in neonatal asphyxia. Free Radic Biol Med. 142:113–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez-Rodríguez A, Egea-Guerrero JJ,
Murillo-Cabezas F and Carrillo-Vico A: Oxidative stress in
traumatic brain injury. Curr Med Chem. 21:1201–1211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kovalčíková A, Gyurászová M,
Vavrincová-Yaghi D, Vavrinec P, Tóthová Ľ, Boor P, Šebeková K and
Celec P: Oxidative stress in the brain caused by acute kidney
injury. Metab Brain Dis. 33:961–967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khatri N, Thakur M, Pareek V, Kumar S,
Sharma S and Datusalia AK: Oxidative stress: Major threat in
traumatic brain injury. CNS Neurol Disord Drug Targets. 17:689–695.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zonis S, Ljubimov VA, Mahgerefteh M,
Pechnick RN, Wawrowsky K and Chesnokova V: p21Cip restrains
hippocampal neurogenesis and protects neuronal progenitors from
apoptosis during acute systemic inflammation. Hippocampus.
23:1383–1394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerkhoff C, Klempt M and Sorg C: Novel
insights into structure and function of MRP8 (S100A8) and MRP14
(S100A9). Biochim Biophys Acta. 1448:200–211. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebhardt C, Nemeth J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun P, Li Q, Zhang Q, Xu L and Han JY:
Upregulated expression of S100A8 in mice brain after focal cerebral
ischemia reperfusion. World J Emerg Med. 4:210–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dessing MC, Tammaro A, Pulskens WP, Teske
GJ, Butter LM, Claessen N, van Eijk M, van der Poll T, Vogl T, Roth
J, et al: The calcium-binding protein complex S100A8/A9 has a
crucial role in controlling macrophage-mediated renal repair
following ischemia/reperfusion. Kidney Int. 87:85–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Chen B, Yang X, Zhang C, Jiao Y, Li
P, Liu Y, Li Z, Qiao B, Bond Lau W, et al: S100a8/a9 signaling
causes mitochondrial dysfunction and cardiomyocyte death in
response to ischemic/reperfusion injury. Circulation. 140:751–764.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakaguchi M, Yamamoto M, Miyai M, Maeda T,
Hiruma J, Murata H, Kinoshita R, Winarsa Ruma IM, Putranto EW,
Inoue Y, et al: Identification of an S100A8 receptor neuroplastin-β
and its heterodimer formation with EMMPRIN. J Invest Dermatol.
136:2240–2250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Yin M, Deng H, Jin FQ, Xu S, Lu Y,
Mastrangelo MA, Luo H and Jin ZG: Cardiac Gab1 deletion leads to
dilated cardiomyopathy associated with mitochondrial damage and
cardiomyocyte apoptosis. Cell Death Differ. 23:695–706. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun L, Chen C, Jiang B, Li Y, Deng Q, Sun
M, An X, Yang X, Yang Y, Zhang R, et al: Grb2-associated binder 1
is essential for cardioprotection against ischemia/reperfusion
injury. Basic Res Cardiol. 109:4202014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holgado-Madruga M and Wong AJ: Gab1 is an
integrator of cell death versus cell survival signals in oxidative
stress. Mol Cell Biol. 23:4471–4484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu T, Wang Z, Prativa S, Xu Y, Wang T,
Zhang Y, Yu L, Xu N, Tang J, You W, et al: Macrophage stimulating
protein preserves blood brain barrier integrity after intracerebral
hemorrhage through recepteur d'origine nantais dependent
GAB1/Src/β-catenin pathway activation in a mouse model. J
Neurochem. 148:114–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Thompson H, Hemphill C, Hong F,
Forrester J, Johnson RH, Zhang W and Meldrum DR: An improved
one-tube RT-PCR protocol for analyzing single-cell gene expression
in individual mammalian cells. Anal Bioanal Chem. 397:1853–1859.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rocha-Ferreira E, Vincent A, Bright S,
Peebles DM and Hristova M: The duration of hypothermia affects
short-term neuroprotection in a mouse model of neonatal hypoxic
ischaemic injury. PLoS One. 13:e01998902018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lundgren C, Brudin L, Wanby AS and
Blomberg M: Ante- and intrapartum risk factors for neonatal hypoxic
ischemic encephalopathy. J Matern Fetal Neonatal Med. 31:1595–1601.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang SY, Tai SH, Chang CC, Tu YF, Chang
CH and Lee EJ: Magnolol protects against ischemic-reperfusion brain
damage following oxygen-glucose deprivation and transient focal
cerebral ischemia. Int J Mol Med. 41:2252–2262. 2018.PubMed/NCBI
|
|
26
|
Zhang J, Xiao F, Zhang L, Wang X, Lai X,
Shen Y, Zhang M, Zhou B, Lang H, Yu P and Hua F: Alpha-Lipoic acid
preconditioning and ischaemic postconditioning synergistically
protect rats from cerebral injury induced by ischemia and
reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling
pathway. Cell Physiol Biochem. 51:1448–1460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma M, Uekawa K, Hasegawa Y, Nakagawa T,
Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Kuratsu J and
Kim-Mitsuyama S: Pretreatment with rosuvastatin protects against
focal cerebral ischemia/reperfusion injury in rats through
attenuation of oxidative stress and inflammation. Brain Res.
1519:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue
XD, Xu YL, Zhang J, Xiao X, Han JS, et al: Melatonin protects
diabetic heart against ischemia-reperfusion injury, role of
membrane receptor-dependent cGMP-PKG activation. Biochim Biophys
Acta Mol Basis Dis. 1864:563–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S,
Yang Y and Gu C: AMPK: Potential therapeutic target for ischemic
stroke. Theranostics. 8:4535–4551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hwang JH, Kumar VR, Kang SY, Jung HW and
Park YK: Effects of flower buds extract of tussilago farfara on
focal cerebral ischemia in rats and inflammatory response in BV2
microglia. Chin J Integr Med. 24:844–852. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo Y, Wang C, Li WH, Liu J, He HH, Long
JH, Yang J, Sui X, Wang S, You Z and Wang YA: Madecassoside
protects BV2 microglial cells from oxygen-glucose
deprivation/reperfusion-induced injury via inhibition of the
toll-like receptor 4 signaling pathway. Brain Res. 1679:144–154.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Zhang DM, Feng X, Wang J, Qin YY,
Zhang T, Huang Q, Sheng R, Chen Z, Li M and Qin ZH: TIGAR inhibits
ischemia/reperfusion-induced inflammatory response of astrocytes.
Neuropharmacology. 131:377–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Cheng S, Hu H, Zhang X, Xu J, Wang R
and Zhang P: Progranulin protects against cerebral
ischemia-reperfusion (I/R) injury by inhibiting necroptosis and
oxidative stress. Biochem Biophys Res Commun. 521:569–576. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ayala A, Munoz MF and Arguelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo M, Lu H, Qin J, Qu S, Wang W, Guo Y,
Liao W, Song M, Chen J and Wang Y: Biochanin a provides
neuroprotection against cerebral ischemia/reperfusion injury by
Nrf2-Mediated inhibition of oxidative stress and inflammation
signaling pathway in rats. Med Sci Monit. 25:8975–8983. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cui Y, Wang JQ, Shi XH, Wang YY, Liu HY,
Li Z, Dong Y, Mang J and Xu ZX: Nodal mitigates cerebral
ischemia-reperfusion injury via inhibiting oxidative stress and
inflammation. Eur Rev Med Pharmacol Sci. 23:5923–5933.
2019.PubMed/NCBI
|
|
37
|
Pruenster M, Vogl T, Roth J and Sperandio
M: S100A8/A9: From basic science to clinical application. Pharmacol
Ther. 167:120–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujita Y, Khateb A, Li Y, Tinoco R, Zhang
T, Bar-Yoseph H, Tam MA, Chowers Y, Sabo E, Gerassy-Vainberg S, et
al: Regulation of S100A8 Stability by RNF5 in intestinal epithelial
cells determines intestinal inflammation and severity of colitis.
Cell Rep. 24:3296–3311.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma L, Sun P, Zhang JC, Zhang Q and Yao SL:
Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling
pathways in BV-2 microglial cells. Int J Mol Med. 40:31–38. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simard JC, Cesaro A, Chapeton-Montes J,
Tardif M, Antoine F, Girard D and Tessier PA: S100A8 and S100A9
induce cytokine expression and regulate the NLRP3 inflammasome via
ROS-dependent activation of NF-KB(1). PLoS One. 8:e721382013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ren Z, Chen L, Wang Y, Wei X, Zeng S,
Zheng Y, Gao C and Liu H: Activation of the Omega-3 Fatty Acid
Receptor GPR120 protects against focal cerebral ischemic injury by
preventing inflammation and apoptosis in mice. J Immunol.
202:747–759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Wu X, Luo J, Wang X, Guo H, Feng D,
Zhao L, Bai H, Song M, Liu X, et al: Pterostilbene attenuates
astrocytic inflammation and neuronal oxidative injury after
Ischemia-Reperfusion by inhibiting NF-KB phosphorylation. Front
Immunol. 10:24082019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weng C, Chen Y, Wu Y, Liu X, Mao H, Fang
X, Li B, Wang L, Guan M, Liu G, et al: Silencing UBE4B induces
nasopharyngeal carcinoma apoptosis through the activation of
caspase3 and p53. Onco Targets Ther. 12:2553–2561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He JT, Li HQ, Li GF and Yang L: Hyperoside
protects against cerebral ischemia-reperfusion injury by
alleviating oxidative stress, inflammation and apoptosis in rats.
Biotechnol Biotechnol Equipment. 33:798–806. 2019. View Article : Google Scholar
|
|
45
|
Ouyang YB and Giffard RG: MicroRNAs affect
BCL-2 family proteins in the setting of cerebral ischemia.
Neurochem Int. 77:2–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta R and Ghosh S: Putative roles of
mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family
proteins and c-Jun N-terminal Kinases in ischemic stroke associated
apoptosis. Biochim Open. 4:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kerkhoff C, Voss A, Scholzen TE, Averill
MM, Zänker KS and Bornfeldt KE: Novel insights into the role of
S100A8/A9 in skin biology. Exp Dermatol. 21:822–826. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu Y, Fan B, Zhang LH, Cheng XJ, Niu ZJ
and Ji JF: Clinical significance of S100A8 and S100A9 expression in
gastric cancer. Zhonghua Yi Xue Za Zhi. 93:3369–3374. 2013.(In
Chinese). PubMed/NCBI
|
|
49
|
Ghavami S, Eshragi M, Ande SR, Chazin WJ,
Klonisch T, Halayko AJ, McNeill KD, Hashemi M, Kerkhoff C and Los
M: S100A8/A9 induces autophagy and apoptosis via ROS-mediated
cross-talk between mitochondria and lysosomes that involves BNIP3.
Cell Res. 20:314–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jianrong S, Yanjun Z, Chen Y and Jianwen
X: DUSP14 rescues cerebral ischemia/reperfusion (IR) injury by
reducing inflammation and apoptosis via the activation of Nrf-2.
Biochem Biophys Res Commun. 509:713–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hao MQ, Xie LJ, Leng W and Xue RW: Trim47
is a critical regulator of cerebral ischemia-reperfusion injury
through regulating apoptosis and inflammation. Biochem Biophys Res
Commun. 515:651–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Higuchi K, Nakaoka Y, Shioyama W, Arita Y,
Hashimoto T, Yasui T, Ikeoka K, Kuroda T, Minami T, Nishida K, et
al: Endothelial Gab1 deletion accelerates angiotensin II-dependent
vascular inflammation and atherosclerosis in apolipoprotein E
knockout mice. Circ J. 76:2031–2040. 2012. View Article : Google Scholar : PubMed/NCBI
|