Introduction
Cutaneous squamous cell carcinoma (cSCC) is a
malignant tumor that originates from epidermal or appendage
keratinocytes (1). cSCC primarily
occurs in the elderly population in light-exposed areas, including
the scalp, face and back of the hands (2). cSCC is not life threatening, but if
left untreated can grow larger or spread to other organs causing
serious complications, local lymph node metastases and distant
metastases (3). Previous studies
have reported that cSCC accounts for ~20% of all skin malignant
tumors (4,5). For the majority of patients with cSCC,
a good prognosis can be achieved if the lesion is completely
removed by surgery. However, for patients who cannot undergo
surgery or have metastatic disease, radiotherapy and chemotherapy
are common treatment strategies used. Although radiotherapy and
chemotherapy have been widely used for the treatment of cSCC, these
strategies are not recommended due to their side effects and
inconsistent therapeutic effects (6,7).
Targeted molecular therapy has become a recent trend in cSCC
therapy (8), thus investigating the
molecular mechanism underlying cSCC progression is important for
the identification of novel therapeutic targets.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
that are >200 nucleotides in length (9). Previous studies have demonstrated that
lncRNAs serve important roles in numerous biological functions,
including the dose compensation effect, epigenetic regulation, cell
cycle regulation and cell differentiation regulation (10,11).
In recent years, lncRNAs have become a focus of research and
increasing evidence has demonstrated that lncRNAs regulate human
cancer cell proliferation, apoptosis, migration and invasion
(12,13). Recent research has reported that
certain lncRNAs that might be involved in tumorigenesis and
development are differentially expressed in tumor tissues compared
with healthy tissues (14). lncRNA
ezrin antisense RNA 1 (EZR-AS1) is a natural antisense lncRNA
transcribed from the opposite strand at the EZR gene locus, which
is located on chromosome 6q25.3, with a length of 362 bp (15,16).
Ghaffari et al (17)
demonstrated that EZR inhibition impedes breast cancer cell
migration and lymph node metastasis. Xie et al (18,19)
reported that EZR expression is related to poor overall survival of
patients with esophageal squamous cell carcinoma (ESCC).
Furthermore, EZR-AS1 knockdown significantly suppresses human
breast cancer MCF7 and MDA-MB-231 cell proliferation and cell cycle
progression (20). Zhang et
al (21) reported that EZR-AS1
knockout significantly inhibited ESCC cell migration, reduced tumor
volume and weight, and decreased the number of metastatic lymph
nodes in mice (21). However, the
biological function of lncRNA EZR-AS1 in cSCC cells and the
underlying molecular mechanism are not completely understood.
PI3K/AKT is a classical signaling pathway that
serves a vital role in carcinogenesis (22). The PI3K/AKT signaling pathway
regulates a variety of physiological functions, including cell
survival, proliferation, migration, invasion and protein
translation (23,24). More importantly, it has been
reported that the PI3K/AKT signaling pathway critically controls
cSCC cell survival, and molecular alterations to the signaling
pathway, including phosphorylation of PI3K and AKT, have been
widely reported in cancer (22,25).
Liu et al (15) have
indicated that silencing of lncRNA EZR-AS1 inhibits proliferation,
invasion, and migration of colorectal cancer cells through blocking
TGF-β signaling. However, whether EZR-AS1 could regulate the
PI3K/Akt signaling pathway in the progression of cSCC remains
unclear.
In the present study, we aimed to investigate
whether lncRNA EZR-AS1 promote the progression of cSCC by
regulating the PI3K/Akt signaling pathway, thereby further
revealing the regulatory role of lncRNA EZR-AS1 in cSCC and helping
to find new targets for the cSCC therapy.
Materials and methods
Clinical sample collection
A total of 66 cSCC tissues and healthy adjacent
non-cancerous tissues (1–2 cm from cSCC tissues) were obtained from
patients with cSCC (37 males and 29 females; age range, 25–79
years) who underwent surgery at the Second Affiliated Hospital of
Shandong First Medical University (Tai'an, China) between January
2014 and September 2019. All tissues were immediately frozen in
liquid nitrogen and stored at −80°C until further analysis. Written
informed consent was obtained from all patients. The present study
was approved by the Ethics Committee of The Second Affiliated
Hospital of Shandong First Medical University.
Cell culture
CSCC cell lines (SCL-1, SCC13, A431 and HSC-5) and a
human immortalized keratinocytes line (HaCaT; cat. no. CC-Y1177)
were obtained from EK-Biosciences. Cells were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Sigma-Aldrich; Merck KGaA) in a humidified environment with 5%
CO2 at 37°C. The medium was changed every 2–3 days.
Cell transfection
At 70–80% confluence, SCC13, SCL-1 and A431 cells
were inoculated into a 6-cm culture dish. SCC13 and SCL-1 cells
were divided into the following groups: i) Control, untreated; ii)
si-NC, transfected with 0.5 µg siRNA NC (scrambled control); iii)
si-EZR-AS1-1, transfected with 0.5 µg siRNA-EZR-AS1-1; iv)
si-EZR-AS1-2, transfected with 0.5 µg siRNA-EZR-AS1-2; v) pc-NC,
transfected with 1 µg pcDNA3.1 NC (empty vector; Invitrogen; Thermo
Fisher Scientific, Inc.); vi) pc-FAK, transfected with 1 µg
pcDNA3.1-FAK (Invitrogen; Thermo Fisher Scientific, Inc.); and vii)
pc-FAK + si-EZR-AS1-1, co-transfected with 1 µg pc-FAK and 0.5 µg
si-EZR-AS1-1. All siRNAs were purchased from Shanghai GenePharma,
Co., Ltd., whereas plasmids were from Invitrogen (Thermo Fisher
Scientific, Inc.). A431 cells were divided into three groups: i)
Control, untreated; ii) pc-NC, transfected with 1 µg pcDNA3.1 NC;
and iii) pc-FAK, transfected with 1 µg pcDNA3.1-FAK.
Cells were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, si-NC- and
si-EZR-AS1-1-transfected SCC13 cells were cultured in RPMI-1640
containing 20 µM 740Y-P (a PI3K agonist; MedChem Express) for 2 h
at 37°C to establish the following groups: i) Control,
si-NC-transfected SCC13 cells; ii) 740-YP, si-NC-transfected SCC13
cells treated with 740Y-P; iii) si-EZR-AS1-1, si-EZR-AS1-1
transfected SCC13 cells; and iv) 740-YP + si-EZR-AS1-1,
si-EZR-AS1-1 transfected SCC13 cells treated with 740Y-P. After
48-h transfection at 37°C, cells were used for subsequent
experiments. The sequences of the EZR-AS1 siRNAs were as follows:
i) si-EZR-AS1-1, 5′-AAAUAAUACUACAAUUAAA-3′; ii) si-EZR-AS1-2,
5′-UUUAAUUGUAGUAUUAUUU-3′; and iii) si-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from of cells (SCL-1, SCC13,
A431, HSC-5 and HaCaT cells) and tissues (healthy and cSCC tissues)
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using the PrimeScript™ RT Reagent Kit (Takara Bio, Inc.). Reverse
transcription was performed at 16°C for 30 min, 42°C for 30 min and
85°C for 5 min. Subsequently, SYBR® Premix Ex Taq™
(Takara Biotechnology Co., Ltd.) was used to examine the expression
of lncRNA and mRNA. qPCR was performed using the following
thermocycling conditions: 94°C for 6 min; 35 cycles of 96°C for 20
sec, 58°C for 40 sec and extension at 72°C for 2 min. The relative
gene expression was determined using the 2−ΔΔCq method
(26). The following primers were
used for qPCR: EZR-AS1 forward, 5′-CCCTCTCCAATGAAGCCTCTC-3′ and
reverse, 5′-ACCGAAAATGCCGAAACCAG-3′; EZR forward,
5′-TGATCACGCTGTAAGGCACA-3′ and reverse, 5′-AGGCCTCATGTACCCCTCTT-3′;
FAK forward, 5′-CTATGGTGAAGGAAGTCGGCTTGG-3′ and reverse,
5′-TGTACTCTTGCTGGAGGCTGGTC-3′; and GAPDH forward,
5′-TCCTCTGACTTCAACAGCGACAC-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAATTC-3′. mRNA expression levels were
normalized to the internal reference gene GAPDH.
Western blotting
At 48 h post-transfection, total protein was
extracted from cells from each group using a Total protein
extraction kit (BestBio Science). Protein concentration was
determined using a bicinchoninic acid kit (Abcam). Proteins (50 µg
per lane) were separated by SDS-PAGE on 12% gels and transferred to
PVDF membranes. After blocking with 5% dry skimmed milk for 2.5 h
at room temperature, the membranes were incubated with the primary
antibodies overnight at 4°C. The primary antibodies including PI3K
(1:1,000; cat. no. 4257), phosphorylated (p)-PI3K (1:1,000; cat.
no. 17366), AKT (1:1,000; cat. no. 4685), p-AKT (1:1,000; cat. no.
4060), FAK (1:1,000; cat. no. 13009), matrix metallopeptidase
(MMP)-2 (1:1,000; cat. no. 40994), MMP-9 (1:1,000; cat. no. 13667),
Bcl-2 (1:1,000; cat. no. 4223) and Bax (1:1,000, cat. no. 14796)
and β-actin (1:1,000; cat. no. 4970). All primary antibodies were
purchased form Cell Signaling Technology, Inc. Subsequently, the
membranes were incubated with a horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:1,000; cat. no. A0277;
Beyotime Institute of Biotechnology) at room temperature for 45
min. Protein bands were visualized using electrochemiluminescent
solution (Thermo Fisher Scientific, Inc.). Protein expression
levels were semi-quantified using ImageJ software (version 1.8.0;
National Institutes of Health).
LncRNA-EZR-AS1 binding prediction with
LncTar
To predict the targeted mRNAs of lncRNA EZR-AS1, an
ensemble classifier-based predictor, lncLocator (http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/) was
performed in this study. The operational process of lncLocator was
conducted as previously described (27). Briefly, both k-mer features and
high-level abstraction features generated by unsupervised deep
models were used to construct four classifiers for support vector
machine (SVM) and random forest (RF), respectively. A stacked
ensemble strategy was then used to combine the four classifiers for
final prediction results.
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using the Magna RIP kit
(EMD Millipore) to verify the binding relationship between EZR-AS1
and FAK. SCC13 and SCL-1 cells were lysed using RIP lysis buffer
and then incubated with anti-FAK or anti-IgG antibodies overnight
at 4°C, followed by incubation with protein A magnetic beads at 4°C
for 4 h. The co-precipitated RNAs were isolated to detect the
expression levels of EZR-AS1 and FAK via RT-qPCR.
Cell viability analysis
At 24, 48, 72 and 96 h, cell viability in each group
was assessed by performing the MTT assay. Cells were seeded into a
96-well plate at a density of 5×103 cells/well and
cultured at 37°C with 5% CO2. Subsequently, 20 µl MTT
solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well and incubated at 37°C with 5% CO2. Following
incubation for 4 h, 150 µl DMSO was added to each well for 10 min
with gentle agitation to dissolve the formazan crystals. Absorbance
was measured at a wavelength of 570 nm using a microplate
reader.
Plate cloning experiment
Cells in the logarithmic growth phase of each group
were collected and seeded (5×102 cells/well) into 6-well
plates. Cells were cultured for 14 days and the medium was changed
every 2 to 3 days. After washing twice with PBS, cells were fixed
with 4% formaldehyde for 15 min at 37°C and stained with crystal
violet for 10–20 min at room temperature. Following washing with
distilled water, cell colonies were observed and counted using a
light microscope (Carl Zeiss AG). Cell colonies were defined as
cell clusters containing ≥50 cells. Images of the cell colonies
were directly obtained using a camera.
Wound healing assay
Cells were seeded into a 6-well plate and cultured
in RPMI-1640 supplemented with 10% FBS until the cells were grown
to 100% confluence. A 10 µl pipette tip was used to create a
scratch wound in the cell monolayer. The medium was aspirated to
remove the detached cells. Serum-free medium (Gibco; Thermo Fisher
Scientific, Inc.) was added to the 6-well plate. At 0 and 48 h,
cell migration was observed using an inverted light microscope
(magnification, ×100; Carl Zeiss AG) and photographed using a
Cybershot camera (Sony Corporation). Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.) was used to quantify the wound distance
at 0 and 48 h. The percentage of gap closure was used as an
indicator of cell migration. The percentage of gap closure is
calculated as (average distance at 0 h-average distance at 48
h)/(average distance at 0 h) ×100%.
Transwell invasion assay
At 48 h post-transfection, cells were trypsinized
and resuspended in serum-free medium. Matrigel™ (BD Biosciences)
was used to precoat the membrane of the upper chambers at 37°C for
2 h. Cells (1×105) in serum-free medium were plated into
the upper chamber and medium containing 15% FBS was plated into the
lower chamber. Following incubation at 37°C for 24 h, cells were
rinsed with PBS, fixed with 4% paraformaldehyde at room temperature
for 15 min and stained with 0.1% crystal violet at 37°C for 30 min.
Invading cells were observed in five randomly selected fields of
view using an light microscope (magnification, ×200; Carl Zeiss
AG).
Cell apoptosis assay
Cell apoptosis was detected via flow cytometry using
the Annexin V-PI kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Briefly, cells were cultured at 37°C with 5% CO2 for 48
h. Cells were collected by centrifugation at 850 × g for 5 min at
room temperature. A total of 1×105 cells were incubated
with Annexin V in the dark at 4°C for 20 min and then 10 µl PI in
the dark for 15 min. Cell apoptosis was acquired using a
FACSCalibur flow cytometer (BD Biosciences) and analyzed on FlowJo
7.0 software (FlowJo LLC). The sum of early apoptotic cells and
late apoptotic cells was assessed.
Statistical analysis
Data are presented as the mean ± SD of at least
three independent experiments. Statistical analyses were performed
using GraphPad Prism software (version 7.0; GraphPad Software,
Inc.). Comparisons between two matched groups were analyzed using
paired Student's t-tests. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Tukey's post hoc test. The
data presented in Table I was
analyzed using the χ2 test. P<0.05 was considered to
indicate a statistically significant difference. Experiments were
repeated at least three times.
 | Table I.Correlation between EZR-AS1
expression and clinicopathological parameters. |
Table I.
Correlation between EZR-AS1
expression and clinicopathological parameters.
|
|
| EZR-AS1
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.445 |
|
<55 | 30 | 13 | 17 |
|
|
≥55 | 36 | 19 | 17 |
|
| Sex |
|
|
| 0.457 |
|
Male | 37 | 20 | 17 |
|
|
Female | 29 | 13 | 16 |
|
| Lymph node
metastasis |
|
|
| 0.037a |
|
Present | 22 | 7 | 15 |
|
|
Absent | 44 | 26 | 18 |
|
| Histopathological
grade |
|
|
| 0.041a |
| Well to
moderate | 42 | 25 | 17 |
|
|
Poor | 24 | 8 | 16 |
|
Results
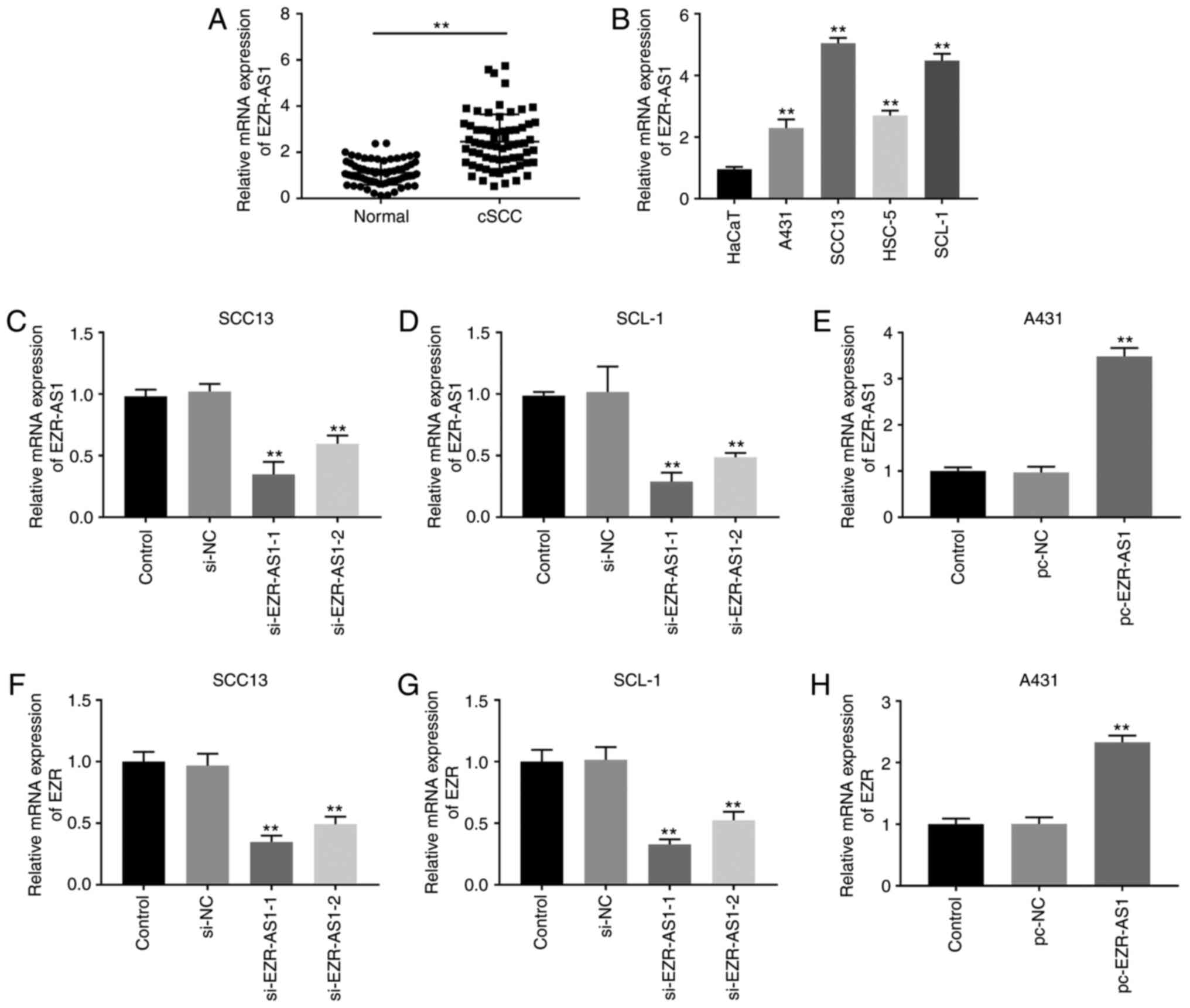
lncRNA EZR-AS1 expression is
upregulated in cSCC cells
EZR-AS1 mRNA expression levels were significantly
increased in cSCC tissues compared with adjacent healthy tissues
(P<0.01; Fig. 1A). EZR-AS1
expression levels in cSCC cell lines (A431, SCC13, HSC-5 and SCL-1)
and the human immortalized keratinocyte cell line (HaCaT) were
measured. EZR-AS1 expression levels were significantly increased in
cSCC cell lines compared with HaCaT cells, especially in SCC13 and
SCL-1 cells (P<0.01; Fig. 1B).
In addition, based on the median expression level (2.33) of
EZR-AS1, patients with cSCC were divided into two groups: i) High
expression; and ii) low expression. The associations between
EZR-AS1 expression and clinicopathological characteristics are
presented in Table I. EZR-AS1
expression was significantly associated with histopathological
grade and lymph node metastasis (P<0.05), but there was no
significant association with age and sex (P>0.05).
si-EZR-AS1 inhibits cSCC cell
proliferation
To further investigate the effect of EZR-AS1 on cSCC
progression, SCC13 and SCL-1 cells were transfected with si-EZR-AS1
or si-NC, and A431 cells were transfected with pc-EZR-AS1 or pc-NC.
In SCC13 and SCL-1 cells, EZR-AS1 expression was significantly
decreased in the si-EZR-AS1-1 and si-EZR-AS1-2 groups compared with
the si-NC group, especially in the si-EZR-AS1-1 group (P<0.01;
Fig. 1C and D). In A431 cells,
EZR-AS1 expression in the pc-EZR-AS1 group was significantly higher
compared with the pc-NC group (P<0.01; Fig. 1E). Similar results were observed for
EZR expression. In SCC13 and SCL-1 cells, EZR mRNA expression
levels in the si-EZR-AS1-1 and si-EZR-AS1-2 groups were
significantly lower compared with the si-NC group (P<0.01;
Fig. 1F and G). In A431 cells, EZR
mRNA expression levels in the pcEZR-AS1 group were significantly
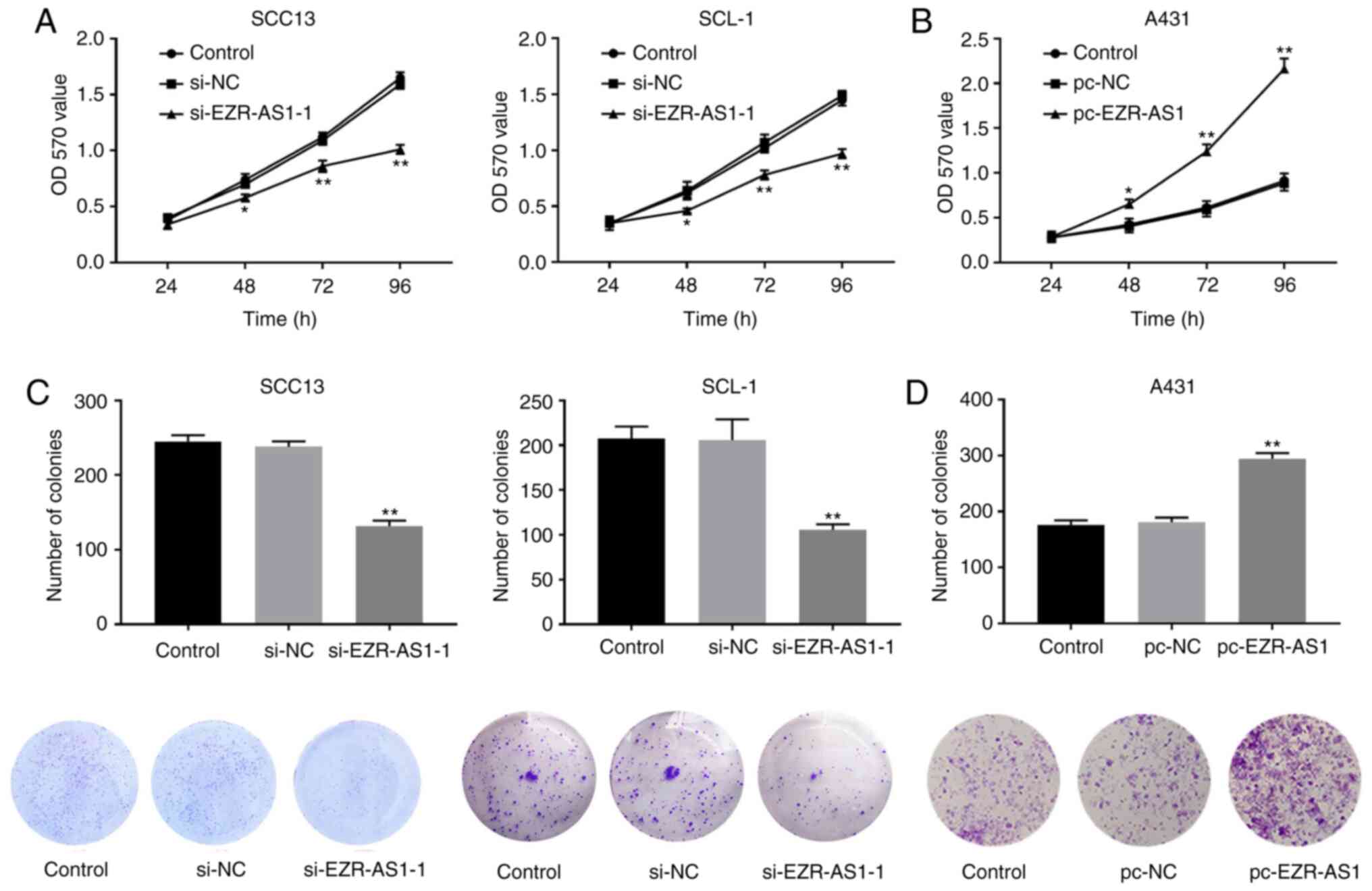
increased compared with the pc-NC group (P<0.01; Fig. 1H). Subsequently, MTT and plate
cloning assays were performed to detect the effects of EZR-AS1 on
cSCC cell proliferation. The MTT and plate cloning assay results
indicated that EZR-AS1 knockdown significantly decreased cell
viability and colony formation compared with the si-NC group,
whereas EZR-AS1 overexpression significantly increased cell
viability and colony formation compared with the pc-NC group (all
P<0.01; Fig. 2). Therefore, the
results suggested that si-EZR-AS1 inhibited SCC13 and SCL-1 cell
viability and proliferation.
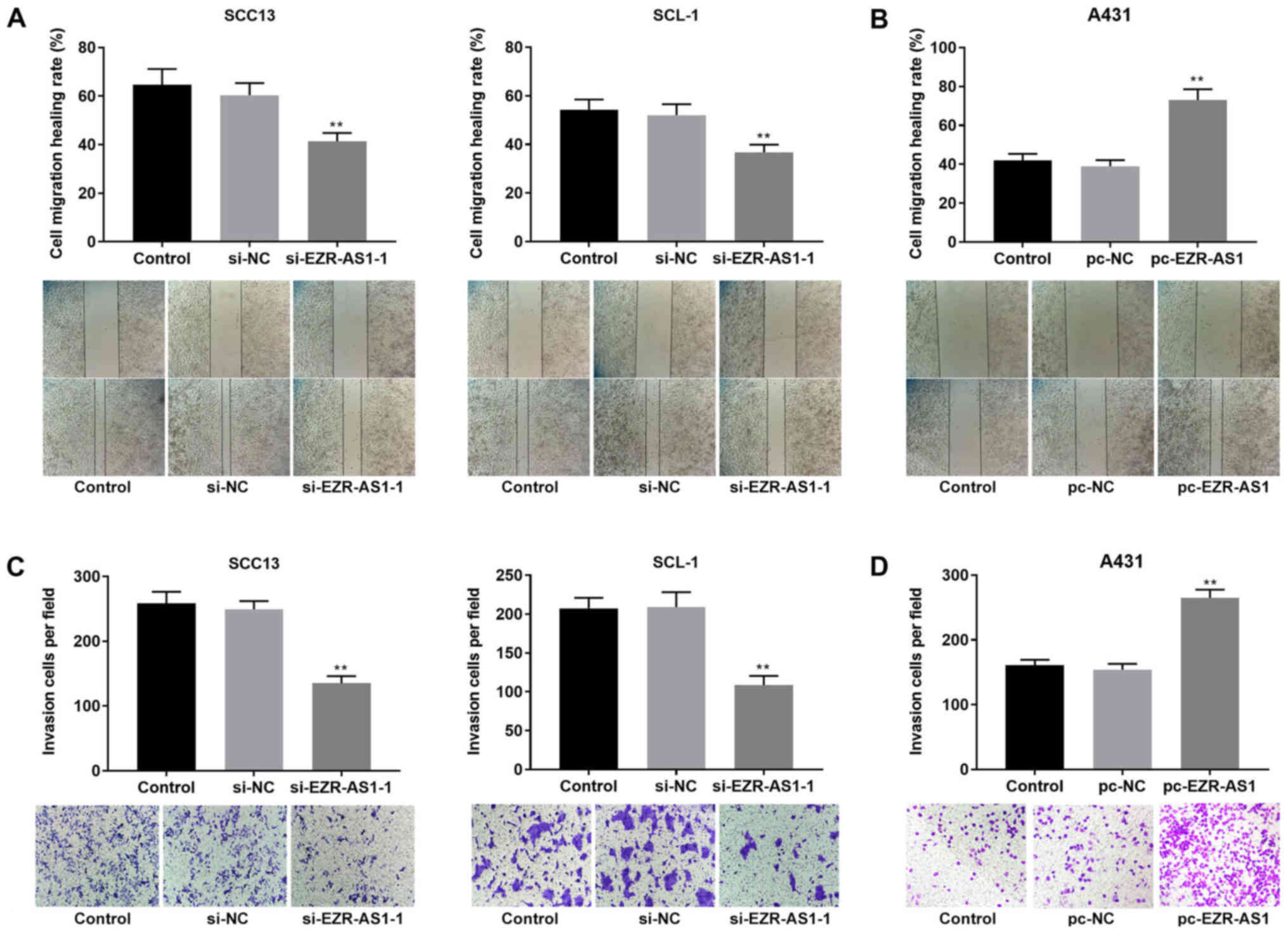
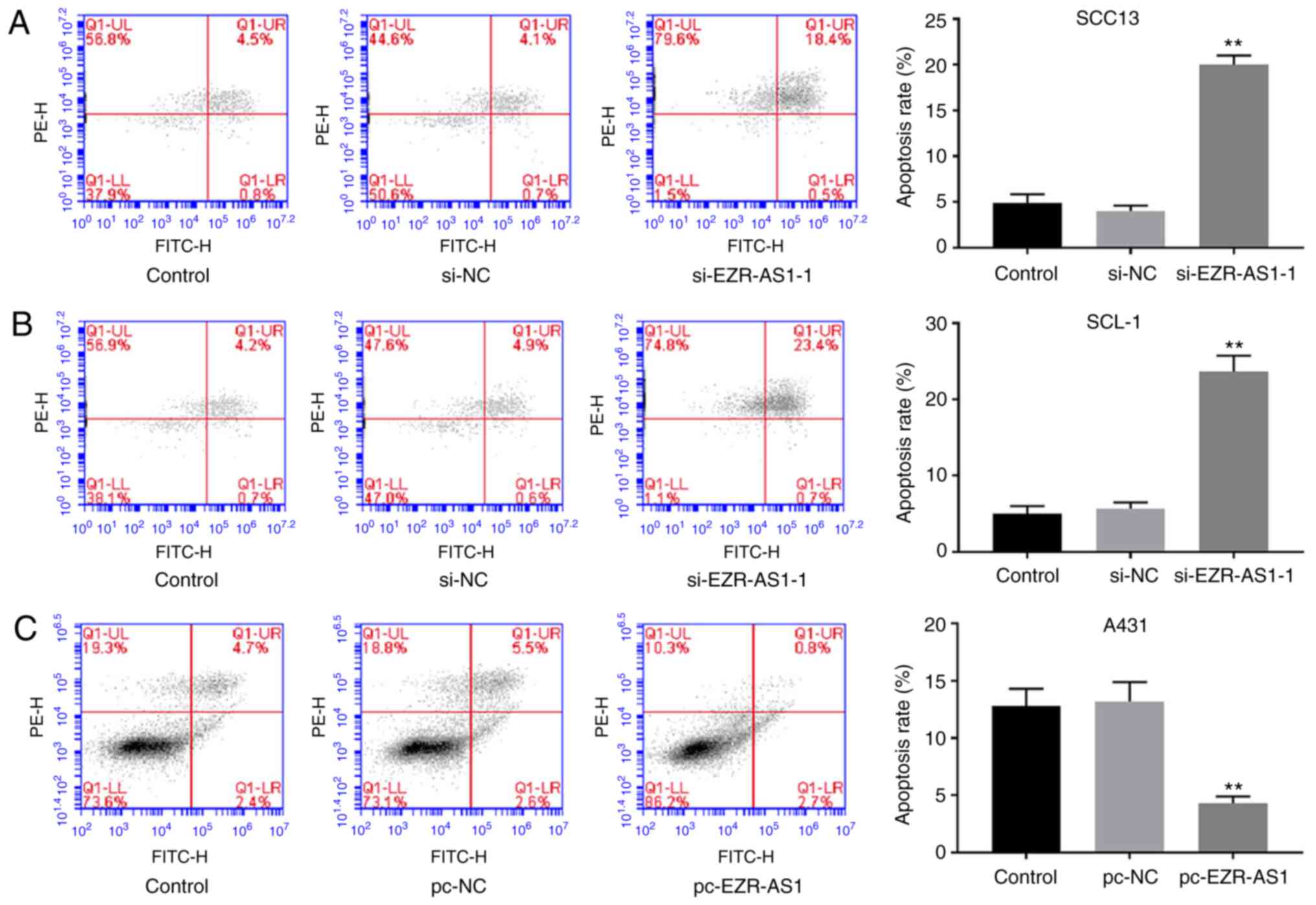
si-EZR-AS1 inhibits cSCC cell
migration and invasion, and promotes apoptosis
To investigate the role of EZR-AS1 in cSCC cell
migration, invasion and apoptosis, wound healing, Transwell
invasion and flow cytometry assays were performed, respectively. At
48 h, the relative wound width of the si-EZR-AS1-1 group was
significantly increased compared with the si-NC group in both SCC13
and SCL-1 cells (P<0.01; Fig.
3A). Compared with the pc-NC group, the relative wound width
was significantly decreased in the pc-EZR-AS1 group in A431 cells
(P<0.01; Fig. 3B). The Transwell
invasion assay results indicated that compared with the si-NC
group, the number of invading cells in the si-EZR-AS1-1 group was
significantly decreased in SCC13 and SLC-1 cells (P<0.01;
Fig. 3C). In A431 cells, the number
of invading cells in the pc-EZR-AS1 group was significantly
increased compared with the pc-NC group (P<0.01; Fig. 3D). The flow cytometry results
demonstrated that EZR-AS1 knockdown significantly increased the
rate of apoptosis in SCC13 and SCL-1 cells compared with the si-NC
group, whereas EZR-AS1 overexpression significantly decreased the
rate of apoptosis in A431 cells compared with the pc-NC group
(P<0.01; Fig. 4). Collectively,
the results indicated that EZR-AS1 knockdown inhibited cSCC cell
migration and invasion, and promoted cell apoptosis.
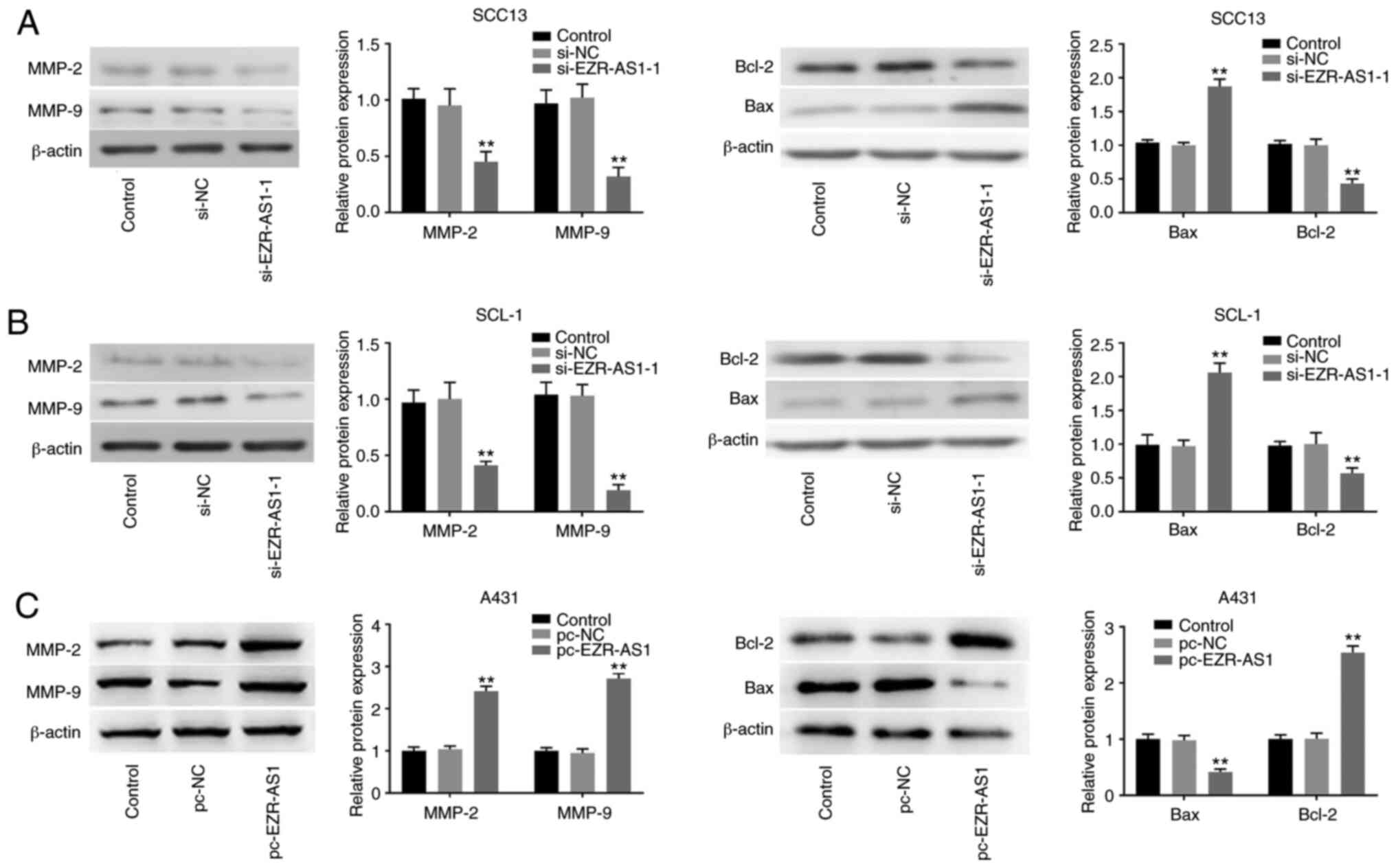
si-EZR-AS1 downregulates MMP-2, MMP-9
and Bcl-2 protein expression levels, and increases Bax protein
expression in cSCC cells
MMP-2 and MMP-9 belong to the MMP family and serve
an important role in tumor cell invasion and migration (28), whereas Bax and Bcl-2 belong to the
Bcl-2 family and are two important factors that regulate apoptosis
(29). Western blotting was
performed to detect protein expression levels. Compared with the
si-NC group, EZR-AS1 knockdown significantly reduced the protein
expression levels of MMP-2, MMP-9 and Bcl-2, but significantly
increased the protein expression levels of Bax (P<0.01; Fig. 5A and B). Compared with the pc-NC
group, EZR-AS1 overexpression significantly increased the protein
expression levels of MMP-2, MMP-9 and Bcl-2, and significantly
reduced the protein expression levels of Bax (P<0.01; Fig. 5C). The results suggested that
EZR-AS1 knockdown decreased the protein expression levels of MMP-2,
MMP-9 and Bcl-2 and increased Bax protein expression levels.
si-EZR-AS1 inhibits the PI3K/AKT
signaling pathway in cSCC cells
PI3K/AKT is a classic signaling pathway that is
often overexpressed in cancer cells (30). To further explore the mechanism
underlying EZR-AS1 in cSCC progression, western blotting was
performed to detect the effect of EZR-AS1 on the PI3K/AKT signaling
pathway. Compared with the si-NC group, EZR-AS1 knockdown
significantly decreased the protein expression levels of
p-PI3K/PI3K and p-AKT/AKT in SCC13 and SCL-1 cells (P<0.001;
Fig. 6A and B). By contrast,
compared with the pc-NC group, EZR-AS1 overexpression significantly
increased the protein expression levels of p-PI3K/PI3K and
p-AKT/AKT in A431 cells (P<0.01; Fig. 6C). FAK is a critical gene that
regulates the PI3K/AKT signaling pathway (31). The analysis of LncTar identified FAK
as a target of EZR-AS1 (Fig. 6D).
The RIP assay results verified the target relationship between
EZR-AS1 and FAK (Fig. 6E). The
western blotting results demonstrated that EZR-AS1 knockdown
significantly decreased the expression levels of FAK compared with
the si-NC group (P<0.01; Fig.
6F). Furthermore, the RT-qPCR results suggested that FAK
expression was significantly higher in cSCC tissues compared with
healthy tissues (P<0.01; Fig.
6G). To confirm the regulatory effect of EZR-AS1 and FAK on the
PI3K/AKT signaling pathway, the protein expression levels of
p-PI3K/PI3K and p-AKT/AKT in SCC13 and SCL-1 cells were measured
following co-transfection with si-EZR-AS1-1 and pc-FAK. FAK protein
expression levels in the pc-FAK group were significantly higher
compared with the pc-NC group (P<0.01; Fig. 6H). Compared with the pc-NC group,
the protein expression levels of p-PI3K/PI3K and p-AKT/AKT in the
pc-FAK group were significantly increased in SCC13 and SCL-1 cells
(P<0.01; Fig. 6H). However, the
effects of FAK overexpression on FAK, p-PI3K/PI3K and p-AKT/AKT
expression levels were significantly reversed by EZR-AS1 knockdown
(P<0.01; Fig. 6H). In summary,
EZR-AS1 knockdown inhibited the PI3K/AKT signaling pathway by
suppressing FAK expression in cSCC cells.
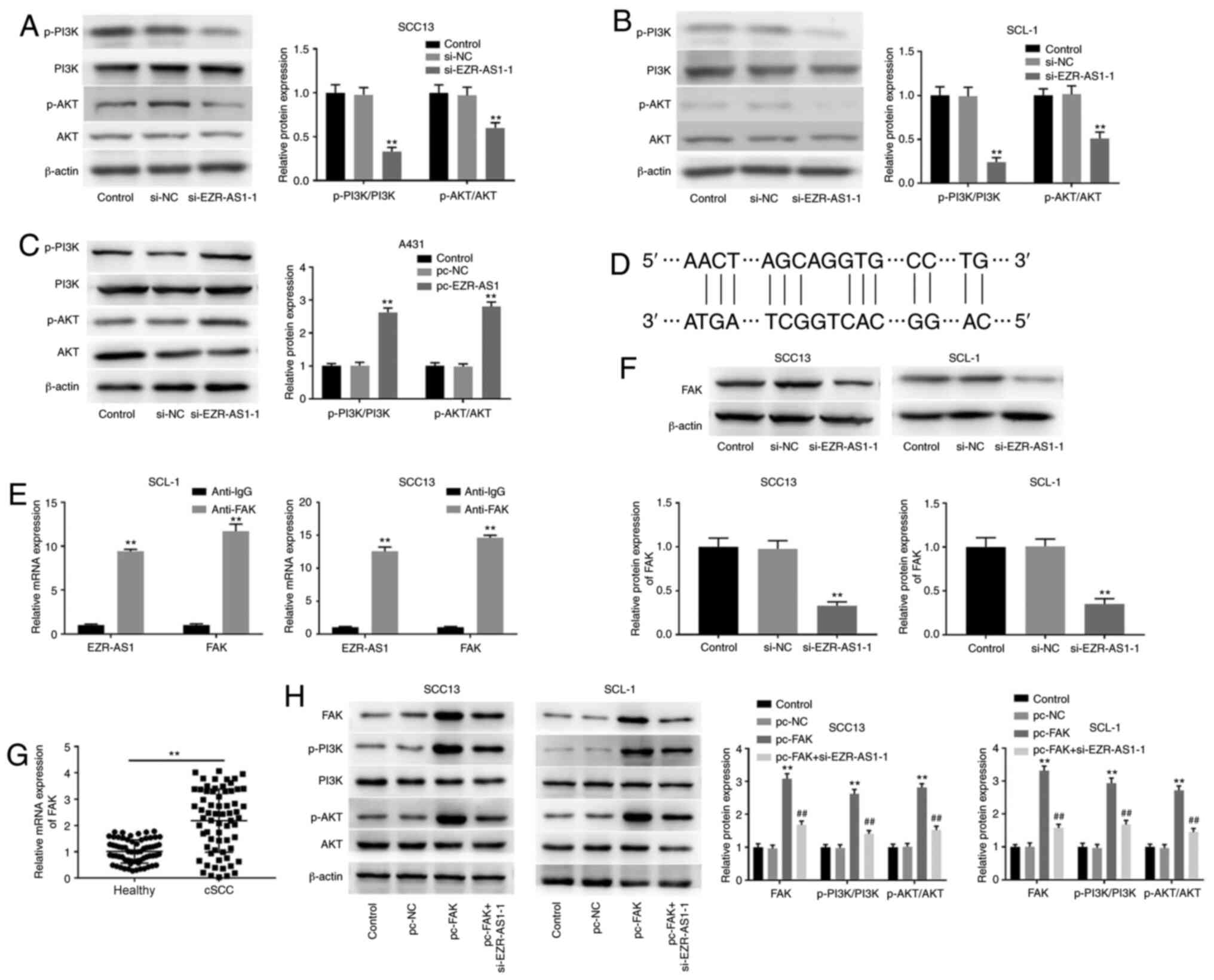 | Figure 6.si-EZR-AS1 inhibits the PI3K/AKT
signaling pathway in cSCC cells. Western blotting was performed to
measure the protein expression levels of p-PI3K/PI3K and p-AKT/AKT
in (A) SCC13, (B) SCL-1 and (C) A431 cells. (D) LncTar analysis
identified FAK as a target of EZR-AS1. (E) The RNA
immunoprecipitation assay was performed to confirm FAK as a target
of EZR-AS1. (F) Effect of EZR-AS1 knockdown on FAK protein
expression levels in SCC13 and SCL-1 cells. (G) FAK mRNA expression
levels in cSCC tissues and healthy adjacent tissues. (H) Western
blotting was performed to measure the protein expression levels of
FAK, p-PI3K/PI3K and p-AKT/AKT in pc-FAK- and
si-EZR-AS1-1-transfected SCC13 and SLC-1 cells. **P<0.01 vs.
si-NC, pc-NC, anti-IgG or healthy; ##P<0.01 vs.
pc-FAK. si, small interfering RNA; EZR-AS1, ezrin antisense RNA 1;
cSCC, cutaneous squamous cell carcinoma; p, phosphorylated; FAK,
focal adhesion kinase; NC, negative control. |
EZR-AS1 affects cSCC cell
proliferation, migration, invasion and apoptosis via the PI3K/AKT
signaling pathway
To further investigate the relationship between
EZR-AS1 and the PI3K/AKT signaling pathway in cSCC progression,
740Y-P (a PI3K agonist) was used. Compared with the control group,
740Y-P significantly increased the expression levels of p-AKT/AKT
and p-PI3K/PI3K, and significantly reversed si-EZR-AS1-1-mediated
effects on p-AKT/AKT and p-PI3K/PI3K expression levels (all
P<0.01; Fig. 7A). Compared with
the control group, EZR-AS1 knockdown significantly reduced colony
formation, whereas 740Y-P significantly reversed the inhibitory
effects of si-EZR-AS1 on SCC13 cell colony formation (all
P<0.01; Fig. 7B). Similarly,
740Y-P significantly reversed si-EZR-AS1-mediated effects on SCC13
cell migration, invasion and apoptosis (P<0.01; Fig. 7C-E). Collectively, the
aforementioned results indicated that EZR-AS1 knockdown inhibited
cSCC cell proliferation, migration and invasion, and promoted cSCC
cell apoptosis via inhibiting the PI3K/AKT signaling pathway.
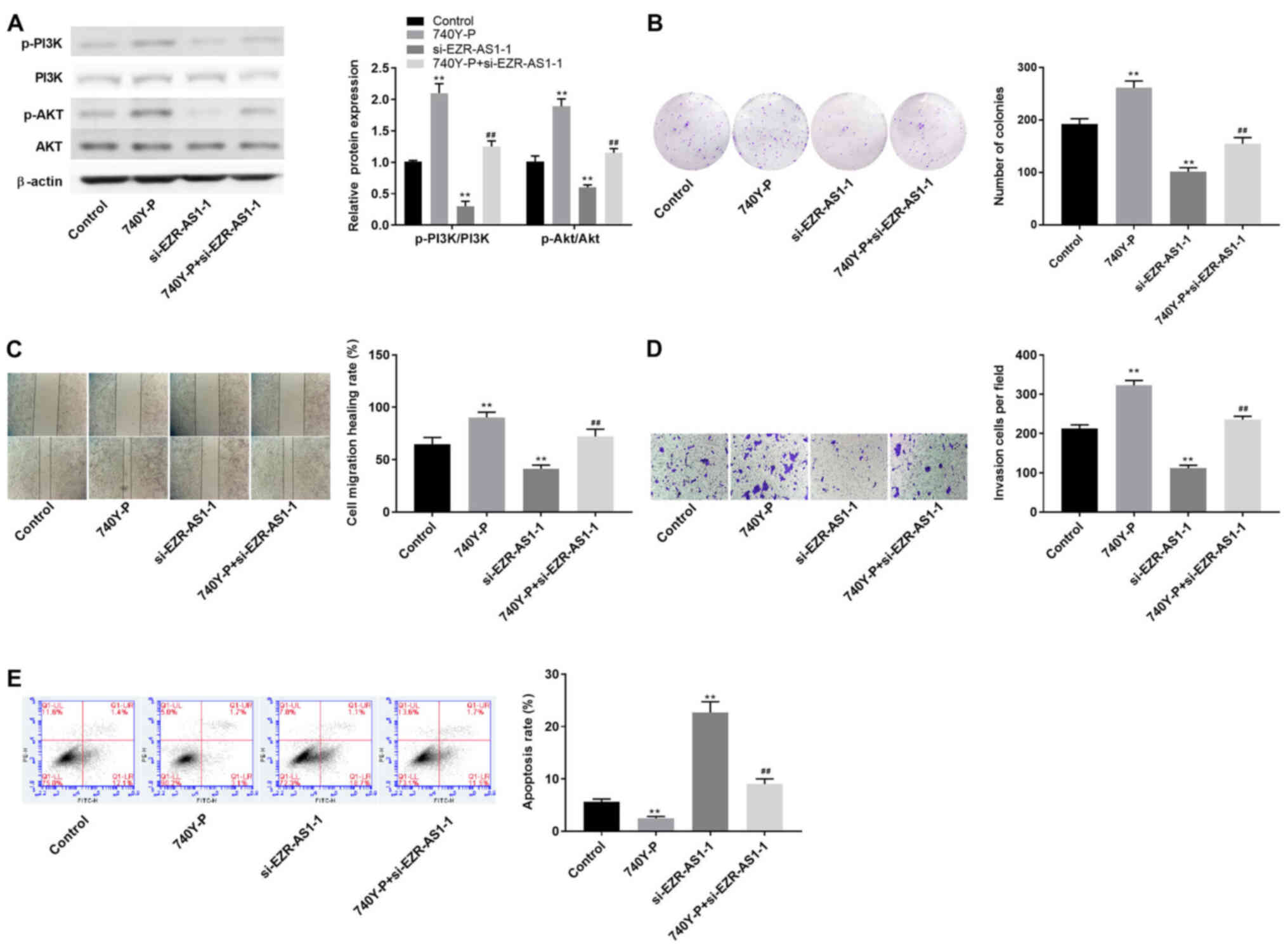 | Figure 7.EZR-AS1 regulates cutaneous squamous
cell carcinoma cell proliferation, migration, invasion and
apoptosis via the PI3K/AKT signaling pathway. (A) Western blotting
was performed to assess the effect of 740Y-P on the protein
expression levels of p-AKT/AKT and p-PI3K/PI3K. (B) The effect of
740Y-P on SCC13 cell colony formation was assessed by performing
the plate cloning assay (magnification, ×40). The effect of 740Y-P
on SCC13 cell migration and invasion was assessed by performing (C)
wound healing (scale bar, 100 µm) and (D) Transwell invasion
(magnification, ×200) assays, respectively. (E) The effect of
740Y-P on SCC13 cell apoptosis was determined by performing flow
cytometry. **P<0.01 vs. control or si-NC; ##P<0.01
vs. si-EZR-AS1-1. EZR-AS1, ezrin antisense RNA 1; p,
phosphorylated; si, small interfering RNA; NC, negative
control. |
Discussion
In recent years, lncRNAs have been reported to
regulate various cellular processes, including cell proliferation,
metastasis, differentiation and metabolism, leading to the
progression of numerous diseases, including malignant tumors
(32). In the present study, lncRNA
EZR-AS1 expression was significantly upregulated in cSCC cells
compared with HaCaT cells. In addition, compared with the si-NC
group, EZR-AS1 knockdown significantly inhibited SCC13 and SCL-1
cell proliferation, migration and invasion, and promoted cell
apoptosis. Mechanistically, the results indicated that lncRNA
EZR-AS1 might exert its molecular function via the PI3K/AKT
signaling pathway, thus affecting cSCC progression.
lncRNA EZR-AS1 is a key regulator of disease
progression in numerous types of cancer, including ESCC, breast
cancer and colorectal cancer, but the biological function of
EZR-AS1 is not completely understood (15,20,21). A
previous study have demonstrated that lncRNA EZR-AS1 knockdown can
inhibit TGF-β signaling, thereby inhibiting colorectal cancer cell
proliferation, invasion, migration and epithelial-mesenchymal
transition, and promoting apoptosis (15). In addition, EZR-AS1 knockdown
significantly inhibited ESCC cell migration, reduced tumor volume
and weight, and reduced the number of metastatic lymph nodes in
mice (21). The specific regulatory
effects and mechanisms underlying lncRNA EZR-AS1 in cSCC are not
completely understood. In the present study, EZR-AS1 expression was
significantly increased in cSCC cells compared with HaCaT cells.
Compared with the si-NC group, EZR-AS1 knockdown significantly
inhibited SCC13 and SCL-1 cell proliferation, migration and
invasion, and induced apoptosis. Moreover, EZR-AS1 targeted FAK in
cSCC cells. Moreover, compared with the si-NC group, EZR-AS1
knockdown significantly decreased FAK expression, and FAK
expression levels were significantly higher in cSCC tissues
compared with healthy tissues.
The basic features of the metastasis process include
tumor cell invasion and migration (33). In recent years, MMPs have been
considered as important proteases related to tumor invasion and
migration, especially the two metal proteins, MMP-2 and MMP-9
(28). MMP-2 and MMP-9 promote the
progression of ovarian cancer, and their expression level increases
with the increased malignant potential of ovarian tumors (34). In addition, Li et al
(35) also reported that
MMP-2/MMP-9 induction promoted hepatocellular carcinoma cell
metastasis. Consistently, the present study demonstrated that
EZR-AS1 knockdown significantly decreased MMP-2 and MMP-9 protein
expression levels compared with the si-NC group. Apoptosis is
regulated by specific proteins, which regulates tumorigenesis and
development (36). Among the
specific apoptosis-related proteins, Bax can promote cancer cell
apoptosis, whereas Bcl-2 can interact with Bax to regulate the
occurrence of apoptosis (29). The
present study demonstrated that EZR-AS1 knockdown significantly
upregulated Bax expression and significantly downregulated Bcl-2
expression compared with the si-NC group. Collectively, the results
indicated that EZR-AS1 may serve as an oncogene and contribute to
cSCC malignancy.
PI3K/AKT is an important intracellular signaling
pathway directly related to cell dormancy, proliferation,
canceration and longevity (37).
PI3K is a downstream mediator of the cell membrane tyrosine kinase
receptor, which can phosphorylate phosphatidylinositol
4,5-bisphosphate to form phosphatidylinositol [3-5]-triphosphate.
PIP3 activates the serine/threonine kinase AKT, which regulates
cellular functions by phosphorylating downstream factors, including
various enzymes, kinases and transcription factors (38,39).
An increasing number of studies have demonstrated that the PI3K/AKT
signaling pathway serves a critical role in cSCC cell survival
(25,40), and alterations in the molecular
function of the signaling pathway primarily involves
phosphorylation of PI3K and AKT (22). In the present study, the expression
levels of p-PI3K/PI3K and p-AKT/AKT were significantly reduced by
EZR-AS1 knockdown compared with the si-NC group. As the key
regulator of the PI3K/AKT signaling pathway, FAK overexpression
significantly increased the protein expression levels of
p-PI3K/PI3K and p-AKT/AKT in cSCC cells compared with the pc-NC
group. However, EZR-AS1 knockdown partly reversed FAK
overexpression-mediated effects on the PI3K/AKT signaling pathway.
To further investigate the relationship between EZR-AS1 and the
PI3K/AKT signaling pathway in cSCC progression, 740Y-P (a PI3K
agonist) was used. Compared with the control group, 740Y-P
significantly increased the expression levels of p-AKT/AKT and
p-PI3K/PI3K, and reversed si-EZR-AS1-mediated effects on the
expression levels of p-AKT/AKT and p-PI3K/PI3K. Moreover, 740Y-P
partially reversed the inhibitory effect of si-EZR-AS1 on SCC13
cell proliferation. Similarly, SCC13 cell migration, invasion and
apoptosis were assessed, and the results indicated that 740Y-P
reversed si-EZR-AS1-mediated effects on SCC13 cells.
To conclude, the present study demonstrated that
EZR-AS1 was upregulated in cSCC cells compared with HaCaT cells.
Furthermore, si-EZR-AS1 inhibited cSCC cell proliferation,
migration and invasion, and promoted cell apoptosis compared with
the si-NC group. The molecular mechanisms underlying EZR-AS1 might
be associated with the PI3K/AKT signaling pathway. The results
provided novel insights into the diagnosis and treatment of
cSCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81602756), the National
Natural Science Foundation of Shan Dong University (grant no.
ZR2016HQ37) and the Jinan Science and Technology Bureau of Shandong
Province (grant no. 201821053).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
DL, LS and ZL performed the experiments. ZM provided
technical support. DL and ZM analyzed the data and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Shandong First
Medical University (approval no. 2020-181; Tai'an, China). All
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu S, Chen M, Li P, Wu Y, Chang C, Qiu Y,
Cao L, Liu Z and Jia C: Ginsenoside rh2 inhibits cancer stem-like
cells in skin squamous cell carcinoma. Cell Physiol Biochem.
36:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ou C, Liu H, Ding Z and Zhou L:
Chloroquine promotes gefitinib-induced apoptosis by inhibiting
protective autophagy in cutaneous squamous cell carcinoma. Mol Med
Rep. 20:4855–4866. 2019.PubMed/NCBI
|
|
3
|
Mei XL and Zhong S: Long noncoding RNA
LINC00520 prevents the progression of cutaneous squamous cell
carcinoma through the inactivation of the PI3K/Akt signaling
pathway by downregulating EGFR. Chin Med J (Engl). 132:454–465.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toll A, Gimeno-Beltrán J, Ferrandiz-Pulido
C, Masferrer E, Yébenes M, Jucglà A, Abal L, Martí RM, Sanmartín O,
Baró T, et al: D2-40 immunohistochemical overexpression in
cutaneous squamous cell carcinomas: A marker of metastatic risk. J
Am Acad Dermatol. 67:1310–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Huang C and Yang X: Characterization
of TCF4-mediated oncogenic role in cutaneous squamous cell
carcinoma. Int J Clin Exp Pathol. 12:3583–3594. 2019.PubMed/NCBI
|
|
6
|
Ci C, Wu C, Lyu D, Chang X, He C, Liu W,
Chen L and Ding W: Downregulation of kynureninase restrains
cutaneous squamous cell carcinoma proliferation and represses the
PI3K/AKT pathway. Clin Exp Dermatol. 45:194–201. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous squamous cell carcinoma: A review of high-risk and
metastatic disease. Am J Clin Dermatol. 17:491–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zilberg C, Lee MW, Kraitsek S, Ashford B,
Ranson M, Shannon K, Iyer NG, Ch'ng S, Low TH, Palme C, et al: Is
high-risk cutaneous squamous cell carcinoma of the head and neck a
suitable candidate for current targeted therapies? J Clin Pathol.
73:17–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uszczynska-Ratajczak B, Lagarde J,
Frankish A, Guigo R and Johnson R: Towards a complete map of the
human long non-coding RNA transcriptome. Nat Rev Genet. 19:535–548.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi D, Zhang C and Liu X: Long noncoding
RNAs in cervical cancer. J Cancer Res Ther. 14:745–753. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hosseini ES, Meryet-Figuiere M,
Sabzalipoor H, Kashani HH, Nikzad H and Asemi Z: Dysregulated
expression of long noncoding RNAs in gynecologic cancers. Mol
Cancer. 16:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Yao H, Wang K and Liu X: Long
Non-coding RNA MALAT1 regulates ZEB1 expression by sponging
miR-143-3p and promotes hepatocellular carcinoma progression. J
Cell Biochem. 118:4836–4843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Fu L, Sun A, Tang D, Xu Y, Li Z,
Chen M and Zhang G: C/EBPβ contributes to transcriptional
activation of long non-coding RNA NEAT1 during APL cell
differentiation. Biochem Biophys Res Commun. 499:99–104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Wang N, Wang F, Zhang S and Ding J:
Silencing of lncRNA EZR-AS1 inhibits proliferation, invasion, and
migration of colorectal cancer cells through blocking transforming
growth factor β signaling. Biosci Rep. 39:BSR201911992019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You G, Long X, Song F, Huang J, Tian M,
Xiao Y, Deng S and Wu Q: Long noncoding RNA EZR-AS1 regulates the
proliferation, migration, and apoptosis of human venous endothelial
cells via SMYD3. Biomed Res Int. 2020:68402342020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaffari A, Hoskin V, Turashvili G, et al:
Intravital imaging reveals systemic ezrin inhibition impedes cancer
cell migration and lymph node metastasis in breast cancer. Breast
Cancer Res. 21:122019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie JJ, Xu LY, Wu ZY, Zhao Q, Xu XE, Wu
JY, Huang Q and Li EM: Prognostic implication of ezrin expression
in esophageal squamous cell carcinoma. J Surg Oncol. 104:538–543.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie YH, Li LY, He JZ, Xu XE, Liao LD,
Zhang Q, Xie JJ, Xu LY and Li EM: Heat shock protein family B
member 1 facilitates ezrin activation to control cell migration in
esophageal squamous cell carcinoma. Int J Biochem Cell Biol.
112:79–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai Y, Zhou X, Huang L, Wan Y, Li X and
Wang Y: Long noncoding RNA EZR-AS1 promotes tumor growth and
metastasis by modulating Wnt/β-catenin pathway in breast cancer.
Exp Thera Med. 16:2235–2242. 2018.
|
|
21
|
Zhang XD, Huang GW, Xie YH, He JZ, Guo JC,
Xu XE, Liao LD, Xie YM, Song YM, Li EM and Xu LY: The interaction
of lncRNA EZR-AS1 with SMYD3 maintains overexpression of EZR in
ESCC cells. Nucleic Acids Res. 46:1793–1809. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gou XJ, Bai HH, Liu LW, Chen HY, Shi Q,
Chang LS, Ding MM, Shi Q, Zhou MX, Chen WL and Zhang LM: Asiatic
acid interferes with invasion and proliferation of breast cancer
cells by inhibiting WAVE3 activation through PI3K/AKT signaling
pathway. Biomed Res Int. 2020:18743872020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Qin Y, Zhong Y, Qin Y, Wei Y, Li L
and Xie Y: Fentanyl inhibits the progression of gastric cancer
through the suppression of MMP-9 via the PI3K/Akt signaling
pathway. Ann Transl Med. 8:1182020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Troiano A, Lomoriello IS, di Martino O,
Fusco S, Pollice A, Vivo M, La Mantia G and Calabro V: Y-box
binding protein-1 is part of a complex molecular network linking
ΔNp63α to the PI3K/akt pathway in cutaneous squamous cell
carcinoma. J Cell Physiol. 230:2067–2074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Z, Pan X, Yang Y, Huang Y and Shen HB:
The lncLocator: A subcellular localization predictor for long
non-coding RNAs based on a stacked ensemble classifier.
Bioinformatics. 34:2185–2194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Bao C, Ma Z, Xu B, Ying X, Liu X and
Zhang X: Perfluorooctanoic acid stimulates ovarian cancer cell
migration, invasion via ERK/NF-κB/MMP-2/-9 pathway. Toxicol Lett.
294:44–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jarskog LF, Selinger ES, Lieberman JA and
Gilmore JH: Apoptotic proteins in the temporal cortex in
schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation.
Am J Psychiatry. 161:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sathe A and Nawroth R: Targeting the
PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol.
1655:335–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang SL, Ma L, Zhao J, You SP, Ma XT, Ye
XY and Liu T: The phenylethanol glycoside liposome inhibits
PDGF-induced HSC activation via regulation of the FAK/PI3K/Akt
signaling pathway. Molecules. 24:32822019. View Article : Google Scholar
|
|
32
|
Duan H, Li X, Chen Y, Wang Y and Li Z:
LncRNA RHPN1-AS1 promoted cell proliferation, invasion and
migration in cervical cancer via the modulation of miR-299-3p/FGF2
axis. Life Sci. 239:1168562019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao CL, Chu YL, Lin HY, Chen CY, Hsu MJ,
Liu KC, Lai KC, Huang AC and Chung JG: Bisdemethoxycurcumin
suppresses migration and invasion of human cervical cancer HeLa
cells via inhibition of NF-kB, MMP-2 and −9 pathways. Anticancer
Res. 38:3989–3997. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rasool M, Malik A, Basit Ashraf MA,
Parveen G, Iqbal S, Ali I, Qazi MH, Asif M, Kamran K, Iqbal A, et
al: Evaluation of matrix metalloproteinases, cytokines and their
potential role in the development of ovarian cancer. PLoS One.
11:e01671492016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Yang Z, Song W, et al:
Overexpression of Bmi-1 contributes to the invasion and metastasis
of hepatocellular carcinoma by increasing the expression of matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor via the PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu F, Jiang D, Zhang M and Zhao B:
2,4-Dihydroxy-3′-methoxy-4′-ethoxychalcone suppresses cell
proliferation and induces apoptosis of multiple myeloma via the
PI3K/akt/mTOR signaling pathway. Pharm Biol. 57:641–648. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanz Ressel BL, Massone AR and Barbeito
CG: Immunohistochemical expression of selected phosphoproteins of
the mTOR signalling pathway in canine cutaneous squamous cell
carcinoma. Vet J. 245:41–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu XY, Tian F, Su MH, Wu M, Huang Y, Hu
LH, Jin L and Zhu XJ: BF211, a derivative of bufalin, enhances the
cytocidal effects in multiple myeloma cells by inhibiting the
IL-6/JAK2/STAT3 pathway. Int Immunopharmacol. 64:24–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yao M, Shang YY, Zhou ZW, Yang YX, Wu YS,
Guan LF, Wang XY, Zhou SF and Wei X: The research on lapatinib in
autophagy, cell cycle arrest and epithelial to mesenchymal
transition via Wnt/ErK/PI3K-AKT signaling pathway in human
cutaneous squamous cell carcinoma. J Cancer. 8:220–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|