Introduction
Childhood acute myeloid leukaemia (AML) is a common
malignancy in children, accounting for one-third of all childhood
cancer types (1). AML, a common
type of leukaemia, is caused by the uncontrolled proliferation,
apoptosis, and differentiation of haematopoietic cells in the bone
marrow and other haematopoietic tissues (2). AML is known for its high rates of
morbidity, recurrence and mortality (3). Although there are many treatment
options available, most patients with AML relapse and succumb to
remission, and the prognosis remains unsatisfactory (4). Therefore, to develop more effective
monitoring and treatment strategies, it is imperative to identify
novel biomarkers that can be used to improve the diagnosis and
prognosis of AML.
Long non-coding RNAs (lncRNAs) have been reported to
function as important regulators of biological and pathological
processes in diverse human cancers, including AML (5–7). For
example, myocardial infarction associated transcript knockdown was
shown to repress the proliferation and promote the apoptosis of AML
cells by regulating microRNA (miR)-495 (8). Silencing long intergenic non-protein
coding RNA (LINC)00152 was found to inhibit proliferation, induce
apoptosis and enhance cycle arrest in AML cells by sponging
miR-193a (9). Small nucleolar RNA
host gene (SNHG)5 knockdown increases the sensitivity of AML cells
to chemotherapy by targeting the miR-32/DNAJ heat shock protein
family (hsp40) member B9 axis (10). SNHG14 has been reported to induce
oncogenic function by regulating the proliferation, migration,
invasion, and chemical resistance ability of various malignant
tumour types, including non-small cell lung (11), cervical (12) and gastric (13) cancer. Recently, Wang et al
(14) reported that SNHG14
functions as an antitumour gene in glioma. However, the biological
function of SNHG14 in AML and the potential mechanism of its action
remain unclear.
miRNAs have been identified to function as oncogenic
or tumour-suppressive genes, regulating diverse biological
processes in cancer, (15) and it
has been well documented that they are related to the occurrence
and development of AML (16).
miR-193b-3p has been shown to function as an antitumour gene in
numerous malignant tumour types, including ovarian (17), gastric (18) and breast (19) cancer. Additionally, a recent study
indicated that miR-193b serves an anticancer role in homeobox
A9/meis homeobox 11-induced leukaemia in vivo (20). Notably, increasing lines of evidence
support a novel mechanism by which lncRNAs act as competitive
endogenous RNAs (ceRNAs) to mediate the expression and functions of
specific miRNAs involved in tumour progression (21). For example, Wang et al
(22) demonstrated that silencing
lncRNA linc00152 impeded the progression of gastric cancer by
modulating miR-193b-3p. Xie et al (23) reported that SNHG14 contributes to
the tumorigenesis of breast cancer by sponging miR-193a-3p.
Nevertheless, the regulatory mechanism involved in the interaction
between SNHG14 and miR-193b-3p in AML remains unclear.
In the present study, first, SNHG14 expression in
bone marrow tissues from patients with AML was measured. Next, the
effect of SNHG14 on AML cell viability and apoptosis was evaluated.
Furthermore, the potential mechanism involved in the interaction
among SNHG14, miR-193b-3p and MCL1 apoptosis regulator BCL2 family
member (MCL1) in AML cells was explored. The results of the current
study may aid in better understanding the pathogenesis of AML and
provide a promising therapeutic target for AML treatment.
Materials and methods
Patients and specimens
A total of 57 children with AML (28 male, 29 female;
10 months-14 years old) were identified after screening potential
participants at the East Hospital of Shouguang People's Hospital
(Shouguang, China) between January 2017 and January 2018. The
inclusion criteria included a first-time diagnosis and no history
of antitumour treatment. The exclusion criteria included the
presence of other malignant tumours and/or organ dysfunctions. AML
was diagnosed according to the World Health Organization
classification of tumours of haematopoietic and lymphoid tissues
(Version 2008) (24). A total of 57
age- and sex-matched children with normal bone marrow morphology,
and without malignancy who were examined at the hospital at the
same time were enrolled as the control group. The clinical
characteristics of patients with AML and controls listed in
Table I. The bone marrow samples
were obtained by puncture and were stored at −80°C until use.
Informed consent was obtained from the legal guardians of children
<18 years old. This study was approved by the Ethics Committee
of the East Hospital of Shouguang People's Hospital in accordance
with the Declaration of Helsinki.
 | Table I.Clinical characteristics of patients
with AML and healthy controls. |
Table I.
Clinical characteristics of patients
with AML and healthy controls.
|
Characteristics | AML | Healthy
controls |
|---|
| Age, years
olda | 8±5a | 9±5a |
| Sex, n |
|
|
|
Male | 28 | 28 |
|
Female | 29 | 29 |
| FAB classification,
n |
|
|
| M0 | 0 | – |
| M1 | 13 | – |
| M2 | 16 | – |
| M3 | 11 | – |
| M4 | 7 | – |
| M5 | 5 | – |
| M6 | 1 | – |
| M7 | 4 | – |
| Bone marrow blasts
(non-M3, n=46), % [mean ± standard deviation (range)] | 54.5±22.9
(30.3–97.8) | – |
| Cytogenetics,
n |
|
|
|
Favourable | 15 | – |
|
Intermediate | 33 | – |
|
Unfavourable | 9 | – |
Cell culture and transfection
Human normal bone marrow CD34+ cells and
AML cell lines (MV-4-11, AML-193, HL-60, and KG-1 cells) were
obtained from the American Type Culture Collection and cultured in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C.
The small interfering RNA targeting SNHG14 (si-SNHG14)
(5′-CAGCAUAUGUAAGUGGAACUCAGAA-3′), corresponding negative control
(si-NC) (5′-AUCUUCAUUGGCACCGAACGUGUCACGUUU-3′), miR-193b-3p mimics
(5′-AACUGGCCCUCAAAGUCCCGCU-3′), miR-193b-3p inhibitor
(5′-AGCGGGACUUUGAGGGCCAGUU-3′), miR-NC
(5′-UUUGUACUACACAAAAGUACUG-3′), pcDNA-SNHG14, pcDNA-NC, and
pcDNA-MCL1 were all purchased from Shanghai GenePharma Co., Ltd.
MV-4–11 and AML-193 cells were transfected with the aforementioned
oligonucleotides (50 nM) or plasmids (50 ng) using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Following an incubation period of 48 h at 37°C,
the cells were utilised in the subsequent experiments.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from the bone marrow samples
and cells using an miRNeasy Mini kit (Qiagen GmbH). A PrimeScript™
IV 1st strand cDNA Synthesis Mix (Takara Bio, Inc.) was used to
reverse transcribe the isolated RNA (2 µg) into cDNA according to
the manufacturer's protocol. qPCR analyses were conducted using an
SYBR Green PCR Master mix (Qiagen GmbH) on an ABI 7500HT Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc). The reaction conditions were as follows: 95°C for 3 min, and
40 cycles at 95°C for 10 sec, 60°C for 20 sec and 72°C for 34 sec.
The melting curve had a single peak. The 2−ΔΔCq method
was used to analyse the relative mRNA expression levels (25). GAPDH or U6 was utilised as an
internal control. The primer sequences were shown as follows:
SNHG14 forward, 5′-GGGTGTTTACGTAGACCAGAACC-3′ and reverse,
5′-CTTCCAAAAGCCTTCTGCCTTAG-3′; miR-193b-3p forward,
5′-TCTACAGTGCACGTGTCTCCAG-3′ and reverse,
5′-ACCTGCGTAGGTAGTTTCATGT-3′; MCL1 forward,
5′-GGACATCAAAAACGAAGACG-3′ and reverse, 5′-GCAGCTTTCTTGGTTTATGG-3′;
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-AGGGATCTCGCTCCTGGAA-3′; U6 forward, 5′-AGTACCAGTCTGTTGCTGG-3′
and reverse, 5′-TAATAGACCCGGATGTCTGGT-3′.
Target predicting
The targets of SNHG14 and miR-193b-3p were predicted
by a starBase online database (starbase.sysu.edu.cn/agoClipRNA.php?). miR-193-3p was
identified as a target of SNHG14, and MCL1 was identified as a
target of miR-193b-3p.
RNA-binding protein
immunoprecipitation (RIP)
The target association between SNHG14 and
miR-193b-3p was analysed using the RIP assay with a Magna RIP
RNA-binding protein immunoprecipitation kit (#17-700, EMD
Millipore) in accordance with the manufacturer's instructions.
Briefly, MV-4-11 and AML-193 cells were lysed in NP-40 lysis buffer
containing 1% protease inhibitor (Sigma-Aldrich; Merck KGaA). After
10 min of centrifugation at 200 g and 4°C, 100 µl supernatant was
conjugated with human anti-protein argonaute-2 (ab186733, 1:1,000,
AGO2) antibody (Abcam) for 10 h at 4°C. Mouse IgG (M8642, 1:1,000,
EMD Millipore) was used as a negative control. After Coprecipitated
RNA was isolated and measured using RT-qPCR for direct binding as
aforementioned.
Dual luciferase reporter gene (DLR)
assay
The wild-type (wt) or mutated (mut) fragments of
SNHG14 (SNHG14 wt/mut) and MCL1 (MCL1 wt/mut) were cloned into a
pmiRGLO vector (Promega Corporation). To investigate the
association among SNHG14, miR-193b-3p, and MCL1, MCL1 wt/mut or
SNHG14 wt/mut was co-transfected into MV-4-11 and AML-193 cells
with miR-NC/miR-193b-3p mimics using Lipofectamine® 3000
reagent. After transfection for 48 h at 37°C, the
Dual-Luciferase® Reporter assay system (Promega
Corporation) was used to measure the luciferase activity.
Renilla luciferase activity was measured as the internal
control.
MTT assay
MV-4-11 and AML-193 cells (1×104
cells/well) were seeded in 96-well plates and cultured for 24, 48,
72 or 96 h. Cells were then incubated with 20 µl MTT (0.5 mg/ml)
for 4 h at 37°C, followed by the addition of 150 µl dimethyl
sulfoxide to terminate the reaction. The absorbance at 450 nm
(A450) was measured using a microplate reader.
Flow cytometry analysis
The cell apoptosis assay (MV-4-11 and AML-193 cells)
was performed using an Annexin V-FITC/PI Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, the cells were double-stained with 5 µl Annexin
V/FITC and 10 µl PI. The cells were then incubated for 15 min at
25°C in the dark. The stained cells were detected using a flow
cytometer (FACSAria II, Becton Dickinson, Franklin Lakes, NJ, USA)
with CellQuest software (v1.5, Becton Dickinson).
Western blot analysis
Total proteins were isolated from MV-4-11 cells
using RIPA lysis buffer (Thermo Fisher Scientific, Inc.) and
quantified using a BCA Protein assay kit (Thermo Fisher Scientific,
Inc.). The proteins (30–50 µg/lane) were separated using 10%
SDS-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene membrane. Following blocking with 5% non-fat milk
for 2 h at 25°C, the membrane was incubated with rabbit anti-MCL1
(1:5,000; cat. no. ab246684; Abcam) and rabbit anti-β-actin
(1:10,000; cat. no. 4970; Cell Signaling Technology, Inc.) primary
antibodies at 4°C overnight. Then, the membrane was incubated with
HRP-conjugated goat anti-rabbit IgG (A0545, 1:10,000;
Sigma-Aldrich) for 1 h at room temperature. Finally, the protein
bands were visualised using enhanced chemiluminescent substrate
reagent kit (WP20005, Thermo Fisher Scientific, Inc.), and
quantified using ImageJ software (v1.5, National Institutes of
Health). β-actin was used as an internal control.
Statistical analysis
Each experiment was repeated at least three times.
SPSS 22.0 statistical software (SPSS, Inc.) and GraphPad Prism
v7.01 (GraphPad Software, Inc.) were used for all statistical
analyses. Data are presented as the mean ± standard deviation.
Student's t-test was used to compare significant differences
between two groups and one-way ANOVA followed by Tukey's post hoc
test was applied when analysing >two groups. Spearman's
correlation analysis was performed to evaluate the correlation
between SNHG14 and miR-193b-3p expression. P<0.05 was considered
to indicate a statistically significant difference.
Results
SNHG14 is overexpressed in the bone
marrow tissues of patients with AML
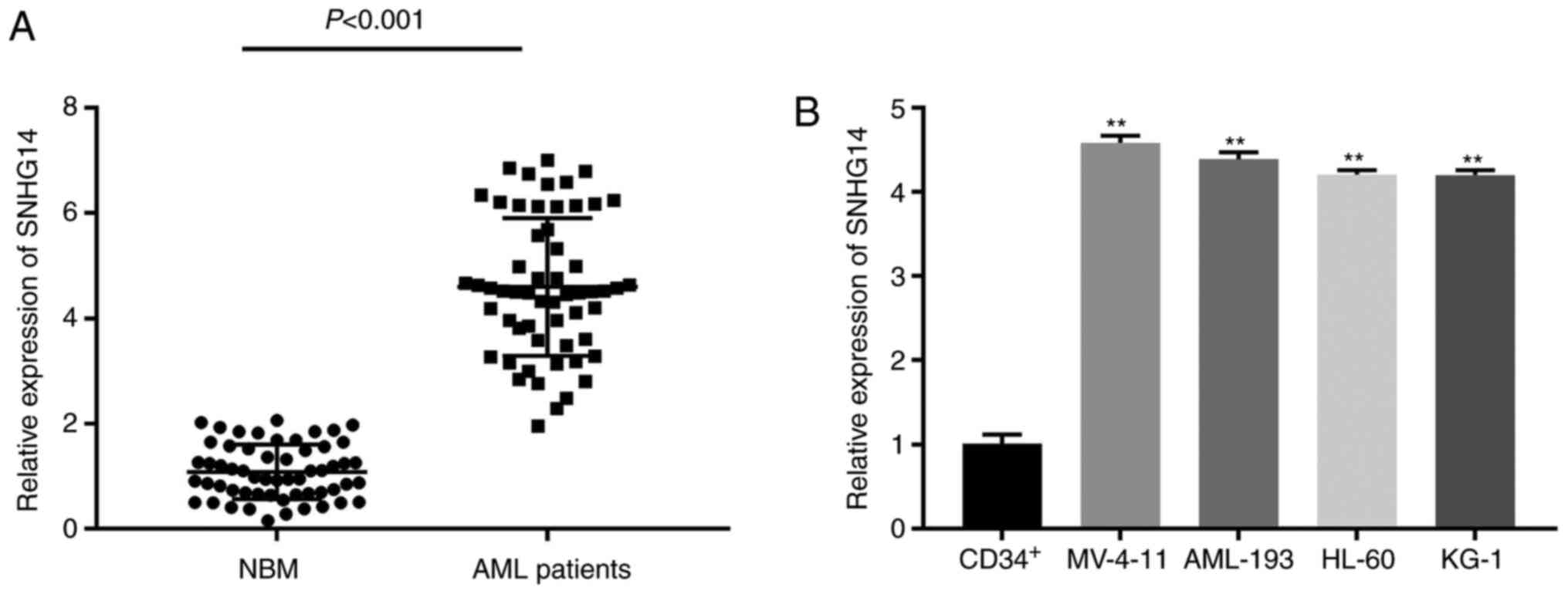
To investigate the role of SNHG14, the gene
expression of SNHG14 was measured first. SNHG14 was found to be
significantly overexpressed in AML bone marrow tissue compared with
the level in normal marrow tissue (P<0.001; Fig. 1A). The AML bone marrow tissues were
divided into two groups according to the expression of SNHG14: High
expression, SNHG14 expression level ≥median and low expression,
SNHG14 expression level <median. The clinicopathological
features of the patients with AML are shown in Table II. The analysis of the data
revealed that the French-American-British classification (P=0.348)
and cytogenetics (P<0.001) were significantly different between
the high and low expression groups (Table II). In addition, SNHG14 gene
expression was significantly upregulated in AML cell lines
(MV-4-11, AML-193, HL-60 and KG-1) compared with that in human
normal bone marrow CD34+ cells (P<0.01; Fig. 1B). MV-4-11 and AML-193 cells, which
had relatively higher SNHG14 gene expression, were utilised in the
subsequent experiments.
 | Table II.Clinicopathologic characteristics of
AML patients with low and high expression of SNHG14. |
Table II.
Clinicopathologic characteristics of
AML patients with low and high expression of SNHG14.
|
| No. of
patients |
|
|---|
|
|
|
|
|---|
|
Characteristics | Total | Low SNHG14
expression | High SNHG14
expression | P-value |
|---|
| Age, years |
|
|
| 0.681 |
| ≤7 | 26 | 12 | 14 |
|
|
8–14 | 31 | 16 | 15 |
|
| Sex |
|
|
| 0.896 |
|
Male | 28 | 14 | 14 |
|
|
Female | 29 | 14 | 15 |
|
| Leukocytes, µl |
|
|
| 0.661 |
|
>10,000 | 35 | 18 | 17 |
|
|
≤10,000 | 22 | 10 | 12 |
|
| Bone marrow blasts
(non-M3, n=46), % |
|
|
| 0.203 |
|
30–55 | 26 | 14 | 12 |
|
|
≥55 | 20 | 7 | 13 |
|
| FAB
classification |
|
|
| 0.042a |
|
M1-6 | 53 | 28 | 25 |
|
| M7 | 4 | 0 | 4 |
|
| Cytogenetics |
|
|
|
<0.001b |
|
Favourable | 15 | 15 | 0 |
|
|
Intermediate | 33 | 13 | 20 |
|
|
Unfavourable | 9 | 0 | 9 |
|
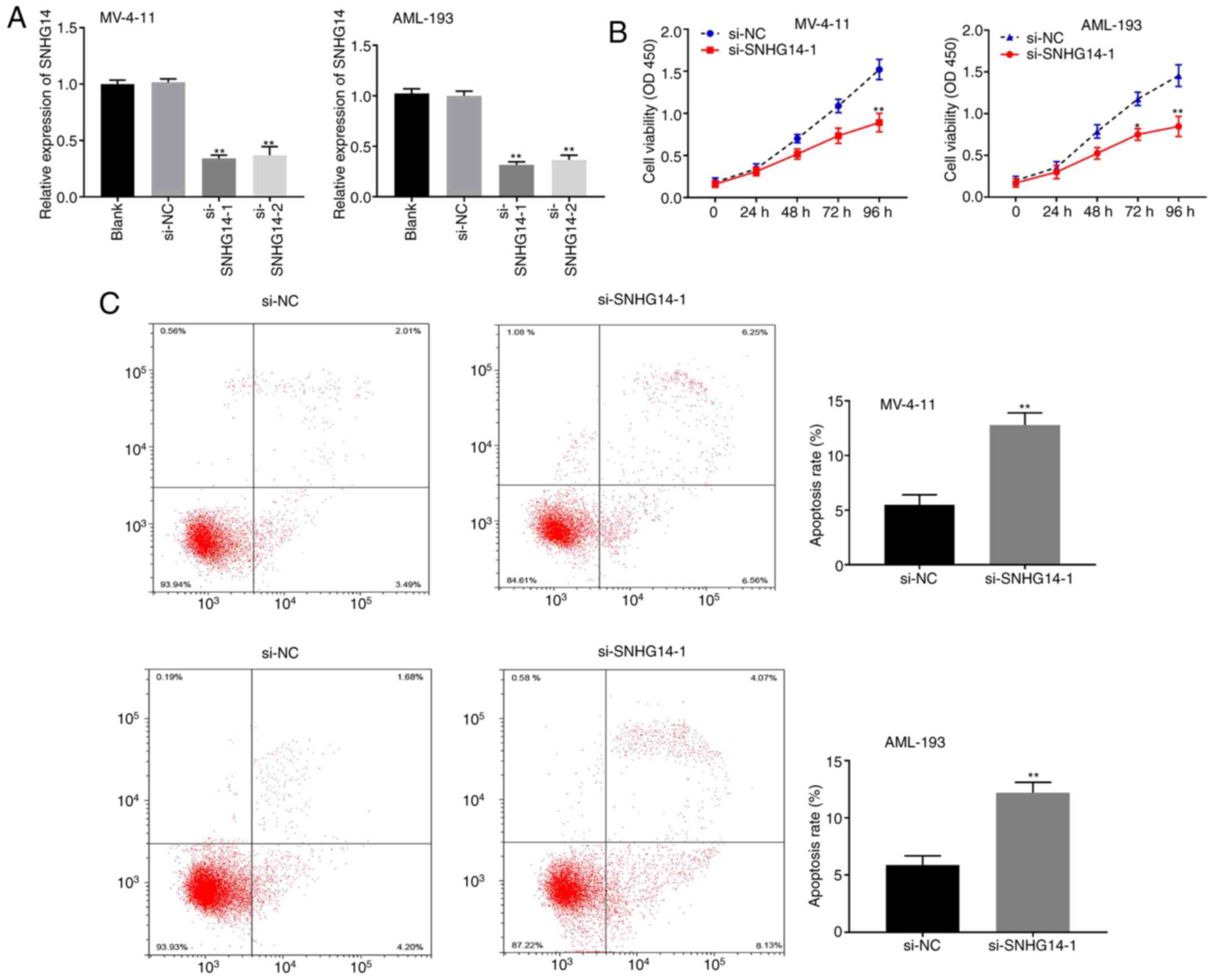
Silencing SNHG14 inhibits the
proliferation and facilitates the apoptosis of AML cells
To explore the effect of SNHG14 on AML progression,
SNHG14 gene expression was knocked down by transfection of MV-4-11
and AML-193 cells with SNHG14 siRNA. RT-qPCR analysis revealed that
SNHG14 expression was significantly reduced in the si-SNHG14-1 and
si-SNHG14-2 groups compared with that in the blank control group
(P<0.01; Fig. 2A). si-SNHG14-1
was utilised in the subsequent tests as it resulted in markedly
lower SNHG14 expression compared with si-SNHG14-2. Cell viability
was significantly lower in the si-SNHG14-1 group compared with that
in the si-NC group for both cell lines (both P<0.05; Fig. 2B). Conversely, the apoptosis rate
was significantly higher in the si-SNHG14-1 group compared with the
si-NC group (P<0.01; Fig.
2C).
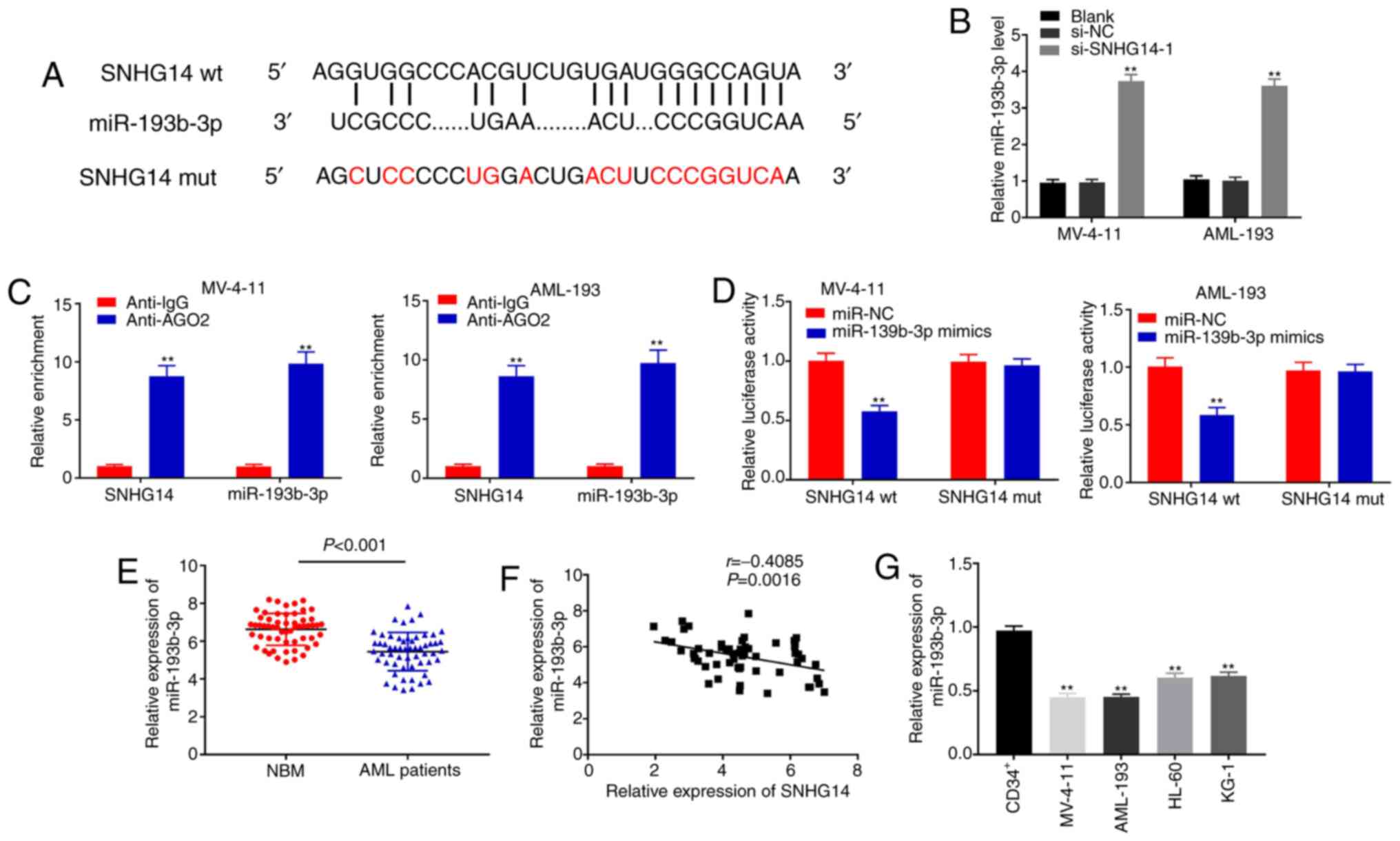
SNHG14 acts as a sponge for
miR-193b-3p in AML cells
The starBase online database was used to predict the
potential binding site between miR-193-3p and SNHG14 (Fig. 3A). As illustrated in Fig. 3B, SNHG14 knockdown significantly
elevated miR-193-3p expression in MV-4-11 and AML-193 cells
compared with the si-NC and blank control groups (all P<0.01).
The results of the RIP assay indicated that the expression of
SNHG14 and miR-193-3p were significantly enriched in the AGO2
immunoprecipitate compared with that in the IgG immunoprecipitate
in both cell lines (all P<0.01; Fig.
3C). The DLR assay revealed that miR-193-3p mimics
significantly decreased the luciferase activity of SNHG14 wt but
did not affect that of SNHG14 mut in MV-4-11 and AML-193 cells
(P<0.01; Fig. 3D). As shown in
Fig. 3E, miR-193-3p expression was
markedly decreased in bone marrow tissues from patients with AML
compared to that in the NBM group (P<0.01). Additionally, a
negative correlation was observed between the expression of
miR-193-3p and SNHG14 (r=−0.4085; P=0.0016; Fig. 3F). miR-193-3p expression was
significantly decreased in AML cell lines compared with that in
human NBM CD34+ cells (P<0.01; Fig. 3G). Together, these results indicate
that SNHG14 functions as a sponge for miR-193b-3p in AML cells.
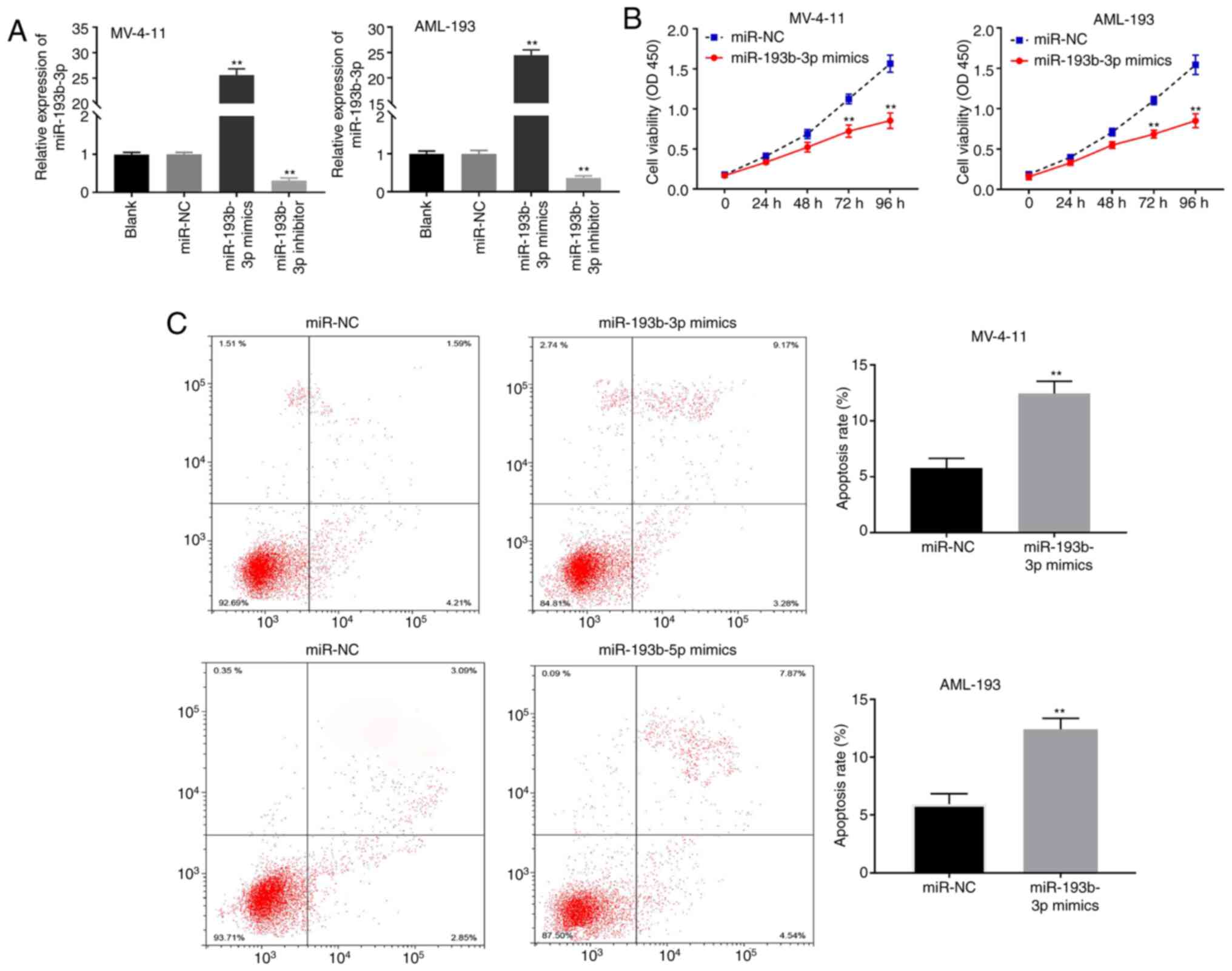
miR-193b-3p suppresses the viability
and induces the apoptosis of AML cells
As indicated in Fig.
4A, miR-193b-3p expression was markedly enhanced in the
miR-193b-3p mimics group but was markedly decreased in the
miR-193b-3p inhibitor group compared with that in the blank control
group in both cell lines (both P<0.01). The MTT analysis
revealed that miR-193b-3p mimics markedly reduced the viability and
promoted the apoptosis of MV-4-11 and AML-193 cells compared with
that in the miR-NC group (both P<0.01; Fig. 4B and C).
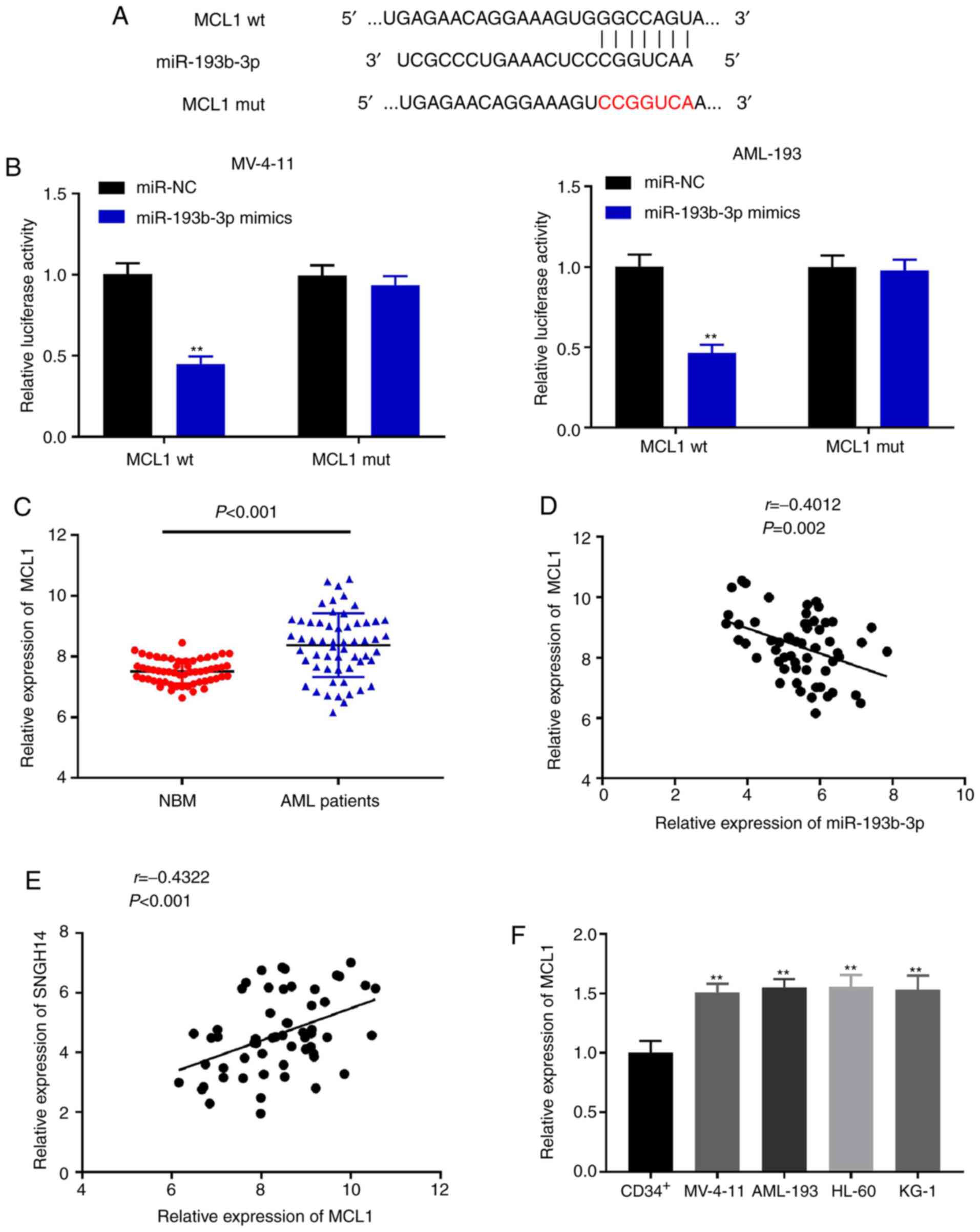
miR-193b-3p targets MCL1
The starBase online database was used to first
predict the possible binding site between miR-193b-3p and MCL1
(Fig. 5A). As shown in Fig. 5B, the DLR assay revealed that
miR-193b-3p mimics significantly reduced the luciferase activity of
MCL1 wt (P<0.05), whereas there was no notable change in the
MCL1 mut in both cell lines. The expression of MCL1 was markedly
elevated in bone marrow tissues from patients with AML compared
with that in the NBM group (P<0.001; Fig. 5C). As shown in Fig. 5D and E, miR-193b-3p expression was
negatively correlated with MCL1 (r=−0.4012; P=0.002), whereas
SNHG14 expression was positively correlated with MCL1 (r=0.4322;
P<0.001). Finally, the relative expression of MCL1 was
significantly higher in all AML cell lines compared with that in
CD34+ cells (P<0.01; Fig.
5F). Together, these results indicate that miR-193b-3p targets
MCL1 and that MCL1 is highly expressed in AML.
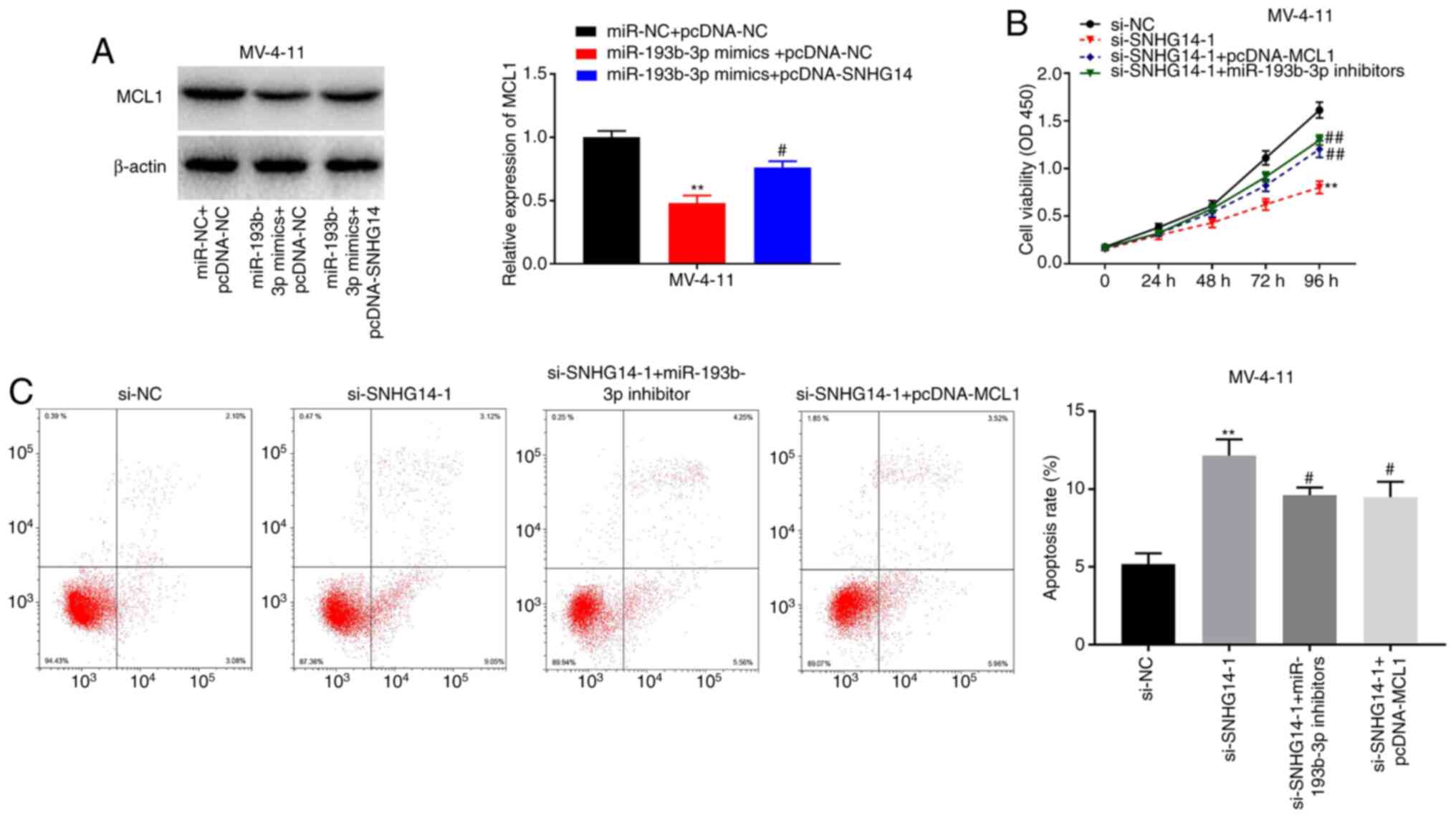
Silencing SNHG14 inhibits the
viability and induces the apoptosis of AML cells by modulating the
miR-193b-3p/MCL1 axis
The western blotting results indicated that the
protein expression of MCL1 was notably decreased in the miR-193b-3p
mimics + pcDNA-NC group compared with that in the miR-NC + PCDNA-NC
group, and the decrease in MCL1 expression caused by miR-193b-3p
was partially rescued by the overexpression of SNHG14 (P<0.05;
Fig. 6A). To further investigate
the underlying mechanism involved in the interaction among SNHG14,
miR-193b-3p, and MCL1 in AML progression, MV-4-11 cells were
co-transfected with si-SNHG14-1 and pcDNA-MCL1 or miR-193b-3p
inhibitors. As shown in Fig 6B,
MV-4-11 cell viability was significantly decreased in the
si-SNHG14-1 group, and the decrease in cell viability induced by
SNHG14 knockdown was recovered by transfection with pcDNA-MCL1 or
miR-193b-3p inhibitors (P<0.05). Conversely, MV-4-11 cell
apoptosis was markedly enhanced in the si-SNHG14-1 group, and
pcDNA-MCL1 or miR-193b-3p inhibitors partially eliminated the
increase in cell apoptosis caused by SNHG14 knockdown (P<0.05;
Fig. 6C). In summary, these results
indicate that SNHG14 may act as ceRNA to modulate AML cell
viability and apoptosis via the miR-193b-3p/MCL axis.
Discussion
Numerous lines of evidence indicate that lncRNAs are
involved in the biological and pathological processes of AML. For
example, UCA1 and LINC00662 are overexpressed in AML tissues, and
their knockdown has been shown to decrease cell proliferation and
promote cell apoptosis in AML (26,27).
In the present study, SNHG14 was found to be overexpressed in bone
marrow tissues from patients with AML and AML cell lines,
suggesting that SNHG14 may be associated with the development of
AML. Previous studies have shown that SNHG14 functioned as an
oncogenic gene in the progression of various human cancer types,
including gastric cancer (13),
colorectal cancer (28), cervical
cancer (29) and hepatocellular
carcinoma (30). Notably, SNHG14
silencing decreased the proliferation and facilitated the apoptosis
of AML cells in the present study. These findings demonstrate that
SNHG14 may function as an oncogenic gene in AML progression, which
is consistent with previous data on the role of SNHG14 in
tumours.
Numerous studies have shown that SNHG14 is involved
in various types of human cancers via acting as a competitive ceRNA
for different miRNAs. For example, SNHG14 accelerates the
progression of cervical cancer by sponging miR-206 to regulate the
expression of YWHAZ (29). SNHG14
facilitates the development of gastric cancer by targeting miR-145
to modulate SOX9 expression (13).
The results of the present study demonstrated that SNHG14 acted as
a sponge for miR-193b-3p and that the expression of miR-193b-3p in
AML cells was negatively modulated by SNHG14, further clarifying
the mechanism by which SNHG14 affects the progression of AML.
miR-193b-3p has been reported to be underexpressed in tumour
tissues and identified as an antitumour gene in human malignancies
(17,18,31).
Notably, Bhayadia et al (20) found that miR-193b-3p was
underexpressed in patients with AML. Furthermore, Mets et al
(32) revealed that miR-193b-3p
acts as a tumour suppressor in T-cell AML. Consistent with previous
studies, a significant decrease in miR-193-3p expression in
patients with AML was observed in the present study. Additionally,
these results demonstrated that miR-193b-3p inhibited the viability
and induced the apoptosis of AML cells, suggesting that miR-193b-3p
might function as an antitumour gene in AML. Xie et al
(23) reported that SNHG14 promotes
the proliferation and invasion of breast cancer cells by sponging
miR-193a-3p. Similarly, the current findings indicate that SNHG14
might exert its oncogenic function in AML progression via sponging
miR-193b-3p.
In the present study, starBase and DLR assays were
performed and demonstrated that MCL1 was a direct target of
miR-193b-3p. MCL1 has been reported to be overexpressed in numerous
human cancer types, including malignant melanoma (33) liver cancer (34) and ovarian adenocarcinoma (35). In this study, MCL1 was highly
expressed in AML bone marrow tissues and cells. Previous studies
have shown that several miRNAs are involved in the progression of
human cancer by targeting MCL1. For example, miR-107 impedes the
progression of cervical cancer by directly targeting MCL1 (36). miR-125b impedes cell proliferation
and invasion by negatively regulating MCL1 expression in gastric
cancer (37). Notably, Huang et
al (38) demonstrated that
LINC00152 accelerated the cell proliferation rate in gastric cancer
by modulating miR-193a-3p and its target gene MCL1. In the present
study, the data indicated that miR-193b-3p targets MCL1, and the
antitumour effect of SNHG14 knockdown on AML cells was rescued by
overexpressing MCL1 or inhibiting miR-193b-3p, suggesting that
SNHG14 acts as a ceRNA, modulating AML progression via the
miR-193b-3p/MCL axis.
In conclusion, SNHG14 was found to be overexpressed
in bone marrow tissues from patients with AML and AML cell lines.
Furthermore, the silencing of SNHG14 decreased the proliferation
and facilitated the apoptosis of AML cells. Additionally,
miR-193b-3p was identified as a target of SNHG14, and suppressed
the viability and increased the apoptosis rate of AML cells. More
importantly, miR-193b-3p targeted MCL1, and the effects of SNHG14
knockdown on AML cell viability and apoptosis were abrogated by
miR-193b-3p inhibition or MCL1 overexpression. In summary, the
results of the current study indicate that silencing SNHG14
decreased the viability and induced the apoptosis of AML cells by
modulating the miR-193b-3p/MCL1 axis, which suggests that
SNHG14/miR-193b-3p/MCL1 may be a promising target for AML
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
XW and WL made substantial contributions to
conception and design, acquisition of data, and analysis and
interpretation of data. YC and LZ took part in drafting the article
and revising it critically for important intellectual content, they
are responsible for the experimental operation and the analysis of
the experimental data, software application processing, the whole
project management. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the East Hospital of Shouguang People's Hospital in accordance with
the Declaration of Helsinki. Informed consents were obtained from
legal guardians of children <18 years old.
Patient consent for publication
Informed consent was obtained for the release of
patient data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukaemia
|
|
RIP
|
RNA-binding protein
immunoprecipitation
|
|
DLR
|
dual luciferase reporter gene
|
|
PVDF
|
polyvinylidene fluoride
|
|
lncRNAs
|
long non-coding RNAs
|
|
SNHG14
|
small nucleolar RNA host gene 14
|
|
miRNAs
|
microRNAs
|
|
ceRNAs
|
competitive endogenous RNA
|
|
NBM
|
normal marrow tissues
|
References
|
1
|
Saletta F, Wadham C, Ziegler DS, Marshall
GM, Haber M, McCowage G, Norris MD and Byrne JA: Molecular
profiling of childhood cancer: Biomarkers and novel therapies. BBA
Clin. 1:59–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kadia TM, Ravandi F, Cortes J and
Kantarjian H: New drugs in acute myeloid leukemia. Ann Oncol.
27:770–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burnett A, Wetzler M and Löwenberg B:
Therapeutic advances in acute myeloid leukemia. J Clin Oncol.
29:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gugnoni M and Ciarrocchi A: Long noncoding
RNA and epithelial mesenchymal transition in cancer. Int J Mol Sci.
20:19242019. View Article : Google Scholar
|
|
7
|
Cruz-Miranda GM, Hidalgo-Miranda A,
Bárcenas-López DA, Núñez-Enríquez JC, Ramírez-Bello J,
Mejía-Aranguré JM and Jiménez-Morales S: Long non-coding RNA and
acute leukemia. Int J Mol Sci. 20:7352019. View Article : Google Scholar
|
|
8
|
Wang G, Li X, Song L, Pan H, Jiang J and
Sun L: Long noncoding RNA MIAT promotes the progression of acute
myeloid leukemia by negatively regulating miR-495. Leuk Res.
87:1062652019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X and Tao W: Long noncoding RNA
LINC00152 facilitates the leukemogenesis of acute myeloid leukemia
by promoting CDK9 through miR-193a. DNA Cell Biol. 38:236–242.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D, Zeng T, Lin Z, Wang F, Tang L,
Wang L, Tang D, Chen P and Yang M: Long non-coding RNA SNHG5
regulates chemotherapy resistance through the miR-32/DNAJB9 axis in
acute myeloid leukemia. Biomed Pharmacother. 123:1098022020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu K, Li J, Qi Y, Zhang C, Zhu D, Liu D
and Zhao S: SNHG14 confers gefitinib resistance in non-small cell
lung cancer by up-regulating ABCB1 via sponging miR-206-3p. Biomed
Pharmacother. 116:1089952019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang YY, Li M, Xu YD and Shang J: LncRNA
SNHG14 promotes the development of cervical cancer and predicts
poor prognosis. Eur Rev Med Pharmacol Sci. 23:3664–3671.
2019.PubMed/NCBI
|
|
13
|
Liu Z, Yan Y, Cao S and Chen Y: Long
non-coding RNA SNHG14 contributes to gastric cancer development
through targeting miR-145/SOX9 axis. J Cell Biochem. 119:6905–6913.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Teng Y, Wang R, Deng D, You Y,
Peng Y, Shao N and Zhi F: The long non-coding RNA SNHG14 inhibits
cell proliferation and invasion and promotes apoptosis by sponging
miR-92a-3p in glioma. Oncotarget. 9:12112–12124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wallace JA and O'Connell RM: MicroRNAs and
acute myeloid leukemia: Therapeutic implications and emerging
concepts. Blood. 130:1290–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Qin J and Su Y: miR-193b-3p
possesses anti-tumor activity in ovarian carcinoma cells by
targeting p21-activated kinase 3. Biomed Pharmacother.
96:1275–1282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Du J, Song DF, Ren JC and Feng Y:
Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and
miR-1179 may contribute to the progression of gastric cancer. Biol
Res. 51:442018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Zhuang Q, Hu G and Geng S: MORC4
is a novel breast cancer oncogene regulated by miR-193b-3p. J Cell
Biochem. 120:4634–4643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhayadia R, Krowiorz K, Haetscher N,
Jammal R, Emmrich S, Obulkasim A, Fiedler J, Schwarzer A, Rouhi A,
Heuser M, et al: Endogenous tumor suppressor microRNA-193b:
Therapeutic and prognostic value in acute myeloid leukemia. J Clin
Oncol. 36:1007–1016. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Chen W, Yang P, Zhou J, Wang K and
Tao Q: Knockdown of linc00152 inhibits the progression of gastric
cancer by regulating microRNA-193b-3p/ETS1 axis. Cancer Biol Ther.
20:461–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie SD, Qin C, Jin LD, Wang QC, Shen J,
Zhou JC, Chen YX, Huang AH, Zhao WH and Wang LB: Long noncoding RNA
SNHG14 promotes breast cancer cell proliferation and invasion via
sponging miR-193a-3p. Eur Rev Med Pharmacol Sci. 23:2461–2468.
2019.PubMed/NCBI
|
|
24
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO Classification of Tumours of
HaematoPoietic and Lymphoid Tissues, 4th edition. WHO Press;
Geneva: 2008
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Y, Li E, Zhang H, Zhang L, Tang Y
and Wanyan Y: Silencing of lncRNA UCA1 curbs proliferation and
accelerates apoptosis by repressing SIRT1 signals by targeting
miR-204 in pediatric AML. J Biochem Mol Toxicol. 34:e224352020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Gao X and Tian X: High expression
of long intergenic non-coding RNA LINC00662 contributes to
malignant growth of acute myeloid leukemia cells by upregulating
ROCK1 via sponging microRNA-340-5p. Eur J Pharmacol.
859:1725352019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di W, Weinan X, Xin L, Zhiwei Y, Xinyue G,
Jinxue T and Mingqi L: Long noncoding RNA SNHG14 facilitates
colorectal cancer metastasis through targeting EZH2-regulated
EPHA7. Cell Death Dis. 10:5142019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji N, Wang Y, Bao G, Yan J and Ji S:
LncRNA SNHG14 promotes the progression of cervical cancer by
regulating miR-206/YWHAZ. Pathol Res Pract. 215:668–675. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pu J, Wei H, Tan C, Qin B, Zhang Y, Wang A
and Wang J: Long noncoding RNA SNHG14 facilitates hepatocellular
carcinoma progression through regulating miR-4673/SOCS1. Am J
Transl Res. 11:5897–5904. 2019.PubMed/NCBI
|
|
31
|
Choi KH, Shin CH, Lee WJ, Ji H and Kim HH:
Dual-strand tumor suppressor miR-193b-3p and −5p inhibit malignant
phenotypes of lung cancer by suppressing their common targets.
Biosci Rep. 39:BSR201906342019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mets E, Van der Meulen J, Van Peer G,
Boice M, Mestdagh P, Van de Walle I, Lammens T, Goossens S, De
Moerloose B, Benoit Y, et al: MicroRNA-193b-3p acts as a tumor
suppressor by targeting the MYB oncogene in T-cell acute
lymphoblastic leukemia. Leukemia. 29:798–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Zhang X, Lentz C, Abi-Daoud M,
Paré GC, Yang X, Feilotter HE and Tron VA: miR-193b regulates Mcl-1
in melanoma. Am J Pathol. 179:2162–2168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin W, Nie Y, Chen L, Wang Q, Liu S, He X
and Wang W: Deregulation of microRNA-193b affects the proliferation
of liver cancer via myeloid cell leukemia-1. Oncol Lett.
15:2781–2788. 2018.PubMed/NCBI
|
|
35
|
Li C, Song Y and Li P: MCL1 regulates cell
death, tumor growth and chemosensitivity to sabutoclax in ovarian
adenocarcinoma. Cell Tissue Res. 379:625–633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou C, Li G, Zhou J, Han N, Liu Z and Yin
J: miR-107 activates ATR/Chk1 pathway and suppress cervical cancer
invasion by targeting MCL1. PLoS One. 9:e1118602014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu S, Liu F, Xie L, Peng Y, Lv X, Zhu Y,
Zhang Z and He X: miR-125b Suppresses proliferation and invasion by
targeting MCL1 in gastric cancer. Biomed Res Int. 2015:3652732015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang Y, Luo H, Li F, Yang Y, Ou G, Ye X
and Li N: LINC00152 down-regulated miR-193a-3p to enhance MCL1
expression and promote gastric cancer cells proliferation. Biosci
Rep. 38:BSR201716072018. View Article : Google Scholar : PubMed/NCBI
|