Introduction
Hilar cholangiocarcinomas (HCs) are the most common
type of cholangiocarcinoma and are highly prevalent in Southeast
Asia, although uncommon in the USA (1). The only strategy for treating patients
and prolonging overall survival (OS) is complete resection with
negative surgical margins (2).
However, in most patients, the tumors are unresectable due to
locally advanced or metastatic disease at diagnosis (3). Recently, advances in revealing the
genetic landscape of HC have led to the identification of several
promising systemic therapeutic agents or strategies that could
improve the outcome of patients with HC (4).
Forkhead box K1 (FOXK1) is a member of the FOX
transcription factor family, which plays critical roles in
embryonic development and organogenesis, and regulates a variety of
physiological processes, including metabolism, cell signaling and
cell proliferation (5–7). The dysregulation of FOXK1 expression
and subcellular localization leads to the uncontrolled development
and progression of human solid cancer types. For instance,
increasing evidence has demonstrated that FOXK1 knockdown can
inhibit cell proliferation, migration and invasion in prostate
cancer (8), hepatocellular
carcinoma (9) and esophageal cancer
(10), whereas its enhanced
expression facilitates cell proliferation and metastasis in ovarian
(11) and esophageal cancer
(10). These studies suggest that
FOXK1 has a role in tumorigenesis. However, its expression pattern
and function in HC are not well defined.
The present study investigated the clinical
significance and biological functions of FOXK1 in HC. The results
demonstrated that FOXK1 was upregulated in HC tissues and that its
high expression was significantly associated with neural invasion
and lymph node (LN) metastasis. In addition, the nuclear expression
of FOXK1 was found to be associated with recurrence and poor
outcome in patients with HC. FOXK1 knockdown in vitro
inhibited the proliferation and migration of HC cells. To the best
of the authors knowledge, the present study was the first to reveal
the expression profile of FOXK1 and its function in HC cell
proliferation and metastasis, highlighting its potential as a
therapeutic target for this disease.
Materials and methods
Patient samples and cell culture
Tissue microarrays (TMAs) of 48 resected HC
specimens and 15 matched non-cancerous bile duct tissues (with
>5-mm distance from the primary tumors edge) obtained from the
Eastern Hepatobiliary Hospital and Changhai Hospital (Shanghai,
China) were constructed as previously described (12). Of the patients, 31 were men and 17
women, with a mean age of 62 years (range, 42–78 years). Patient
characteristics are given in Table
I. The median follow-up duration was 16 months (range, 1–59
months). The tissue sample experiments were approved by the Ethics
Committee of The Affiliated Hospital of Qingdao University.
Informed consent written from all participants (or their parent or
legal guardian in the case of children under 16) was obtained to
participate in the study or to use their tissues. Human
cholangiocarcinoma cell lines (FRH-0201, QBC939 and RBE) were
obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and. The expression of FOXK1 in the
four cell lines were detected by western blotting, and it was
identified that RBE cells exhibited the highest expression, so the
RBE cell line was selected as the experimental cells. These cells
were maintained in Dulbeccos modified Eagles medium (Invitrogen;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and cultured at 37°C
with 5% CO2 (12).
 | Table I.Association between the expression of
FOXK1 and clinicopathological parameters of hilar
cholangiocarcinoma. |
Table I.
Association between the expression of
FOXK1 and clinicopathological parameters of hilar
cholangiocarcinoma.
| Clinicopathological
parameters | N | FOXK1, n (%) | P-value |
|---|
| Tumor size, cm |
|
| 0.135 |
| ≤3 | 17 | 8 (47.1) |
|
| 3 | 31 | 23 (74.2) |
|
| Nerve invasion |
|
| 0.838 |
| Yes | 25 | 17 (68.0) |
|
| No | 23 | 15 (65.2) |
|
| T stage |
|
| 0.005 |
| T1-3 | 6 | 1 (16.7) |
|
| T4 | 42 | 31 (73.8) |
|
| N stage |
|
| 0.008 |
| N0 | 15 | 6 (40.0) |
|
|
N1-2 | 33 | 26 (78.8) |
|
|
Differentiation |
|
| 0.480 |
|
High/moderate | 36 | 23 (63.9) |
|
|
Low/undifferentiated | 12 | 9 (75.0) |
|
| TNM |
|
| 0.095 |
|
I/II | 19 | 10 (52.6) |
|
|
III/IV | 29 | 22 (75.9) |
|
Immunohistochemistry and evaluation of
HC specimens
Sections (4 µm) of TMAs were prepared and processed
for the immunohistochemical analysis of FOXK1 (1:100; cat. no.
ab18196; Abcam), which was carried out according to a previous
study (13). A streptavidin-biotin
kit (cat. no. KIT-9720; Fuzhou Maixin Biotech Co., Ltd.) was used
to visualize antibody binding in these sections. Immunostaining of
FOXK1 was evaluated by two individuals (ZGB and YJF) and a
semi-quantitative scoring system was used in the present study, as
previously reported (12). A
weighted score was generated for each case ranging from 0 (0% of
cells stained) to 300 (100% of cells stained at >3 intensity) as
previous study described, a score of <75 was defined as low
expression and that of ≥75 was defined as high expression (12).
Cell transfection
FOXK1 short hairpin (sh)RNA (cat. no. PR6021) and
scrambled shRNA plasmids (pLent-U6-GFP-Puro) were constructed by
Shanghai GeneChem Co., Ltd. Next, 1×105 RBE cells were
seeded in 6-well plates and transfected with FOXK1 shRNA (shFOXK1)
or scrambled shRNA (shNC) at 5 ng shRNA plasmid per well using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturers instructions.
These cells were then subcultured and selected in the presence of
puromycin (1 µg/ml) for 3 days between transfection and
experimentation at 37°C to generate stable NC and FOXK1-knockdown
cells.
Cell Counting Kit-8 (CCK-8)
analysis
After obtaining stable FOXK1-knockdown or NC RBE
lines, 5,000 cells per well were seeded in 96-well plates. At the
indicated times (24, 48 and 72 h), the CCK-8 assay (Dojindo
Molecular Technologies, Inc.) was performed to assess the results.
This experiment was performed in triplicate.
Cell viability assay
FOXK1-knockdown and NC RBE cells were seeded in
96-well plates and treated with different concentrations (0, 0.25,
0.5, 1, 2.5, 5, 10, 25, 50 and 100 µg/ml) of 5-FU and cisplatin
(DDP) when the cell density reached 60–70%. Cell viability was
assayed using an MTT assay (Dojindo Molecular Technologies, Inc.)
according to the manufacturers instructions.
Transwell invasion assay
Transwell chambers (8 µm pore size; Corning, Inc.)
were used to assess the effect of FOXK1 knockdown on cell invasion.
After stable transfection with shFOXK1 or shNC, 10,000 cells were
resuspended in serum-free DMEM and placed in the upper well of the
chamber coated with 25 µg Matrigel (BD Biosciences) for the
invasion assay. The lower well was filled with DMEM containing 10%
FBS. The chambers were maintained at 37°C for 24 h, and then
removed. The cells on the upper surface of the chambers were
removed using a cotton swab, while the cells on the lower surface
were stained with 0.1% crystal violet for 30 min at room
temperature and counted in five representative (magnification,
×200) fields per insert under an Inversion Microscope (Zeiss AG) by
two individuals (YJF and ZGB), who were blinded to the study.
Wound healing assay
The indicated cells were cultured in 6-well plates
in monolayers and were pre-incubated with Mitomycin-C (10 µg/ml)
for 1 h at 37°C to suppress cell proliferation. Then, cells were
plated in serum-starved medium. A sterile 200-µl pipette tip was
used to create wounds and the areas of the wound fields were
observed and images (magnification, ×200) were taken at 0, 24, 48
and 72 h following wound creation using an inverted microscope
(Carl Zeiss AG).
Western blotting
Total protein was extracted from the cells. Western
blotting was performed as previously described (14). All antibodies used are as follows:
Anti-FOXK1 (1:1,000; cat. no. ab18196; Abcam), anti-matrix
metallopeptidase (MMP)-9 (1:500; cat. no. ab228402; Abcam),
anti-Twist (1:1,000; cat. no. ab50581; Abcam), anti-E-cadherin
(1:1,000; cat. no. 3195; Cell Signaling Technology, Inc.),
anti-vimentin (1:1,000; cat. no. 5741; Cell Signaling Technology,
Inc.), anti-glutathione S-transferase (GST)-π (1:500; cat. no.
66001-2-Ig; ProteinTech Group, Inc.), anti-MDR-1 (1:200; cat. no.
sc13131; anti-Santa Cruz Biotechnology, Inc.), anti-P-glycoprotein
(P-gp; 1:500; cat. no. ab170904; Abcam), and anti-GAPDH (1:1,000;
cat. no. 5174; Cell Signaling Technology, Inc.). The signals were
detected using horseradish peroxidase-based chemiluminescence
analysis.
Statistical analyses
Statistical analyses were carried out using SPSS
statistical software (version 16.0; SPSS, Inc.) and Prism software
(version 5.0; GraphPad Software, Inc.). Data are presented as the
mean ± standard error of the mean. The differences between two
groups were evaluated with a Students t-test. Categorical data were
analyzed using χ2 tests. The Kaplan-Meier log-rank
method was used to estimate survival rates, and the Cox
proportional hazards model for multivariate survival analysis was
used to assess predictors related to recurrence or survival.
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression of FOXK1 in patients
with HC
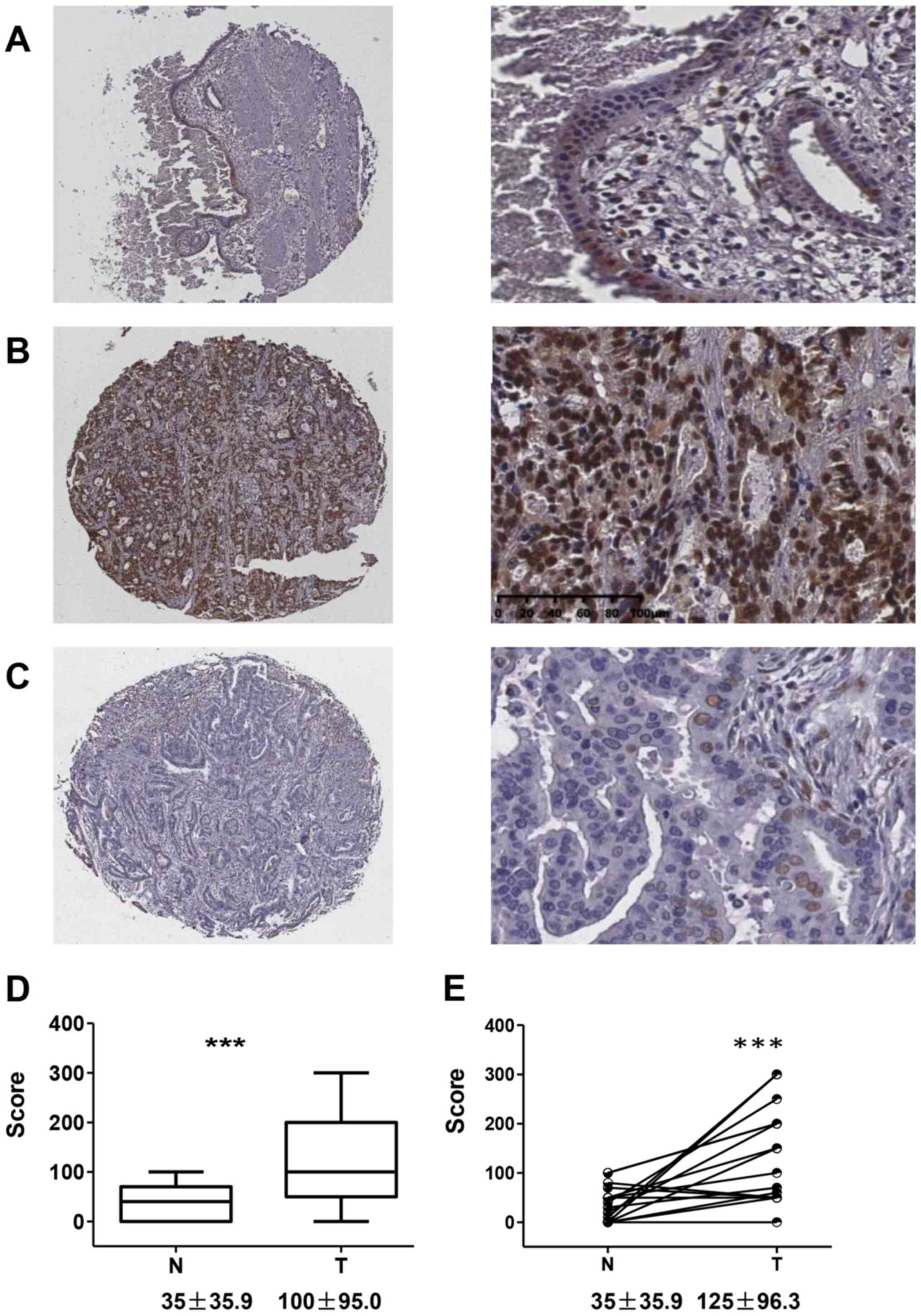
Immunostaining revealed that FOXK1 was primarily
localized to the nucleus of the normal bile duct epithelium and
cancer cells (Fig. 1). The
epithelium in the adjacent non-cancerous tissues showed low
expression of FOXK1, with an average score of 35±35.9 (Fig. 1A and D). Furthermore, 32 cases
(66.7%) showed high expression (Fig.
1B) and the other 16 cases presented low expression (Fig. 1C). The average score for FOXK1 in HC
was 100±95.0, which was significantly higher than that in the
non-cancerous bile duct epithelium (P=0.0003). Similarly, in the
matched cases with both HC and adjacent non-cancerous epithelium,
the score was 125±96.3 and 35±35.9, respectively (P=0.0006;
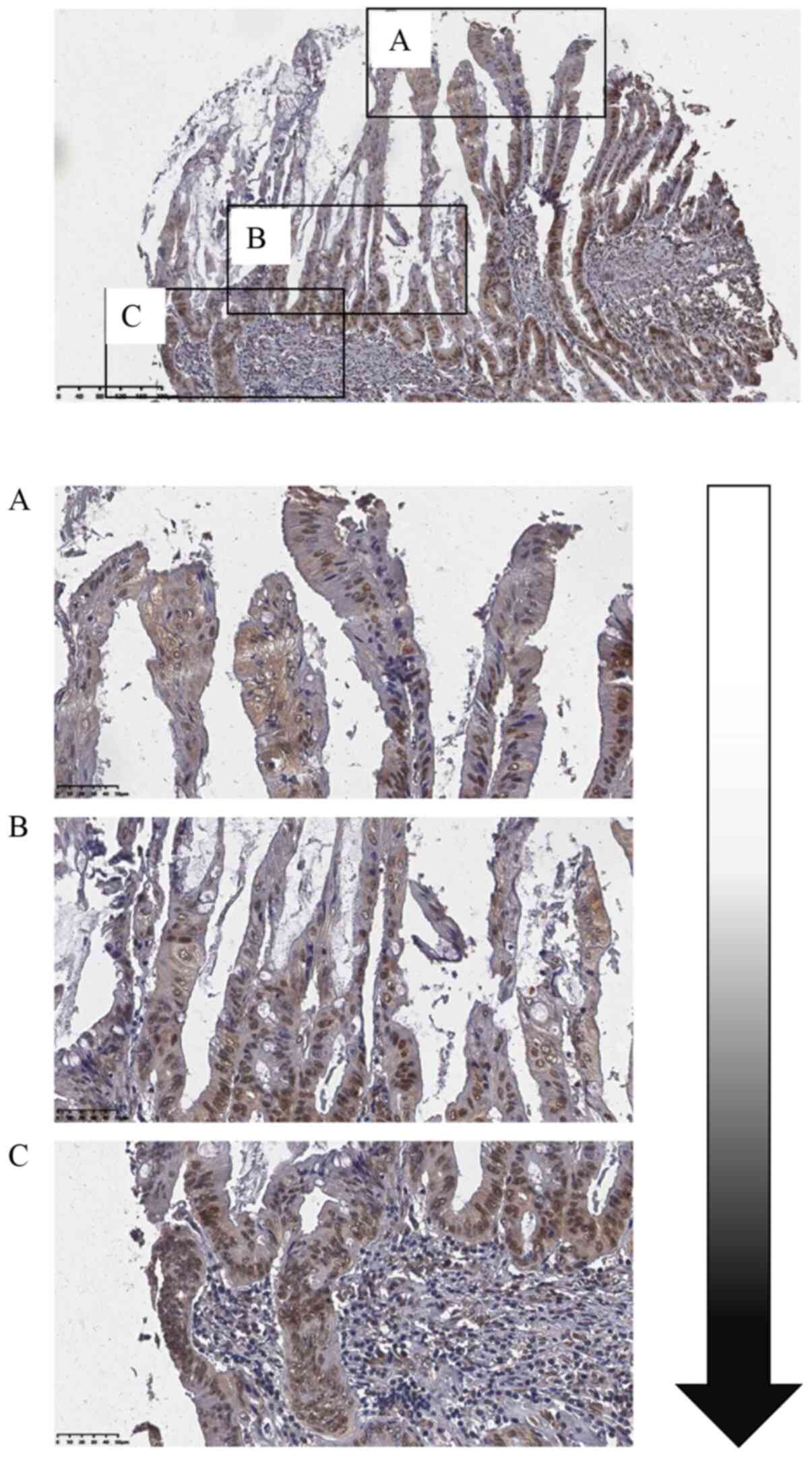
Fig. 1E). In one intracholangial
papillary tumor (Fig. 2), ~50% of
the cells on the surface showed FOXK1 expression (Fig. 2A), whereas the tumor cells at the
front edge of the invasive front showed 100% FOXK1 expression
(Fig. 2B and C), indicating that
FOXK1 might be involved in the progression of HC.
Association between high FOXK1
expression and clinicopathological variables of HC
Table I summarizes
the association between high FOXK1 expression and
clinicopathological variables of HC. A statistically significant
association was observed between high FOXK1 expression and tumor
invasion and regional LN metastasis. Furthermore, high FOXK1
expression occurred more frequently in highly invasive tumors
(invasion level T4, 73.8%) than in less invasive tumors (levels
T1-T3, 16.7%; P=0.005). With regard to the N stage, FOXK1 was
highly expressed in HCs with regional LN metastasis (78.8%)
compared with that in HCs without LN metastasis (40%) (P=0.008).
This indicated that the expression of FOXK1 is associated with the
prognosis of HC.
Relationship between high FOXK1
expression and HC tumor recurrence or outcome
For this assessment, the cohort consisted of 34 male
(70.8%) and 14 female (29.2%) patients with a median age of 55
years (range, 31–79 years). The median disease-free survival (DFS)
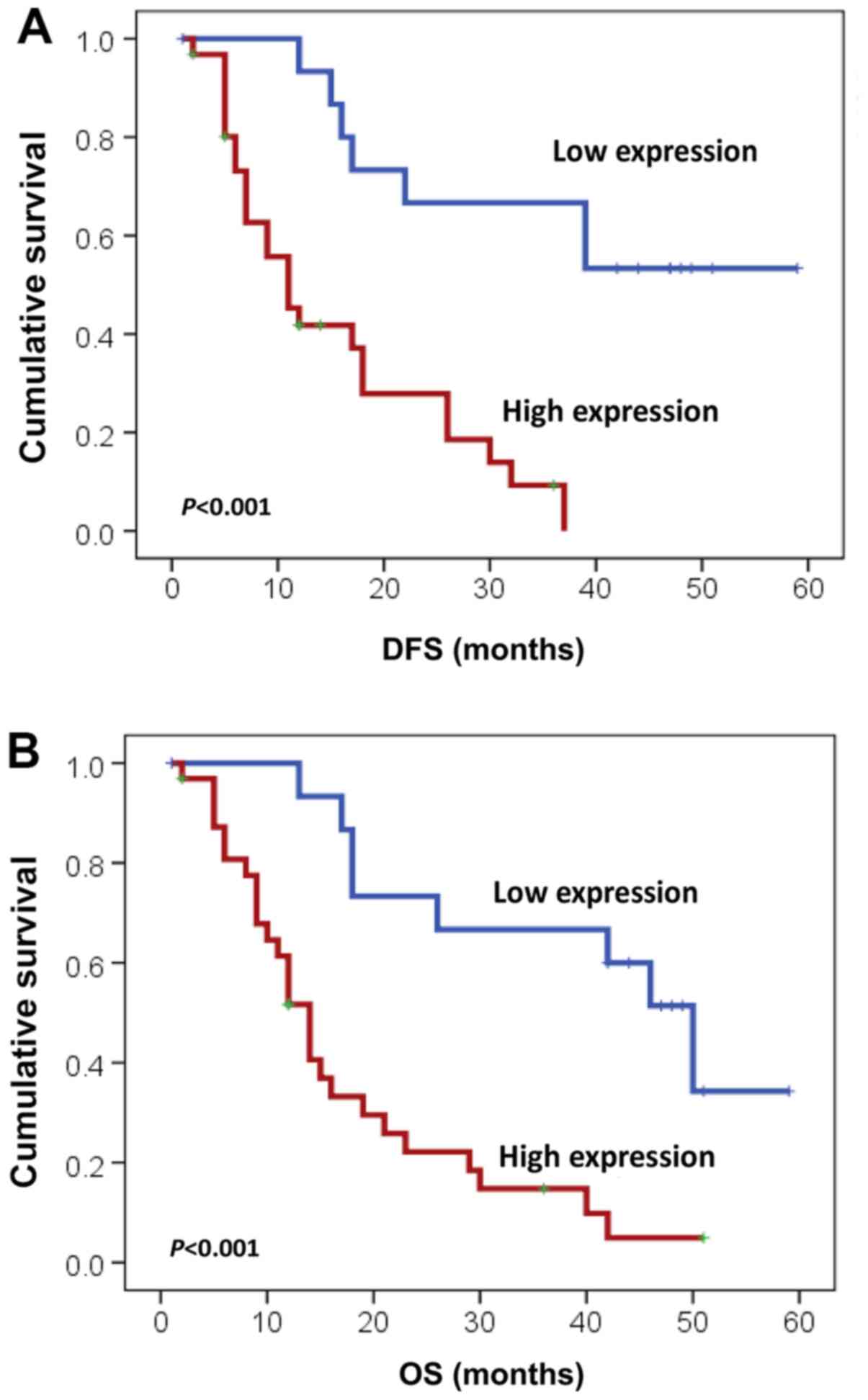
in patients with resected HC was 14 months. Patients with tumors
exhibiting high FOXK1 expression had a significantly shorter DFS
than that in patients with low FOXK1 expression (11 vs. 42 months;
P<0.001; Fig. 3A). Additionally,
it was noted that tumor invasion (P=0.006) and regional LN
metastasis (P=0.05) significantly affected DFS based on the
univariate analysis. In contrast to the univariate analysis, the
multivariate analysis using the Cox proportional hazards model
showed that high FOXK1 expression was an independent predictor of
tumor recurrence (P=0.014; Table
II).
 | Table II.Univariate and multivariate analysis
of variables associated with disease-free survival in patients with
hilar cholangiocarcinoma. |
Table II.
Univariate and multivariate analysis
of variables associated with disease-free survival in patients with
hilar cholangiocarcinoma.
|
|
|
| P-value |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | N | DFS, months | Univariate
analysis | Multivariate
analysis | HR | 95% CI |
|---|
| Tumor invasion |
|
| 0.006 | 0.065 | 0.146 | 0.019–1.125 |
|
T1-T3 | 6 | 51.2 |
|
|
|
|
| T4 | 42 | 16.0 |
|
|
|
|
| Regional LN
positive |
|
| 0.05 | 0.423 | 0.690 | 0.279–1.710 |
| No | 15 | 37.0 |
|
|
|
|
|
Yes | 33 | 15.0 |
|
|
|
|
| FOXK1 |
|
| <0.001 | 0.014 | 0.253 | 0.084–0.759 |
|
Low | 16 | 42 |
|
|
|
|
|
High | 32 | 11 |
|
|
|
|
With respect to OS, the median for patients with
resected HC was 16 months. However, patients with high FOXK1
expression in tumors had a significantly worse OS than those with
low FOXK1 expression (14 months vs. 50 months; P<0.001; Fig. 3B). Additionally, tumor invasion
(P=0.014) and regional LN metastasis (P=0.033) also significantly
influenced OS based on the univariate analysis (Table III). The multivariate analysis
also showed that high FOXK1 expression was an independent
prognostic factor (P=0.037; Table
III).
 | Table III.Univariate and multivariate analysis
of variables associated with OS in patients with hilar
cholangiocarcinoma. |
Table III.
Univariate and multivariate analysis
of variables associated with OS in patients with hilar
cholangiocarcinoma.
|
|
|
| P-value |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | N | DFS, months | Univariate
analysis | Multivariate
analysis | HR | 95% CI |
|---|
| Tumor invasion |
|
| 0.014 | 0.114 | 0.297 | 0.066–1.340 |
|
T1-T3 | 6 | 50 |
|
|
|
|
| T4 | 42 | 16 |
|
|
|
|
| Regional LN
positive |
|
| 0.033 | 0.390 | 0.678 | 0.279–1.646 |
| No | 15 | 42 |
|
|
|
|
|
Yes | 33 | 14 |
|
|
|
|
| FOXK1 |
|
| <0.001 | 0.037 | 0.357 | 0.136–0.940 |
|
Low | 16 | 50 |
|
|
|
|
|
High | 32 | 14 |
|
|
|
|
FOXK1 knockdown attenuates cell
invasion and migration in vitro
To provide direct evidence for the critical role of
FOXK1 in HC, its expression was knocked down in HC cells in
vitro using shRNA. HC cells exhibited high endogenous FOXK1
expression (Fig. 4A). Fig. 4B shows that the silencing of FOXK1
expression using shRNA was successful in RBE cells (Fig. 4B). Silencing endogenous FOXK1 did
not affect cell proliferation (Fig.
4B), but it significantly inhibited cell invasion, as revealed
by the Transwell assay (P=0.0013; Fig.
4C), and cell migration, as revealed by the wound healing assay
(Fig. 4D). Western blotting
revealed that FOXK1 knockdown resulted in the upregulation of
E-cadherin and the downregulation of vimentin, MMP-9 and Twist
(Fig. 4E).
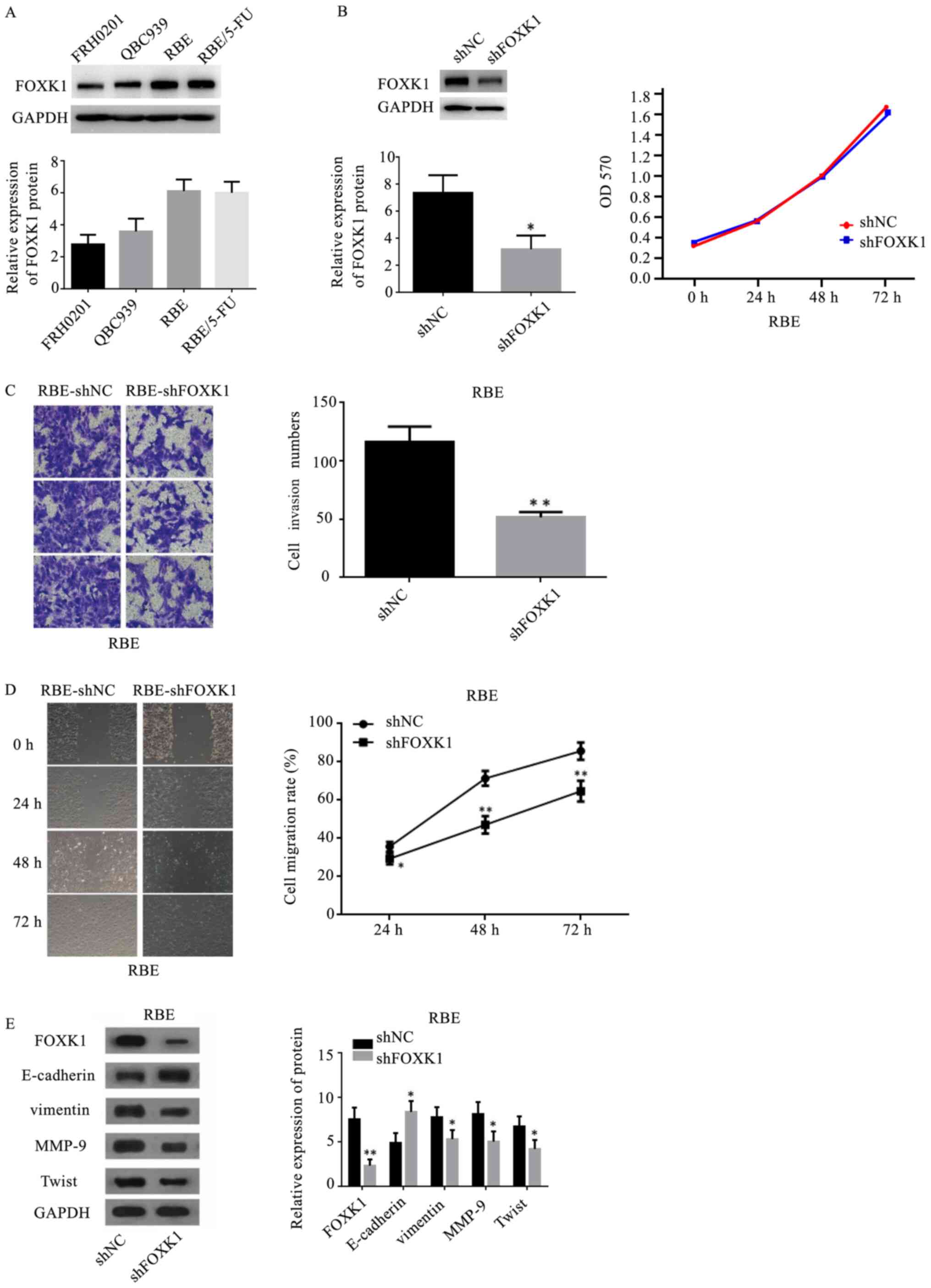 | Figure 4.Inhibitory role of silencing FOXK1 on
hilar cholangiocarcinoma cells. (A) Endogenous levels of FOXK1 in
FRH0201, QBC939, RBE and RBE/5-FU cells. (B) RBE cells were
infected with shFOXK1 or shNC and examined by western blotting.
Cells infected with the shNC or shFOXK1 lentivirus were seeded into
96-well plates and cell growth was assessed by performing Cell
Counting Kit-8 assays. (C) Transwell assay was performed to
evaluate the effect of FOXK1 on cell invasion (magnification, ×200)
in RBE cells transfected with shFOXK1 compared with that in the
sh-NC group. (D) Wound healing assay was used to evaluate the
effect of FOXK1 on the migration (magnification, ×200) of RBE cells
transfected with shFOXK1 compared with the sh-NC group. (E) Levels
of E-cadherin, vimentin, MMP-9 and Twist were measured by western
blotting in FOXK1-silenced RBE cells. *P<0.05, **P<0.01 vs.
shNC group. FOXK1, forkhead box K1; sh, short hairpin RNA; NC,
negative control; MMP, metallopeptidase. |
FOXK1 knockdown reduces drug
resistance in vitro
To explore chemoresistance in HC, FOXK1-knockdown
RBE cells were treated with 5-FU and DDP. It was found that
silencing endogenous FOXK1 expression in RBE cells increased their
sensitivity to 5-FU and DDP (Fig.
5A). In addition, western blotting revealed that markers
related to drug resistance, such as GST-π, MDR1 and P-gp, were
downregulated in RBE cells in vitro when FOXK1 was
suppressed (Fig. 5B).
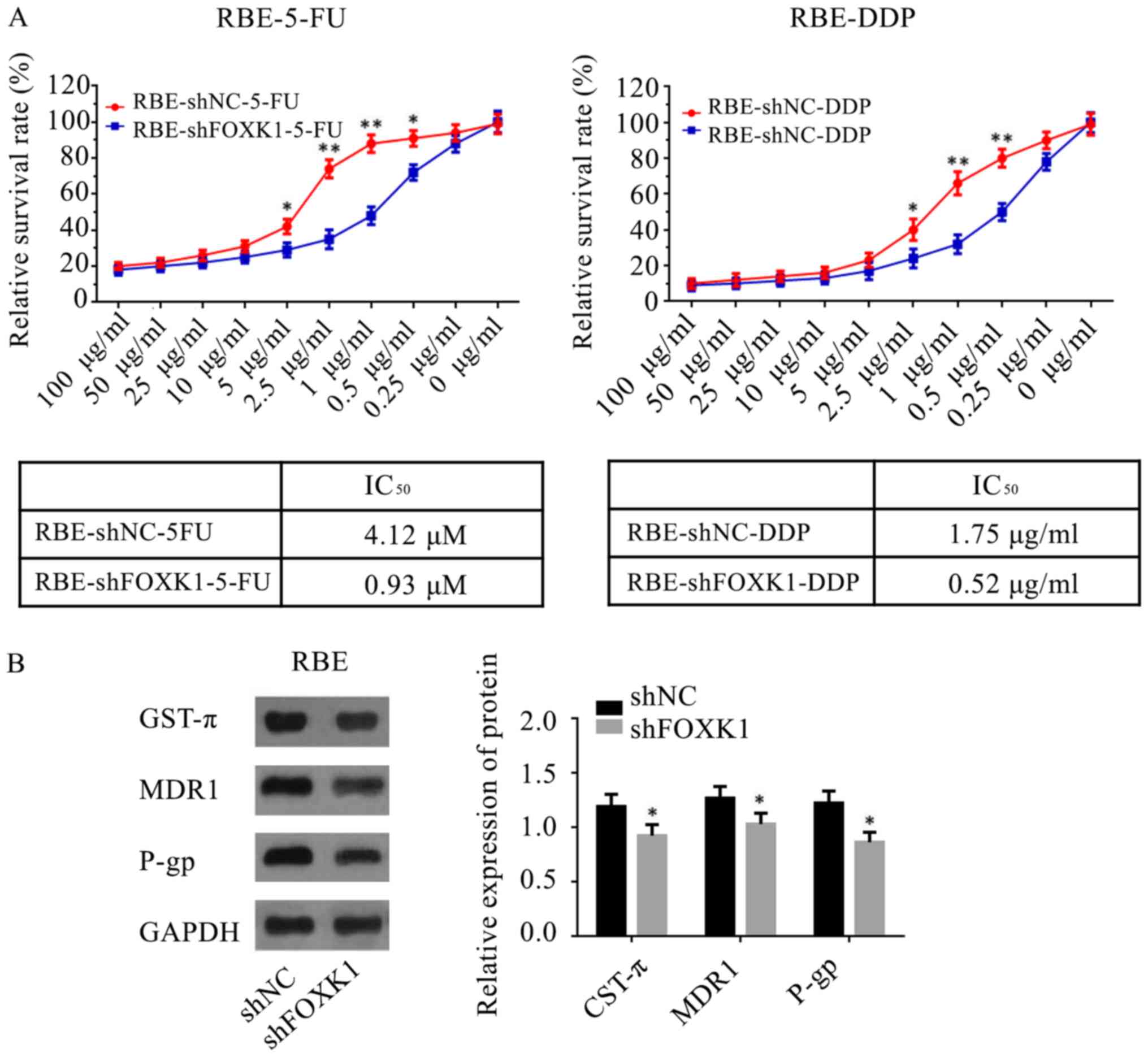 | Figure 5.Role of FOXK1 in chemotherapy
resistance of hilar cholangiocarcinoma cells. (A) RBE cells
transfected with shFOXK1 have a lower IC50 of 5-FU and
DDP compared with the sh-NC group. (B) Levels of GST-π, MDR1 and
P-gp were measured by western blotting in FOXK1-silenced RBE cells
and the expression of GST-π, MDR1 and P-gp decreased in the shFOXK1
group compared with that in the sh-NC group. *P<0.05,
**P<0.01 vs. shNC group. FOXK1, forkhead box K1; sh, short
hairpin RNA; 5-FU, 5-fluorouracil; DDP, cisplatin; GST, glutathione
S-transferase; MDR1, multidrug resistance mutation 1; P-gp,
P-glycoprotein; NC, negative control. |
Discussion
The present study determined the expression profile
of FOXK1 in patients with HC and identified that it was
considerably increased and significantly associated with tumor
invasion and metastasis. However, it had no effect on the
proliferation of HC. The data of survival follow-up revealed that
its high expression was an independent predictor of tumor
recurrence and OS after HC resection. However, the migratory and
invasive abilities of RBE cells were inhibited and the expression
of several epithelial-mesenchymal transition (EMT)-associated
proteins were influenced following knockdown of FOXK1, which
provided a novel molecular basis for the key role of FOXK1 in HC
development and progression.
FOXK1 plays an oncogenic role in a number of solid
tumors, and it has been observed to be upregulated in
hepatocellular carcinoma, and gastric, colorectal, prostate,
esophageal and ovarian cancer (7–11,15–17).
This high expression of FOXK1 is also found to correlate
significantly with malignant behaviors, including poor
differentiation of esophageal cancer (10), size and metastasis of ovarian cancer
(9), and differentiation, LN
metastasis and AJCC stage of colorectal cancer (18). Consistent with these studies, the
present study confirmed that FOXK1 was highly expressed in HC
tissues, using resected HC specimens and matched specimens. High
expression was identified to be associated with tumor invasion and
metastasis. All these data supported the role of FOXK1 as an
oncogene and suggested that it might serve as a potential
therapeutic target for patients with HC.
A number of clinicopathological variables have been
identified as associated with tumor recurrence and OS for HC,
including tumor invasion, nerve invasion, regional LN metastasis,
curative resection and disease progression (19–21).
In the small HC cohort of the present study, tumor invasion and LN
metastasis significantly affected disease progression and OS.
However, these factors did not independently predict tumor
recurrence and patient outcome. Possible reasons for this
discrepancy are the limited number of subjects in the cohort and
the fact that this cohort involved a number of patients with HC
with advanced-stage disease. A number of studies have introduced
biological markers into the Cox regression model, including amino
acid transporter A1, proline-rich protein 11, pyruvate kinase PKM
and Annexin A1 (12,13,22–24).
The survival analysis from the present study showed that high FOXK1
expression in tumors was associated with shorter PFS and worse
outcome. In addition, FOXK1 was an independent predictor of tumor
recurrence and OS in patients with HC. These findings suggested
that this protein might be a potential biomarker to predict disease
progression and outcome in patients with HC.
Locally advanced or metastatic HC at diagnosis makes
patients ineligible for surgical resection and thus limits their
overall 5-year survival. Given the association between high FOXK1
expression and tumor invasion or LN metastasis, the present study
aimed to detect the role of FOXK1 in cell migration by suppressing
its expression and exploring a possible associated mechanism. The
present study used shRNA interference transfection to construct an
effective FOXK1-knockdown cell line. It was identified that
silencing endogenous FOXK1 significantly inhibited HC cell
migration and invasion, as revealed by the Transwell and wound
healing assays. Overall, the data indicated that FOXK1 played a
critical role in disease progression. Several previous studies have
revealed that EMT confers properties that are critical for invasion
and distant metastasis to neoplastic epithelial cells (25,26).
It has been demonstrated that the knockdown of FOXK1 inhibits
transforming growth factor β-induced EMT (27). Its overexpression induces this
process by upregulating cysteine-rich angiogenic inducer 61 in
colorectal cancer (7), whereas
knockdown prevents an EMT phenotype through the upregulation of
E-cadherin and downregulation of N-cadherin in prostate cancer
cells (28). Therefore, the present
study determined the expression of EMT-related proteins, such as
E-cadherin, vimentin, MMP-9 and Twist, by western blotting in
FOXK1-knockdown cell lines. The loss of E-cadherin and the
upregulation of vimentin and Twist represent the EMT process, while
MMP-9 is a key component that mediates cell adhesion. According to
previous studies (7,25–28)
and the results of the present study, FOXK1 could be a critical
inducer of EMT and it is proposed that FOXK1 plays a critical
oncogenic role by promoting the EMT process. Although further
research is needed to explore the molecular mechanisms, the results
of the present study provided a novel molecular basis for the key
role of FOXK1 in HC development and progression.
In addition to the lack of effective biomarkers,
chemoresistance is also an important factor in the high mortality
of HC. Silencing FOXK1 increased sensitivity to 5-FU and DDP, and
downregulated the expression of GST-π, MDR1 and P-gp, which are
related to drug resistance, in RBE cells in vitro. Thus,
FOXK1 may serve as a putative target for HC.
In summary, FOXK1 was highly expressed in HC and
associated with tumor invasion and LN metastasis in Chinese
patients with HC. Furthermore, high FOXK1 expression was an
independent predictor of tumor recurrence and OS. Thus, these
results indicated that FOXK1 can facilitate cancer metastasis by
regulating EMT-associated proteins in HC and plays a role in
chemoresistance.
Acknowledgements
The authors would like to acknowledge Dr Wenlong Yu
from the Eastern Hepatobiliary Hospital (Shanghai, China) and Dr
Ying Chen from Changhai Hospital (Shanghai, China) for providing HC
samples.
Funding
The present study was partially supported by Beijing
Municipal Administration of Hospitals Clinical Medicine Development
of Special Funding Support (grant no. ZYLX201504) and National Key
Technologies R&D Program (grant no. 2015BAI13B09)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
ZZ conceived and designed the study. YF and ZB
acquired the data. YF, ZB and JS were responsible for data analysis
and interpretation. YF and ZZ wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The tissue sample experiments were approved by the
Ethics Committee of The Affiliated Hospital of Qingdao University.
Informed consent written from all participants (or their parent or
legal guardian in the case of children under 16) was obtained to
participate in the study or to use their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poruk KE, Pawlik TM and Weiss MJ:
Perioperative management of hilar cholangiocarcinoma. J
Gastrointest Surg. 19:1889–1899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donati M, Stang A, Stavrou GA, Basile F
and Oldhafer KJ: Extending resectability of hilar
cholangiocarcinomas: How can it be assessed and improved? Future
Oncol. 15:193–205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis HL, Rahnemai-Azar AA, Dillhoff M,
Schmidt CR and Pawlik TM: Current management of perihilar
cholangiocarcinoma and future perspectives. Chirurgia (Bucur).
112:193–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Peng Y, Wu M, Zhang W, Zhang M, Xie
R, Zhang P, Bai Y, Zhao J, Li A, et al: Oncogene FOXK1 enhances
invasion of colorectal carcinoma by inducing epithelial-mesenchymal
transition. Oncotarget. 7:51150–51162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi X, Wallis AM, Gerard RD, Voelker KA,
Grange RW, DePinho RA, Garry MG and Garry DJ: Foxk1 promotes cell
proliferation and represses myogenic differentiation by regulating
Foxo4 and Mef2. J Cell Sci. 125:5329–5337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang X, Xiang L, Li Y, Zhao Y, Zhu H,
Xiao Y, Liu M, Wu X, Wang Z, Jiang P, et al: Snail/FOXK1/Cyr61
signaling axis regulates the epithelial-mesenchymal transition and
metastasis in colorectal cancer. Cell Physiol Biochem. 47:590–603.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen F, Xiong W, Dou K and Ran Q:
Knockdown of FOXK1 Suppresses proliferation, migration, and
invasion in prostate cancer cells. Oncol Res. 25:1261–1267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Yu Z, He L, Zhou D, Xie S, Hou H and
Geng X: Knockdown of FOXK1 inhibited the proliferation, migration
and invasion in hepatocellular carcinoma cells. Biomed
Pharmacother. 92:270–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen D, Wang K, Li X, Jiang M, Ni L, Xu B,
Chu Y, Wang W, Wang H, Kang H, et al: FOXK1 plays an oncogenic role
in the development of esophageal cancer. Biochem Biophys Res
Commun. 494:88–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Gong M, Zhao Y, Zhao X and Li Q:
FOXK1 facilitates cell proliferation through regulating the
expression of p21, and promotes metastasis in ovarian cancer.
Oncotarget. 8:70441–70451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu G, Yu W, Jin G, Xu D, Chen Y, Xia T, Yu
A, Fang W, Zhang X, Li Z, et al: PKM2 regulates neural invasion of
and predicts poor prognosis for human hilar cholangiocarcinoma. Mol
Cancer. 14:1932015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao X, Yu W, Qian J, Chen Y, Wei P, Fang
W and Yu G: ADAM-17 is a poor prognostic indicator for patients
with hilar cholangiocarcinoma and is regulated by FoxM1. BMC
Cancer. 18:5702018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang W, Cui H, Yu D, Chen Y, Wang J and Yu
G: Increased expression of phospho-acetyl-CoA carboxylase protein
is an independent prognostic factor for human gastric cancer
without lymph node metastasis. Med Oncol. 31:152014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, Xie R, Liu X, Wang J, Peng Y, Tang
W, Wu M, Zhang P, Ba Y, Zhao J, et al: Knockdown of FOXK1 alone or
in combination with apoptosis-inducing 5-FU inhibits cell growth in
colorectal cancer. Oncol Rep. 36:2151–2159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie R, Wang J, Liu X, Wu L, Zhang H, Tang
W, Li Y, Xiang L, Peng Y, Huang X, et al: RUFY3 interaction with
FOXK1 promotes invasion and metastasis in colorectal cancer. Sci
Rep. 7:37092017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang P, Tang WM, Zhang H, Li YQ, Peng Y,
Wang J, Liu GN, Huang XT, Zhao JJ, Li G, et al: MiR-646 inhibited
cell proliferation and EMT-induced metastasis by targeting FOXK1 in
gastric cancer. Br J Cancer. 117:525–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu M, Wang J, Tang W, Zhan X, Li Y, Peng
Y, Huang X, Bai Y, Zhao J, Li A, et al: FOXK1 interaction with FHL2
promotes proliferation, invasion and metastasis in colorectal
cancer. Oncogenesis. 5:e2712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergquist A and von Seth E: Epidemiology
of cholangiocarPlentz RR and Malek NP: Clinical presentation, risk
factors and staging systems of cholangiocarcinoma. Best Pract Res
Clin Gastroenterol. 29:245–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Plentz RR and Malek NP: Clinical
presentation, risk factors and staging systems of
cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 29:245–252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Zhang H, Fang Z and Yu G:
Annexin-1 downregulation is associated with clinical outcome in
Chinese patients with hilar cholangiocarcinoma. Eur Surg Res.
45:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu WL, Cong WM, Zhang Y, Chen Y, Wang F
and Yu G: Overexpression of ATA1/SLC38A1 predicts future recurrence
and death in Chinese patients with hilar cholangiocarcinoma. J Surg
Res. 171:663–668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Cha Z, Fang W, Qian B, Yu W, Li W,
Yu G and Gao Y: The prognostic potential and oncogenic effects of
PRR11 expression in hilar cholangiocarcinoma. Oncotarget.
6:20419–20433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen S, Kang X, Liu G, Zhang B, Hu X and
Feng Y: α7-Nicotinic acetylcholine receptor promotes
cholangiocarcinoma progression and epithelial-mesenchymal
transition process. Dig Dis Sci. 64:2843–2853. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Wu X, Xiao Y, Wu L, Peng Y, Tang
W, Liu G, Sun Y, Wang J, Zhu H, et al: Coexpression of FOXK1 and
vimentin promotes EMT, migration, and invasion in gastric cancer
cells. J Mol Med (Berl). 97:163–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Liu G, Liu M, Xiang L, Xiao Y, Zhu
H, Wu X, Peng Y, Zhang W, Jiang P, et al: The FOXK1-CCDC43 axis
promotes the invasion and metastasis of colorectal cancer cells.
Cell Physiol Biochem. 51:2547–2563. 2018. View Article : Google Scholar : PubMed/NCBI
|