Introduction
Chronic neuropathic pain is a chronic and highly
incapacitating complex disease, which is aroused by primary injury
and dysfunction of the nervous system, accompanied by activation of
inflammation signaling pathways (1). Neuropathic pain may result from many
factors including physical injury, metabolic or nutritional nerve
change, virus infection, neurotoxicity of drugs or radiotherapy,
ischemic nerve damage, neurotransmitter dysfunction, etc.
Individuals with neuropathic pain displayed high sensitivity to
mechanical, heat and cold stimulation, and local and/or systemic
inflammation reaction. In order to develop novel therapeutic target
or strategy for neuropathic, it is essential to better understand
its underlying mechanisms. The signal transducer and activator of
transcription 3 (STAT3) pathway is a key pathway mediating
inflammation, which plays an important role in synaptic plasticity,
neural degeneration and memory formation in central nervous system.
Recently, it has been revealed that activation of STAT3 was
important to the development of chronic pain, and inhibition of
STAT3 activation has been regarded as a promising therapy for
neuropathic pain.
The suppressor of cytokine signaling (SOCS) proteins
have previously been reported to negatively regulate the STAT3
signaling, of which SOCS3 are the most effective member (2). SOCS3 can inhibit STAT3 activation and
blocks STAT3 signaling, thereby limiting some of the
pathophysiological consequences (3,4).
Therefore, SOCS3 is a potential target for the effective treatment
of neuropathic pain.
Yin Yang 1 (YY1) is a commonly expressed zinc-finger
DNA-binding transcription factor in a variety of cells, which can
interact with other transcription factors (5). It is reported that YY1 improved tumor
cells growth and suppressed apoptosis through negative regulation
of TP53 (6). YY1 is also involved
in airway inflammation, and is a positive regulator of many
inflammatory cytokines in T cells, including IL-4, IL-5 and IL-13
(7). However, the function of YY1
in neuropathic pain is still unclear. In this study, we detected
the YY1 level in neuropathic pain model of rats and analyzed its
influence on neuropathic pain. Then, we further explored weather
the effect of YY1 on pain is involved in regulation of the
SOCS3/STAT3 signaling pathway.
Materials and methods
Animals
Adult female Sprague-Dawley rats (195–215 g) were
purchased from the Experimental Animal Centre of Shandong
University (China). Animals were housed in individual cages at 23.0
± 1°C under a 12 h light/dark cycle and were given free access to
water and food. All animal protocols were conducted in accordance
with the Institutional Animal Care and Use of the Yantai
Yuhuangding Hospital (Shandong, China). The present study was
approved by the Ethics Committee of Yantai Yuhuangding
Hospital.
Neuropathic pain model
Bilateral Chronic constriction injury (bCCI) of rats
sciatic nerve was a common experimental model for neuropathic pain
(8). The rats were anesthetized
with an intraperitoneal injection of sodium pentobarbital (40
mg/kg). The mid-thigh on both side was incised and the heads of the
biceps femoris muscle was separated to expose the sciatic nerve.
4-0 chromic gut was carried out ligate the sciatic nerve at four
sites. The wound was closed with absorbable sutures. Sham-operated
rats received a similar exposure but without nerve isolation and
ligation. The rats were closely monitored following surgery. Naïve
group did not receive any surgery.
Mechanical allodynia
Rats were acclimatized in suspended cages. The hind
paws were probed with calibrated Electronic von Frey device (IITC
Life) erected to the plantar surface and kept for ~5 sec. A
positive response represented a sharp withdrawal of the paw. The
mechanical withdrawal threshold (MWT) was indicated as the average
of the measurements in each test session.
Thermal hyperalgesia
Thermal hyperalgesia was detected with a Plantar
Test Apparatus for Mice and Rats (IITC). Rats were acclimated on a
glass floor above a radiant heat producer that was aimed at the
plantar surface of the hind paw. In each test session, triple
measurements of latency were done for each hind paw. Each test was
done with intervals greater than 3 min and the device was cutoff
for 30 sec in the intervals of each test to avoid tissue damage.
The thermal withdrawal latency (TWL) was indicated as the average
of the measurements in each test session.
Cold hyperalgesia
A drop of acetone was gently applied to each hind
paw at room temperature. A rapid hind paw withdrawal in response to
the volatilization of acetone was considered as a sign of cold
allodynia. The test was repeated 3 times for every hind paw, with
an interval of 3 min. Times of shaking, lifting or licking the paw
during 1 min were recorded. An increase in the rate of withdrawal
response interpreted as increased cold sensitivity.
Culture of primary spinal cord neurons
and cell transfection
A total of 6 SD rats (3 months old) were
anesthetized intraperitoneal injection with 3% pentobarbital sodium
at a dose of 30 mg/kg body weight. Spinal cord was isolated from
L4-L6 of the rats after sacrificed by cervical dislocation and
digested with 0.25% trypsin for 30 min followed by 0.2% collagenase
II for 2 h. The cells were washed with D-Hank's buffer (Invitrogen;
Thermo Fisher Scientific, Inc.) for 3 times and then seeded in the
plates with DMEM/F12 (containing 4.5 g/l glucose) supplemented with
20% FBS and 4 mM L-glutamine. Cells were cultured in a 37°C
humidified cell culture chamber with 5% CO2.
On reaching 70% confluence, 2 µg/ml pcDNA-YY1
(constructed by GenScript Company, Nanjing, China) and 50 nM YY1
siRNA (designed and synthesized by Ribobio Technology, Guangzhou,
China) were respectively transfected in to the cells with
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) in accordance with the manufacturers' instructions. After 48
h, the cells were harvested.
Cell viability assay
Cell viability was performed by MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide)
assay (Amresco, Solon, OH, USA) (9). After transfection for 48 h, cells were
plated in 96-well plates (Corning, MA, USA) at 1.0 × 104
cells per well and incubated for 12 h. MTT solution (20 µl/well, 5
mg/ml) were added to the well and incubated for 4 h. The media were
removed and 200 µl DMSO per well was added to dissolve formazan
crystals. Finally, the absorbance was measured using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 570 nm
wavelength.
Apoptosis assay
In 60 mm plates, cells were collected by
centrifugation after transfection for 72 h. the Apoptosis Assay was
performed using Apoptosis Detection kit II (BD Biosciences, San
Diego, CA, USA). Cells were resuspended in 500 µl of 1X Binding
Buffer with 5 µl of Annexin V-FITC and 10 µl of propidium iodide
(PI) and incubated at room temperature for 15 min in the dark. The
flow cytometry (BD Biosciences) was used for the evaluation of
percentage of apoptotic cells. The data was from typically 10,000
cells and analyzed in Cell Quest software.
Fluorometry assay
Intracellular reactive oxygen species. (ROS)
generation was evaluated using a fluorometry assay (10). Cell was collected with trypsin and
centrifuged. Cell pellets were exposed to
dichlorodihydrofluorescein diacetate (DCFH-DA) (1:1,000; Sigma,
USA) for 20 min at 37°C in the darkroom. The flow cytometry (BD
Biosciences) was used to measure the ROS level, which was the mean
of the fluorescence for each treatment with typically 10,000 cells
analyzed. All experiments were conducted for three times.
Protein estimation by ELISA
Total protein concentration of samples was
determined with a Bicinchoninic Acid (BCA) protein assay kit
(Pierce, Rockford, IL, USA) IL-1β and IL-6 level were determined
with ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA).
Sample optical densities were measured at 450 nm with a reference
filter set at 570 nm. Cytokine concentrations were calculated
according to the standard curves.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The concentration of
total RNA was determined using a NanoDrop 2000c (Thermo Fisher
Scientific, Inc.). 1 µg of RNA was used to synthesize cDNA with the
PrimeScript™ RT master mix (Takara, Japan). RT-qPCR was
performed using SYBR Premix Ex Taq (Takara, Japan) and the
thermocycling conditions were as followed: 42°C for 30 min, then
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
15 sec and 60°C for 1 min. The relative amount of transcript was
quantified via the comparative threshold cycle method using GAPDH
as the endogenous reference. Transcript levels in each rat were
determined with the formula 2−ΔΔCq (11). The primers for amplifying all the
genes were selected from reported references and are presented in
Table I.
 | Table I.Primers applied in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers applied in reverse
transcription-quantitative polymerase chain reaction analysis.
| Target | Sequences
(5′-3′) | Product size
(bp) |
|---|
| YY1 | F:
GCCCTCATAAAGGCTGCACAAAGAT | 223 |
|
| R:
GTGCGCAAATTGAAGTCCAGTGAA |
|
| SOCS3 | F:
TCACGGCTGCCAACATCTGG | 228 |
|
| R:
CGGCGGCGGGAAACTTG |
|
| COX2 | F:
TCTTTGCCCAGCACTTCACTCA | 367 |
|
| R:
TCAGGATGCTCCTGTTTGA |
|
| CCL2 | F:
ATGCAGTTAATGCCCCACTC | 167 |
|
| R:
TTCCTTATTGGGGTCAGCAC |
|
| IL-1β | F:
CACCTTCTTTTCCTTCATCTT | 238 |
|
| R:
GTCGTTGCTTGTCTCTCCTTGTA |
|
| IL-6 | F:
AAGTTTCTCTCCGCAAGAGACTTCCAG | 326 |
|
| R:
AGGCAAATTTCCTGGTTATATCCAGTT |
|
| TNF-α | F:
GTAGCCCACGTCGTAGCAAAC | 196 |
|
| R:
TGTGGGTGAGGAGCACATAGTC |
|
| GAPDH | F:
ATCCCATCACCATCTTCCAG | 322 |
|
| R:
CCATCACGCCACAGTTTCC |
|
Western blot analysis
Protein concentration was determined with a BCA
protein assay kit (Pierce). Extracted proteins were separated by
SDS-PAGE, transferred on a nitrocellulose membrane (NC; Millipore,
USA). Anti-YY1 (AV100899; Sigma, USA), anti-SOCS3 (sc-9023; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-STAT3 (sc-482;
Santa Cruz Biotechnology, Inc.), anti-phosphorylated STAT3
(sc-135649; Santa Cruz Biotechnology, Inc.), anti-CCL2 (66272-2-lg;
ProteinTech, USA), anti-COX2 (SAB5500087; Sigma, USA), anti-GCR
(ab2768; Abcam, UK) and anti-GAPDH (sc-25778; Santa Cruz
Biotechnology, Inc.) antibodies were used to block the membrane
overnight at 4°C. After washing with TBST for 3 times, the membrane
was incubated with horseradish peroxidase conjugated antibodies
(1:2,000; bs-0295-HRP; Bioss, China). Then the membrane was
incubated with western chemiluminescent HRP substrate (Millipore,
USA) to detect the signal. Image J was used for statistical
analysis. Three consistent independent experiments were performed
to analyze the results.
Statistical analysis
All data is presented as the mean ± standard
deviation. Two factors (group and times) repeated measures analysis
of variance (ANOVA) was used to analyze the time course of
bCCI-induced tactile allodynia between groups. Multiple comparisons
were analyzed with ANOVA and Dunnett's post hoc test. Comparisons
between two groups were analyzed by the Student's t-test. Data were
processed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
YY1 was decreased in rats with
bCCI
To investigate the involvement of YY1 in neuropathic
pain, the bCCI rats were established. Before and after surgery, the
recorded scores exhibited no significant differences between left
and right hind paws from the stimulus at any of the time, the mean
scores of left and right hind paws were used to analyze the
results. At days 0, 3, 7, 14 and 21, the indexes of neuropathic
pain including MWT, paw thermal withdrawal latency (PTWL) and paw
frequency in response to cold stimulus were detected in each group,
respectively characterizing the mechanical allodynia, thermal
hyperalgesia and cold hyperalgesia of the rats. Compared with sham
group, MWT and PTWL of bCCI group were significantly decreased from
3 days to 21 days after operation and reached its lowest point on
day 14 (Fig. 1A and B). From 3 days
after surgery, the times of the responses of bCCI rats to cold
acetone was significantly higher than that in sham group and
reached its highest point on day 14 (Fig. 1C). Then we determined the expression
of YY1 in bCCI rats. The results suggested that YY1 mRNA level was
significantly decreased in rats with bCCI compared to the sham
group (Fig. 1D).
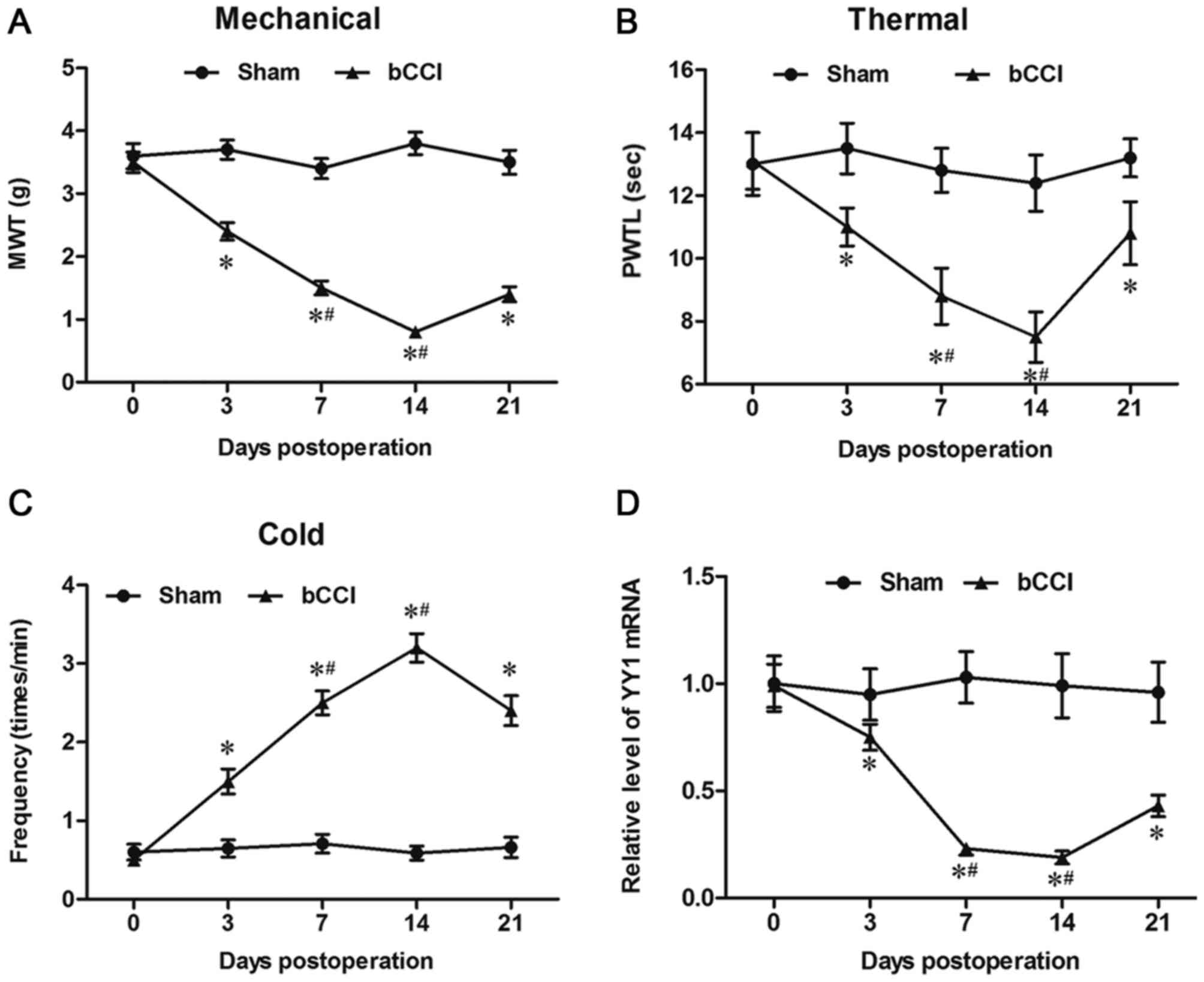 | Figure 1.YY1 is downregulated in the spinal
cord cells of bCCI rats. A neuropathic pain rat model was
established using the bCCI method. On days 0, 3, 7, 14 and 21, the
indexes of neuropathic pain including (A) MWT, (B) PTWL and (C) paw
frequency were detected in response to a cold stimulus in each
group, characterizing the mechanical allodynia, thermal
hyperalgesia and cold hyperalgesia of the rats. (D) In addition,
spinal cord cells were isolated from the L4-L6 of the rats, and the
expression of YY1 was detected by reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± standard deviation (n=6). *P<0.05 vs.
Sham group; #P<0.05 vs. day 3 of the bCCI group. YY1,
Yin Yang 1; bCCI, bilateral chronic constriction injury; MWT,
mechanical withdrawal threshold; PTWL, paw thermal withdrawal
latency. |
YY1 reduced neuropathic pain symptoms
of bCCI rats
Different concentrations of pcDNA-YY1 expression
vectors were injected intrathecally, and expression of YY1 mRNA and
protein was detected with qPCR and Western blotting. The results
showed that, compared with the Vector group, 4 mg/kg pcDNA-YY1
transfection significantly increased and 8 mg/kg pcDNA-YY1 further
increased the level of YY1 mRNA (Fig.
2A). Moreover, in pcDNA-YY1 overexpression groups (4 mg/kg and
8 mg/kg groups), YY1 mRNA displayed a robust increase at day 3 and
a drop at days 7 and 14 (Fig. 2A).
YY1 protein was being increased from day 0 to day 14 in the 8 mg/kg
pcDNA-YY1 group, compared with the Vector group (Fig. 2B). The values of MWT and PMWT of
bCCI group were markedly increased by treatment with 4 mg/kg
pcDNA-YY1 vector, and further increased by treatment with 8 mg/kg
(Fig. 2C and D). Consistently, the
paw frequency in response to cold stimulus of bCCI group was
significantly lowered by treatment with 4 mg/kg pcDNA-YY1 vector,
and further lowered by treatment with 8 mg/kg (Fig. 2E). As a key signaling pathway
regulating neuropathic pain, activation of STAT3 was obviously
suppressed by overexpression of YY1, and expression of SOCS3, a
negative regulator of STAT3 was obviously promoted (Fig. 2F).
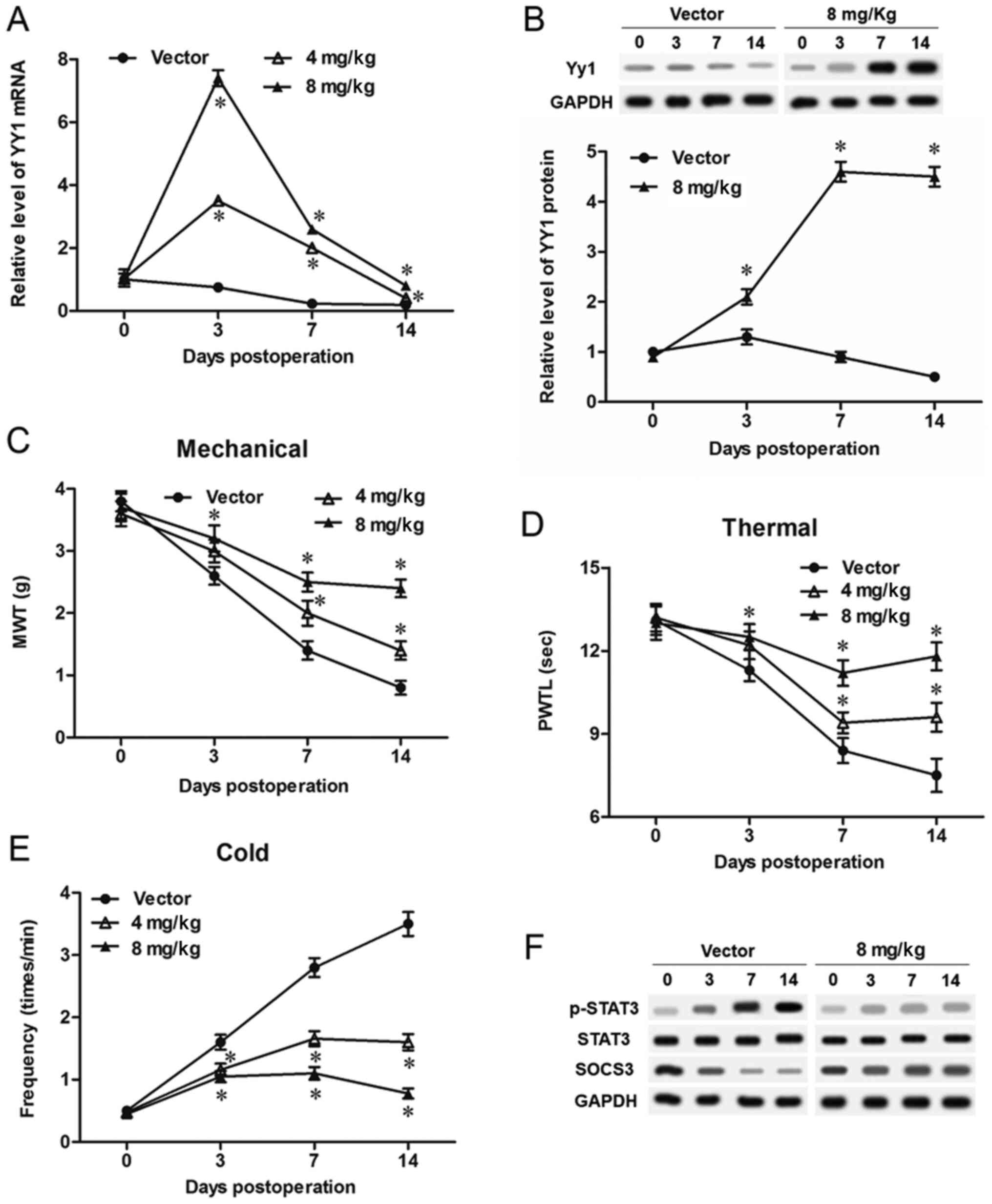 | Figure 2.YY1 relieves neuropathic pain symptoms
in bCCI rats. YY1 was overexpressed in bCCI rats by intrathecally
injecting different doses (4 or 8 mg/kg) of the pcDNA-YY1
expression vector. The levels of YY1 (A) mRNA and (B) protein in
spinal cord cells were detected on days 0, 3, 7 and 14. The effect
of YY1 overexpression on the indexes of neuropathic pain including
(C) MWT, (D) PMWT and (E) paw frequency in response to cold stimuli
were detected on days 0, 3, 7 and 14. (F) YY1 promoted SOCS3
protein expression and suppressed the activation of STAT3. Data are
expressed as the mean ± standard deviation (n=6). *P<0.05 vs.
Vector. YY1, Yin Yang 1; bCCI, bilateral chronic constriction
injury; MWT, mechanical withdrawal threshold; SOCS3, suppressor of
cytokine signaling 3; STAT3, signal transducer and activator of
transcription 3. |
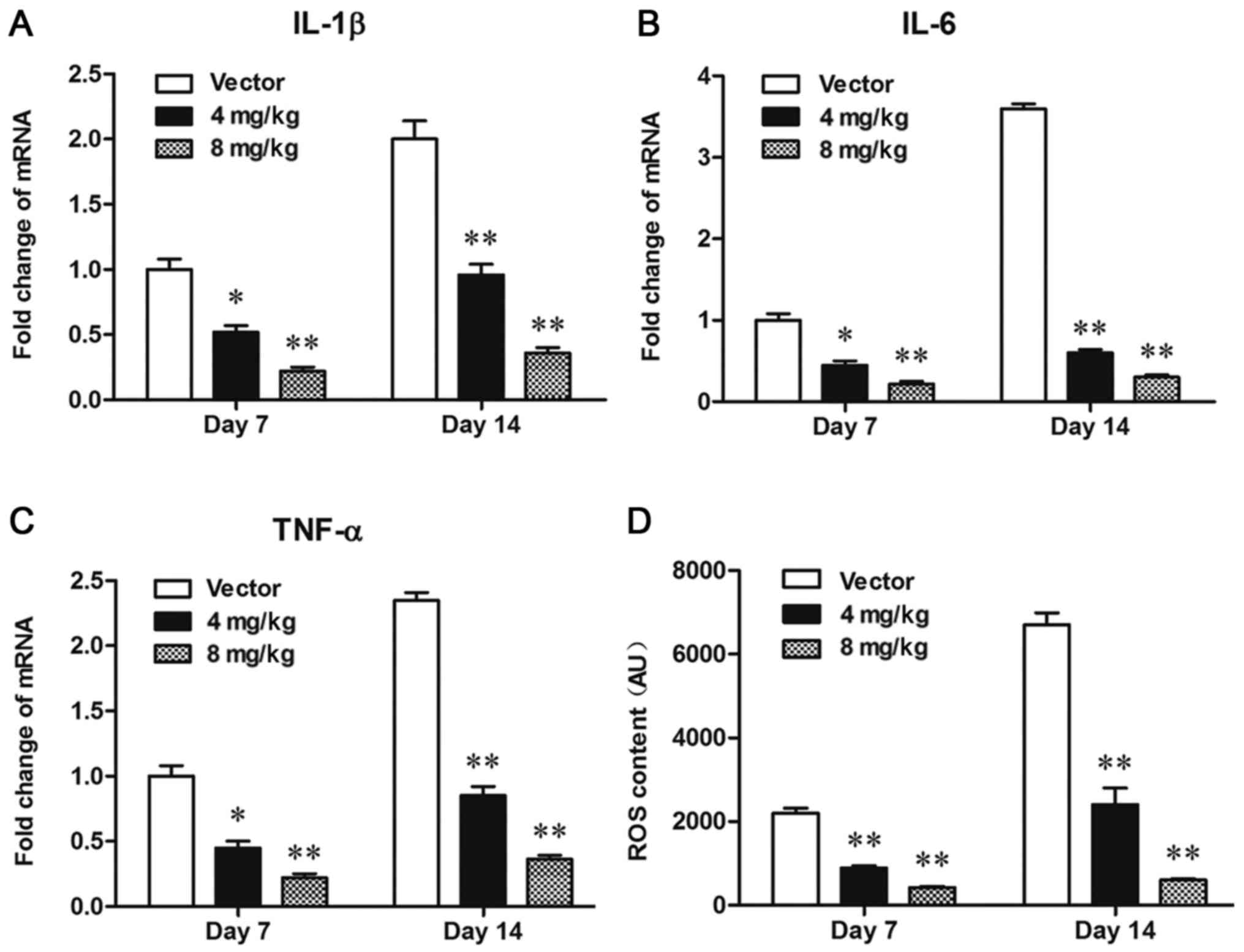
YY1 significantly inhibited
inflammation and ROS overproduction in bCCI rats
Neuroinflammation and oxidative stress are direct
pathological mechanism of neuropathic pain. We checked the contents
of ROS and inflammatory cytokines including IL-1β, IL-6 and TNF-α
in the spinal cord cells from each group. The results from qPCR
showed that the expression levels of IL-1β (Fig. 3A), IL-6 (Fig. 3B), and TNF-α (Fig. 3C) were significantly decreased by
treatment with 4 mg/kg pcDNA-YY1, and further decreased by
treatment with 8 mg/kg pcDNA-YY1. Moreover, YY1 decreased the ROS
content in bCCI rats in a dose-dependent manner (Fig. 3D). These data indicated that
overexpression of YY1 inhibited neuroinflammation and oxidative
stress in the spinal cord cells bCCI rats.
YY1 inhibited STAT3 signaling
activation through upregulating SOCS3 expression
To verify the role of YY1 in STAT3 signaling
pathway, primary spinal cord cells were cultured, and expression of
YY1 was manipulated in the cells by transfection with pcDNA-YY1 or
YY1 siRNA. After 72 h, we detected the changes in the levels of its
downstream gene glucocorticoid receptor (GCR), SOCS3,
phosphorylated STAT3 (p STAT3) and downstream target genes of STAT3
including cyclooxygenase 2 (COX2) and chemokine (C-C motif) ligand
2 (CCL2). The levels of YY1 mRNA and protein were significantly
increased by pcDNA-YY1 and decreased by YY1 siRNA (Fig. 4A and E). In response to YY1
overexpression, expression of SOCS3 and GCR was significantly
promoted, while the levels of COX2, CCL2 and pSTAT3 were
significantly decreased (Fig.
4B-E). In contrast, YY1 knockdown suppressed expression of
SOCS3 and glucocorticoid receptor, and increased the levels of
COX2, CCL2 and phosphorylated STAT3 (Fig. 4B-E). To validate that YY1
deregulated the activation of the STAT3 pathway through
upregulation of SOCS3, SOCS3 antibody was used to neutralizing
SOCS3 when YY1 was overexpressed. Our results showed that blockade
of SOCS3 abrogated the effect of YY1 overexpression on the
upregulation of COX2, CCL2 and pSTAT3 (Fig. 4B-E). These results indicated that
YY1 inhibited STAT3 signaling activation through upregulating the
expression of SOCS3.
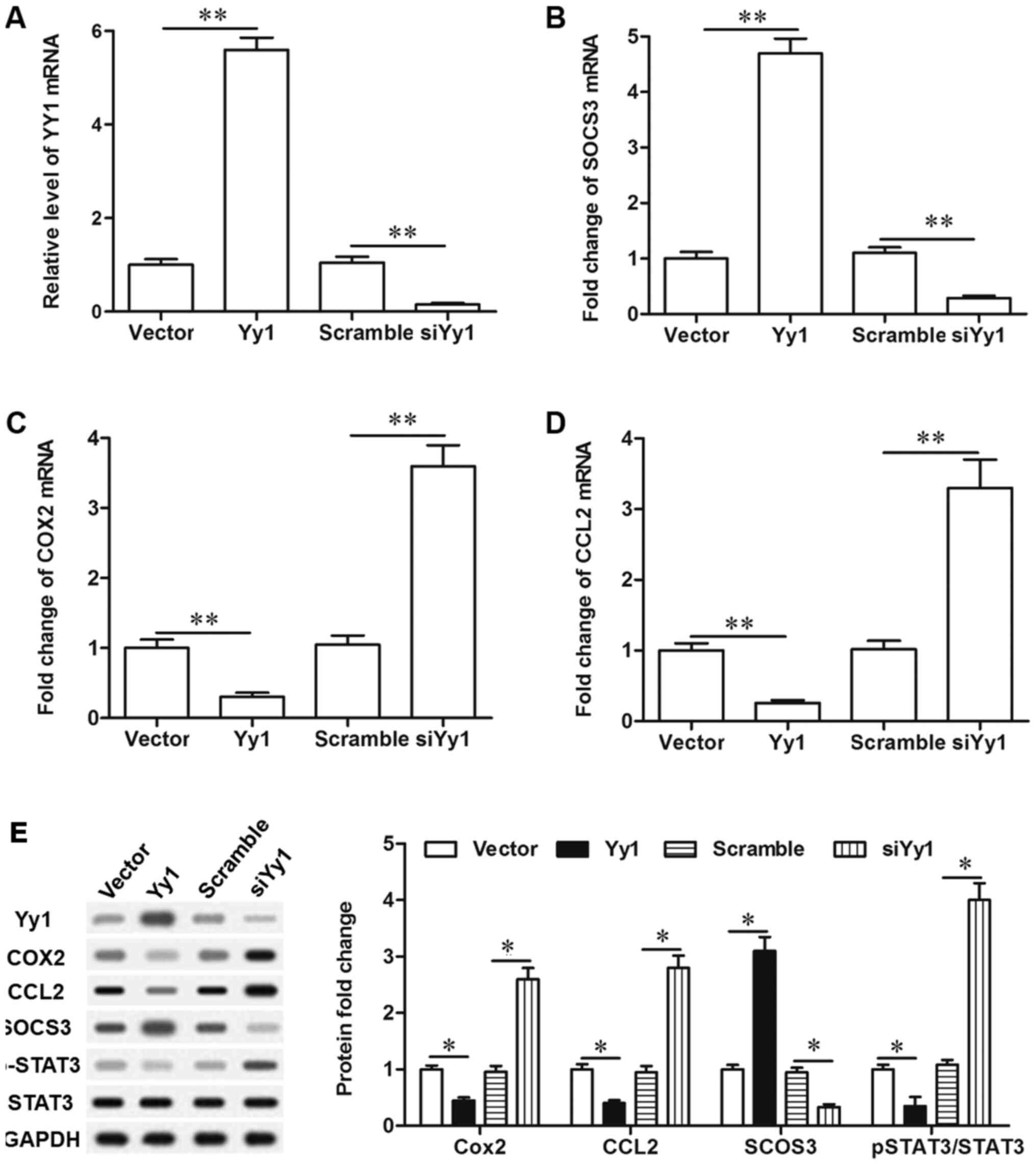 | Figure 4.YY1 negatively regulates the
activation of the STAT3 signaling pathway via upregulation of
SOCS3. (A) The overexpression and knockdown efficiencies of
pcDNA-YY1 and YY1 siRNA. (B) YY1 positively regulated the
expression of SOCS3. (C) YY1 negatively regulated the expression of
Cox-2. (D) YY1 negatively regulated the expression of CCL2. (E) YY1
negatively regulated the activation of the STAT3 signaling pathway
via upregulation of SOCS3. Primary spinal cord cells were isolated
from young rats. Upon reaching ~70% confluence, the cells were
treated with 2 µg/ml pcDNA empty vector, 2 µg/ml pcDNA-YY1, 50 nM
scrambled siRNA, 50 nM YY1 siRNA, or 2 µg/ml pcDNA-YY1 plus 5 µg/ml
SOCS3 neutralizing antibody. Following incubation for 72 h, total
RNA and total protein were extracted from the cells. The mRNA
levels of YY1, SOCS3, Cox-2 and CCL2 were detected with reverse
transcription-quantitative polymerase chain reaction, and the
protein levels of YY1, GCR, SOCS3, Cox-2, CCL2, STAT3 and pSTAT3
were detected with western blotting. Data are expressed as the mean
± standard deviation (n=4). *P<0.05 and **P<0.01. YY1, Yin
Yang 1; SOCS3, suppressor of cytokine signaling 3; STAT3, signal
transducer and activator of transcription 3; GCR, glucocorticoid
receptor; COX2, cyclooxygenase 2; CCL2, chemokine (C-C motif)
ligand 2; p-, phosphorylated. |
YY1 improved cell viability, and
suppressed cell apoptosis and secretion of inflammatory
factors
Finally, the effect of YY1 on cell survival and
inflammation in the spinal cord cells was investigated. Our results
from MTT, flow cytometry and ELISA indicated that overexpression of
YY1 increased cell viability, inhibited apoptosis, and reduced the
secretion of inflammatory factors IL-1β and IL-6, whereas YY1
knockdown displayed an opposite effect (Fig. 5A-D). Moreover, blockade of SOCS3
abrogated the effect of YY1 overexpression on cell viability, cell
apoptosis and inflammation (Fig.
5A-D).
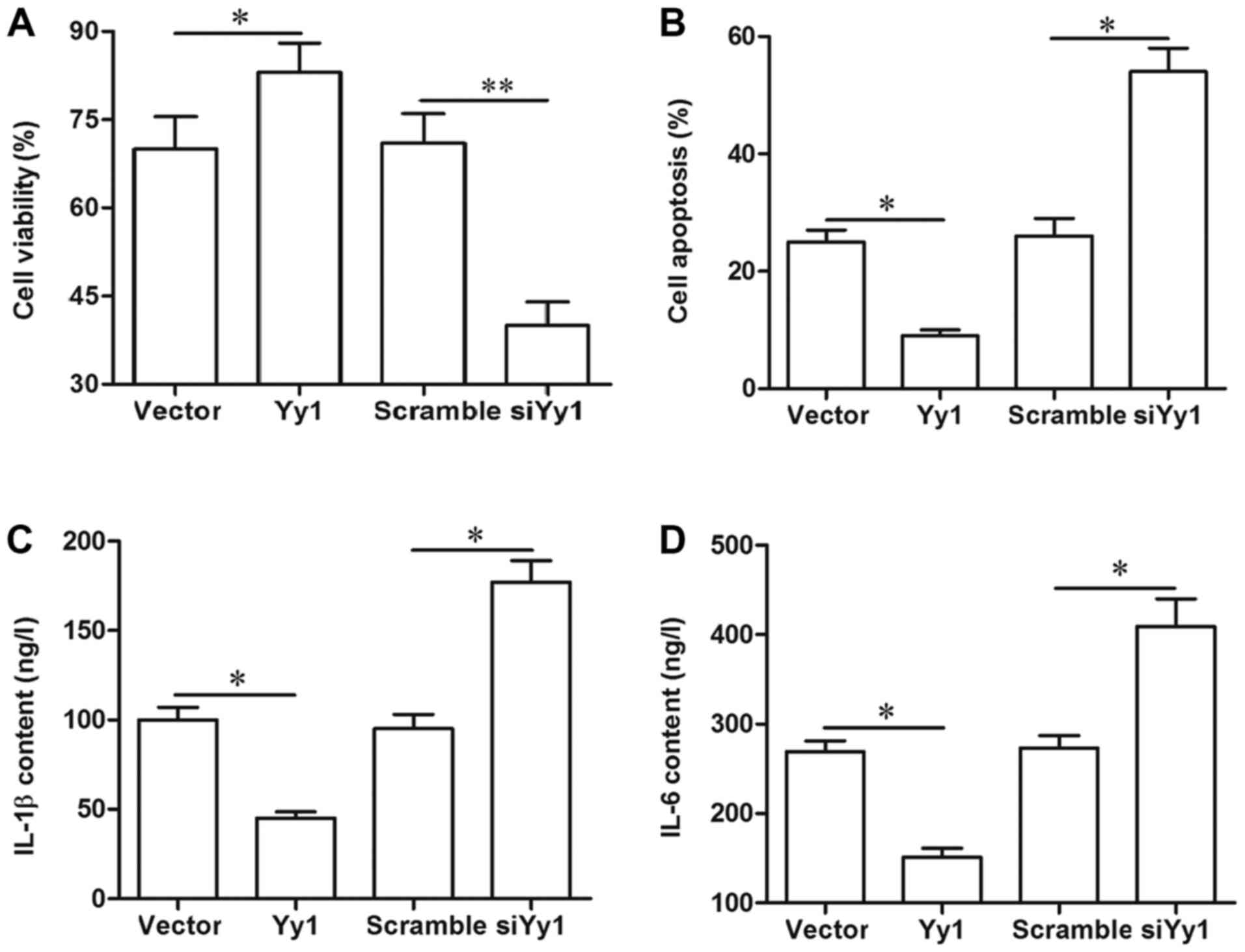 | Figure 5.YY1 improves cell survival and
suppresses inflammation. (A) YY1 could improve the viability of
spinal cord cells, which was antagonized by the blockade of SOCS3.
(B) YY1 negatively regulated the apoptosis of spinal cord cells,
which was antagonized by the blockade of SOCS3. YY1 negatively
regulated the secretion of (C) IL-6 and (D) IL-1β. Primary spinal
cord cells were isolated from young rats. Upon reaching ~70%
confluence, the cells were treated with 2 µg/ml pcDNA empty vector,
2 µg/ml pcDNA-YY1, 50 nM scrambled siRNA, 50 nM YY1 siRNA, or 2
µg/ml pcDNA-YY1 plus 5 µg/ml SOCS3 neutralizing antibody. Following
incubation for 72 h, cell viability was detected via the MTT
method, cell apoptosis was determined by flow cytometry, and the
secretion of IL-6 and IL-1β was evaluated with ELISA in the
supernatant of the spinal cord cells. Data are expressed as the
mean ± standard deviation (n=4). *P<0.05 and **P<0.01. YY1,
Yin Yang 1; SOCS3, suppressor of cytokine signaling 3; IL,
interleukin; si-/siRNA, small interfering RNA; n.s., not
significant. |
Discussion
As a poorly managed clinical problem, neuropathic
pain may also result from immune activation and proinflammatory
cytokine release (11).
Transcription factors act as central regulators of the immune
response to stimuli (12). YY1 is a
commonly expressed zinc-finger DNA-binding transcription factor in
a variety of cells, which can interact with other transcription
factors (5). We all know that YY1
is a ‘versatile’ regulator in gene expression and pathogenesis of
various diseases. For example, YY1 may function as a tumor
suppressor or a tumor promoting gene in different types of cancer.
In this study, a neuropathic pain rat model with the bCCI method
was established, and we found that YY1 was downregulated in bCCI
neuropathic pain model and its overexpression attenuated
neuropathic pain symptoms of bCCI rats. We showed that YY1 can
suppress the activation of STAT3, which is regarded as a key
contributor to neuropathic pain. What is more, upregulation of YY1
significantly inhibited the production of pro-inflammation factors
IL-1β, TNF-α and IL-6 in bCCI rats. These results implicated that
YY1 attenuate neuropathic pain symptoms via suppression of the
STAT3 pathway. Most of existing papers showed that YY1 had
pro-inflammatory effects. However, a few of studies demonstrated
that YY1 could suppress expression of pro-inflammatory genes and
functioned as an anti-inflammatory gene (13–16).
For example, YY1 was shown to strongly repress transcription of the
proinflammatory gene matrix metalloproteinase-9 in brain neurons
(15). Furthermore, in many other
types of cells, YY1 was shown to upregulate glucocorticoid
receptor, the which has been regarded as a therapeutic target for
chronic pains (17–20). In this study, we found that
glucocorticoid receptor was also upregulated by YY1 overexpression
in spinal cord neurons, which may partially contribute to the
anti-neuroinflammation and neuropathic pain alleviation of YY1,
apart from upregulation of SOCS3.
In the overexpression experiments in vivo, 4
mg/kg and 8 mg/kg pcDNA-YY1 transfection significantly increased
the level of YY1 mRNA, compared with the control. Moreover, in
pcDNA-YY1 overexpression groups, YY1 mRNA displayed a robust
increase at day 3 and a drop at days 7 and 14. It is worth
mentioning that, unlike the change of YY1 mRNA expression, YY1
protein expression was being increased from day 0 to day 14 in the
8 mg/kg pcDNA-YY1 group. We carefully checked these results and
found that the expression patterns of YY1 mRNA and its protein
indeed are not consistent, which is probably because the YY1
protein expression is lagged behind the expression of YY1 mRNA. In
fact, it is not uncommon that the expression patterns of mRNA and
protein of a gene are not consistent. For example, during porcine
adipogenesis, levels of the transcription factor PU.1 mRNA
increased at day 2 and then gradually decreased, but it is
interestingly that PU.1 protein level was being increased during
differentiation (21).
Spinal cord neurons play important roles in the
occurrence and maintenance of neuropathic pain during nerve injury
and systematic inflammation (22).
Neuropathic pain, embodied as pain hypersensitivity, was thought to
be mainly resulted from altered neuronal activity in primary
sensory and spinal cord neurons. Improvement of spinal cord neuron
viability and activity has been used to manage pains (23). In the present study, our results
demonstrated YY1 improved the viability and inhibited apoptosis of
spinal cord neurons, which explained why YY1 could alleviate
neuropathic pain in bCCI rats at the cellular level to some extent.
There were rarely reports on the role of YY1 in the behaviors of
spinal cord neuron, but its promotion on cell proliferation and
inhibition on cell apoptosis have been reported. For example,
during skeletal muscle development and regeneration, YY1 was found
to be upregulated, and downregulation of YY1 expression had an
effect on suppressing proliferation of myoblasts (24). YY1 was also proven to promote the
viability of many other types of cells, including many types of
cancer cells and leukomonocytes (25–27).
As for the role of YY1 in apoptosis, it was found to be one of only
a few transcription factors targeted by caspases (28). During many pathological processes,
YY1 was upregulated or activated in response to cell stress to
block cell death signaling pathways (29–31).
The STAT signaling is a key pathway activated by
nerve injury and plays an important role in nerve survival and
regeneration (32). STAT3 was
reported to promote cell damage in the immune response and is
activated by phosphorylation (33).
Persistent activated STAT3 leads to occurrence of many diseases,
including neurological impairment (34). The activation of the STAT3 pathway
is also observed in bCCI rats, during which STAT3 was highly
phosphorylated (8). Activated STAT3
upregulated downstream inflammation biomarkers COX-2 and CCL2,
induced production and release of pro-inflammatory cytokines IL-1,
IL-6 and TNF-α, and then resulted in intense inflammatory response
and then causing histiocytic death, tissue dysfunction and pain
(35–38).
SOCS3 was reported as the most effective negative
regulator of the Janus Kinase2/STAT3 signaling (39). Some recent studies indicated that
SOCS3 has been identified as a therapeutic target for neuropathic
pain (39–41). For example, downregulation of
microRNA-218 caused increase in SOCS3 expression and can relieve
neuropathic pain in bCCI rats (39). Here, our results showed that
upregulation of SOCS3 by YY1 overexpression decreased had a
suppression effect on STAT3 activation in spinal cord cells and
could relieve neuropathic pain bCCI rats. In conclusion, YY1 is
downregulated in bCCI rats, and it upregulates SOCS3 expression to
inhibit STAT3-mediated neuroinflammation and neuropathic pain.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MYS and KZL designed the present study. MYS, YS and
JFM performed the experiments, analyzed the data and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yantai Yuhuangding Hospital (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neville A, Peleg R, Singer Y, Sherf M and
Shvartzman P: Chronic pain: A population-based study. Isr Med Assoc
J. 10:676–680. 2008.
|
|
2
|
Yoshimura A, Naka T and Kubo M: SOCS
proteins, cytokine signalling and immune regulation. Nat Rev
Immunol. 7:454–465. 2007. View
Article : Google Scholar
|
|
3
|
Recio C, Oguiza A, Mallavia B, Lazaro I,
Ortiz-Muñoz G, Lopez-Franco O, Egido J and Gomez-Guerrero C: Gene
delivery of suppressors of cytokine signaling (SOCS) inhibits
inflammation and atherosclerosis development in mice. Basic Res
Cardiol. 110:82015. View Article : Google Scholar
|
|
4
|
Jo D, Liu D, Yao S, Collins RD and Hawiger
J: Intracellular protein therapy with SOCS3 inhibits inflammation
and apoptosis. Nat Med. 11:892–898. 2005. View Article : Google Scholar
|
|
5
|
Shi Y, Lee JS and Galvin KM: Everything
you have ever wanted to know about Yin Yang 1……. Biochim Biophys
Acta. 1332:F49–F66. 1997.
|
|
6
|
Sui G, Affar el B and Shi Y, Brignone C,
Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR and Shi Y:
Yin yang 1 is a negative regulator of p53. Cell. 117:859–872. 2004.
View Article : Google Scholar
|
|
7
|
Guo J, Lin X, Williams MA, Hamid Q and
Georas SN: Yin-yang 1 regulates effector cytokine gene expression
and T(H)2 immune responses. J Allergy Clin Immunol. 122:195–201,
201.e1-e5. 2008. View Article : Google Scholar
|
|
8
|
Xue ZJ, Shen L, Wang ZY, Hui SY, Huang YG
and Ma C: STAT3 inhibitor WP1066 as a novel therapeutic agent for
bCCI neuropathic pain rats. Brain Res. 1583:79–88. 2014. View Article : Google Scholar
|
|
9
|
Soares Nda C, Teodoro AJ, Oliveira FL,
Santos CA, Takiya CM, Junior OS, Bianco M, Junior AP, Nasciutti LE,
Ferreira LB, et al: Influence of lycopene on cell viability cell
cycle and apoptosis of human prostate cancer and benign
hyperplastic cells. Nutr Cancer. 65:1076–1085. 2013. View Article : Google Scholar
|
|
10
|
Sánchez-Fidalgo S, da Silva MS, Cárdeno A,
Aparicio-Soto M, Salvador MJ, Frankland Sawaya AC, Souza-Brito AR
and de la Lastra CA: Abarema cochliacarpos reduces LPS-induced
inflammatory response in murine peritoneal macrophages regulating
ROS-MAPK signal pathway. J Ethnopharmacol. 149:140–147. 2013.
View Article : Google Scholar
|
|
11
|
Watkins LR and Maier SF: Immune regulation
of central nervous system functions: From sickness responses to
pathological pain. J Intern Med. 257:139–155. 2005. View Article : Google Scholar
|
|
12
|
Seiler F, Herr C, Lepper PM, Bals R and
Beisswenger C: FOXO transcription factors regulate innate immune
mechanisms in respiratory epithelial cells during bacterial
infection. American thoracic society 2012 international conference
may 18–23, 2012 • San Francisco, California. ppA42582012.
|
|
13
|
Lu SY, Rodriguez M and Liao WS: YY1
represses rat serum amyloid A1 gene transcription and is
antagonized by NF-kappa B during acute-phase response. Mol Cell
Biol. 14:6253–6263. 1994. View Article : Google Scholar
|
|
14
|
Yan X, Pan J, Xiao W, Cheng M, Sun Y,
Zhang S and Chen Y: Yin Yang 1 (YY1) synergizes with Smad7 to
inhibit TGF-β signaling in the nucleus. Sci China Life Sci.
57:128–136. 2014. View Article : Google Scholar
|
|
15
|
Rylski M, Amborska R, Zybura K,
Mioduszewska B, Michaluk P, Jaworski J and Kaczmarek L: Yin yang 1
is a critical repressor of matrix metalloproteinase-9 expression in
brain neurons. J Biol Chem. 283:35140–35153. 2008. View Article : Google Scholar
|
|
16
|
Yeh TS, Lin YM, Hsieh RH and Tseng MJ:
Association of transcription factor YY1 with the high molecular
weight notch complex suppresses the transactivation activity of
notch. J Biol Chem. 278:41963–41969. 2003. View Article : Google Scholar
|
|
17
|
Takasaki I, Kurihara T, Saegusa H, Zong S
and Tanabe T: Effects of glucocorticoid receptor antagonists on
allodynia and hyperalgesia in mouse model of neuropathic pain. Eur
J Pharmacol. 524:80–83. 2005. View Article : Google Scholar
|
|
18
|
Mao J: Central glucocorticoid receptor: A
new role in the cellular mechanisms of neuropathic pain. Rev
Neurosci. 16:233–238. 2005. View Article : Google Scholar
|
|
19
|
Lu Y, Xiong X, Wang X, Zhang Z, Li J, Shi
G, Yang J, Zhang H, Ning G and Li X: Yin yang 1 promotes hepatic
gluconeogenesis through upregulation of glucocorticoid receptor.
Diabetes. 62:1064–1073. 2013. View Article : Google Scholar
|
|
20
|
Bergad PL, Towle HC and Berry SA: Yin-yang
1 and glucocorticoid receptor participate in the Stat5-mediated
growth hormone response of the serine protease inhibitor 2.1 gene.
J Biol Chem. 275:8114–8120. 2000. View Article : Google Scholar
|
|
21
|
Wei N, Wang Y, Xu RX, Wang GQ, Xiong Y, Yu
TY, Yang GS and Pang WJ: PU.1 antisense lncRNA against its mRNA
translation promotes adipogenesis in porcine preadipocytes. Anim
Genet. 46:133–140. 2015. View Article : Google Scholar
|
|
22
|
Wu J, Zhao Z, Zhu X, Renn CL, Dorsey SG
and Faden AI: Cell cycle inhibition limits development and
maintenance of neuropathic pain following spinal cord injury. Pain.
157:488–503. 2016. View Article : Google Scholar
|
|
23
|
Sato KL, Johanek LM, Sanada LS and Sluka
KA: Spinal cord stimulation reduces mechanical hyperalgesia and
glial cell activation in animals with neuropathic pain. Anesth
Analg. 118:464–472. 2014. View Article : Google Scholar
|
|
24
|
Wang M, Liu C, Su Y, Zhang K, Zhang Y,
Chen M, Ge M, Gu L, Lu T, Li N, et al: miRNA-34c inhibits myoblasts
proliferation by targeting YY1. Cell Cycle. 16:1661–1672. 2017.
View Article : Google Scholar
|
|
25
|
Ramkumar C, Cui H, Kong Y, Jones SN,
Gerstein RM and Zhang H: Smurf2 suppresses B-cell proliferation and
lymphomagenesis by mediating ubiquitination and degradation of YY1.
Nat Commun. 4:25982013. View Article : Google Scholar
|
|
26
|
Lu S, Wang MS, Chen PJ, Ren Q and Bai P:
MiRNA-186 inhibits prostate cancer cell proliferation and tumor
growth by targeting YY1 and CDK6. Exp Ther Med. 13:3309–3314. 2017.
View Article : Google Scholar
|
|
27
|
Wang M, Zhi LI, Jinyu XU and Shanshan XU:
The inhibition of cell proliferation and invasion through miR-34a
regulating YY1 in human renal carcinoma cell. Lab Med. 27:635–640.
2012.
|
|
28
|
Riman S: Mechanisms regulating YY1
cleavage during apoptosis. Dissertations & Theses-Gradworks.
2011.
|
|
29
|
Yakovleva T, Kolesnikova L, Vukojević V,
Gileva I, Tan-No K, Austen M, Lüscher B, Ekström TJ, Terenius L and
Bakalkin G: YY1 binding to a subset of p53 DNA-target sites
regulates p53-dependent transcription. Biochem Biophys Res Commun.
318:615–624. 2004. View Article : Google Scholar
|
|
30
|
Reséndizmartínez J, Asbunbojalil J,
Huertayepez S and Vega M: Correlation of the expression of YY1 and
fas cell surface death receptor with apoptosis of peripheral blood
mononuclear cells and the development of multiple organ dysfunction
in children with sepsis. Mol Med Rep. 15:2433–2442. 2017.
View Article : Google Scholar
|
|
31
|
Trabucco SE, Gerstein RM and Zhang H: YY1
regulates the germinal center reaction by inhibiting apoptosis. J
Immunol. 197:1699–1707. 2016. View Article : Google Scholar
|
|
32
|
Nicolas CS, Mascia A, Bortolotto ZA,
Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge
GL and Peineau S: The role of JAK-STAT signaling within the CNS.
JAKSTAT. 2:e229252013.
|
|
33
|
Planas AM, Gorina R and Chamorro A:
Signalling pathways mediating inflammatory responses in brain
ischaemia. Biochem Soc Trans. 34:1267–1270. 2006. View Article : Google Scholar
|
|
34
|
Cai QW, Li J, Li XQ, Wang JQ and Huang Y:
Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and
correlation with pathological features. Mol Med Rep. 5:1438–1442.
2012.
|
|
35
|
Matsukawa A, Kudo S, Maeda T, Numata K,
Watanabe H, Takeda K, Akira S and Ito T: Stat3 in resident
macrophages inflammatory response. J Immunol. 175:3354–3359. 2005.
View Article : Google Scholar
|
|
36
|
Kiguchi N, Saika F, Kobayashi Y, Ko MC and
Kishioka S: TC-2559, an α4β2 nicotinic acetylcholine receptor
agonist, suppresses the expression of CCL3 and IL-1β through STAT3
inhibition in cultured murine macrophages. J Pharmacol Sci.
128:83–86. 2015. View Article : Google Scholar
|
|
37
|
Rummel C, Sachot C, Poole S and Luheshi
GN: Circulating interleukin-6 induces fever through a STAT3-linked
activation of COX-2 in the brain. Am J Physiol Regul Integ Comp
Physiol. 291:R1316–R1326. 2006. View Article : Google Scholar
|
|
38
|
Aid S, Langenbach R and Bosetti F:
Neuroinflammatory response to lipopolysaccharide is exacerbated in
mice genetically deficient in cyclooxygenase-2. J
Neuroinflammation. 5:1–14. 2008. View Article : Google Scholar
|
|
39
|
Li L and Zhao G: Downregulation of
microRNA-218 relieves neuropathic pain by regulating suppressor of
cytokine signaling 3. International J Mol Med. 37:851–858. 2016.
View Article : Google Scholar
|
|
40
|
Croker BA, Krebs DL, Zhang JG, Wormald S,
Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen
BE, et al: SOCS3 negatively regulates IL-6 signaling in vivo. Nat
Immunol. 4:540–545. 2003. View
Article : Google Scholar
|
|
41
|
Xiang-Mei YU, Yang YZ, Liu JB, Chang-Zheng
LI, Gong DG and Wang ZF: Effects of warming acupuncture therapy on
expressions of IL-6 and SOCS3 in spinal cord in rats with
neuropathic pain. Chin J Inform Tradit Chin Med. 2018.
|