Introduction
Nasopharyngeal carcinoma (NPC) refers to a malignant
type of cancer that occurs at the top and lateral wall of the
nasopharyngeal cavity. Currently, this deleterious tumor has an
extremely unbalanced geographical global distribution, with a high
incidence rate in Asian countries, particularly in south China
(1). The annual survival rate of
patients with NPC is ~76%, whereas the 5-year survival rate is 50%
(2). As most patients with NPC are
sensitive to ionizing radiation, radiotherapy is the primary method
of treating non-metastatic diseases (3). With advances in radiation therapy,
most sensitive cells can be destroyed using ionizing radiation
(4). Nonetheless, tumor cells may
develop strong radiotherapy tolerance, thereby resulting in tumor
recurrence (5). Although several
mechanisms such as circular RNAs (6) and mRNA demethylation (7) underlie the acquisition of tolerance to
radiotherapy, their effects on humans remain to be elucidated.
MicroRNAs (miRNAs/miRs) are small, endogenous,
non-coding RNA molecules, ≤23 nucleotides in length (8). miRNAs bind to the 3′-untranslated
region (UTR) of its target genes, inhibiting its translation or
degrading it altogether, thus decreasing the intracellular
expression levels of the target gene (9–11).
Previous studies have confirmed that miRNAs are involved in altered
radioresistance in NPC cells, including miR-125b, miRNA-17 and
miR-203 (12–14). A commonly known onco-miR,
miR-182-5p, has been reported to be overexpressed in glioma, liver
cancer, lung cancer and medullary thyroid carcinoma (15–18).
However, the contribution of miR-182-5p in NPC and radioresistance
of cancer remains unknown.
BCL2/adenovirus E1B 19 kDa protein-interacting
protein 3 (BNIP3) is a member of the Bcl-2 protein family, and it
is the only BH3 family member that contains the BH-3 domain. BNIP3
is also a pro-apoptotic protein whose structure, intracellular
localization and regulation of cell survival are not identical to
other proteins of the BH3-only subfamily (19). As a pro-apoptotic gene, BNIP3 had
been demonstrated to act as a tumor suppressor in several types of
cancer, such as breast cancer (20), clear cell renal cell carcinoma
(21) and malignant glioma
(22). BNIP3 has also been shown to
be overexpressed in NPC cells when the cells were treated with
SYUIQ-5 (a cell autophagy reagent), thus suggesting that the
overexpression of BNIP3 may be positively associated with autophagy
(23). However, it has also been
reported that the downregulation of BNIP3 inhibits hepatocellular
carcinoma cell progression, with insufficient radiofrequency
ablation that was found to enhance tumor aggression (24).
Nonetheless, the effect of BNIP3 in several other
types of cancer has yet to be comprehensively studied. Thus, the
aim of the present study was to examine the effects of BNIP3 on the
radioresistance of NPC cells and assess the relationship between
BNIP3 and miR-182-5p. It was also hypothesized that overexpression
of BNIP3 may inhibit the radioresistance of NPC cells and that
miR-182-5p could reverse this inhibitory effect. The present study
found that BNIP3 inhibited the radioresistance of NPC cells by
inhibiting viability, invasion and migration and promoting
apoptosis. Therefore, the mechanism of BNIP3 in NPC radioresistance
requires further investigation.
Materials and methods
Bioinformatics analysis
The GEO dataset, GSE48503, was obtained from the
National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48503)
(25). The GEO dataset consisted of
two radio-sensitive samples and two radioresistant samples.
Differentially expressed genes (DEGs) were identified using the
screening criteria of adjusted P<0.05, so the statistically
significant DEGs were selected. Subsequently, the DEGs were
uploaded to Metascape (https://metascape.org/) for enrichment analysis.
Kaplan-Meier plotter (kmplot.com/) was
used to evaluate the prognostic effect of the key genes in patients
with NPC.
Cell culture
5-8F cells were obtained from Sun Yat-sen University
Cancer Center (Guangzhou, China). Radioresistant NPC cells
(5-8F-R), were derived from 5–8F cells, and established at Wuhan
Puren Hospital (Wuhan, China) according to previously reported
methods (26). Cell lines were
authenticated using STR profiling, and cultured at a density of
1×105 cells/6-cm plate for 24 h in an RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) with 5% CO2 at 37°C.
Cells were exposed to 10 Gy irradiation (IR) at 300 cGy/min with a
linear accelerator (Clinac 23EX; Varian Medical Systems, Inc.).
Cells were exposed to radiation for 2 weeks before the 5–8F-R cell
line was established. A Cell Counting Kit-8 (CCK-8) assay was used
to evaluate whether the 5–8F-R cell line was established
successfully. 293T cells (Procell Life Science & Technology
Co., Ltd.) were used for luciferase reporter gene assay and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS.
Cell transfection
miR-182-5p mimic, miR-182-5p inhibitor and
corresponding negative control (NC-mimic, inhibitor-NC), BNIP3
small interfering RNA (si-BNIP3) and non-targeting sequence for
siRNA (si-NC), recombinant BNIP3 overexpression vectors
(constructed using pcDNA3.1 plasmid, BNIP3 OE) and empty vectors
(OE-NC) were purchased from Shanghai GenePharma Co., Ltd. Cells
were cultured in 6-well plates (3×105 cells/well) for 24
h. Subsequently, Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to transfect NC (50 nM), BNIP3
siRNA (50 nM), BNIP3 OE (1 µg/ml), miR-182-5p inhibitor (50 nM) or
miR-182-5p mimic (50 nM) into the cells. For the co-transfection
group, cells were transfected with 1 µg/ml BNIP3 OE and 50 nM
miR-182-5p mimic (OE+mimic) or 50 nM BNIP3 siRNA and 100 nM
miR-182-5p inhibitor (si+inhibitor). Following transfection for 48
h, the follow-up experiments were performed. The sequences of
vectors are given in Table SI.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from 5–8F and
5–8F-R cells. Subsequently, cDNA was synthesized at 50°C for 30 min
and 95°C for 4 min using 1 µg RNA and a cDNA synthesis kit (cat.
no. D6210A, Takara Bio, Inc.). cDNA was quantified using TaqMan™
Universal PCR Master Mix (Thermo Fisher Scientific, Inc.) with a
real-time PCR system. For miRNA quantification, miRNA was purified
using a miRNeasy kit (cat. no. 217604; Qiagen, Inc.). The miRNA was
then reverse transcribed by incubation at 37°C for 1 h and 95°C for
5 min using a miScript II RT kit (cat. no. 218161; Qiagen, Inc.).
Finally, the cDNA was quantified using a miScript SYBR Green PCR
kit (cat. no. 218073; Qiagen, Inc.). The thermocycling conditions
used for amplification were: 10 min at 95°C; followed by 40 cycles
of 30 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C. U6 and GAPDH
were used as the internal controls for miR-182-5p and BNIP3,
respectively. The sequences of the primers used are presented in
Table I. The relative expression
was quantified by the 2−ΔΔCq method (27).
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene | Primer sequences
(5′→3′) |
|---|
| miR-182-5p | F:
TGCGGTTTGGCAATGGTAGAA |
|
| R:
CCAGTGCAGGGTCCGAGGT |
| BNIP3 | F:
GAAACAGATACCCATAGCA |
|
| R:
GAACGCAGCATTTACAGA |
| GAPDH | F:
ATGGAGAAGGCTGGGGCTC |
|
| R:
AAGTTGTCATGGATGACCTTG |
| U6 | F:
TGCGGGTGCTCGCTTCGGCAGC |
|
| R:
CCAGTGCAGGGTCCGAGGT |
Western blotting
Cells were washed with PBS and lysed using RIPA
buffer (Sigma-Aldrich; Merck KGaA) supplemented with a protease
inhibitor. After isolating the cells, the protein concentration was
measured using a bicinchoninic acid assay kit. Proteins (30 µg)
were loaded on a 12% SDS-gel and resolved using SDS-PAGE. The
separated proteins were then transferred to a PVDF membrane and the
membranes were washed with TBS with 0.1% Tween-20 (TBST) and
blocked for 1 h with 5% skimmed milk at room temperature. Membranes
were incubated with primary antibodies against GAPDH (1:5,000; cat.
no. ab8245; Abcam), BNIP3 (1:5,000; cat. no. ab109362; Abcam),
E-cadherin (1:10,000; cat. no. ab40772; Abcam), N-cadherin
(1:5,000; cat. no. ab76011; Abcam), cleaved caspase-3 (1:500; cat.
no. ab32042; Abcam), caspase-3 (1:500; cat. no. ab13847; Abcam),
cleaved caspase-9 (1:5,000; cat. no. ab2324; Abcam), caspase-9
(1:1,000; cat. no. ab32539; Abcam) and Bax (1:1,000; cat. no.
ab32503; Abcam) at 4°C for 12 h. Subsequently, membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:2,000; cat. no. ab205719; Abcam) at 37°C for 2 h.
Signals were visualized using chemiluminescence reagent (Bio-Rad
Laboratories, Inc.), and densitometry analysis was performed using
FlourChem FC2 (ProteinSimple) with AlphaEase FC software version
6.0.2 (ProteinSimple).
CCK-8 assay
A CCK-8 assay was used to evaluate the viability of
transfected 5–8F-R and 5–8F cells following IR treatment. The cells
were first seeded in 96-well plates (2.5×103 cells/well)
and then cultured overnight. Cells were later exposed to five
different doses of IR (0, 2, 4, 6 or 8 Gy). After 4 days of IR
exposure, 10 µl CCK-8 reagent (Dojindo Molecular Technologies,
Inc.) was added to each well and incubated for a further 4 h.
Subsequently, the optical density was measured using a microplate
reader at 450 nm.
Colony formation assays
A total of 2.5×103 transfected 5–8F-R and
5–8F cells were seeded in 6-well plates for 12 h, and subsequently
exposed to 4 Gy IR for 14 days until cell colonies were visible.
Cells were washed with PBS and fixed with 10% paraformaldehyde for
15 min at room temperature. Colonies were washed twice with PBS and
stained with crystal violet for 30 min at room temperature. The
number of visible colonies in each well (≥50 cells) was counted
under a light microscope (Olympus Corporation) at 10×
magnification.
Wound healing assay
5-8F cells and 5–8F-R cells were transfected for 48
h. Following treatment with 4 Gy IR for 4 days, the cells were
plated in 6-well plates at a density of 3×105
cells/well, and grown until they formed a confluent monolayer. A
pipette tip was used to scratch a wound in the middle of each
monolayer, and the medium was replaced with fresh RPMI-1640 medium
without FBS. After 24 h, the wells were imaged using an inverted
microscope (Olympus Corporation) at ×100 magnification. The
migration rate was defined as the ratio of the migrated distance
after 24 h compared with the width of the scratch at the start.
Transwell invasion assay
Matrigel was diluted to 50 mg/l in serum-free
RPMI-1640 medium in a 4°C refrigerator overnight and added to the
upper chamber of the Transwell insert. Following IR treatment, the
1×104/100 µl transfected 5–8F-R and 5–8F cells were
seeded in the upper chamber, and 500 µl RPMI-1640-medium containing
10% FBS was added to the lower chamber. Cells were incubated for 24
h at 37°C, after which, the upper chamber was removed and washed
twice with PBS. Subsequently, the cells on the upper side of the
chamber membrane were removed using cotton buds, and the cells that
had invaded were fixed with 4% paraformaldehyde for 15 min at room
temperature. The cells were subsequently stained with 1% crystal
violet for 5 min at room temperature. Finally, the number of cells
in six randomly selected fields of view were counted under a light
microscope (Olympus Corporation) at ×100 magnification.
Cell apoptosis assay
Apoptosis of transfected IR-treated 5–8F-R and 5–8F
cells was assessed using an Annexin V -FITC/PI apoptosis detection
kit (Invitrogen; Thermo Fisher Scientific, Inc.). A total of
2×105 cells were obtained and re-suspended in 100 µl 1X
binding buffer, and 5 µl PI solution and 2.5 µl Annexin V-FITC were
added to the cell suspension in the dark for 30 min. A BD
FACSCalibur flow cytometer (Becton, Dickinson and Company) was used
to detect cell apoptosis and CellQuest Pro software version 5.1
(Becton, Dickinson and Company) was used to analyze apoptosis rate.
The cells in the right quadrants were considered the apoptotic
cells.
Dual-luciferase reporter assay
TargetScan Human 7.2 (28,29)
was used to predict the binding site between the BNIP3 mRNA and
miR-182-5p. BNIP3 mRNA 3′untranslated region (UTR), which contained
the binding site of miR-182-5p, was mutated to obtain a mutant
BNIP3 mRNA 3′UTR. The mutant and wild-type BNIP3 mRNA 3′UTR were
then cloned into the pGL4 luciferase reporter vector (Promega
Corporation). The constructs were subsequently transfected into
293T cells together with miR-182-5p mimic or mimic NC (Shanghai
GenePharma Co., Ltd.) by Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Renilla
constructs were used as the internal control. A total of 48 h
later, the cells were collected and washed with PBS. A
dual-luciferase reporter system (Promega Corporation) was used to
measure the luciferase activity of cells in each group.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 7.0 (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation of at least three independent
experiments. Differences between groups were compared using a
Student's t-test or an ANOVA followed by Dunnett's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
BNIP3 expression is downregulated in
5–8F-R cells
A total of 53 significant DEGs were identified based
on the GSE48503 dataset. The 53 DEGs are listed in Table SII. Following enrichment analysis,
three genes (cyclin-G2, BNIP3 and homer scaffold protein 2) were
found to be closely associated with the ‘Foxo signaling pathway’,
which has been reported to be involved in cancer development
(Fig. 1A) (30,31).
The 5-year survival analysis of the three genes demonstrated that
BNIP3 expression was significantly associated with an improved
outcome (Fig. 1B). Therefore, BNIP3
was further investigated in NPC radioresistance. The 5–8F and
5–8F-R cells were irradiated with different doses of IR to identify
the IC50 of IR for 5–8F and 5–8F-R cells. 5–8F cells
exhibited significantly reduced viability compared with the 5–8F-R
cells when irradiated (P<0.001). Cell viability was decreased by
50% at 4 Gy in 5–8F cells (Fig.
1C); thus, 4 Gy was used in subsequent experiments.
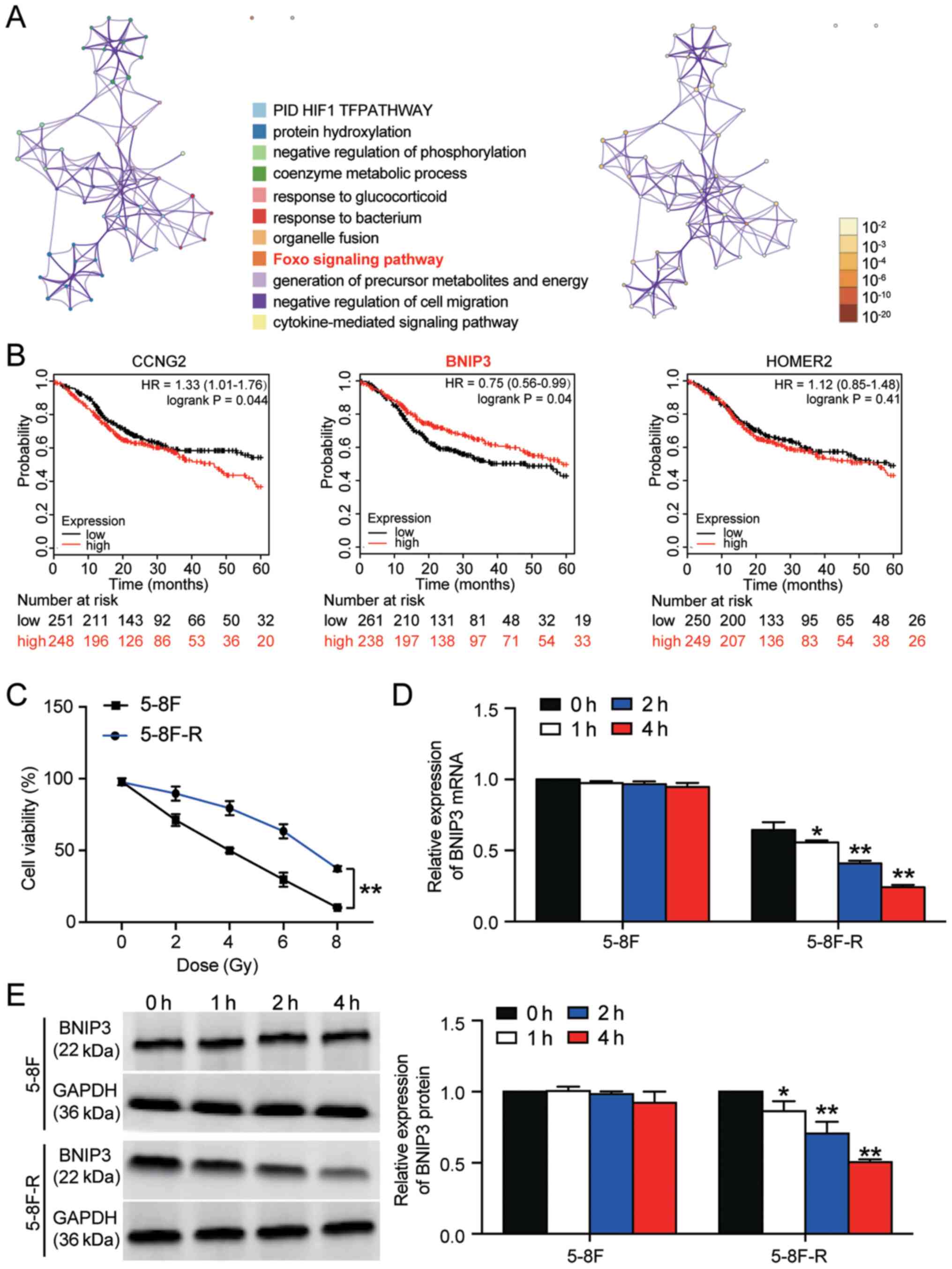 | Figure 1.BNIP3 expression is downregulated in
radioresistant NPC cells. (A) A total of 53 significant DEGs were
identified in the GSE48503 dataset between radioresistant NPC
samples and radio-sensitive NPC samples. Enrichment analysis was
performed on the DEGs. The ‘Foxo signaling pathway’ was shown to be
the key pathway associated with BNIP3, CCNG2 and HOMER2. (B) The
5-year survival rates of patients based on CCNG2, BNIP3 and HOMER2
expression, stratified by expression, was analyzed using
Kaplan-Meier plotter. (C) 5-8F-R and 5-8F cells were exposed to
different doses of IR, and the cell viability was measured. (D)
Expression of BNIP3 mRNA was detected in 5-8F-R cells and 5-8F
cells following IR. (E) Expression of BNIP3 protein was detected in
5-8F-R cells and 5-8F cells following IR treatment. *P<0.05,
**P<0.001 vs. 0 h. DEG, differentially expressed gene; IR,
irradiation; NPC, nasopharyngeal carcinoma; BNIP3, BCL2/adenovirus
E1B 19 kDa protein-interacting protein 3; CCNG2, cyclin-G2; HOMER2,
homer protein homolog 2. |
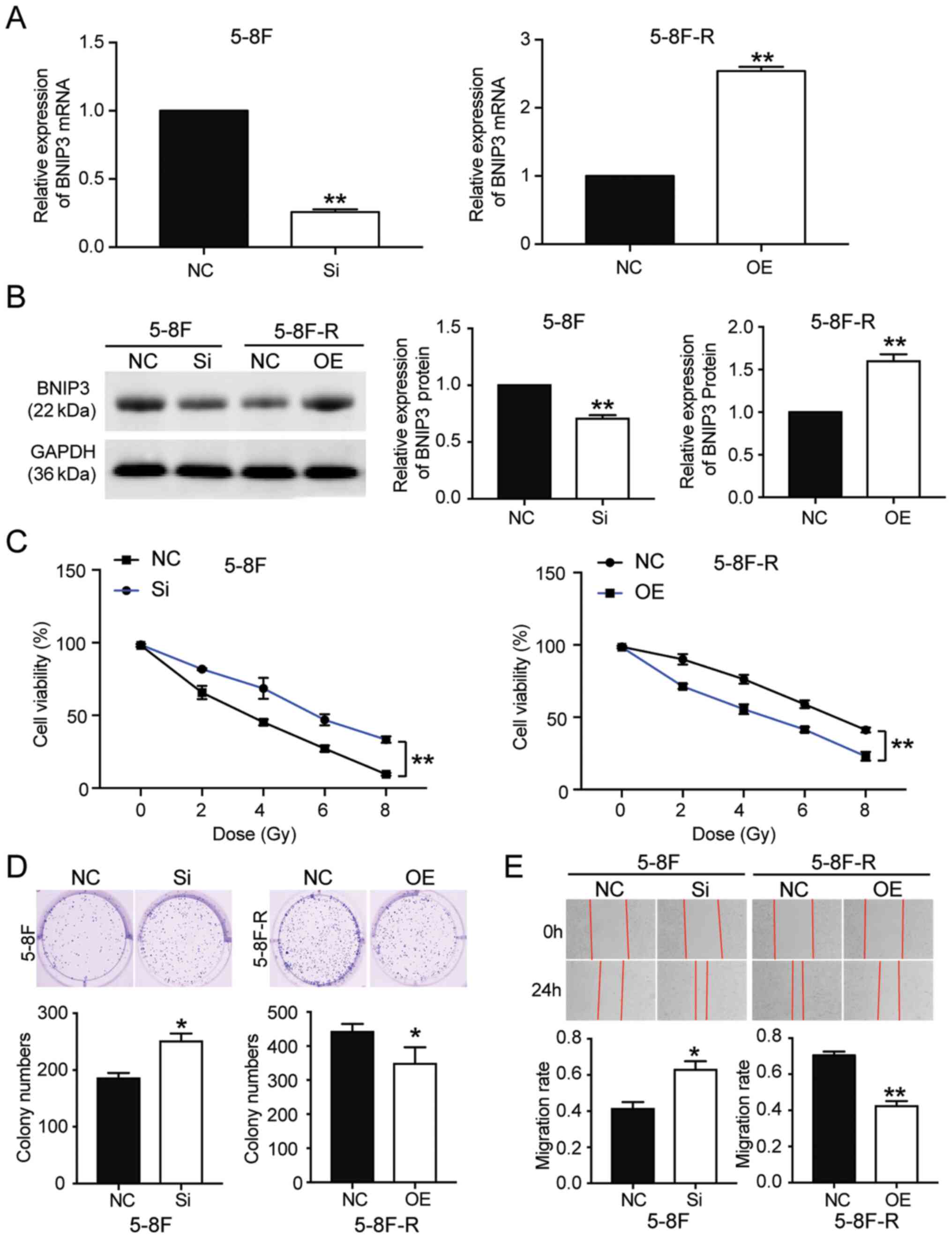
The expression levels of BNIP3 at both the mRNA and
protein level was significantly downregulated in 5–8F-R cells
following treatment with 4 Gy irradiation for 1, 2 and 4 h.
However, the expression of BNIP3 in 5–8F cells was not altered
significantly following irradiation treatment (*P<0.05,
**P<0.001 vs. 0 h; Fig. 1D and
E). Together, the results showed that downregulation of BNIP3
was associated with the radioresistance of 5–8F-R cells.
BNIP3 overexpression attenuates
radioresistance in NPC cells
To further clarify whether BNIP3 could affect the
radioresistance of NPC cells, BNIP3 siRNA and OE constructs were
transfected into the 5–8F cells and 5–8F-R cells, respectively.
RT-qPCR results showed that the BNIP3 mRNA expression levels in
5–8F cells transfected with si-BNIP3 constructs was reduced by 70%,
whereas in the 5–8F-R cells transfected with BNIP3 OE constructs,
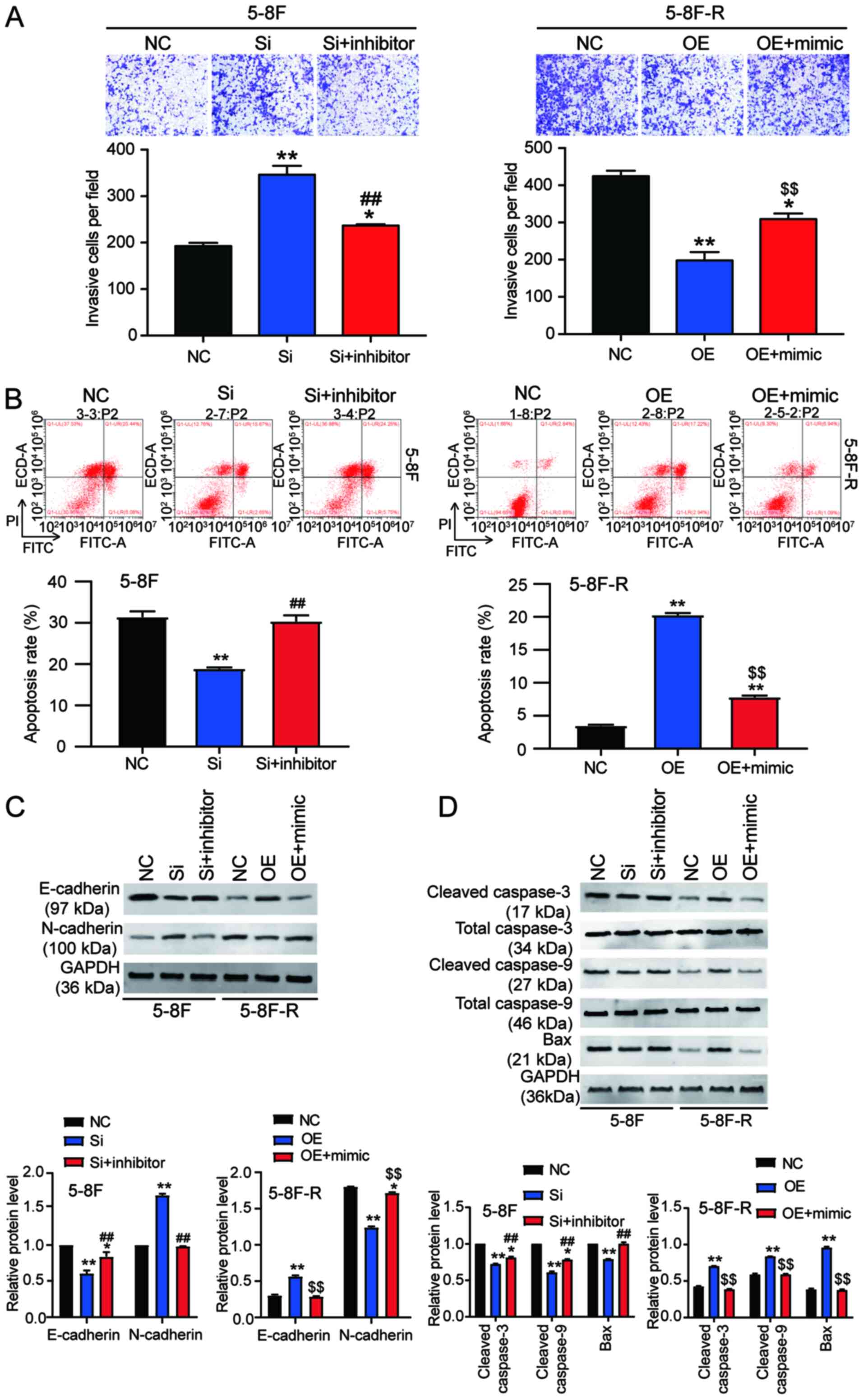
its expression was increased 2.5-fold (P<0.001 vs. NC; Fig. 2A). Western blotting results also
showed a 28% decrease in BNIP3 protein expression levels in the
si-BNIP3 group, as well as a 1.54-fold increase of BNIP3 protein
levels in BNIP3 OE cells (P<0.001 vs. NC; Fig. 2B). Moreover, the CCK-8 assays showed
that silencing BNIP3 increased the cell viability of 5–8F cells
treated with IR. By contrast, BNIP3 overexpression reduced the cell
viability of 5–8F-R cells treated with IR (P<0.001 vs. NC;
Fig. 2C). Colony formation was
increased by 36% in the 5–8F cells transfected with si-BNIP3, and
decreased by 21% in the 5–8F-R BNIP3 OE cells (P<0.05 vs. NC;
Fig. 2D). The 5–8F cells
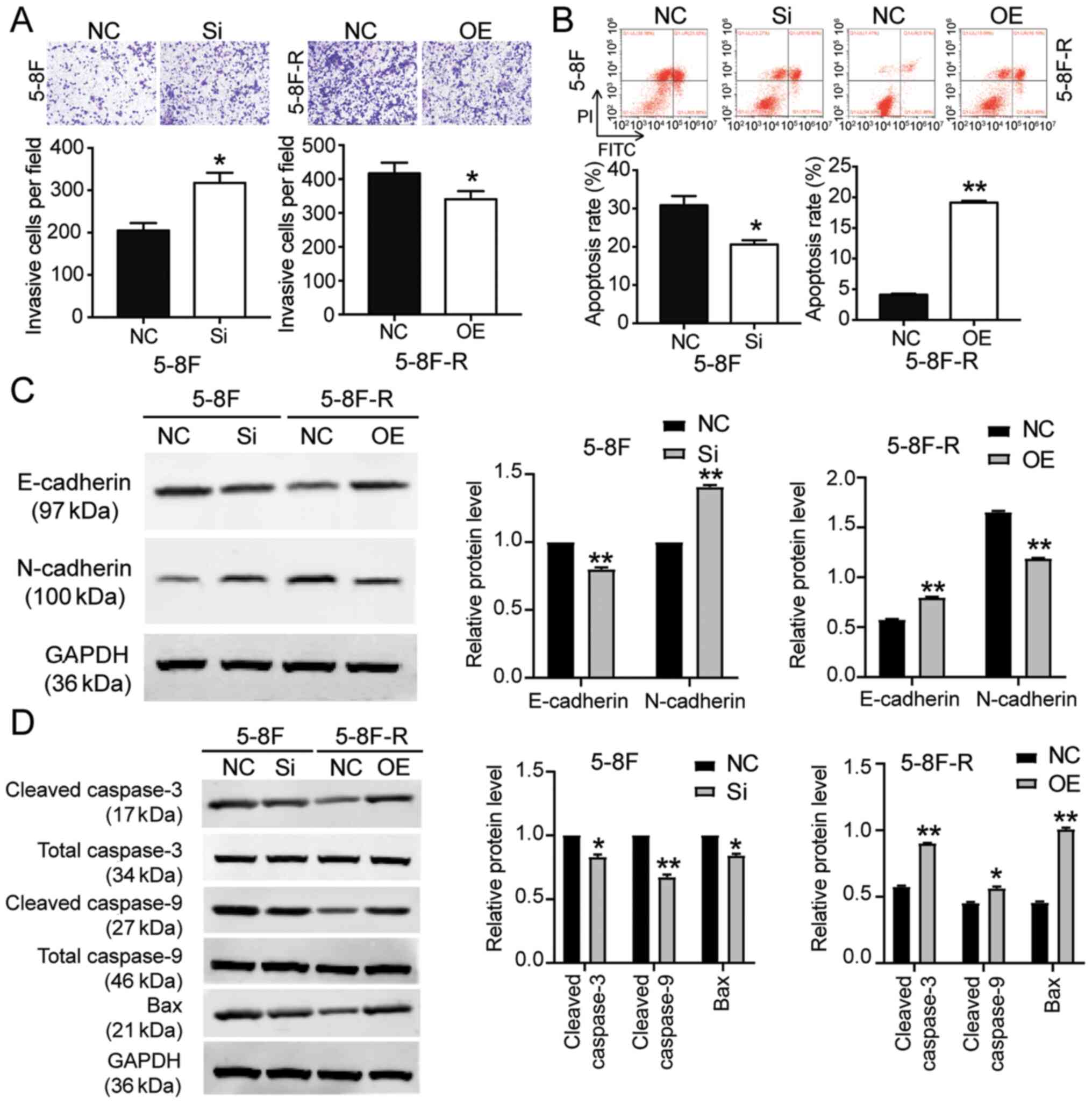
transfected with si-BNIP3 showed enhanced cell migration and
invasion, whereas the 5–8F-R cells overexpressing BNIP3 exhibited
reduced cell invasion and migration following IR treatment
(P<0.05, P<0.001 vs. NC; Figs.
2E and 3A). Knockdown of BNIP3
also resulted in a 33% decrease in the rate of apoptosis in the
5–8F cells, and overexpression of BNIP3 resulted in a 4.66-fold
increase in the rate of apoptosis in 5–8F-R cells following IR
exposure (P<0.05, P<0.001 vs. NC; Fig. 3B). Furthermore, expression of
invasion and apoptosis-related proteins was detected using western
blotting. E-cadherin expression decreased by 20%, and N-cadherin
increased by 45% in 5–8F cells following BNIP3 knockdown.
Conversely, E-cadherin expression increased by 40%, and N-cadherin
decreased by 25% in the 5–8F-R BNIP3 OE cells (P<0.001 vs. NC;
Fig. 3C). These results suggested
that BNIP3 impaired the invasion of NPC cells. After assessing the
expression of apoptosis-related proteins, cleaved caspase-3,
cleaved caspase-9 and Bax, the results showed that cleaved
caspase-3, cleaved caspase-9 and Bax protein expression levels
decreased following knockdown of BNIP3 in the 5–8F cells, and
increased in the 5–8F-R cells following overexpression of BNIP3
(P<0.05, P<0.001 vs. NC; Fig.
3D).
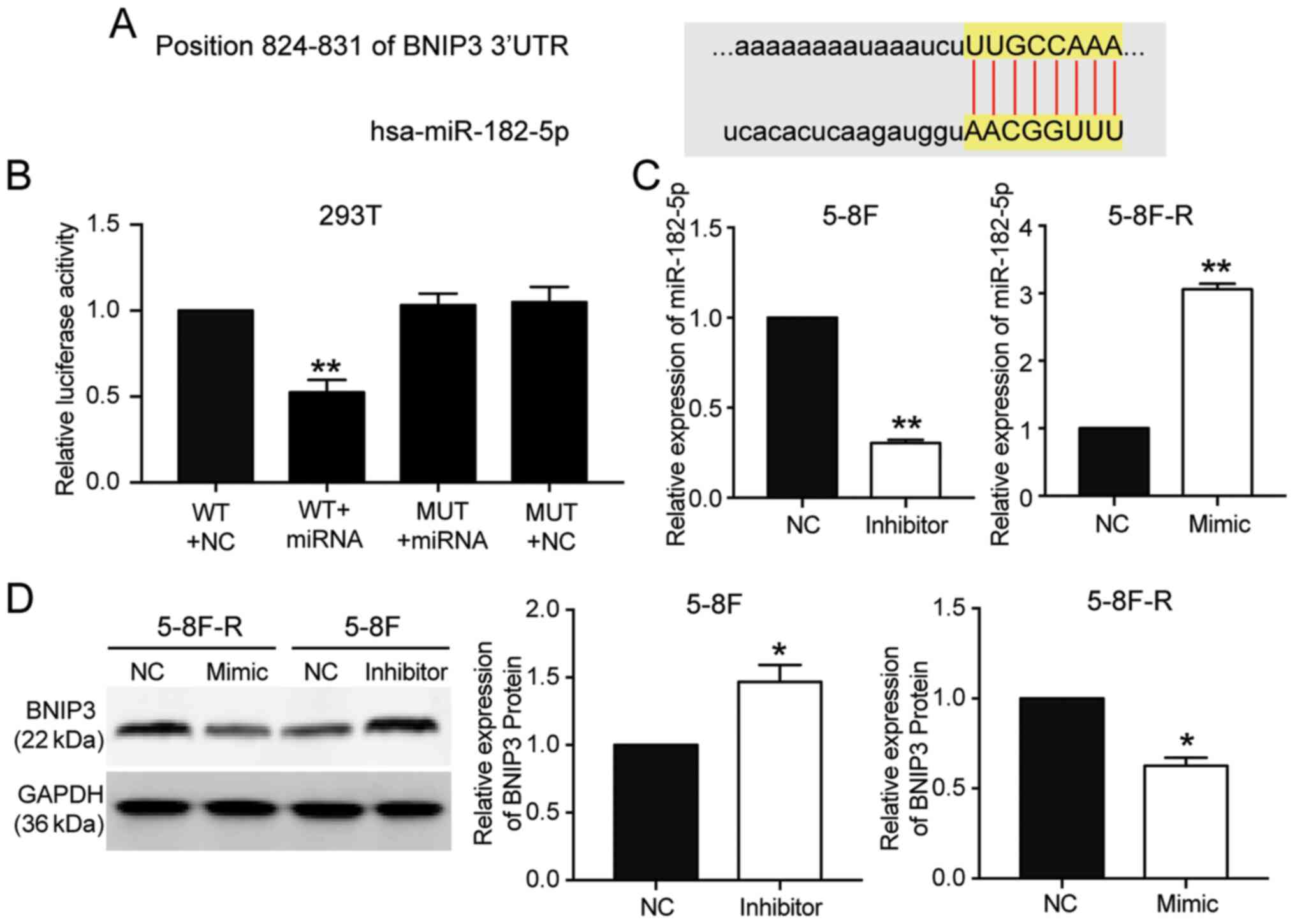
miR-182-5p targets the 3′UTR of
BNIP3
miR-182-5p was predicted to bind to the 3′UTR of
BNIP3 based on TargetScan Human 7.2 (Table SIII). The predicted binding
sequences between BNIP3 mRNA and miR-182-5p are presented in
Fig. 4A. The luciferase activity
assay results showed that transfection with miR-182-5p mimic
significantly reduced the luciferase activity of the wild-type
BNIP3 mRNA 3′UTR luciferase constructs, but not the mutated-type
BNIP3 mRNA 3′UTR luciferase plasmids (P<0.001 vs. WT+NC group;
Fig. 4B). To further support the
hypothesis that miR-182-5p directly targeted and suppressed BNIP3,
miR-182-5p mimic were transfected into 5–8F-R cells and miR-182-5p
inhibitor into 5–8F cells (P<0.001 vs. NC; Fig. 4C). BNIP3 protein expression levels
decreased by 33% in the 5–8F-R cells transfected with miR-182-5p
mimic, and increased 1.62-fold in the 5–8F cells transfected with
miR-182-5p inhibitor (P<0.05 vs. NC; Fig. 4D).
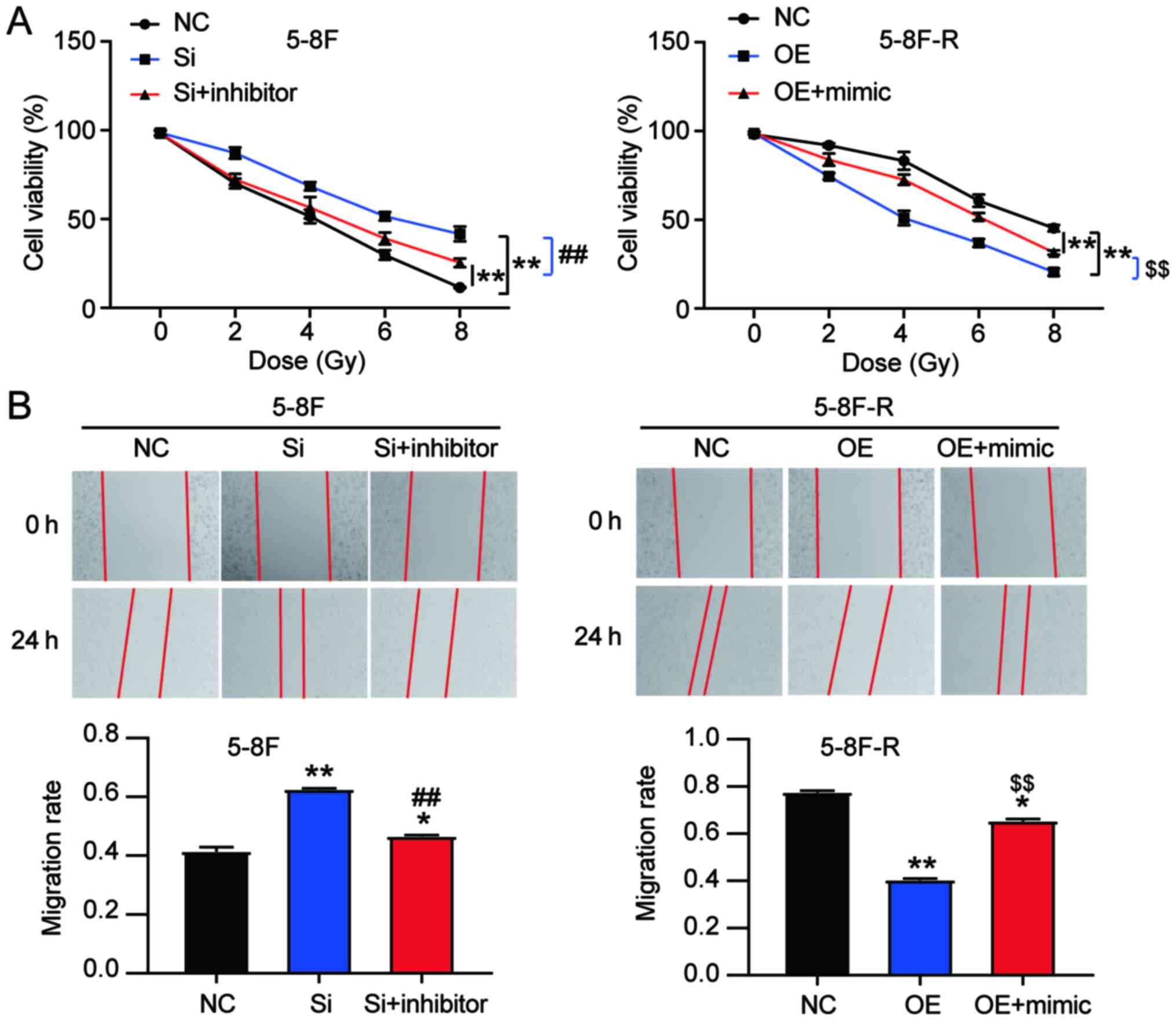
miR182-5p mitigates the effects of
BNIP3 on radioresistance of NPC cells
To explore the effect of miR-182-5p on NPC via
regulation of BNIP3, si-BNIP3 and miR-182-5p inhibitor were
co-transfected into 5–8F cells, and BNIP3 OE and miR-182-5p mimic
were co-transfected into 5–8F-R cells. The viability of 5–8F cells
in the co-transfection group was significantly lower than that of
the si-BNIP3 transfected cells. However, the viability of 5–8F-R
cells in the co-transfection group was significantly higher
compared with the BNIP3 OE group following IR treatment (P<0.001
vs. NC; P<0.001 vs. si-BNIP3 group in 5–8F cells; P<0.001 vs.
OE group in 5–8F-R cells; Fig. 5A).
Wound healing assays showed that knockdown of BNIP3 increased the
migration rate, which was reduced by miR-182-5p inhibitor
co-transfection in the 5–8F cells. Similarly, BNIP3 OE inhibited
the migration of 5-8F-R cells, whereas miR-182-5p mimic
co-transfection resulted in upregulation (P<0.05, P<0.001 vs.
NC; P<0.001 vs. si-BNIP3 in 5-8F cells; P<0.001 vs. OE group
in 5-8F-R cells; Fig. 5B). Compared
with the si-BNIP3 group, co-transfection of si-BNIP3 and miR-182-5p
inhibitor reduced the invasion count of 5-8F cells following IR
exposure (237 vs. 346 cells). In 5-8F-R cells following IR
treatment, the number of cells that had invaded was increased in
the BNIP3 OE+miR-182-5p mimic group compared with the BNIP3 OE
group (309 vs. 198 cells; P<0.05, P<0.001 vs. NC; P<0.001
vs. si-BNIP3 group in 5-8F cells; P<0.001 vs. OE group in 5-8F-R
cells; Fig. 6A).
Cell apoptosis analysis provided additional insights
into the function of BNIP3. si-BNIP3 transfection reduced 5-8F cell
apoptosis, and this reduction was reversed by co-transfection with
a miR-182-5p inhibitor. Moreover, BNIP3 OE increased 5-8F-R cell
apoptosis, which was reversed by co-transfection with miR-182-5p
mimic (P<0.001 vs. NC; P<0.001 vs. si-BNIP3 group in 5-8F
cells; P<0.001 vs. OE group in 5-8F-R cells; Fig. 6B).
The expression levels of invasion and
apoptosis-related proteins were also assessed. The downregulation
of E-cadherin and upregulation of N-cadherin caused by si-BNIP3
transfection were reversed by co-transfection with the miR-182-5p
inhibitor in 5-8F cells. The increased E-cadherin and decreased
N-cadherin observed following BNIP3 OE transfection in 5-8F-R cells
was reversed by co-transfection with the miR-182-5p mimic
(P<0.05, P<0.001 vs. NC; P<0.001 vs. si-BNIP3 group in
5-8F cells; P<0.001 vs. OE group in 5-8F-R cells; Fig. 6C). In addition, the reduced
expression levels of cleaved caspase-3, cleaved caspase-9 and Bax
caused by BNIP3 knockdown in 5-8F cells was reversed by
co-transfection with the miR-182-5p inhibitor. The increased
expression of cleaved caspase-3, cleaved caspase-9 and Bax caused
by BNIP3 OE transfection was reversed by co-transfection with the
miR-182-5p mimic in 5-8F-R cells (P<0.05, P<0.001 vs. NC;
P<0.001 vs. si-BNIP3 group in 5-8F cells; P<0.001 vs. OE
group in 5-8F-R cells; Fig.
6D).
Discussion
The main treatment for NPC is radiotherapy (32). Intensity-modulated radiotherapy
(IMRT) is the most prominently used technique for treating patients
with NPC. Although IMRT significantly improves the survival rates
and local control of NPC in patients, and reduces the toxicity
levels (1,33–36),
radioresistance is the primary cause for the failure of NPC
treatment (37). In the present
study, a radioresistant NPC cell model was established, termed
5-8F-R, to identify the key gene and miRNA involved in the
acquisition of NPC radioresistance. The results showed that
expression of BNIP3 was downregulated in 5-8F-R cells and that
BNIP3 impaired radioresistance in NPC cells. Conversely, miR-182-5p
was upregulated in 5-8F-R cells, and it enhanced the
radioresistance of NPC cells. Additionally, it was shown that
miR-182-5p reversed the effects of BNIP3 on radiation resistance of
NPC cells.
miRNAs have been reported to serve prominent roles
in cancer development. A number of studies have found that miRNAs
contribute to the acquisition of radioresistance and tumor
progression (38–41). Qu et al (39) established a radioresistant NPC cell
line model (CNE-2R) and found that miR-205 enhanced the number of
foci and reduced the apoptosis of CNE-2R cells by directly
targeting the tumor suppressor gene PTEN following IR treatment.
The results of other studies revealed that certain miRNAs increase
the radio-sensitivity and inhibit tumor growth in NPC (42). Qu et al (43) demonstrated that miR-23a decreased
the resistance to irradiation of NPC cells in vivo and in
vitro by activating the IL-8/STAT3 signaling pathway, and may
thus serve as a potential therapeutic target for treatment of NPC.
Although miR-182-5p has been demonstrated to serve as an onco-miR
in several types of cancer, including breast cancer (44), ovarian cancer (45) and melanoma (46), its effects on NPC have not been
explored. Only one study has confirmed that miR-182-5p is
associated with radioresistance in non-small cell lung cancer
(47); where it was shown that by
downregulating miR-182-5p expression, cell proliferation was
inhibited and cell apoptosis was promoted following IR treatment in
non-small cell lung cancer cells. Downregulation of miR-182-5p
resulted in DNA damage, and thus cell-cycle arrest. In the present
study, following successful establishment of the 5-8F-R cell line,
it was shown that miR-182-5p inhibition increased the sensitivity
to irradiation in NPC cells; similar to that observed previously in
non-small cell lung cancer cells.
BNIP3, a member of the Bcl-2 family of proteins, has
been reported to be upregulated in several types of cancer,
including renal cell carcinoma (21), prostate cancer (47) and breast cancer (20). BNIP3 was initially identified as a
pro-cell death protein. However, a previous study revealed that
BNIP3 may also serve as transcriptional corepressor of the
apoptosis-inducing factor gene (48). Furthermore, the role of BNIP3 in
cancer cells is contested. Overexpression of BNIP3 has been found
to reduce cell proliferation and increase apoptosis of renal cell
carcinomas (21), whereas the
opposite result has also been reported (47). Zhou et al (23) used a cell autophagy reagent
(SYUIQ-5) to treat NPC cells and found that the expression of BNIP3
was elevated when SYUIQ-5 dosage was increased. Thus, it was
hypothesized that BNIP3 might decrease radioresistance of NPC
cells. In the present study, it was shown that BNIP3 was
downregulated in radioresistant NPC cells and that the
overexpression of BNIP3 significantly impaired the radioresistance
of NPC cells.
In summary, BNIP3 expression was significantly
downregulated in radioresistant NPC cells. This result indicated
that downregulated BNIP3 expression may decrease the
radioresistance of NPC cells. Additionally, it was also shown that
miR-182-5p reversed the inhibitory effect of BNIP3 on NPC
radioresistance. In future studies, in vivo experiments are
required to improve our understanding of the radiation response of
NPC cells following modulation of miR-182-5p and BNIP3
expression.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and or/analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS designed the study. WH performed the experiments.
HJ and QL analyzed the data and wrote the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang B, Mo Z, Du W, Wang Y, Liu L and Wei
Y: Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for
the treatment of nasopharyngeal carcinoma: A systematic review and
meta-analysis. Oral Oncol. 51:1041–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szatkowska M and Krupa R: Regulation of
DNA damage response and homologous recombination repair by microRNA
in human cells exposed to ionizing radiation. Cancers (Basel).
12:18382020. View Article : Google Scholar
|
|
5
|
Jin H, Ko YS and Kim HJ: P2Y2R-mediated
inflammasome activation is involved in tumor progression in breast
cancer cells and in radiotherapy-resistant breast cancer. Int J
Oncol. 53:1953–1966. 2018.PubMed/NCBI
|
|
6
|
Cui C, Yang J, Li X, Liu D, Fu L and Wang
X: Functions and mechanisms of circular RNAs in cancer radiotherapy
and chemotherapy resistance. Mol Cancer. 19:582020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R,
Wang YY and Zhe H: FTO regulates the chemo-radiotherapy resistance
of cervical squamous cell carcinoma (CSCC) by targeting β-catenin
through mRNA demethylation. Mol Carcinog. 57:590–597. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artzi S, Kiezun A and Shomron N:
miRNAminer: A tool for homologous microRNA gene search. BMC
Bioinformatics. 9:392008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macha MA, Seshacharyulu P, Krishn SR, Pai
P, Rachagani S, Jain M and Batra SK: MicroRNAs (miRNAs) as
biomarker(s) for prognosis and diagnosis of gastrointestinal (GI)
cancers. Curr Pharm Des. 20:5287–5297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tricoli JV and Jacobson JW: MicroRNA:
Potential for cancer detection, diagnosis, and prognosis. Cancer
Res. 67:4553–4555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gartel AL and Kandel ES: miRNAs: Little
known mediators of oncogenesis. Semin Cancer Biol. 18:103–110.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LN, Xiao T, Yi HM, Zheng Z, Qu JQ,
Huang W, Ye X, Yi H, Lu SS, Li XH and Xiao ZQ: miR-125b increases
nasopharyngeal carcinoma radioresistance by targeting A20/NF-kappaB
signaling pathway. Mol Cancer Ther. 16:2094–2106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Z, Zhou S, Luo H, Ji M, Zheng J, Huang
F and Wang F: miRNA-17 promotes nasopharyngeal carcinoma
radioresistance by targeting PTEN/AKT. Int J Clin Exp Pathol.
12:229–240. 2019.PubMed/NCBI
|
|
14
|
Qu JQ, Yi HM, Ye X, Zhu JF, Yi H, Li LN,
Xiao T, Yuan L, Li JY, Wang YY, et al: miRNA-203 reduces
nasopharyngeal carcinoma radioresistance by targeting IL8/AKT
signaling. Mol Cancer Ther. 14:2653–2664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue J, Zhou A, Wu Y, Morris SA, Lin K,
Amin S, Verhaak R, Fuller G, Xie K, Heimberger AB and Huang S:
miR-182-5p Induced by STAT3 activation promotes glioma
tumorigenesis. Cancer Res. 76:4293–4304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo J, Shi K, Yin SY, Tang RX, Chen WJ,
Huang LZ, Gan TQ, Cai ZW and Chen G: Clinical value of miR-182-5p
in lung squamous cell carcinoma: A study combining data from TCGA,
GEO, and RT-qPCR validation. World J Surg Oncol. 16:762018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY,
Zhang SZ, Zhang KW, Cai H, Shi WK, Li XL, et al: miR-182-5p
promotes hepatocellular carcinoma progression by repressing FOXO3a.
J Hematol Oncol. 11:122018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spitschak A, Meier C, Kowtharapu B,
Engelmann D and Putzer BM: miR-182 promotes cancer invasion by
linking RET oncogene activated NF-κB to loss of the HES1/Notch1
regulatory circuit. Mol Cancer. 16:242017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyd JM, Malstrom S, Subramanian T,
Venkatesh LK, Schaeper U, Elangovan B, D'Sa-Eipper C and
Chinnadurai G: Adenovirus E1B 19 kDa and Bcl-2 proteins interact
with a common set of cellular proteins. Cell. 79:341–351. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun
L, Wang Y, Li X, Xiong XF, Wei B, et al: RNA N6-methyladenosine
demethylase FTO promotes breast tumor progression through
inhibiting BNIP3. Mol Cancer. 18:462019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao Y, Liu Z, Liu J, Wang H, Huang L, Lin
T, Liu J, Wei Q, Zeng H, He G and Li X: Expression and epigenetic
regulatory mechanism of BNIP3 in clear cell renal cell carcinoma.
Int J Oncol. 54:348–360. 2019.PubMed/NCBI
|
|
22
|
Daido S, Kanzawa T, Yamamoto A, Takeuchi
H, Kondo Y and Kondo S: Pivotal role of the cell death factor BNIP3
in ceramide-induced autophagic cell death in malignant glioma
cells. Cancer Res. 64:4286–4293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou WJ, Deng R, Feng GK and Zhu XF: A
G-quadruplex ligand SYUIQ-5 induces autophagy by inhibiting the
Akt-FOXO3a pathway in nasopharyngeal cancer cells. Ai Zheng.
28:1049–1053. 2009.(In Chinese). PubMed/NCBI
|
|
24
|
Xu WL, Wang SH, Sun WB, Gao J, Ding XM,
Kong J, Xu L and Ke S: Insufficient radiofrequency ablation-induced
autophagy contributes to the rapid progression of residual
hepatocellular carcinoma through the HIF-1α/BNIP3 signaling
pathway. BMB Rep. 52:277–282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XH, Qu JQ, Yi H, Zhang PF, Yi HM, Wan
XX, He QY, Ye X, Yuan L, Zhu JF, et al: Integrated analysis of
differential miRNA and mRNA expression profiles in human
radioresistant and radiosensitive nasopharyngeal carcinoma cells.
PLoS One. 9:e877672014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng X, Lv W, Wang S and He Q: miR495
enhances the efficacy of radiotherapy by targeting GRP78 to
regulate EMT in nasopharyngeal carcinoma cells. Oncol Rep.
40:1223–1232. 2018.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
30
|
Farhan M, Wang H, Gaur U, Little PJ, Xu J
and Zheng W: FOXO signaling pathways as therapeutic targets in
cancer. Int J Biol Sci. 13:815–827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Gan B, Liu D and Paik JH: FoxO
family members in cancer. Cancer Biol Ther. 12:253–259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shuai M and Huang L: High expression of
hsa_circRNA_001387 in nasopharyngeal carcinoma and the effect on
efficacy of radiotherapy. Onco Targets Ther. 13:3965–3973. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kam MK, Leung SF, Zee B, Chau RM, Suen JJ,
Mo F, Lai M, Ho R, Cheung KY, Yu BK, et al: Prospective randomized
study of intensity-modulated radiotherapy on salivary gland
function in early-stage nasopharyngeal carcinoma patients. J Clin
Oncol. 25:4873–4879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng G, Wang T, Yang KY, Zhang S, Zhang T,
Li Q, Han J and Wu G: A prospective, randomized study comparing
outcomes and toxicities of intensity-modulated radiotherapy vs.
conventional two-dimensional radiotherapy for the treatment of
nasopharyngeal carcinoma. Radiother Oncol. 104:286–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Co J, Mejia MB and Dizon JM: Evidence on
effectiveness of intensity-modulated radiotherapy versus
2-dimensional radiotherapy in the treatment of nasopharyngeal
carcinoma: Meta-analysis and a systematic review of the literature.
Head Neck. 38 (Suppl 1):E2130–E2142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao YP, Tang LL, Chen L, Sun Y, Qi ZY,
Zhou GQ, Liu LZ, Li L, Lin AH and Ma J: Prognostic factors and
failure patterns in non-metastatic nasopharyngeal carcinoma after
intensity-modulated radiotherapy. Chin J Cancer. 35:1032016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang W, Shi G, Yong Z, Li J, Qiu J, Cao
Y, Zhao Y and Yuan L: Downregulation of RKIP promotes
radioresistance of nasopharyngeal carcinoma by activating NRF2/NQO1
axis via downregulating miR-450b-5p. Cell Death Dis. 11:5042020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu W, Chen X, Yu S, Wang R, Zhao R and Du
C: MicroRNA-222 promotes tumor growth and confers radioresistance
in nasopharyngeal carcinoma by targeting PTEN. Mol Med Rep.
17:1305–1310. 2018.PubMed/NCBI
|
|
39
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: miR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li G, Wang Y, Liu Y, Su Z, Liu C, Ren S,
Deng T, Huang D, Tian Y and Qiu Y: miR-185-3p regulates
nasopharyngeal carcinoma radioresistance by targeting WNT2B in
vitro. Cancer Sci. 105:1560–1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang Y, Tan D, Xiao J, Li Q, Zhang X and
Luo Z: miR-150 contributes to the radioresistance in nasopharyngeal
carcinoma cells by targeting glycogen synthase kinase-3β. J Cancer
Res Ther. 14:111–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo Y, Zhai J, Zhang J, Ni C and Zhou H:
Improved radiotherapy sensitivity of nasopharyngeal carcinoma cells
by miR-29-3p targeting COL1A1 3′-UTR. Med Sci Monit. 25:3161–3169.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T,
Yuan L, Li JY, Wang YY, Feng J, et al: miR-23a sensitizes
nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3
pathway. Oncotarget. 6:28341–28356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao YS, Yang WC, Xin HW, Han JX and Ma
SG: miR-182-5p knockdown targeting PTEN inhibits cell proliferation
and invasion of breast cancer cells. Yonsei Med J. 60:148–157.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu X, Ayub B, Liu Z, Serna VA, Qiang W,
Liu Y, Hernando E, Zabludoff S, Kurita T, Kong B and Wei JJ:
Anti-miR182 reduces ovarian cancer burden, invasion, and
metastasis: An in vivo study in orthotopic xenografts of nude mice.
Mol Cancer Ther. 13:1729–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Segura MF, Hanniford D, Menendez S, Reavie
L, Zou X, Alvarez-Diaz S, Zakrzewski J, Blochin E, Rose A,
Bogunovic D, et al: Aberrant miR-182 expression promotes melanoma
metastasis by repressing FOXO3 and microphthalmia-associated
transcription factor. Proc Natl Acad Sci USA. 106:1814–1819. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Gong J, Zeng H, Chen N, Huang R,
Huang Y, Nie L, Xu M, Xia J, Zhao F, et al: MicroRNA145 targets
BNIP3 and suppresses prostate cancer progression. Cancer Res.
70:2728–2738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Burton TR, Eisenstat DD and Gibson SB:
BNIP3 (Bcl-2 19 kDa interacting protein) acts as transcriptional
repressor of apoptosis-inducing factor expression preventing cell
death in human malignant gliomas. J Neurosci. 29:4189–4199. 2009.
View Article : Google Scholar : PubMed/NCBI
|