Introduction
Coronary artery disease, which caused >9 million
deaths in 2016, is a leading cause of death worldwide (1). Early reperfusion therapy is the most
effective strategy in the treatment of myocardial infarction, a
common type of coronary artery disease (2). However, the process of reperfusion can
induce myocardial ischemia/reperfusion (MIR) injury, a complex
pathophysiological process that involves oxidative stress,
Ca2+ overload and inflammatory response (3,4),
eventually leading to programmed cell death of cardiomyocytes
(5–7). In order to improve the beneficial
effects of reperfusion therapy, it is necessary to develop powerful
and safe drugs for the treatment for MIR injury.
Chinese herbal medicine has been used in the
treatment of MIR injury for hundreds of years (8). Numerous herbal formulas have been
determined to protect cardiomyocytes from MIR injury (9–11).
Zenglv Fumai Granule (ZFG), a clinical discovery by Professor Yu
Zuo-Ying, has been used in the treatment of sick sinus syndrome for
>30 years (12). Sick sinus
syndrome is a disorder characterized by abnormal heart rhythms due
to the malfunction of the sinus node (13). Our previous study revealed that ZFG
decreases clinical symptoms and improves heart rate in patients
with sick sinus syndrome (12). A
previous study demonstrated that sick sinus syndrome is associated
with MIR injury (14). Therefore,
it was hypothesized that ZFG may also serve a protective role
against MIR injury.
Tripartite interaction motif (TRIM) proteins are a
class of E3 ubiquitin ligases that contain a ring-finger domain,
one or two B-boxes and a coiled-coil region at the amino-terminal
region (15). They function via the
regulation of protein degradation by ubiquitination, and are
thereby involved in numerous biological processes, such as
proliferation and apoptosis (16).
Previous studies have revealed that TRIM proteins are key
regulators in MIR injury (17,18).
For example, TRIM6 aggravates MIR injury by inducing cardiomyocyte
apoptosis (17) and TRIM59
alleviates MIR injury via the inhibition of myocardial apoptosis
and inflammatory response (18). A
recent study demonstrated aberrant expression levels of multiple
TRIM proteins in the heart during myocardial infarction, including
TRIM7, TRIM14, TRIM22 and TRIM28 (19); however, it is unclear whether they
serve a role in MIR injury.
The present study aimed to investigate the effect of
ZFG on MIR-induced myocardial apoptosis, and to determine whether
it functions via the regulation of TRIM proteins. An in
vitro MIR injury model was established by treating human
cardiomyocyte cell line AC16 with hypoxia/reoxygenation (H/R).
Using this model, the hypothesis that ZFG had a protective role in
MIR injury by regulating the TRIM28/GPX1 axis was tested.
Materials and methods
Extraction of ZFG
ZFG consists of eight types of herbal medicines,
including Panax ginseng (radix), Astragalus
propinquus (radix), Cinnamomum cassia (ramulus),
Epimedium brevicornum (aerial part), Polygonatum
sibiricum (rhizoma), Salvia miltiorrhiza (radix),
Ligusticum chuanxiong (rhizome) and Ophiopogon
japonicas (radix) (12). These
herbs were mixed in the following ratio: 4:6:3:4:4:4:3:4. Herbs
were decocted twice (2 h each time) with 10 times the amount of
water, as previously described (20). The extracts were made into
lyophilized powder for subsequent experiments.
Cell culture
Human cardiomyocyte cell line AC16 was purchased
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. AC16 cells were cultured in DMEM (HyClone;
Cytiva) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (Beijing
Solarbio Science & Technology Co., Ltd.) in a humidified
incubator at 5% CO2 at 37°C.
H/R treatment
H/R treatment was performed as previously described
by Benoist et al (21). In
brief, AC16 cells were cultured in glucose/serum-free DMEM (Gibco;
Thermo Fisher Scientific, Inc.) under hypoxia (95% N2
and 5% CO2) for 5 h at 37°C. Then, cells were
transferred to normal medium and cultured under reoxygenation (5%
CO2 and 95% O2) for 1 h at 37°C. Cells that
were cultured under normal conditions only were used as the
control. Following H/R treatment, various concentrations of ZFG
(0.0, 0.1, 0.2 and 0.4 mg/ml) were added and cells were incubated
for 24 h at 37°C.
Lentivirus construction and
transduction
Lentivirus overexpression (oe)TRIM28 and small
interfering (si)TRIM28 were constructed in order to overexpress and
knock down TRIM28, respectively. For constructing lentivirus
oeTRIM28, DNA fragments of TRIM28 were cloned into 1,000 ng vector
plasmid pLVX-Puro (Clontech Laboratories, Inc.). Then, the
second-generation packaging plasmids psPAX2 (100 ng) and pMD2G (900
ng; both Addgene, Inc.) were used to co-transfect the vector
plasmid into 293T cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C. After 6 h,
the DMEM was replaced with complete medium. High-titer recombinant
lentiviruses were obtained at 48 and 72 h after transfection. In
order to construct lentivirus siTRIM28, the designed TRIM28 siRNAs
(Generay Biotech Co., Ltd.) were cloned into the vector plasmid
PLKO.1 (Addgene, Inc.), which was then transfected into 293T cells
as aforementioned. The sequences of TRIM28 siRNAs were as follows:
siTRIM28-1, 5′-GCAACAGTGCTTCTCCAAA-3′; siTRIM28-2,
5′-GGAGATGATCCCTACTCAA-3′; and siTRIM28-3′,
5′-GGACTACAACCTTATTGTT-3′.
For lentivirus transduction, AC16 cells were
inoculated into 6-well plates at a density of 2×105
cells/well. At 60–70% confluence, the cells were transduced with
lentivirus at a multiplicity of infection of 10 at 37°C for 6 h,
followed by culture with fresh medium for a further 24 h at 37°C.
Cells transduced with lentivirus-containing vector plasmid or siNC
sequence (5′-TCGCTGCATCAGATGAGAC-3′) were used as negative
controls. For subsequent experiments, cells were also cultured with
l mmol/l ROS inhibitor N-Acetyl-L-cysteine (NAC) for 24 h.
Measurement of apoptotic cells
AC16 cells were digested using 0.25% trypsin-EDTA
solution (Beijing Solarbio Science & Technology Co., Ltd.) and
suspended in PBS for counting. Then, 1×105 cells were
centrifuged at 500 × g for 5 min at 4°C. The supernatant was
discarded and cells were resuspended with 195 µl Annexin V-FITC
(Beyotime Institute of Biotechnology). Then, cells were incubated
with 5 µl Annexin V-FITC for 15 min in the dark at 4°C, followed by
incubation with 5 µl propidium iodide for 5 min at 4°C. Apoptotic
cells were detected using BD Accuri™ C6 flow cytometer (BD
Biosciences), and the data were analyzed by FlowJo software
(v10.2.8; FlowJo LLC).
Reverse transcription-quantitative
(RT-qPCR)
Total RNA in AC16 cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and the residual DNA was digested using DNase I enzymes. The
cDNA was synthesized using RevertAid First Strand cDNA Synthesis
Kit (cat. no. K1622; Fermentas; Thermo Fisher Scientific, Inc.),
according to the manufacturers instructions. RT-qPCR was performed
with SYBR-Green Mix (Thermo Fisher Scientific, Inc.) in an ABI 7300
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 5 min, followed by 45 cycles of 95°C for
15 sec and 60°C for 45 sec. GAPDH was selected as the internal
control. The relative mRNA levels of target genes (TRIM7, TRIM14,
TRIM22, TRIM28 and GPX1) were calculated using the
2−∆∆Cq method (22). The
primers are listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5→3) |
|---|
| GAPDH | F:
AATCCCATCACCATCTTC |
|
| R:
AGGCTGTTGTCATACTTC |
| TRIM7 | F:
CTGAGGGCTTTCCTGGTG |
|
| R:
GAGACTGTGGTTGGCTTGG |
| TRIM14 | F:
GAGCTTGTCGAGGGATGCG |
|
| R:
CTGGGTTATGTTGTCAATGTGC |
| TRIM22 | F:
CAAACATTCCGCATAAAC |
|
| R:
ATCCAGCACATTCACCTCAC |
| TRIM28 | F:
CCCGTCTTCAAGGTCTTCC |
|
| R:
GAGCCATAAGCACAGGTTTG |
| GPX1 | F:
TCGGTGTATGCCTTCTCGG |
|
| R:
CTTGGCGTTCTCCTGATGC |
Western blotting
Total protein in AC16 cells was extracted using
super RIPA lysis buffer (cat. no. R0010; Beijing Solarbio Science
& Technology Co., Ltd.), and its concentration was determined
with a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Then, 25 µg protein was separated via SDS-PAGE on a 10% gel, and
then transferred onto a PVDF membrane (EMD Millipore). After being
blocked with 5% skimmed milk for 1 h at room temperature, the
membrane was incubated with primary antibodies overnight at 4°C,
followed by incubation with HRP-conjugated secondary antibody
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) for
1 h at 37°C. The primary antibodies included antibodies against
TRIM28 (1:1,000; cat. no. ab10484; Abcam), GPX1 (1:2,000; cat. no.
ab108427; Abcam), cleaved caspase-3′ (1:5,000; cat. no. ab214430;
Abcam) and GAPDH (1:5,000; cat. no. 60004-1-1G; ProteinTech Group,
Inc.). Finally, the PVDF membrane was washed with enhanced
chemiluminescent detection reagent (EMD Millipore) and visualized
using a Tanon-5200 system (Tanon Science and Technology Co., Ltd.).
Densitometric analysis was performed using ImageJ software (v1.44;
National Institutes of Health).
Detection of ROS levels
AC16 cells were incubated with 10 µM
27-dichlorofluorescin diacetate probe (Beyotime Institute of
Biotechnology) for 20 min at 37°C in the dark. The fluorescence of
AC16 cells was detected using a BD Accuri™ C6 flow cytometer.
Wavelengths of 480 and 525 nm were selected as the excitation and
emission wavelengths, respectively.
Co-immunoprecipitation and
ubiquitination assays
Total protein was extracted and its concentration
was determined using BCA protein assay kit. Then, 2 mg protein was
incubated with 1 µg antibodies against TRIM28, GPX1 or IgG (cat.
no. 10283-1-AP; ProteinTech Group, Inc.) overnight at 4°C.
Thereafter, these samples were incubated with 30 µl Protein A/G
Plus-Agarose (Santa Cruz Biotechnology, Inc.) for 2 h at 4°C. The
supernatant was discarded following centrifugation at 4,500 × g for
5 min at 4°C. Then, the A/G Plus-Agarose beads were washed four
times with 1 ml RIPA lysis buffer, and subsequently boiled with 2X
loading buffer for 5 min. Following centrifugation at 500 × g for 1
min at 4°C, the supernatant was collected and western blotting was
performed. Antibodies against TRIM28 or GPX1 were used as the
primary antibodies.
For the ubiquitination detection of GPX1 protein,
anti-GPX1 antibody was used to pull down immunocomplex. Subsequent
western blotting was performed according to the same method
outlined above, but using anti-ubiquitin antibody (1:2,000; cat.
no. ab7780; Abcam) as the primary antibody.
Statistical analysis
All experiments were repeated three times, and the
experimental data are presented as the mean ± SD. Statistical
analysis was performed using Prism software (version 8.0.2;
GraphPad Software Inc.). Statistical differences were evaluated by
one-way ANOVA and post hoc Tukey's test. P<0.05 was considered
to indicate a statistically significant difference.
Results
ZFG inhibits H/R-induced apoptosis in
AC16 cells
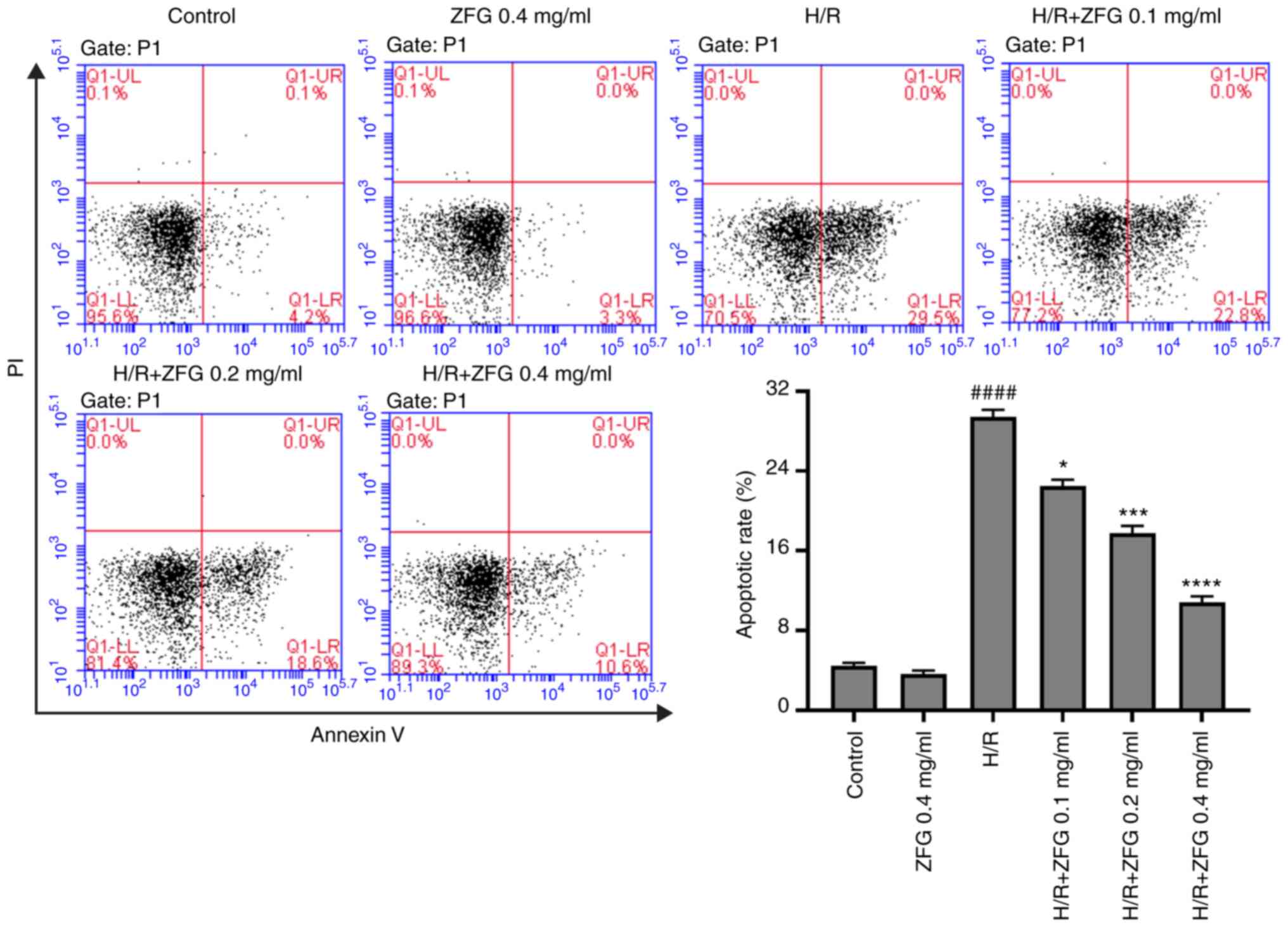
In order to investigate the effect of ZFG on
myocardial apoptosis during H/R injury, H/R-injured AC16 cells were
treated with various ZFG dosages. The results revealed that H/R
treatment significantly increased apoptosis in AC16 cells, while
subsequent ZFG administration significantly inhibited H/R-induced
apoptosis in a dose-dependent manner (Fig. 1). In addition, 0.4 mg/ml ZFG
treatment-alone had no significant effect on apoptosis in AC16
cells (Fig. 1).
ZFG inhibits H/R-induced TRIM28
expression in AC16 cells
In order to determine whether TRIM proteins
contributed to the protective effect exerted by ZFG, the expression
levels of TRIM7, TRIM14, TRIM22 and TRIM28 in H/R-injured AC16
cells were assessed. H/R treatment significantly increased the mRNA
expression levels of TRIM7, TRIM14, TRIM22 and TRIM28; these
effects were eliminated by subsequent administration of ZFG
(Fig. 2). Among the TRIM proteins,
TRIM28 induced the greatest change in expression levels; therefore,
it was selected for subsequent experiments. RT-qPCR and western
blotting assays showed that TRIM28 mRNA and protein expression
levels in H/R-injured AC16 cells significantly increased in a
time-dependent manner (Fig. 3A and
B), and subsequent ZFG treatment attenuated H/R-induced
upregulation of TRIM28 expression levels in a dose-dependent manner
(Fig. 3C and D).
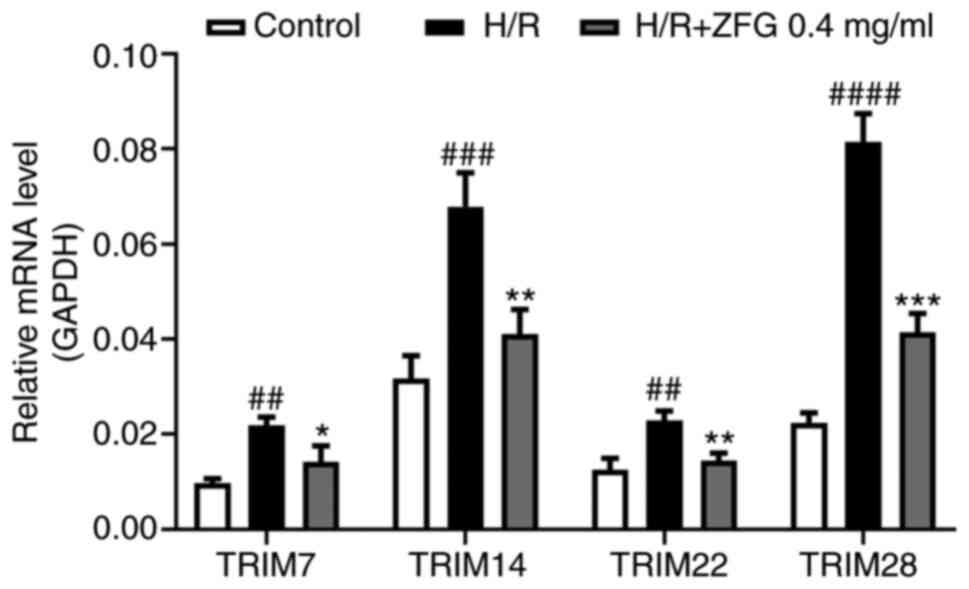 | Figure 2.ZFG treatment inhibits H/R-induced
upregulation of TRIM7, TRIM14, TRIM22 and TRIM28. H/R-induced AC16
cells were treated with 0.4 mg/ml ZFG. After 24 h, the mRNA levels
of TRIM7, TRIM14, TRIM22 and TRIM28 were detected by reverse
transcription-quantitative PCR. ##P<0.01,
###P<0.001 and ####P<0.0001 vs.
Control; *P<0.05, **P<0.01 and ***P<0.001 vs. H/R. ZFG,
Zengly Fumai Granule; H/R, hypoxia/reoxygenation; TRIM, tripartite
interaction motif. |
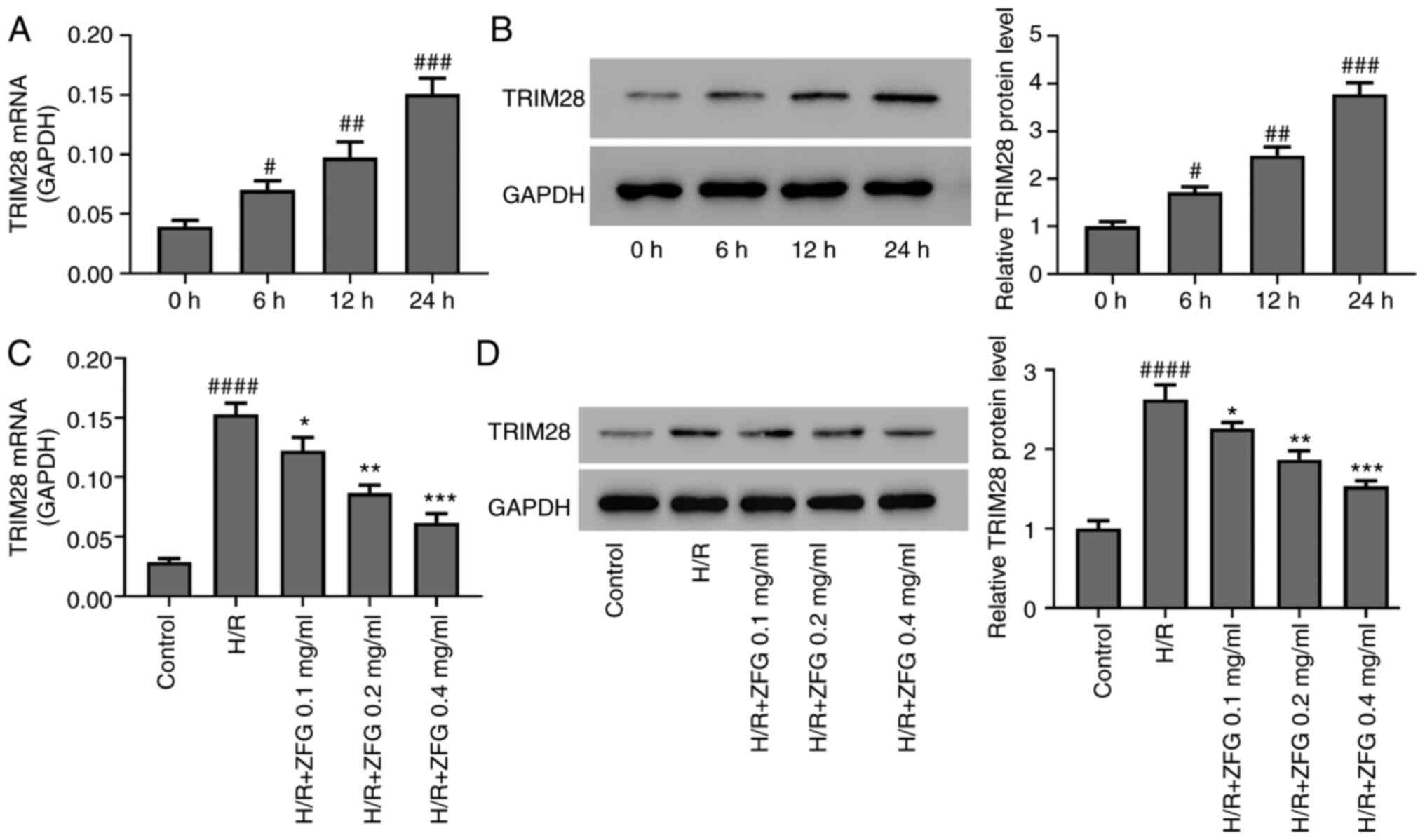 | Figure 3.ZFG inhibits H/R-induced TRIM28
expression in AC16 cells. (A) mRNA and (B) protein levels of TRIM28
were measured by RT-qPCR and western blotting, respectively, at 0,
6, 12 and 24 h after H/R treatment. #P<0.05,
##P<0.01 and ###P<0.001 vs. 0 h.
H/R-induced AC16 cells were treated with various concentrations of
ZFG (0.0, 0.1, 0.2 and 0.4 mg/ml). After 24 h, the (C) mRNA and (D)
protein levels of TRIM28 were measured by RT-qPCR and western
blotting, respectively. ####P<0.0001 vs. Control;
*P<0.05, **P<0.01 and ***P<0.0001 vs. H/R. ZFG, Zengly
Fumai Granule; H/R, hypoxia/reoxygenation; TRIM, tripartite
interaction motif; RT-q, reverse transcription-quantitative. |
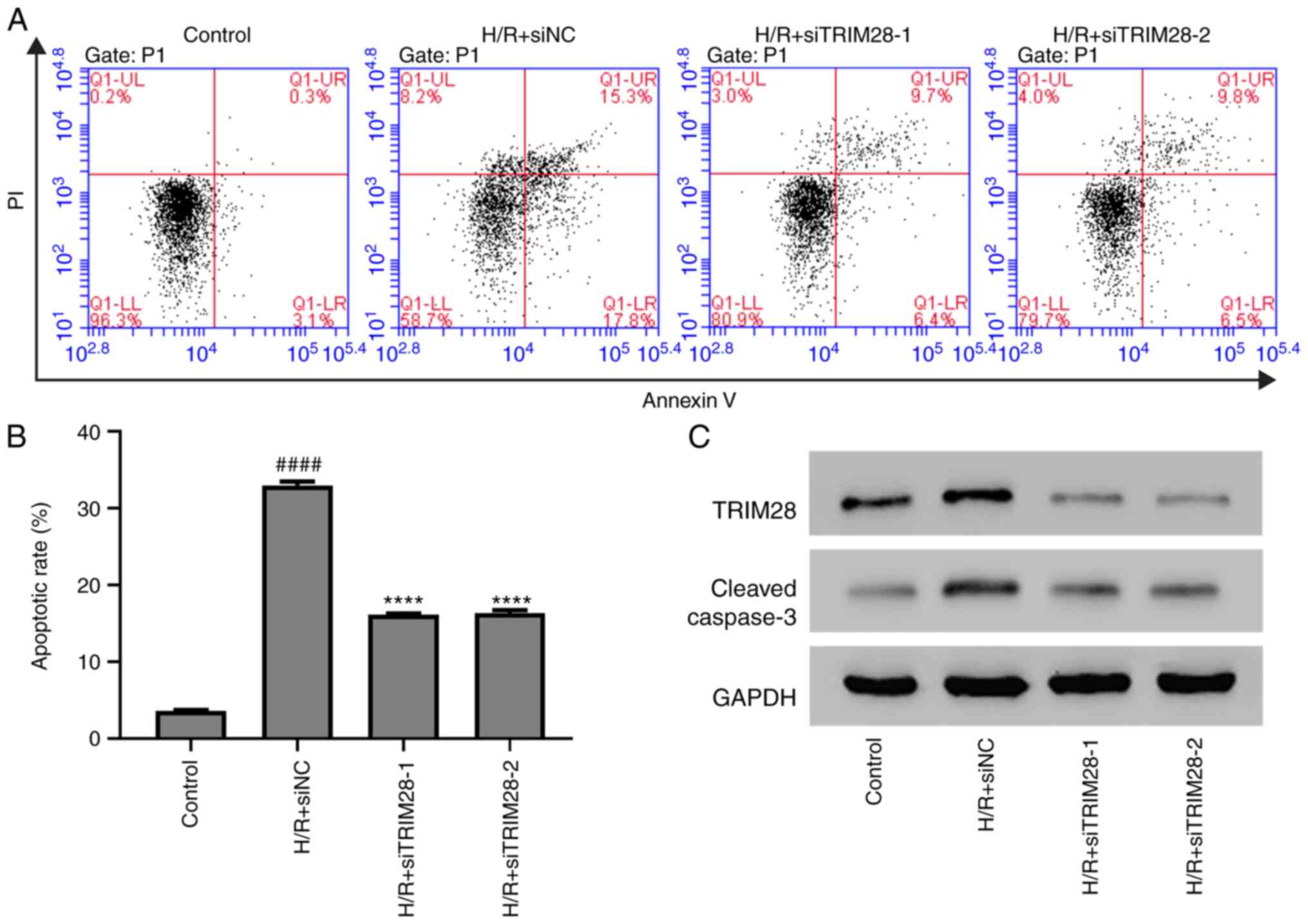
Knockdown of TRIM28 inhibits
H/R-induced apoptosis in AC16 cells
Lentivirus siTRIM28 was constructed to knock down
TRIM28 expression in AC16 cells. All three siTRIM28 lentiviruses
significantly decreased the mRNA and protein levels of TRIM28
(Fig. S1A and B). The lentiviruses
siTRIM28-1 and siTRIM28-2 were then transduced into H/R-injured
AC16 cells. Flow cytometric analysis revealed that TRIM28 knockdown
attenuated H/R-induced upregulation of apoptotic rate in AC16 cells
(Fig. 4A and B). Western blotting
revealed that TRIM28 knockdown inhibited H/R-induced upregulation
of cleaved caspase-3 in AC16 cells (Fig. 4C). Thus, TRIM28 knockdown inhibited
H/R-induced apoptosis in AC16 cells.
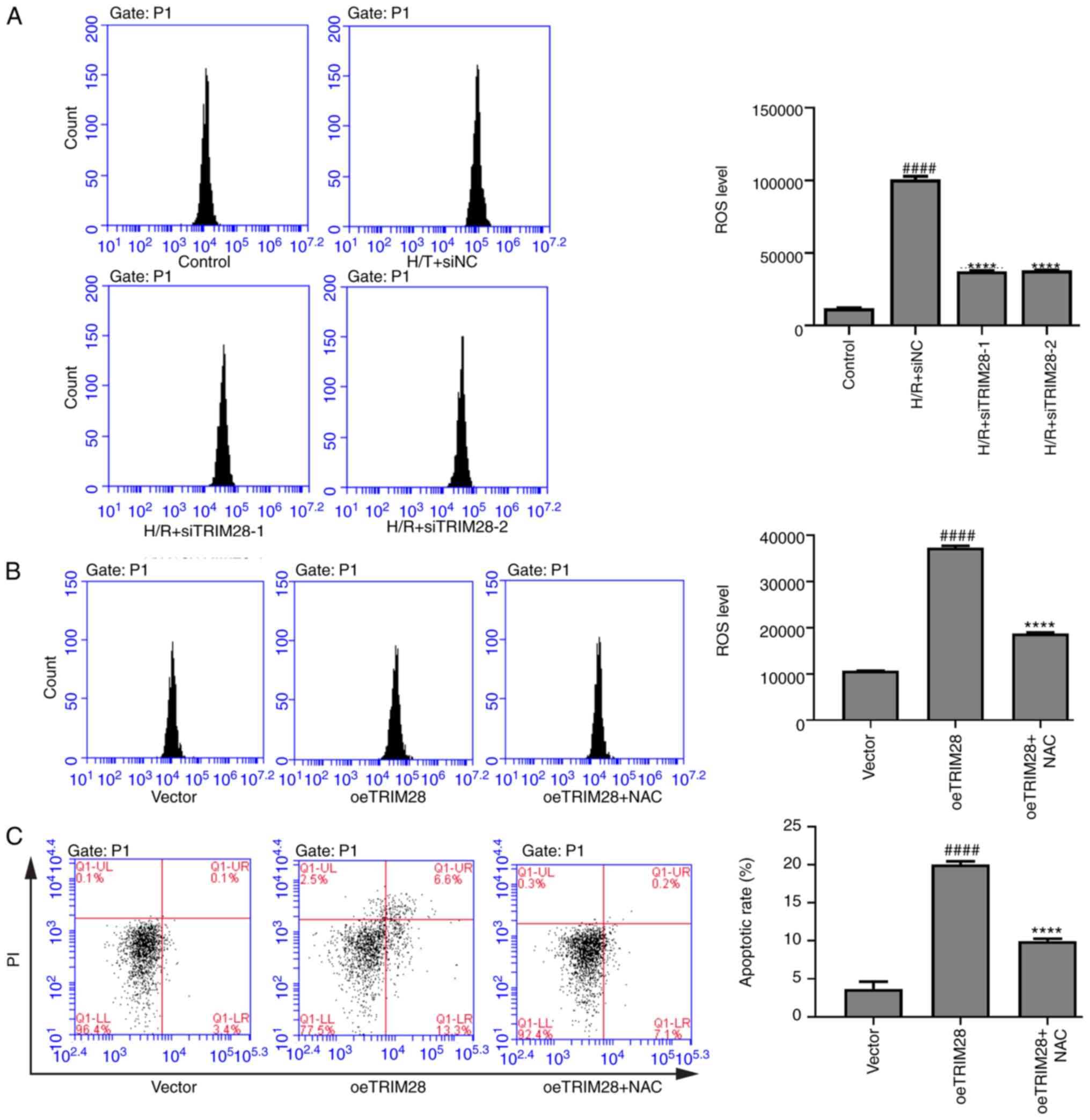
TRIM28 induces apoptosis in
H/R-injured AC16 cells by activating ROS generation
ROS production is a cause of myocardial apoptosis
during MIR injury (23). Therefore,
it was investigated whether TRIM28 promoted apoptosis by regulating
ROS generation. The lentiviruses siTRIM28-1 and siTRIM28-2 were
transduced into H/R-injured AC16 cells. H/R treatment upregulated
ROS levels in AC16 cells, while TRIM28 knockdown inhibited
H/R-induced upregulation of ROS levels (Fig. 5A). In addition, lentivirus oeTRIM28
was used to overexpress TRIM28 in AC16 cells (Fig. S1A and B). TRIM28 overexpression
increased the ROS levels and apoptotic rate in AC16 cells (Fig. 5B and C). However, the effects of
oeTRIM28 on ROS and apoptosis were attenuated by ROS inhibitor NAC
(Fig. 5B and C). Collectively,
these data indicated that TRIM28 may promote apoptosis in
H/R-injured AC16 cells via inducing ROS generation.
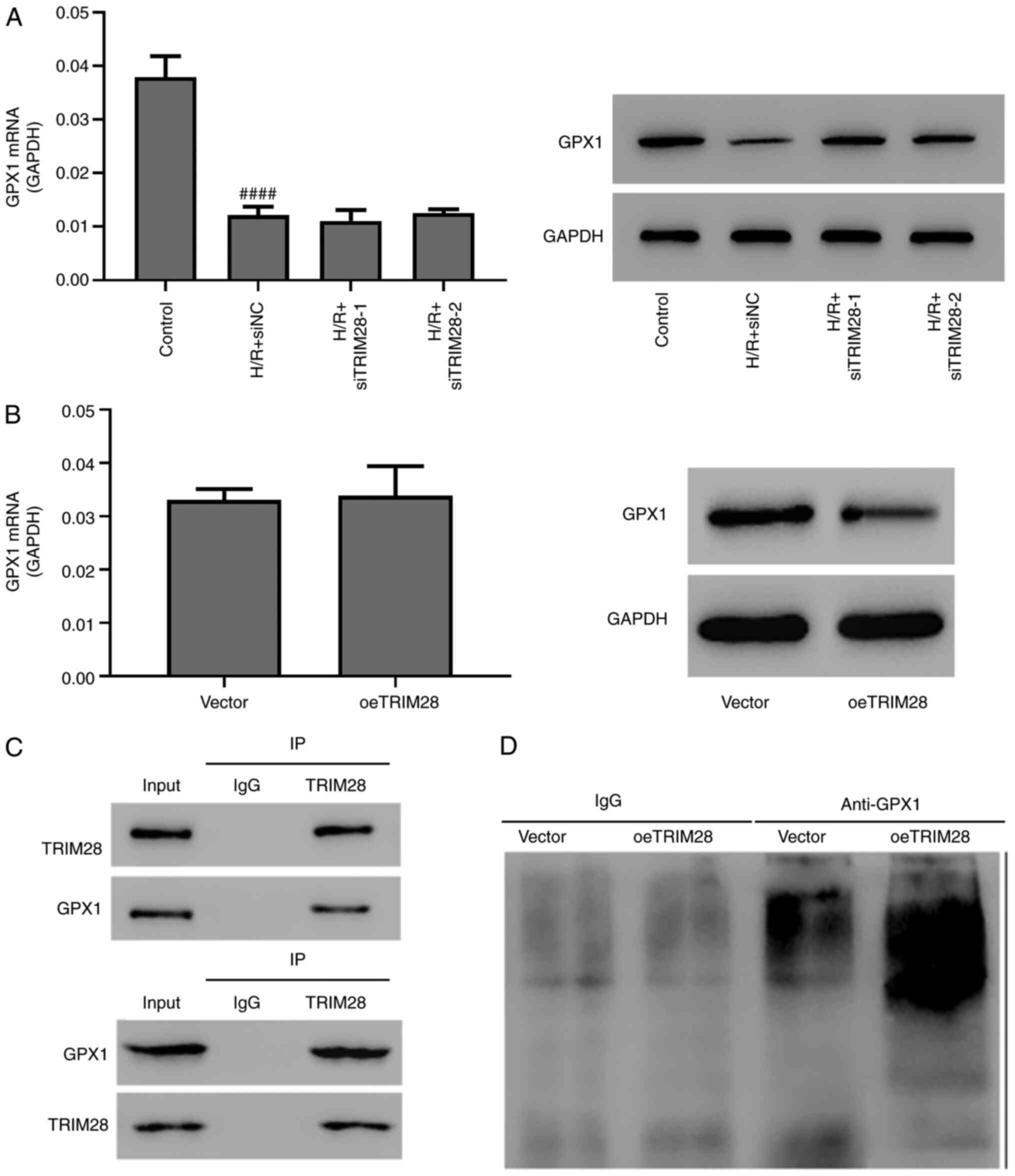
TRIM28 negatively regulates GPX1
stability via ubiquitination
GPX1 is an antioxidant enzyme that scavenges free
radicals in cells and serves a protective role in MIR injury
(24). In H/R-induced AC16 cells,
the mRNA and protein levels of GPX1 were significantly
downregulated (Fig. 6A). TRIM28
knockdown restored GPX1 protein levels but had no effect on GPX1
mRNA expression levels in H/R-induced AC16 cells (Fig. 6A). oeTRIM28 caused similar effects
(Fig. 6B). Co-immunoprecipitation
analysis revealed that TRIM28 interacted with GPX1 (Fig. 6C). Ubiquitination assays revealed
that oeTRIM28 promoted the ubiquitination of GPX1 in AC16 cells
(Fig. 6D). Collectively, these data
indicated that TRIM28 negatively regulated GPX1 via
ubiquitination.
Discussion
ZFG is a clinical prescription for the treatment of
sick sinus syndrome (12). Previous
studies have revealed a close association between sick sinus
syndrome and MIR injury (14,25).
Therefore, it was speculated that ZFG may exert a protective effect
on MIR injury. The present study revealed that ZFG significantly
inhibited H/R-induced cardiomyocyte apoptosis, supporting this
hypothesis.
A recent study demonstrated that Tongxinluo capsule,
a Chinese herbal compound, attenuates MIR injury by downregulating
the ubiquitin-proteasome system (26). Previous studies have also revealed
that the ubiquitin-proteasome system serves an important regulatory
role in MIR injury (17,18). Hence, it was hypothesized that ZFG
may also exert a protective effect by regulating the
ubiquitin-proteasome system. TRIM proteins, which mediate the
ubiquitin-dependent protein degradation pathway, are the primary
members of ubiquitin-proteasome system (27). A number of TRIM proteins are
expressed abnormally in the heart in patients with myocardial
infarction, including TRIM7, TRIM14, TRIM22 and TRIM28 (19). The present study assessed the
expression levels of these four proteins in cardiomyocytes
following H/R and ZFG treatment. These proteins were significantly
upregulated in H/R-injured cardiomyocytes, whereas their expression
was inhibited by subsequent administration of ZFG. Of these four
proteins, TRIM28 induced the greatest change in expression levels
and was therefore selected for subsequent analysis. Silencing
TRIM28 attenuated H/R-induced apoptosis. Therefore, ZFG may protect
cardiomyocytes against H/R-induced apoptosis partly by regulating
TRIM28. Further research is necessary to confirm the involvement of
TRIM7, TRIM14 and TRIM22 in the protective role of ZFG in MIR
injury.
TRIM28, also called TIF1β or KAP1, is involved in
multiple cellular processes, such as proliferation, migration and
apoptosis (28,29). It has also been revealed to regulate
apoptosis in a cell type-dependent manner. For example, TRIM28
serves an anti-apoptotic function in immature erythroid, lymphoma
and lung cancer cells (30–32) but exerts a pro-apoptotic effect on
bovine fibroblasts and embryonic kidney cells (33,34).
The present study revealed that oeTRIM28 promoted apoptosis in AC16
cells, suggesting a pro-apoptotic role of TRIM28 in cardiomyocytes.
Previous research has primarily focused on the role of TRIM28 in
cancer (35,36). To the best of our knowledge, the
role of TRIM28 in MIR injury has not been studied. The present
study, reported the involvement of TRIM28 in an in vitro MIR
model. TRIM28 was also revealed to be upregulated and induce
apoptosis in H/R-injured cardiomyocytes.
During MIR injury, excessive ROS are produced,
triggering a series of pathological processes, including
cardiomyocyte apoptosis and the inflammatory response (23). Therefore, ROS have been recognized
as critical regulators in MIR injury. As anticipated, ROS levels in
H/R-injured cardiomyocytes were significantly upregulated in the
present study. However, TRIM28 knockdown attenuated H/R-induced
accumulation of ROS in cardiomyocytes. Previous studies have
revealed that numerous TRIM proteins, such as TRIM10 and TRIM69,
promote cell apoptosis via enhancing ROS generation (37,38).
Consistent with these results, in the present study, TRIM28
promoted cardiomyocyte apoptosis by regulating ROS production. GPX1
is an antioxidant enzyme that can directly scavenge ROS in cells
(39), and thereby negatively
regulate ROS. The present study revealed that GPX1 was
significantly decreased in H/R-injured cardiomyocytes, which is
consistent with previous studies (40,41).
TRIM28 knockdown restored GPX1 protein levels but had no effect on
GPX1 mRNA levels in H/R-injured cardiomyocytes. A previous study
reported that TRIM33 was involved in MIR injury by ubiquitinating
GPX1 (24). In the present study,
TRIM28 negatively regulated GPX1 via ubiquitination. Collectively,
the present results indicated that TRIM28 may serve a pro-apoptotic
role in H/R-injured cardiomyocytes by regulating the GPX1/ROS
pathway.
In summary, the present study demonstrated that ZFG
attenuated H/R-induced cardiomyocyte apoptosis via the inhibition
of TRIM28 expression levels. TRIM28 served a pro-apoptotic role in
cardiomyocytes by enhancing ROS generation via GPX1 ubiquitination.
These results suggested that ZFG and TRIM28 are potential drug and
therapeutic targets for MIR injury treatment, respectively. Further
in vivo research is necessary to confirm the present
findings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Major Projects of Jilin Province: Study on the treatment
of sick sinus syndrome by Zenglv Fumai Granule (grant no.
20160204024YY).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
XHZ and SRL designed the study. HYZ, XYL and FQ
performed the experiments. XHZ and SRL confirmed the authenticity
of all the raw data. YW and LD analyzed and interpreted the data.
All authors wrote the manuscript and read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MIR
|
myocardial ischemia/reperfusion
|
|
TRIM
|
tripartite interaction motif
|
|
H/R
|
hypoxia/reoxygenation
|
|
NAC
|
N-acetyl-L-cysteine
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
WHO, . The Top 10 Causes of Death. World
Health Organization, Geneva, 2018. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye G, Fu Q, Jiang L and Li Z: Vascular
smooth muscle cells activate PI3K/Akt pathway to attenuate
myocardial ischemia/reperfusion-induced apoptosis and autophagy by
secreting bFGF. Biomed Pharmacother. 107:1779–1785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao D, Feng P, Sun Y, Qin Z, Zhang Z, Tan
Y, Gao E, Lau WB, Ma X, Yang J, et al: Cardiac-derived CTRP9
protects against myocardial ischemia/reperfusion injury via
calreticulin-dependent inhibition of apoptosis. Cell Death Dis.
9:7232018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D,
Hu S, Ren J, Cao F and Chen Y: Ripk3 induces mitochondrial
apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury.
Redox Biol. 13:498–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Q, Li J, Wang J, Li J, Janicki JS and
Fan D: Effects and mechanisms of chinese herbal medicine in
ameliorating myocardial ischemia-reperfusion injury. Evid Based
Complement Alternat Med. 2013:9256252013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vincent A, Covinhes A, Barrère C, Gallot
L, Thoumala S, Piot C, Heurteaux C, Lazdunski M, Nargeot J and
Barrère-Lemaire S: Acute and long-term cardioprotective effects of
the traditional Chinese medicine MLC901 against myocardial
ischemia-reperfusion injury in mice. Sci Rep. 7:147012017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Huang X, Tang W, Ren P, Xing Z,
Tian X, Zhu Z and Wang Y: Effect of oriental herbal prescription
Guan-Xin-Er-Hao on coronary flow in healthy volunteers and
antiapoptosis on myocardial ischemia-reperfusion in rat models.
Phytother Res. 21:926–931. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Xu L, Qiao Z, Gao L, Ding S, Ying
X, Su Y, Lin N, He B and Pu J: YiXin-Shu, a ShengMai-San-based
traditional Chinese medicine formula, attenuates myocardial
ischemia/reperfusion injury by suppressing mitochondrial mediated
apoptosis and upregulating liver-X-receptor α. Sci Rep.
6:230252016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Yu D, Qian F and Liu S: Zenglv
Fumai Granule on 120 cases of sick sinus syndrome. J Tradit Chin
Med. 26:1926–1928. 2017.
|
|
13
|
Jou CJ, Arrington CB, Barnett S, Shen J,
Cho S, Sheng X, McCullagh PC, Bowles NE, Pribble CM, Saarel EV, et
al: A functional assay for sick sinus syndrome genetic variants.
Cell Physiol Biochem. 42:2021–2029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Zhu B, Ren J, Lu F, Qi Y, Weng W
and Gao R: Two methods for modeling of sick sinus syndrome in rats:
Ischemia reperfusion and sodium hydroxide induced injury. Biomed
Pharmacother. 111:778–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe M and Hatakeyama S: TRIM proteins
and diseases. J Biochem. 161:135–144. 2017.PubMed/NCBI
|
|
16
|
Yamada Y, Takayama KI, Fujimura T,
Ashikari D, Obinata D, Takahashi S, Ikeda K, Kakutani S, Urano T,
Fukuhara H, et al: A novel prognostic factor TRIM44 promotes cell
proliferation and migration, and inhibits apoptosis in testicular
germ cell tumor. Cancer Sci. 108:32–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng G, Lian C, Yang P, Zheng M, Ren H and
Wang H: E3-ubiquitin ligase TRIM6 aggravates myocardial
ischemia/reperfusion injury via promoting STAT1-dependent
cardiomyocyte apoptosis. Aging (Albany NY). 11:3536–3550. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv ZQ, Yang CY and Xing QS: TRIM59
attenuates inflammation and apoptosis caused by myocardial ischemia
reperfusion injury by activating the PI3K/Akt signaling pathway.
Eur Rev Med Pharmacol Sci. 24:5192. 2020.PubMed/NCBI
|
|
19
|
Borlepawar A, Frey N and Rangrez AY: A
systematic view on E3 ligase Ring TRIMmers with a focus on cardiac
function and disease. Trends Cardiovasc Med. 29:1–8. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Zhang X and Wang Z: Optimization
of extraction techenology of Zengmaifulv Granules by orthogonal
test. Shandong Chem Ind. 45:28–30. 2016.
|
|
21
|
Benoist L, Chadet S, Genet T, Lefort C,
Heraud A, Danila MD, Muntean DM, Baron C, Angoulvant D, Babuty D,
et al: Stimulation of P2Y11 receptor protects human cardiomyocytes
against Hypoxia/Reoxygenation injury and involves PKCε signaling
pathway. Sci Rep. 9:116132019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jian Z, Liang B, Pan X, Xu G, Guo SS, Li
T, Zhou T, Xiao YB and Li AL: CUEDC2 modulates cardiomyocyte
oxidative capacity by regulating GPX1 stability. EMBO Mol Med.
8:813–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu RX, Tan S, Liu M, Peng J, Wang YL and
Liu ZM: Effects of Chinese herbal medicine serum on the apoptosis
of sinoatrial node cells induced by simulated ischemia-reperfusion.
J Tradit Chin Med. 31:224–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang HX, Wang P, Wang NN, Li SD and Yang
MH: Tongxinluo ameliorates myocardial ischemia-reperfusion injury
mainly via activating parkin-mediated mitophagy and downregulating
ubiquitin-proteasome system. Chin J Integr Med. June;2019.DOI:
10.1007/s11655-019-3166-8. View Article : Google Scholar
|
|
27
|
Hatakeyama S: TRIM family proteins: Roles
in autophagy, immunity, and carcinogenesis. Trends Biochem Sci.
42:297–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Chen H, Deng X, Peng Y, Zeng Q,
Song Z, He W, Zhang L, Xiao T, Gao G, et al: Knockdown of TRIM28
inhibits PDGF-BB-induced vascular smooth muscle cell proliferation
and migration. Chem Biol Interact. 311:1087722019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng Y, Zhang M, Jiang Z and Jiang Y:
TRIM28 activates autophagy and promotes cell proliferation in
glioblastoma. OncoTargets Ther. 12:397–404. 2019. View Article : Google Scholar
|
|
30
|
Zhang P-P, Ding D-Z, Shi B, Zhang SQ, Gu
LL, Wang YC and Cheng C: Expression of TRIM28 correlates with
proliferation and Bortezomib-induced apoptosis in B-cell
non-Hodgkin lymphoma. Leuk Lymphoma. 59:2639–2649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Zhang L, Wang J, Zhao X, Xu Q, Lu
Y, Zuo Y, Chen L, Du J, Lian Y, et al: Downregulation of TRIM28
inhibits growth and increases apoptosis of nude mice with non-small
cell lung cancer xenografts. Mol Med Rep. 17:835–842.
2018.PubMed/NCBI
|
|
32
|
Hosoya T, Clifford M, Losson R, Tanabe O
and Engel JD: TRIM28 is essential for erythroblast differentiation
in the mouse. Blood. 122:3798–3807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma P, Man X, Yang S, Dong X, Su L, Luan W
and Ma X: Effect of TRIM28 on proliferation, apoptosis and histone
H3K9 trimethylation in bovine fibroblasts. Indian J Animal Res.
53:724–730. 2019.
|
|
34
|
Lionnard L, Duc P, Brennan MS, Kueh AJ,
Pal M, Guardia F, Mojsa B, Damiano MA, Mora S, Lassot I, et al:
TRIM17 and TRIM28 antagonistically regulate the ubiquitination and
anti-apoptotic activity of BCL2A1. Cell Death Differ. 26:902–917.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei C, Cheng J, Zhou B, Zhu L, Khan MA, He
T, Zhou S, He J, Lu X, Chen H, et al: Tripartite motif containing
28 (TRIM28) promotes breast cancer metastasis by stabilizing TWIST1
protein. Sci Rep. 6:298222016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fong KW, Zhao JC, Song B, Zheng B and Yu
J: TRIM28 protects TRIM24 from SPOP-mediated degradation and
promotes prostate cancer progression. Nat Commun. 9:50072018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Q, Zhu X and Xu M: Silencing of
TRIM10 alleviates apoptosis in cellular model of Parkinsons
disease. Biochem Biophys Res Commun. 518:451–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rong X, Rao J, Li D, Jing Q, Lu Y and Ji
Y: TRIM69 inhibits cataractogenesis by negatively regulating p53.
Redox Biol. 22:1011572019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sies H: Role of metabolic H2O2 generation:
Redox signaling and oxidative stress. J Biol Chem. 289:8735–8741.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seara FAC, Maciel L, Barbosa RAQ,
Rodrigues NC, Silveira ALB, Marassi MP, Carvalho AB, Nascimento JHM
and Olivares EL: Cardiac ischemia/reperfusion injury is inversely
affected by thyroid hormones excess or deficiency in male Wistar
rats. PLoS One. 13:e01903552018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jian Z, Liang B, Pan X, Xu G, Guo SS, Li
T, Zhou T, Xiao YB and Li AL: CUEDC2 modulates cardiomyocyte
oxidative capacity by regulating GPX1 stability. EMBO Mol Med.
8:813–829. 2016. View Article : Google Scholar : PubMed/NCBI
|