Introduction
Type 2 diabetes mellitus (T2DM) is a long-term
metabolic disorder characterized by a reduction in insulin
secretion and an increase in insulin resistance. T2DM is regarded
as one of the most common chronic diseases that can advance to
various organ-associated complications, resulting in an increase in
morbidity and mortality. Persistent glycemic dysfunction promotes
numerous pathological changes and causes functional damage to
organs, including the kidney, eye, nervous and cardiovascular
systems (1). Chronic diabetes may
also promote pulmonary dysfunction, and widespread attention
surrounds the concept of ‘diabetic lungs’ (2). Epidemiological, experimental and
clinical evidence has suggested that high glucose-induced oxidative
stress and inflammation are implicated in the diabetic lung, and
accelerate the decline in respiratory function (3,4).
Several studies have also demonstrated the subclinical significance
of the diabetic lung, and that patients with diabetes are prone to
severe respiratory disorders in the event of acute or chronic lung
and/or cardiac disease (2,5). Further clinical and experimental data
have indicated that inflammatory responses and metabolic
abnormalities in diabetes aggravate the acute lung injury (ALI) via
autophagy (6,7). Myocardial infarction is considered to
be one of the more common complications underlying
diabetes-associated mortality. Myocardial ischemia/reperfusion
(I/R) has been reported to promote the injury of distant organs,
including the lung (8,9). Further studies have confirmed that ALI
caused by myocardial I/R is more severe in patients with diabetes
than in those without diabetes (10). However, there are few reports
concerning the potential underlying mechanisms of ALI in
association with myocardial I/R, and under diabetic conditions.
Autophagy is a catabolic cellular process that
conservatively maintains cell homeostasis and survival. The key
phases of autophagy, such as autophagosome biogenesis, lysosomal
fusion and product degradation, are collectively termed ‘autophagy
flux’, which is elevated during ischemia and markedly enhanced
following tissue reperfusion. Inhibiting excessive autophagy may
alleviate tissue damage under various pathological conditions.
Numerous studies have demonstrated that autophagic dysfunction is
closely associated with diabetic complications (11,12). A
significant time-related effect of lung injury has been associated
with the diabetic condition, demonstrating the involvement of
autophagy in ALI caused by pulmonary I/R in patients with diabetes
(13). Another study suggested that
pulmonary I/R impairment of the autophagic state, and a moderate
increase in autophagy-associated injury, are beneficial for
attenuating lung I/R injury (14).
Collectively, these findings suggest that maintaining a moderate
level of autophagy may help to reduce I/R-induced lung injury and
promote cell survival in vivo. However, in diabetes, the
role of autophagy in ALI caused by myocardial I/R has not been
fully elucidated.
Oxymatrine (OMT) is a quinolizidine alkaloid derived
from Sophora flavescens Ait., which is commonly used to
treat a variety of pathological states, including inflammation,
fibrosis and autophagy. OMT has been used to treat inflammatory
diseases, damage to the heart caused by myocardial ischemia
(15) and hypertension (16), and previous studies have
demonstrated its protective effects against I/R injury (17). The underlying mechanism by which OMT
operates may be associated with the inhibition of oxidative stress
and inflammation, and the induction of apoptosis. However, there
are limited relevant reports concerning the effect of OMT on
autophagy in myocardial I/R-associated ALI in diabetic rats.
Therefore, the present study may improve our understanding of how
OMT ameliorates autophagy in myocardial I/R-induced ALI under
diabetic conditions.
Materials and methods
Animal experiments
A total of 100 male Sprague-Dawley rats (weight,
250–300 g) were purchased from the Hunan SJA Laboratory Animal Co.,
Ltd. The animals were maintained under a 12 h light/dark cycle at
22–25°C, with 50–65% humidity. All animals had free access to food
and water. The present study was performed following the Guide for
the Care and Use of Laboratory Animals, published by the National
Institutes of Health (NIH publication no. 85-23, revised in 1996)
(18), and the experiments were
approved by the Huazhong University of Science and Technology
Medicine Animal Care and Use Committee (Wuhan, China), according to
the regulation of the study of pain, and the Guide for the Care and
Use of Laboratory Animals.
Reagents and antibodies
OMT was purchased from Xi'an Aladdin Biological
Technology Co., Ltd., (cat. no. 16837-52-8) and dissolved in PBS.
Assay kits for the assessment of blood glucose, cytokines [tumor
necrosis factor (TNF)-α (cat. no. H052), interleukin (IL)-6 (cat.
no. H007) and IL-8 (cat. no. H008; Beyotime Institute of
Biotechnology)], leukocyte counts, myocardial enzymes [lactate
dehydrogenase (LDH) (cat. no. A020-2-2) and creatine kinase
isoenzyme MB (CK-MB; cat. no. H197)], superoxide dismutase (SOD;
cat. no. A001-1-2;) and 15-F2t-isoprostane (15-F2t-IsoP; cat. no.
A151-1-1) were purchased from Nanjing Jiancheng Bioengineering
Institute. Antibodies against LC3I (cat. no. 4108), LC3II (cat. no.
3868), beclin-1 (cat. no. 4122), p62 (cat. no. 48768) and autophagy
protein 5 (Atg5; cat. no. 9980) were purchased from Cell Signaling
Technology, Inc. Anti-β-actin (cat. no. BM3873), as well as
anti-rabbit (cat. no. BA1041) and anti-mouse secondary antibodies
(cat. no. BM2020) were purchased from Wuhan Boster Biological
Technology, Ltd. The autophagy inhibitor 3-Methyladenine (3-MA;
cat. no. M9281) and the autophagy inducer rapamycin (Rap; cat. no.
V900930) were purchased from Sigma-Aldrich (Merck KGaA).
Induction and assessment of
diabetes
Male Sprague-Dawley rats were randomly divided into
following groups: i) Control group (n=8); ii) sham group (surgery
with no ischemia) (n=8); and iii) diabetes mellitus (DM) group
(n=40). DM group were fed a high-glucose, high-fat diet consisting
of 70% standard laboratory chow, 15% carbohydrate, 10% lard and 5%
yolk powder. After 4 weeks, the rats received a single
intraperitoneal injection of streptozocin (STZ, 35 mg/kg) and the
high-glucose, high-fat diet was continued. At week 8, blood glucose
was assessed using a sample from the tail vein; a fasting blood
glucose level ≥7.0 mmol/l, or a random blood glucose level ≥11.0
mmol/l, was defined as diabetic, and these rats were selected for
further investigation (n=40).
Establishment of myocardial I/R
injury
The coronary artery ligation method was used to
establish the myocardial I/R injury model. Control and DM rats were
anesthetized with an intraperitoneal injection of sodium
pentobarbital (30 mg/kg), and subsequently received endotracheal
intubation and artificial ventilation. Following exposure of the
heart, a thread was passed through the left coronary artery, and
another two threads were drawn from the knot to loosen the
ligature. The left coronary artery was ligated to induce ischemia,
resulting in a cyanotic appearance to the local myocardium. After 1
h of ischemic induction, the ligature was loosened to restore blood
flow and initiate reperfusion, which was continued for an
additional 1 h.
The DM group were randomly divided into eight groups
as follows (n=8 per group): i) Sham group, surgery with no
ischemia, DM without ischemia; ii) I/R model group, DM and
myocardial I/R; iii) I/R+OMT (12.5 mg/kg) group, DM and myocardial
I/R with 12.5 mg/kg OMT; iv) IR+OMT (25 mg/kg) group, DM and
myocardial I/R with 25 mg/kg OMT; v) IR+OMT (50 mg/kg) group, DM
and myocardial I/R with 50 mg/kg OMT; vi) I/R+Rap group, DM and
myocardial I/R with 15 mg/kg Rap; and vii) I/R+3-MA group, DM and
myocardial I/R with 10 mg/kg 3-MA. OMT was dissolved in isotonic
saline according to previous reports (13,14),
and the rats were administered OMT by gavage 10 min prior to
occlusion of the left coronary artery. After 25 min of reperfusion,
Rap and 3-MA were administered to the appropriate groups to compare
the effects of these compounds on I/R. At the end of the study,
rats were anesthetized by an intraperitoneal injection of
pentobarbital sodium (30 mg/kg) until they lost consciousness.
Blood samples (5 ml) were collected from the abdominal aorta for
subsequent analysis. All the animals were sacrificed following
anesthesia by exsanguination, and their heart and lung tissues were
collected for further experiments.
Blood glucose detection
Blood samples were obtained from the abdominal
aorta, allowed to clot for 30 min at 4°C, and centrifuged at 3,500
× g for 10 min (4°C). The supernatant was used to assess the level
of blood glucose, which was determined using a commercially
available glucose kit.
Blood gas analysis, WET/DRY ratio, and
hematoxylin and eosin (H&E) staining
Carotid blood gas analysis [partial pressure of
oxygen (PO2)] was conducted following 2 h of
reperfusion; the WET/DRY ratio and H&E staining were used to
assess the degree of pulmonary injury. The lung tissues were
weighed and dried for 48 h in an oven at a constant temperature of
60°C; when a dehydrated consistency was achieved, the tissues were
weighed once again. The twice weight ratio was calculated as an
indicator of edema. Specimens from the left lower lobe of the lung
were fixed at 25°C in 4% paraformaldehyde for 24 h, sectioned (2-µm
thick) and stained with hematoxylin for 2 min at 25°C followed by
eosin for 2 min at 25°C. Light microscopic examination was
conducted to determine the following: i) Pulmonary interstitial
edema; ii) airway epithelial injury; iii) the degree of
hyalinization; and vi) the lung injury score. The criteria for the
lung injury score was membrane formation, neutrophil infiltration
and alveolar hemorrhage (7,19). The indices were graded as follows:
i) Normal=0′; ii) minimal change=1′; iii) mild change=2′; iv)
moderate change=3′; and v) severe change=4′. Collectively, each
section received five scores, and the scores for each criterion
were used to evaluate the degree of lung tissue injury.
Detection of myocardial enzyme
levels
Following sacrifice, the blood of each rat was
collected via the tail vein and the serum was isolated as
aforementioned. Using commercially available kits (per the
manufacturers' instructions), LDH and CK-MB were detected to
evaluate the level of damage to the heart muscle.
Determination of SOD, GSH and
15-F2t-IsoP levels
Lung tissues were homogenized, centrifuged at 4,000
× g, 4°C for 10 min, and the supernatant was collected and stored
at −80°C. The levels of SOD, GSH and 15-F2t-IsoP were determined
using commercially available kits per the manufacturers'
protocols.
Detection of leukocyte count, and the
levels of TNF-α, IL-6 and IL-8 in the bronchoalveolar lavage (BAL)
fluid
The leukocyte count, and the levels of TNF-α, IL-6
and IL-8 in the BAL fluid of diabetic rats were evaluated using
commercially available kits.
Western blotting
Total protein was extracted from lung tissues using
RIPA buffer (containing 0.1% PMSF; cat. no. R0278; Sigma-Aldrich;
Merck KGaA). Equal amounts of protein from each sample (50 mg) were
separated via SDS-PAGE on 10% or 12% gels, and subsequently
transferred to polyvinylidene fluoride membranes. The membranes
were then incubated with primary antibodies overnight at 4°C, and
washed prior to a second incubation with horseradish peroxidase
(HRP)-coupled goat anti-mouse (1:1,000) or goat anti-rabbit
secondary antibodies (1:1,000) for 1 h at room temperature. The
chemiluminescence signals were detected using the
EasySee® Western Blot Kit (Beijing TransGen Biotech Co.,
Ltd.), and densitometric analysis was conducted using ImageJ 1.43
(National Institutes of Health). The primary antibodies and their
dilutions were as follows: Anti-LC3I (1:1,000), LC3II (1:1,000),
beclin-1 (1:1,000), p62 (1:1,000), Atg5 (1:1,000) and β-actin
(1:1,000; cat. no. BM3873; Wuhan Boster Biological Technology,
Ltd).
Statistical analysis
SPSS 21.0 (IBM Corp.) was used for statistical
analysis, and all data are presented as the mean ± standard
deviation (SD). For gene expression, one-way ANOVA followed by
Tukey's post hoc test was used to test for differences among
groups. To analyze lung injury scores, Kruskal-Wallis test was
performed, followed by Dunn's multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Myocardial injury following myocardial
I/R in diabetic rats
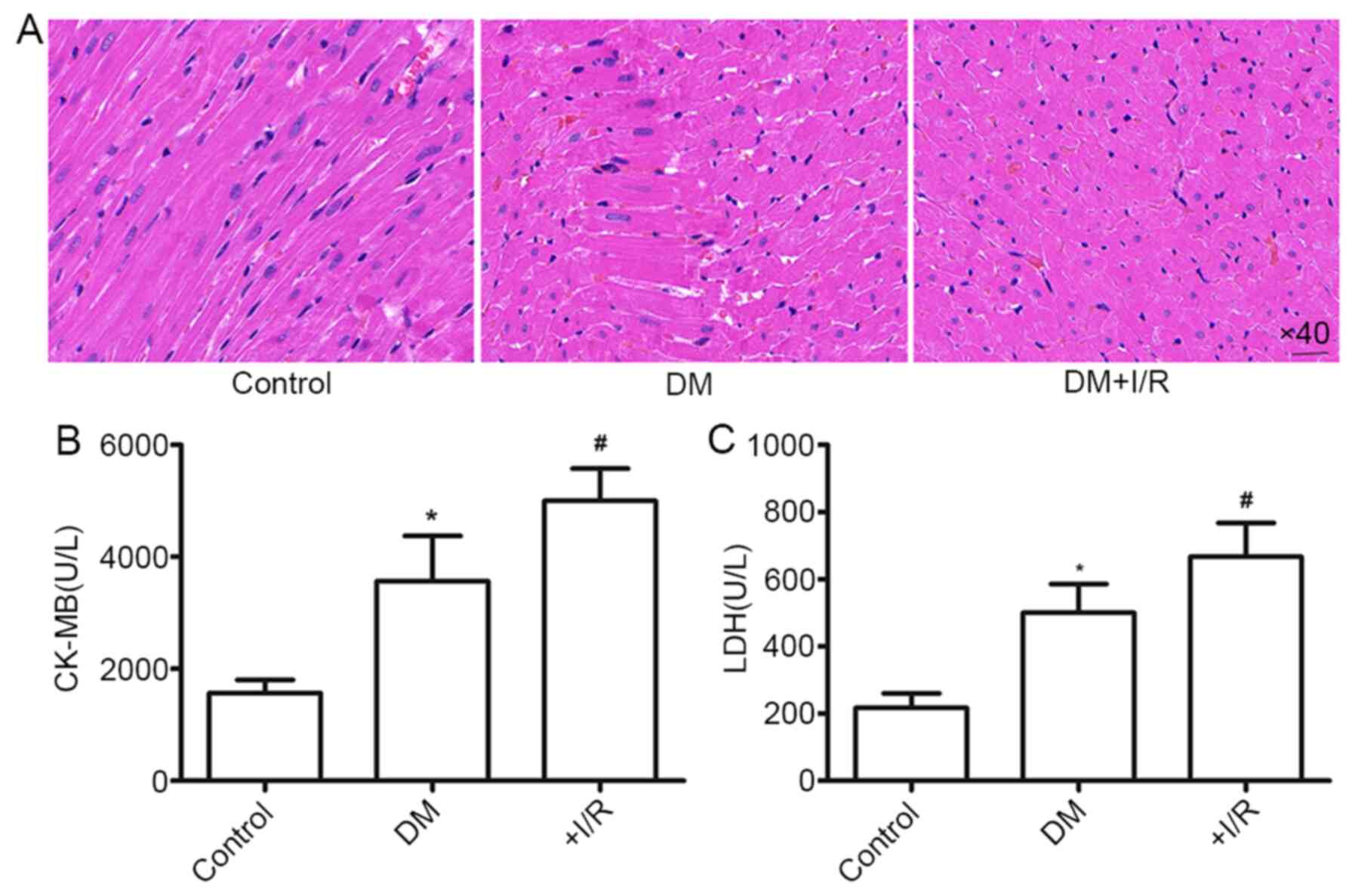
Following myocardial I/R, the morphology of the
myocardial tissue of diabetic rats was notably altered; myocardial
fiber arrangement was disordered and even partially dissolved
(Fig. 1A). Furthermore, the levels
of CK-MB and LDH, markers of myocardial tissue damage, were
significantly higher in the DM+myocardial I/R group than those in
the Sham group (P<0.05; Fig. 1B and
C). These findings suggested that hyperglycemic rats are prone
to myocardial injury, and that I/R accelerates diabetic myocardial
injury.
Diabetes aggravates ALI following
myocardial I/R, which is accompanied by an impaired autophagy
status
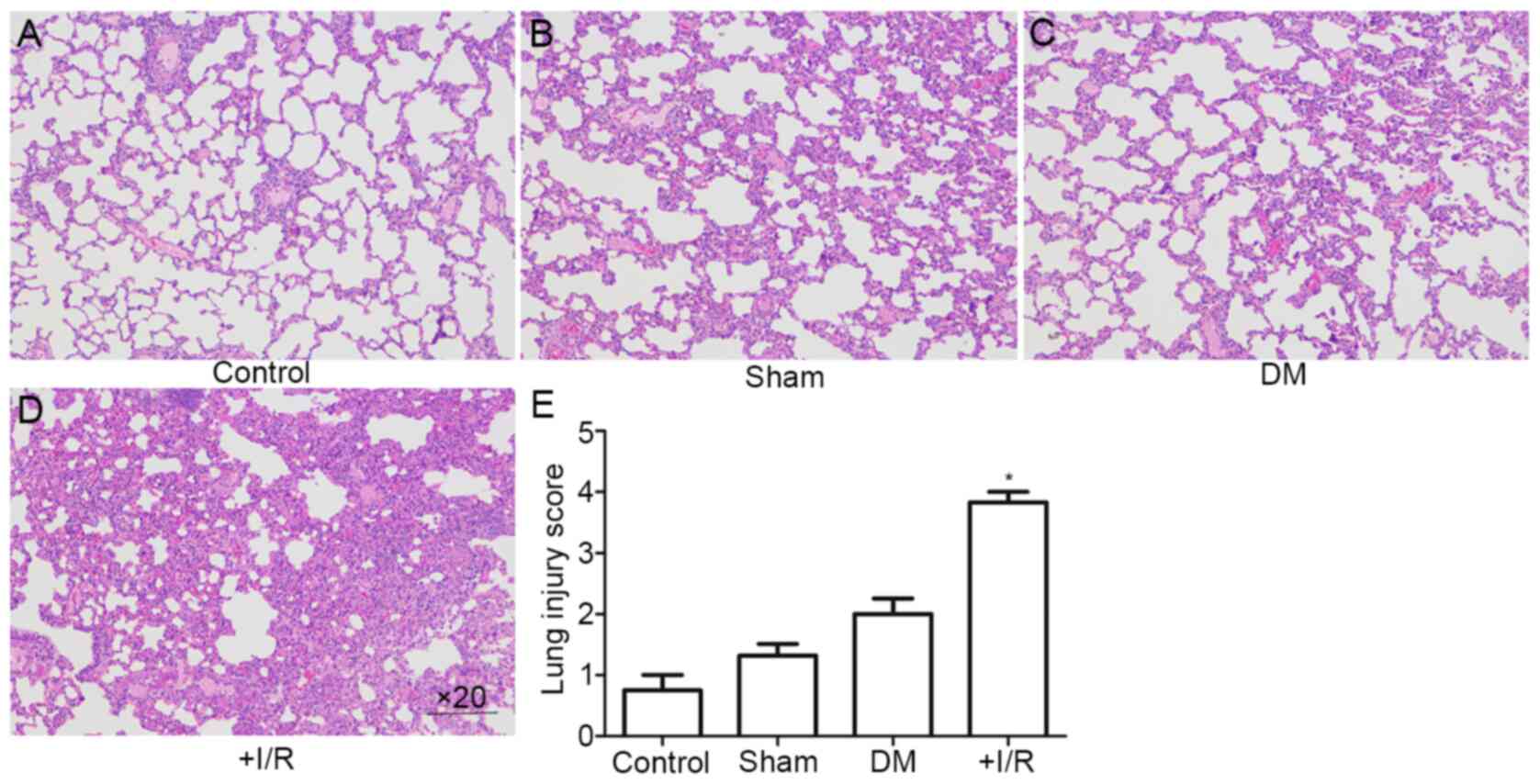
Present experimentation demonstrated that ALI occurs
in diabetic rats following myocardial I/R. As shown in Fig. 2A-D, H&E staining of the lung
tissues revealed ALI with alveolar and interstitial edema,
hemorrhage and inflammatory cell infiltration. These pathological
changes were more severe in diabetic rats. Compared with the Sham
group, the degree of lung injury in the DM+myocardial I/R groups
was significantly increased compared with the control and sham
groups (P<0.05; Fig. 2E). These
results indicated that diabetic rats with myocardial I/R exhibited
a greater degree of lung injury than those in the Sham group.
Effects of Rap and 3-MA on ALI
following myocardial I/R in diabetic rats
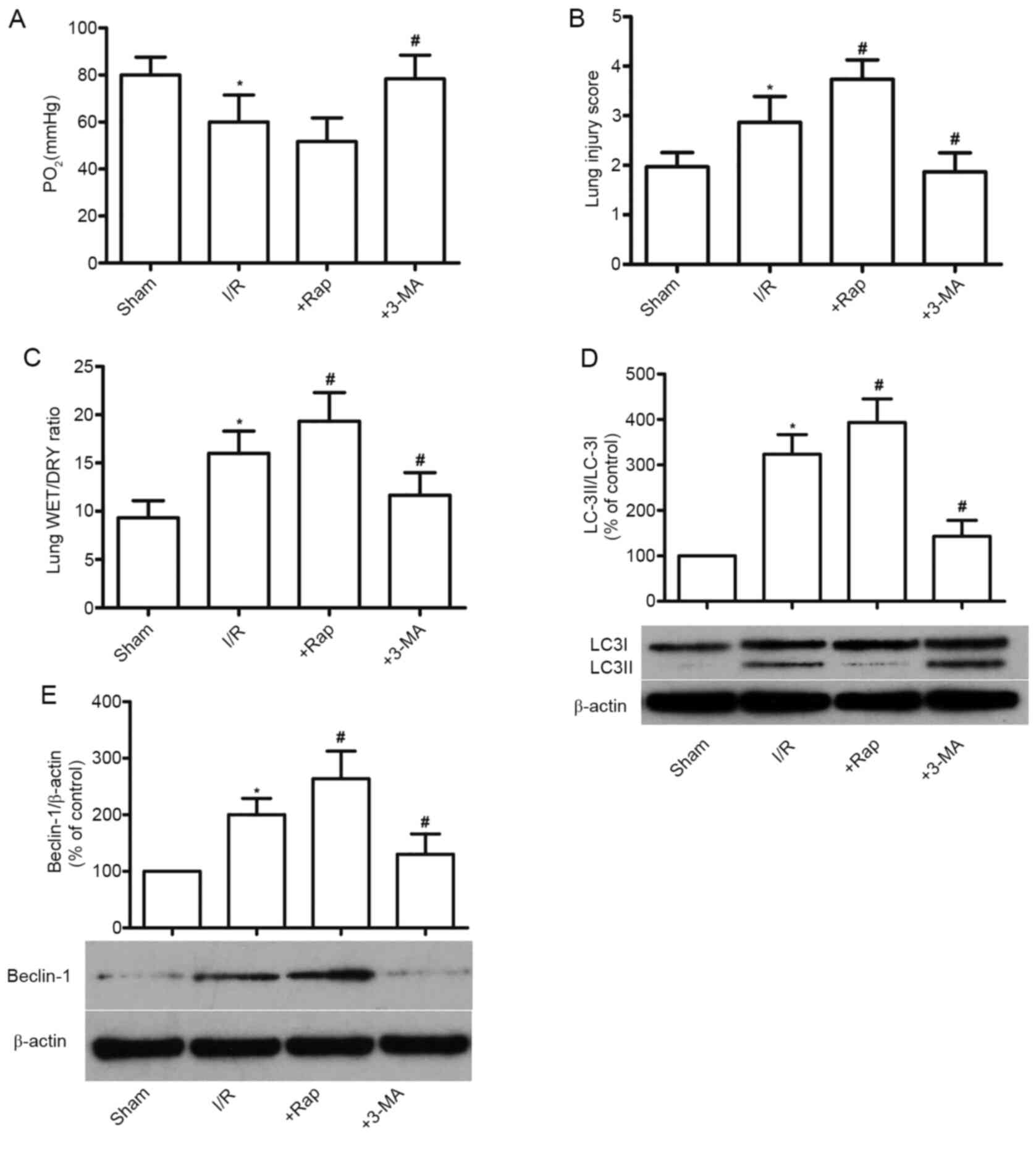
As shown in Fig. 3,
compared with the Sham group, myocardial I/R significantly
decreased lung function in rats (P<0.05; Fig. 3A); lung tissue damage was
significantly enhanced (P<0.05; Fig.
3B), and the WET/DRY ratio of the lung tissue was increased
(P<0.05; Fig. 3C), indicating
that myocardial I/R injury induces and accelerates ALI in diabetic
rats. The autophagy inducer Rap further enhanced I/R-induced ALI
damage, and by contrast, the inhibitor 3-MA alleviated ALI compared
with the DM+myocardial I/R group (P<0.05; Fig. 3A-C).
Autophagy is reportedly initiated by the activation
of beclin-1 and the binding of LC-3II to autophagosomes. Thus,
beclin-1 and LC-3II expression are considered as specific markers
of autophagic initiation (20). As
shown in Fig. 3D and E, the
expression levels of LC-3II/LC-3I and beclin-1 were significantly
increased in diabetic rats compared with those in the Sham group
(P<0.05), which was enhanced by Rap and reversed by 3-MA
treatment. These results indicated that diabetes accompanied by
myocardial I/R impairs the autophagic state.
Effects of Rap and 3-MA on pulmonary
inflammation and oxidative stress in diabetic rats subjected to
myocardial I/R
Myocardial I/R aggravates inflammation and oxidative
stress in diabetic rats, which promotes autophagy-associated
injury. The leukocyte count, and the levels of TNF-α, IL-6 and IL-8
have been regarded as common indicators of the extent of lung
injury. Thus, the BAL fluid was collected and analyzed to assess
the severity of ALI in diabetic rats. As shown in Fig. 4A-D, the leukocyte counts and levels
of TNF-α, IL-6 and IL-8 in the BAL fluid of diabetic rats were
significantly higher than those in the Sham group (P<0.05).
These results suggested a severe inflammatory reaction in diabetic
rats following myocardial I/R. The autophagy inducer Rap
significantly increased the leukocyte count and levels of
inflammatory cytokines, whereas the autophagy inhibitor 3-MA had
the opposite effects (P<0.05). The influence of autophagy on
pulmonary oxidative stress in diabetic rats was also investigated
following myocardial I/R. The results also showed that ALI
significantly decreased SOD activity and increased 15-F2t-IsoP
formation, as compared with the Sham group (P<0.05; Fig. 4E and F). All pulmonary inflammatory
changes were increased by Rap, but were reversed by 3-MA
administration.
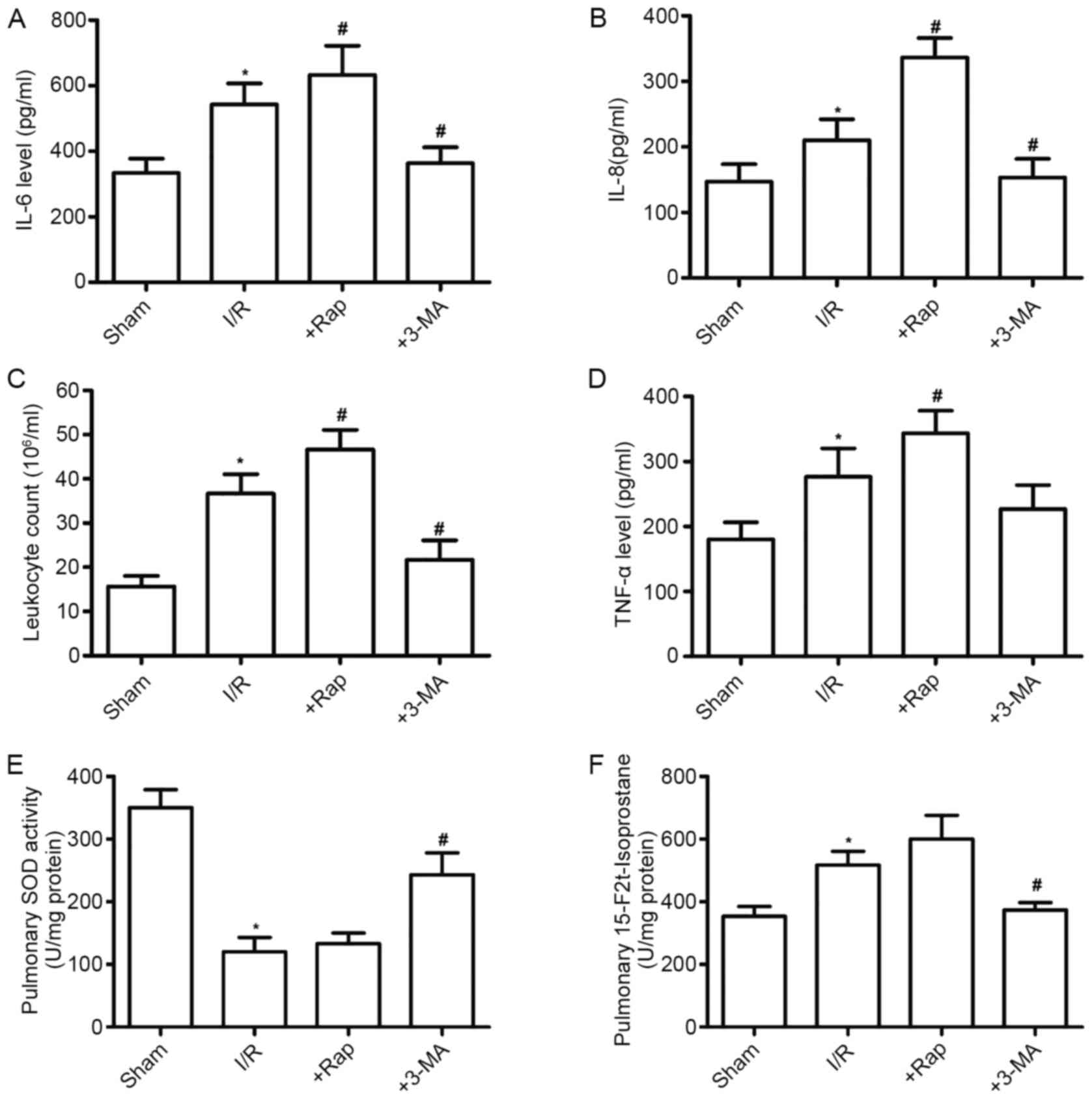 | Figure 4.Effects of Rap and 3-MA on pulmonary
inflammation in DM rats subjected to myocardial I/R. Effects of Rap
and 3-MA on (A) IL-6, (B) IL-8, (C) leukocyte counts and (D) TNF-α
in the bronchoalveolar lavage fluid. Effects of Rap and 3-MA on (E)
SOD and (F) 15-F2t-Isop in lung tissues from control and DM rats
following myocardial I/R. All values are expressed as the mean ±
SD, n=8. *P<0.05 vs. the Sham group; #P<0.05 vs.
the I/R group. Rap, rapamycin; 3-MA, 3-Methyladenine; I/R,
ischemia/reperfusion; IL, interleukin; TNF-α, tumor necrosis
factor-α; SOD, superoxide dismutase; 15-F2t-Isop,
15-F2t-isoprostane; DM, diabetes mellitus. |
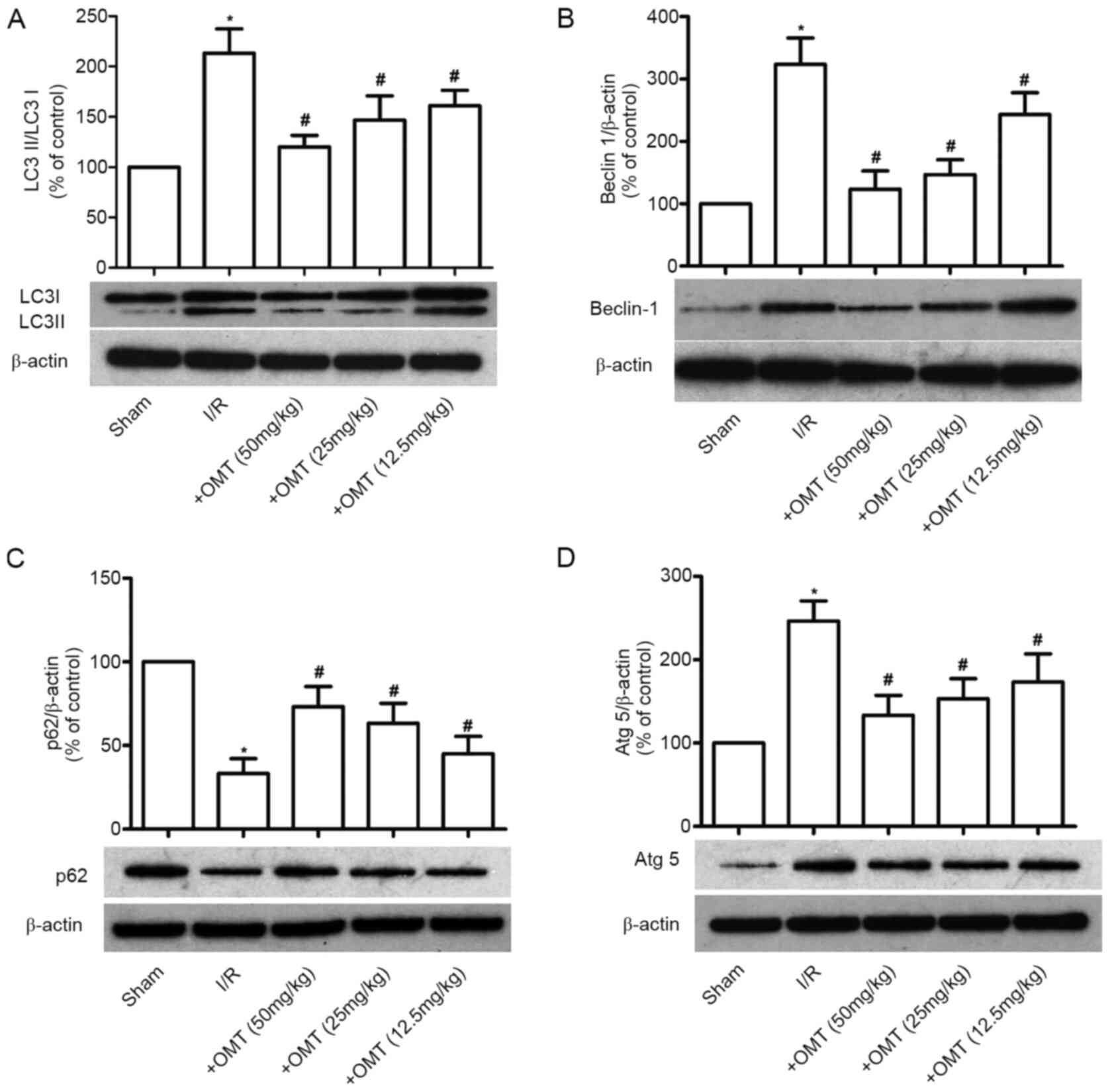
Effect of OMT on autophagy in
myocardial I/R-induced lung injury in diabetic rats
Autophagy is activated when myocardial I/R in
diabetic rats induces ALI. To investigate the effect of OMT on
autophagy in myocardial I/R-induced ALI in diabetic rats, the
expression of LC-3II/LC-3I, Beclin-1, p62 and Atg5 was evaluated.
Compared with the Sham group, the expression levels of
LC-3II/LC-3I, Beclin-1 and Atg5 were significantly upregulated, and
the expression of p62 was downregulated following myocardial I/R in
diabetic rats (P<0.05; Fig.
5A-D). Furthermore, these effects were reversed following
treatment with OMT, in a dose-dependent manner (P<0.05). These
findings suggested that OMT inhibits ALI-induced autophagy in
diabetic rats.
Discussion
At present, there are an increasing number of
studies focusing on diabetic complications, including those of the
heart and kidney, as well as retinopathic injuries (21,22).
However, relatively few reports have focused on the influence of
diabetes on the lungs, especially in ALI induced by diabetic
myocardial infarction. The results of the present study revealed
that the diabetic lung is more susceptible to myocardial I/R, and
provided further evidence that ALI following myocardial I/R is
associated with aberrant autophagy. Using the autophagy inducer Rap
or the autophagy inhibitor 3-MA, it was further confirmed that
autophagy is implicated in ALI caused by myocardial I/R under
diabetic conditions, which is consistent with previous findings
(5,7). To the best of our knowledge, the
present study was the first to investigate the protective effects
of OMT on myocardial I/R-associated ALI, via the inhibition of
autophagy in diabetic rats.
Diabetes remains a major global health issue, and
persistent hyperglycemia causes systemic damage to various organs;
such complications include diabetic cardiomyopathy, diabetic
nephropathy, retinopathy, neuropathy and diabetic foot (1,23).
Changes in the normal anatomy and biological characteristics of the
lung during diabetes significantly accelerates the deterioration in
respiratory function (2). Previous
studies have demonstrated that diabetes disrupts ventilation by
reducing respiratory muscle strength (24). Additionally, abnormal
saccharification leads to thickening of the basement membrane and
proliferation of vascular smooth muscle cells, reducing ventilation
capacity and blood flow to the area. Experiencing acute or chronic
lung and/or heart disease during diabetes confers vulnerability to
the diabetic lung, and patients with diabetes are at a higher risk
of developing cardiovascular diseases, and thus have a poor
prognosis following myocardial infarction (25,26).
Acute myocardial ischemia is regarded to be the primary cause of
ALI, resulting in acute severe pulmonary hypertension (27) and pulmonary hemodynamics (28). Previous studies have demonstrated
several mechanisms of ALI that are associated with the pulmonary
dysfunction induced by myocardial I/R (29,30).
Under diabetic conditions, the lung tissue is characterized by a
large number of oxygen free radicals (primarily reactive oxygen
species) accompanied by a decrease in its antioxidant capacity,
including activation of NADPH oxidase, decreased SOD activity and
reduced GSH levels. In addition, diabetes promotes the release of
various proinflammatory cytokines, which further exacerbate reduced
lung function and ALI. Numerous studies have demonstrated that
inhibiting the pulmonary inflammatory response and reducing
oxidative stress alleviates lung injury in diabetic conditions
(31,32). In vivo animal experiments
have also indicated that myocardial I/R aggravates lung tissue
injury, which is involved in oxidative stress and the inflammatory
response in diabetes (33). The
present study demonstrated that oxidative damage and inflammation
accelerate diabetic lung injury, especially when accompanied by
myocardial I/R. The results of the present study also revealed a
significant decrease in the antioxidant capacity of lung tissue
(including decreased SOD activity and increased formation of
15-F2t-IsoP), and an increase in inflammatory cytokines (leukocyte
count, TNF-α, IL-6 and IL-8) in diabetic rats, accompanied by
higher lung injury scores and WET/DRY ratios, and a lower PO2;
these findings are consistent with those previously reported
(10), and indicate that myocardial
I/R accelerates the loss of pulmonary function and results in
severe lung injury in diabetic rats.
Autophagy is a eukaryotic cellular self-degradation
and recycling process in which intracellular membrane structures
package protein complexes and organelles to degrade and recycle
intracellular proteins and damaged organelles, maintaining the
normal cellular function. Under normal circumstances, autophagy is
a homeostatic mechanism for cell survival and maintenance via the
lysosomal degradation of cytoplasmic constituents. However,
accumulating evidence indicates that under pathological conditions,
such as inflammation, diabetes, a high-fat diet and oxidative
stress, autophagic dysfunction is linked to cell death processes
(34,35). Autophagy has been demonstrated to
play an important role in regulating normal function of pancreatic
β cells and insulin-target tissues in the liver, and adipose
tissue. Enhanced autophagy also acts as a protective mechanism
against oxidative stress in these tissues. Altered autophagic
activity has been implicated in the progression of obesity to T2DM
through impaired β cell function and development of insulin
resistance (36). Moreover, there
is ample evidence to suggest that lung I/R injury promotes
excessive autophagy (13), and cold
ischemia preservation for lung transplantation has been shown to
induce autophagy to varying degrees over different time periods
(37). Based on this evidence, it
was speculated that the severity and duration of I/R could
influence the degree of autophagy, promoting either cell survival
or death. The results of the present study further confirmed the
fact that the pulmonary autophagy state was excessive in both
control and diabetic rats subjected to 30 min ischemia, followed by
2 h reperfusion. Defective autophagy was also observed in lung
tissues subjected to I/R, which is consistent with a previous
report (16). The expression levels
of markers of autophagy, such as of LC3-II/LC3-I and Beclin-1 were
therefore investigated in the present study, and were found to be
increased in diabetic rats following myocardial I/R, suggesting
that myocardial I/R induces autophagy. Furthermore, an upregulation
in Atg5 and a downregulation in p62 were observed in the diabetic
rats, and these effects were reduced by OMT. Autophagy selectively
degrades damaged cellular organelles and protein aggregates, while
apoptosis removes damaged or aged cells. Maintaining a balance
between autophagy and apoptosis is critical for cell fate. I/R
accelerates autophagy and then contributes to cell apoptosis, which
is widely accepted. In present study, it was found that I/R
disturbed the balance between autophagy and cell apoptosis, which
exacerbated tissue damage.
In order to clarify whether diabetes induced by STZ
or a high-sugar, high-fat diet promotes autophagy, the
phosphoinositide 3-kinase inhibitor, 3-MA, was utilized. Rap was
also used to induce autophagy. During treatment with OMT, LC3-II
expression was significantly decreased in diabetic rats in a
concentration-dependent manner. The effect of OMT was similar to
that of 3-MA. To the best of our knowledge, the present study is
the first to demonstrate that OMT ameliorates myocardial
I/R-induced ALI by inhibiting autophagy in diabetic rats.
In conclusion, the present study demonstrated that
diabetes-impaired autophagic status is associated with ALI
following myocardial I/R in diabetic rats. Blocking autophagy may
improve myocardial I/R-induced ALI by inhibiting inflammatory and
oxidative stress responses. Furthermore, OMT inhibits
ALI-associated autophagy following diabetic myocardial I/R. These
results suggested that OMT treatment may be an effective strategy
for maintaining autophagic homeostasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Hubei National
Science Funds (grant no. 2017JJ2343).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX designed the study, performed the experiments,
and analyzed, interpreted and presented results for group
discussions. ZX, XL and JX designed the study, performed the
experiments and wrote the manuscript. XL provided rationale,
background, framework and feedback. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was performed following the Guide
for the Care and Use of Laboratory Animals, published by the
National Institutes of Health (NIH publication no. 85-23, revised
in 1996), and the experiments were approved by the Huazhong
University of Science and Technology Medicine Animal Care and Use
Committee (Wuhan, China; approval no. 2019-0013), according to the
regulation of the study of pain, and the Guide for the Care and Use
of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gregg EW, Sattar N and Ali MK: The
changing face of diabetes complications. Lancet Diabetes
Endocrinol. 4:537–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pitocco D, Fuso L, Conte EG, Zaccardi F,
Condoluci C, Scavone G, Incalzi RA and Ghirlanda G: The diabetic
lung-a new target organ? Rev Diabet Stud. 9:23–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anhê FF, Roy D, Pilon G, Dudonné S,
Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E
and Marette A: A polyphenol-rich cranberry extract protects from
diet-induced obesity, insulin resistance and intestinal
inflammation in association with increased Akkermansia spp.
population in the gut microbiota of mice. Gut. 64:872–883. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khateeb J, Fuchs E and Khamaisi M:
Diabetes and lung disease: A neglected relationship. Rev Diabet
Stud. 15:1–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh F, Dixon AE, Marion S, Schaefer C,
Zhang Y, Best LG, Calhoun D, Rhoades ER and Lee ET: Obesity in
adults is associated with reduced lung function in metabolic
syndrome and diabetes: The strong heart study. Diabetes Care.
34:2306–2313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li K, Li M, Li W, Yu H, Sun X, Zhang Q, Li
Y, Li X, Li Y, Abel ED, et al: Airway epithelial regeneration
requires autophagy and glucose metabolism. Cell Death Dis.
10:8752019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan L, Zhang Y, Su W, Zhang Q, Chen R,
Zhao B, Li W, Xue R, Xia Z and Lei S: The roles of autophagy in
acute lung injury induced by myocardial ischemia reperfusion in
diabetic rats. J Diabetes Res. 2018:50475262018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Ji M, Chen L, Wu X and Wang L:
Breviscapine reduces acute lung injury induced by left heart
ischemic reperfusion in rats by inhibiting the expression of ICAM-1
and IL-18. Exp Ther Med. 6:1322–1326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Guo Y, Yu S, Wei J and Jin J:
Effects of edaravone on the expression of β-defensin-2 mRNA in lung
tissue of rats with myocardial ischemia reperfusion. Mol Med Rep.
7:1683–1687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alkan M, Celik A, Bilge M, Kiraz HA, Kip
G, Ozer A, Sivgin V, Erdem O, Arslan M and Kavutcu M: The effect of
levosimend an on lung damage after myocardial ischemia reperfusion
in rats in which experimental diabetes was induced. J Surg Res.
193:920–925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto S, Kazama JJ and Fukagawa M:
Autophagy: A two edged swordin diabetes mellitus. Biochem J.
456:e1–e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Jia JY, Yan TK, Yu YM, Shang WY,
Wei L, Zheng ZF, Fang P, Chang BC and Lin S: Effects of ammonium
pyrrolidine dithiocarbamate (PDTC) on osteopontin expression and
autophagy in tubular cells in streptozotocin-induced diabetic
nephropathy rat. Zhonghua Yi Xue Za Zhi. 96:3590–3595. 2016.(In
Chinese). PubMed/NCBI
|
|
13
|
Zhang J, Wang JS, Zheng ZK, Tang J, Fan K,
Guo H and Wang JJ: Participation of autophagy in lung
ischemia-reperfusion injury in vivo. J Surg Res. 182:e79–e87. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang D, Li C, Zhou J, Song Y, Fang X, Ou
J, Li J and Bai C: Autophagy protects against
ischemia/reperfusion-induced lung injury through alleviating
blood-air barrier damage. J Heart Lung Transplant. 34:746–755.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D,
Guo-Fen Q, Yan L, Wen-Feng C and Bao-Feng Y: Cardioprotective
effects and underlying mechanisms of oxymatrine against ischemic
myocardial injuries of rats. Phytother Res. 22:985–989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao TT, Wang YY, Zhang Y, Bai CH and Shen
XC: Similar to spironolactone, oxymatrine is protective in
aldosterone-induced cardiomyocyte injury via inhibition of calpain
and apoptosis-inducing factor signaling. PLoS One. 9:e888562014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li RS, Yu CL and Jin XZ: Effect of
oxymatrine on beating of cultured myocardial cells in vitro.
Zhongguo Yao Li Xue Bao. 10:530–532. 1989.(In Chinese). PubMed/NCBI
|
|
18
|
Institute of Laboratory Animal Resources
(US), . National Research Council. National Academy Press;
Washington, DC: 1996
|
|
19
|
Liu R, Fang X, Meng C, Xing J, Liu J, Yang
W, Li W and Zhou H: Lung inflation with hydrogen during the cold
ischemia phase decreases lung graft injury in rats. Exp Biol Med
(Maywood). 240:1214–1222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuma A, Komatsu M and Mizushima N:
Autophagy-monitoring and autophagy-deficient mice. Autophagy.
13:1619–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Marco E, Jha JC, Sharma A,
Wilkinson-Berka JL, Jandeleit-Dahm KA and de Haan JB: Are reactive
oxygen species still the basis for diabetic complications? Clin Sci
(Lond). 129:199–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Li S and Sun D: Calcium dobesilate
and micro-vascular diseases. Life Sci. 221:348–353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flemming NB, Gallo LA, Ward MS and Forbes
JM: Tapping into mitochondria to find novel targets for diabetes
complications. Curr Drug Targets. 17:1341–1349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tai H, Wang MY, Zhao YP, Li LB, Jiang XL,
Dong Z, Lv XN, Liu J, Dong QY, Liu XG and Kuang JS: Pulmonary
function and retrobulbar hemodynamics in subjects with type 2
diabetes mellitus. Respir Care. 62:602–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strojek K, Raz I, Jermendy G, Gitt AK, Liu
R, Zhang Q, Jacober SJ and Milicevic Z: Factors associated with
cardiovascular events in patients with type 2 diabetes and acute
myocardial infarction. J Clin Endocrinol Metab. 101:243–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbarash O, Gruzdeva O, Uchasova E, Belik
E, Dyleva Y and Karetnikova V: Biochemical markers of type 2
diabetes as a late complication of myocardial infarction: A
case-control study. Arch Med Sci. 13:311–320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khatib D, Boettcher BT, Freed JK and Pagel
PS: Acute, severe pulmonary arterial hypertension during off-pump
coronary artery surgery: Is new myocardial ischemia, cardiac
repositioning, or external mitral valve compression the culprit? J
Cardiothorac Vasc Anesth. 30:1744–1747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Evlakhov VI and Poiasov IZ: Pulmonary
hemodynamics following experimental myocardial ischemia after the
blockade of adrenergic receptors. Ross Fiziol Zh Im I M Sechenova.
101:44–53. 2015.(In Russian). PubMed/NCBI
|
|
29
|
Wang M, Verhaegh R, Tsagakis K, Brencher
L, Zwanziger D, Jakob HG, Groot H and Dohle DS: Impact of acute
intestinal ischemia and reperfusion injury on hemodynamics and
remote organs in a rat model. Thorac Cardiovasc Surg. 66:99–108.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palladini G, Ferrigno A, Rizzo V,
Tarantola E, Bertone V, Freitas I, Perlini S, Richelmi P and
Vairetti M: Lung matrix metalloproteinase activation following
partial hepatic ischemia/reperfusion injury in rats.
ScientificWorldJournal. 2014:8675482014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo W, Jin Y, Wu G, Zhu W, Qian Y, Zhang
Y, Li J, Zhu A and Liang G: Blockage of ROS and MAPKs-mediated
inflammation via restoring SIRT1 by a new compound LF10 prevents
type 1 diabetic cardiomyopathy. Toxicol Appl Pharmacol. 370:24–35.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F, Yang F, Zhao H and An Y: Curcumin
alleviates lung injury in diabetic rats by inhibiting nuclear
factor-κB pathway. Clin Exp Pharmacol Physiol. 42:956–963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conklin DJ, Guo Y, Jagatheesan G, Kilfoil
PJ, Haberzettl P, Hill BG, Baba SP, Guo L, Wetzelberger K, Obal D,
et al: Genetic deficiency of glutathione S-transferase p increases
myocardialsensitivity to ischemia-reperfusion injury. Circ Res.
117:437–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dehdashtian E, Mehrzadi S, Yousefi B,
Hosseinzadeh A, Reiter RJ, Safa M, Ghaznavi H and Naseripour M:
Diabetic retinopathy pathogenesis and the ameliorating effects of
melatonin; involvement of autophagy, inflammation and oxidative
stress. Life Sci. 193:20–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Montane J, Cadavez L and Novials A: Stress
and the inflammatory process: A major cause of pancreatic cell
death in type 2 diabetes. Diabetes Metab Syndr Obes. 7:25–34.
2014.PubMed/NCBI
|
|
36
|
Barlow AD and Thomas DC: Autophagy in
diabetes: β-cell dysfunction, insulin resistance, and
complications. DNA Cell Biol. 34:252–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Wu JX, You XJ, Zhu HW, Wei JL and
Xu MY: Cold ischemia-induced autophagy in rat lung tissue. Mol Med
Rep. 11:2513–2519. 2015. View Article : Google Scholar : PubMed/NCBI
|