Introduction
Parkinson's disease is characterized by motor
symptoms such as tremor, postural instability, and bradykinesias
caused by the progressive loss of dopaminergic neurons in the
substantia nigra (1,2). Although the motor symptoms of PD can
be managed with dopamine replacement therapy using L-DOPA (3), long-term levodopa treatment leads to
motor complications involving dyskinesia and motor fluctuations
tend to occur within a few years of L-DOPA treatment initiation
(4). L-DOPA-induced dyskinesia
affects quality of life and requires intervention.
The neurological mechanisms of L-DOPA-induced
dyskinesia (LID) remain largely unclear. Among the several factors
that play a role in the onset and severity of LID, abnormalities in
connectivity between the striatum and the motor cortex induced by
the loss of dopaminergic neurons are considered to be critical
elements (5). Numerous studies have
reported alterations in the basal ganglia circuitry involving
excessive release of dopamine (DA) and hyper-activation of striatal
DA receptors and related signaling pathways (5–7). In
addition to neuronal targets for normalizing DA D1 receptor
signaling, such as extracellular signaling-regulated kinase 1/2
(ERK1/2), mTOR, ΔFosB, and the M4 muscarinic receptor (7–9),
non-neuronal mechanisms for regulating glial cells have also been
suggested to contribute to the development of LID (10). Reactive microglia and astrocytes
have been observed in postmortem brain samples of PD patients
(11,12), and an upregulation of astrocytosis
in the striatum of PD animal models displaying LID has been
reported (13). Higher DA induced
by prolonged L-DOPA treatment results in the production of toxic
products in astrocytes (10). With
these premises, modulating glial-mediated neuroinflammation may be
a new promising target for treating LID in PD.
β-Lapachone
(3,4-dihydro-2,2-dimetyl-2H-naphthol[1,2-b]pyran-5,6-dione) is a
quinone-containing compound that was originally isolated from a
lapacho tree in South America (14). The therapeutic effects of
β-Lapachone on rheumatoid arthritis and metabolic syndrome have
been reported (15,16). Recently, in neurological disorders
such as cerebral ischemia, multiple sclerosis, and Huntington's
disease, the neuroprotective effects of β-Lapachone have also been
reported (17–20). β-Lapachone is known as an anticancer
drug candidate that can facilitate quinone oxidoreductase-1
(NQO1)-dependent oxidation of NADP(H) (21). NQO1, a phase II antioxidant enzyme,
is involved in cytoprotective and detoxification processes
(22). In addition, NQO1 has also
been reported to play a regulatory role in the dopaminergic system
of rodents (22,23). β-Lapachone has strong
anti-inflammatory and anti-oxidative effects in vitro and
in vivo (24,25). The neuroprotective effect of
β-Lapachone, which works by upregulating the pAMPK/NRF/HO-1
signaling pathway in astrocytes, has been shown in an MPTP-induced
PD mouse model (20). However, the
effect of β-Lapachone cotreatment on L-DOPA therapy in PD has not
been elucidated. We hypothesized that β-Lapachone co-treatment with
L-DOPA alleviates the dyskinesia induced by chronic L-DOPA
treatment. In the present study, we examined the behavioral AIMs
associated with LID in an animal model of PD and the effects of
β-Lapachone on the D1R signaling pathway, astrocyte activation, and
GSK-3β phosphorylation in the 6-OHDA mouse model of PD.
Materials and methods
Animals
C57BL/6J mice (8–9 weeks-old male, 22–27 g) used in
the experiment were purchased from Laboratory Animal Resource
Center of KRIBB (Korea). The living environment of mice is
controlled with day and night cycles (light on at 7:00 A.M. and
light off at 7:00 P.M) and the temperature (21–23°C) and humidity
(50–60%) are kept constant. Gamma-irradiated laboratory chow
(Envigo Teklad) and autoclaved water were provided. The animal room
was maintained under specific pathogen-free conditions. The mice
were habituated for 7 days before surgery. 6-hydroxydopamine
(6-OHDA) was injected into the substantia nigra pars compacta (SNc)
of mice. To investigate the effect of β-Lapachone on L-DOPA-induced
dyskinesia in PD, we generated a PD mouse model by inducing
unilateral 6-OHDA lesions (n=25 animals). Two weeks after inducing
the 6-OHDA lesion, ipsilateral turning behavior, induced by
d-amphetamine, was observed in all unilateral 6-OHDA-lesioned mice.
Based on the number of d-amphetamine-induced rotations, mice were
randomly assigned to two groups: Vehicle (0.9% NaCl)-treated group
(n=12 animals), and β-Lapachone (10 mg/kg/day)-treated group (n=13
animals). The threshold number of ipsilateral rotations induced by
d-amphetamine was 300. β-Lapachone was orally administered 30 min
before L-DOPA injection for 11 days (Fig. 1A). Finally, only mice with at least
an 80% reduction in tyrosine hydroxylase (TH)-positive cells in the
6-OHDA-lesioned SNc and TH-immunoreactive fibers in the
6-OHDA-lesioned striata relative to the striata without lesions
were included in the analyses. All animal experiments were approved
by the Institutional Animal Care and Use Committee of the
KRIBB.
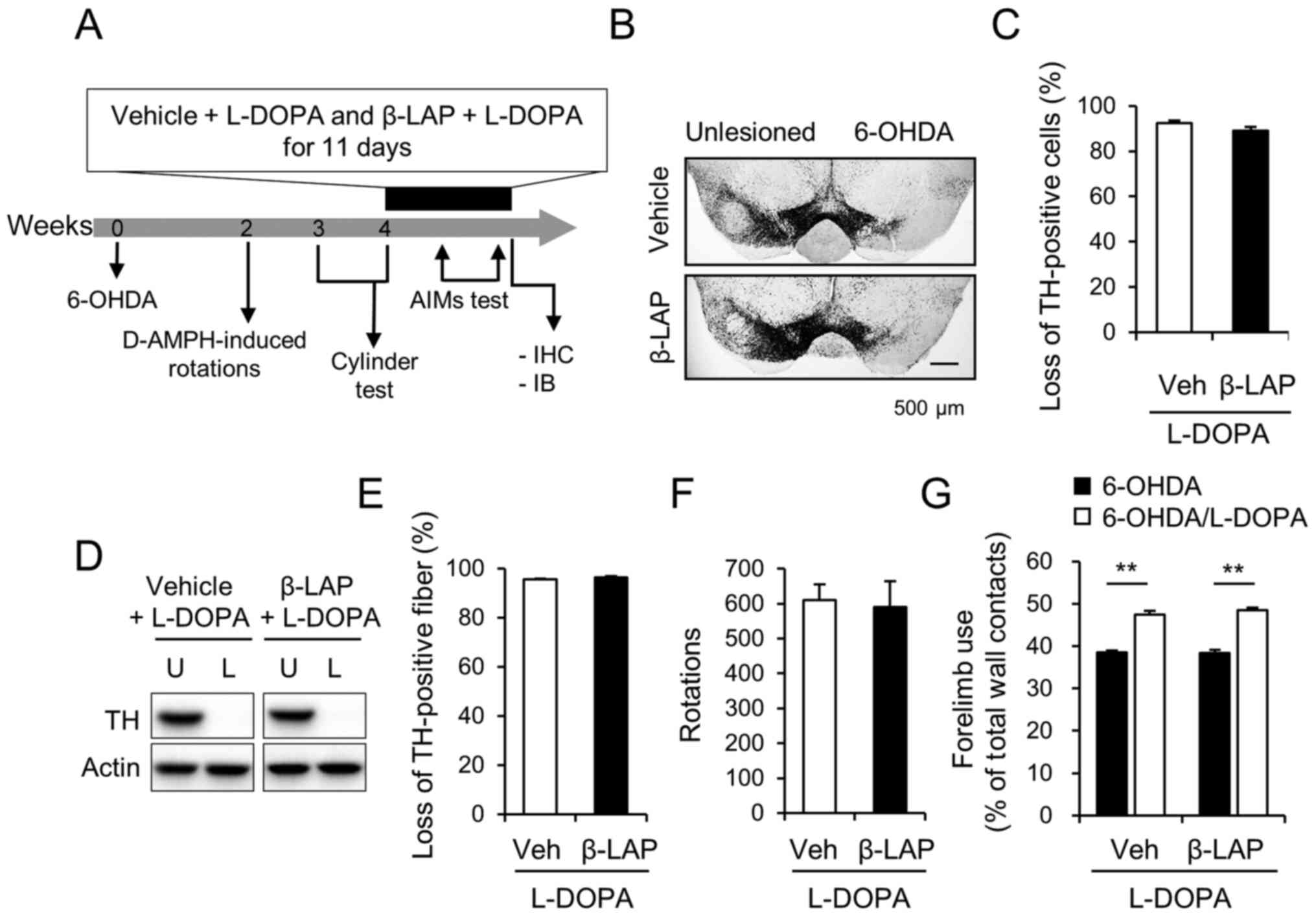 | Figure 1.Unilateral 6-OHDA-lesioned model of
Parkinson's disease was established in C57BL/6J mice. (A) A
schematic of the experimental procedure. (B) Photomicrograph
showing TH-immunoreactive cells in the SNc and (C) % loss of
TH-positive cells in the lesioned side compared with those in the
intact side of the SNc (n=11 animals/group). Scale bar, 500 µm. The
protein extract from the striatum of vehicle + L-DOPA group and 10
mg/kg β-Lapachone + L-DOPA group were subjected to western blot
analyses using TH antibody (n=11 animals/group) and (D)
representative blots are provided. (E) Semi-quantification of the
western blotting data. (F) D-amphetamine-induced rotations for 60
min in the vehicle + L-DOPA (n=11 animals) and 10 mg/kg β-Lapachone
+ L-DOPA (n=11 animals) groups. (G) Right forelimb use in the
cylinder test after 6-OHDA lesioning (6-OHDA) and 30 min after the
first treatment with L-DOPA (6-OHDA/L-DOPA) and vehicle or 10 mg/kg
β-Lapachone. **P<0.01 (Student's t-test). Data are presented as
the mean ± SEM. 6-OHDA, 6-hydroxydopamine; TH, tyrosine
hydroxylase; SNc, substantia nigra pars compacta; Veh, vehicle +
L-DOPA; β-LAP, β-Lapachone + L-DOPA; AIMs, abnormal involuntary
movements; L-DOPA, 3,4-dihydroxyphenyl-l-alanine; U, unlesioned; L,
6-OHDA-lesioned; IHC, immunohistochemistry; IB, immunoblot. |
Drugs
6-Hydroxydopamine (6-OHDA), desipramine
hydrochloride, 3,4-Dihydroxy-L-phenylalanine (L-DOPA), and
benserazide hydrochloride (peripheral dopa decarboxylase inhibitor)
were purchased from Sigma-Aldrich Co. LLC; Merck KGaA. 6-OHDA was
dissolved in 0.2% ascorbic acid in 0.9% NaCl, stored at −20°C and
diluted to 5 µg/µl with 0.2% ascorbic acid before use. Desipramine,
L-DOPA, and benserazide hydrochloride were dissolved in 0.9% NaCl.
β-Lapachone was purchased from Tocris Bioscience and dissolved in
0.9% NaCl. D-amphetamine (D-AMPH) was purchased from United States
Pharmacopeia and then diluted in 0.9% NaCl and stored at −20°C.
Intra-nigral injection of 6-OHDA
The injection of 6-OHDA was performed as described
previously (26). 25 min after the
intraperitoneal administration of desipramine (25 mg/kg), a mixed
anesthetic of ketamine hydrochloride (71.34 mg/kg) and xylazine
hydrochloride (6.14 mg/kg) was administered intraperitoneally
(26,27). After anesthesia, mice were placed in
a stereotactic frame (Stoleting Europe) with a mouse warming pad,
as previously described (26). Mice
were injected with 3 µl of 6-OHDA (5 µg/µl, at the injection speed
of 1 µl/min) into the left SNc at the following coordinates:
Anteroposterior, −3.0 mm; median lateral, −1.3 mm; and
dorsoventral, −4.7 mm. Mice were left on the warmer (37°C) until
they woke up from anesthesia. To avoid dehydration, the mice were
subcutaneously administered sterile glucose-saline solution (50
mg/ml, 0.1 ml/10 g body weight) immediately after surgery and once
a day for 3 days. Food pellets were mixed with 15% sugar/water
solution and placed in a shallow vessel on the floor of the cage
for 7 days.
Cylinder test
After 3 weeks of 6-OHDA injection and the first
injection of L-DOPA, a cylinder test was performed to determine the
sensorimotor abnormalities manifested by unilateral 6-OHDA
injection and the protective effects of L-DOPA on sensorimotor
function. On the first day of L-DOPA, the cylinder test was
performed after 1 h of vehicle or β-Lapachone treatment and 30 min
after injection of L-DOPA. Mice were placed in a transparent
acrylic cylinder (diameter, 15 cm; height, 27 cm). The number of
contacts with both forelimbs touching the wall was counted for 5
min. The use of the impaired (right) forelimb was expressed as a
percentage of the total number of supporting wall contacts.
D-amphetamine-induced rotation
test
A d-amphetamine [5 mg/kg, intraperitoneally
(i.p.)]-induced rotation test was used to measure the unilateral
6-OHDA-lesion-induced asymmetry of mice. The unilateral lesion of
the nigro-striatal dopamine system induced a profound asymmetry in
motor performance. The amphetamine-induced rotation reflects
dopaminergic cell loss in the 6-OHDA-lesioned side of the brain
(26,27). After 2 weeks of 6-OHDA injections,
d-amphetamine-induced rotations of mice were recorded in a cylinder
(diameter, 20 cm; height, 13 cm) for 60 min. The number of
ipsilateral rotations was analyzed using the SMART video tracking
program (Panlab).
Abnormal involuntary movement
test
To determine the effects of β-Lapachone cotreatment
with L-DOPA in dyskinesia, both the vehicle and 10 mg/kg
β-Lapachone groups were cotreated with L-DOPA in 6-OHDA-lesioned
mice 4 weeks after the 6-OHDA lesion was induced. 6-OHDA injected
mice were treated with β-Lapachone and L-DOPA (20 mg/kg, i.p.) and
benserazide (12 mg/kg, i.p., a selective inhibitor of the
peripheral dopa decarboxylase) for 11 days after 4 weeks of 6-OHDA
injection. Mice were individually placed in a separate glass
cylinder, and dyskinetic behaviors were scored for 1 min
(monitoring period) every 20 mins block for a period of 120 min on
days 5 and after L-DOPA injection. The AIM score corresponds to the
sum of the individual scores for each AIM subtype. A composite
score was obtained by the adding the scores for axial, limb, and
orofacial (ALO) AIMs in consideration of the report that composite
AIM scores more closely reflect human dyskinetic behavior compared
with the locomotive (LOC) AIM score. The score was measured from 1
to 4 points for each subtype. 0 point indicates no abnormal
behavior, 1 point means that abnormal behavior appears once or
twice, 2 point means that abnormal behavior appears repeatedly more
than 2 times, 3 point means that abnormal behavior is repeated for
more than 30 sec in 1 min, and 4 point means that abnormal behavior
is repeated for more than 30 sec in 1 min and it does not stop even
if a stimulation, such as sound, is given (8).
Immunohistochemistry
Immunohistochemistry was conducted as previously
described (26). Briefly, 1 h after
vehicle or β-Lapachone administration and 30 min after chronic
L-DOPA injections, mice were euthanized by quick cervical
dislocation and their brains are removed. The mouse brains were
fixed with 4% paraformaldehyde in PBS for more than one day and
then carefully cut into 40 µm coronal sections on a vibratome
(Vibratome VT1000A). Free-floating sections were blocked with 5%
horse serum for 1 h at room temperature. Samples were incubated in
primary antibodies overnight at 4°C. The primary antibodies used
were rabbit polyclonal antibodies for tyrosine hydroxylase (TH;
Pel-Freez), ionized calcium-binding adapter molecule 1 (Iba-1), and
astrocytes (GFAP, Dako). The secondary antibody, a biotinylated
secondary anti-rabbit IgG (1:200, Vector Laboratories), was
administered for 1 h at room temperature, and then samples were
rinsed 3 times in 1X TBST. Immunohistochemistry was subsequently
performed using avidin-biotinylated peroxidase complex (ABC kit,
Vector Laboratories) for 1 h at room temperature and then rinsed 3
times in 1X TBST, followed by incubation in 3,3′-diaminobenzidine
(Sigma-Aldrich Co.; Merck KGaA) for 10 min at room temperature;
samples were then attached to the slide. Since the levels of TH
depletion in the striatum and SNc could affect the behavioral
analysis, only mice that showed TH depletion levels of >80% were
included in the final analysis of this study. In our previous
study, TH depletions >80% in the SNc and striatum showed the
absence of a correlation between TH depletion and AIM scores in
6-OHDA-lesioned mice (8). When
6-OHDA is directly injected into the SNc, a significant loss of
dopaminergic neurons occurs 2–3 days later (28,29).
In this study, dopaminergic cell death was assessed at the end of
all experiments. TH-stained neurons in the left and right SNc (−3.6
to −3.0 mm from the bregma) were counted for two sections per
animal. GFAP-stained astrocytes and Iba-1-stained microglia in the
left and right SNc (−3.6 to −3.0 mm from the bregma) were counted
for one section per animal. To avoid double counting of neurons
with unusual shapes, TH-stained cells were counted only when their
nuclei were visualized in a focal plane using the MetaMorph image
analyzer (Molecular Devices Inc.). Qualitative evaluations of
immunoreactive cells were performed in a blinded manner.
Western blot analysis
Western blot analysis was performed as described
previously (8). 30 min after
completion of the L-DOPA with β-Lapachone treatment schedule, brain
tissue was quickly removed and homogenized in homogenization buffer
(50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS,
and 0.1% sodium deoxycholate) containing a cocktail of protease
inhibitors (Roche Diagnostics GmbH). Equal protein samples were
resolved by SDS-PAGE and then transferred onto a PVDF membrane
(Bio-Rad Laboratories, Inc.) using a semi-dry transfer system
(Trans-Blot SD, Bio-Rad Laboratories, Inc.). The blots were
incubated overnight at 4°C with the following primary antibodies
(unless otherwise stated, all antibodies were used at 1:1,000
dilution); rabbit polyclonal antibodies for TH (1:2,000,
Pel-Freez), extracellular signal-regulated kinases 1/2 (ERK1/2,
1:2,000, Cell Signaling Technology, Inc.), pERK1/2 (Thr202/Tyr204;
Cell Signaling Technology, Inc.), GSK3β (Cell Signaling Technology,
Inc.), pGSK3β (Ser9, Cell Signaling Technology, Inc.), AMPA
receptor subunit GluR1 (Abcam), pGluR1 (Ser845, Millipore), FosB
(Cell Signaling Technology, Inc.), c-Fos (Santa-Cruz Biotechnology,
Inc.), GFAP (Dako; Agilent Technologies, Inc.), and actin
(1:10,000, Millipore), respectively. After incubation with
horseradish peroxidase-conjugated secondary antibodies (Jax
ImmunoResearch), the blots were developed using an enhanced
chemiluminescence kit (ATTO Corporation) and quantified using
Quantity One 1-D analysis software, version 4.6.1 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
GraphPad PRISM (GraphPad Software, Inc.) software
was used to perform the statistical analyses. Two-sample
comparisons were carried out using Student's t-test (unpaired
t-test), while multiple comparisons were made using one-way ANOVA
followed by Tukey-Kramer's post hoc test and two-way ANOVA followed
by bonferroni's post hoc test. All data were presented as the mean
± standard error of the mean (SEM) and statistical differences were
accepted at the 5% level unless otherwise indicated.
Results
Generation of 6-OHDA-induced mouse
model of PD
Based on the ipsilateral rotations and forelimb use,
the mice were separated into the vehicle/L-DPA group, and 10 mg/kg
β-Lapachone/L-DOPA group. The ipsilateral rotation and forelimb use
did not differ between the two groups (Fig. 1F; 609.73±45.00% in vehicle/L-DOPA
and 589.70±73.96% in 10 mg/kg β-Lapachone/L-DOPA, and G;
38.48±0.52% in vehicle/L-DOPA, and 38.41±0.66% in 10 mg/kg
β-Lapachone/L-DOPA). The nigral dopaminergic cell bodies were
depleted by 92.08±1.44% in vehicle/L-DOPA group and 95.60±0.33% in
10 mg/kg β-Lapachone/L-DOPA group (Fig.
1B and C). The striatal dopaminergic dendritic fibers were
depleted by 93.20±1.02% in vehicle/L-DOPA group and 96.24±0.62% in
10 mg/kg β-Lapachone/L-DOPA group (Fig.
1D and E).
β-Lapachone mitigates the development
of LID in the 6-OHDA mouse model
On the first day of β-Lapachone and L-DOPA
treatment, the therapeutic effect of L-DOA on motor deficits was
not affected by β-Lapachone treatment (Fig. 1G; 47.45±0.86% in vehicle/L-DOPA
group and 48.57±0.58% in the 10 mg/kg β-Lapachone/L-DOPA group).
The forelimb use was significantly improved by L-DOPA treatment in
all groups (P<0.01).
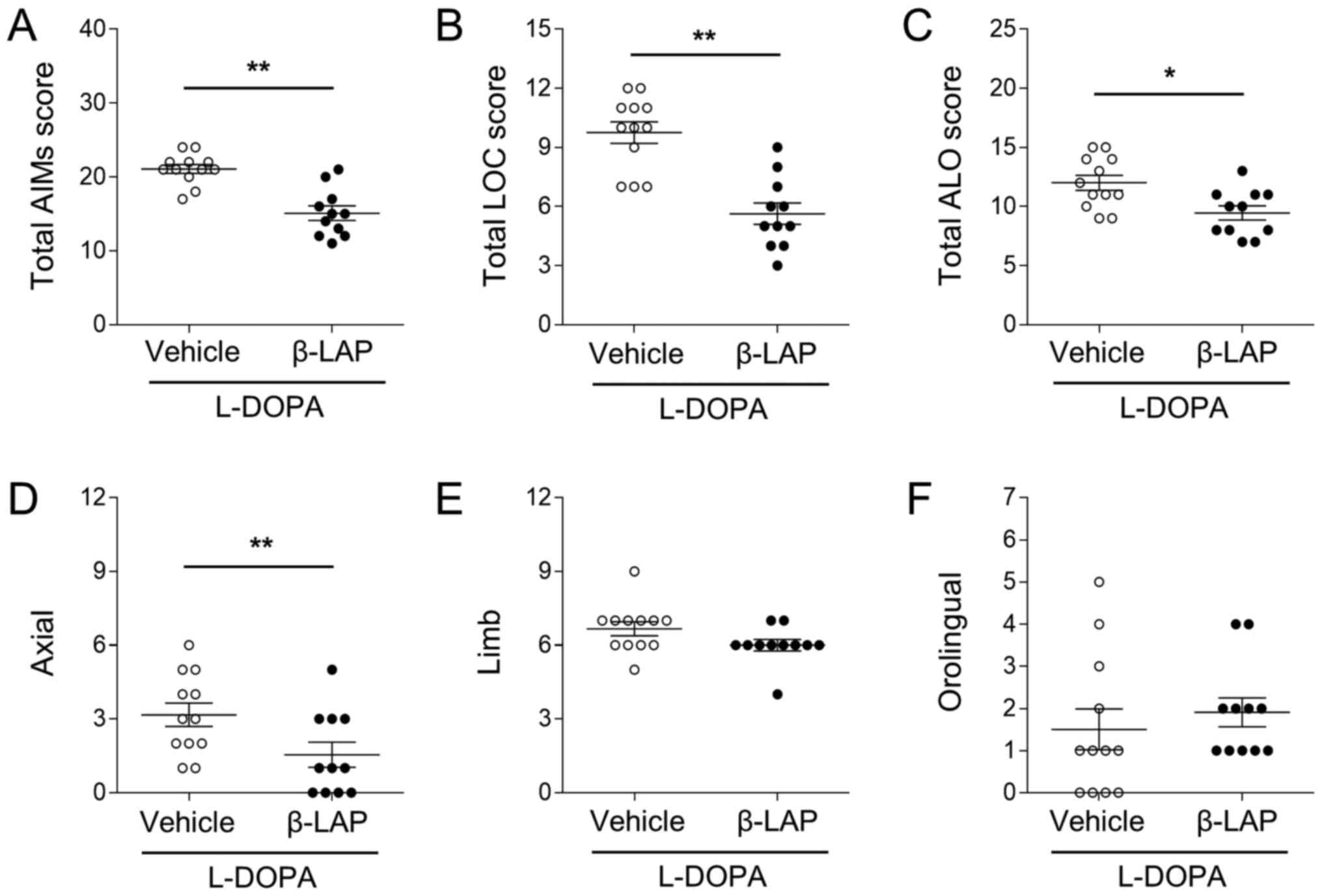
To compare dyskinesia in vehicle-treated and
β-Lapachone-treated mice, the AIMs test was performed on days 5 and
10. On day 5, the total AIM scores were 20.82±0.58% in the
vehicle/L-DOPA group, and 15.09±0.98% in the 10 mg/kg
β-Lapachone/L-DOPA group (Fig. 2A).
β-Lapachone treatment significantly decreased total AIM scores
(t(21)=5.339, P<0.01). In addition, both LOC and ALO
scores were also decreased by β-Lapachone treatment (Fig. 2B; t(21)=5.367, P<0.01,
and Fig. 2C;
t(21)=2.934, P<0.01). In the ALO subtypes, axial AIMs
were significantly decreased by β-Lapachone treatment (Fig. 2D; t(21)=2.329,
P<0.05), but not limb (t(21)=1.793, P>0.05) or
orolingual (t(21)=0.6778, P>0.05) scores (Fig. 2E and F).
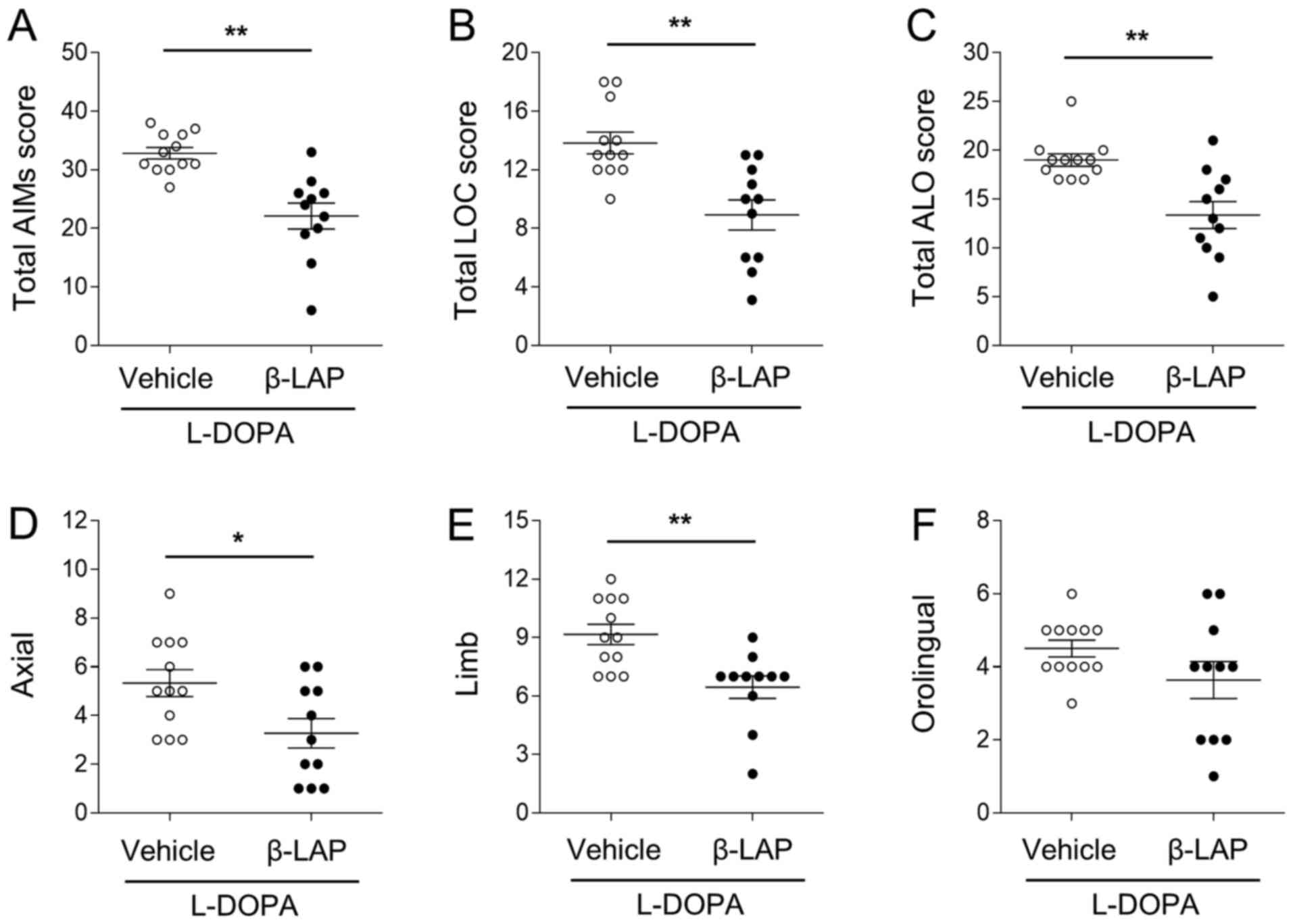
On day 10, the total AIM scores were 32.36±1.26% in
the vehicle group, and 22.09±2.21% in the 10 mg/kg
β-Lapachone/L-DOPA group (Fig. 3A).
β-Lapachone treatment significantly decreased total AIM scores
(Fig. 3B; t(21)=4.589,
P<0.01). In addition, both LOC and ALO scores were also
decreased by β-Lapachone treatment (Fig. 3A; t(21)=3.942, P<0.01,
and Fig. 3C;
t(21)=3.819, P<0.01). In the ALO subtypes, axial and
limb AIMs were significantly decreased by β-Lapachone treatment
(Fig. 3D; axial,
t(21)=2.516, P<0.05, and Fig. 3E; limb, t(21)=3.499,
P<0.01), but not orolingual (t(21)=1.590, P>0.05)
scores (Fig. 3F).
β-Lapachone does not alter
hyperactivation of the D1R signaling pathway in LID
To examine the possible involvement of the DA D1
receptor and ERK1/2 signaling in the beneficial role of β-Lapachone
treatment in LID, we performed western blotting using the
unlesioned and 6-OHDA lesioned striata of vehicle/L-DOPA-treated
and 10 mg/kg β-Lapachone/L-DOPA-treated group. The phosphorylation
of GluR1 at Ser845 and ERK1/2 at Thr202/Tyr204 and the expression
of ∆FosB and c-Fos were increased by chronic treatment with L-DOPA
in the 6-OHDA-lesioned side of the striatum compared to the
unlesioned side of striatum (Fig. 4A
and B). However, the enhanced level of phosphorylation and
expression was not decreased by co-administration of β-Lapachone
and L-DOPA. The pERK1/2 level was increased rather than reduced on
both sides of the striatum in the β-Lapachone-treated group
compared to vehicle-treated group (Fig.
4A and B, P<0.01).
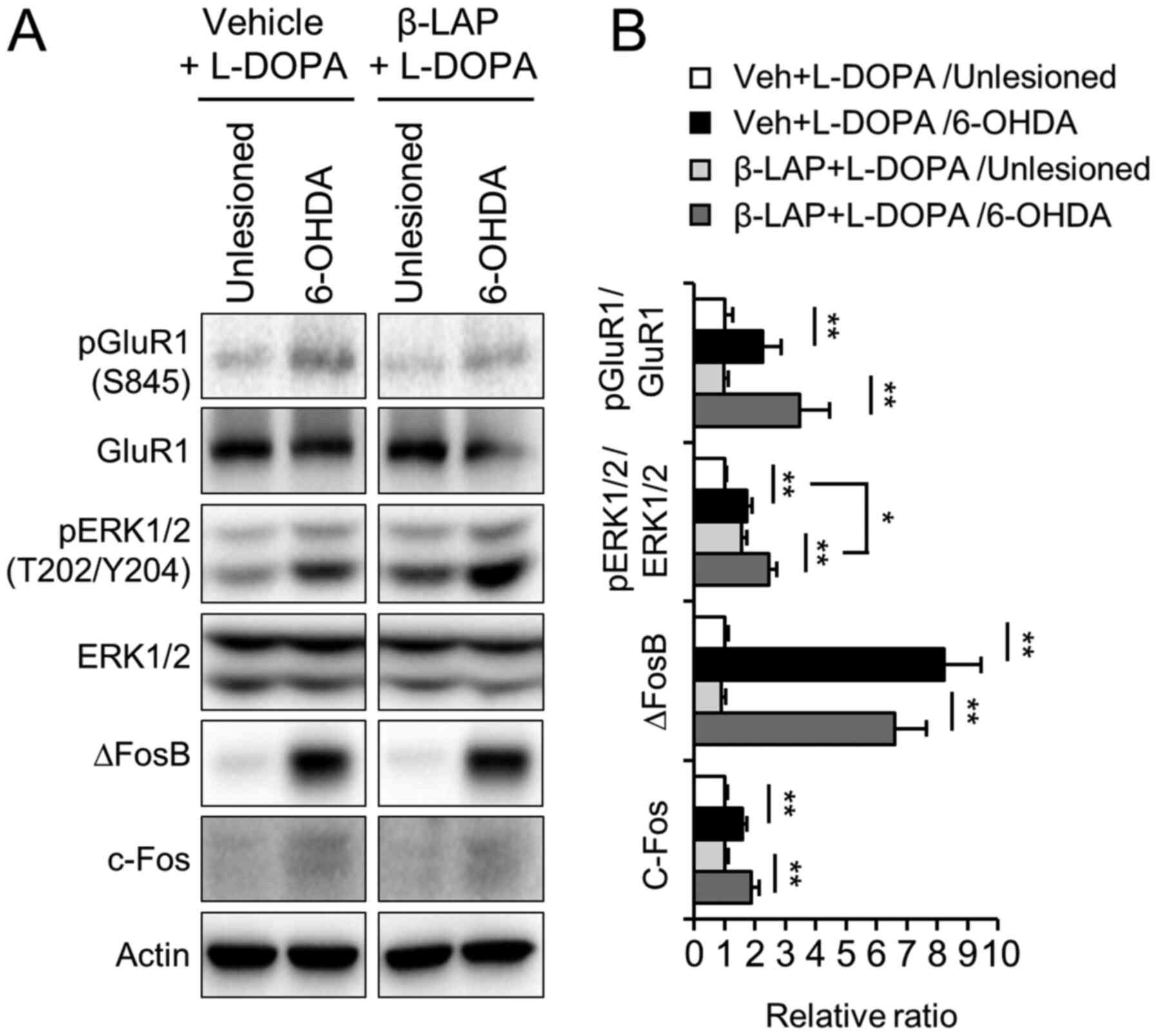 | Figure 4.Effects of β-Lapachone on the D1
receptor and ERK1/2 signaling in the L-DOPA-induced dyskinesia.
Protein extracts from the striatum of the vehicle + L-DOPA and 10
mg/kg β-Lapachone + L-DOPA groups were subjected to western blot
analysis using pGluR1, GluR1, pERK1/2, ERK1/2, ∆FosB and c-Fos
antibodies (n=11 animals) and (A) representative blots are shown.
(B) Semi-quantification of western blotting data. Values were
normalized to actin values, and are presented relative to vehicle +
L-DOPA in the unlesioned striatum. *P<0.05, **P<0.01 (n=11
animals; Student's t-test and one-way ANOVA). Data are presented as
the mean ± SEM. 6-OHDA, 6-hydroxydopamine; β-LAP, β-Lapachone;
L-DOPA, 3,4-dihydroxyphenyl-l-alanine; GluR1, AMPAR subunit
glutamate receptor 1; pGluR1, phospho-GluR1 at Ser845; ERK1/2,
extracellular signal regulated kinase 1/2; pERK1/2, phospho-ERK1/2
at Thr202/Tyr204; ∆FosB, deltaFosB; c-Fos, proto-oncogene
c-fos. |
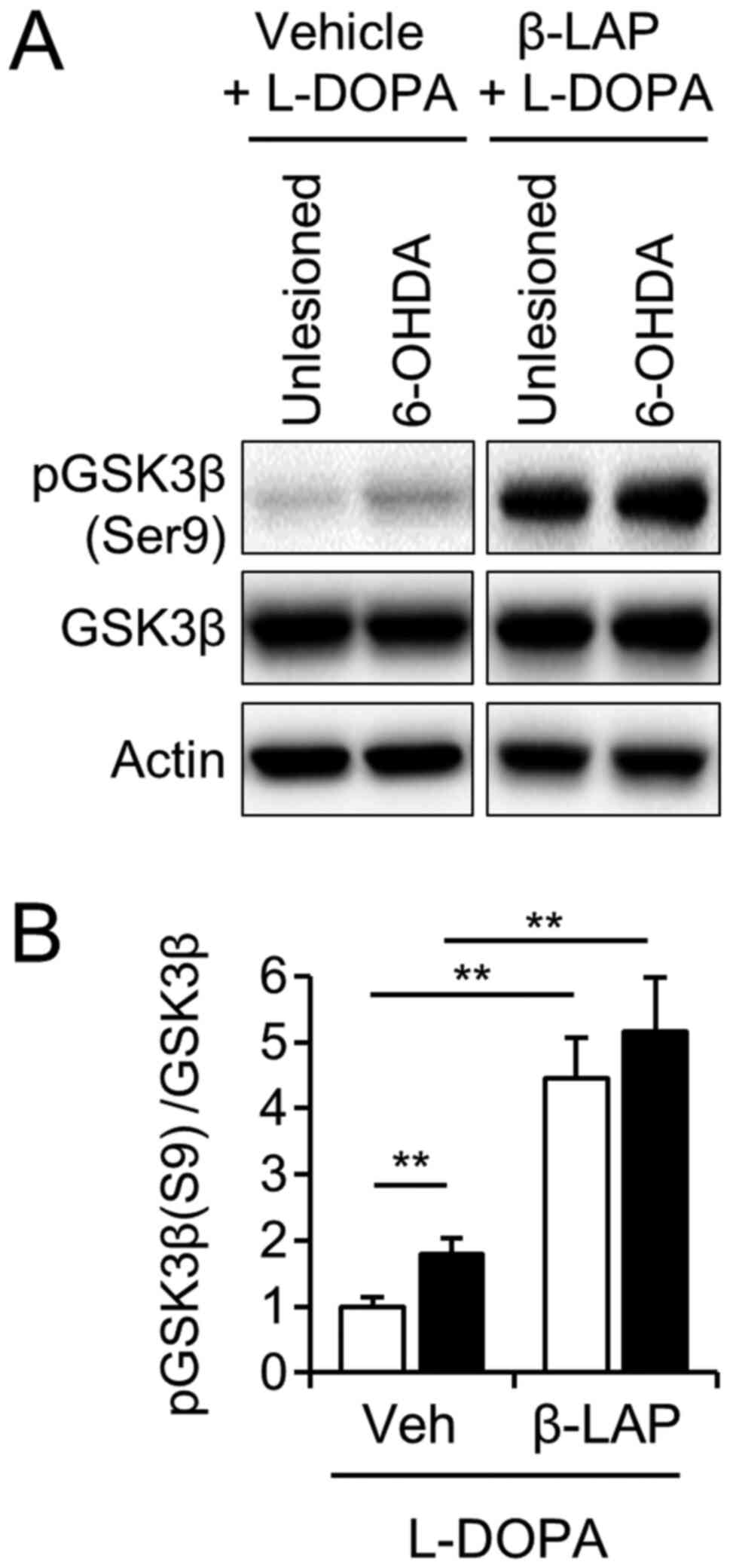
β-Lapachone regulated phosphorylation
of GSK3β in both the intact and 6-OHDA-lesioned striatum
Accumulating evidence suggests that glycogen
synthase kinase-3β (GSK-3β) is involved in the development of LID
(30,31). GSK-3β is inactivated by
phosphorylation at the N-terminal Ser9 (32). However, the regulatory effect of
β-Lapachone on GSK-3β in the brain has not previously been
investigated. We examined whether co-treatment with β-Lapachone and
L-DOPA affected GSK-3β phosphorylation in the LID of the 6-OHDA
mouse model. The phosphorylation of GSK-3β was significantly
increased in the 6-OHDA lesioned side of the striatum compared to
the unlesioned side of the striatum in the vehicle-treated group
(Fig. 5A and B,
t(10)=4.046, P<0.01, paired t-test). Two-way ANOVA
showed significant main effects on the 6-OHDA lesion
(F(1,20)=6.35, P<0.05) and β-Lapachone
(F(1,20)=20.06, P<0.01), but not on the 6-OHDA lesion
or β-Lapachone interaction (F(1,20)=0.03,
P>0.05).
β-lapachone relieves astrocyte
activation in dopamine depleted regions
To determine the effect of β-Lapachone on astrocyte
and microglia activation, we performed immunohistochemistry and
immunoblotting using GFAP and Iba-1 antibodies, markers of
astrocyte and microglia activation respectively.
Immunohistochemistry revealed that, the percentage of the areas
occupied by GFAP immunoreactive astrocytes was significantly
increased by 6-OHDA lesions in both groups in the SNc (Fig. 6A and B). Of note, the percentage of
area occupied by GFAP immunoreactive astrocytes was significantly
decreased by β-Lapachone co-treatment with L-DOPA compared to
vehicle treatment with L-DOPA in the 6-OHDA-lesioned SNc (Fig. 6A and B). In addition, western blot
results showed a significant decrease in the protein expression of
GFAP in the β-Lapachone + L-DOPA group compared to the vehicle +
L-DOPA group in the 6-OHDA-lesioned striatum (Fig. 6C and D, two-way ANOVA showed the
main effect of lesion: F(1,20)=3.71, P>0.05; drug
effect: F(1,20)=12.63, P<0.01, interaction,
F(1,20)=12.63, P<0.01). However, the activation of
microglia was not decreased by β-Lapachone treatment in either the
intact or lesioned side of SNc (Fig. 6E
and F, two-way ANOVA showed a main effect of lesion:
F(1,20)=118.78, P<0.01; drug effect:
F(1,20)=0.20, P>0.05, interaction,
F(1,20)=1.14, P<0.05).
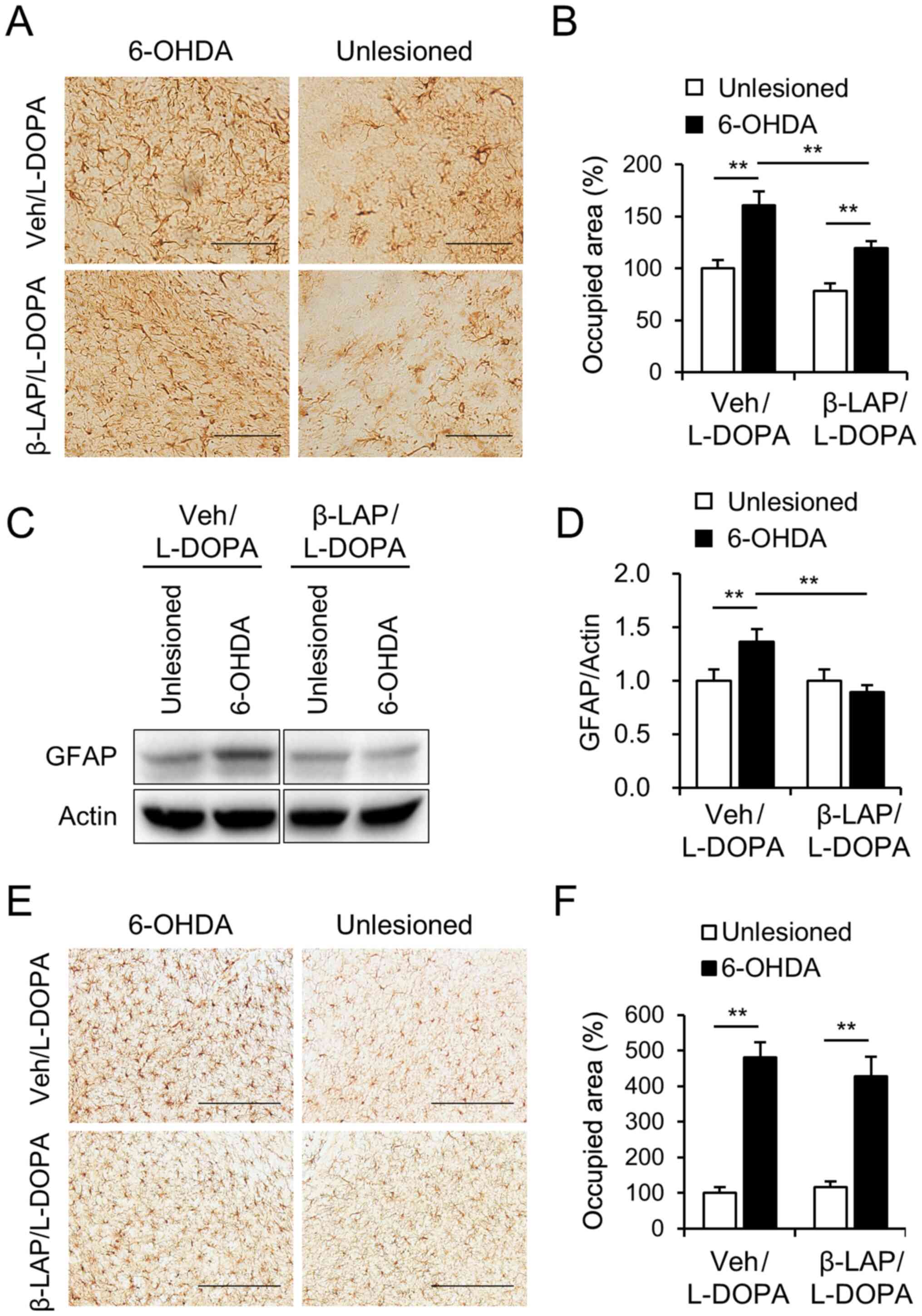 | Figure 6.Effects of β-Lapachone on the
activation of the astrocyte and microglia. (A) Photomicrograph
showing GFAP-immunoreactive cells in the SNc and (B) the percentage
of the areas occupied by GFAP-immunoreactive astrocytes in intact
and 6-OHDA lesioned SNc of the Veh + L-DOPA and β-Lapachone +
L-DOPA groups (n=11 animals). Quantification of GFAP-positive
astrocytes reactivity was measured, and was presented relative to
veh + L-DOPA in unlesioned SNc. Scale bar, 100 µm. The protein
extracts from the striatum of the vehicle + L-DOPA and 10 mg/kg
β-LAP + L-DOPA groups were subjected to western blot analysis using
the GFAP antibody (n=11 animals) and (C) representative blots are
provided. (D) Expression levels of GFAP in dorsal striatum were
analyzed using western blotting. Values were normalized using
actin, and are presented relative to vehicle + L-DOPA in unlesioned
striatum. (E) Photomicrograph showing Iba-1 immunoreactive cells in
the SNc and the (F) percentage of the areas occupied by Iba-1
immunoreactive astrocytes in intact and 6-OHDA lesioned SNc of the
vehicle + L-DOPA group and β-Lapachone + L-DOPA group (n=11
animals). Quantification of Iba-1-positive microglia reactivity was
measured, and was presented relative to veh + L-DOPA in unlesioned
SNc. Scale bar, 200 µm. **P<0.01 (two-way ANOVA followed by a
post hoc test). Data are presented as the mean ± SEM. 6-OHDA,
6-hydroxydopamine; β-LAP, β-Lapachone; L-DOPA,
3,4-dihydroxyphenyl-l-alanine; GFAP, glial fibrillary acidic
protein; SNc, substantia nigra pars compacta; Veh, vehicle; Iba-1,
ionized calcium-binding adapter molecule 1. |
Discussion
In the present study, we found that β-Lapachone
treatment with L-DOPA can suppress the development of dyskinesia
associated with long-term treatment of L-DOPA in a mouse model of
PD. The hyperactivation of dopamine D1 receptor signaling pathway
caused by repeated administration of L-DOPA in a dopamine depletion
situation was not regulated by β-Lapachone co-treatment. Of note,
the inhibitory effect of β-Lapachone on GSK3 activation was
revealed in LID. Moreover, we demonstrated that β-Lapachone
co-treatment with L-DOPA could suppress astrocyte activation in the
chronic treatment of L-DOPA in the 6-OHDA lesioned striatum and
SNc.
Dyskinesia is a serious motor complication that
occurs when L-DOPA is administered to patients with PD over a long
period (5). The striatum is thought
to be important for dyskinesia development. A direct output pathway
expressing the D1 dopamine receptor in the striatum is
overstimulated by L-DOPA treatment, and the modulation of these
signaling pathways can effectively suppress LID in animal models
(7,33,34).
The phosphorylation of GluR1 and ERK1/2 and the expression of ∆FosB
and c-Fos mediating the D1R signaling pathway was not suppressed by
β-Lapachone. Rather, ERK1/2 activation was enhanced by β-Lapachone
cotreatment in both the intact and 6-OHDA-lesioned striata. These
results indicate that the effects of β-Lapachone on LID were not
due to down-regulation of the D1 receptor signaling pathway.
It was reported that the inhibition of GSK-3β
promotes the induction of long-term potentiation in neurons
(35). Moreover, the role of GSK3β
in the phosphorylation of several substrates involved in
synaptogenesis and neurite stabilization has been suggested
(36,37). In PD, an abnormal increase in GSK3β
activity can affect dendrite degeneration (38). The protective effects in GSK-3β
inhibition on LID have been reported (30). Ser9-phosphorylation of GSK3β
enhanced by a single treatment with L-DOPA but decreased in
response to repeated administration of L-DOPA indicating
enhancement of GSK3β activity in prolonged L-DOPA treatment
(26). In our study, β-Lapachone
co-treatment with L-DOPA markedly increased phosphorylation of
GSK3β at Ser9 in the striatum. These data suggest that prolonged
treatment with β-Lapachone and L-DOPA can inactivate GSK3-β in the
striatum of LID in PD.
Anti-inflammatory effects of β-Lapachone on
lipopolysaccharide-activated in vivo and in vitro
models have been reported (1,24).
β-Lapachone showed anti-inflammatory properties by inhibiting NF-kB
activation, blocking IkappaBalpha degradation in microglia
(1). β-Lapachone co-treatment with
L-DOPA did not alter microglial activation in the 6-OHDA-lesioned
SNc, indicating that microglia have no effect on LID. Accumulating
evidence suggests that both PD-related inflammation and LID-related
inflammation states could lead to astrogliosis (10). Astrogliosis, which is caused by
increasing neuroinflammation in the brain, can be modulated by LID
(39,40). The activation of astrocytes was
markedly inhibited by β-Lapachone co-treatment with L-DOPA in the
6-OHDA-lesioned striatum and SNc in LID. Increases in astrocyte
activation and number were restricted to the hemisphere ipsilateral
to the 6-OHDA-lesioned side in mice receiving L-DOPA. These results
indicate that astrocyte activity can be regulated by β-Lapachone
co-treatment with L-DOPA in the striatum and SNc in LID of PD.
This work provides the first evidence for the
therapeutic utility of β-Lapachone in the LID of PD. Altogether,
our results indicate that dyskinesia caused by prolonged L-DOPA
treatment in 6-OHDA-lesioned mice is attenuated by β-Lapachone with
L-DOPA. Therefore, the results collectively suggest that
β-Lapachone may be a candidate for treating dyskinesia in PD. These
behavioral and neurobiological studies in a 6-OHDA-lesioned mouse
model of PD should be further investigated using other in
vivo and in vitro systems and patients with PD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the KRIBB
Research Initiative Program of the Republic of Korea, and by the
National Research Foundation of Korea grant funded by the Korea
government (MSIT) (grant no. 2018R1C1B6005079).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YKR, CHL and KSK designed the research. YKR, HYP,
JG, IBL and YKC performed the experiments. YKR, HYP, JG and KSK
analyzed data. YKR, CHL and KSK interpreted data and wrote the
paper. YKR, CHL, and KSK critically revised the manuscript. YKR and
KSK assessed and confirmed the authenticity of the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moon DO, Choi YH, Kim ND, Park YM and Kim
GY: Anti-inflammatory effects of beta-lapachone in
lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol.
7:506–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Den Eeden SK, Tanner CM, Bernstein AL,
Fross RD, Leimpeter A, Bloch DA and Nelson LM: Incidence of
Parkinson's disease: Variation by age, gender, and race/ethnicity.
Am J Epidemiol. 157:1015–1022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cotzias GC: L-Dopa for Parkinsonism. N
Engl J Med. 278:6301968. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran TN, Vo TNN, Frei K and Truong DD:
Levodopa-induced dyskinesia: Clinical features, incidence, and risk
factors. J Neural Transm (Vienna). 125:1109–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jenner P: Molecular mechanisms of
L-DOPA-induced dyskinesia. Nat Rev Neurosci. 9:665–677. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santini E, Heiman M, Greengard P, Valjent
E and Fisone G: Inhibition of mTOR signaling in Parkinson's disease
prevents L-DOPA-induced dyskinesia. Sci Signal. 2:ra362009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santini E, Alcacer C, Cacciatore S, Heiman
M, Hervé D, Greengard P, Girault JA, Valjent E and Fisone G: L-DOPA
activates ERK signaling and phosphorylates histone H3 in the
striatonigral medium spiny neurons of hemiparkinsonian mice. J
Neurochem. 108:621–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park HY, Kang YM, Kang Y, Park TS, Ryu YK,
Hwang JH, Kim YH, Chung BH, Nam KH, Kim MR, et al: Inhibition of
adenylyl cyclase type 5 prevents L-DOPA-induced dyskinesia in an
animal model of Parkinson's disease. J Neurosci. 34:11744–11753.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen W, Plotkin JL, Francardo V, Ko WK,
Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, et al:
M4 muscarinic receptor signaling ameliorates striatal plasticity
deficits in models of L-DOPA-induced dyskinesia. Neuron.
88:762–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carta AR, Mulas G, Bortolanza M, Duarte T,
Pillai E, Fisone G, Vozari RR and Del-Bel E: l-DOPA-induced
dyskinesia and neuroinflammation: Do microglia and astrocytes play
a role? Eur J Neurosci. 45:73–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGeer PL, Itagaki S, Boyes BE and McGeer
EG: Reactive microglia are positive for HLA-DR in the substantia
nigra of Parkinson's and Alzheimer's disease brains. Neurology.
38:1285–1291. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teismann P, Tieu K, Cohen O, Choi DK, Wu
DC, Marks D, Vila M, Jackson-Lewis V and Przedborski S: Pathogenic
role of glial cells in Parkinson's disease. Mov Disord. 18:121–129.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bortolanza M, Cavalcanti-Kiwiatkoski R,
Padovan-Neto FE, da-Silva CA, Mitkovski M, Raisman-Vozari R and
Del-Bel E: Glial activation is associated with l-DOPA induced
dyskinesia and blocked by a nitric oxide synthase inhibitor in a
rat model of Parkinson's disease. Neurobiol Dis. 73:377–387. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaffner-Sabba K, Schmidt-Ruppin KH,
Wehrli W, Schuerch AR and Wasley JW: beta-Lapachone: Synthesis of
derivatives and activities in tumor models. J Med Chem. 27:990–994.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomez Castellanos JR, Prieto JM and
Heinrich M: Red lapacho (Tabebuia impetiginosa)-a global
ethnopharmacological commodity? J Ethnopharmacol. 121:1–13. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hussain H and Green IR: Lapachol and
lapachone analogs: A journey of two decades of patent research
(1997–2016). Expert Opin Ther Pat. 27:1111–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Wagoner G, Douglas JC and Drew PD:
β-Lapachone ameliorization of experimental autoimmune
encephalomyelitis. J Neuroimmunol. 254:46–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KH, Le TH, Oh HK, Heo B, Moon J, Shin
S and Jeong SH: Protective microencapsulation of β-lapachone using
porous glass membrane technique based on experimental optimisation.
J Microencapsul. 34:545–559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee M, Ban JJ, Chung JY, Im W and Kim M:
Amelioration of Huntington's disease phenotypes by Beta-Lapachone
is associated with increases in Sirt1 expression, CREB
phosphorylation and PGC-1α deacetylation. PLoS One.
13:e01959682018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JS, Leem YH, Park JE, Kim DY and Kim
HS: Neuroprotective effect of β-lapachone in MPTP-induced
parkinson's disease mouse model: Involvement of astroglial
p-AMPK/Nrf2/HO-1 signaling pathways. Biomol Ther (Seoul).
27:178–184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pink JJ, Planchon SM, Tagliarino C, Varnes
ME, Siegel D and Boothman DA: NAD(P)H:Quinone oxidoreductase
activity is the principal determinant of beta-lapachone
cytotoxicity. J Biol Chem. 275:5416–5424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beaver SK, Mesa-Torres N, Pey AL and
Timson DJ: NQO1: A target for the treatment of cancer and
neurological diseases, and a model to understand loss of function
disease mechanisms. Biochim Biophys Acta Proteins Proteom.
1867:663–676. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Go J, Ryu YK, Park HY, Choi DH, Choi YK,
Hwang DY, Lee CH and Kim KS: NQO1 regulates pharmaco-behavioral
effects of d-amphetamine in striatal dopaminergic system in mice.
Neuropharmacology. 170:1080392020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee EJ, Ko HM, Jeong YH, Park EM and Kim
HS: β-Lapachone suppresses neuroinflammation by modulating the
expression of cytokines and matrix metalloproteinases in activated
microglia. J Neuroinflammation. 12:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JS, Lee YY, Kim J, Seo H and Kim HS:
β-Lapachone increases phase II antioxidant enzyme expression via
NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes. Free
Radic Biol Med. 97:168–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryu YK, Park HY, Go J, Choi DH, Kim YH,
Hwang JH, Noh JR, Lee TG, Lee CH and Kim KS: Metformin inhibits the
development of L-DOPA-induced dyskinesia in a murine model of
Parkinson's disease. Mol Neurobiol. 55:5715–5726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu YK, Go J, Park HY, Choi YK, Seo YJ,
Choi JH, Rhee M, Lee TG, Lee CH and Kim KS: Metformin regulates
astrocyte reactivity in Parkinson's disease and normal aging.
Neuropharmacology. 175:1081732020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Faull RL and Laverty R: Changes in
dopamine levels in the corpus striatum following lesions in the
substantia nigra. Exp Neurol. 23:332–340. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeon BS, Jackson-Lewis V and Burke RE:
6-Hydroxydopamine lesion of the rat substantia nigra: Time course
and morphology of cell death. Neurodegeneration. 4:131–137. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie CL, Lin JY, Wang MH, Zhang Y, Zhang
SF, Wang XJ and Liu ZG: Inhibition of glycogen synthase kinase-3β
(GSK-3β) as potent therapeutic strategy to ameliorates
L-dopa-induced dyskinesia in 6-OHDA parkinsonian rats. Sci Rep.
6:235272016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Georgievska B, Sandin J, Doherty J,
Mörtberg A, Neelissen J, Andersson A, Gruber S, Nilsson Y, Schött
P, Arvidsson PI, et al: AZD1080, a novel GSK3 inhibitor, rescues
synaptic plasticity deficits in rodent brain and exhibits
peripheral target engagement in humans. J Neurochem. 125:446–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frame S and Cohen P: GSK3 takes centre
stage more than 20 years after its discovery. Biochem J. 359:1–16.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pavon N, Martin AB, Mendialdua A and
Moratalla R: ERK phosphorylation and FosB expression are associated
with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol
Psychiatry. 59:64–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santini E, Valjent E, Usiello A, Carta M,
Borgkvist A, Girault JA, Hervé D, Greengard P and Fisone G:
Critical involvement of cAMP/DARPP-32 and extracellular
signal-regulated protein kinase signaling in L-DOPA-induced
dyskinesia. J Neurosci. 27:6995–7005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peineau S, Bradley C, Taghibiglou C,
Doherty A, Bortolotto ZA, Wang YT and Collingridge GL: The role of
GSK-3 in synaptic plasticity. Br J Pharmacol. 153 (Suppl
1):S428–S437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucas FR, Goold RG, Gordon-Weeks PR and
Salinas PC: Inhibition of GSK-3beta leading to the loss of
phosphorylated MAP-1B is an early event in axonal remodelling
induced by WNT-7a or lithium. J Cell Sci. 111:1351–1361.
1998.PubMed/NCBI
|
|
37
|
Rui Y, Myers KR, Yu K, Wise A, De Blas AL,
Hartzell HC and Zheng JQ: Activity-dependent regulation of
dendritic growth and maintenance by glycogen synthase kinase 3β.
Nat Commun. 4:26282013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golpich M, Amini E, Hemmati F, Ibrahim NM,
Rahmani B, Mohamed Z, Raymond AA, Dargahi L, Ghasemi R and
Ahmadiani A: Glycogen synthase kinase-3 beta (GSK-3 β) signaling:
Implications for Parkinson's disease. Pharmacol Res. 97:16–26.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mulas G, Espa E, Fenu S, Spiga S, Cossu G,
Pillai E, Carboni E, Simbula G, Jadžić D, Angius F, et al:
Differential induction of dyskinesia and neuroinflammation by
pulsatile versus continuous l-DOPA delivery in the 6-OHDA model of
Parkinson's disease. Exp Neurol. 286:83–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barnum CJ, Eskow KL, Dupre K, Blandino P
Jr, Deak T and Bishop C: Exogenous corticosterone reduces
L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: Role for
interleukin-1beta. Neuroscience. 156:30–41. 2008. View Article : Google Scholar : PubMed/NCBI
|