Introduction
Skin flaps, also known as pedicled skin grafts, are
organs with blood vessels and attached subcutaneous fat tissue, and
can be transferred from one part of the body to another (1). Flap transplantation is widely used to
repair large areas of skin defects caused by trauma or burns and to
reconstruct organs (2,3). However, the choice of source and size
of the flap, as well as the initial state of the area covered by
the graft, such as presence of infection and diabetes, amongst
others, can affect the survival of the flap (3). Among all factors that influence the
survival of flap transplantation, up to 85% of the complications
were caused by blood flow disruption, involving outflows and
inflows (4). In severe cases, this
can cause necrosis of large tissue grafts or complete failure of
surgery (5). Therefore, the
continuous improvement of surgical methods, and the search for
novel alternative therapies, such as the development of new
targeted drugs, or extraction of effective drug ingredients from
traditional Chinese medicine, are required to improve the survival
rate of skin grafts.
Astragalus mongholicus and Astragalus
membranaceus, both known as Huangqi in China, are perennial
herbs used in the treatment of a variety of diseases, such as
dyspepsia, diarrhea, heart diseases, hepatitis and anemia (6). Astragaloside IV (AS-IV) is an active
constituent extracted from the plant A. membranaceus which
exhibits roles in improving diabetic nephropathy, inhibiting
cardiac fibrosis, promoting functional recovery following spinal
cord injury and inhibiting hepatic fibrosis, as well as serving
anticancer functions (7–11). Results from in vivo studies
demonstrated that AS-IV is able to prevent cognitive deficits
induced by transient cerebral ischemia and reperfusion (12,13).
In addition, AS-IV exerts protective effects against endothelial
damage caused by hyperglycemia via inhibiting oxidative stress and
calpain-1 activation (14).
However, to the best of our knowledge, whether AS-IV also improves
angiogenesis under hypoxia has not been reported.
Hypoxia-inducible factors (HIFs) are transcription
factors that respond to changes in available oxygen in the cellular
environment, specifically to a decrease in oxygen, or hypoxia
(15). HIF-1α regulates the
transcription of >40 genes, including erythropoietin (16), glucose transporters (17), glycolytic enzymes (18), vascular endothelial growth factor
(VEGF) (19) and other genes whose
protein products increase oxygen delivery (20) or facilitate metabolic adaptation to
hypoxia (21). In these complex
pathophysiological mechanisms, the stable presence of HIF-1α in the
nucleus and the continued activation of the HIF-1α/VEGF signaling
pathway depend on the balance of ubiquitination and small
ubiquitin-related modifier (SUMO) modification of HIF-1α (22,23).
At normal oxygen levels, HIF-1α protein is degraded by
ubiquitination-mediated proteolysis (24), whereas under hypoxic conditions
HIF-1α preferentially undergoes SUMO modification and the
ubiquitination degradation pathway is inhibited (25). Through this molecular mechanism,
HIF-1α is continuously and stably expressed in the nucleus and
promotes neovascularization via the VEGF pathway. The aim of the
present study was to investigate whether AS-IV affects the balance
of ubiquitination and SUMOylation of HIF-1α and the regulation of
its downstream VEGF pathway, promotes neovascularization and
improves the survival rate of skin grafts, and therefore to provide
novel ideas for the clinical treatment of large-scale skin
defects.
Materials and methods
Cell lines and culture
Human umbilical vein endothelial cells (HUVECs) were
obtained from American Type Culture Collection (ATCC). The cells
were cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. For hypoxia treatment, cells were cultured in an
atmosphere of 3% O2 for 7 days. Cells cultured in
normoxic conditions (21% O2) were used as controls, and
referred to as the normoxic group.
Lentiviral plasmid and gene
transfection
Lentiviral (pWPXLD-GFP-SUMO1) or control plasmids
(pWPXLD-GFP) were synthesized by Biogot Technology, Co. Ltd. and
were transfected into 293T cells (ATCC) for production of
lentiviral particles. All constructs were verified via nucleic acid
sequencing. HUVECs were cultured to 60–70% confluence in normoxic
and aforementioned cell culture conditions, and 5 µl viral
suspension (1×108 titer) was placed on the cell
monolayers. Flasks were then incubated at 37°C and 5%
CO2 for 6 h, after which the viral suspension was
removed and replaced with fresh medium. Gene transduction
efficiency was verified via western blotting.
Drug preparation and treatment
AS-IV (≥98%) (CAS no. 84687-43-4; cat. no. CS-4272;
≥99.15% purity) was purchased from Chengdu Biopurify Phytochemicals
Ltd. AS-IV was dissolved in DMSO (<0.1%), then diluted with DMEM
to a final concentration of 5 ng/ml. Subsequently, stable SUMO1
gene-transfected or control cells cultured under hypoxic or normal
conditions were treated with AS-IV for 7 days. Untreated cells were
considered the control group.
Western blotting
Total protein was extracted from cells using RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.) with 1
mM PMSF and 20 mM N-ethylmaleimide, and protein concentration was
determined using the BCA method (Thermo Fisher Scientific, Inc.).
Total protein (100 µg/lane) was separated by 4–12% SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore). Subsequently,
membranes were blocked in 5% BSA (Sigma-Aldrich; Merck KGaA) at
room temperature for 1 h and incubated with antibodies for specific
target proteins overnight at 4°C. Antibodies used are listed in
Table I. The membranes were then
incubated for 1 h at room temperature with anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (1:2,000; cat.
no. Sc-516087; Santa Cruz Biotechnology, Inc.). β actin served as
an internal control and was probed on the same membrane of the
proteins of interest. A Super Signal protein detection kit (cat.
no. 34095; Pierce; Thermo Fisher Scientific, Inc.) was used to
detect protein signals according to the manufacturer's protocols.
Data were evaluated using image analysis software (ImageJ; version
1.48; National Institutes of Health). All experiments were repeated
three times.
 | Table I.Antibodies used for western
blotting. |
Table I.
Antibodies used for western
blotting.
| Antibody | Dilution | Cat. no. | Supplier |
|---|
| Small
ubiquitin-related modifier 1 | 1:1,000 | ab11672 | Abcam |
| Ubiquitin | 1:1,000 | 3936 | Cell Signaling
Technology, Inc. |
| Hypoxia inducible
factor-1α | 1:500 | ab216842 | Abcam |
| Vascular
endothelial growth factor | 1:500 | ab2350 | Abcam |
| β-actin | 1:1,000 | ab8227 | Abcam |
Angiogenesis assays
A 2-mm-thick layer of semi-solid Matrigel (1:3; BD
Biosciences) was pre-coated on the bottom of 96-well plates at 37°C
overnight. Then, 0.25×106 HUVECs transfected with SUMO1
or control plasmid were added to the surface of the gel in each
well and inoculated with 0.1 ml DMEM supplemented with FBS as
aforementioned in the presence or absence of AS-IV. The formation
of blood vessels was observed under an inverted microscope
(magnification, ×200; Olympus cellSens Entry 1.16; Olympus
Corporation) after 24 h.
Lactate dehydrogenase (LDH) activity
detection
HUVECs (5×103) transfected with SUMO1 or
control plasmid were seeded into 96-well plates and incubated in
the presence or absence of AS-IV for 7 days. The cells were then
collected and sonicated at 5 MHz for 30 sec at 4°C, followed by
centrifugation at 12,000 × g for 10 min at 4°C. The LDH content of
the conditioned medium was measured via an ELISA-based LDH activity
assay kit (cat. no. YM-ME0351; Shanghai Yuan Mu Biotechnology Co.,
Ltd.) in accordance with the manufacturer's instructions.
Statistical analysis
Data were analyzed using GraphPad Prism software
(version 6; GraphPad Software, Inc.) and are presented as the mean
± SD of ≥3 independent experimental repeats. The statistical
significance of differences in results were determined via one-way
ANOVA or unpaired Student's t-test. Differences among multiple
groups were analyzed by one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
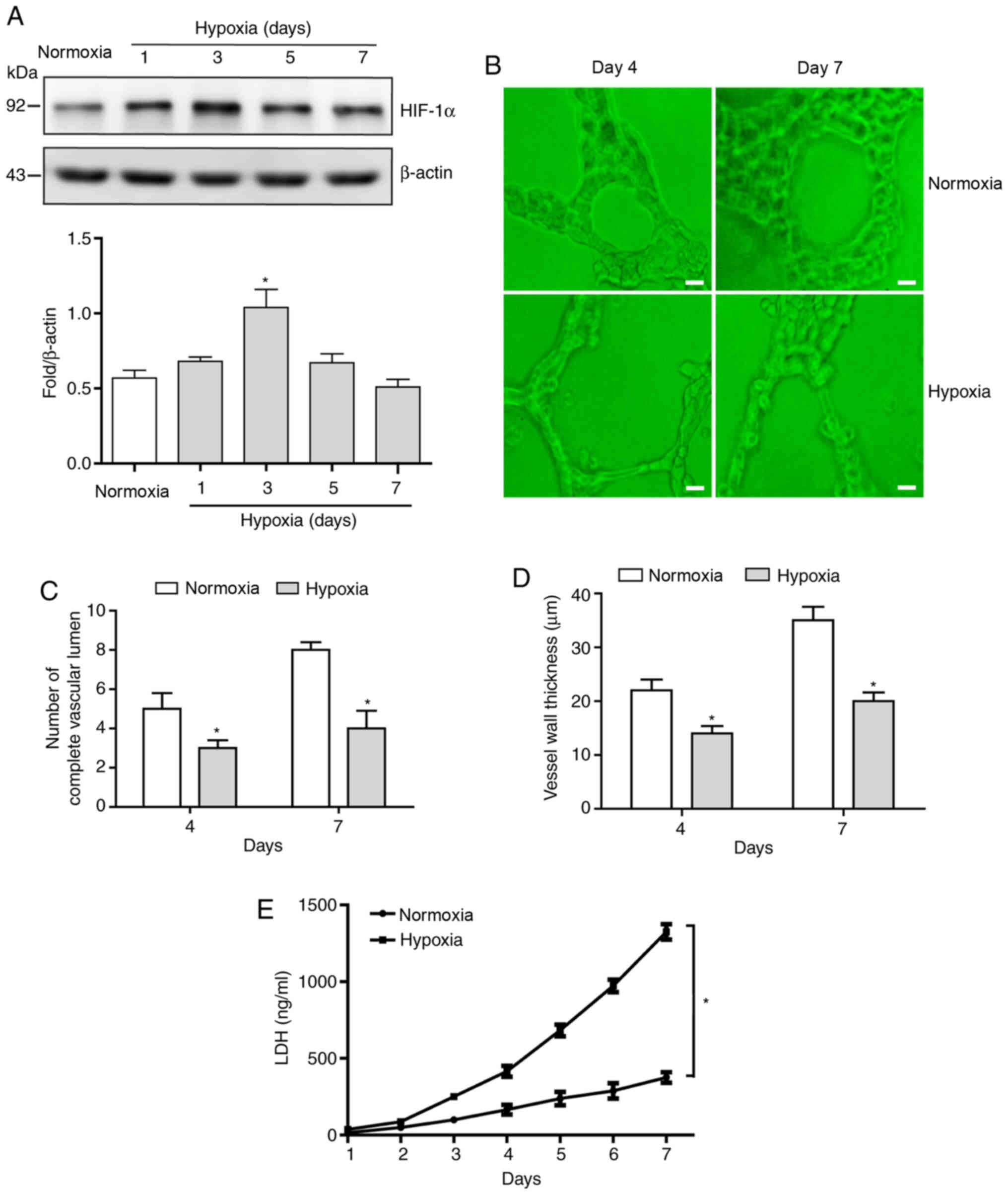
Sustained hypoxia leads to waveform
expression levels of HIF-1α protein and increases the proportion of
abnormal blood vessels
In order to investigate the effect of sustained
hypoxia on the expression levels of HIF-1α protein, the levels of
HIF-1α protein in HUVECs cultured under hypoxic conditions for 7
days was evaluated. The expression levels of HIF-1α protein
exhibited a notable pattern of an initial increase and then a
decrease, with peak expression levels appearing on day 4 of hypoxia
treatment (Fig. 1A). Observation of
simulated blood vessel morphology on days 4 and 7 using
angiogenesis assays demonstrated that, compared with the normoxic
group, significantly fewer intact blood vessels formed in the
hypoxic group (Fig. 1B and C) and
their walls were significantly thinner (Fig. 1B and D) at both time points.
Since LDH content in the medium directly reflects
the degree of cell damage, LDH levels in the conditioned medium
were assessed following culturing of HUVECs under hypoxic or
normoxic conditions. The results demonstrated that LDH levels in
the normoxic group remained low and did not change significantly
over time (Fig. 1E); however,
gradually increasing levels of LDH were detected in the conditioned
medium of the hypoxia group (Fig.
1E), which indicated that the degree of cell damage increases
with the prolongation of hypoxia time.
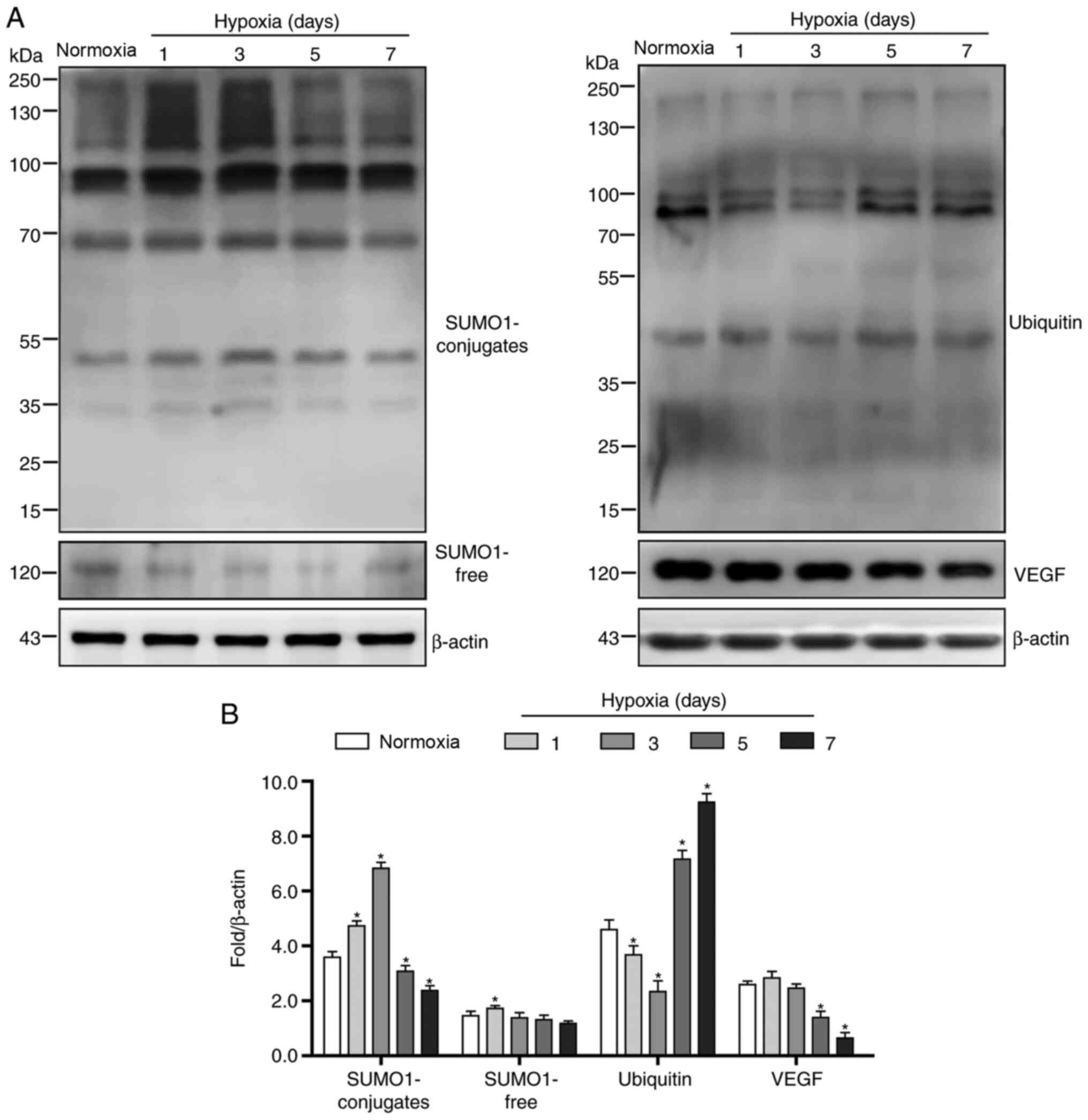
Rapid depletion and slow compensation
of SUMO1 protein are key factors for HIF-1α protein
degradation
In order to investigate whether persistent hypoxia
affects the HIF-1α/VEGF pathway via the regulation of HIF-1α
SUMOylation and thereby alters angiogenesis, the expression levels
of SUMO1 and VEGF were assessed. The results demonstrated that
HUVECs expressed basal levels of SUMO1 (Fig. 2A and B). At the initial stage of
exposure to hypoxic conditions, the levels of covalently modified
SUMO1 significantly increased, accompanied by a rapid decline of
free SUMO1 (Fig. 2A and B). With
the prolongation of hypoxia time, levels of both covalently
modified SUMO1 and free SUMO1 decreased significantly until day 7
(Fig. 2A and B). Correspondingly,
VEGF protein levels began to decline between days 3 and 7 (Fig. 2A and B). However, ubiquitin
conjugates showed the opposite trend to SUMO1; ubiquitin levels
initially decreased and then increased rapidly between days 3 and 7
(Fig. 2A and B).
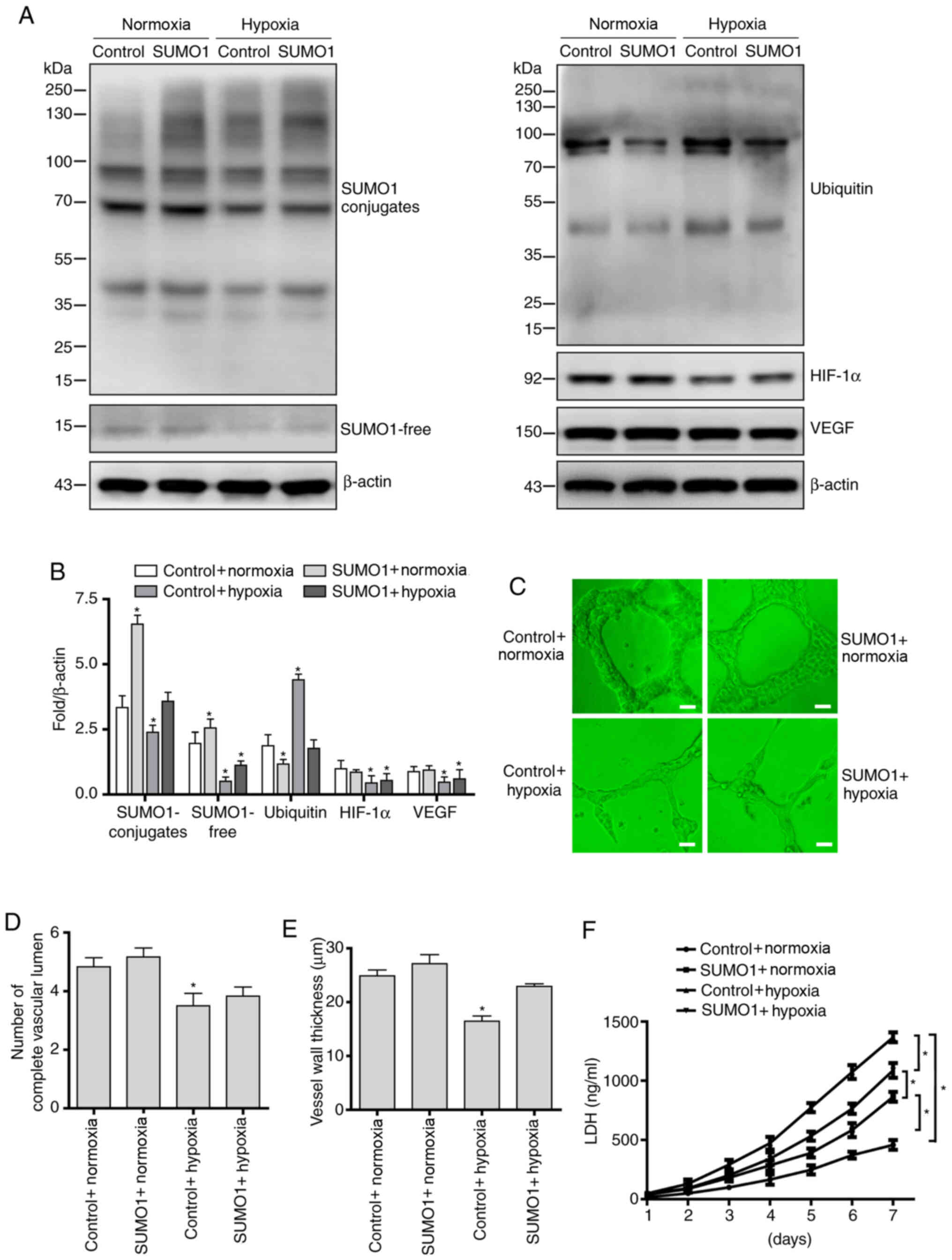
Overexpression of SUMO1 stabilizes
HIF-1α protein and partially restores vascular morphology
In order to further investigate the effect of
SUMOylation on HIF-1α protein expression levels and angiogenesis in
continuous hypoxic conditions, lentiviral-mediated gene
transfection was used to achieve SUMO1 overexpression in HUVECs.
Western blotting results confirmed that SUMO1 was overexpressed in
HUVECs following gene transfection (Fig. 3A and B). Further results showed that
overexpression of SUMO1 increased the covalent binding of SUMO1 to
HIF-1α protein, antagonized the degradation of HIF-1α protein
induced by ubiquitination modification, and further regulated
downstream function via the HIF-1α/VEGF pathway under hypoxic
conditions (Fig. 3A and B).
Next, the effects of SUMO1 overexpression on
angiogenesis and cell damage under hypoxic conditions were
evaluated. The results demonstrated that SUMO1 overexpression
significantly improved the angiogenic potential of HUVECs under
hypoxic conditions, with cells overexpressing SUMO1 showing a more
complete vessel lumen structure (Fig.
3C and D) and thicker vessel walls (Fig. 3C and E) than the empty
control-transfected cells. Finally, cytotoxicity was assessed via
testing LDH levels. The results showed that overexpression of SUMO1
decreased LDH levels in conditioned medium in both normoxic and
hypoxic cells compared with empty control-transfected cells
(Fig. 3F).
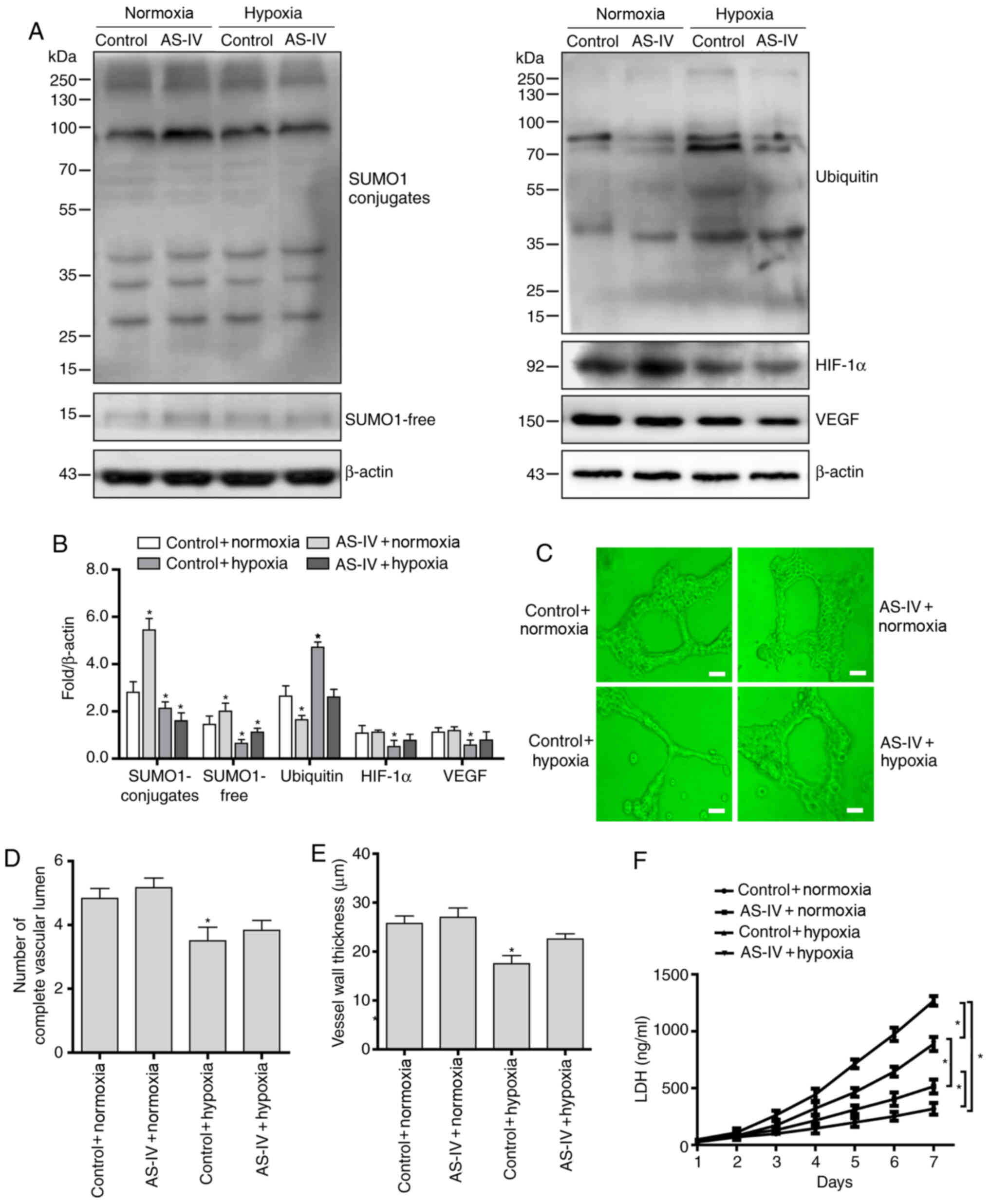
AS-IV activates HVUECs to continuously
express SUMO1 and improves angiogenesis in a hypoxic
environment
In order to determine whether AS-IV exerts its
antagonistic effect on hypoxia-induced endothelial cell injury via
affecting the SUMO1 modification of HIF-1α and the HIF-1α/VEGF
pathway, HUVECs were treated with AS-IV under hypoxic conditions.
The present results demonstrated that AS-IV continuously induced
SUMO1 expression levels, increased SUMOylation of HIF-1α protein,
decreased its ubiquitination modification and stabilized HIF-1α
protein levels, leading to activation of the HIF-1α/VEGF signaling
pathway (Fig. 4A and B).
Next, the effect of AS-IV on the angiogenesis
potential and cytotoxicity of HUVECs under hypoxic conditions was
evaluated. The results demonstrated that AS-IV significantly
improved the angiogenic potential of HUVECs under hypoxic
conditions, with AS-IV-treated cells exhibiting a relatively intact
vascular lumen (Fig. 4C and D) and
thicker vessel walls (Fig. 4C and
E) than those of the untreated cells. Finally, the results of
the cytotoxicity assay showed that AS-IV decreased the LDH content
of HUVECs under hypoxic conditions (Fig. 4F).
Discussion
In orthopedics and plastic surgery, flap
transplantation is a common method for repairing large-scale skin
wounds (26,27). Factors affecting the quality of flap
survival include arterial blood supply (28), venous return (29), tissue damage (30) and ischemia-reperfusion injury
(31). Among these, ischemic injury
is the key factor leading to flap necrosis following surgery
(32). Certain drugs targeting
ischemic injury have been proven effective, such as L-arginine
(33) and estrogen (34) to relax micro-vessels, recombinant
connective tissue growth factor (35), erythropoietin (36) and VEGF (37) to promote vascular regeneration, and
others to control inflammation and scavenge oxygen free radicals.
However, the safety and effectiveness of these treatments in humans
need to be further verified by clinical studies.
Traditional Chinese medicine and herbal medicine
have proven effective through thousands of years of practice,
although active ingredients and underlying mechanisms of action
have not been fully elucidated. Among these, AS-IV, the primary
active component of A. membranaceus, has been reported to
exhibit numerous effects including improvement in the function of
endothelial cells (38) and
neovascularization (39),
anti-inflammatory (40) and
antioxidant effects (41),
regulation of energy metabolism (42), protection of the nervous system
(43) and anticancer effects
(44). However, the specific
mechanism underlying the promotion of vascular regeneration by
AS-IV, particularly under hypoxic conditions, remains unclear.
HIF-1 is a key transcription factor for the cellular
adaptive response to hypoxia (45).
Structurally, HIFs are heterodimers composed of two different
subunits, HIF-α and HIF-β. The HIF-1α subunits accumulate in the
cytoplasm and translocate to the nucleus to form heterodimers with
a β subunit. Then, the heterodimers associate with co-activators
and bind to hypoxia response elements in gene promoters to initiate
gene transcription (46). Hypoxia
induces epithelial-to-mesenchymal transition and metastasis,
mediated by various signaling pathways such as TGF-β, PI3K/AKT, Wnt
and Jagged/Notch. Concomitantly, the hypoxic environment stimulates
vessel growth via the HIF-1/VEGF axis and other secondary factors,
including angiopoietin 2, fibroblast growth factor and hepatocyte
growth factor (47). The
transcriptional activity, protein stabilization, protein-protein
interactions and cellular localization of HIF-1α, the
oxygen-sensitive subunit of HIF-1, are modulated by various
post-translational modifications, including phosphorylation,
acetylation, methylation, and alkylation, as well as the covalent
linkage of fatty acids, saccharides or small proteins such as
ubiquitin and SUMO (48,49). A recent study demonstrated that
SUMOylation, the covalent attachment of SUMOs to proteins, is
involved in activation of the hypoxic response and the ensuing
signaling cascade (50). The stable
presence of HIF-1α in the nucleus under hypoxic conditions depends
on the balance between its SUMOylation and ubiquitination
modification (51). The present
study investigated how sustained hypoxic treatment (7 days) affects
the protein presence of HIF-1α and angiogenesis. The results showed
that the expression levels of SUMO1 protein gradually decreased
with the extension of hypoxia time, and its compensation cycle was
slow, and the SUMOylation state of HIF-1α protein was not
maintained. The relative deficiency of HIF-1α was insufficient to
activate its downstream VEGF pathway, leading to abnormal vascular
morphology.
In order to determine the effect of SUMO1 on
maintaining the stability of HIF-1α protein, gene transfection was
used to achieve overexpression of SUMO1 in HUVECs. As expected,
overexpression of SUMO1 inhibited the degradation of HIF-1α by
ubiquitinase, maintained HIF-1α at a high level, stimulated the
HIF-1α/VEGF signaling pathway and improved angiogenesis in hypoxic
environments. These results indicated that inducing HUVECs to
continuously express SUMO1 protein via the administration of drugs
may improve angiogenesis under hypoxia, and that this strategy
could be used to treat conditions that result in vascular
abnormalities due to hypoxia.
A number of traditional Chinese medicine monomer
components were screened in preliminary experiments, which
demonstrated that AS-IV induced HUVECs to continuously produce
SUMO1 (data not shown). Therefore, it was hypothesized that AS-IV
may improve angiogenesis via promoting the stability of HIF-1α and
its downstream VEGF signaling pathway. The present results
validated this hypothesis. Under sustained exposure to AS-IV,
HUVECs overexpressed SUMO1, continuously activated the HIF-1α/VEGF
pathway and promoted angiogenesis. Nie et al (52) found that administration of AS-IV
significantly improved endothelial dysfunction associated with
diabetes in diabetic rats by decreasing oxidative stress and levels
of calpain-1. Leng et al (53) demonstrated that AS-IV improved
vascular endothelial dysfunction induced by hyperglycemia, and that
the protective effect of AS-IV may be achieved via the toll-like
receptor4/NF-κB signaling pathway. Zhang et al (54) confirmed that AS-IV exhibited vessel
dilatation properties via the endothelium-dependent NO-cGMP pathway
in normal and hypertensive rats; this blocks extracellular calcium
influx and results in vessel relaxation, partly via phenylephrine
and angiotensin II inhibition, when perivascular fat is present
(54). These results provide
understanding of the potential molecular mechanisms underlying the
protective effect of AS-IV on vascular endothelial cells.
In conclusion, the present study identified a novel
mechanism by which AS-IV drugs improve angiogenesis under hypoxic
conditions, which may be useful to develop future treatments of
numerous diseases associated with flap transplantation. More
detailed assessments of the molecular mechanisms, as well as
further animal experiments, should be performed in future
investigations.
Acknowledgements
The authors would like to thank Dr Melony Black
(Liwen Bianji, Edanz Editing China), for his language editing of
this manuscript.
Funding
The present study was supported by grants from the
Tianjin Natural Science Foundation of China (grant nos.
18JCQNJC12800 and 19JCZDJC35200), Tianjin Special Project of New
Generation Artificial Intelligence Technology (grant no.
18ZXZNSY00260) and Binhai Health and Family Planning Commission
Science and Technology Projects (grant no. 2014BWKZ008 and
2019BWKQ030).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH designed the experiments. BW, CZ, DC, XM, TY and
XL performed the experiments and collected and analysed the data.
BW and XL drafted the manuscript. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lucas JB: The Physiology and Biomechanics
of Skin Flaps. Facial Plast Surg Clin North Am. 25:303–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Starkman SJ, Williams CT and Sherris DA:
Flap Basics I: Rotation and Transposition Flaps. Facial Plast Surg
Clin North Am. 25:313–321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang YJ, Chen G, Guan H and Hu DH:
Advances in the research of poststernotomy dehiscence and repair
with tissue flap transplantation. Zhonghua Shao Shang Za Zhi.
35:879–883. 2019.(In Chinese). PubMed/NCBI
|
|
4
|
Sigaux N, Philouze P, Boucher F,
Jacquemart M, Frobert P and Breton P: Efficacy of the postoperative
management after microsurgical free tissue transfer. J Stomatol
Oral Maxillofac Surg. 118:173–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polito F, Bitto A, Galeano M, Irrera N,
Marini H, Calò M, Squadrito F and Altavilla D:
Polydeoxyribonucleotide restores blood flow in an experimental
model of ischemic skin flaps. J Vasc Surg. 55:479–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Liu L, Gao C, Chen W, Vong CT, Yao
P, Yang Y, Li X, Tang X, Wang S, et al: Astragali Radix (Huangqi):
A promising edible immunomodulatory herbal medicine. J
Ethnopharmacol. 258:1128952020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du N, Xu Z, Gao M, Liu P, Sun B and Cao X:
Combination of Ginsenoside Rg1 and Astragaloside IV reduces
oxidative stress and inhibits TGF-β1/Smads signaling cascade on
renal fibrosis in rats with diabetic nephropathy. Drug Des Devel
Ther. 12:3517–3524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Y, Wu Y, Feng K, Zhao Y, Tao R, Xu H
and Tang Y: Astragaloside IV inhibits cardiac fibrosis via
miR-135a-TRPM7-TGF-β/Smads pathway. J Ethnopharmacol.
249:1124042020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Pan X, Huang C, Gu M, Chen X, Zheng
X, Shao Z, Hu S, Wang B, Lin H, et al: Dual regulation of microglia
and neurons by Astragaloside IV-mediated mTORC1 suppression
promotes functional recovery after acute spinal cord injury. J Cell
Mol Med. 24:671–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Wei W, Sun WY and Li X: Protective
effects of astragaloside IV on porcine-serum-induced hepatic
fibrosis in rats and in vitro effects on hepatic stellate cells. J
Ethnopharmacol. 122:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He CS, Liu YC, Xu ZP, Dai PC, Chen XW and
Jin DH: Astragaloside IV Enhances Cisplatin Chemosensitivity in
Non-Small Cell Lung Cancer Cells Through Inhibition of B7-H3. Cell
Physiol Biochem. 40:1221–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Gan H, Jin H, Fang Y, Yang Y, Zhang
J, Hu X and Chu L: Astragaloside IV promotes microglia/macrophages
M2 polarization and enhances neurogenesis and angiogenesis through
PPARγ pathway after cerebral ischemia/reperfusion injury in rats.
Int Immunopharmacol. 92:1073352021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Li H, Fang F, Deng X and Ma S:
Astragaloside IV attenuates cognitive impairments induced by
transient cerebral ischemia and reperfusion in mice via
anti-inflammatory mechanisms. Neurosci Lett. 639:114–119. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie Q, Zhu L, Zhang L, Leng B and Wang H:
Astragaloside IV protects against hyperglycemia-induced vascular
endothelial dysfunction by inhibiting oxidative stress and
Calpain-1 activation. Life Sci. 232:1166622019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodríguez-Jiménez FJ and Moreno-Manzano V:
Modulation of hypoxia-inducible factors (HIF) from an integrative
pharmacological perspective. Cell Mol Life Sci. 69:519–534. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Souvenir R, Flores JJ, Ostrowski RP,
Manaenko A, Duris K and Tang J: Erythropoietin inhibits HIF-1α
expression via upregulation of PHD-2 transcription and translation
in an in vitro model of hypoxia-ischemia. Transl Stroke Res.
5:118–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Ma WQ, Han XQ, Wang Y, Wang X and
Liu NF: Advanced glycation end products accelerate calcification in
VSMCs through HIF-1α/PDK4 activation and suppress glucose
metabolism. Sci Rep. 8:137302018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Cui B, Fan Y, Li X, Li Q, Du Y,
Feng Y and Zhang P: Protein kinase D1 regulates hypoxic metabolism
through HIF-1α and glycolytic enzymes incancer cells. Oncol Rep.
40:1073–1082. 2018.PubMed/NCBI
|
|
19
|
Li Y, Liu Y, Wang C, Xia WR, Zheng JY,
Yang J, Liu B, Liu JQ and Liu LF: Succinate induces synovial
angiogenesis in rheumatoid arthritis through metabolic remodeling
and HIF-1α/VEGF axis. Free Radic Biol Med. 126:1–14. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malgoyre A, Chabert C, Tonini J, Koulmann
N, Bigard X and Sanchez H: Alterations to mitochondrial fatty-acid
use in skeletal muscle after chronic exposure to hypoxia depend on
metabolic phenotype. J Appl Physiol (1985). 122:666–674. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thiersch M, Rimann M, Panagiotopoulou V,
Öztürk E, Biedermann T, Textor M, Lühmann TC and Hall H: The
angiogenic response to PLL-g-PEG-mediated HIF-1α plasmid DNA
delivery in healthy and diabetic rats. Biomaterials. 34:4173–4182.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan JT, Prosser HC, Vanags LZ, Monger SA,
Ng MK and Bursill CA: High-density lipoproteins augment
hypoxia-induced angiogenesis via regulation of post-translational
modulation of hypoxia-inducible factor 1α. FASEB J. 28:206–217.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z,
Liu M, Yeh ET, Chen G and Cheng J: Induction of SENP1 in
endothelial cells contributes to hypoxia-driven VEGF expression and
angiogenesis. J Biol Chem. 285:36682–36688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spirli C, Villani A, Mariotti V, Fabris L,
Fiorotto R and Strazzabosco M: Posttranslational regulation of
polycystin-2 protein expression as a novel mechanism of
cholangiocyte reaction and repair from biliary damage. Hepatology.
62:1828–1839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Liang X, Liang H and Wang B:
SENP1/HIF-1α feedback loop modulates hypoxia-induced cell
proliferation, invasion, and EMT in human osteosarcoma cells. J
Cell Biochem. 119:1819–1826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao H and Xu G: Soft tissue repair for
tibialis anterior tendon ruptures using plate and screw fixation
technique in combination with anterolateral thigh flaps
transplantation. J Orthop Surg Res. 10:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blume PA, Donegan R and Schmidt BM: The
role of plastic surgery for soft tissue coverage of the diabetic
foot and ankle. Clin Podiatr Med Surg. 31:127–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Chen SY, Gao WY, Ding J, Shi W,
Feng XL, Tao XY, Wang L and Ling DS: Experimental study of survival
of pedicled perforator flap with flow-through and flow-end blood
supply. Br J Surg. 102:375–381. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xi S, Cheng S, Lou J, Qiu L, Yang Q, Yu W,
Mei J and Tang M: A Preliminary Study of the Effects of Venous
Drainage Position on Arterial Blood Supply and Venous Return within
the Conjoined Flap. Plast Reconstr Surg. 143:322e–328e. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kocak OF, Bozan N, Oksuz M, Yuce S, Demir
CY, Bulut G and Ragbetli MC: The Effect of the Active Ingredient
Thymoquinone on Flap Viability in Random Pattern Flaps in Rats. J
Membr Biol. 249:513–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang EW, Fang T, Arnold PB, Songcharoen
SJ, Lineaweaver WC and Zhang F: The Effect of Activated Protein C
on Attenuation of Ischemia-Reperfusion Injury in a Rat Muscle Flap
Model. Ann Plast Surg. 75:448–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu CE, Shyu VB, Wen CJ, Wei FC, Huang XT
and Cheng HY: The rat groin flap model redesigned for evaluating
treatment effects on ischemia-reperfusion injury. J Surg Res.
222:160–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venardos KM, Rajapakse NW, Williams D, Hoe
LS, Peart JN and Kaye DM: Cardio-protective effects of combined
l-arginine and insulin: Mechanism and therapeutic actions in
myocardial ischemia-reperfusion injury. Eur J Pharmacol. 769:64–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Menazza S, Sun J, Appachi S, Chambliss KL,
Kim SH, Aponte A, Khan S, Katzenellenbogen JA, Katzenellenbogen BS,
Shaul PW, et al: Non-nuclear estrogen receptor alpha activation in
endothelium reduces cardiac ischemia-reperfusion injury in mice. J
Mol Cell Cardiol. 107:41–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu DM, Liu Y, Duan WQ and Cen Y: Effects
of connective tissue growth factor on angiogenesis of random skin
flaps in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 39:111–113.
2008.(In Chinese). PubMed/NCBI
|
|
36
|
Chen F, Liu Q, Zhang ZD and Zhu XH:
Co-delivery of G-CSF and EPO released from fibrin gel for
therapeutic neovascularization in rat hindlimb ischemia model.
Microcirculation. 20:416–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Esposito E, Hayakawa K, Ahn BJ, Chan SJ,
Xing C, Liang AC, Kim KW, Arai K and Lo EH: Effects of ischemic
post-conditioning on neuronal VEGF regulation and microglial
polarization in a rat model of focal cerebral ischemia. J
Neurochem. 146:160–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You L, Fang Z, Shen G, Wang Q, He Y, Ye S,
Wang L, Hu M, Lin Y, Liu M, et al: Astragaloside IV prevents high
glucose induced cell apoptosis and inflammatory reactions through
inhibition of the JNK pathway in human umbilical vein endothelial
cells. Mol Med Rep. 19:1603–1612. 2019.PubMed/NCBI
|
|
39
|
Cheng S, Zhang X, Feng Q, Chen J, Shen L,
Yu P, Yang L, Chen D, Zhang H, Sun W, et al: Astragaloside IV
exerts angiogenesis and cardioprotection after myocardial
infarction via regulating PTEN/PI3K/Akt signaling pathway. Life
Sci. 227:82–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu R, Jiang H, Tian Y, Zhao W and Wu X:
Astragaloside IV protects against polymicrobial sepsis through
inhibiting inflammatory response and apoptosis of lymphocytes. J
Surg Res. 200:315–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Wang P, Huang F, Jin J, Wu H, Zhang
B, Wang Z, Shi H and Wu X: Astragaloside IV protects blood-brain
barrier integrity from LPS-induced disruption via activating Nrf2
antioxidant signaling pathway in mice. Toxicol Appl Pharmacol.
340:58–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang XG, Sun K, Liu YY, Yan L, Wang MX,
Fan JY, Mu HN, Li C, Chen YY, Wang CS, et al: Astragaloside IV
ameliorates 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced
colitis implicating regulation of energy metabolism. Sci Rep.
7:418322017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Costa IM, Lima FOV, Fernandes LCB, Norrara
B, Neta FI, Alves RD, Cavalcanti JRLP, Lucena EES, Cavalcante JS,
Rego ACM, et al: Astragaloside IV Supplementation Promotes A
Neuroprotective Effect in Experimental Models of Neurological
Disorders: A Systematic Review. Curr Neuropharmacol. 17:648–665.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Menendez MT, Teygong C, Wade K, Florimond
C and Blader IJ: siRNA Screening Identifies the Host Hexokinase 2
(HK2) Gene as an Important Hypoxia-Inducible Transcription Factor 1
(HIF-1) Target Gene in Toxoplasma gondii-Infected Cells. MBio.
6:e004622015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiong A and Liu Y: Targeting Hypoxia
Inducible Factors-1α As a Novel Therapy in Fibrosis. Front
Pharmacol. 8:3262017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tirpe AA, Gulei D, Ciortea SM, Crivii C
and Berindan-Neagoe I: Hypoxia: Overview on Hypoxia-Mediated
Mechanisms with a Focus on the Role of HIF Genes. Int J Mol Sci.
20:61402019. View Article : Google Scholar
|
|
48
|
Kuschel A, Simon P and Tug S: Functional
regulation of HIF-1α under normoxia--is there more than
post-translational regulation? J Cell Physiol. 227:514–524. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Albanese A, Daly LA, Mennerich D,
Kietzmann T and Sée V: The Role of Hypoxia-Inducible Factor
Post-Translational Modifications in Regulating Its Localisation,
Stability, and Activity. Int J Mol Sci. 22:222020. View Article : Google Scholar
|
|
50
|
Chachami G, Stankovic-Valentin N,
Karagiota A, Basagianni A, Plessmann U, Urlaub H, Melchior F and
Simos G: Hypoxia-induced Changes in SUMO Conjugation Affect
Transcriptional Regulation Under Low Oxygen. Mol Cell Proteomics.
18:1197–1209. 2019. View Article : Google Scholar
|
|
51
|
van Hagen M, Overmeer RM, Abolvardi SS and
Vertegaal AC: RNF4 and VHL regulate the proteasomal degradation of
SUMO-conjugated Hypoxia-Inducible Factor-2alpha. Nucleic Acids Res.
38:1922–1931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nie Q, Zhu L, Zhang L, Leng B and Wang H:
Astragaloside IV protects against hyperglycemia-induced vascular
endothelial dysfunction by inhibiting oxidative stress and
Calpain-1 activation. Life Sci. 232:1166622019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Leng B, Tang F, Lu M, Zhang Z, Wang H and
Zhang Y: Astragaloside IV improves vascular endothelial dysfunction
by inhibiting the TLR4/NF-κB signaling pathway. Life Sci.
209:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang W-D, Zhang C, Wang XH, Gao PJ, Zhu
DL, Chen H, Liu RH and Li HL: Astragaloside IV dilates aortic
vessels from normal and spontaneously hypertensive rats through
endothelium-dependent and endothelium-independent ways. Planta Med.
72:621–626. 2006. View Article : Google Scholar : PubMed/NCBI
|