Introduction
Gastric cancer accounts for a large percentage of
cancer-related deaths (1). Recent
advances in diagnostic techniques, including radiation tests,
endoscopy, and biopsy, as well as the heightened public awareness
of cancer have steadily improved survival rates and facilitated the
early detection of gastric cancer over time (2). In general, surgery, chemotherapy, and
radiation therapy are regularly employed as treatment methods, and
many anticancer drugs have recently been developed and utilized to
complement chemotherapy according to postoperative treatment, but
the resulting survival rates remain unsatisfactory (3). As a result, many studies have been
conducted to identify effective anticancer drugs; however, many of
these therapeutics displayed negative side effects in clinical
trials (4). Therefore, it is
necessary to develop a new anticancer drug that has no side effects
on normal cells.
Natural products can be used in combination
therapies against various diseases as a mixture of different
ingredients that interact with each other (5). Because it affects multiple targets
in vivo, new medicines are being developed using natural
components as medicines or by synthesizing individual components or
derivatives (5). Naturally derived
anticancer drugs are expected to continue to be developed for the
current pharmaceutical market (6).
Therefore, the development of novel anticancer drugs will likely
focus on the utilization of existing natural products, the special
mechanism of which will be revealed afterwards. Most chemotherapies
are closely linked to apoptosis (7)
and in the field of anticancer research targeting apoptosis, a
variety of drugs are being developed, especially via chemical and
radiotherapeutic mechanisms, and many studies are currently being
conducted on anticancer supplements (8). The study of anticancer drugs related
to these natural products mainly focuses on factors involved in
signal transduction related to apoptosis (8).
In recent years, molecules separated from natural
substances have been found to be useful in various diseases
treatment as alternative medicines. Alisol B 23-acetate (AB23A) is
a major ingredient isolated from Alismatis rhizome (9); it has been reported to have various
pharmacological activities, including anti-hepatic (10), antibacterial (11), diuretic (12), hyperlipidemic (13), hepatoprotective (14) and anti-inflammatory effects
(15). Furthermore, it is known to
have a cell death effect on some cancer cells, including SGC7901
stomach cancer cells (16), HEY
ovarian cancer cells (17), HCT116
colon cancer cells (18), A549 lung
cancer cells (19,20) and HepG2 or SK-HEP-1 hepatoma cells
(21,22). However, the detailed anticancer
efficacy mechanisms of AB23A remain largely unknown in gastric
cancer cells. Therefore, we investigated the anticancer mechanisms
of AB23A in AGS gastric cancer cells.
Materials and methods
Cell proliferation
The gastric cancer cell line AGS were obtained from
the American Type Culture Collection (ATCC). AGS cells were
cultured in RPMI-1640 medium (Gibco-BRL; Thermo Fisher Scientific,
Inc.) supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C and seeded onto 12-well plates at a density of
3×104 cells/well. Cell viability was determined using
the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
(MTT) assay for 24, 48 and 72 h. Also, to identify the effects of
AB23A on the mitogen-activated protein kinase (MAPK) pathways,
PD98059 (p42/44 MAPK inhibitor; 10 µM), SB203580 (p38 MAPK
inhibitor; 10 µM), or SP600125 (JNK inhibitor; 10 µM) was used with
the MTT assay for 24 h.
Cell cycle measurement
After 24 h of treatment with AB23A, AGS cells were
treated with ethyl alcohol (3 ml; 100%) and vortexed prior to
overnight incubation at 4°C. Samples were centrifuged for 5 min and
the supernatant was discarded. Cell pellets were resuspended in
propidium iodine (PI) staining solution (5 mg/ml; 2 µl) containing
RNase (2 µl), spun at 2,0000 × g for 10 sec and incubated for 40
min in the dark at room temperature. Samples were analyzed using a
fluorescence-activated cell sorter (FACScan; Becton-Dickinson).
Mitochondrial membrane depolarization
assay
After 24 h of treatment with AB23A, AGS cells were
treated with 50 nM tetramethylrhodamine methyl ester (TMRM;
Sigma-Aldrich; Merck KGaA) for 30 min. The fluorescence intensities
were measured using a BD FACSCanto II (BD Biosciences) at the
excitation and emission wavelengths of 510 and 580 nm,
respectively.
Western blot analysis
The Bradford method (Bio-Rad Laboratories) was used
to extract the total protein. The protein samples were separated
via 8 or 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and probed with specific antibodies. Antibodies
against survivin (cat. no. 2808), extracellular signal-regulated
kinase (ERK; cat. no. 9102), phosphorylated (p) ERK (cat. no.
9106), c-Jun N-terminal kinase (JNK; cat. no. 9252), pJNK (cat. no.
9251), p38 (cat. no. 9212), and pp38 (cat. no. 9216) were purchased
from Cell Signaling Technology, and antibodies against B cell
lymphoma 2 (Bcl-2; cat. no. sc-783), Bax (cat. no. sc-493),
caspase-3 (cat. no. sc-7148), caspase-9 (cat. no. sc-7885), poly
(ADP-ribose) polymerase (PARP; cat. no. sc-7150), β-actin (cat. no.
sc-47778) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat.
no. sc-32233) were procured from Santa Cruz Biotechnology. After 24
h of treatment with AB23A, survivin, Bcl-2, Bax, caspase-3,
caspase-9 and PARP experiments were conducted. In case of ERK,
p-ERK, JNK, p-JNK, p38 and pp38, experiments were conducted after
0.5, 1, 2 and 4 h of treatment.
Caspase assay
Caspase-3 and −9 assay kits (Cellular Activity Assay
kit Plus; BioMol Research Laboratories, Inc.) were used. After 24 h
of treatment with AB23A, caspase experiments were conducted. After
resuspending the cells in ice-cold cell lysis buffer, the
supernatant was removed. Supernatant samples were incubated with
caspase substrate (400-lM Ac-DEVD-pNA; 50 µl) at 37°C and then,
samples were read at 405 nm.
Reactive oxygen species (ROS)
measurement
After 24 h of treatment with AB23A, AGS cells were
treated with 20 µl using DCF-DA (2′,7′-dichlorodihydrofluorescein
diacetate: Molecular Probes) at 37°C for 30 m in and washed with
PBS. Fluorescence was measured using FACS (Becton-Dickinson), at
excitation/emission wavelengths of 488/525 nm, respectively
(23).
Statistical analysis
Two-way analysis of variance (ANOVA) or one-way
ANOVA with Tukey's post hoc comparison method were used for
multiple comparisons. The analysis was performed using the Prism
6.0 (GraphPad Software, Inc.) and Origin 8.0 (OriginLab
Corporation) software. Data are expressed as the mean ± standard
error of the mean (SEM), and P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of AB23A on AGS gastric cancer
cell viability
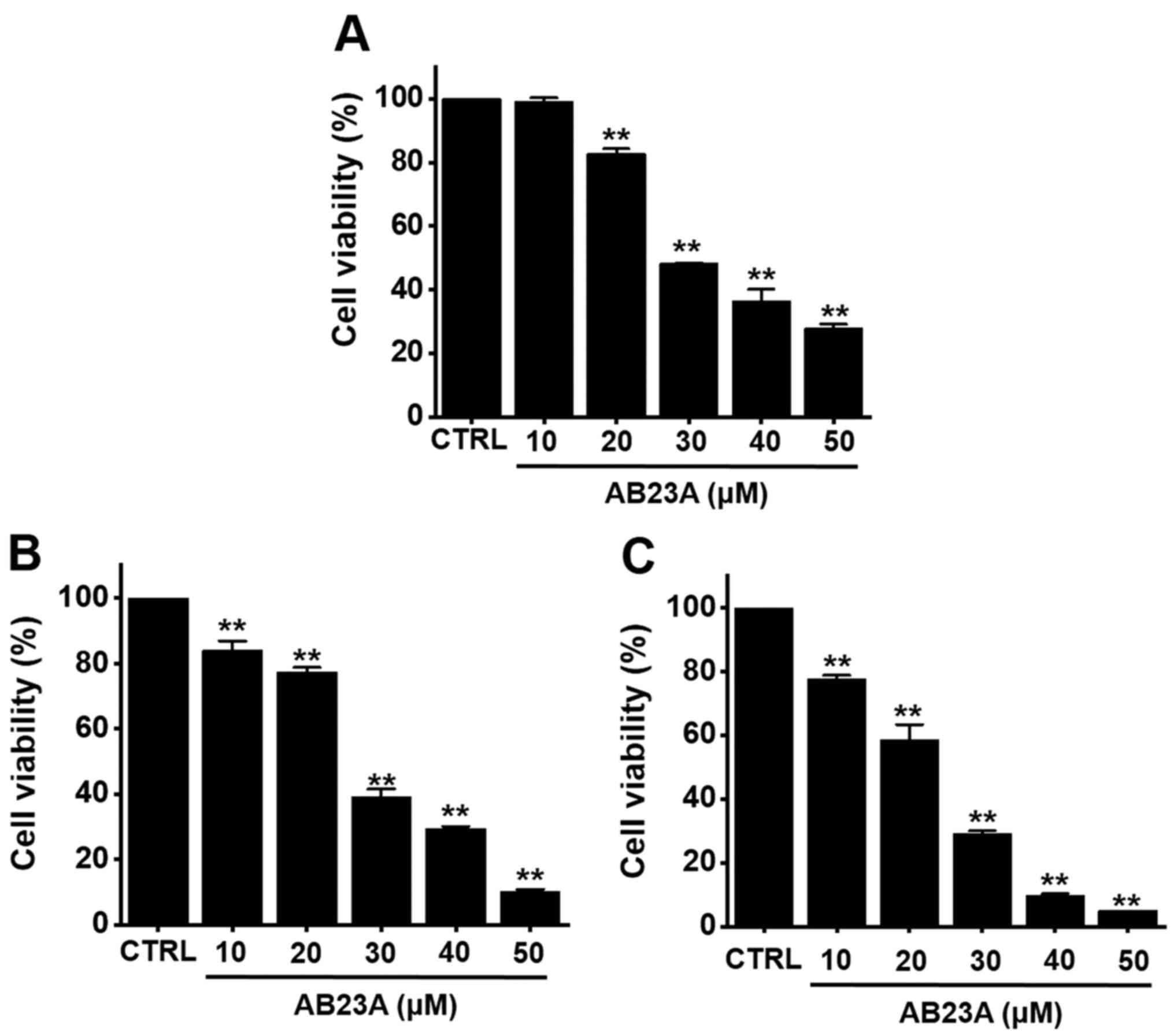
The MTT method was used to evaluate the effects of
AB23A on cell viability in AGS cells. AB23A (10, 20, 30, 40 or 50
µM) reduced the cell viability by 99.3±1.1, 82.8±1.6% (P<0.01),
48.3±0.2% (P<0.01), 36.6±3.6% (P<0.01) and 27.9±1.3%
(P<0.01), respectively, at 24 h (Fig. 1A), by 84.1±2.9% (P<0.01),
77.5±1.3% (P<0.01), 39.3±2.2% (P<0.01), 29.5±0.6% (P<0.01)
and 10.1±0.8% (P<0.01) at 48 h (Fig.
1B) and by 77.7±1.2% (P<0.01), 58.7±4.7% (P<0.01),
29.1±1.0% (P<0.01), 9.7±0.6% (P<0.01) and 5.1±0.1%
(P<0.01) at 72 h (Fig. 1C) as
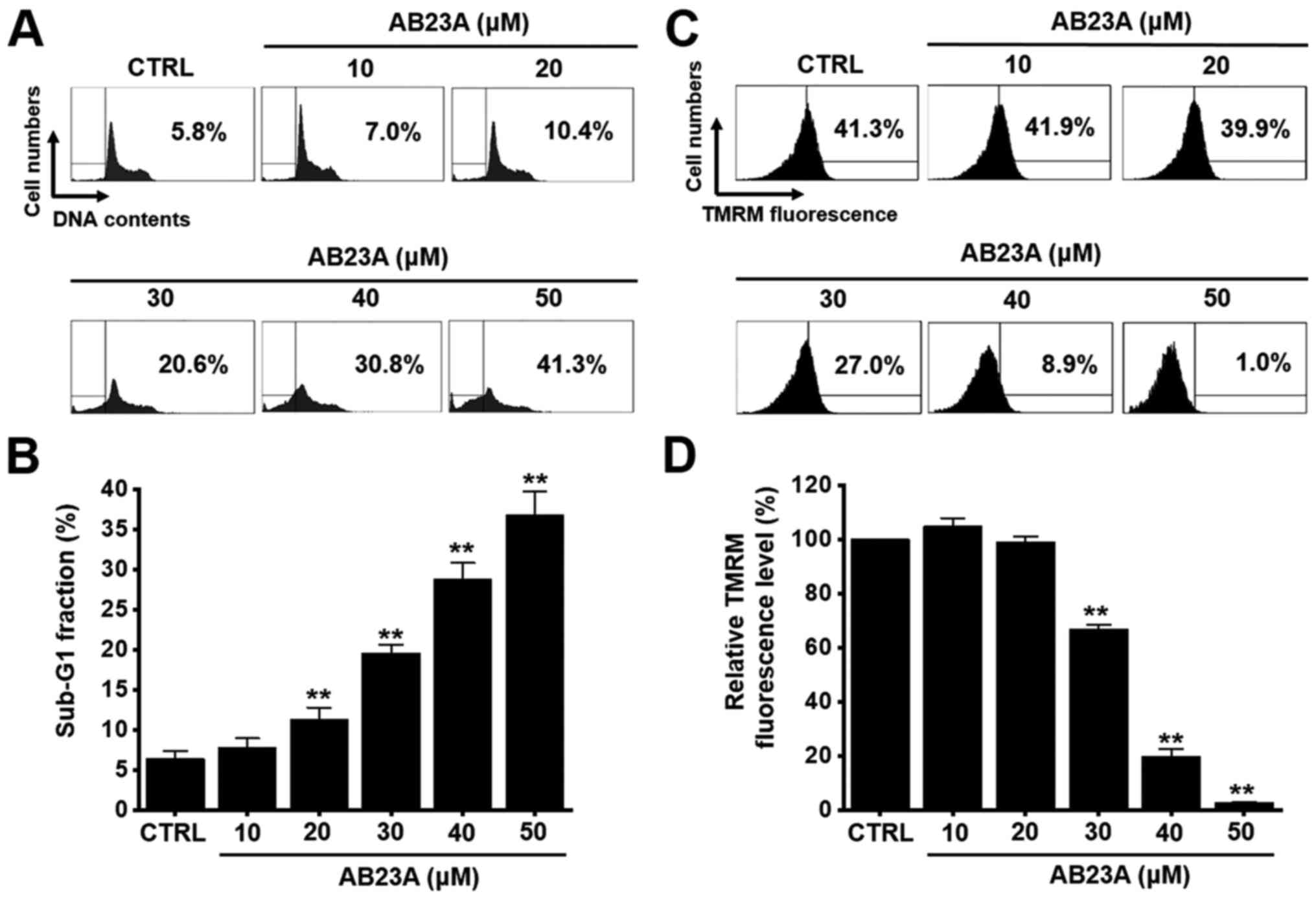
determined by MTT assay. In addition, cell cycle analysis and
mitochondrial membrane depolarization experiments were conducted to
assess the apoptotic effects of AB23A. The sub-G1 phase ratios were
increased by 7.8±1.2% at 10 µM, 11.3±1.4% (P<0.01) at 20 µM,
19.6±1.1% (P<0.01) at 30 µM, 28.8±2.0% (P<0.01) at 40 µM, and
36.8±2.9% (P<0.01) at 50 µM for 24 h (Fig. 2A and B). Mitochondrial membrane
depolarization was examined via TMRM staining, and the
mitochondrial membrane was indeed depolarized by AB23A (Fig. 2C). The TMRM fluorescence level was
decreased by 104.8±3.0% at 10 µM, 99.0±2.1% at 20 µM, 66.9±1.6%
(P<0.01) at 30 µM, 19.8±2.6% (P<0.01) at 40 µM, and 2.6±0.2%
(P<0.01) at 50 µM for 24 h (Fig.
2D). These results suggest that AB23A inhibits the
proliferation of AGS cells and that these effects are related to
apoptosis.
Effects of AB23A on the
mitochondria-dependent pathway in AGS gastric cancer cells
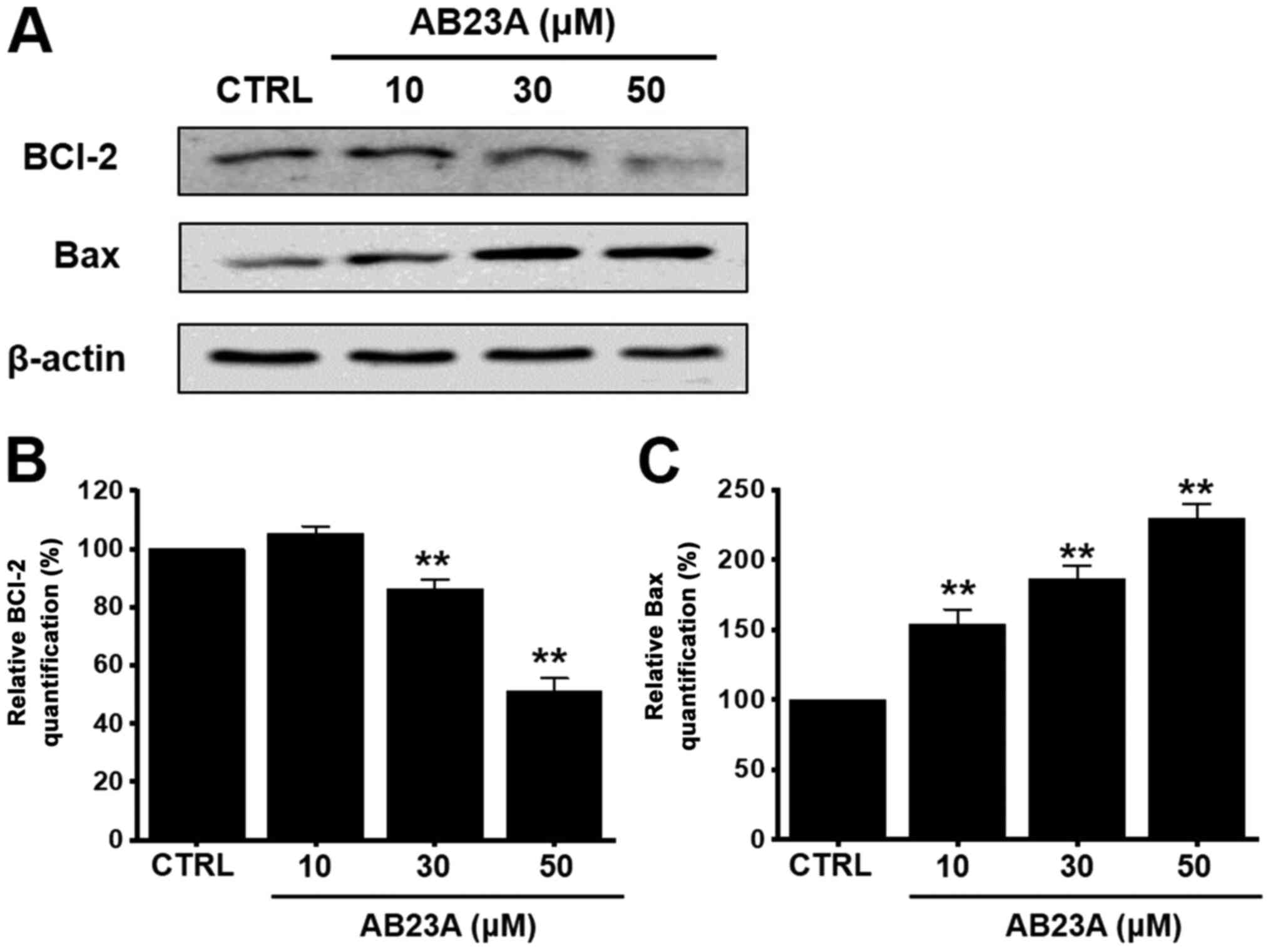
We investigated whether the Bcl-2 (anti-apoptotic)
and the Bax (pro-apoptotic) proteins were involved in the apoptosis
induced by AB23A. Using the western blot method, it was observed
that the Bcl-2 level was decreased by 105.3±2.5% at 10 µM,
86.2±3.1% (P<0.01) at 30 µM, and 51.3±4.2% (P<0.01) at 50 µM
for 24 h (Fig. 3A and B), whereas
the Bax level was increased by 154.1±10.2% (P<0.01) at 10 µM,
186.3±9.3% (P<0.01) at 30 µM, and 229.5±10.1% (P<0.01) at 50
µM for 24 h (Fig. 3A and C). These
results suggest that the AB23A-induced apoptosis in AGS cells is
related to the mitochondria-dependent pathway.
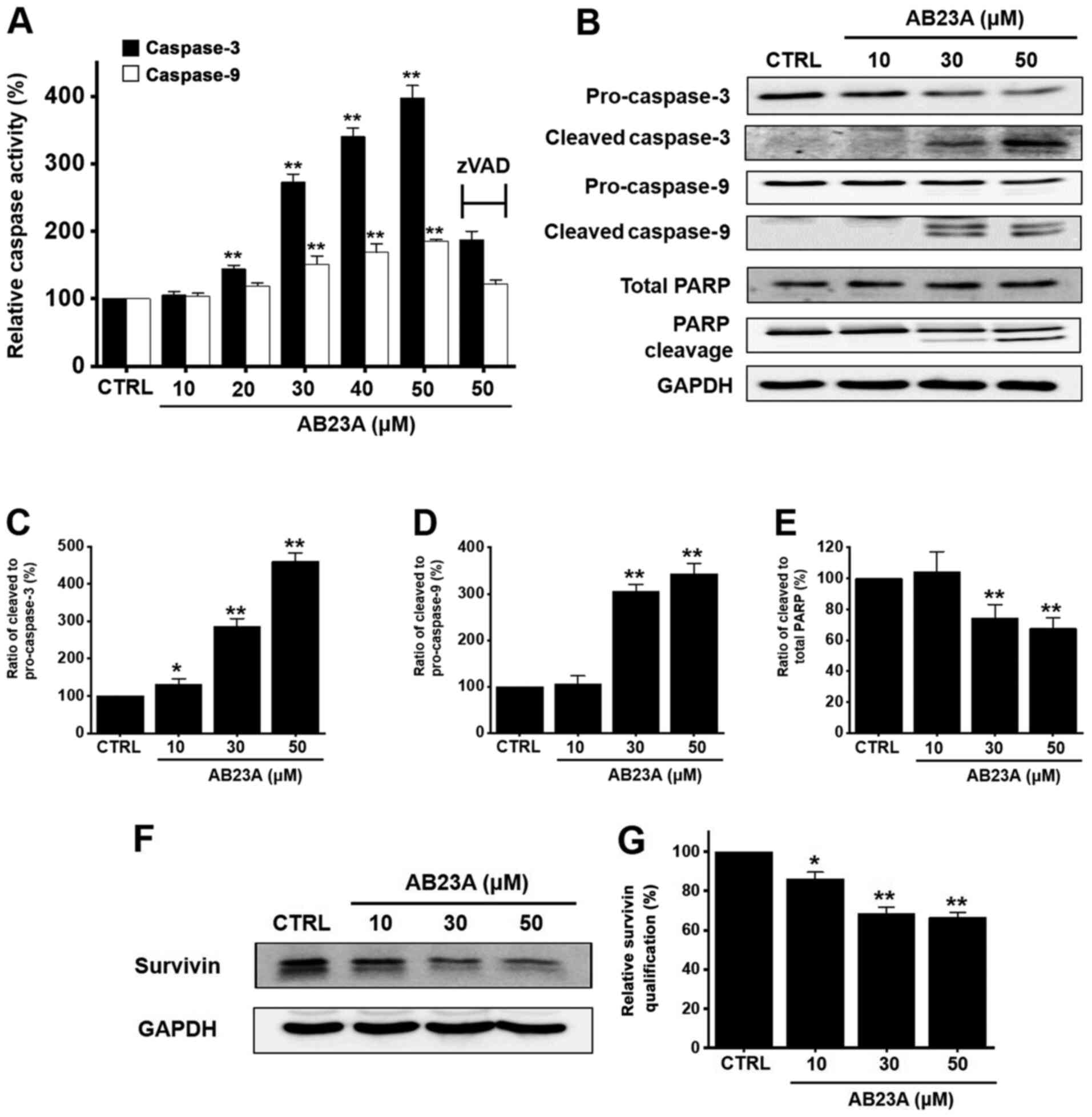
Effects of AB23A on the caspase
pathway in AGS gastric cancer cells
Apoptosis typically takes place via the extrinsic or
intrinsic apoptotic pathway (24).
Caspases represent some of the important genes that regulate
apoptosis to maintain homeostasis via the intrinsic and extrinsic
apoptotic pathways (25). AB23A
increased caspase-3 activation by 105.7±4.9% at 10 µM, 144.3±5.3%
(P<0.01) at 20 µM, 272.5±12.1% (P<0.01) at 30 µM, 340.1±12.9%
(P<0.01) at 40 µM, and 397.1±18.7% (P<0.01) at 50 µM for 24 h
(Fig. 4A) as well as caspase-9
activation by 104.2±4.6% at 10 µM, 119.2±4.4% at 20 µM, 151.2±12.1%
(P<0.01) at 30 µM, 168.9±12.6% (P<0.01) at 40 µM, and
185.0±2.8% (P<0.01) at 50 µM for 24 h (Fig. 4A). In addition, Z-VAD-FMK inhibited
this activation by 187.4±12.7% for caspase-3 and 121.7±6.3% for
caspase-9 at 50 µM for 24 h (Fig.
4A). Using the western blot method, it was observed that the
expression levels of pro-caspase-3 and −9 were reduced by AB23A,
and the expression levels of the active forms were increased. PARP
cleavage levels were also increased for 24 h (Fig. 4B). The ratio of cleaved caspase-3 to
pro-caspase-3 was increased for 24 h (Fig. 4C) and the ratio of cleaved caspase-9
to pro-caspase-9 was also increased for 24 h (Fig. 4D). However, the ratio of cleaved
PARP to total PARP was decreased for 24 h (Fig. 4E). In addition, survivin, an
inhibitor of the apoptosis protein, was decreased by 86.1±3.5%
(P<0.05) at 10 µM, 68.3±3.4% (P<0.01) at 30 µM, and 66.4±2.6%
(P<0.01) at 50 µM for 24 h (Fig. 4F
and G). These results suggest that the AB23A-induced apoptosis
is related to caspase activation in AGS cells.
Effects of AB23A on the MAPK pathways
in AGS gastric cancer cells
To identify the effects of AB23A on the MAPK
pathways in AGS cells, PD98059, SB203580, or SP600125 was applied
along with AB23A using the MTT assay to investigate the effects on
cell viability. Co-treatment with AB23A (10, 20, 30, 40, or 50 µM)
and PD98059 reduced cell viability by 94.8±3.1, 86.4±2.3, 74.4±2.5%
(P<0.001), 69.8±1.9% (P<0.001) and 49.6±2.7% (P<0.01),
respectively, for 24 h (Fig. 5A),
and co-treatment with AB23A and SB203580 reduced cell viability by
87.3±2.2, 77.1±2.6% (P<0.01), 64.6±4.4, 42.4±1.0% (P<0.001)
and 33.1±3.1%, respectively, for 24 h (Fig. 5B). In addition, co-treatment with
AB23A and SP600125 reduced cell viability by 89.7±3.9, 80.9±6.0,
69.4±1.1, 55.6±3.6 and 37.1±1.6%, respectively, for 24 h (Fig. 5C). To find out more about the
efficacy of AB23A on the MAPK pathways, we investigated the
AB23A-induced phosphorylation of MAPK proteins (ERK, JNK and p38)
using the western blot. The phosphorylation of these proteins
increased with AB23A treatment for 0.5, 1, 2 or 4 h (Fig. 6A). The ratio of phosphorylated ERK
to ERK was increased at 0.5, 1, 2 or 4 h (Fig. 6B-a) and the ratio of phosphorylated
JNK to JNK was also increased at 0.5, 1, 2 or 4 h (Fig. 6B-b). In addition, the ratio of
phosphorylated p38 to p38 was increased at 0.5, 1, 2 or 4 h
(Fig. 6B-c). These results suggest
that AB23A induces apoptosis by regulating the MAPK signaling
pathways in AGS cells.
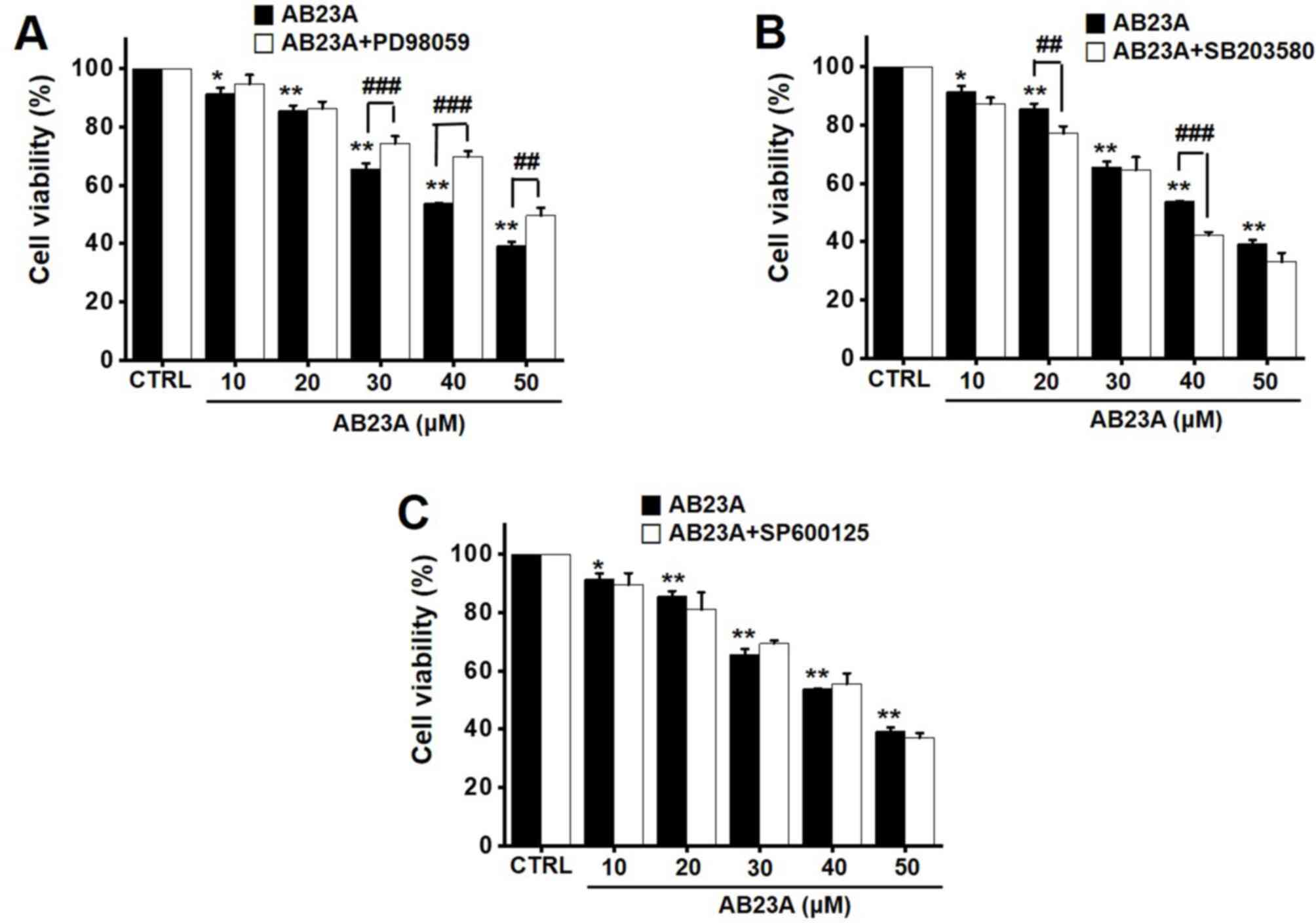 | Figure 5.Effects of AB23A on MAPK pathway
inhibitors in AGS cells. Cell viabilities were determined after
co-treating the cells with AB23A plus (A) PD98059, (B) SB203580, or
(C) SP600125 at 24 h. The change after 24 h with 100% of control (0
h) was organized. The results are presented as the means ± SEM.
*P<0.05, **P<0.01 vs. CTRL.; ##P<0.01,
###P<0.001, as indicated. AB23A, alisol B 23-acetate;
CTRL, control; MAPK, mitogen-activated protein kinase; MTT,
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; SEM,
standard error of the mean. |
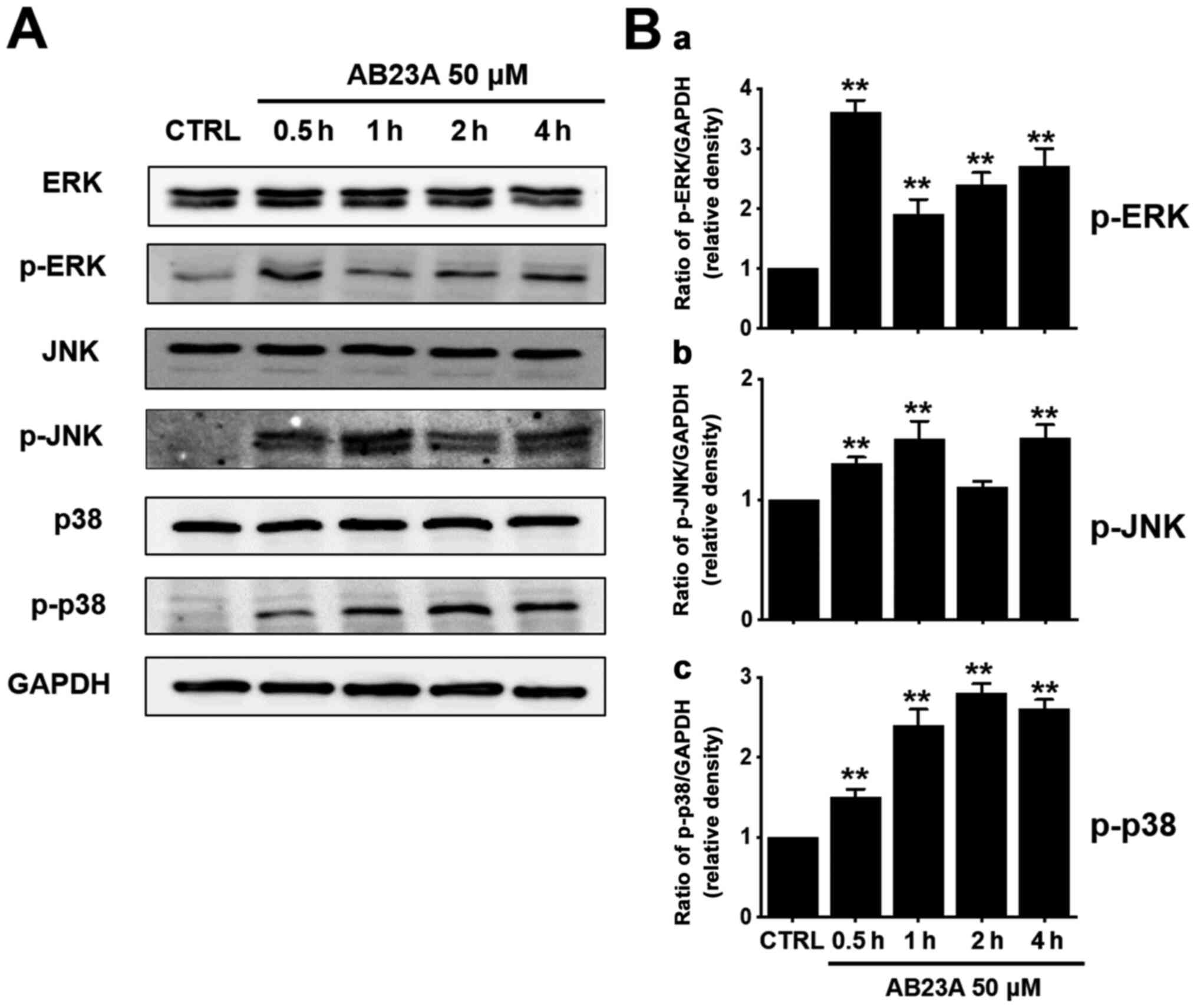 | Figure 6.Effects of AB23A on ERK, JNK, and p38
MAPK pathway activation in AGS cells. (A) The phosphorylation of
ERK, JNK and p38 was confirmed following AB23A treatment using the
western blot method. (B) Phosphorylated levels of these proteins
[(a) ERK, (b) JNK and (c) p38] are indicated as band densities
relative to that of GAPDH. The results are presented as the means ±
SEM. **P<0.01 vs. CTRL. AB23A, alisol B 23-acetate; CTRL,
control; ERK, extracellular signal-regulated kinase; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; JNK, c-Jun N-terminal
kinase; MAPK, mitogen-activated protein kinase; SEM, standard error
of the mean. |
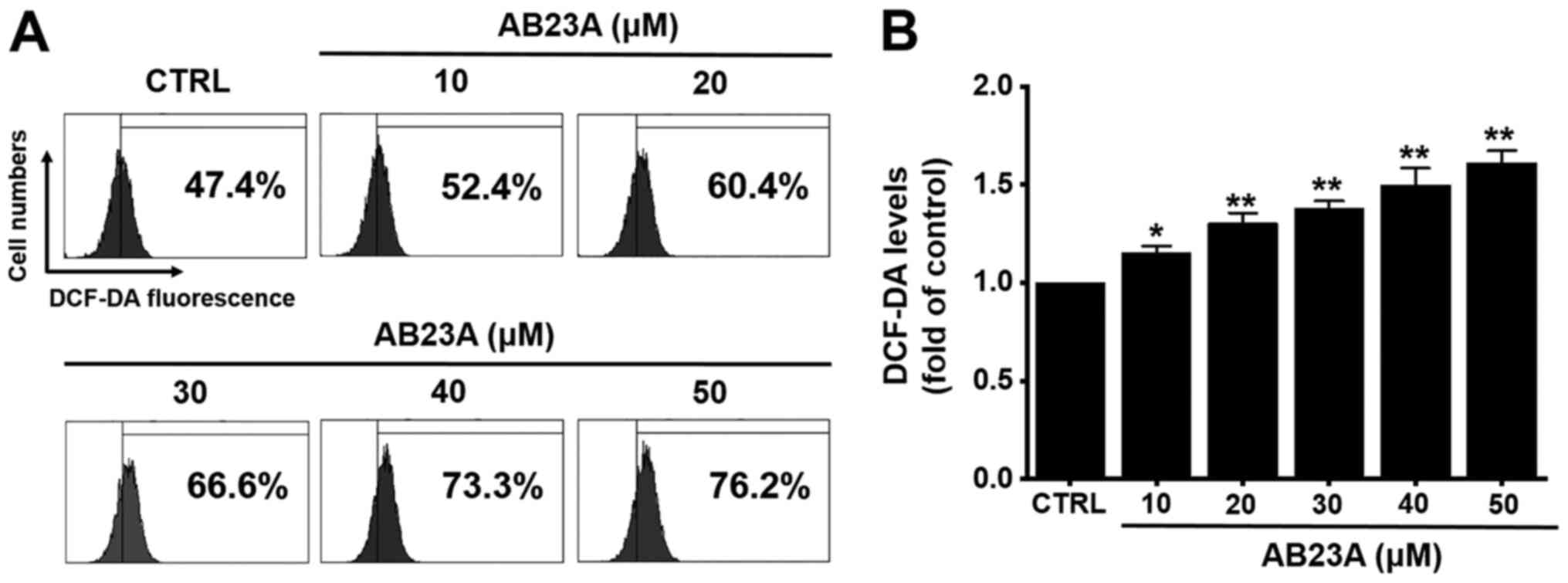
Effects of AB23A on ROS generation in
AGS gastric cancer cells
Many reports have suggested that ROS also plays a
key role in apoptosis (26).
Therefore, we investigated whether DCF-DA levels was increased by
AB23A. AB23A increased the DCF-DA levels by the flow cytometry
method for 24 h (Fig. 7). These
results suggest that AB23A may induce apoptosis via ROS generation
in AGS cells.
Discussion
Cancer is one of the major causes of death around
the world, and the incidence of cancer is expected to increase
further in the future owing to environmental problems and higher
life expectancy (27). Research is
being conducted on effective anticancer drugs, but since anticancer
drugs are equally applied to normal cells as well as cancer cells,
damage to normal tissues, toxicity and side effects are inevitable
when these drugs are administered (28). Therefore, efforts are being made to
identify and isolate cancer-preventing substances from natural
products to develop effective anticancer drugs with minimal side
effects.
AB23A, isolated from A. rhizome (9), has various pharmacological activities
(10–15). It also has anticancer effects on
various cancer cells. AB23A causes apoptosis via mitochondria and
phosphatidylinositol 3-kinase (PI3K)/Akt mechanisms in SGC7901
gastric cancer cells (16) and
SK-HEP-1 hepatocellular carcinoma (22) while blocking the G1 phase in HEY
ovarian cancer cells, resulting in a decrease in related proteins
and thus inhibiting cell growth (17). It also generates ROS and activates
JNK to cause apoptosis in HCT116 human colon cancer cells (18) while promoting apoptosis in human
lung cancer cells through intrinsic mechanisms associated with the
mitochondria (20) or PIK/AKT/mTOR
signaling (19). In addition, AB23A
blocks the G1 phase in HepG2 hepatoma cells, again causing
apoptosis (21). Furthermore, the
present study demonstrates that AB23A induces apoptosis of AGS
gastric cancer cells.
Factors belonging to the Bcl-2 family act as
important modulating factors in the intrinsic apoptosis pathway
associated with mitochondrial dysfunction (29). If the expression of the
pro-apoptotic protein Bax increases relative to that of the
anti-apoptotic protein Bcl-2, Bax moves to the mitochondria,
inducing the loss of matrix metalloproteinase and the movement of
cytochrome c to the cytoplasm, thus activating the intrinsic
apoptosis pathway (30). In
addition, the inhibitor of apoptosis protein (IAP) family is known
to suppress caspase activity, thus inhibiting the induction of
apoptosis (31). In the present
study, Bcl-2 decreased and Bax increased following AB23A treatment
(Fig. 3A-C). Among the IAP family
proteins treated with AB23A, the expression of survivin reduced
significantly (Fig. 4C and D).
These observations indicate that mitochondrial damage due to the
change in the Bcl-2 family protein expression is involved in the
AB23A-induced apoptosis, which suggests that the intrinsic
apoptosis pathway is activated rather than the extrinsic
pathway.
Apoptosis is induced via the extrinsic and intrinsic
pathways in which caspase activation plays a key role (24). The extrinsic pathway is initiated
via the activation of caspase-8 by the death receptor present in
the cell membrane, through the activity of caspase-3 and −7
(25). In contrast, in the
intrinsic pathway, the cytochrome c protein of the
mitochondria promotes caspase-9, −3, and −7 activity, causing
apoptosis (25). According to the
results shown in Fig. 4A, the
activity of caspase-3 and −9 increased with increased
concentrations of AB23A, and the activity expression of caspase-9
and −3 decreased with decreased concentrations of AB23A (Fig. 4B). Therefore, it can be seen that
the activation of the intrinsic pathway is involved in the AB23A
induced apoptosis.
Ion channels are engaged in the mechanism of killing
cancer cells (32). Various ion
channels such as TRPM7, TRPM2, and TRPC6 are involved in the
killing of gastric cancer cells (33–35).
The TRPM7 ion channel is involved in AGS cell survival (34), and the TRPM2 ion channel is involved
in gastric cancer cell penetration (35). Furthermore, the TRPC6 ion channel is
involved in gastric cancer formation (33). However, no studies have assessed the
effects of AB23A on these ion channels. Therefore, in the future,
it is necessary to study whether ion channels are related with the
apoptosis of AGS gastric cancer cells induced by AB23A.
MAPKs mediate intracellular signal transmission in
response to external stimuli (36).
MAPKs have previously been known to be involved in various
physiological mechanisms such as cell growth and differentiation
(36). MAPK activation also
involves apoptosis via three main mechanisms: ERK, JNK and p38
kinase (37). In the present study,
we found that AB23A co-treatment with PD98059 increases cell
viability (Fig. 5) and that AB23A
activates the ERK, p38 and JNK pathways (Fig. 6). Thus, it appears that the
activation of the MAPK mechanism by AB23A plays a role in
preventing the growth of AGS cells.
Apoptosis can be caused by various stimuli,
including ROS, reactive nitrogen species and hormones (38). In the present study, we found that
AB23A increased DCF-DA levels (Fig.
7).
Therefore, ROS generation may be also involved in
the apoptosis induced by AB23A. ROS accumulation can cause chronic
cell damage and has been associated with apoptosis in cancer cells
(38). High levels of ROS can also
be generated abruptly as part of the immune response to pathogens
and several enzymes such as superoxide dismutase (SOD), glutathione
peroxidase and catalase are involved in ROS detoxification
(39). AB-induced ROS generation
can be clearly determined by administering the catalase or
antioxidant with AB23A and checking that apoptosis is
suppressed.
In conclusion, AB23A inhibits AGS cell
proliferation. It increased the sub-G1 proportion and depolarized
the mitochondrial membrane. In addition, the AB23A-induced
apoptosis was related to the downregulation of Bcl-2 and survivin
as well as the upregulation of Bax. It activated caspase-3 and −9
and the MAPK cascades. AB23A also increased ROS generation. Thus,
it is hoped that various natural products like AB23A will be
developed into novel treatments for gastric cancer.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education of South Korea (grant no.
NRF-2019R1I1A3A01041391).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BJK and JHN designed the research. MJK and JNK
conducted the experiments. MJK, JNK, MJL, WKK, JHN and BJK analyzed
the data. BJK and JHN wrote the manuscript. BJK and JHN confirm the
authenticity of all the raw data. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
You MW, Park S, Kang HJ and Lee DH:
Radiologic serosal invasion sign as a new criterion of T4a gastric
cancer on computed tomography: Diagnostic performance and
prognostic significance in patients with advanced gastric cancer.
Abdom Radiol (NY). 45:2950–2959. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ooi SL, McMullen D, Golombick T, Nut D and
Pakm SC: Evidence-based review of BioBran/MGN-3 arabinoxylan
compound as a complementary therapy for conventional cancer
treatment. Integr Cancer Ther. 17:165–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernsen EC, Hagleitner MM, Kouwenberg TW
and Hanff LM: Pharmacogenomics as a tool to limit acute and
long-term adverse effects of chemotherapeutics: An update in
pediatric oncology. Front Pharmacol. 11:11842020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamberlin SR, Blucher A, Wu G, Shinto L,
Choonoo G, Kulesz-Martin M and McWeeney S: Natural product target
network reveals potential for cancer combination therapies. Front
Pharmacol. 10:5572019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdulridha MK, Al-Marzoqi AH, Al-Awsi GRL,
Mubarak SMH, Heidarifard M and Ghasemian A: Anticancer effects of
herbal medicine compounds and novel formulations: A literature
review. J Gastrointest Cancer. 51:765–773. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci MS and Zong WX: Chemotherapeutic
approaches for targeting cell death pathways. Oncologist.
11:342–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar
|
|
9
|
Wang C, Feng L, Ma L, Chen H, Tan X, Hou
X, Song J, Cui L, Liu D, Chen J, et al: Alisol a 24-acetate and
Alisol b 23-acetate induced autophagy mediates apoptosis and
nephrotoxicity in human renal proximal tubular cells. Front
Pharmacol. 8:1722017.PubMed/NCBI
|
|
10
|
Jiang ZY, Zhang XM, Zhang FX, Liu N, Zhao
F, Zhou J and Chen JJ: A new triterpene and anti-hepatitis B virus
active compounds from Alisma orientalis. Planta Med.
72:951–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin HG, Jin Q, Ryun Kim A, Choi H, Lee JH,
Kim YS, Lee DG and Woo ER: A new triterpenoid from Alisma
orientale and their antibacterial effect. Arch Pharm Res.
35:1919–1926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng YL, Chen H, Tian T, Chen DQ, Zhao YY
and Lin RC: Diuretic and anti-diuretic activities of the ethanol
and aqueous extracts of Alismatis rhizoma. J Ethnopharmacol.
154:386–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan Y, Gao H, Wand J and Zhao J:
Separated prescription research on black currant seed and Zexie
decoction, Crataegi fructus combination about depressurization and
adjusting blood lipid. Chin J Mod Appl Pharm. 33:414–419. 2016.
|
|
14
|
Meng Q, Chen X, Wang C, Liu Q, Sun H, Sun
P, Huo X, Liu Z, Yao J and Liu K: Protective effects of Alisol B
23-acetate via Farnesoid X receptor-mediated regulation of
transporters and enzymes in estrogen-induced cholestatic liver
injury in mice. Pharm Res. 32:3688–3698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li HM, Fan M, Xue Y, Peng LY, Wu XD, Liu
D, Li RT and Zhao QS: Guaiane-type sesquiterpenoids from Alismatis
Rhizoma and their anti-inflammatory activity. Chem Pharm Bull
(Tokyo). 65:403–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu YH, Zhao LJ and Li Y: Alisol B acetate
induces apoptosis of SGC7901 cells via mitochondrial and
phosphatidylinositol 3-kinases/Akt signaling pathways. World J
Gastroenterol. 15:2870–2877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang LL, Xu YL, Tang ZH, Xu XH, Chen X,
Li T, Ding CY, Huang MQ, Chen XD, Wang YT, et al: Effects of alisol
B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest,
apoptosis, migration and invasion inhibition. Phytomedicine.
23:800–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Li ETS and Wang M: Alisol B
23-acetate induces autophagic-dependent apoptosis in human colon
cancer cells via ROS generation and JNK activation. Oncotarget.
8:70239–70249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Xia XC, Meng LY, Wang Y and Li YM:
Alisol B 23-acetate inhibits the viability and induces apoptosis of
non-small cell lung cancer cells via PI3K/AKT/mTOR signal pathway.
Mol Med Rep. 20:1187–1195. 2019.PubMed/NCBI
|
|
20
|
Wang J, Li H, Wang X, Shen T, Wang S and
Ren D: Alisol B-23-acetate, a tetracyclic triterpenoid isolated
from Alisma orientale, induces apoptosis in human lung
cancer cells via the mitochondrial pathway. Biochem Biophys Res
Commun. 505:1015–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia J, Luo Q, Huang S, Jiang F, Wang L,
Wang G, Xie J, Liu J and Xu Y: Alisol B 23-acetate-induced HepG2
hepatoma cell death through mTOR signaling-initiated G1
cell cycle arrest and apoptosis: A quantitative proteomic study.
Chin J Cancer Res. 31:375–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Cheng J, Zhu D, Shi X, Wei Y, Chen
S, Wang Z and Yuan D: The effects of Alisol B 23-acetate in
hepatocellular carcinoma via inducing cell apoptosis and inhibiting
cell migration and invasion. Gen Physiol Biophys. 39:219–228. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jo G, Kwon MJ, Kim JN and Kim BJ: Radix
sophorae flavescentis induces apoptosis through by caspase,
MAPK activation and ROS signaling pathways in 5637 human bladder
cancer cells. Int J Med Sci. 17:1474–1481. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q and Wang HG: Anti-cancer drug
discovery and development. Bcl-2 family small molecule inhibitors.
Commun Integr Biol. 5:557–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hata AN, Engelman JA and Faber AC: The
BCL-2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berthelet J and Dubrez L: Regulation of
apoptosis by inhibitors of apoptosis (IAPs). Cells. 2:163–187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Litan A and Langhans SA: Cancer as a
channelopathy: Ion channels and pumps in tumor development and
progression. Front Cell Neurosci. 9:862015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G,
Jin Y and Wang Y: Blockade of TRPC6 channels induced G2/M phase
arrest and suppressed growth in human gastric cancer cells. Int J
Cancer. 125:2281–2287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim MC, Lee HJ, Lim B, Ha KT, Kim SY, So I
and Kim BJ: Quercetin induces apoptosis by inhibiting MAPKs and
TRPM7 channels in AGS cells. Int J Mol Med. 33:1657–1663. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Almasi S, Sterea AM, Fernando W, Clements
DR, Marcato P, Hoskin DW, Gujar S and El Hiani Y: TRPM2 ion channel
promotes gastric cancer migration, invasion and tumor growth
through the AKT signaling pathway. Sci Rep. 9:41822019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci. 21:23462020.
View Article : Google Scholar
|
|
38
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harman D: The free radical theory of
aging. Antioxid Redox Signal. 5:557–561. 2003. View Article : Google Scholar : PubMed/NCBI
|