Introduction
Pulmonary tuberculosis (TB) is a chronic respiratory
infectious disease caused by Mycobacterium tuberculosis
(M. tuberculosis), and has become a key public health issue
worldwide (1). The World Health
Organization reported 10 million new TB cases and 1.6 million
TB-related mortalities worldwide in 2017 (2). TB is the ninth leading cause of
mortality globally, second only to HIV/AIDS as the leading cause of
mortality for a single infectious disease (3). An accurate, rapid and easy method for
diagnosis is the key to controlling TB. At present, the gold
standard for diagnosing TB still relies on old bacteriological
tests, which are laborious and time-consuming, with a positive rate
of only 30% (4,5). Therefore, it is important to identify
novel diagnostic biomarkers with higher sensitivity and
specificity.
MicroRNAs (miRNAs/miRs) are a class of small
endogenous non-coding RNAs that are 18–22 nucleotides in length,
which regulate gene expression by binding to the 3′-untranslated
regions (3′-UTRs) of the target gene (6). miRNAs have been widely studied as
sensitive diagnostic markers in the occurrence and development of
various diseases, including cancer (7) and TB (8). Previous studies have reported that the
expression levels of numerous host miRNAs are altered in the
peripheral blood mononuclear cells (PBMCs) (9), serum (10) and macrophages (11) of patients with TB. For example,
elevated miR-423-5p expression has been revealed to serve an
important role in TB by inhibiting autophagosome-lysosome fusion
(12). Furthermore, Fu et al
(13) have shown that miR-206 could
regulate the secretion of inflammatory cytokines and the expression
of MMP9 by targeting TIMP metallopeptidase inhibitor 3 in M.
tuberculosis infected THP-1 macrophages. Wang et al
(14) have also confirmed that
miR-31 could be a potential diagnostic marker in patients with TB
by inhibiting the secretion of inflammatory cytokines. However, the
specific regulatory role of miR-125b in TB remains unknown.
The present study investigated the expression levels
of miR-125b and RAF1 in PBMCs from patients with TB, as well as the
regulatory mechanism between miR-125b and RAF1 in TB progression
in vitro. The present findings may provide new theoretical
foundation for investigating novel diagnostic biomarkers with
higher sensitivity and specificity for TB.
Materials and methods
Study subjects
A total of 40 patients with TB (23 women and 17 men)
were recruited for the study in the Shanxi Provincial Institute for
Tuberculosis Control and Prevention between December 2017 and June
2018. Among the patients with TB, 18 cases were under the age of 18
years and 22 cases were ≥18 years of age. In addition, 19 cases had
a positive sputum smear and 21 were negative. The TB diagnostic
criteria referred to were those of the ‘Clinical diagnostic
criteria and treatment guide for TB’ (15). A total of 40 healthy volunteers (23
female and 17 male; age <18 years, n=18; age ≥18 years, n=22)
with a background of Bacillus Calmette-Guérin vaccination were
recruited in the same period. None of patients with TB or the
healthy volunteers had other viral infections, autoimmune diseases,
respiratory diseases or diseases of the vital organs, such as the
kidney, heart and liver. The project was approved by the Ethics
Committee of the Shanxi Provincial Institute for Tuberculosis
Control and Prevention and it was performed in accordance with the
Declaration of Helsinki. All the recruited patients and volunteers
provided signed informed consent. The parents or guardians of
patients who were <18 years old provided this consent.
Mononuclear cell isolation
Peripheral venous blood (2 ml) from patients with TB
and healthy volunteers was drawn and placed in a tube containing
the anticoagulant EDTA. Then, PBMCs were isolated via Fycoll-Paque
plus density gradient centrifugation (450 × g, at 20°C for 20 min)
according to the manufacturer's instructions. Following three
washes with Hank's Balanced Salt Solution, PBMCs were collected,
resuspended in PBS (1 ml) and stored at −80°C until subsequent
experimentation.
Cell transfection
The miR-125b mimics (forward,
5′-UCCCUGAGACCCUAACUUG-3′ and reverse,
5′-ACAAGUUAGGGUCUCAGGGAUU-3′), miR-125b inhibitor
(5′-UCACAAGUUAGGGUCUCAGGGA-3′) and their corresponding negative
control [miR-125b mimics NC (forward, 5′-UUCUCCGAACGUGUCACGUTT-3′
and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′) and miR-125b inhibitor NC
(5′-CAGUACUUUUGUGUAGUACAA-3′)] were purchased from Shanghai
GenePharma Co., Ltd. RAF1 small interfering (si)RNA (si-RAF1-1
forward, 5′-CAUGGUAGUCACUAACAUA-3′ and reverse,
5′-UAUGUUAGUGACUACCAUG-3′; si-RAF1-2 forward,
5′-GUCAAUAAAAUGCGGGUUU-3′ and reverse, 5′-AUUAUCCUUUGGAUUCCCG-3′;
si-RAF1-3 forward, 5′-GGGUAGCACCAUCUGAAA-3′ and reverse,
5′-CAGUGCGUGUCCUGGAGU-3′) and RAF1 siRNA NC (forward,
5′-UUCUCCGAACGUGUCACGU-3′ and reverse, 5′-ACGUGACACGUUCGGAGAA-3′)
were supplied by Guangzhou RiboBio Co., Ltd. PBMCs were transfected
with the different oligonucleotides (50 nM) using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The transfected PBMCs were
randomly divided into the following groups: Blank group
(no-treatment group), miR-NC group (transfected with miR-125b
mimics NC), miR-125b mimics group (transfected with miR-125b
mimics), anti-miR-NC group (transfected with miR-125b inhibitor
NC), miR-125b inhibitor group (transfected with miR-125b
inhibitor), anti-miR-NC + si-NC group (transfected with miR-125b
inhibitor NC and siRNA NC), anti-miR-NC + si-RAF1 group
(transfected with miR-125b inhibitor NC and RAF1 siRNA), miR-125b
inhibitor + si-NC group (transfected with miR-125b inhibitor and
siRNA NC) and miR-125b inhibitor + si-RAF1 group (transfected with
miR-125b inhibitor and RAF1 siRNA). All the cells were cultured at
37°C in an incubator for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen, USA) was
used to extract total RNA from the PBMCs. Then, total RNA was
reverse-transcribed into cDNA at 42°C for 45 min using the Revert
Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific,
Inc.), and measured using a StepOne RealTime PCR (Thermo Fisher
Scientific, Inc.) with SYBR green qPCR Master mix (Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows: 95°C
for 3 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 30
sec, and final extension at 72°C for 1 min. The primers used for
RT-qPCR analysis were as follows: miR-125b forward,
5′-GCCGTAAAGTGCTGACAGT-3′ and reverse, 5′-GTGCAGGGTCCGAGGTAT-3′; U6
forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-ACGCTTCACGAATTTGCGT-3′; RAF1 forward, 5′-CCTCCAGTCCCTCATCTGAA-3′
and reverse, 5′-CTCAATCATCCTGCTGTCCA-3′; and GADPH forward,
5′-ATTGTCAGCAATGCATCCTG-3′ and reverse, 5′-GTAGGCCATGAGGTCCACCA-3′.
Expression levels were quantified using the 2−ΔΔCq
method (16). U6 was used as the
internal control in the quantitative analysis of miR-125b,
si-RAF1-1, si-RAF1-2, si-RAF1-3 and si-NC expression levels, and
GADPH was used as the internal control in the quantitative analysis
of RAF1 expression.
Western blot analysis
PBMCs were extracted using lysis buffer (Beyotime
Institute of Biotechnology) and the protein concentration was
measured using the BCA kit (Invitrogen; Thermo Fisher Scientific,
Inc.). The total proteins (50 µg) were separated via SDS-PAGE on
10% polyacrylamide gels, and transferred to nitrocellulose
membranes. Following blocking with 5% skimmed milk for 2 h at 25°C,
the membranes were incubated with the specific primary antibody,
including RAF1 (1:1,000; cat. no. ab137435; Abcam) and GAPDH
(1:1,000; cat. no. 100242-MM05; Sinopharm Chemical Reagent Co.,
Ltd.) at 4°C overnight. Subsequently, the peroxidase-labeled
secondary antibody (anti-rabbit IgG; 1:5,000; cat. no. 14708; Cell
Signaling Technology, Inc.) was used for incubation for 1 h at
37°C. The protein blots were visualized with an ECL kit (Thermo
Fisher Scientific, Inc.). Finally, the density of western blotting
bands was analyzed using a Gel-Pro analyzer (version 4.0; Media
Cybernetics, Inc.).
ELISA
The levels of TNF-α (cat. no. PDTA00D; R&D
Systems), IL-6 (cat. no. PD6050; R&D Systems), NF-κB (cat. no.
ab176647; Abcam) and IFN-γ (cat. no. ab174443; Abcam) were measured
using ELISA kits (according to manufacturer's instructions. The
absorbance of each well was measured at 450 nm using a microplate
reader (Molecular Devices LLC).
Dual luciferase reporter gene
assay
The targeted relationships between miR-125b and RAF1
was analysed using the TargetScan software (version 5.2; targetscan.org). The 3′-UTR fragment of RAF1 was
cloned and ligated into Psi-CHECK2 reporter vector (Promega
Corporation) to construct wild-type (WT) Psi-CHECK2-WT-RAF1-3′-UTR
(RAF1-WT) and mutant (MUT) Psi-CHECK2-MUT-RAF1-3′-UTR (RAF1-MUT).
Subsequently, miR-125b mimics or miR-125b mimics NC (80 ng) were
co-transfected with the reporter plasmids into PBMCs
(2×105 cells/well) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). Based on the
differences in the transfected sequences, the PBMCs were grouped as
follows: MUT + miR-125b mimics group (transfected with RAF1-MUT and
miR-125b mimics), MUT + NC group (transfected with RAF1-MUT and
miR-125b mimics NC), WT + miR-125b mimics group (transfected with
RAF1-WT and miR-125b mimics) and WT + NC group (transfected with
RAF1-WT and miR-125b mimics NC). Following 48 h transfection at
37°C, Renilla and firefly luciferase activities were
detected using a Dual-Luciferase Reporter assay system (Promega
Corporation), according to the manufacturer's protocol. The
activity of firefly luciferase was normalized to the activity of
Renilla luciferase.
Statistical analysis
All statistical analyses were performed using the
SPSS 22.0 statistical software (IBM Corp.). Data are presented as
the mean ± SD. The two-tailed t-test was used for comparison
between two groups, while one-way ANOVA followed by followed by
Tukey's post hoc test was used for comparison among multiple
groups. Pearson's correlation analysis was used to determine the
correlations between the expression levels of miR-125b and
IL-6/TNF-α/NF-κB/IFN-γ in/RAF1 in patients with TB. The diagnostic
analysis was performed via receiver operating characteristic (ROC)
curve analysis with healthy controls as true negative cases and
patients with TB as true positive cases. All experiments were
repeated three times in this study. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-125b is downregulated in the PBMCs
of patients with TB, and is a potential biomarker for TB
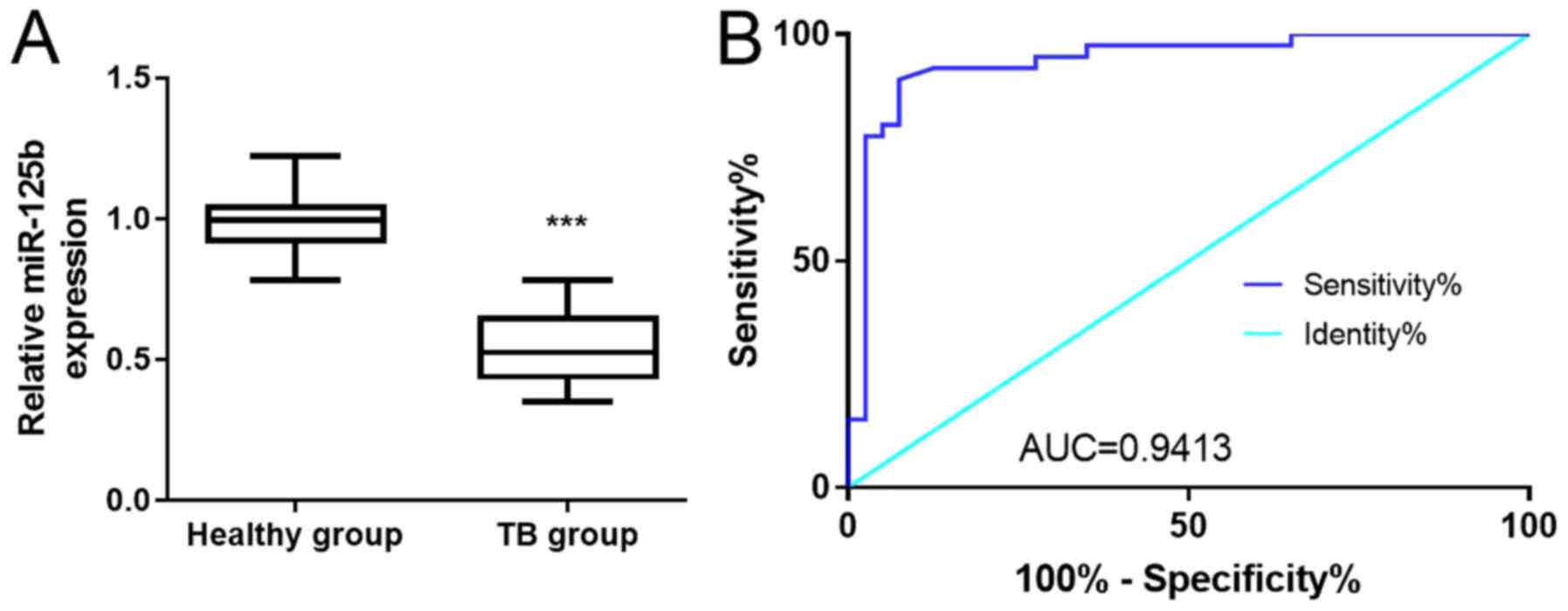
The expression of miR-125b in the PBMCs of patients
with TB and healthy volunteers was analyzed using RT-qPCR (Fig. 1A). When compared with healthy
volunteers, miR-125b expression was significantly decreased in the
patients with TB (P<0.001). Subsequently, it was assessed
whether miR-125b was a potential biomarker for TB via ROC curve
analysis (Fig. 1B). The area under
the curve (AUC) of miR-125b was 0.9413 (95% CI=88.55–99.7%). The
sensitivity and specificity of miR-125b expression for TB were 90
and 92.5%, respectively, suggesting that it possessed a high
diagnostic value. In addition, the correlation between the
expression of miR-125b and the clinical indicators in TB patients
was investigated (Table I). The
results demonstrated that the expression of miR-125b was
significantly decreased in sputum smear positive group compared
with sputum smear negative group (P<0.01). However, other
clinical factors such as age, sex and clinical classification had
no significant association with the miR-125b expression
(P>0.05). All these results suggested that miR-125b was
downregulated in the PBMCs of patients with TB, and thus, may be a
potential biomarker for TB.
 | Table I.Analysis of expression of miR-125b
and clinical parameters in patients with TB. |
Table I.
Analysis of expression of miR-125b
and clinical parameters in patients with TB.
| Parameter | Cases | miR-125b
expression | P-value |
|---|
| Age, years |
|
|
|
|
<18 | 18 | 0.558±0.139 | 0.9488 |
|
≥18 | 22 | 0.545±0.142 |
|
| Sex |
|
|
|
|
Male | 17 | 0.580±0.141 | 0.7887 |
|
Female | 23 | 0.527±0.135 |
|
| Sputum acid-fast
bacillus smear |
|
|
|
|
Negative | 21 | 0.657±0.066 | 0.0078a |
|
Positive | 19 | 0.428±0.045 |
|
| Clinical
classification |
|
|
|
| Primary
pulmonary tuberculosis | 22 | 0.510±0.110 | 0.7711 |
|
Hematogenous tuberculosis | 7 | 0.656±0.100 |
|
|
Secondary pulmonary
tuberculosis | 11 | 0.549±0.149 |
|
Levels of IL-6, TNF-α, NF-κB and IFN-γ
are negatively correlated with the expression of miR-125b in
PBMCs
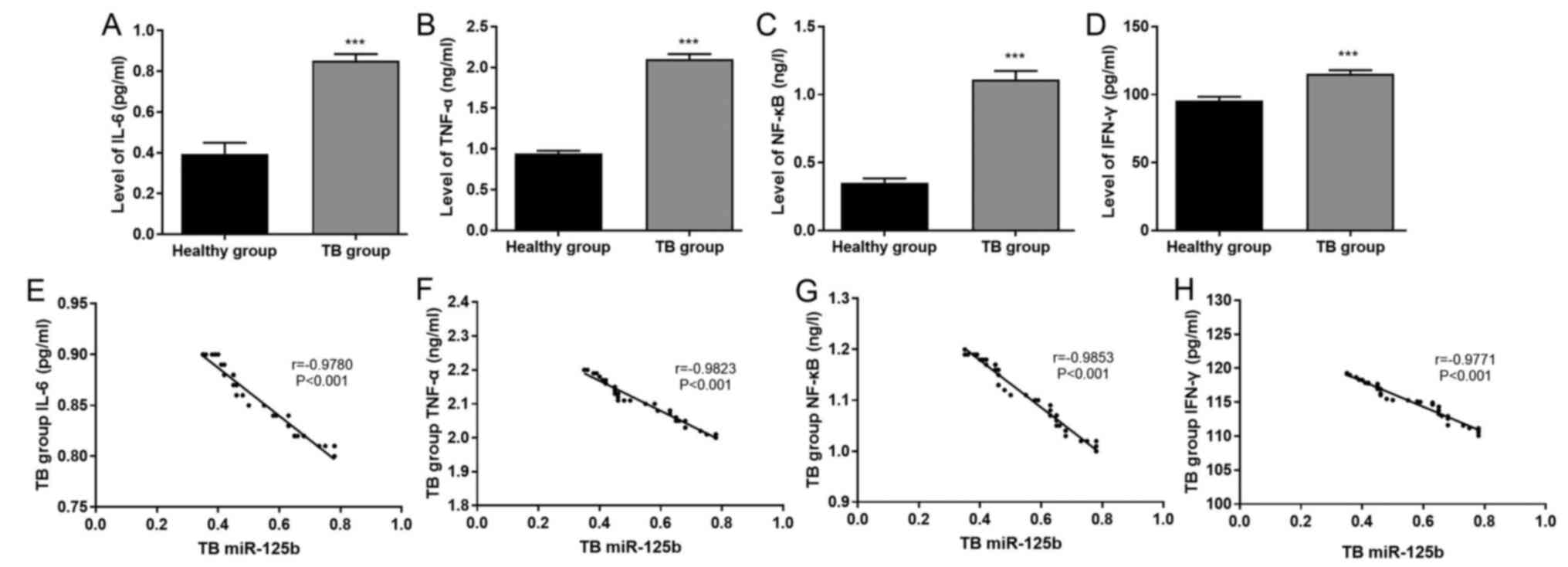
The results of ELISA demonstrated that the levels of
IL-6, TNF-α, NF-κB and IFN-γ in the TB group were significantly
increased compared with those in the healthy group (P<0.001;
Fig. 2A-D). Very strong negative
correlations were identified between miR-125b expression and the
levels of IL-6 (r=−0.9780; P<0.001), TNF-α (r=−0.9823;
P<0.001), NF-κB (r=−0.9853; P<0.001) and IFN-γ (r=−0.9771;
P<0.001) in patients with TB (Fig.
2E-H).
miR-125b decreases the levels of IL-6,
TNF-α, NF-κB and IFN-γ in PBMCs
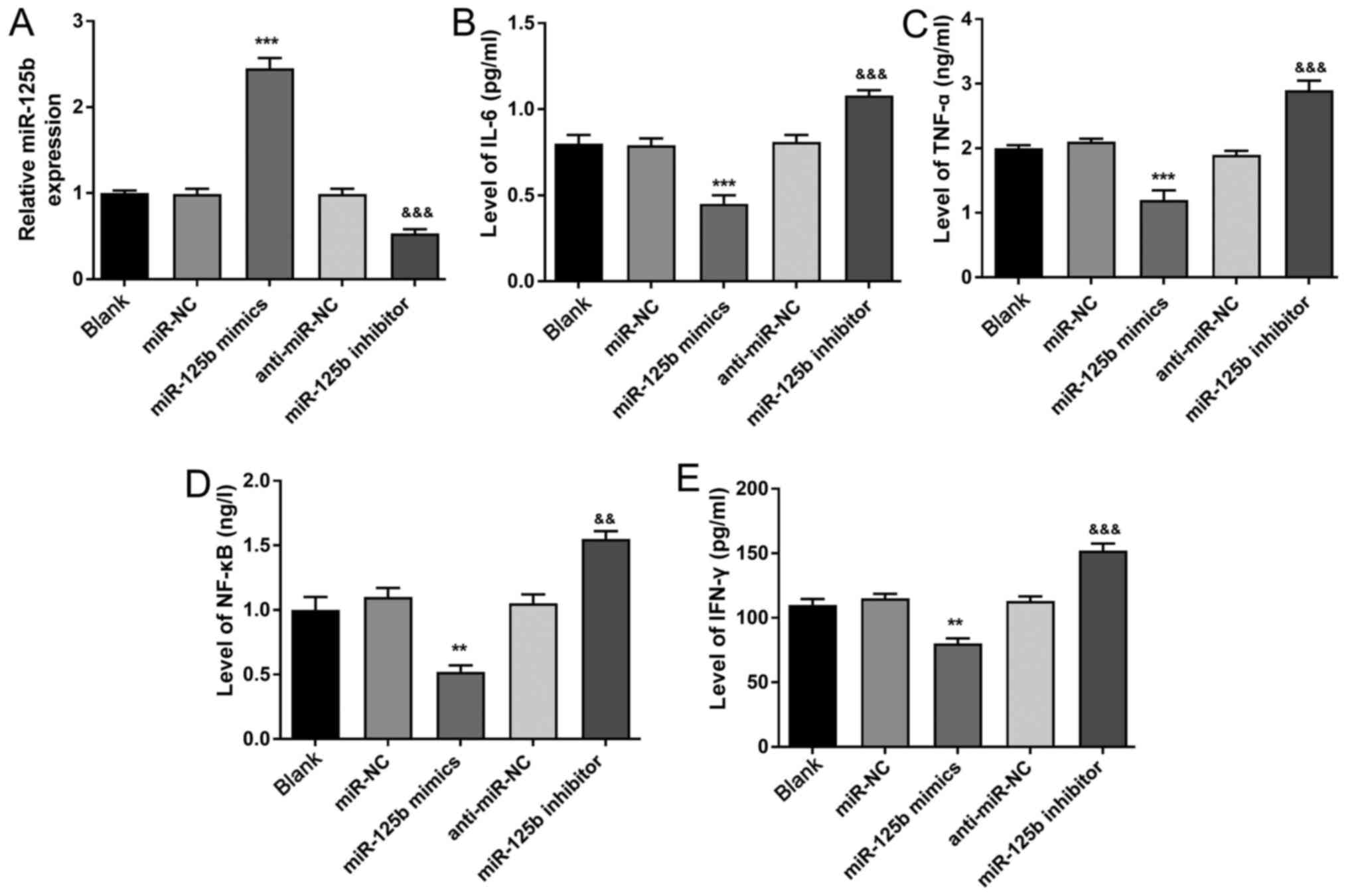
As presented in Fig.
3A, the expression of miR-125b was significantly increased in
the miR-125b mimics group compared with the miR-NC group
(P<0.001). On the contrary, miR-125b expression in miR-125b
inhibitor group was significantly lower compared with that in the
anti-miR-NC group (P<0.001), suggesting that the transfection
method was successful. The results of ELISA demonstrated that the
levels of IL-6, TNF-α, NF-κB and IFN-γ were significantly decreased
in the miR-125b mimics group compared with the miR-NC group
(P<0.01 or P<0.001). Moreover, IL-6, TNF-α, NF-κB and IFN-γ
levels in the miR-125b inhibitor group were significantly increased
compared with the anti-miR-NC group (P<0.01 or P<0.001)
(Fig. 3B-E). These results
indicated that miR-125b could decrease the levels of IL-6, TNF-α,
NF-κB, and IFN-γ in the PBMCs.
miR-125b targets the inhibition of the
expression of RAF1 in PBMCs
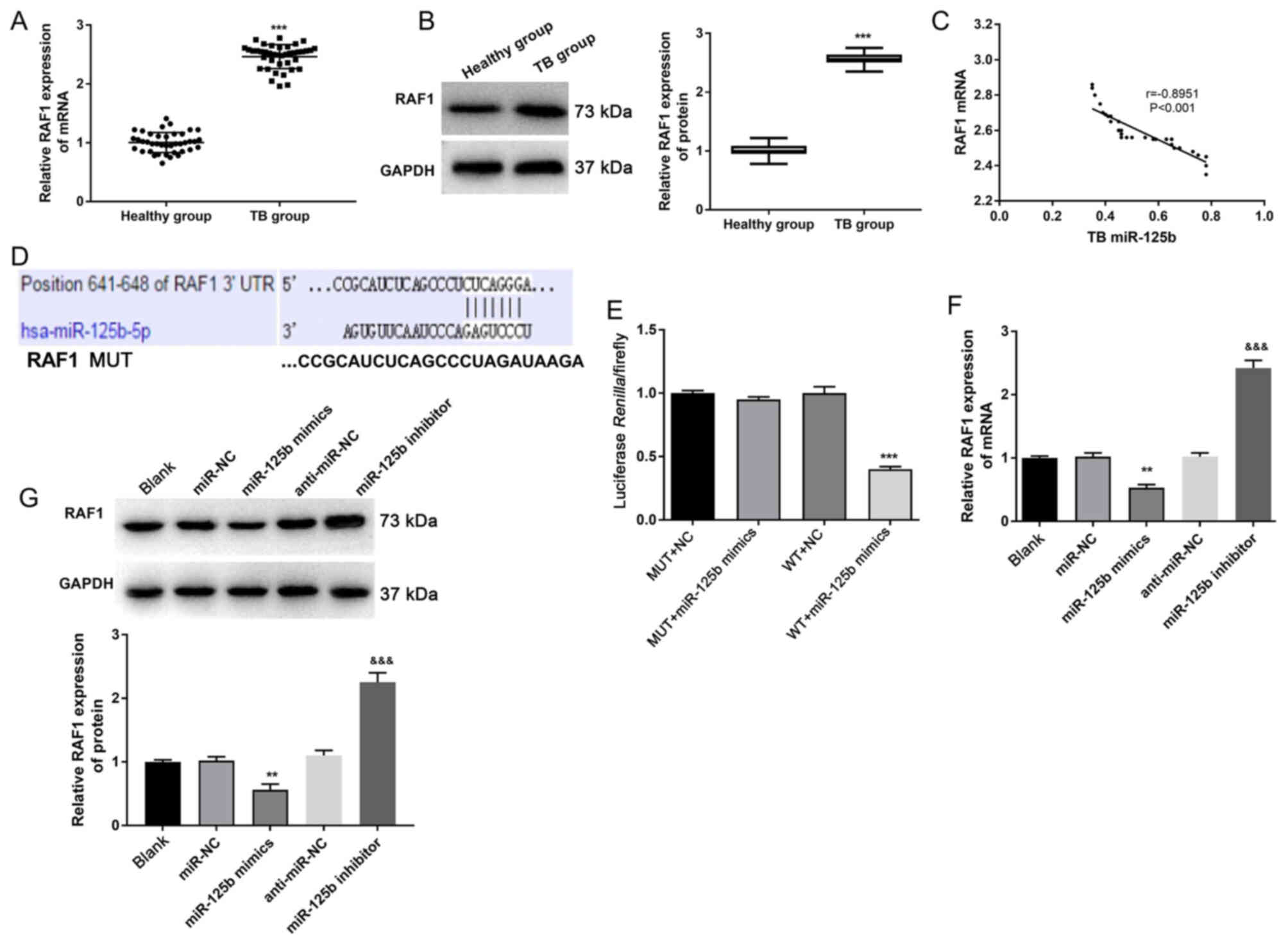
The results of both RT-qPCR and western blotting
indicated that the expression of RAF1 in the TB group was
significantly increased compared with the healthy group
(P<0.001; Fig. 4A and B).
Moreover, Pearson's correlation analysis demonstrated that the
expression of miR-125b in the PBMCs was strongly negatively
correlated with the expression of RAF1 (r=−0.8951; P<0.001;
Fig. 4C). TargetScan predicted that
the binding site of RAF1 to miR-125b was in the 3′-UTR region
(Fig. 4D). According to the
luciferase reporter assay, the luciferase activity in the WT +
miR-125b mimics group was significantly lower compared with that in
WT + NC group (P<0.001), while the difference in luciferase
activity between MUT + NC and MUT + miR-125b mimics group was not
statistically significant (Fig.
4E). Therefore, it was suggested that miR-125b directly
regulated RAF1 expression.
To further investigate whether miR-125b regulated
the expression of RAF1, RT-qPCR and western blotting were performed
(Fig. 4F and G). Both the mRNA and
protein expression levels of RAF1 in the miR-125b mimics group were
significantly lower compared with those in the miR-NC group
(P<0.01). When compared with the anti-miR-NC group, RAF1 mRNA
and protein expression levels were significantly increased in the
miR-125b inhibitor group (P<0.001). All these results indicated
that miR-125b could target the inhibition of the expression of RAF1
in PBMCs.
RAF1 reverses the roles of miR-125b in
attenuating the levels of IL-6, TNF-α, NF-κB, and IFN-γ in
PBMCs
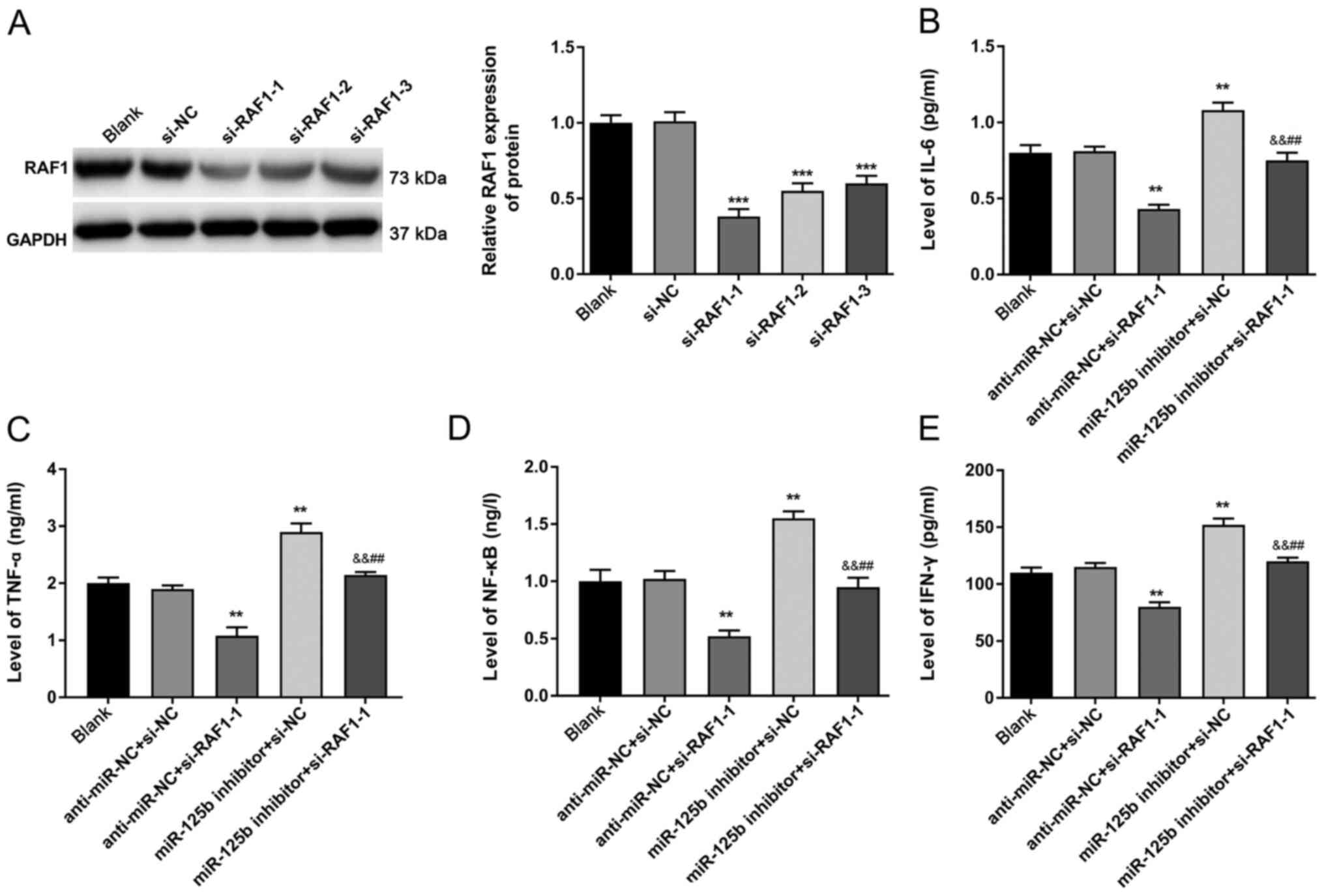
The protein expression of RAF1 was detected via
western blotting (Fig. 5A).
Compared with the si-NC group, the protein expression of RAF1 was
significantly decreased in the si-RAF1-2, si-RAF1-3 and, especially
in the si-RAF1-1 group (P<0.001). Therefore, si-RAF1 could
successfully interfere with RAF1 expression, and si-RAF1-1 was
selected for subsequent experiments.
The ELISA results (Fig.
5B-E) demonstrated that, while the levels of IL-6, TNF-α, NF-κB
and IFN-γ were significantly decreased in the anti-miR-NC +
si-RAF1-1 group compared with those in the anti-miR-NC + si-NC
group (P<0.01), their levels were significantly increased in
miR-125b inhibitor + si-NC group (P<0.01). In addition, the
levels of IL-6, TNF-α, NF-κB, and IFN-γ in the miR-125b inhibitor +
si-RAF1-1 group were significantly higher compared with those in
the anti-miR-NC + si-RAF1-1 group (P<0.01). The levels of IL-6,
TNF-α, NF-κB and IFN-γ in the miR-125b inhibitor + si-RAF1-1 group
were significantly lower compared with those in the miR-125b
inhibitor + si-NC group (P<0.01), suggesting that RAF1 could
reverse the roles of miR-125b in attenuating the levels of IL-6,
TNF-α, NF-κB and IFN-γ in PBMCs.
Discussion
China has the second largest TB incidence rate in
the world, with 4.99 million active TB cases (15). Currently, in the absence of a quick
and effective method to detect TB, 14% of patients are discharged
without complete treatment (17).
Therefore, the identification of novel diagnostic biomarkers for TB
is a priority. The present study demonstrated that miR-125b could
serve an important role in the occurrence and development of TB by
inhibiting RAF1.
Some miRNAs have been previously investigated as
potential TB biomarkers (10,18).
Peripheral venous blood is a commonly used sample in clinical
practice, and there is low risk of infection in using the
peripheral venous blood of patients with TB (19). Thus, miRNAs in the PBMCs could be
used as effective biomarkers for the diagnosis of TB (14). In addition, the expression levels of
miRNAs in the PBMCs have revealed differences between patients with
TB and healthy participants, which supports the potential
application of miRNAs in TB diagnosis (20). For instance, miR-21 and miR-223 are
differentially expressed in patients with TB compared with healthy
participants (21,22). miRNAs have been demonstrated to
serve a critical role in TB. For example, miR-423-5p serves a vital
role in the occurrence of TB by inhibiting the fusion of
autophagosomes and lysosomes (12).
In the present study, miR-125b expression was significantly
decreased in patients with TB compared with healthy volunteers,
which was consistent with previous research (21,22).
The potential of a biomarker can be assessed by analyzing the AUC
value for differentiating the test subjects as compared with the
controls (23). Previous research
has evaluated the AUC values of some miRNAs and found that miR-155
has an AUC value of 0.970 (23),
while miR-29a-3p and miR-889 have AUC values of 0.814 and 0.765,
respectively (3,10). In the present study, the ROC
analysis identified that miR-125b has an AUC value of 0.9413.
However, it is acknowledged that the sample size of the present
study was a limitation and that the sampling was not been adjusted
for confounding factors, such as age and sex. Therefore, it can
only be concluded that miR-125b has the potential as a biomarker
for TB.
NF-κB is the primarily intracellular signaling
pathway involved in inflammatory responses (24). Inflammatory cytokines including
TNF-α and IL-6 can be induced via the NF-κB signaling pathway
(25). In addition, IL-6 is
secreted during early M. tuberculosis infection, and is also
involved in anti-tuberculosis immunity (26). Yang et al (27) reported that M. tuberculosis
activates inflammatory mediators, including TNF-α. IFN-γ is a
pivotal factor in the control of M. tuberculosis infection
in murine models, and IFN-γ gene knockout mice are highly sensitive
to M. tuberculosis (28).
However, the function of IFN-γ remains unknown in humans. Previous
studies have confirmed that IFN-γ is highly expressed in the
pleural effusion of patients with TB (29). IFN-γ induces autophagy in
mycobacteria-infected cells, which is associated with protection
against M. tuberculosis (30). In the present study, the levels of
IL-6, TNF-α, NF-κB and IFN-γ were significantly increased in
patients with TB compared with healthy participants.
Accumulating evidence supports the notion that
miRNAs serve an important role in the host response to
mycobacterium-induced inflammation and immune responses, and that
they could also affect the inflammatory function in patients with
TB (31,32). Previous studies have reported that
miR-125b could protect the liver from hepatic ischemia and
reperfusion injury by inhibiting the NF-κB signaling pathway
(33). Xiao et al (34) have revealed that miR-125b could
suppress the carcinogenesis of osteosarcoma cells via the
MAPK/STAT3 signaling pathway. miR-206 has also been reported to
regulate the secretion of the inflammatory cytokines, such as IL-6,
IFN-γ, IL-1β and TNF-α, and MMP9 in TB (13). Furthermore, Wang et al
(14) observed that the expression
of miR-31 was negatively correlated with the levels of IL-6, TNF-α,
NF-κB and IFN-γ in PBMCs. However, whether miR-125b regulates
mycobacterium-induced inflammatory responses in TB is yet to be
elucidated. The present results suggested that the levels of IL-6,
TNF-α, NF-κB, and IFN-γ were negatively correlated with the
expression of miR-125b in the PBMCs of patients with TB. Moreover,
the high levels of IL-6, TNF-α, NF-κB, and IFN-γ in patients with
TB were reversed by miR-125b. These aforementioned findings are
consistent with those of previous studies (13,33).
RAF1 acts as a part of the RAF/MEK/ERK pathway, and
regulates cell cycle, migration, apoptosis and proliferation
(35). In addition, a previous
study demonstrated that RAF1 serves an important role in regulating
the transactivation property of NF-κB (36). Both the NF-κB and ERK pathways are
crucial to the expression of the inflammatory mediators, including
IL-6 and TNF-α (24,27). In the current study, the dual
luciferase reporter gene assay demonstrated that RAF1 was the
target gene of miR-125b. Furthermore, it was identified that
miR-125b targeted the inhibition of the expression of RAF1 in
PBMCs. It was also demonstrated that RAF1 reversed the roles of
miR-125b in attenuating the levels of IL-6, TNF-α, NF-κB and IFN-γ
in PBMCs.
There are, however, some limitations to the present
study. Firstly, the sample size of the study was relatively small
and limited. Next, the levels of miR-125b between the serum, plasma
and the PBMCs, and the diagnostic values of miR-125b from serum and
plasma for TB were not investigated. The experiments were conducted
with PBMC from patients with TB, followed by detection of
inflammatory factors after transfection of miR-125b mimics and
miR-125b inhibitor in vitro. However, detection of the
expression levels of the inflammatory factors of transfected PBMC
with M. tuberculosis was not performed. These limitations
will be addressed in additional investigations in the future.
In conclusion, the present study demonstrated that
miR-125b was downregulated and RAF1 was upregulated in patients
with TB. Moreover, the levels of IL-6, TNF-α, NF-κB and IFN-γ were
negatively correlated with the expression of miR-125b in the PBMCs.
The results indicated that miR-125b served an important role in the
occurrence and development of TB by decreasing the levels of IL-6,
TNF-α, NF-κB and IFN-γ via the inhibition of RAF1. Collectively,
the present study provides a new theoretical foundation for
investigating novel diagnostic biomarkers with higher sensitivity
and specificity for TB.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Health and
Family Planning Research Fund project of Shaanxi Province (grant
no. 2016D097).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and KL were involved in the conception, design
and analysis of data, as well as performed the data analyses and
wrote the manuscript. XW and TZ contributed to the conception of
the study. XL and YZ contributed significantly to data analysis and
manuscript preparation. All authors performed the experiments and
read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was conducted after obtaining
approval from the Shanxi Provincial Institute for Tuberculosis
Control and Prevention's Ethical Committee (approval no. 202008).
Written informed consent was provided by all subjects. The parents
or guardians of patients <18 years of age provided this
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giorgia S, Alberto R, Alberto M and
Raviglione MC: Tuberculosis: Epidemiology and control. Mediterr J
Hematol Infect Dis. 6:e20140702014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization (WHO), . Global
tuberculosis report 2018. WHO; Geneva: 2018
|
|
3
|
Ndzi EN, Nkenfou CN, Mekue LM, Zentilin L,
Tamgue O, Pefura EWY, Kuiaté JR, Giacca M and Ndjolo A: MicroRNA
hsa-miR-29a-3p is a plasma biomarker for the differential diagnosis
and monitoring of tuberculosis. Tuberculosis (Edinb). 114:69–76.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Small PM: Tuberculosis: A new vision for
the 21st century. Kekkaku. 84:721–726. 2009.PubMed/NCBI
|
|
5
|
Heydari AA, Movahhede Danesh MR and
Ghazvini K: Urine PCR evaluation to diagnose pulmonary
tuberculosis. Jundishapur J Microbiol. 7:e93112014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harapan H, Fitra F, Ichsan I, Mulyadi M,
Miotto P, Hasan NA, Calado M and Cirillo DM: The roles of microRNAs
on tuberculosis infection: Meaning or myth? Tuberculosis (Edinb).
93:596–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spinelli SV, Diaz A, D'Attilio L,
Marchesini MM, Bogue C, Bay ML and Bottasso OA: Altered microRNA
expression levels in mononuclear cells of patients with pulmonary
and pleural tuberculosis and their relation with components of the
immune response. Mol Immunol. 53:265–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi Y, Cui L, Ge Y, Shi Z, Zhao K, Guo X,
Yang D, Yu H, Cui L, Shan Y, et al: Altered serum microRNAs as
biomarkers for the early diagnosis of pulmonary tuberculosis
infection. BMC Infect Dis. 12:3842012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furci L, Schena E, Miotto P and Cirillo
DM: Alteration of human macrophages microRNA expression profile
upon infection with mycobacterium tuberculosis. Int J
Mycobacteriol. 2:128–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu H, Yang S, Jiang T, Wei L, Shi L, Liu
C, Wang C, Huang H, Hu Y, Chen Z, et al: Elevated pulmonary
tuberculosis biomarker miR-423-5p plays critical role in the
occurrence of active TB by inhibiting autophagosome-lysosome
fusion. Emerg Microbes Infect. 8:448–460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu X, Zeng L, Liu Z, Ke X, Lei L and Li G:
MicroRNA-206 regulates the secretion of inflammatory cytokines and
MMP9 expression by targeting TIMP3 in mycobacterium
tuberculosis-infected THP-1 human macrophages. Biochem Biophys Res
Commun. 477:167–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JX, Xu J, Han YF, Zhu YB and Zhang
WJ: Diagnostic values of microRNA-31 in peripheral blood
mononuclear cells for pediatric pulmonary tuberculosis in Chinese
patients. Genet Mol Res. 14:17235–17243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
No authors listed, . Diagnostic Standards
and Classification of Tuberculosis in Adults and Children. This
official statement of the American Thoracic Society and the Centers
for Disease Control and Prevention was adopted by the ATS Board of
Directors, July 1999. This statement was endorsed by the Council of
the Infectious Disease Society of America, September 1999. Am J
Respir Crit Care Med. 161((4 Pt 1)): 1376–1395. 2000.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Yang S, Liu CM, Jiang TT, Chen ZL,
Tu HH, Mao LG, Li ZJ and Li JC: Screening and identification of
four serum miRNAs as novel potential biomarkers for cured pulmonary
tuberculosis. Tuberculosis (Edinb). 108:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Guo J, Fan S, Li Y, Wei L, Yang
X, Jiang T, Chen Z, Wang C, Liu J, et al: Screening and
identification of six serum microRNAs as novel potential
combination biomarkers for pulmonary tuberculosis diagnosis. PLoS
One. 8:e810762013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bloom BM, Grundling J, Bestwick JP and
Harris T: The role of venous blood gas in the emergency department:
A systematic review and meta-analysis. Eur J Emerg Med. 21:81–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Yang S, Sun G, Tang X, Lu S,
Neyrolles O and Gao Q: Comparative miRNA expression profiles in
individuals with latent and active tuberculosis. PLoS One.
6:e258322011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kleinsteuber K, Heesch K, Schattling S,
Kohns M, Sander-Jülch C, Walzl G, Hesseling A, Mayatepek E,
Fleischer B, Marx FM and Jacobsen M: Decreased expression of
miR-21, miR-26a, miR-29a, and miR-142-3p in CD4(+) T cells and
peripheral blood from tuberculosis patients. PLoS One.
8:e616092013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Wang R, Jiang J, Yang B, Cao Z and
Cheng X: miR-223 is upregulated in monocytes from patients with
tuberculosis and regulates function of monocyte-derived
macrophages. Mol Immunol. 67:475–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagh V, Urhekar A and Modi D: Levels of
microRNA miR-16 and miR-155 are altered in serum of patients with
tuberculosis and associate with responses to therapy. Tuberculosis
(Edinb). 102:24–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HL, Li YX, Niu YT, Zheng J, Wu J, Shi
GJ, Ma L, Niu Y, Sun T and Yu JQ: Observing anti-inflammatory and
anti-nociceptive activities of glycyrrhizin through regulating
COX-2 and pro-inflammatory cytokines expressions in mice.
Inflammation. 38:2269–2278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Ning LP, Wang YH, Zhang Y, Ding
XL, Ge HY, Arendt-Nielsen L and Yue SW: Nuclear factor-kappa B
mediates TRPV4-NO pathway involved in thermal hyperalgesia
following chronic compression of the dorsal root ganglion in rats.
Behav Brain Res. 221:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng FM, Liu XX, Sun YH, Zhang P, Sun SF,
Zhang B, Wang XT and Lu LJ: Independent and joint effects of the
IL-6 and IL-10 gene polymorphisms in pulmonary tuberculosis among
the Chinese Han population. Genet Mol Res. 13:7766–7772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ,
Shin DM, Lee YH, Lee DS, El-Benna J and Jo EK: Reactive oxygen
species and p47phox activation are essential for the mycobacterium
tuberculosis-induced pro-inflammatory response in murine microglia.
J Neuroinflammation. 4:272007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Afum-Adjei Awuah A, Ueberberg B,
Owusu-Dabo E, Frempong M and Jacobsen M: Dynamics of T-cell IFN-γ
and miR-29a expression during active pulmonary tuberculosis. Int
Immunol. 26:579–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ribera E, Ocana I, Martinez-Vazquez JM,
Rossell M, Espanol T and Ruibal A: High level of interferon gamma
in tuberculous pleural effusion. Chest. 93:308–311. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dutta RK, Kathania M, Raje M and Majumdar
S: IL-6 inhibits IFN-γ induced autophagy in mycobacterium
tuberculosis H37Rv infected macrophages. Int J Biochem Cell Biol.
44:942–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song Q, Li H, Shao H, Li C and Lu X:
MicroRNA-365 in macrophages regulates mycobacterium
tuberculosis-induced active pulmonary tuberculosis via
interleukin-6. Int J Clin Exp Med. 8:15458–15465. 2015.PubMed/NCBI
|
|
32
|
Ma C, Li Y, Li M, Deng G, Wu X, Zeng J,
Hao X, Wang X, Liu J, Cho WCS, et al: microRNA-124 negatively
regulates TLR signaling in alveolar macrophages in response to
mycobacterial infection. Mol Immunol. 62:150–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Z, Zheng D, Pu J, Dai J, Zhang Y,
Zhang W and Wu Z: MicroRNA-125b protects liver from
ischemia/reperfusion injury via inhibiting TRAF6 and NF-κB pathway.
Biosci Biotechnol Biochem. 83:829–835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao T, Zhou Y, Li H, Xiong L, Wang J,
Wang ZH and Liu LH: MiR-125b suppresses the carcinogenesis of
osteosarcoma cells via the MAPK-STAT3 pathway. J Cell Biochem.
2018.(Epub ahead of print).
|
|
35
|
Tian H, Yin L, Ding K, Xia YY, Wang XH, Wu
JZ and He X: Raf1 is a prognostic factor for progression in
patients with nonsmall cell lung cancer after radiotherapy. Oncol
Rep. 39:1966–1974. 2018.PubMed/NCBI
|
|
36
|
Baumann B, Weber CK, Troppmair J,
Whiteside S, Israel A, Rapp UR and Wirth T: Raf induces NF-kappaB
by membrane shuttle kinase MEKK1, a signaling pathway critical for
transformation. Proc Natl Acad Sci USA. 97:4615–4620. 2000.
View Article : Google Scholar : PubMed/NCBI
|