Introduction
As a chronic degenerative joint disease, the
characteristics of osteoarthritis (OA) are degeneration of
articular cartilage, subchondral bone sclerosis and bone
hyperplasia (1). OA affects an
estimated 10% of men and 18% of women >60 years of age,
worldwide (2). OA is affected by
multiple factors, such as age, sex, trauma history, obesity,
heredity and joint deformity (3).
At present, the pathogenesis of OA remains largely unknown. It is
currently considered that the degeneration of articular cartilage
in OA is due to a decrease in the number of chondrocytes, as well
as a degradation of cartilage extracellular matrix stimulated by
cytokines and growth factors (4).
With the destruction of articular cartilage, the patient develops
joint pain, stiffness and ultimately loss of mobility. Early drug
relief symptoms and advanced joint replacement are the main
treatments for OA (5). However,
there are disadvantages, such as it is not applicable to all
patients, and complications include instability, dislocation,
infection, loosening, lysis and fracture, as well as adverse
reactions to these treatments. Therefore, it is critical to
investigate the molecular mechanisms underlying the development of
OA and to provide markers of early OA diagnosis and bio-therapeutic
targets.
MicroRNAs (miRNAs/miRs) are a class of non-coding
short sequence RNAs of 18–25 nucleotides in length and without an
open reading frame, which are widely found in eukaryotes (6). With the progress of research, the
miRNA maturation process and its functional roles have gradually
been identified. Incomplete base pairing between the miRNA and the
3′-untranslated region (3′-UTR) of the target mRNA can inhibit the
expression of the target mRNA, and a complete complementary
interaction between miRNA and target mRNA can result in mRNA
degradation (7). miRNA also serves
a regulatory role in the pathophysiology of human life, such as
viral defense, cell proliferation and apoptosis, tumorigenesis,
invasion and migration (8). Studies
have reported that miRNAs serve key roles in maintaining
homeostasis in the cartilage (9,10).
There is a significant difference of miRNA expression (such as
miR-146a-5p, miR-140 and miR-27b) in chondrocytes between healthy
individuals and patients with OA, which causes an imbalance of
chondrocyte synthesis and catabolism that leads to the development
of OA (11–13). These differentially expressed miRNAs
may serve as predictive biomarkers for OA or potential targets for
targeted therapies (13,14).
miR-186-5p has been reported to be associated with
numerous physiological processes, including migration, invasion,
proliferation and inflammation, as well as the development of a
number of diseases, such as cancer, ischemia stroke and diabetic
cardiomyopathy (15–17). miR-186-5p has been studied in
several malignancies, including non-small cell lung cancer
(15), glioma (18), hepatocellular carcinoma (19), prostate cancer (20), lung adenocarcinoma (21), osteosarcoma (22) and ovarian cancer (23). A previous study revealed that
miR-186 is downregulated in OA and its inhibition could block
chondrocyte apoptosis in mice with OA (24). However, to the best of our
knowledge, the role of miR-186-5p in OA development is yet to be
fully elucidated.
The aim of the present study was to investigate the
roles of miR-186-5p in the development of OA, as well as identify
the potential molecular mechanisms involved, in order to provide a
theoretical basis for OA treatment and a novel perspective for
clinical therapy. Currently, IL-1β-induced inflammatory injury has
been widely used to investigate OA in vitro, and the human
chondrocyte cell line CHON-001 is often used to establish a model
of chondrocyte inflammation injury (25–27).
Thus, in the present study, the role of miR-186-5p in IL-1β-induced
CHON-001 cell inflammatory injury was examined, in order to
evaluate the role of miR-186-5p in OA development.
Materials and methods
Cell culture and treatment
The human chondrocyte cell line CHON-001, derived
from healthy human articular cartilage, was obtained from the
American Type Culture Collection. Cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 0.1
mg/ml G-418 (Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), in a humidified atmosphere
with 5% CO2 at 37°C and passaged at a ratio of 1:5.
The recombinant human IL-1β (R&D Systems, Inc.;
0.1, 2, 5 and 10 ng/ml) was used to treat the CHON-001 cells at
37°C for 12 h to induce cell inflammatory injury.
Cell transfection
CHON-001 cells were transfected with 100 nM
miR-186-5p inhibitor (5′-AGCCCAAAAGGAGAAUUCUUUG-3′; Guangzhou
RiboBio Co., Ltd.), 100 nM inhibitor control
(5′-GCCUCCGGCUUCGCACCUCU-3′; Guangzhou RiboBio Co., Ltd.), 0.2 µM
MAPK1-small interfering RNA (siRNA; cat no. sc-35335; Santa Cruz
Biotechnology, Inc.), 0.2 µM control-siRNA (cat no. sc-36869; Santa
Cruz Biotechnology, Inc.), 100 nM miR-186-5p inhibitor + 0.2 µM
control-siRNA or 100 nM miR-186-5p inhibitor + 0.2 µM MAPK1-siRNA,
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. At 48
h post-transfection, reverse transcription-quantitative PCR
(RT-qPCR) was performed to detect the transfection efficiency. A
total of 24 h after cell transfection, the cells were subjected to
treatment with 10 ng/ml IL-1β at 37°C for 12 h, and further
experiments were then performed.
Cell viability assay
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay according
to the manufacturer's instructions. CHON-001 cells were seeded in a
96-well plate at a density of 5×103 cells per well.
Following administration, the CCK-8 solution (10 µl) was added to
the culture medium, and cells were incubated for 1 h at 37°C in
humidified 95% air and 5% CO2. The absorbance was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Cell apoptosis assay
Cell apoptosis analysis was performed using an
Annexin V-FITC and PI apoptosis detection kit (Beyotime Institute
of Biotechnology) according to the manufacturer's instructions.
Following treatment, cells (106) were collected and
washed in PBS. Cells were then suspended in binding buffer
containing 10 µl Annexin V-FITC and 5 µl PI, which was followed by
incubation for 1 h at room temperature in the dark. Flow cytometry
analysis was performed using a FACScan flow cytometer (Beckman
Coulter, Inc.) to detect cell apoptosis (the percentage of early +
late apoptotic cells). Data were analyzed using FlowJo software
(version 7.6.1; FlowJo LLC).
ELISA
ELISA was used to detect the levels of TNF-α, IL-6
and IL-8 in the supernatant of CHON-001 cell culture medium. The
supernatant of CHON-001 cell culture medium was collected via
centrifugation (500 × g; 5 min; 4°C). ELISA kits (Beyotime
Institute of Biotechnology) were used to detect TNF-α (cat. no.
PT518), IL-6 (cat. no. PI330) and IL-8 (cat. no. PI640) release in
the supernatant of cells, following the manufacturer's instructions
of each kit. To calculate the corresponding concentration of the
sample, the A450 value was detected using the FLUOstar®
Omega Microplate reader (BMG Labtech GmbH) (28).
Determination of caspase-3
activity
Trypsin was used to detach the treated CHON-001
cells from the culture medium. CHON-001 cells were collected via
centrifugation (600 × g; 4°C; 5 min). Subsequently, caspase-3
activity was determined using caspase-3 activity assay kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. To evaluate the caspase-3 activity, the
wavelength at 405 nm was detected using an automatic microplate
reader (ELX800; BioTek Instruments Inc.). In total, one unit is the
amount of enzyme that will cleave 1.0 nmol of the colorimetric
substrate Ac-DEVD-pNA
[L-Asparagine,N-acetyl-L-a-aspartyl-L-a-glutamyl-L-valyl-N-(4-nitrophenyl)-(9CI)]
per h at 37°C under saturated substrate concentrations (29).
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate the total RNA from
treated CHON-001 cells, following the manufacturer's instructions.
miScript II Reverse Transcription kit (Qiagen GmbH) and miSCRIPT
SYBR-Green PCR kit (Qiagen GmbH) were used to analyze miRNA
expression as per the manufacturer's protocols. For mRNA detection,
PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used
for RT, and then qPCR analysis was performed using the SYBR Premix
Ex Taq™ II (Tli RNaseH Plus) kit (Takara Bio, Inc.), according to
the manufacturer's protocol. The reaction conditions for RT were as
follows: 70°C for 5 min, 37°C for 5 min and 42°C for 60 min. The
following thermocycling conditions were used for the qPCR: Initial
denaturation for 5 min at 95°C; followed by 37 cycles of
denaturation at 95°C for 10 sec, annealing at 60°C for 30 sec and
extension at 72°C for 34 sec. U6 was used to normalize the
expression of miR-186-5p, and GAPDH was used to normalize MAPK1
mRNA expression. The primer sequences used for the PCR were listed
as follows: U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and
reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′; miR-186-5p forward,
5′-AAGAATTCTCCTTTTGGGCT-3′ and reverse, 5′-GTGCGTGTCGTGGAGTCG-3′;
and MAPK1 forward, 5′-GTCGCCATCAAGAAAATCAGC-3′, and reverse
5′-GGAAGGTTTGAGGTCACGGT-3′. The 2−ΔΔCq method (30) was used to calculate the expression
of target genes.
Dual-luciferase reporter gene
assay
TargetScan 7.2 (http://www.targetscan.org/vert_72/) was used to
predict the target of miR-186-5p, and the results suggested a
binding site of miR-186-5p in the 3′-UTR of the MAPK1 gene.
Subsequently, it was verified that MAPK1 was a target gene of
miR-186-5p using the dual-luciferase reporter gene assay. The
pmirGLO vector (1 ng; Promega Corporation) containing a mutant type
or wild-type 3′-UTR of MAPK1 was co-transfected with the 100 nM
miR-186-5p mimic or 100 nM mimic control into CHON-001 cells for 48
h using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
relative luciferase activity was measured using a Dual-Luciferase
reporter assay system (Promega Corporation), according to the
manufacturer's protocols. Luciferase activity was normalized to the
Renilla luciferase activity.
Western blot analysis
RIPA lysis buffer (Beyotime Institute of
Biotechnology) and protease inhibitor (Beyotime Institute of
Biotechnology) were used to extract the proteins from cells. A BCA
protein assay was then used to quantify the proteins. Equal amounts
of protein (35 µg/lane) were separated via 12% SDS-PAGE and then
transferred to PVDF membranes. Subsequently, the membranes were
blocked with 5% non-fat milk at room temperature for 90 min and
incubated with primary antibodies: MAPK1 (cat. no. ab205819;
1:1,000; Abcam) and GAPDH (cat. no. 5174; 1:1,000; Cell Signaling
Technology, Inc.) at 4°C overnight. This was followed by incubation
with anti-rabbit horseradish peroxidase-conjugated immunoglobulin G
secondary antibody (cat no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) at room temperature for 2 h. Finally, an enhanced
chemiluminescence detection system (Applygen Technologies, Inc.)
was used to observe the protein bands. For densitometry detection,
analysis with ImageJ 1.38X software (National Institutes of Health)
was performed.
Statistical analysis
All experiments were repeated three times, and data
are presented as the mean ± SD. GraphPad 6.0 (Graph Pad Software,
Inc.) was used to perform the statistical analyses, and unpaired
Student's t-test or one-way ANOVA with Tukey's post hoc test were
used to analyze differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
IL-1β induces chondrocyte inflammatory
injury and promotes miR-186-5p expression in vitro
Different concentrations of IL-1β (0.1, 2, 5 and 10
ng/ml) were used to treat human chondrocyte CHON-001 cells for 12
h. The results demonstrated that the treatments with 5 and 10 ng/ml
IL-1β significantly decreased CHON-001 cell viability (Fig. 1A). Therefore, 5 and 10 ng/ml IL-1β
were used as the effective concentrations for the further
experiments. Subsequently, cell apoptosis assay results suggested
that the treatment with 5 and 10 ng/ml IL-1β significantly induced
CHON-001 cell apoptosis (Fig. 1B and
C), as well as promoted the activity of caspase-3 in CHON-001
cells (Fig. 1D).
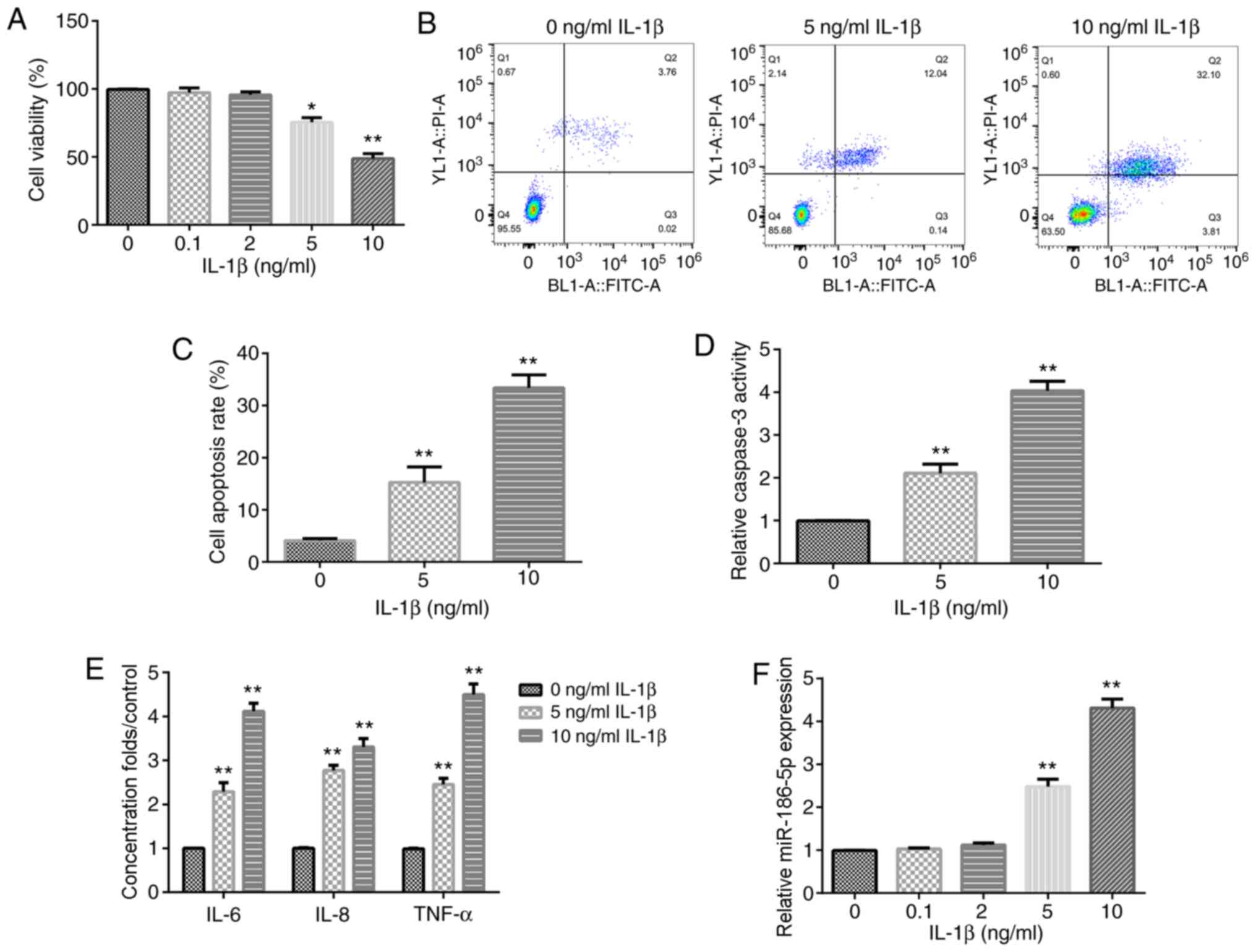 | Figure 1.Effect of IL-1β on chondrocyte
inflammation and miR-186-5p expression. Different concentrations of
IL-1β (0.1, 2, 5 and 10 ng/ml) were used to treat CHON-001 cells
for 12 h, and then Cell Counting Kit-8 analysis was used to detect
cell viability. (A) Effect of different concentrations of IL-1β on
CHON-001 viability. (B) Flow cytometric analysis was used to
examine the (C) effect of 5 and 10 ng/ml IL-1β on CHON-001 cell
apoptosis. (D) Effect of 5 and 10 ng/ml IL-1β on the activity of
caspase-3. (E) Effect of 5 and 10 ng/ml IL-1β on the levels of
three inflammation cytokines, TNF-α, IL-8 and IL-6, was determined
using ELISA. (F) Reverse transcription-quantitative PCR was used to
detect the effect of IL-1β (0.1, 2, 5 and 10 ng/ml) on miR-186-5p
expression. *P<0.05, **P<0.01 vs. 0 ng/ml IL-1β. miR-186-5p,
microRNA-186-5p. |
To confirm that treatment with IL-1β could induce
the inflammatory response of CHON-001 cells, the levels of TNF-α,
IL-8 and IL-6 were then examined. It was found that following
exposure to IL-1β (5 or 10 ng/ml), the levels of TNF-α, IL-8 and
IL-6 were increased (Fig. 1E).
These results indicated that treatment with IL-1β induced
inflammatory injury of CHON-0001 cells. In addition, it was
identified that the expression of miR-186-5p was significantly
increased in IL-1β-treated CHON-0001 cells at concentrations of 5
and 10 ng/ml (Fig. 1F).
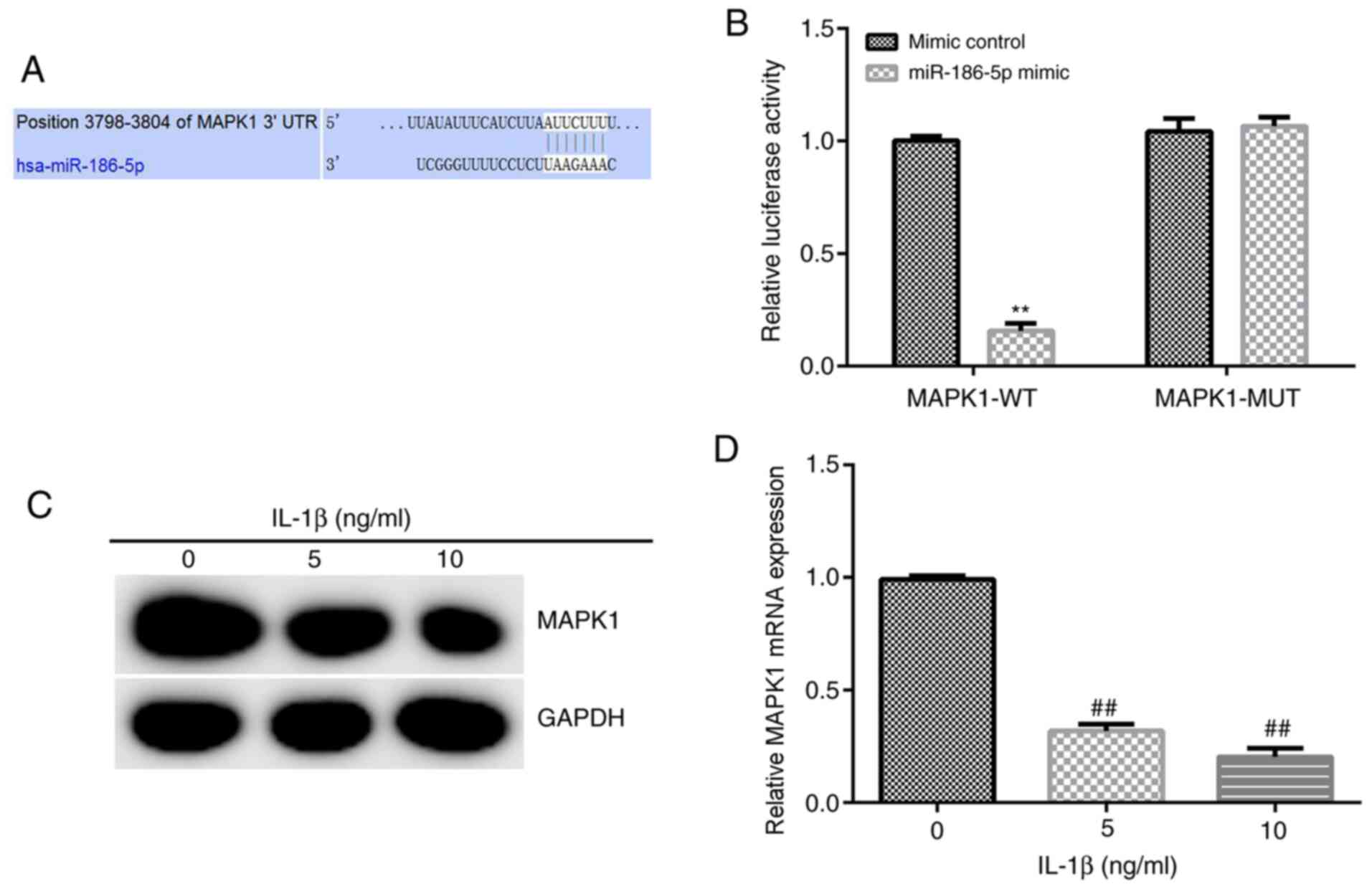
MAPK1 is a target gene of miR-186-5p,
and its expression is decreased significantly in chondrocytes
treated with IL-1β
Based on the information provided by TargetScan,
binding sites between miR-186-5p and MAPK1 were identified
(Fig. 2A), and then a
Dual-Luciferase reporter gene assay was used to verify these
results. miR-186-5p overexpression significantly decreased the
luciferase activity of the wild-type MAPK1 3′-UTR reporter.
However, miR-186-5p overexpression had no significant effect on the
luciferase activity of the mutant MAPK1 3′-UTR reporter (Fig. 2B). Overall, these results suggested
that MAPK1 was a direct target gene of miR-186-5p.
Subsequently, 5 and 10 ng/ml IL-1β was used to treat
CHON-001 cells for 12 h, and the expression of MAPK1 was detected
using RT-qPCR and western blot analysis. It was demonstrated that 5
and 10 ng/ml IL-1β significantly decreased the expression of MAPK1
in CHON-001 cells (Fig. 2C and
D).
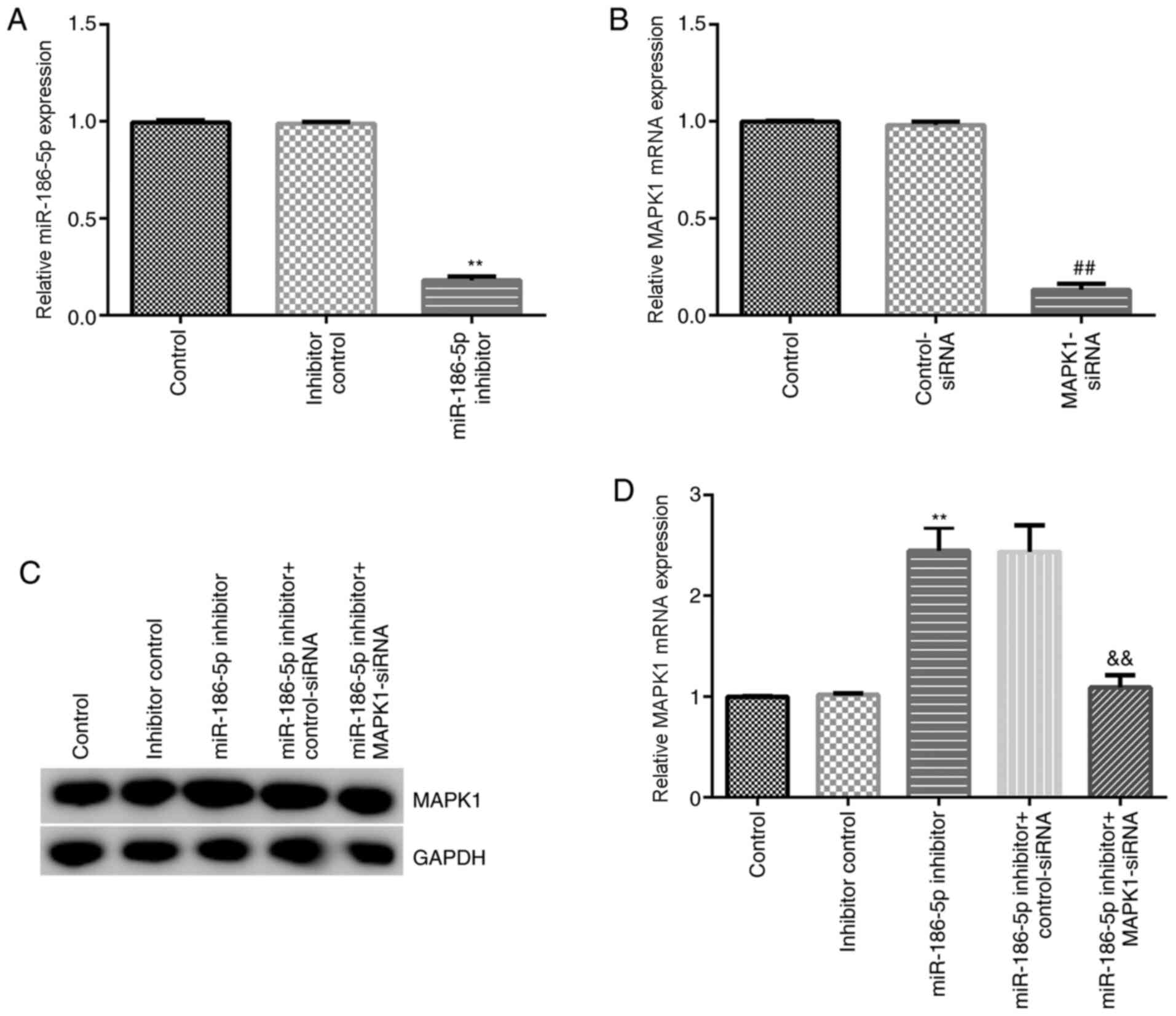
Inhibition of miR-186-5p decreases the
chondrocyte inflammatory injury induced by IL-1β
The effect of miR-186-5p inhibition on chondrocytes
induced by IL-1β (10 ng/ml) was then investigated. Inhibitor
control, miR-186-5p inhibitor, MAPK1-siRNA, control-siRNA,
miR-186-5p inhibitor + control-siRNA and miR-186-5p inhibitor +
MAPK1-siRNA were transfected into CHON-001 cells. RT-qPCR results
demonstrated that miR-186-5p inhibitor significantly decreased the
expression of miR-186-5p in CHON-001 cells (Fig. 3A). MAPK1-siRNA significantly
inhibited MAPK1 mRNA expression in CHON-001 cells (Fig. 3B). Furthermore, the miR-186-5p
inhibitor significantly increased protein and mRNA expression
levels of MAPK1 in CHON-001 cells, which was reversed by
MAPK1-siRNA (Fig. 3C and D).
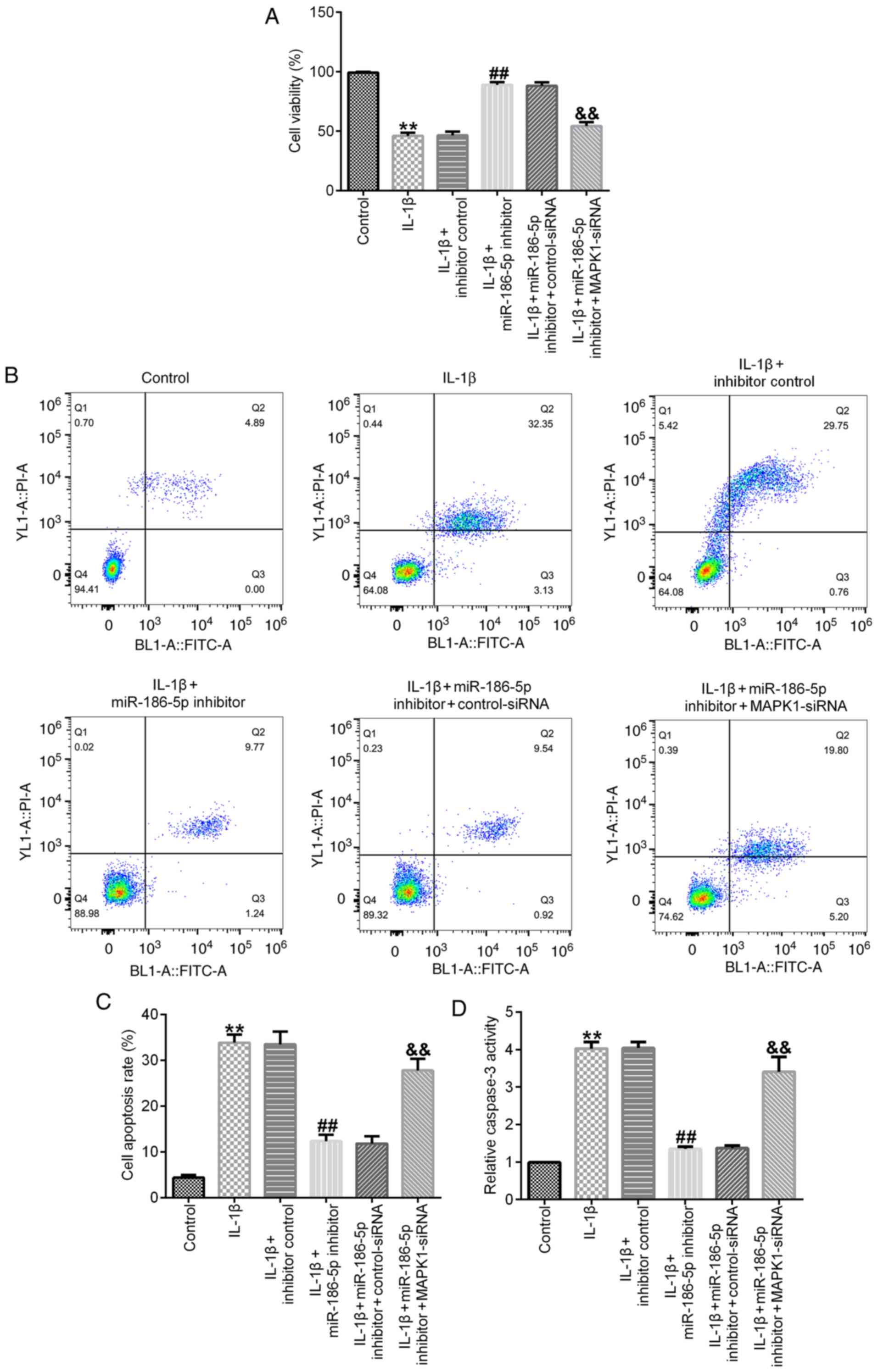
Results of the cell viability assay demonstrated
that, compared with the IL-1β (10 ng/ml) treatment group,
transfection with miR-186-5p inhibitor significantly increased cell
viability (Fig. 4A), decreased cell
apoptosis (Fig. 4B and C) and
inhibited the activity of caspase-3 (Fig. 4D). All these changes were
significantly reversed by MAPK1-siRNA (Fig. 4A-D).
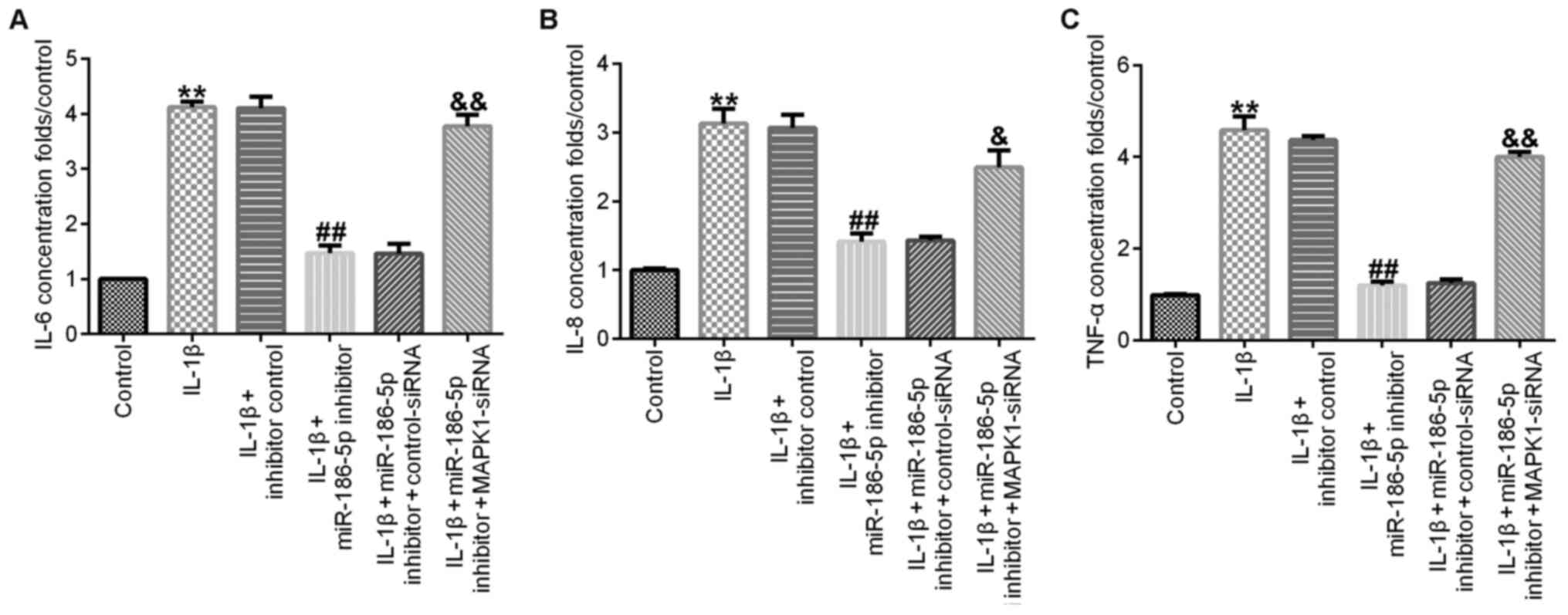
Compared with the IL-1β (10 ng/ml) treatment group,
transfection with miR-186-5p inhibitor significantly decreased the
release of IL-6 (Fig. 5A), IL-8
(Fig. 5B) and TNF-α (Fig. 5C) in CHON-001 cell culture medium,
and this effect was significantly reversed by MAPK1-siRNA.
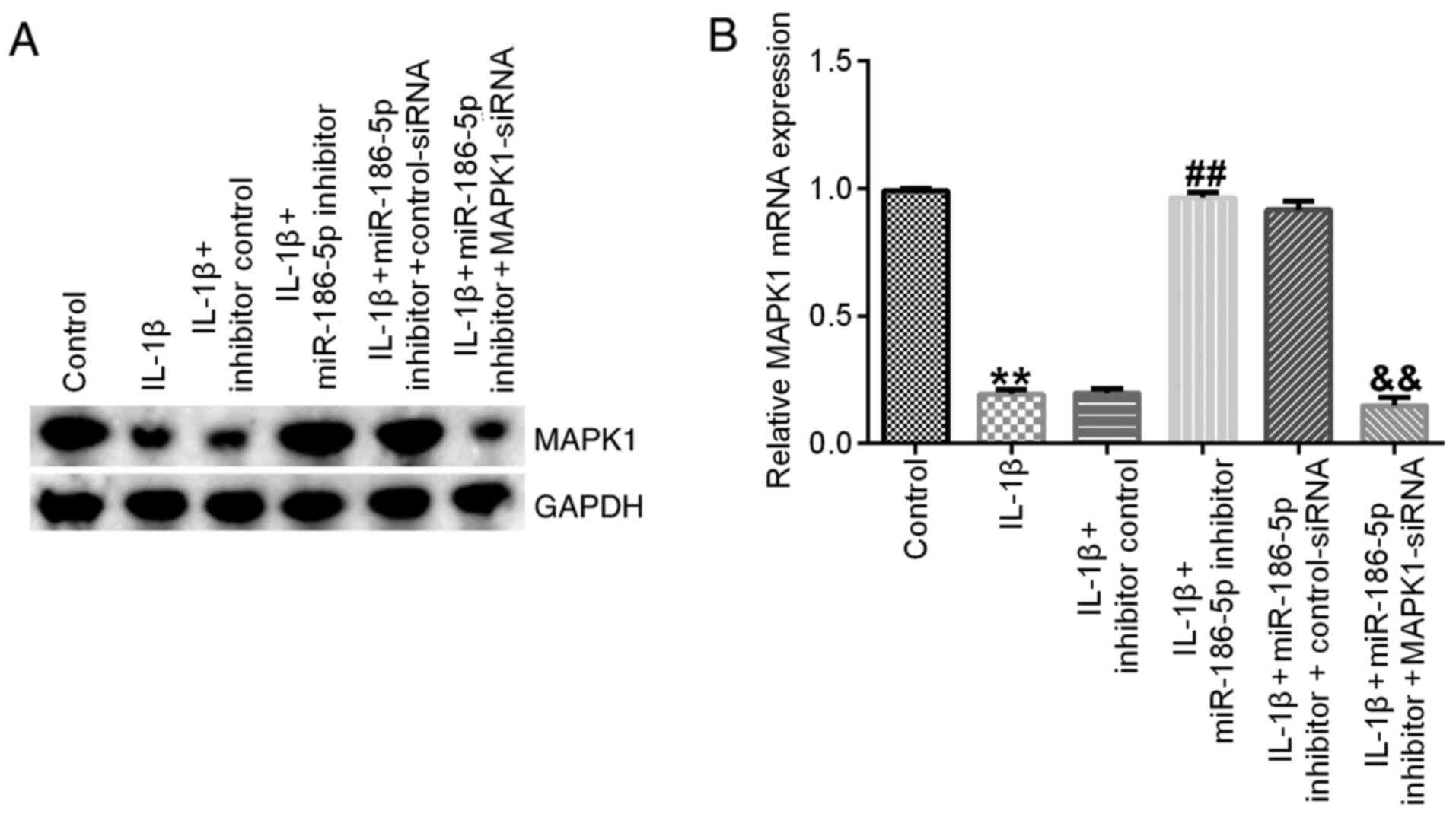
Finally, it was identified that, compared with the
IL-1β (10 ng/ml) treatment group, miR-186-5p inhibitor transfection
significantly increased the expression of MAPK1, which was
significantly reversed by MAPK1-siRNA (Fig. 6).
Discussion
A key pathogenic factor of OA is inflammation; it
has been reported that there is a positive correlation between OA
severity and the expression of the pro-inflammatory cytokine IL-1β
(31).
The present study established an OA model using
CHON-001 chondrocytes induced by IL-1β in vitro. The results
suggested that 5 and 10 ng/ml IL-1β could induce effects of cell
inflammation, such as decreasing cell viability, as well as
promoting cell apoptosis and the expression levels of inflammatory
factors.
A previous study reported that miRNAs regulate 100s
of genes, such as ADAM metallopeptidase with thrombospondin type 1
motif 5, MMP-13 and insulin like growth factor binding protein 5)
that are involved in homeostasis, cartilage development and OA
pathology (32). Due to their
ability to regulate cell apoptosis and reactive oxygen species,
miRNAs serve important roles in the abnormal autophagy response of
OA chondrocytes (33). Wu et
al (33) reviewed numerous
studies and revealed that >25 types of miRNAs are involved in
the development of cartilage and OA, particularly in regulating
proteolytic enzyme synthesis and chondrocyte hypertrophy. In
addition, certain OA cartilage signal transduction pathways are
regulated by miRNAs, such as the TGF-β, bone morphogenetic protein
family, inducible nitric oxide synthase IL-1, MMP and TNF-α
pathways (34). Cong et al
(35) reviewed published reports
and reported that numerous miRNAs are differentially expressed in
OA, where upregulated miRNAs are mainly involved in biological
processes occurring in the nucleus, and downregulated miRNAs are
primary involved in the transcriptional process, indicating that
miRNAs exert key roles in the beginning and development of OA.
Specifically, miR-140, miR-9, miR-34a, miR-558, miR-27, miR-602 and
miR-146a are abnormally expressed in OA and serve important roles
in the pathological processes of OA (36).
The present study identified that in chondrocytes
administrated IL-1β, miR-186-5p expression was upregulated. This
aberrant expression suggested that miR-186-5p may regulate the
inflammatory response in chondrocytes. miR-186-5p is tumor
specific, and it serves a carcinogenic or inhibitory role in
different tumors (19,21–23).
miR-186-5p has different effects on the regulation of apoptosis in
multiple types of cells or under varying conditions (16,17,24,37).
For example, miR-186-5p promotes apoptosis in an oxygen and glucose
deprivation/reperfusion cell model (16), while miR-186-5p attenuates high
glucose-induced apoptosis by regulating Toll-like receptor 3 in
cardiomyocytes (37). Inhibition of
miR-186-5p contributes to high glucose-induced cytotoxicity and
apoptosis in AC16 cardiomyocytes (17). Moreover, miR-186 has been reported
to inhibit primary mouse chondrocyte apoptosis (24). The present study also investigated
the effect of miR-186-5p on the inflammatory injury of chondrocytes
induced by IL-1β. Contrary to previous findings that miR-186
upregulation inhibits chondrocyte apoptosis (24), the present results suggested that
the miR-186-5p inhibitor suppressed chondrocyte apoptosis induced
by IL-1β. This opposite result may be due to the different
environmental conditions of the chondrocytes. The previous study
focused on investigating the effect of miR-186 on primary mouse
chondrocyte apoptosis (24), while
the current study examined the effects of miR-186-5p on IL-1β
induced human chondrocyte cell (CHON-001) apoptosis. This
controversy requires additional in-depth study.
The present results indicated that the miR-186-5p
inhibitor repressed the inflammatory response in IL-1β induced
CHON-001 cells, suggesting that a treatment strategy for OA may be
the downregulation of miR-186-5p. Moreover, the present findings
demonstrated that miR-186-5p may negatively regulate MAPK1
expression in the inflammatory response of chondrocytes, ultimately
affecting OA. It is worth noting that IL-1β can induce production
of inflammatory cytokines, such as IL-6 and TNF-α, by activating
p38 MAPK signaling in chondrocytes (38,39).
Park et al (38) reported
that IL-1β-induced MAPKs activation in SW1353 chondrocytes, while
Sun et al (40) revealed
that IL-1β treatment significantly activated the p38, JNK and ERK
pathway in primary chondrocytes. However, the present study
demonstrated IL-1β inhibited MAPK1 expression in CHON-001 cell
line, and this effect was reversed by miR-186-5p inhibitor. In
fact, CHON-001 has different features from the primary chondrocytes
(41). The present study only used
the CHON-001 cell line, and >1 cell line, such as primary
chondrocytes, should be included in future studies to further
validate the current results; this was a limitation of the present
study. Moreover, chondrocytes may change their phenotype during
culturing, and whether CHON-001 cells remained as chondrocytes was
not identified in the current study, which was another
limitation.
In conclusion, the present study used IL-1β to
stimulate human inflammatory chondrocytes in vitro, and it
was identified that upregulated miR-186-5p may regulate the
inflammatory response. Furthermore, it was demonstrated that
suppression of miR-186-5p could treat OA by increasing MAPK1
expression.
Acknowledgements
Not applicable.
Funding
This study was supported by the Sanming Project of
Medicine in Shenzhen (grant no. SZSM201612019), the Shenzhen Key
Laboratory of Digital Surgical Printing Project (grant no.
ZDSYS201707311542415) and the Southern Medical University Clinical
Start-up Fund (grant no. LC2016ZD036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL contributed to the study design, the data
collection, data interpretation and the manuscript preparation. MW,
GF, KL, WC and LL contributed to the data collection, the
statistical analysis and the data interpretation. XL, JW and YC
contributed to the manuscript preparation and statistical analysis,
data interpretation and literature search. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xia B, Chen D, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang W, Zhong B, Zhang C, Luo C and Zhan
Y: miR-373 regulates inflammatory cytokine-mediated chondrocyte
proliferation in osteoarthritis by targeting the P2X7 receptor.
FEBS Open Bio. 8:325–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L,
Zhou C and Ao Y: Long noncoding RNA related to cartilage injury
promotes chondrocyte extracellular matrix degradation in
osteoarthritis. Arthritis Rheumatol. 66:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun T, Yu J, Han L, Tian S, Xu B, Gong X,
Zhao Q and Wang Y: Knockdown of long non-coding RNA RP11-445H22.4
alleviates LPS-induced injuries by regulation of MiR-301a in
osteoarthritis. Cell Physiol Biochem. 45:832–843. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He W and Cheng Y: Inhibition of miR-20
promotes proliferation and autophagy in articular chondrocytes by
PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 97:607–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Towler BP, Jones CI and Newbury SF:
Mechanisms of regulation of mature miRNAs. Biochem Soc Trans.
43:1208–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Pan X, Zhang J, Ma A, Yang S, Ma J
and Xie A: Plasma levels of miR-137 and miR-124 are associated with
Parkinson's disease but not with Parkinson's disease with
depression. Neurol Sci. 38:761–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le TT, Swingler TE, Crowe N, Vincent TL,
Barter MJ, Donell ST, Delany AM, Dalmay T, Young DA and Clark IM:
The microRNA-29 family in cartilage homeostasis and osteoarthritis.
J Mol Med (Berl). 94:583–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endisha H, Rockel J, Jurisica I and Kapoor
M: The complex landscape of microRNAs in articular cartilage:
Biology, pathology, and therapeutic targets. JCI Insight.
3:e1216302018. View Article : Google Scholar
|
|
11
|
Swingler TE, Niu L, Smith P, Paddy P, Le
L, Barter MJ, Young DA and Clark IM: The function of microRNAs in
cartilage and osteoarthritis. Clin Exp Rheumatol. 37 (Suppl
120):S40–S47. 2019.
|
|
12
|
Akhtar N, Rasheed Z, Ramamurthy S,
Anbazhagan AN, Voss FR and Haqqi TM: MicroRNA-27b regulates the
expression of matrix metalloproteinase 13 in human osteoarthritis
chondrocytes. Arthritis Rheum. 62:1361–1371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rousseau JC, Millet M, Croset M,
Sornay-Rendu E, Borel O and Chapurlat R: Association of circulating
microRNAs with prevalent and incident knee osteoarthritis in women:
The OFELY study. Arthritis Res Ther. 22:22020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malemud CJ: MicroRNAs and osteoarthritis.
Cells. 7:922018. View Article : Google Scholar
|
|
15
|
Liu X, Zhou X, Chen Y, Huang Y, He J and
Luo H: miR-186-5p targeting SIX1 inhibits cisplatin resistance in
non-small-cell lung cancer cells (NSCLCs). Neoplasma. 67:147–157.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Bao H, Zhang S, Li R, Chen L and
Zhu Y: miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y
OGD/R model. Int J Biol Sci. 14:1791–1799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang J, Mo H, Liu C, Wu B, Wu Z, Li X, Li
T, He S, Li S, You Q, et al: Inhibition of miR-186-5p contributes
to high glucose-induced injury in AC16 cardiomyocytes. Exp Ther
Med. 15:627–632. 2018.PubMed/NCBI
|
|
18
|
Xie Z, Li X, Chen H, Zeng A, Shi Y and
Tang Y: The lncRNA-DLEU2/miR-186-5p/PDK3 axis promotes the progress
of glioma cells. Am J Transl Res. 11:4922–4934. 2019.PubMed/NCBI
|
|
19
|
Shan Y and Li P: Long intergenic
non-protein coding RNA 665 regulates viability, apoptosis, and
autophagy via the MiR-186-5p/MAP4K3 axis in hepatocellular
carcinoma. Yonsei Med J. 60:842–853. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin C, Zhao W, Zhang Z and Liu W:
Silencing circular RNA circZNF609 restrains growth, migration and
invasion by up-regulating microRNA-186-5p in prostate cancer. Artif
Cells Nanomed Biotechnol. 47:3350–3358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng H, Zhang Z, Qing X, French SW and Liu
D: miR-186-5p promotes cell growth, migration and invasion of lung
adenocarcinoma by targeting PTEN. Exp Mol Pathol. 108:105–113.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Zhang W, Mao J, Xu Z and Fan M:
miR-186-5p functions as a tumor suppressor in human osteosarcoma by
targeting FOXK1. Cell Physiol Biochem. 52:553–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong S, Wang R, Wang H, Ding Q, Zhou X,
Wang J, Zhang K, Long Y, Lu S, Hong T, et al: HOXD-AS1 promotes the
epithelial to mesenchymal transition of ovarian cancer cells by
regulating miR-186-5p and PIK3R3. J Exp Clin Cancer Res.
38:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng L, Tian XY, Huang XY, He LL and Xu F:
microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits
chondrocyte apoptosis in mice with osteoarthritis. J Cell Physiol.
234:6042–6053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Fan J, Ding X, Sun Y, Cui Z and
Liu W: Tanshinone I inhibits IL-1β-induced apoptosis, inflammation
and extracellular matrix degradation in chondrocytes CHON-001 cells
and attenuates murine osteoarthritis. Drug Des Devel Ther.
13:3559–3568. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Zhang Q, Gao Z, Yu C and Zhang L:
Baicalin alleviates IL-1β-induced inflammatory injury via
down-regulating miR-126 in chondrocytes. Biomed Pharmacother.
99:184–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Q, Zhou Y, Cai P, Fu W, Wang J, Wei Q
and Li X: Downregulation of microRNA-23b-3p alleviates
IL-1β-induced injury in chondrogenic CHON-001 cells. Drug Des Devel
Ther. 13:2503–2512. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao H and Liu Z: Effects of microRNA-217
on high glucose-induced inflammation and apoptosis of human retinal
pigment epithelial cells (ARPE-19) and its underlying mechanism.
Mol Med Rep. 20:5125–5133. 2019.PubMed/NCBI
|
|
29
|
Zhang J, Zhang Z, Shu B, Cui G and Zhong
G: Cytotoxic and apoptotic activity of the novel harmine derivative
ZC-14 in Sf9 cells. Int J Mol Sci. 19:8112018. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei GH, Gao SG, Li KH, Zeng KB and Li LJ:
Correlation of substance P and interleukin-1 beta with pathogenesis
of human osteoarthritis. J Clin Rehabil Tissue Eng Res.
12:7237–7240. 2008.
|
|
32
|
Yu C, Chen WP and Wang XH: MicroRNA in
osteoarthritis. J Int Med Res. 39:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (Review). Int J Mol Med. 34:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sondag GR and Haqqi TM: The role of
MicroRNAs and their targets in osteoarthritis. Curr Rheumatol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cong L, Zhu Y and Tu G: A bioinformatic
analysis of microRNAs role in osteoarthritis. Osteoarthritis
Cartilage. 25:1362–1371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nugent M: MicroRNAs: Exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Zheng W, Pan Y and Hu J: Low
expression of miR-186-5p regulates cell apoptosis by targeting
toll-like receptor 3 in high glucose-induced cardiomyocytes. J Cell
Biochem. 120:9532–9538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park C, Jeong JW, Lee DS, Yim MJ, Lee JM,
Han MH, Kim S, Kim HS, Kim GY, Park EK, et al: Sargassum
serratifolium extract attenuates interleukin-1β-induced
oxidative stress and inflammatory response in chondrocytes by
suppressing the activation of NF-κB, p38 MAPK, and PI3K/Akt. Int J
Mol Sci. 19:23082018. View Article : Google Scholar
|
|
39
|
Ashraf S, Cha BH, Kim JS, Ahn J, Han I,
Park H and Lee SH: Regulation of senescence associated signaling
mechanisms in chondrocytes for cartilage tissue regeneration.
Osteoarthritis Cartilage. 24:196–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun FF, Hu PF, Xiong Y, Bao JP, Qian J and
Wu LD: Tricetin protects rat chondrocytes against IL-1β-induced
inflammation and apoptosis. Oxid Med Cell Longev. 2019:46953812019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zignego DL, Hilmer JK, Bothner B, Schell
WJ and June RK: Primary human chondrocytes respond to compression
with phosphoproteomic signatures that include microtubule
activation. J Biomech. 97:1093672019. View Article : Google Scholar : PubMed/NCBI
|