Introduction
The incidence of stroke around the world is high and
continues to increase. Between 1988 and 2010, the incidence of
stroke in men in Tianjin, China increased from 136.8/100,000 to
387.0/100,000 and the incidence in women increased from
65.9/100,000 to 249.7/100,000 (1).
In the United States >795,000 stroke cases occur each year and
ischemic stroke accounts for 87% of all strokes (2). Ischemic stroke, the most common type
of stroke, is usually caused by a sudden interruption of blood flow
due to a thrombosis or embolism blocking a blood vessel, resulting
in brain dysfunction (3). Hence,
the most effective method to treat cerebral ischemic stroke is to
restore blood flow quickly and completely (4). However, the restoration of blood flow
inevitably causes a series of pathophysiological changes, including
oxidative stress, mitochondrial dysfunction, excitatory amino acid
toxicity, inflammation and infarct formation (5). This process, known as ‘cerebral
ischemia-reperfusion injury’ (CIRI), remains to be fully understood
at a mechanistic level (6).
Endoplasmic reticulum stress (ERS) is involved in
CIRI, which can be alleviated by inhibiting ERS-induced apoptosis
(7,8). As the main site responsible for
protein folding and secretion, the endoplasmic reticulum (ER)
serves a vital role in maintaining homeostasis within the cellular
microenvironment (9,10). In some pathological states, such as
ischemia and hypoxia, the homeostasis of the ER is disrupted,
causing large quantities of unfolded or misfolded proteins to
accumulate in the ER, further exacerbating ERS (9,10). ERS
can be maintained within certain limits via the unfolded protein
reaction (UPR), but an excessive or long-term activation of the UPR
causes ERS-related apoptosis (9,10). ERS
causes the molecular chaperone GRP78 to separate from three
membrane proteins, PERK, inositol requiring enzyme 1α (IRE1α) and
activating transcription factor 6 (ATF6), which then activate a
series of downstream signal responses (11–14).
When the stimulus is intense or persists for a long time, these
responses eventually lead to apoptosis. Phosphorylated (p)-JNK,
CHOP and caspase-12 are activated by IRE1α (11–14).
Neuronal apoptosis is a major pathophysiological change associated
with CIRI (15), and attenuating
cell death is an important strategy for alleviating permanent
damage.
Orexin-A (OXA), a neuropeptide that is primarily
secreted by orexin-containing neurons located in the lateral
hypothalamus, exerts a neuroprotective effect against CIRI
(16). Our previous study initially
confirmed that OXA can prevent CIRI in Sprague-Dawley (SD) rats
subjected to middle cerebral artery occlusion (MCAO) model and
reperfusion (17). In an
oxygen/glucose deprivation and reoxygenation model in vitro,
it was also revealed that OXA exerts its protective role by
inhibiting ERS-mediated apoptosis (18). In the present study, an MCAO model
was established to simulate CIRI and investigated whether OXA can
exert neuroprotective effects by inhibiting ERS-mediated apoptosis
in vivo. The present findings will help clarify the
mechanism underlying the neuroprotective effect of OXA and provide
an experimental basis for the treatment of ischemic stroke.
Materials and methods
Animals
A total of 90 male SD rats (age, 8–9 weeks; weight,
250±10 g) were purchased from Jinan Pengyue Experimental Animal
Breeding, Co., Ltd. All animals were given water and food ad
libitum, and were maintained on a 12-h light/dark cycle in a
temperature-controlled room at 24–26°C with humidity of 50–65%. All
rats acclimated for 1 week before experimental procedures. All
animal experiments were approved by the Animal Ethics Committee of
Jining Medical University, and performed were in accordance with
the National Experimental Animal Feeding Guidelines (19). The rats were sacrificed with
decapitation after deep anesthesia causing rapid and unconscious
death without pain.
MCAO model
SD rats were fasted for 12 h before the experiment.
Rats were anesthetized using 10% chloral hydrate (300 mg/kg) via
intraperitoneal injection for up to 2 h after reperfusion. After
intraperitoneal injection of 10% chloral hydrate, the rats did not
show symptoms of peritonitis. After a midline incision was
introduced in the neck, the right common carotid artery, right
external carotid artery and internal carotid artery (ICA) were
exposed and separated. A 2.5 nylon mono-filament (0.265 mm in
diameter) was inserted through the common carotid artery into the
lumen of the ICA and advanced 18–22 mm from the bifurcation until
it blocked the origin of the right middle cerebral artery. In the
drug and model groups, the filament remained in the lumen for 2 h,
and was subsequently withdrawn to allow reperfusion for 3, 6, 12,
24 and 48 h. The sham-operated group was treated the same as the
model group except for the occlusion of the middle cerebral
arteries after the neck incision. A total of 30 rats were randomly
divided into 6 groups: sham operation group (Sham), MCAO model
group and reperfusion for 3 h (CIRI 3h), 6 h (CIRI 6h), 12 h (CIRI
12h), 24 h (CIRI 24h) and 48 h (CIRI 48h). Based on the expression
of ERS-related proteins, CIRI 24h was selected for OXA
intervention. Therefore, 20 rats were randomly divided into the
sham operation group (Sham), the MCAO model group and reperfusion
for 24 h (CIRI), CIRI with intracerebroventricular (ICV) injection
of normal saline (NS; CIRI + NS) and CIRI with
intracerebroventricular (ICV) injection of OXA (CIRI + OXA).
Another 40 rats were used for TTC and TUNEL staining. In addition,
there was ~1/3 mortality and model failure rate.
Intracerebroventricular (ICV)
injection
At the beginning of reperfusion, the rats were
anesthetized via intraperitoneal injection with 10% chloral hydrate
(300 mg/kg). A burr hole for ICV administration was carefully made
in the skull at 0.8-mm dorsal and 1.6-mm lateral to the right from
Bregma using a Dremel drill. A total of 10 µl OXA (30 µg/kg)
(20) from Phoenix Pharmaceuticals,
Inc. in 0.9% NaCl or 10 µl NS (0.9% NaCl) were injected at 2 µl/min
for 5 min.
Neurological score
After reperfusion for 24 h, neurological function of
all rats was evaluated according to the Longa five-point scale
(21): 0, no neurological deficit;
1, failed to fully extend their left forepaw; 2, circling to the
left when walking; 3, falling to the left when walking; and 4,
failure to walk spontaneously or stroke-related mortality. Only
rats scoring 1, 2 or 3 were selected for use in subsequent
experiments. The test was performed in a blinded manner.
2,3,5-Triphenyl-2H-tetrazolium chloride (TTC)
staining. Rats were anesthetized with 10% chloral hydrate (300
mg/kg), and brains were collected and incubated on ice for 30 min.
Coronal thin slices (2 mm) of brain were stained with 1% TTC
(Sigma-Aldrich; Merck KGaA) and incubated at 37°C in the dark for
15–20 min. Viable tissues were stained deep red, whereas infarcts
were pale white. TTC-stained brains were then fixed with 4%
formaldehyde for 24 h at room temperature and imaged with a digital
camera. The infarct areas were measured using the ImageJ 2 software
(National Institutes of Health).
TUNEL assay
TUNEL staining was performed according to the
manufacturer's instructions (Promega Corporation). Briefly, coronal
slices (30 µm) were fixed with 4% paraformaldehyde for 30 min at
room temperature and permeabilized with 0.3% Triton X-100 for 10
min at room temperature. After equilibration for 10 min, the slices
were incubated with rTdT reaction buffer for 60 min at a constant
temperature of 37°C and then incubated with 2X SSC for 15 min to
stop the rTdT reaction at a constant temperature of 37°C. Finally,
the nuclei were counterstained with DAPI (100 ng/ml) for 30 min at
room temperature, and then the anti-fluorescence quencher was added
dropwise for mounting. Images of three fields of view were randomly
capture under an optical microscope with a magnification of ×200
(Olympus IX 71; Olympus Corporation) and the percentages of
TUNEL-positive cells vs. total cells were calculated
Western blot analysis
Brain tissues of rats were homogenized in RIPA lysis
buffer (Beyotime Institute of Biotechnology) supplemented with PMSF
(Beyotime Institute of Biotechnology), and protein concentrations
were quantified using the BCA method (Beyotime Institute of
Biotechnology). Equal amounts of protein (25 µg) from each sample
were separated via 10% SDS-PAGE and transferred to PVDF membranes
at 4°C. The membranes were blocked with 5% non-fat milk powder in
TBS-Tween-20 (0.1%) for 2 h at room temperature and then incubated
overnight at 4°C with the following primary antibodies:
Anti-cleaved caspase-3 (cat. no. 9661S), anti-GRP78 (cat. no.
3177S), anti-IRE1α (cat. no. 3294S), anti-PERK (cat. no. 5683S),
anti-p-PERK (cat. no. 3179S), anti-CHOP (cat. no. 2895S),
anti-eIF2α (cat. no. 5324S), anti-p-eIF2α (cat. no. 9721S),
anti-JNK (cat. no. 9252T), anti-p-JNK (cat. no. 9255S),
anti-cleaved caspase-12 (cat. no. 35965S) (all 1:1,000; Cell
Signaling Technology, Inc.), anti-p-IRE1α (cat. no. ab48187;
1:1000; Abcam) and anti-β-actin (cat. no. TA-09; 1:1,000; OriGene
Technologies, Inc.). Next, the membranes were incubated for 1 h at
room temperature with horseradish peroxidase-conjugated anti-rabbit
IgG or anti-mouse IgG secondary antibodies (1:5,000; OriGene
Technologies, Inc.), and visualized using an ECL system
(MultiSciences Biotech Co,. Ltd. The grayscale intensities of
protein bands were quantified using ImageJ 2 software (National
Institutes of Health).
Statistical analysis
All data are representative of ≥3 independent
experiments and were analyzed using the GraphPad Prism 5 software
(GraphPad Software, Inc.). Data are presented as the mean ± SEM.
One-way ANOVA followed by Tukey's test were performed. P<0.05
was considered to indicate a statistically significant
difference.
Results
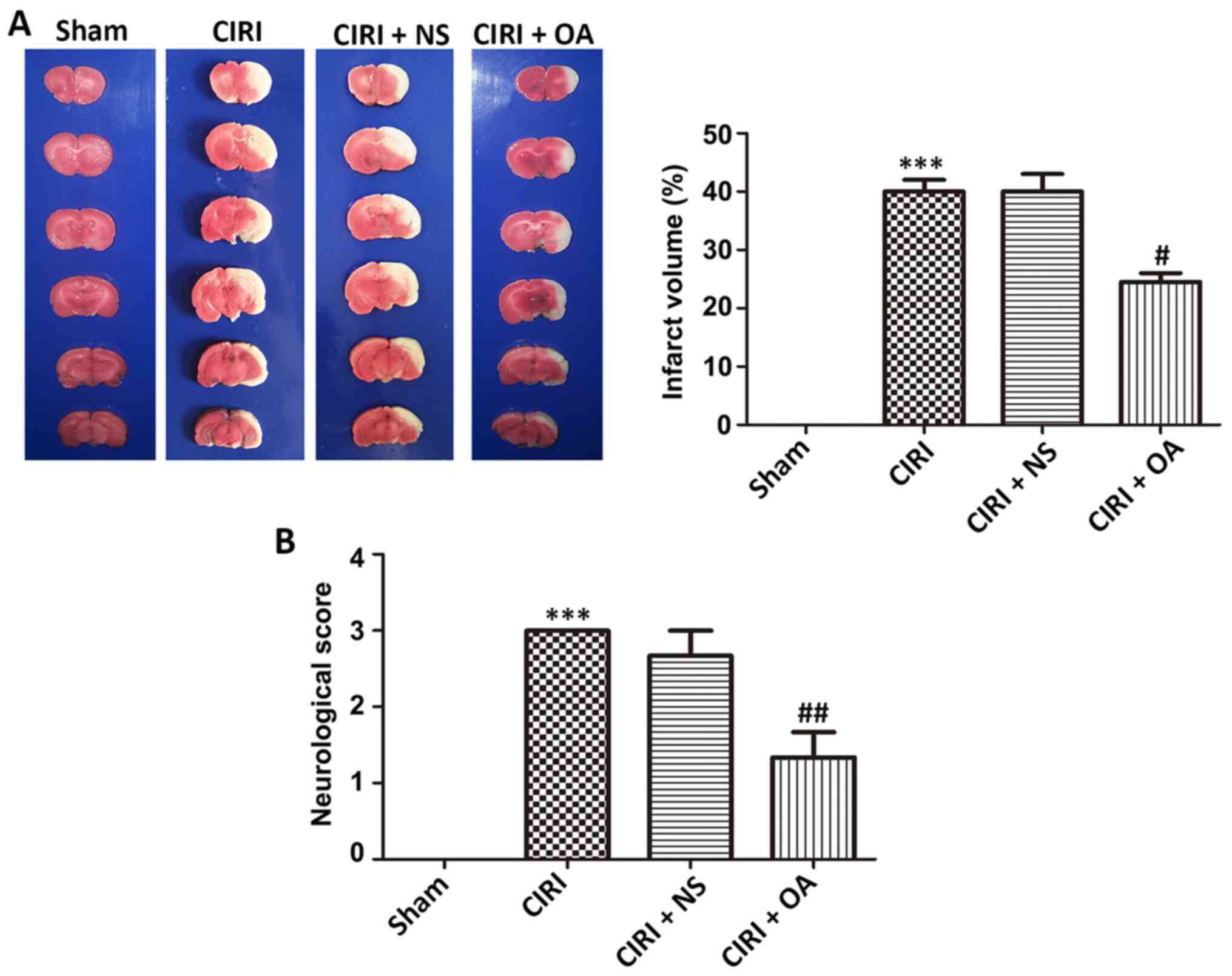
OXA decreases infarct volume and
improves neurological deficit score in brain damage caused by
CIRI
To evaluate the neuroprotective effect of OXA in the
CIRI model, infarct volume and neurological deficit score were
measured, respectively. Healthy cerebral tissues were stained red,
whereas infarcted areas were stained white (Fig. 1A). No infarction areas were detected
in the sham group, whereas the CIRI group had a significantly
higher rate of infarction (40±2%) compared with the sham group
(P<0.001), demonstrating that CIRI-induced cerebral infarct had
been successfully introduced. However, the OXA group had a
significantly lower rate of infarction (24.5±1.5%) compared with
the CIRI group (P<0.05), demonstrating that intervention with
OXA significantly decreased the infarct area.
As presented in Fig.
1B, the average neurological deficit scores of the CIRI model
rats was 3 compared with the sham group, according to the Longa
score scale (P<0.001). After OXA intervention, neurological
deficit scores were significantly decreased (P<0.01), indicating
that OXA has the potential ability to improve neurological deficit
scores. Thus, OXA exhibited a notable neuroprotective effect on
infarct volume and neurological deficit score in CIRI model
rats.
Expression levels of ERS-related
proteins are elevated in the cortex after CIRI
To determine whether CIRI was accompanied by ERS,
western blot analysis was conducted to evaluate the expression
levels of ERS-related proteins in the cerebral cortex on the
ischemic side at different times post-reperfusion. The expression
patterns of five proteins exhibited significant differences at
various times during the post-reperfusion period (Fig. 2). After IR, the expression level of
HSPA5 was increased to a peak at 6 h and then declined (P<0.01
and P<0.001). The phosphorylation level of PERK was elevated at
3 and 6 h, then decreased before increasing again from 24 h
(P<0.01 and P<0.001). The expression of p-eIF2α was highest
at 6 h post-reperfusion, and then gradually decreased (P<0.05
and P<0.01). p-IRE1α reached its highest levels at 3 h
post-reperfusion, and then decreased (P<0.01 and P<0.001).
The expression of p-JNK remained at a high level for 48 h
post-reperfusion (P<0.05 and P<0.01). These observations
demonstrate that the patterns of gene expression are complex and
diverse, increasing the challenge of understanding the molecular
pathology of CIRI. Taken together, however, the present findings
suggested that these proteins were activated in CIRI, indicating
that ERS is accompanied by CIRI.
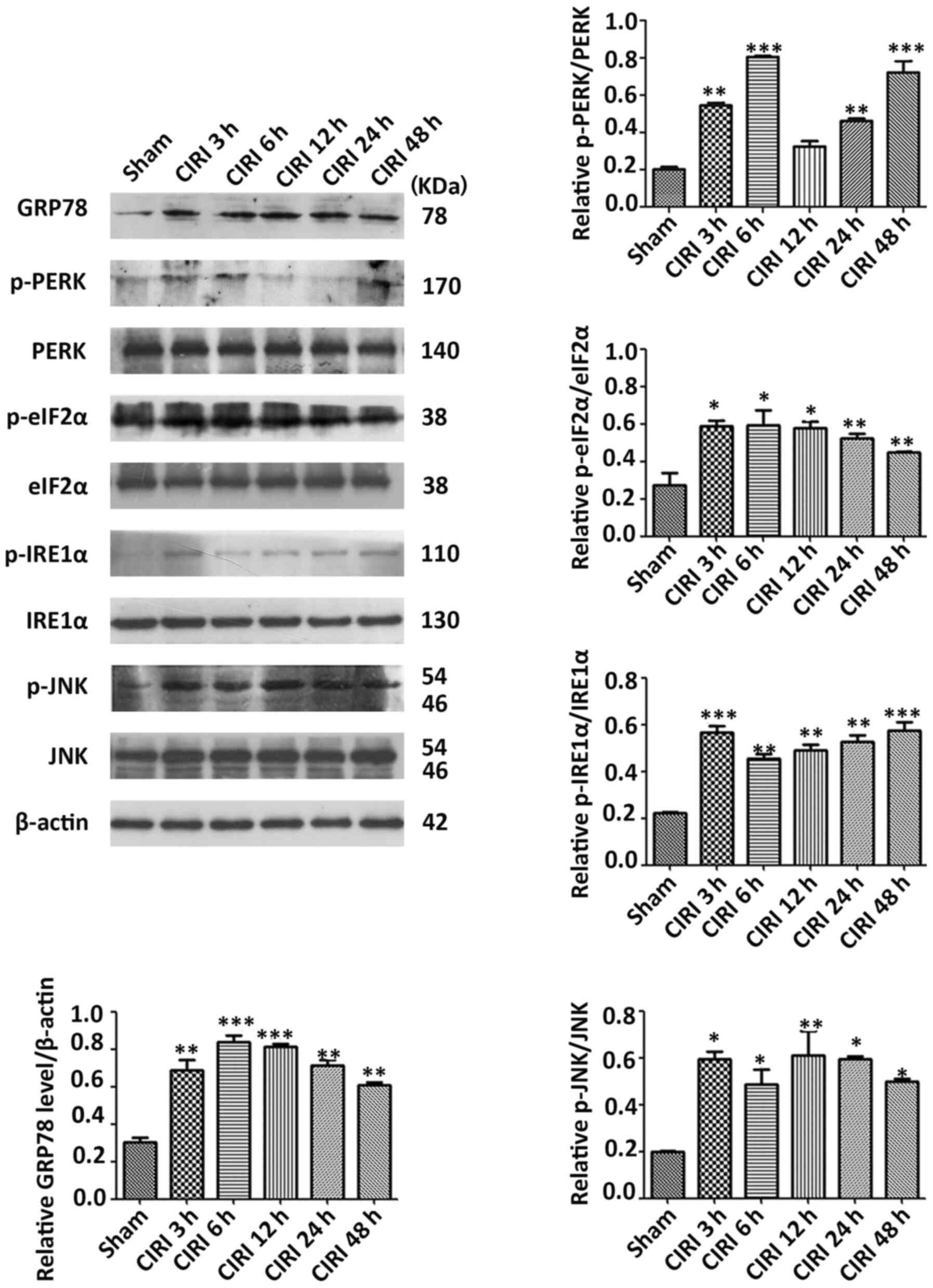 | Figure 2.Expression levels of ERS-related
proteins induced by cerebral ischemia–reperfusion. Western blot
analysis results of ERS-related proteins in cortex of rats after
the indicated durations of CIRI. Semi-quantitative analysis is
presented for HSPA5, p-PERK, PERK, p-eIF2α, eIF2α, p-IRE1α, IRE1α,
p-JNK and JNK. Data are presented as mean ± SEM (n=3). *P<0.05,
**P<0.01 and ***P<0.001 vs. Sham group. CIRI, cerebral
ischemia-reperfusion injury; p-, phosphorylated; HSPA5, heat shock
protein family A (Hsp70) member 5; eIF2α, eukaryotic translation
initiation factor 2; IRE1α, inositol requiring enzyme 1α. |
OXA significantly decreases the
expression of ERS-related protein in the cortex caused by CIRI
To investigate the effect of OXA on the expression
of ERS-related proteins, CIR was simulated by 2 h MCAO followed by
24 h of reperfusion, and OXA was administered via ICV injection
during reperfusion. Next, the expression levels of ERS-related
protein were detected via western blotting (Fig. 3A). Compared with Sham group, the
fold changes of the expression levels of HSPA5, p-PERK, p-eIF2α,
p-IRE1α, and p-JNK in CIRI group and CIRI + OA group were uneven.
GRP78 expression was significantly higher in the CIRI group
(22.6-fold) compared with the Sham model (Fig. 3B), but significantly lower in the
OXA model compared with the CIRI group (P<0.01). No
statistically significant difference was observed between CIRI and
CIRI + NS models. The phosphorylation levels of PERK, eIF2α, IRE1α
and JNK were upregulated in the CIRI group compared with the Sham
group (P<0.05, P<0.01 and P<0.001), but all were
downregulated following intervention with OXA (P<0.05, P<0.01
and P<0.001). In addition, only the phosphorylation levels of
eIF2α and JNK were statistically significant between the Sham and
OXA models (P<0.05 and P<0.01). In brief, these results
indicated that CIRI activated ERS-related proteins in the rat
cortex, and that OXA could decrease their activities.
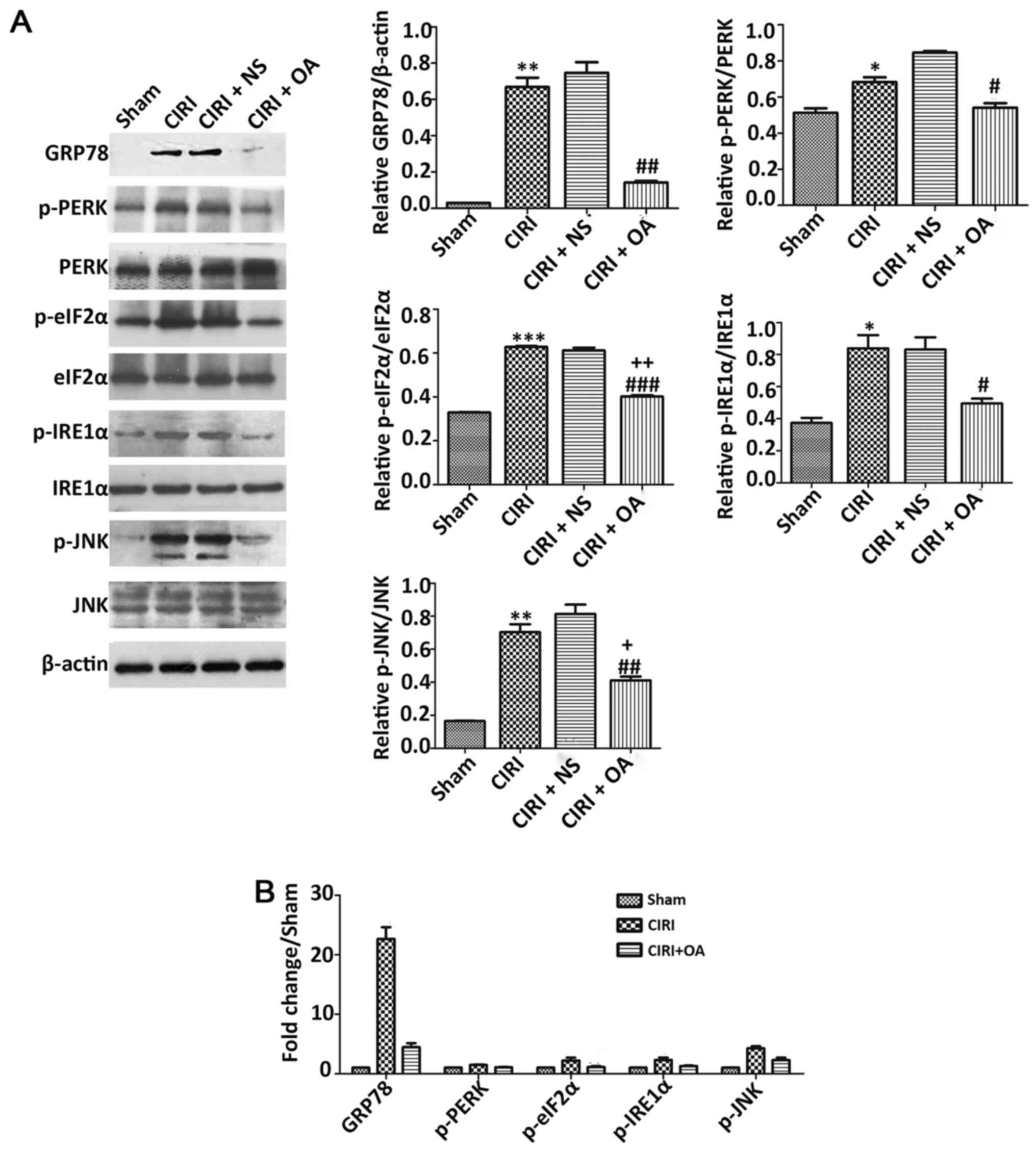 | Figure 3.Effect of OXA on expression levels of
ERS-related proteins induced by CIR. (A) Western blotting and
semi-quantitative analyses of HSPA5, p-PERK, PERK, p-eIF2α, eIF2α,
p-IRE1α, IRE1α, p-JNK and JNK. Treatment with OXA significantly
decreased the expression levels of HSPA5, p-PERK, p-eIF2α, p-IRE1α
and p-JNK. (B) Compared with Sham group, the fold changes of the
expression levels of HSPA5, p-PERK, p-eIF2α, p-IRE1α and p-JNK in
CIRI group and CIRI + OA group were uneven. Data are presented as
mean ± SEM (n=3). *P<0.05, **P<0.01 and ***P<0.001 vs.
Sham group; #P<0.05, ##P<0.01 and
###P<0.001 vs. CIRI group; +P<0.05 and
++P<0.01 vs. sham group. CIRI, cerebral
ischemia-reperfusion injury; NS, normal saline (0.9% NaCl); OA,
Orexin-A; p-, phosphorylated; HSPA5, heat shock protein family A
(Hsp70) member 5; eIF2α, eukaryotic translation initiation factor
2; IRE1α, inositol requiring enzyme 1α. |
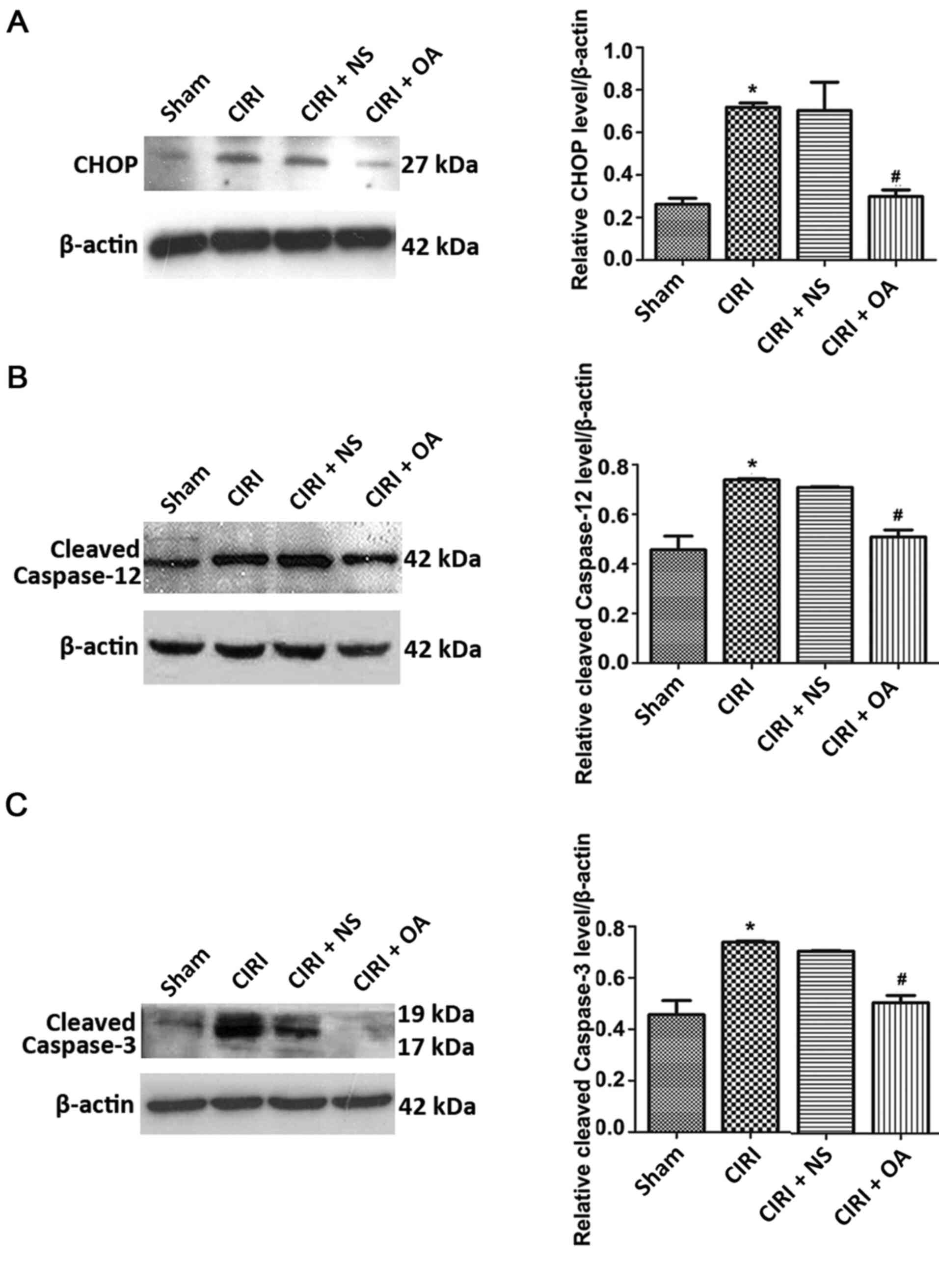
OXA significantly decreases the
expression levels of CHOP, cleaved caspase-12 and cleaved caspase-3
in the cortex following CIRI
The caspase-12/caspase-9/caspase-3 or CHOP apoptotic
pathways are mediated by ERS (22).
Moreover, caspase-12 is regarded as a representative molecule in
ERS-mediated apoptosis, and caspase-3 serves a key role in
regulating apoptosis, which can directly lead to cell death
(22,23). Therefore, the expression levels of
CHOP, cleaved caspase-12 and cleaved caspase-3 were measured using
western blotting (Fig. 4). CIRI
upregulated the expression of all three proteins (all P<0.05).
Moreover, treatment with OXA significantly decreased the expression
of CIRI-induced CHOP, cleaved caspase-12 and cleaved caspase-3 (all
P<0.05). However, the expression levels of these proteins did
not differ significantly between the CIRI + NS and CIRI groups.
These data suggested that OXA exerted an anti-apoptotic effect on
CIRI by inhibiting the expression of apoptosis-related genes.
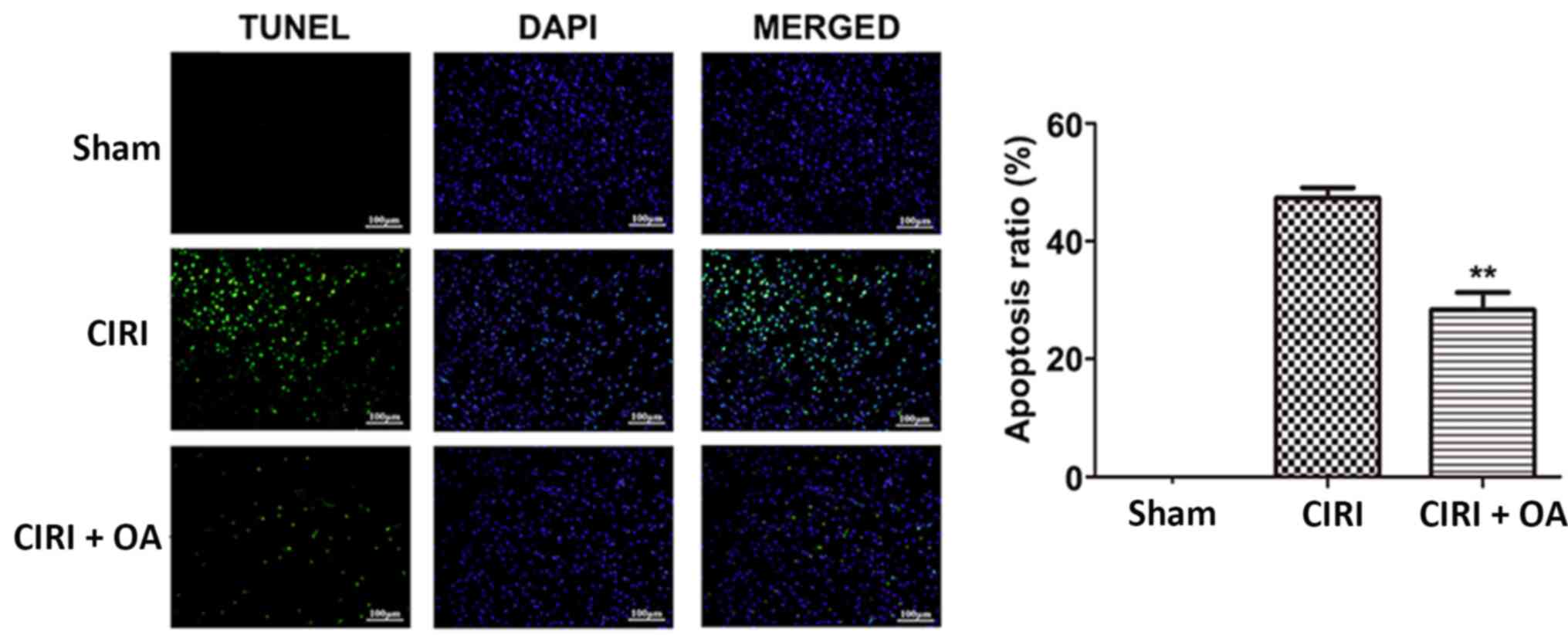
OXA significantly decreases apoptosis
in CIRI models
To determine the effect of OXA on apoptosis in the
CIRI model, apoptotic cells in brain tissues were detected using
TUNEL staining. TUNEL-positive cells were barely visible in the
Sham group, whereas substantial levels of TUNEL-positive neurons
(47.33%) were detected in the CIRI group (Fig. 5). However, the number of
TUNEL-positive cells in the CIRI + OXA group was significantly
decreased (28.33%) compared with the CIRI group (P<0.01). These
results demonstrated that OXA exerted an anti-apoptotic effect in
the cortex following CIR.
Discussion
Previous research established the neuroprotective
effect of OXA on CIRI (16), but
the underlying mechanism remained unknown. Moreover, our previous
study demonstrated that the neuroprotective effect of OXA was
achieved by inhibiting ERS-mediated apoptosis in vitro
(18). The present study
demonstrated that, in rats, OXA protected the brain against CIRI by
attenuating ERS-mediated apoptosis.
The prevention and treatment of CIRI have gained
increased attention in the field of global IR research (24,25).
Blocking neuronal apoptosis is the most important step in
alleviating CIRI (26). Apoptosis
is a major mode of neuron death following CIRI, particular
apoptosis induced by the ERS pathway (8). ER is the main site of protein
synthesis, processing and transport. Accordingly, the ERS response
can be induced by a series of pathophysiological changes that occur
in CIRI, such as Ca2+ overload, oxidative stress,
metabolic disorders and inflammatory responses, all of which
disrupt the homeostasis of the ER (5–7). The
present results study confirm these effects. In the current model,
CIRI was induced in rats by MCAO for 2 h, followed reperfusion for
various times. Time-dependent changes in the expression of
molecular markers of ERS, including HSPA5, p-PERK, p-eIF2α, p-IRE1α
and p-JNK, indicated that ERS serves a critical role in brain
damage after CIR.
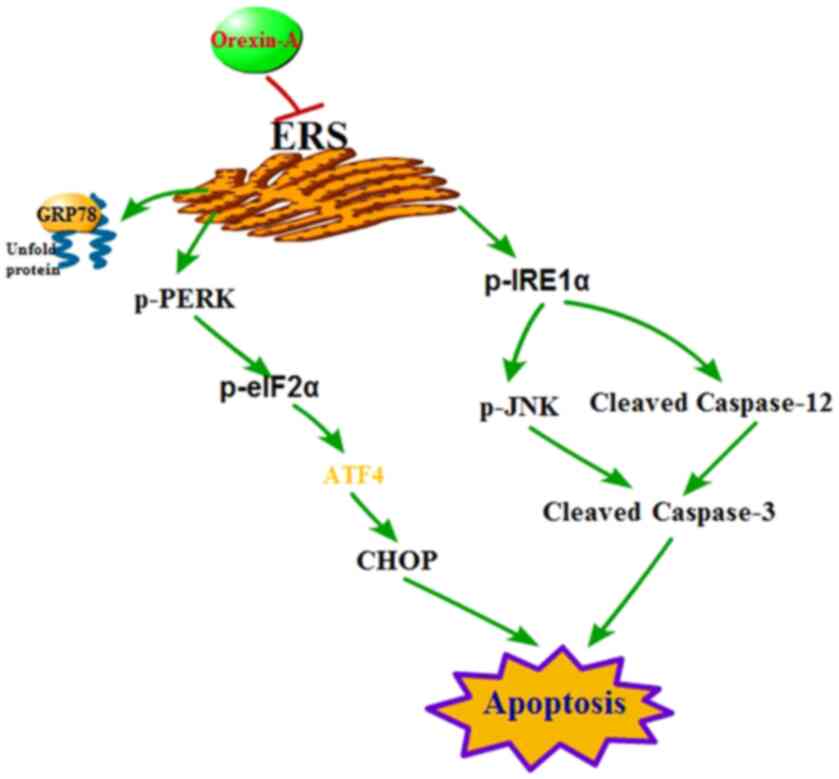
The ERS response promotes the processing of
misfolded or unfolded proteins accumulated in the ER, which helps
to maintain the physiological function of cells, but excessively
long or strong ERS can cause apoptosis (9,10). A
type of ERS response, the UPR, has been studied extensively, and
the mechanisms have been described. The signal transduction
pathways of UPR are 3-fold, comprising the PERK, ATF6 and IRE1α
pathways. In a stress-free state, these three transmembrane
proteins are restrained by binding to HSPA5. HSPA5 itself is a
well-established hallmark of ERS, and the gene that encodes it is
specifically activated during ERS (27). After CIR, HSPA5 dissociates from the
three sensors, resulting in their activation, ultimately triggering
the ERS-mediated caspase-12/caspase-9/caspase-3 or CHOP apoptosis
pathways (11–14). Under ERS, activated PERK
specifically phosphorylates eIF2α, inhibits translation of nascent
proteins and downregulates the overall level of intracellular
protein synthesis (28). In
addition, PERK-mediated eIF2α phosphorylation upregulates ATF4,
which activates the pro-apoptotic protein CHOP after ATF4 enters
the nucleus (29) (Fig. 6). In the early stage of ERS,
phosphorylation of eIF2α will, to some extent, decrease the load on
the ER and promote efficient folding and assembly of proteins,
thereby maintaining the steady state of the ER and cellular
homeostasis (30). Moreover, when
ERS is prolonged, ATF4 transcriptional activity is activated in the
late phase; this factor helps to initiate apoptosis by driving the
expression of the proapoptotic protein CHOP (31) (Fig.
6). Under excessive or long-lasting ERS, the IRE1α pathway can
induce apoptosis by activating JNK and caspase-12, as well as by
upregulating transcription of CHOP (32,33).
The present study identified that CIRI upregulated the expression
levels of components of the PERK and IRE1 pathways, including
p-PERK, p-eIF2α, p-IRE1α, p-JNK, cleaved caspase-12, CHOP, HSPA5
and the apoptotic protein caspase-3. After OXA treatment, all of
these proteins were downregulated, resulting in a lower rate of
apoptosis. Thus, OXA exerted a neuroprotective effect following
CIRI by inhibiting ERS-mediated apoptosis, which provides a novel
method for the treatment of stroke.
The current study has a few limitations. First, the
role of Ca2+ in the ERS/caspase-12/caspase-3 apoptosis
pathway under OXA treatment was not investigated. The ER is the
main Ca2+ reservoir in the cell, and consequently serves
a key role in controlling the intracellular Ca2+
concentration. In CIRI, intracellular Ca2+ overload and
ER Ca2+ depletion are both pathophysiological changes
worthy of attention, and both cause ERS (34). Under ERS, an increase in
intracellular Ca2+ levels results in activation of
cytoplasmic calpain and translocation of the ER membrane,
activating the caspase-12 precursor and ultimately leading to
apoptosis (34). These issues will
be addressed in follow-up studies.
In conclusion, the present study demonstrated that
OXA exerted a neuroprotective effect against CIRI by inhibiting
ERS-mediated apoptosis. Thus, OXA represents a promising basis for
a novel treatment strategy for stroke.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from National
Nature Science Foundation of China (grant nos. 81501018, 81671276
and 31271243), the Natural Science Foundation of Shandong Province
(grant no. ZR2018MC005) and the Scientific Research Support Fund
for teachers of Jining Medical University (grant nos. JYFC2018JS003
and JYFC2018JS008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and JC conceived of and designed the experiments.
DX and TK conducted the experiments and wrote the manuscript. RZ,
CY and BC performed the data analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals and approved by the Animal Ethics Committee
of Jining Medical University, China (approval no. 2018-JS-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang G, Li W, Wang D, Shen C, Ji Y and
Zheng W: Epidemiological transition and distribution of stroke
incidence in Tianjin, China, 1988–2010. Public Health. 131:11–19.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barthels D and Das H: Current advances in
ischemic stroke research and therapies. Biochim Biophys Acta Mol
Basis Dis. 1866:1652602020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo
C, Peng J, Li J, Yung KK and Mo Z: Neuroprotective effects of
bilobalide on cerebral ischemia and reperfusion injury are
associated with inhibition of pro-inflammatory mediator production
and down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chien A and Viñuela F: Analyzing circle of
Willis blood flow in ischemic stroke patients through 3D stroke
arterial flow estimation. Interv Neuroradiol. 23:427–432. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakthavachalam P and Shanmugam PST:
Mitochondrial dysfunction - Silent killer in cerebral ischemia. J
Neurol Sci. 375:417–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma XD, Song JN, Zhang M, An JY, Zhao YL
and Zhang BF: Advances in research of the neuroprotective
mechanisms of cerebral ischemic postconditioning. Int J Neurosci.
125:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu YQ, Chen W, Yan MH, Lai JJ, Tang N and
Wu L: Ischemic preconditioning protects brain from
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress-induced apoptosis through PERK pathway. Eur Rev Med
Pharmacol Sci. 21:5736–5744. 2017.PubMed/NCBI
|
|
8
|
Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF
and Du GH: Pinocembrin protects brain against ischemia/reperfusion
injury by attenuating endoplasmic reticulum stress induced
apoptosis. Neurosci Lett. 546:57–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv Z, Liu C, Zhai M, Zhang Q, Li J, Zheng
F and Peng M: LPS pretreatment attenuates cerebral
ischaemia/reperfusion injury by inhibiting inflammation and
apoptosis. Cell Physiol Biochem. 45:2246–2256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu J, Wang X, Wu F, Wan L, Cheng B, Wu Y
and Bai B: Low dose of Apelin-36 attenuates ER stress-associated
apoptosis in rats with ischemic stroke. Front Neurol. 8:5562017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
García de la Cadena S and Massieu L:
Caspases and their role in inflammation and ischemic neuronal
death. Focus on caspase-12. Apoptosis. 21:763–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maier PJ, Zemoura K, Acuña MA, Yévenes GE,
Zeilhofer HU and Benke D: Ischemia-like oxygen and glucose
deprivation mediates down-regulation of cell surface γ-aminobutyric
acid B receptors via the endoplasmic reticulum (ER) stress-induced
transcription factor CCAAT/enhancer-binding protein
(C/EBP)-homologous protein (CHOP). J Biol Chem. 289:12896–12907.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paschen W and Mengesdorf T: Endoplasmic
reticulum stress response and neurodegeneration. Cell Calcium.
38:409–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mattson MP, Duan W, Pedersen WA and
Culmsee C: Neurodegenerative disorders and ischemic brain diseases.
Apoptosis. 6:69–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitamura E, Hamada J, Kanazawa N, Yonekura
J, Masuda R, Sakai F and Mochizuki H: The effect of orexin-A on the
pathological mechanism in the rat focal cerebral ischemia. Neurosci
Res. 68:154–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CM, Pan YY, Liu MH, Cheng BH, Bai B
and Chen J: RNA-seq expression profiling of rat MCAO model
following reperfusion Orexin-A. Oncotarget. 8:113066–113081. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong T, Qiu K, Liu M, Cheng B, Pan Y, Yang
C, Chen J and Wang C: Orexin-A protects against oxygen-glucose
deprivation/reoxygenation-induced cell damage by inhibiting
endoplasmic reticulum stress-mediated apoptosis via the Gi and PI3K
signaling pathways. Cell Signal. 62:1093482019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US), .
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals: Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press; Washington, DC:
2011
|
|
20
|
Yuan LB, Dong HL, Zhang HP, Zhao RN, Gong
G, Chen XM, Zhang LN and Xiong L: Neuroprotective effect of
orexin-A is mediated by an increase of hypoxia-inducible factor-1
activity in rat. Anesthesiology. 114:340–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagner DC, Riegelsberger UM, Michalk S,
Härtig W, Kranz A and Boltze J: Cleaved caspase-3 expression after
experimental stroke exhibits different phenotypes and is
predominantly non-apoptotic. Brain Res. 1381:237–242. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan J, Lv H, Li J, Che Y, Xu B, Tao Z and
Jiang W: Roles of Nrf2/HO-1 and HIF-1α/VEGF in lung tissue injury
and repair following cerebral ischemia/reperfusion injury. J Cell
Physiol. 234:7695–7707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Chen S, Zhang Z, Xu H, Zhang W,
Xu D, Lin B and Mei Y: Asiaticoside alleviates cerebral
ischemia-reperfusion injury via NOD2/mitogen-activated protein
kinase (MAPK)/nuclear factor kappa B (NF-κB) signaling pathway. Med
Sci Monit. 26:e9203252020.PubMed/NCBI
|
|
26
|
El Khashab IH, Abdelsalam RM, Elbrairy AI
and Attia AS: Chrysin attenuates global cerebral ischemic
reperfusion injury via suppression of oxidative stress,
inflammation and apoptosis. Biomed Pharmacother. 112:1086192019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeGracia DJ, Kumar R, Owen CR, Krause GS
and White BC: Molecular pathways of protein synthesis inhibition
during brain reperfusion: Implications for neuronal survival or
death. J Cereb Blood Flow Metab. 22:127–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marciniak SJ, Garcia-Bonilla L, Hu J,
Harding HP and Ron D: Activation-dependent substrate recruitment by
the eukaryotic translation initiation factor 2 kinase PERK. J Cell
Biol. 172:201–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
DeGracia DJ and Montie HL: Cerebral
ischemia and the unfolded protein response. J Neurochem. 91:1–8.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scheuner D, Song B, McEwen E, Liu C,
Laybutt R, Gillespie P, Saunders T, Bonner-Weir S and Kaufman RJ:
Translational control is required for the unfolded protein response
and in vivo glucose homeostasis. Mol Cell. 7:1165–1176. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta S, Deepti A, Deegan S, Lisbona F,
Hetz C and Samali A: HSP72 protects cells from ER stress-induced
apoptosis via enhancement of IRE1alpha-XBP1 signaling through a
physical interaction. PLoS Biol. 8:e10004102010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberson EC, Tully JE, Guala AS, Reiss JN,
Godburn KE, Pociask DA, Alcorn JF, Riches DW, Dienz O,
Janssen-Heininger YM, et al: Influenza induces endoplasmic
reticulum stress, caspase-12-dependent apoptosis, and c-Jun
N-terminal kinase-mediated transforming growth factor-β release in
lung epithelial cells. Am J Respir Cell Mol Biol. 46:573–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verkhratsky A and Petersen OH: The
endoplasmic reticulum as an integrating signalling organelle: From
neuronal signalling to neuronal death. Eur J Pharmacol.
447:141–154. 2002. View Article : Google Scholar : PubMed/NCBI
|