Introduction
Periodontal disease is a chronic, inflammatory
disease characterized by the loss of supporting bone and
periodontal tissue around the teeth, and is highly prevalent and
can affect up to 90% of the worldwide population (1,2).
Chronic inflammation reduces the regeneration of periodontal
tissues and inhibits the osteogenic differentiation of periodontal
ligament stem cells (PDLSCs) (3).
Human PDLSCs (hPDLSCs) are the most common cells in periodontal
membranes, which constantly form new fibers and bones, resulting in
remodeling of the alveolar bone (4). hPDLSCs serve a critical function in
repairing periodontal tissues and are among the vital seed cells
required for periodontal regeneration (5). However, inflammation can reduce the
bone regeneration ability of hPDLSCs, leading to irreversible
damage (6–8). Multiple factors have been reported to
be involved in the osteogenic differentiation of hPDLSCs, including
bone morphogenetic protein 2 (BMP2), alkaline phosphatase (ALP),
microRNAs (miRNAs/miRs) and runt-related transcription factor 2
(Runx2) (9–17). In addition to miRNAs, long
non-coding RNAs (lncRNAs) also display regulatory effects on
hPDLSCs (1,8,11,18).
Non-coding RNAs (ncRNAs), which comprise ~98% of
transcriptomes, display a variety of biological functions,
including modulating gene expression and DNA synthesis (19,20).
Conventionally, ncRNAs refer to ribosomal RNAs and transfer RNAs,
which serve a vital role in protein synthesis (21). A number of other types of ncRNAs,
including small nuclear RNAs, miRNAs and lncRNAs, have also been
discovered and investigated (20).
lncRNAs serve different roles in gene expression via complex
molecular mechanisms (20). For
example, lncRNA-POIR, maternally expressed 3, XR_111050 and H19
imprinted maternally expressed transcript regulate the osteogenic
differentiation of multiple types of stem cells (18,22–24).
In addition to their functions in cells, lncRNAs also act as
sponges of miRNAs, displaying the hierarchical regulatory potency
of one ncRNA over another (25,26).
For example, lncRNA-malate dehydrogenase acts as a sponge for
miR-133 and miR-135 by preventing the miRNAs from targeting
mastermind like transcriptional coactivator 1 and myocyte enhancer
factor 2C (27). By binding to the
3′-untranslated regions of mRNAs, miRNAs regulate their expression,
whereas lncRNAs serve as sponges of miRNAs by by preventing the
aforementioned process (8).
The role of Prader Willi/Angelman region RNA 6
(PWAR6) in cancer and other diseases has not yet been fully
elucidated. lncRNA PWAR6 has been recognized as a potential
imprinted gene (28). A previous
study verified its importance in the pathogenesis of Prader-Willi
syndrome (29), but it is also
associated with patient survival during glioma progression
(30).
The present study investigated the expression of
PWAR6 in hPDLSCs and its influence on the osteogenic
differentiation of hPDLSCs. The results indicated that PWAR6
could promote hPDLSC osteogenesis and suggested that the
interaction between miR-106a-5p and PWAR6 affected the
osteogenic differentiation of hPDLSCs.
Materials and methods
Cell culture and characterization
hPDLSCs were isolated from premolars extracted from
patients (Age: 11–18 years old, four male and five female) who had
not received pharmacological treatment for orthodontic treatment at
Jingmen No. 1 People's Hospital (Jingmen, China) between September
2018 to May 2019. The present study was approved by the Ethics
Committee of Jingmen No. 1 People's Hospital (approval no.
Y20180831) and written consent was obtained from each
participant.
Premolars were washed with PBS (cat. no. 10010023;
Thermo Fisher Scientific, Inc.) supplemented with 1%
penicillin-streptomycin (100 U/ml penicillin; 100 µg/ml
streptomycin; cat. no. 15140122; Thermo Fisher Scientific, Inc.).
Periodontal ligament tissues were scraped, cut into small pieces
and digested using collagenase I (3 mg/ml; Sigma-Aldrich; Merck
KGaA) and dispase II (4 mg/ml; Sigma-Aldrich; Merck KGaA) for 1 h
at 37°C. To further isolate and purify the stem cells, single-cell
suspensions of primary cells were cloned using the
limiting-dilution method, as previously described (31,32).
Following digestion, the single-cell suspension was filtered
through a 70 µm strainer. Subsequently, half of the single-cell
suspension was seeded (60 cells/cm2) into 10-cm tissue
culture dishes in DMEM supplemented with 10% FBS (cat. no.
10100147; Thermo Fisher Scientific, Inc.), 50 mg/ml streptomycin
and 50 U/ml penicillin at 37°C with 5% CO2. The
non-adherent cells were removed 3 days later, and the culture
medium was changed three times per week. When cells reached 80%
confluency, the supernatant was removed, the cells were washed
twice using PBS twice and 0.5% 10X Trypsin-EDTA (cat. no. 15400054;
Thermo Fisher Scientific, Inc.) was added to the cells for 2 min at
room temperature. 2 ml DMEM/F12 with 10% FBS was added to terminate
dissociation. After centrifugation at 1,500 × g for 3 min at room
temperature, 2–3 ml DMEM/F12 supplemented with 1%
penicillin-streptomycin and 10% FBS was added to the cells for
subculture. P3 cells were used for subsequent experiments. Cell
morphology was observed using an inverted phase contrast light
microscope (DSZ2000X; Chongqing UOP Photoelectric Technology, Co.,
Ltd.).
For osteogenic induction, hPDLSCs were cultured in
osteogenic medium (OM) consisting of 10 mmol/l β-glycerophosphate
sodium (cat. no. 50020; Sigma-Aldrich; Merck KGaA), 50 µg/ml
L-ascorbic acid (cat. no. 795437; Sigma-Aldrich; Merck KGaA) and
100 mmol/l dexamethasone (Sigma-Aldrich; Merck KGaA) at 37°C with
5% CO2. For the control group, hPDLSCs were cultured in
growth medium (GM; cat. no. 112-500, Sigma-Aldrich; Merck KGaA).
The medium was changed every two days and osteogenic induction
duration was 21 days.
The cells were fixed with 4% paraformaldehyde (10
min) at 4°C and blocked in 1X PBS containing 10% normal goat serum
(Thermo Fisher Scientific, Inc.) and 0.3 M glycine for 1 h at room
temperature. To analyze mesenchymal stem cell marker expression,
passage 3 cells were incubated at room temperature for 45 min with
the following FITC or PE-conjugated monoclonal antibodies:
Anti-Cluster of differentiation (CD)73 (Clone AD2, cat. no. 561254,
1:60), anti-CD90 (Clone 5E10, cat. no. 555595, 1:50), anti-CD105
(Clone 266, cat. no. 561443, 1:1,000), anti-CD34 (Clone 581, cat.
no. 560710, 1:50) and anti-CD45 (Clone HI30, cat. no. 562312, 1:50,
all BD Biosciences). Subsequently, the cells were examined using a
FACS Canto II flow cytometer (BD Biosciences) and analyzed using BD
CellQuest Pro Software V1.2 (BD Biosciences).
Immunohistochemical staining
hPDLSCs were cultured in a 24-well plate. At 60%
confluence, 0.5 ml 4% paraformaldehyde (cat. no. MFCD00133991;
Thermo Fisher Scientific, Inc.) was added into each well for 20 min
at room temperature. After washing the cells with PBS (cat. no.
10010031; Thermo Fisher Scientific, Inc.) three times, 0.2% Triton
X-100 (cat. no. HFH10; Thermo Fisher Scientific, Inc.) was added to
the cells for 30 min, and subsequently, the cells were incubated
with 3% H2O2 for 10 min at room temperature.
Cells were blocked in 10% normal serum (Thermo Fisher Scientific,
Inc.) with 1% BSA (Thermo Fisher Scientific, Inc.) in TBS for 2 h
at room temperature. Each well was incubated with 50 µl
anti-vimentin (cat. no. ab193555; 1:200; Abcam) antibody for 2 h at
37°C. After washing with PBS three times for 2 min each time, 50 µl
goat anti-rabbit IgG H&L preadsorption secondary antibody (cat.
no. ab96899; Abcam, 1:20,000) was added to each well for 20 min at
37°C. The cells were then washed with PBS three times.
Subsequently, a DAB Reagent kit (cat. no. PW017; Shanghai Sangong
Pharmaceutical Co., Ltd.) was used and the cells were observed
under a fluorescence microscope at ×400 magnification).
Cell transfection
hPDLSCs (4×105) were transfected with 50
nmol of PWAR6 overexpression vector (PWAR6-OE), empty
negative vector control (NC) (pCMV6-XL4-PWAR6, cat. no. SC127195
and its empty control vector were purchased from OriGene
Technologies, Inc.), short hairpin (sh)RNA negative control (cat.
no. C03002, shNC, Suzhou GenePharma Co., Ltd.), shPWAR6
(TGCTATCCTATTCATTTAGTATA, Suzhou GenePharma Co., Ltd.), miR-106a-5p
mimic (GAUGGACGUGACAUUCGUGAAAA, cat. no. miR10000103-1-5, Guangzhou
RiboBio Co., Ltd.) or mimic control (MC,
5′-UUCUCCGAACGUGUCACGUTT-3′, Guangzhou RiboBio Co., Ltd.) using
Lipofectamine® 2000 transfection reagent (cat. no.
11668019; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Briefly, 0.8–1.0 µg of overexpression
vectors, shRNAs miRNA mimics or their respective controls were
diluted in 50 µl DMEM medium (cat. no. 12491015; Thermo Fisher
Scientific, Inc.). Similarly, 1–3 µl Lipofectamine® 2000
reagent was diluted in 50 µl DMEM medium and maintained at room
temperature for 5 min. Subsequently, the diluted DNA and
Lipofectamine 2000® reagent were mixed together and
incubated at room temperature for 20 min. The mixed solution was
added to the cells for 2 h. Following a further incubation in DMEM
at 37°C with 5% CO2 for 24 or 48 h, transfection
efficiency was assessed.
ALP activity assay
ALP activity was detected using the Alkaline
Phosphatase Assay kit (cat. no. ab83369; Abcam). hPDLSCs
(1×105) were incubated with OM in 24-well plates for 7
days at room temperature. According to the manufacturer's protocol,
10 µl ALP enzyme solution and 50 µl 5 mM para-nitrophenyl
phosphate solution were added into each well and incubated at 25°C
for 60 min in the dark. The reaction was terminated by adding 20 µl
stop solution. The absorbance of each well was measured at a
wavelength of 405 nm using a microplate reader (cat. no.
11-120-533; Thermo Fisher Scientific, Inc.).
Alizarin red staining
Alizarin red staining was performed using the
Alizarin Red S Staining kit (cat. no. 0223; ScienCell Research
Laboratories, Inc.). According to the manufacturer's protocol,
following aspiration of the culture medium, 1×105 cells
were washed twice with 1 ml PBS and fixed in 4% paraformaldehyde
for 15 min at room temperature. Before cell staining, the fixative
was removed, and cells were washed three times with
diH2O. After removing diH2O, 1 ml 2% Alizarin
Red S Stain solution was added to each well and incubated for 20–30
min at room temperature. Subsequently, the solution was removed,
and cells were washed three to five times using diH2O.
To prevent the cells from drying out, 1 ml diH2O was
added to each well. The samples were observed under a light
microscope at ×400 magnification. Quantification of mineralization
indicated by Alizarin red staining was performed using Image-Pro
Plus (version 6.0; Media Cybernetics, Inc.).
Bioinformatics and dual-luciferase
reporter assay
StarBase V2.0 (starbase.sysu.edu.cn) (33) was used to predict the target gene of
PWAR6 and the dual-luciferase reporter assay was performed
to investigate the findings. To assess whether miR-106a-5p targets
PWAR6 and BMP2, this was examined using the
luciferase pGL3-Basic vector (Promega Corporation). miR-106a-5p
binding sites in wild-type (WT) and mutant (Mut) PWAR6 and
BMP2 were analyzed by performing a dual-luciferase reporter
assay using the Dual-Luciferase Reporter assay system (cat. no.
E1910; Promega Corporation). Briefly, after aspirating the culture
medium, 2×105 cells were washed two to three times using
1X PBS. Subsequently, 100–150 µl 1X passive lysis buffer (PLB) was
added to the cells to ensure complete and even coverage of the cell
monolayer. The plates were incubated for 15 min at room temperature
with gentle agitation. After 24 h, the cells were added to the
luminometer tubes. Then, to each luminometer tube, 100 µl
Luciferase Assay Reagent II was added and 20 µl PLB was carefully
transferred. The tube was placed in the luminometer (cat. no.
E5311; Promega Corporation) and the reading was initiated. Finally,
100 µl Stop & Glo® Reagent was added to measure the
activities. miR-106a-5p mimic was co-transfected with WT or MUT
luciferase vector into 293T cells (American Type Culture
Collection) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Renilla luciferase activity was
detected in the same method described above. After 48 h of
transfection, activity was measured.
Western blotting
Following cell culture in OM for 7 days, hPDLSCs
were assessed by western blotting. Total protein (20 µg/lane) was
extracted from hPDLSCs (4×105) using Mammalian Protein
Extraction Reagent (cat. no. 78501; Thermo Fisher Scientific,
Inc.). The protein concentration was determined using a BCA Protein
Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. Subsequently, 20 µg
protein lysate was separated via 12% SDS-PAGE (cat. no. P0012A;
Beyotime Institute of Biotechnology) and transferred to PVDF
membranes (cat. no. FFP28; Beyotime Institute of Biotechnology).
The membranes were blocked using 5% bovine serum albumin (cat. no.
A1933; Sigma-Aldrich; Merck KGaA) for 2 h at room temperature.
Subsequently, the membranes were incubated at 4°C overnight with
the following primary antibodies: Anti-Osteocalcin (OCN; cat. no.
ab93876; 1:500; Abcam), anti-Runx2 (cat. no. ab23981; 1:1,000;
Abcam), anti-BMP2 (cat. no. ab214821; 1:1,000; Abcam) and
anti-GADPH (cat. no. ab8245 or ab205719; 1:1,000; Abcam). Following
primary incubation, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. ab6721; 1:2,000;
Abcam) or goat anti-mouse IgG H&L (cat. no. ab150113, 1:200,
Abcam) secondary antibody at room temperature for 1 h. Protein
bands were visualized using enhanced chemiluminescence reagent
(cat. no. FD8000; Fdbio Science). Protein expression levels were
quantified using ImageJ software (Version 1.8.0; National
Institutes of Health) with GAPDH as the loading control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the nuclei and
cytoplasm of hPDLSCs using the Cytoplasmic and Nuclear RNA
Purification kit (cat. no. 21000; Norgen Biotek Corp.). Total RNA
was extracted from hPDLSCs using TRIzol® reagent (cat.
no. 15596018; Thermo Fisher Scientific, Inc.). RNA concentration
and optical density (1.8–2.1) were detected using a Nanodrop 8000
spectrophotometer (cat. no. ND-8000-GL; Thermo Fisher Scientific,
Inc.) and RNA integrity was assessed via 1% agarose gel
electrophoresis (cat. no. G442001; Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using the PrimeScript
RT Reagent kit with gDNA Eraser (cat. no. RR047B; Takara
Biotechnology Co., Ltd.). Briefly, 2 µl 5X gDNA Eraser Buffer, 1 µl
gDNA Eraser, 1 µg total RNA and 10 µl RNase-free dH20
were mixed together and incubated using an Applied Biosystems
Veriti PCR system (cat. no. 4484073; Thermo Fisher Scientific,
Inc.) for 2 min at 42°C. Subsequently, 1 µl PrimeScript RT Enzyme
Mix I, 4 µl RT primer Mix, 4 µl 5X PrimeScript Buffer 2 and 1 µl
RNase Free dH20 were added to 10 µl sample and incubated
at 37°C for 15 min, followed by incubation at 85°C for 5 sec. qPCR
was performed using the 7500 Real-Time PCR system (cat. no.
4351105; Thermo Fisher Scientific, Inc.) using SYBR green
(Invitrogen; Thermo Fisher Scientific, Inc.) with the following
thermocycling conditions: 95°C for 1 min, 95°C for 5 sec and 60°C
for 30 sec, for a total of 40 cycles, 72°C for 30 sec, with a final
extension at 72°C for 90 sec. Primers used were purchased from
Guangzhou RiboBio Co., Ltd. and are listed in Table I. Expression levels were quantified
using the 2−ΔΔCq method (34) and normalized to the internal
reference genes U6 and GAPDH.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| PWAR6 | F:
GCCTACATGATGGGCAGTTT |
|
| R:
ACAACCAAAAGCAAGCCAAC |
| RUNX2 | F:
AGTAGCCAGGTTCAACGATCTGA |
|
| R:
GACTGTTATGGTCAAGGTGAAACTCTT |
| OCN | F:
CGCTACCTGTATCAATGGCTGG |
|
| R:
ATGTGGTCAGCCAACTCGTCA |
| BMP2 | F:
GCCTGCTTCGCCATCT |
|
| R:
TGCCTCCTCCTTCTCCC |
| GAPDH | F:
TGGATTTGGACGCATTGGTC |
|
| R:
TTTGCACTGGTACGTGTTGAT |
| ALP | F:
AACACCAATGTAGCCAAG |
|
| R:
TCGGGCAGCGGTTACTGT |
| U6 | F:
GCTTCGGCAGCACATATACTAAAAT |
|
| R:
CGCTTCACGAATTTGCGTGTCAT |
| microRNA- | F:
GATGCTCAAAAAGTGCTTACAGTGCA |
| 106a-5p | R:
TATGGTTGTTCTGCTCTCTGTCTC |
Statistical analysis
Statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc.). Data are presented as the mean
± SEM. All experiments were performed in triplicate. Comparisons
among multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. The unpaired Student's t test was used for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cellular morphology and identification
of hPDLSCs
hPDLSC cellular morphology was observed using P3
cells. Growing adherent cells were long and spindle-shaped and
appeared in a radial or vortex-shaped close arrangement.
Immunohistochemical staining results indicated that vimentin was
expressed, which indicated that hPDLSCs were vimentin positive
(Fig. 1A). hPDLSCs were CD73
(99.34%), CD90 (99.88%) and CD105 (99.67%) positive, but CD34
(0.41%) and CD45 (0.58%) negative (Fig.
1B).
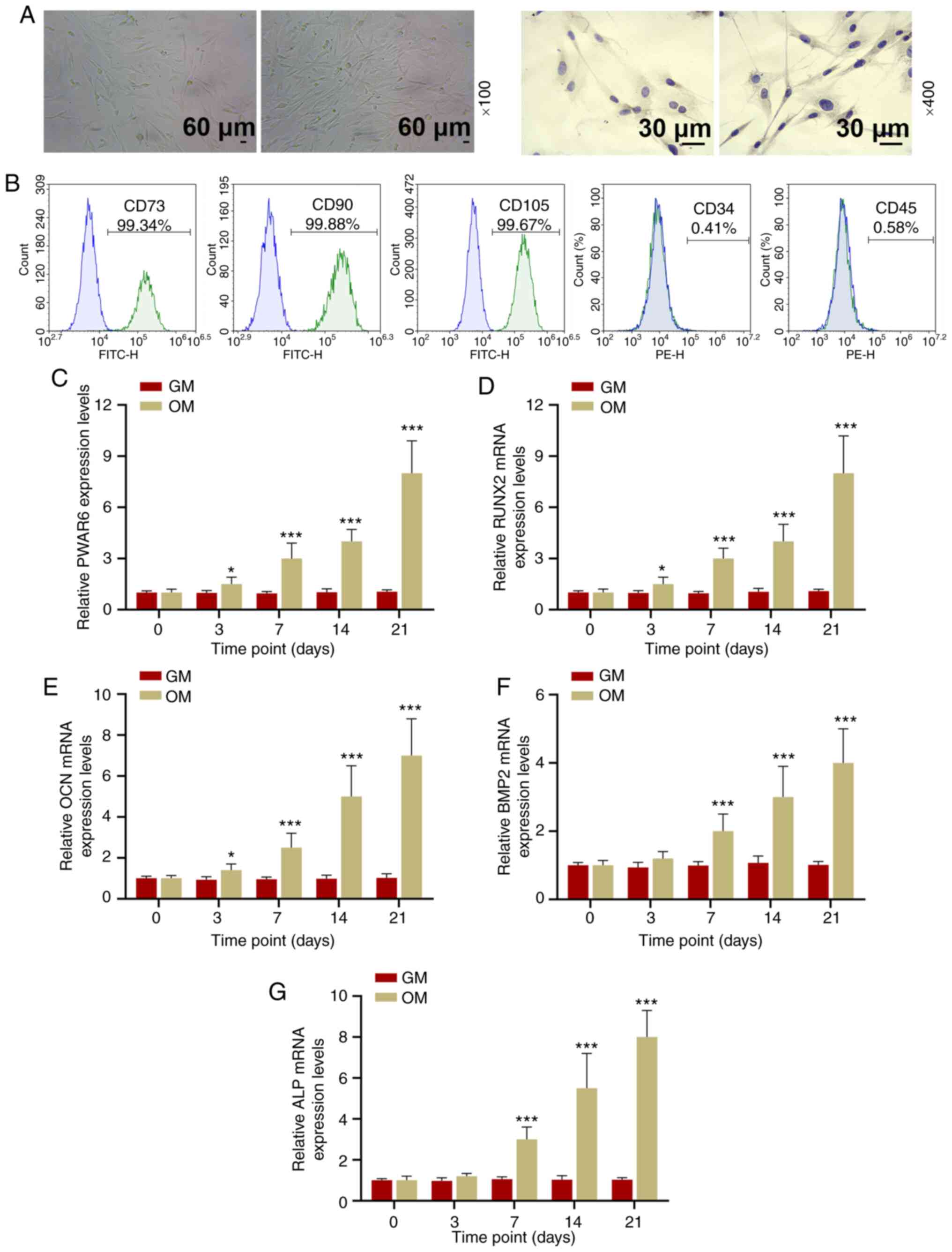 | Figure 1.PWAR6 is upregulated during
the osteogenic induction of hPDLSCs. (A) hPDLSC cell morphology was
detected by microscopy (left:light microscopy, right:
Immunohistochemical staining observed using fluorescence
microscopy). (B) Flow cytometric analysis indicated that hPDLSCs
were CD73+, CD90+, CD105+, CD34- and CD45-. Reverse
transcription-quantitative PCR was performed to detect the
expression levels of (C) PWAR6, (D) RUNX2, (E)
OCN, (F) BMP2 and (G) ALP in hPDLSCs cultured
in OM or GM for 0–21 days. *P<0.05 and ***P<0.001 vs. GM.
hPDLSCs, human periodontal ligament stem cells; CD, cluster of
differentiation; GM, growth medium; OM, osteogenic medium. |
PWAR6 expression during the osteogenic
induction and differentiation of hPDLSCs
hPDLSCs were cultured with OM to induce osteogenic
differentiation. The expression levels of PWAR6 and
osteogenic markers, including RUNX2, OCN, BMP2 and
ALP, in hPDLSCs were measured at 0, 3, 7, 14 and 21 days
following osteogenic induction. The expression levels of
PWAR6 and the osteogenic markers gradually increased in a
time-dependent manner in the OM-incubated group. By contrast,
following treatment with GM, the expression levels of PWAR6
and the osteogenic markers were not significantly altered among
hPDLSCs incubated for different periods of time (Fig. 1C-G).
PWAR6 promotes the osteogenic
differentiation of hPDLSCs
Based on the observation that the expression levels
of PWAR6 and the osteogenic markers displayed a similar trend
during hPDLSC osteogenic induction, the possible role of
PWAR6 in hPDLSC osteogenic differentiation was investigated.
A PWAR6 overexpression lentiviral plasmid (PWAR6-OE)
and a PWAR6 knockdown lentiviral plasmid (shPWAR6)
were constructed. Transfection efficiency was assessed by
performing RT-qPCR, which indicated that PWAR6-OE
significantly increased PWAR6 expression, whereas
shPWAR6 significantly reduced PWAR6 expression in
hPDLSCs compared with the corresponding negative control groups
(Fig. 2A). Based on the finding
that significant differences in gene expression were observed at 7
days post-osteogenic induction, the expression levels of RUNX2,
OCN and BMP2 in the transfected hPDLSCs were detected
after 7 days of culture in OM. The results indicated that
PWAR6-OE significantly upregulated RUNX2, OCN and
BMP2 expression levels in hPDLSCs, whereas PWAR6
knockdown significantly downregulated the mRNA expression levels of
osteogenic marker genes compared with the corresponding negative
control groups (Fig. 2B). Similar
but less significant regulatory effects of PWAR6 on Runx2,
OCN and BMP2 expression levels were observed at the protein level
(Fig. 2C). The effect of
PWAR6 on hPDLSC osteogenic differentiation was investigated
by performing Alizarin red staining and ALP activity assays. The
PWAR6-OE group displayed significantly increased
mineralization and ALP activity compared with the negative control
group. By contrast, mineralization and ALP activity were
significantly decreased in the PWAR6 knockdown group compared with
the shNC group (Fig. 2D-F).
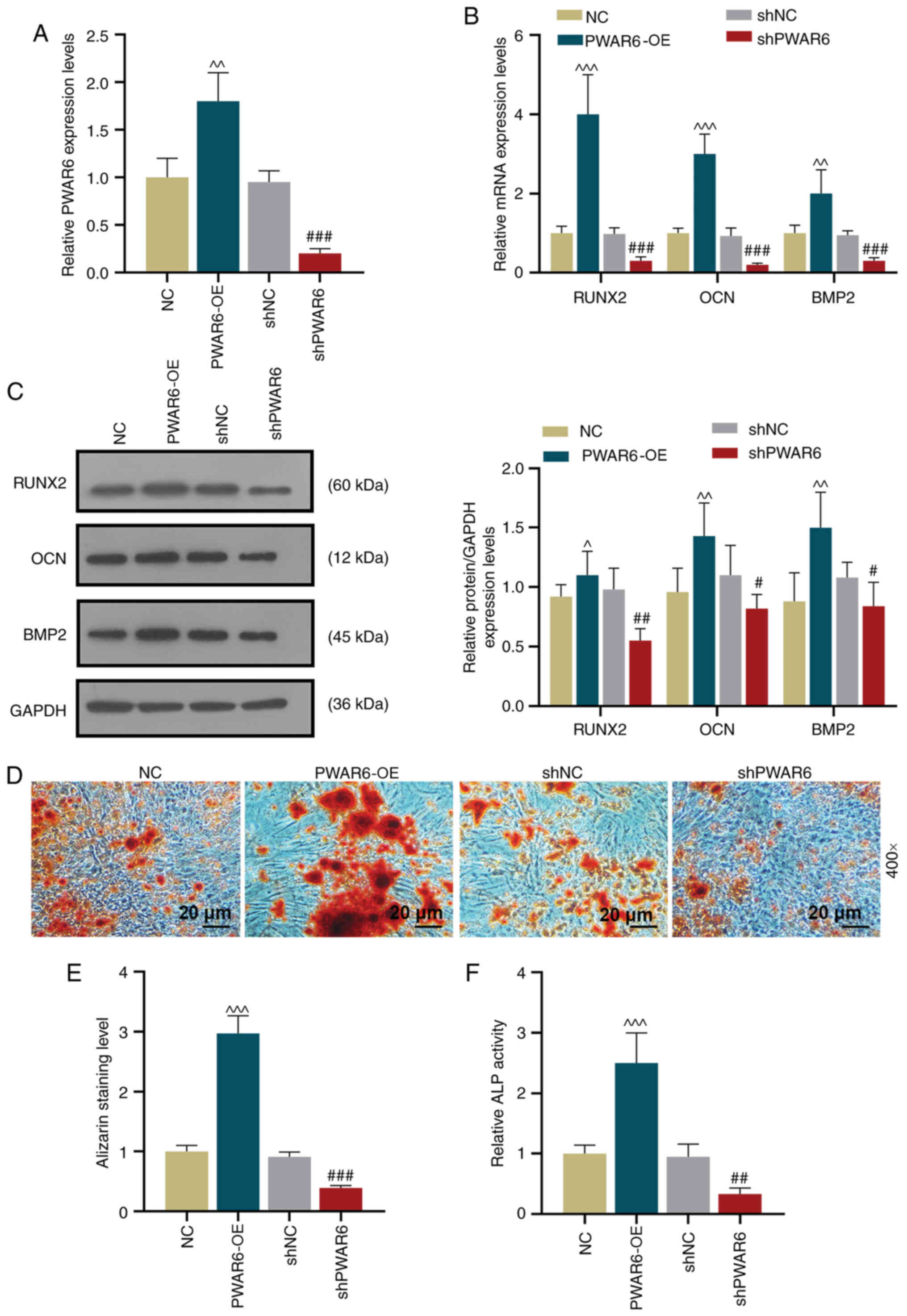 | Figure 2.PWAR6 overexpression increases
the osteogenic differentiation and mineralization of human
periodontal ligament stem cells. (A) RT-qPCR was performed to
detect PWAR6 expression level following transfection with
PWAR6-OE, shPWAR6, NC or shNC (^^P<0.01
vs. NC; ###P<0.001 vs. shNC). (B) RT-qPCR was
performed to detect RUNX2, OCN and BMP2 mRNA expression levels
following transfection. (C) Following transfection, protein
expression levels were determined by western blotting for Runx2,
OCN and BMP2 (^P<0.05, ^^P<0.01 and
^^^P<0.001 vs. NC; #P<0.05,
##P<0.01 and ###P<0.001 vs. shNC).
Osteogenesis was (D) determined by Alizarin red staining and (E)
quantified. (F) Mineralization was assessed by performing an ALP
activity assay. (^^^P<0.001 vs. NC;
##P<0.01 and ###P<0.01 vs. shNC).
PWAR6-OE, PWAR6 overexpression vector; NC, negative
control; sh, short hairpin RNA; RT-qPCR, reverse
transcription-quantitative PCR; Runx2, runt-related transcription
factor 2; OCN, osteocalcin; BMP2, bone morphogenetic protein 2;
ALP, alkaline phosphatase. |
PWAR6 acts as a sponge of
miR-106a-5p
To determine whether PWAR6 acted as a sponge
of miR-106a-5p, StarBase was used to predict the binding sites
between miR-106a-5p and PWAR6 (Fig. 3A). Subsequently, the expression of
PWAR6 in the cytoplasm and nuclei of hPDLSCs was detected,
and the results indicated that PWAR6 expression was
significantly higher in the cytoplasm compared with the nucleus
(Fig. 3B). A dual-luciferase
reporter assay was performed, which suggested that miR-106a-5p
mimic significantly inhibited the luciferase activity of the
PWAR6-WT reporter, but displayed a limited effect on the
PWAR6-Mut reporter compared with the negative control group
(Fig. 3C). Furthermore,
PWAR6 expression was negatively associated with miR-106a-5p
expression in hPDLSCs, as indicated by PWAR6 overexpression
reducing miR-106a-5p expression levels and PWAR6 knockdown
increasing miR-105a-5p expression levels in hPDLSCs compared with
the corresponding negative control groups (Fig. 3D). In addition, miR-106a-5p
expression levels were significantly downregulated in hPDLSCs on
days 7–21 of osteogenic induction compared with the GM-incubated
group (Fig. 3E).
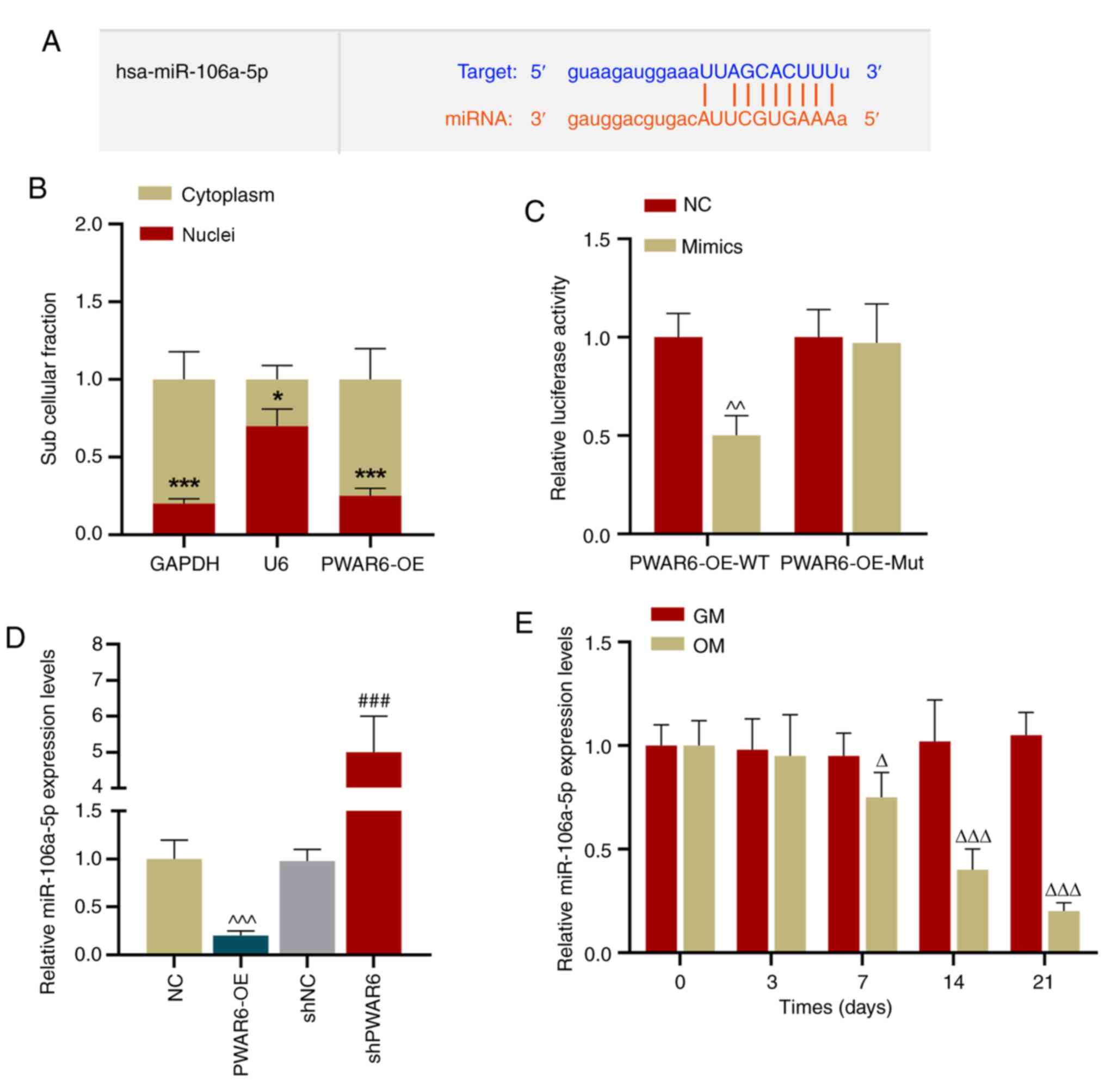 | Figure 3.PWAR6 serves as a sponge of
miR-106a-5p. (A) StarBase was used to predict the binding site
between miR-106a-5p and PWAR6. (B) RT-qPCR was performed to
detect PWAR6 expression levels in the cytoplasm and nuclei
of hPDLSCs (*P<0.05 and ***P<0.01 vs. cytoplasm). (C) A
dual-luciferase reporter assay was performed to detect luciferase
activities of PWAR6-WT and PWAR6-Mut in miR-106a-5p mimic-
and mimic control-transfected cells (^^P<0.01 vs.
NC). (D) RT-qPCR was performed to detect miR-106a-5p expression
levels in PWAR6-OE-, shPWAR6-, NC- and shNC-
transfected hPDLSCs (^^^P<0.001 vs. NC;
###P<0.001 vs. shNC). (E) RT-qPCR was performed to
detect miR-106a-5p expression levels in hPDLSCs cultured in GM or
OM for 0–21 days (ΔP<0.05 and
ΔΔΔP<0.001 vs. GM). miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; hPDLSCs, human periodontal ligament
stem cells; PWAR6-OE, PWAR6 overexpression vector;
sh, short hairpin RNA; NC, negative control; GM, growth medium; OM,
osteogenic medium; WT, wild-type; Mut, mutant. |
PWAR6 negatively regulates miR-106a-5p
during osteogenic differentiation
Based on the finding that PWAR6 sponged
miR-106a-5p, the present study further analyzed how the
relationship between PWAR6 and miR-106a-5p altered hPDLSCs
osteogenesis. The results demonstrated that miR-106a-5p expression
was significantly increased in miR-106a-5p mimic-transfected cells
compared with control and mimic control-transfected cells (Fig. 4A). In addition, the results
indicated that miR-106a-5p mimic significantly increased the
expression level of miR-106a-5p compared with the corresponding
control group, but PWAR6-OE attenuated the effect of
miR-106a-5p mimic on miR-106a-5p expression levels (Fig. 4B). Similarly, at day 7 of osteogenic
induction, miR-106a-5p mimic significantly downregulated
PWAR6-OE-induced RUNX2, OCN and BMP2 mRNA
expression levels in hPDLSCs compared with the MC + PWAR6-OE
group (Fig. 4C). Similar effects of
miR-106a-5p mimic and PWAR6-OE on Runx2, OCN and BMP2
expression were also observed at the protein level; however, the
effects of PWAR6-OE on OCN expression were significantly
altered by miR-106a-5p mimic (Fig.
4D). In addition, hPDLSCs were analyzed by Alizarin red
staining and ALP activity assays. The results indicated that
miR-106a-5p mimic attenuated PWAR6-OE-induced mineralization
and ALP activity in hPDLSCs (Fig.
4E-G).
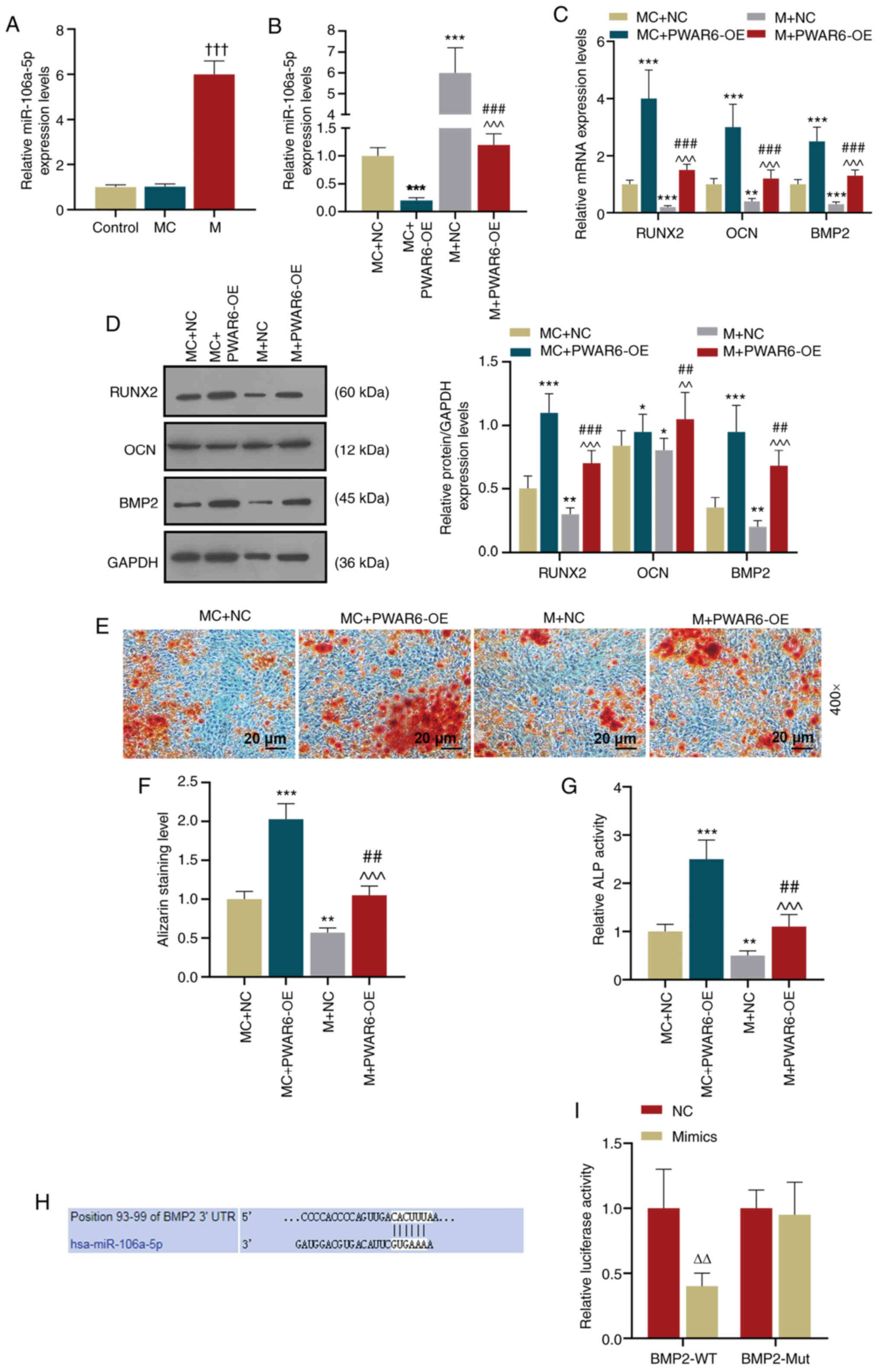 | Figure 4.PWAR6 negatively regulates
miR-106a-5p. BMP2 was identified as a target gene of miR-106a-5p.
(A) RT-qPCR was performed to detect miR-106a-5p expression levels
in transfected hPDLSCs (†††P<0.001 vs. MC). (B)
RT-qPCR was performed to detect miR-106a-5p expression levels in
co-transfected hPDLSCs (***P<0.001 vs. MC + NC;
###P<0.001, vs. MC + PWAR6-OE;
^^^P<0.001 vs. M + NC). (C) RT-qPCR was performed to
detect RUNX2, OCN and BMP2 expression levels in hPDLSCs following
transfection (**P<0.01 and ***P<0.001 vs. MC + NC;
###P<0.001 vs. MC + PWAR6-OE;
^^^P<0.001 vs. M + NC). Protein expression levels
were (D) determined by western blotting and (E) semi-quantified for
Runx2, OCN and BMP2 in hPDLSCs following transfection (*P<0.05,
**P<0.01 and ***P<0.001 vs. MC + NC; ##P<0.01
and ###P<0.001 vs. MC + PWAR6-OE;
^^P<0.01 and ^^^P<0.001 vs. M + NC).
Osteogenesis was (E) determined by Alizarin red staining and (F)
quantified. (G) Mineralization was determined by performing an ALP
activity assay (**P<0.01 and ***P<0.001 vs. MC + NC;
##P<0.01 vs. MC + PWAR6-OE; ^^^P<0.001
vs. M + NC). (H) StarBase was used to predict the binding site
between BMP2 and miR-106a-5p. (I) A dual-luciferase reporter assay
was performed to detect the luciferase activities of BMP2-WT and
BMP2-Mut in miR-106a-5p mimic- and mimic control-transfected cells
(ΔΔP<0.01 vs. NC). miR, microRNA; RT-qPCR, Reverse
transcription-quantitative PCR; hPDLSCs, human periodontal ligament
stem cells; MC, miR-106a-5p mimic control; NC, negative control; M,
miR-106a-5p mimic; Runx2, runt-related transcription factor 2; OCN,
osteocalcin; BMP2, bone morphogenetic protein 2; ALP, alkaline
phosphatase; WT, wild-type; Mut, mutant; 3′UTR, 3′-untranslated
region; PWAR6-OE, PWAR6 overexpression vector. |
BMP2 is the target gene of
miR-106a-5p
The downstream target of miR-106a-5p was predicted
using StarBase, and the binding site between BMP2 and
miR-106a-5p was predicted (Fig.
4H). A dual-luciferase reporter assay was conducted to verify
the interaction between BMP2 and miR-106a-5p, which
indicated that miR-106a-5p mimic significantly inhibited the
luciferase activity of the BMP2-WT reporter, but displayed a
limited effect on the luciferase activity of the BMP2-Mut
reporter compared with the negative control group (Fig. 4I).
Discussion
Inhibition of hPDLSC osteogenic differentiation
under stimuli such as inflammation and hypoxia may result in the
loss of periodontal connective tissues (35). The present study aimed to improve
the current understanding of the regulation of hPDLSC osteogenesis
and the mechanism underlying periodontal tissue loss. The results
indicated that in OM-incubated cells, the expression levels of
PWAR6 and four osteogenic-related proteins (Runx2, OCN, BMP2
and ALP) were increased compared with GM-incubated cells. Secondly,
compared with the corresponding control groups, PWAR6
overexpression significantly increased osteogenesis, whereas
PWAR6 knockdown led to the opposite result, which indicated
that PWAR6 may serve a potential regulatory role during the
osteogenesis of hPDLSCs.
Previous studies have investigated the role of
PWAR6 in cancer and other diseases (29,30).
Lei et al (29) demonstrated
that gene telomeric expression of the PWAR6 breakpoint
significantly affects the pathogenesis of Prader-Willi syndrome. In
addition, PWAR6 has a notable influence on the survival of
patients with glioma and is involved in the immune response, DNA
repair and epithelial-mesenchymal transition (30). However, the effects of PWAR6
on the osteogenesis of hPDLSCs have not been previously reported;
therefore, to the best of our knowledge, the present study
indicated for the first time that PWAR6 may positively
regulate the osteogenic differentiation of hPDLSCs and their ALP
activity by regulating osteogenic factors, such as Runx2, OCN and
BMP2.
It has been reported that multiple lncRNAs serve as
miRNAs sponges (36–41). Wu et al (36) revealed that lncRNA PAGBC displays a
sponge effect on miR-133b and miR-511, which promotes metastasis
and tumor growth of gallbladder cancer. Previous studies have
demonstrated that the differentiation of mesenchymal stem cells
into osteoblasts is regulated by miRNAs (42–44).
The expression levels of miR-42, miR-106a, miR-148a, let-7i and
miR-99a are specific in human mesenchymal stem cells, whereas the
expression levels of miR-15b, miR-24, miR-130b, miR-30c and
miR-130a are specific in differentiated osteoblasts (45). Vimalraj and Selvamurugan (45) indicated that miR-15b promotes
adipogenesis and myogenesis lineages. By considering the sponging
effect of lncRNAs, the present study further investigated the
relationship between PWAR6 and miRNAs. The small non-coding
RNA miR-106a-5p is involved in colorectal cancer, osteosarcoma and
astrocytoma (46). Regarding the
association of miR-106a-5p with osteogenesis, miR-106a-5p promotes
apoptosis and suppresses cell proliferation (47). In the present study, miR-106a-5p
expression was decreased in OM-incubated cells compared with
GM-incubated cells. Furthermore, the results indicated that
PWAR6 may serve as a sponge of miR-106a-5p to promote
osteogenesis via upregulating osteogenic markers. Although other
miRNAs, such as miR-1827, miR-145 and miR-5100, and their
association with osteogenic differentiation have been previously
reported (48–50), at present, little is known about the
association between miR-106a-5p and osteogenesis. Based on the
results of the present study, it was hypothesized that PWAR6
may regulate the osteogenesis of hPDLSCs by serving as a sponge of
miR-106a-5p.
BMP2 induces chondrogenic differentiation,
osteogenic differentiation and endochondral ossification of stem
cells (51). BMP2 can influence the
osteogenic differentiation of mesenchymal stem cells (52). BMP2-modified injectable hydrogel can
be used for the osteogenic differentiation of human periodontal
ligament stem cells (53). The
results of the present study suggested that BMP2 was a
target gene of miR-106a-5p, which was supported by Li et al
(54), who demonstrated that
miR-106a regulates osteogenesis and adipogenic lineage commitment
of human mesenchymal stem cells by directly targeting BMP2.
BMP2 is a vital differentiation-related factor that belongs to the
transforming growth factor β family, and can promote bone healing
and induce bone growth (55,56).
The effect of PWAR6 on the osteogenic differentiation of
hPDLSCs was preliminarily investigated in the present study, but
the effects of proinflammatory factors on PWAR6 and miR-106a
require further investigation.
In conclusion, the present study identified a
possible interaction network between lncRNA PWAR6 and
miR-106a-5p. PWAR6 may promote the osteogenic
differentiation and mineralization of hPDLSCs and serve as a sponge
of miR-106a-5p. The present study indicated that modulating the
osteogenic differentiation of hPDLSCs may serve as a potential
therapeutic strategy for periodontal disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and YB made substantial contributions to the
conception and design of the study, acquired, analyzed and
interpreted the data, and drafted or critically revised the article
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jingmen No. 1 People's Hospital (approval no.
Y20180831) and written consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Zeng X, Miao J, Liu C, Wei F, Liu
D, Zheng Z, Ting K, Wang C and Guo J: Upregulation of long
noncoding RNA MEG3 inhibits the osteogenic differentiation of
periodontal ligament cells. J Cell Physiol. 234:4617–4626. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xue P, Li B, An Y, Sun J, He X, Hou R,
Dong G, Fei D, Jin F, Wang Q and Jin Y: Decreased MORF leads to
prolonged endoplasmic reticulum stress in periodontitis-associated
chronic inflammation. Cell Death Differ. 23:1862–1872. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H and Zhang D: Effects of
periodontal ligament cells on alveolar bone metabolism under the
action of force and inflammatory factors and its molecular
mechanisms. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 39:432–437.
2017.PubMed/NCBI
|
|
5
|
Nagata M, Iwasaki K, Akazawa K, Komaki M,
Yokoyama N, Izumi Y and Morita I: Conditioned medium from
periodontal ligament stem cells enhances periodontal regeneration.
Tissue Eng Part A. 23:367–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu
N, Ding B, Liu W, Liu Y, Shi H, et al: High levels of β-catenin
signaling reduce osteogenic differentiation of stem cells in
inflammatory microenvironments through inhibition of the
noncanonical Wnt pathway. J Bone Miner Res. 26:2082–2095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Li ZG, Si YM, Chen B and Meng J:
The difference on the osteogenic differentiation between
periodontal ligament stem cells and bone marrow mesenchymal stem
cells under inflammatory microenviroments. Differentiation.
88:97–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia B, Qiu X, Chen J, Sun X, Zheng X, Zhao
J, Li Q and Wang Z: A feed-forward regulatory network
lncPCAT1/miR-106a-5p/E2F5 regulates the osteogenic differentiation
of periodontal ligament stem cells. J Cell Physiol.
234:19523–19538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei F, Yang S, Guo Q, Zhang X, Ren D, Lv T
and Xu X: MicroRNA-21 regulates osteogenic differentiation of
periodontal ligament stem cells by targeting Smad5. Sci Rep.
7:166082017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan GQ, Wang X, Yang F, Yang ML, Zhang GR,
Wang GK and Zhou Q: MicroRNA-22 promoted osteogenic differentiation
of human periodontal ligament stem cells by targeting HDAC6. J Cell
Biochem. 118:1653–1658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu X, Li M, Jin Y, Liu D and Wei F:
Identification and integrated analysis of differentially expressed
lncRNAs and circRNAs reveal the potential ceRNA networks during
PDLSC osteogenic differentiation. BMC Genet. 18:1002017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ugawa Y, Yamamoto T, Kawamura M, Yamashiro
K, Shimoe M, Tomikawa K, Hongo S, Maeda H and Takashiba S:
Rho-kinase regulates extracellular matrix-mediated osteogenic
differentiation of periodontal ligament cells. Cell Biol Int.
41:651–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang W, Liang Q, Du L, Shang L, Wang T and
Ge S: Sequential application of bFGF and BMP-2 facilitates
osteogenic differentiation of human periodontal ligament stem
cells. J Periodontal Res. 54:424–434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JS, Lee JC and Heo JS:
Polydopamine-assisted BMP-2 immobilization on titanium surface
enhances the osteogenic potential of periodontal ligament stem
cells via integrin-mediated cell-matrix adhesion. J Cell Commun
Signal. 12:661–672. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao F, Zhan J, Chen X, Zhang K, Lai R and
Feng Z: miR-214 promotes periodontal ligament stem cell
osteoblastic differentiation by modulating Wnt/β-catenin signaling.
Mol Med Rep. 16:9301–9308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Shao J, Zhou Y, Friis T, Yao J, Shi
B and Xiao Y: The impact of Wnt signalling and hypoxia on
osteogenic and cementogenic differentiation in human periodontal
ligament cells. Mol Med Rep. 14:4975–4982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LJ, Hu BB, Shi XL, Ren MM, Yu WB, Cen
SD, Hu RD and Deng H: Baicalein enhances the osteogenic
differentiation of human periodontal ligament cells by activating
the Wnt/β-catenin signaling pathway. Arch Oral Biol. 78:100–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Wu F, Song Y, Li X, Wu Q, Duan Y
and Jin Z: Long noncoding RNA related to periodontitis interacts
with miR-182 to upregulate osteogenic differentiation in
periodontal mesenchymal stem cells of periodontitis patients. Cell
Death Dis. 7:e23272016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Wang P, Wan L, Xu S and Pang D:
The emergence of noncoding RNAs as Heracles in autophagy.
Autophagy. 13:1004–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cech TR and Steitz JA: The noncoding RNA
revolution-trashing old rules to forge new ones. Cell. 157:77–94.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharp PA: The centrality of RNA. Cell.
136:577–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhuang W, Ge X, Yang S, Huang M, Zhuang W,
Chen P, Zhang X, Fu J, Qu J and Li B: Upregulation of lncRNA MEG3
promotes osteogenic differentiation of mesenchymal stem cells from
multiple myeloma patients by targeting BMP4 transcription. Stem
Cells. 33:1985–1997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Dong R, Diao S, Du J, Fan Z and
Wang F: Differential long noncoding RNA/mRNA expression profiling
and functional network analysis during osteogenic differentiation
of human bone marrow mesenchymal stem cells. Stem Cell Res Ther.
8:302017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao J, Yu X, Hu X, Fan J, Wang J, Zhang
Z, Zhao C, Zeng Z, Shu Y, Zhang R, et al: lncRNA H19 mediates
BMP9-induced osteogenic differentiation of mesenchymal stem cells
(MSCs) through Notch signaling. Oncotarget. 8:53581–53601. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mozaffari SV, Stein MM, Magnaye KM,
Nicolae DL and Ober C: Parent of origin gene expression in a
founder population identifies two new candidate imprinted genes at
known imprinted regions. PLoS One. 13:e02039062018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei M, Mitsuhashi S, Miyake N, Ohta T,
Liang D, Wu L and Matsumoto N: Translocation breakpoint disrupting
the host SNHG14 gene but not coding genes or snoRNAs in typical
Prader-Willi syndrome. J Hum Genet. 64:647–652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin X, Jiang T, Bai J, Li J, Wang T, Xiao
J, Tian Y, Jin X, Shao T, Xu J, et al: Characterization of
transcriptome transition associates long noncoding RNAs with glioma
progression. Mol Ther Nucleic Acids. 13:620–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du L, Yang P and Ge S: Isolation and
characterization of human gingiva-derived mesenchymal stem cells
using limiting dilution method. J Dent Sci. 11:304–314. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Kang W, Du L and Ge S: Rho-kinase
inhibitor Y-27632 facilitates the proliferation, migration and
pluripotency of human periodontal ligament stem cells. J Cell Mol
Med. 21:3100–3112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bae WJ, Shin MR, Kang SK, Zhang-Jun, Kim
JY, Lee SC and Kim EC: HIF-2 inhibition supresses inflammatory
responses and osteoclastic differentiation in human periodontal
ligament cells. J Cell Biochem. 116:1241–1255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu XS, Wang F, Li HF, Hu YP, Jiang L,
Zhang F, Li ML, Wang XA, Jin YP, Zhang YJ, et al: LncRNA-PAGBC acts
as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO
Rep. 18:1837–1853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ballantyne MD, McDonald RA and Baker AH:
lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol
Ther. 99:494–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang K, Jin W, Song Y and Fei X: LncRNA
RP11-436H11.5, functioning as a competitive endogenous RNA,
upregulates BCL-W expression by sponging miR-335-5p and promotes
proliferation and invasion in renal cell carcinoma. Mol Cancer.
16:1662017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kallen AN, Zhou XB, Xu J, Qiao C, Ma J,
Yan L, Lu L, Liu C, Yi JS, Zhang H, et al: The imprinted H19 lncRNA
antagonizes let-7 microRNAs. Mol Cell. 52:101–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu C, Li L, Xie F, Guo S, Liu F, Dong N
and Wang Y: LncRNA TUG1 sponges miR-204-5p to promote osteoblast
differentiation through upregulating Runx2 in aortic valve
calcification. Cardiovasc Res. 114:168–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Abdelmohsen K, Yang X, De S,
Grammatikakis I, Noh JH and Gorospe M: LncRNA OIP5-AS1/cyrano
sponges RNA-binding protein HuR. Nucleic Acids Res. 44:2378–2392.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo L, Zhao RC and Wu Y: The role of
microRNAs in self-renewal and differentiation of mesenchymal stem
cells. Exp Hematol. 39:608–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Liu J, Zeng Z, Fan J, Huang S,
Zhang L, Zhang B, Wang X, Feng Y, Ye Z, et al: lncRNA Rmst acts as
an important mediator of BMP9-induced osteogenic differentiation of
mesenchymal stem cells (MSCs) by antagonizing Notch-targeting
microRNAs. Aging (Albany NY). 11:12476–12496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seenprachawong K, Nuchnoi P, Nantasenamat
C, Prachayasittikul V and Supokawej A: Computational identification
of miRNAs that modulate the differentiation of mesenchymal stem
cells to osteoblasts. PeerJ. 4:e19762016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vimalraj S and Selvamurugan N: MicroRNAs
expression and their regulatory networks during mesenchymal stem
cells differentiation toward osteoblasts. Int J Biol Macromol.
66:194–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan YJ, Wei LL, Wu XJ, Huo FC, Mou J and
Pei DS: MiR-106a-5p inhibits the cell migration and invasion of
renal cell carcinoma through targeting PAK5. Cell Death Dis.
8:e31552017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hai B, Ma Y, Pan X, Yong L, Liang C, He G,
Yang C, Zhu B and Liu X: Melatonin benefits to the growth of human
annulus fibrosus cells through inhibiting miR-106a-5p/ATG7
signaling pathway. Clin Interv Aging. 14:621–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hao W, Liu H, Zhou L, Sun Y, Su H, Ni J,
He T, Shi P and Wang X: MiR-145 regulates osteogenic
differentiation of human adipose-derived mesenchymal stem cells
through targeting FoxO1. Exp Biol Med (Maywood). 243:386–393. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu S, Peng W, Li X, Weng J, Zhang X, Guo
J, Huang D, Rong Q and Chen S: miR-1827 inhibits osteogenic
differentiation by targeting IGF1 in MSMSCs. Sci Rep. 7:461362017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang H, Cui Y, Luan J, Zhou X, Li C, Li H,
Shi L and Han J: MiR-5100 promotes osteogenic differentiation by
targeting Tob2. J Bone Miner Metab. 35:608–615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou N, Li Q, Lin X, Hu N, Liao JY, Lin
LB, Zhao C, Hu ZM, Liang X, Xu W, et al: BMP2 induces chondrogenic
differentiation, osteogenic differentiation and endochondral
ossification in stem cells. Cell Tissue Res. 366:101–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun MH, Wang WJ, Li Q, Yuan T and Weng WJ:
Autologous oxygen release nano bionic scaffold composite miR-106a
induced BMSCs enhances osteoblast conversion and promotes bone
repair through regulating BMP-2. Eur Rev Med Pharmacol Sci.
22:7148–7155. 2018.PubMed/NCBI
|
|
53
|
Park SH, Kwon JS, Lee BS, Park JH, Lee BK,
Yun JH, Lee BY, Kim JH, Min BH, Yoo TH and Kim MS: BMP2-modified
injectable hydrogel for osteogenic differentiation of human
periodontal ligament stem cells. Sci Rep. 7:66032017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li H, Li T, Wang S, Wei J, Fan J, Li J,
Han Q, Liao L, Shao C and Zhao RC: miR-17-5p and miR-106a are
involved in the balance between osteogenic and adipogenic
differentiation of adipose-derived mesenchymal stem cells. Stem
Cell Res. 10:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oliveira OR, Martins SP, Lima WG and Gomes
MM: The use of bone morphogenetic proteins (BMP) and
pseudarthrosis, a literature review. Rev Bras Ortop. 52:124–140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bais MV, Wigner N, Young M, Toholka R,
Graves DT, Morgan EF, Gerstenfeld LC and Einhorn TA: BMP2 is
essential for post natal osteogenesis but not for recruitment of
osteogenic stem cells. Bone. 45:254–266. 2009. View Article : Google Scholar : PubMed/NCBI
|


















