Introduction
Skeletal remodeling and integrity, as well as bone
homeostasis, are strictly controlled by the coordinated
interactions of osteoblast-mediated ossification and
osteoclast-regulated bone resorption (1). The abnormal osteoclastic activation
and/or defective osteoblastic function may contribute to the
decline in bone quality and lead to internal microstructure damage
to bone, ultimately leading to the development of bone metabolic
disorders, such as rheumatoid arthritis and osteoporosis.
Osteoclasts are hematopoietic stem cell-derived, multinucleated
giant cells that have a unique role in bone-mineralized matrix
degradation (2). Osteoclast
formation is a sophisticated multiple-stage process that comprises
proliferation, differentiation, and the fusion of
monocyte/macrophage precursors (3).
There are two cytokines that are essential for the regulation of
osteoclastogenesis-related cell behavior. Specifically, osteoclast
precursors rely on macrophage colony-stimulating factor (M-CSF) for
survival and proliferation and receptor activator of NF-κB ligand
(RANKL) binding for osteoclast differentiation (4–6). Upon
RANK/RANKL interaction, the adaptor protein tumor necrosis factor
receptor-associated factor 6 (TRAF6) is recruited to the
cytoplasmic tail of RANK for activation of several signal
transduction pathways. TRAF6-induced downstream events include
initially the activation of distinct signaling pathways that are
mediated by NF-κB and MAPK, and subsequently the synthesis of
transcription factors, such as nuclear factor of activated T-cells
cytoplasmic 1 (NFATc1) and Fos proto-oncogene AP-1 transcription
factor subunit (c-Fos). These transcription factors serve as
definitive regulators and modulate the expression of multiple
specific genes that regulate terminal osteoclastogenesis, including
tartrate resistant acid phosphatase (TRAP), osteoclast-associated
receptor (OSCAR), integrin β3 (ITGB3) and cathepsin K (CTSK), thus,
driving osteoclast differentiation and fusion (2,7,8).
Notably, osteoclastic bone resorption is dependent
not only on the number of osteoclasts formed but also on the extent
of osteoclast activation. Activated mature osteoclasts closely
adhere to the bone surface, followed by a ruffled border, and thus
form a sealing chamber from the extracellular environment. The
process of bone resorption takes place in Howship's lacunae. Inside
the segregated chamber, calcium hydroxyapatite crystals are
dissolved in an acidic environment due to the acidification by
hydrogen ion pumps on the cell surface, resulting in exposure of
the organic bone matrix (9,10). Additionally, different proteolytic
enzymes, particularly cysteine proteinases and matrix
metalloproteinases, are also activated at low pH and are involved
in digestion of the organic bone matrix. Although the exact
cysteine proteinases that are in charge of osteoclast-mediated bone
degradation are not fully understood, substantial evidence has
shown that, as one member of the papain-cysteine proteinase family,
cathepsin K is abundantly expressed in mammalian osteoclasts and
serves a dominant role in efficient cleavage of collagenous bone
matrix (11,12). Single-point mutations in the human
cathepsin K gene have been associated with a rare autosomal
recessive lysosomal storage disorder termed as pycnodysostosis, a
specific osteopetrotic-like phenotype, characterized by dwarfism
and facial deformity, as well as a predisposition to fragility
fractures (13). Similarly, the
phenotype of pycnodysostosis was later reproduced in cathepsin
K-deficient mice, which display moderate osteopetrosis with
hypermineralization, as well as many undigested collagen fragments
in the subosteoclastic area, mainly in their long bones (14). Consequently, these observations
support the hypothesis that cathepsin K may be a potential
antiresorptive target for pharmacologic intervention in bone
metabolic disorders.
Cystatins, endogenous, reversible, and tight-binding
inhibitors of cysteine proteinases, have been successfully
extracted, cloned, and characterized in different taxonomic groups,
from viruses to vertebrates (15).
Based on amino acid sequence similarity and crystal structure
variation, the cystatin family is categorized into three distinct
subgroups. Type I cystatins (stefins) are primarily intracellular
but have been seldomly reported in body fluids. Stefins are
unglycosylated proteins consisting of ~100 amino acids without
disulfide bonds or carbohydrate chains. Type II cystatins, which
are secretory proteins with nearly 120 amino acids and possess 2
conserved disulfide bridges toward the carboxyl terminus, are
mostly extracellular and contain cystatins C, D, E/M, F, G, S, SA,
and SN. Type III cystatins (kininogens), multidomain glycoproteins
composed of ~350 amino acids, are synthesized in the liver and
circulate predominantly in blood plasma (16,17).
To date, cystatins have been widely researched and confirmed to
participate in various physiopathological processes. Mammalian
cystatins serve vital roles in immunomodulation, tumorigenesis, and
bone remodeling, whereas parasite cystatins significantly
contribute to immunosuppression in humans and may be biotherapeutic
candidates for autoimmune or allergic diseases, such as asthma and
inflammatory colitis (15,18–20).
Schistosoma japonicum is a digenetic blood
fluke with a widespread distribution in mainland China. Cystatins
from S. japonicum (Sj-Cys) are similar to other parasite
cystatins and have also been demonstrated in recent years to serve
a vital role in modulation of the host immune response. Yang et
al (21) reported that Sj-Cys
could restrain the production of tumor necrosis factor (TNF)-α and
interleukin (IL-) 6 in lipopolysaccharide-induced RAW264.7 cells
in vitro. Furthermore, our previous study demonstrated that
Sj-Cys alleviated severe inflammation and ameliorated
sepsis-induced multiple organ failure, by reducing pro-inflammatory
cytokines (22). Although
widespread reports of Sj-Cys have focused on the immunoregulation
of inflammation and inhibitory effects on cysteine proteinase,
little is known regarding the functional diversification. Given
that both Sj-Cys and stefin B (cystatin B) belong to the homologous
type I cystatins class, they may have common features to some
extent (23). Stefin B, a
single-chain protein, is ubiquitously expressed by multiple cell
types, including osteoclasts. Stefin B administration restrains
osteoclastic bone resorption via blocking the activity of cathepsin
K (24–26). However, the specific effect of
Sj-Cys on regulation of the formation and activation of osteoclasts
remains unclear. The current study investigated whether Sj-Cys
could affect M-CSF and RANKL-induced osteoclastogenesis and bone
resorption in vitro, and explored the underlying molecular
mechanisms.
Materials and methods
Production and purification of the
recombinant Sj-Cys (rSj-Cys) protein
The expression and purification of rSj-Cys has been
described previously (27).
Briefly, DNA encoding Sj-Cys was cloned into the pET-28a vector
(Promega Corporation). The sequence was confirmed to be inserted
successfully before transformation of the restructured plasmid into
Escherichia coli (BL 21). Next, the transformants were
induced for large-scale protein expression using 1 mM
isopropyl-β-D-1-thiogalactopyranoside (Sigma-Aldrich; Merck KGaA)
at 37°C for 5 h. After purification with the HisPur™ Ni-NTA Spin
Column (Thermo Fisher Scientific, Inc.), endotoxin contamination
was mostly eliminated using the ToxOut™ High-Capacity Endotoxin
Removal kit (BioVision, Inc.), and residual endotoxin detection was
performed using the ToxinSensor™ Chromogenic Limulus Amebocyte
Lysate Endotoxin Assay kit (GenScript), in accordance with the
manufacturer's protocols. Finally, a bicinchoninic acid (BCA)
protein assay kit (Beyotime Institute of Biotechnology) was used to
determine the concentration of rSj-Cys.
Cell culture and in vitro
osteoclastogenesis assay
RAW264.7 cells (American Type Culture Collection)
were cultured in Dulbecco's modified Eagle medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotics (Gibco; Thermo Fisher Scientific, Inc.).
Cells were incubated at 37°C in a humidified 5% CO2
atmosphere. Prior to induction into osteoclasts, the cells were
seeded in 96-well plates at a density of 3×103
cells/well with α-minimal essential medium (α-MEM; Gibco; Thermo
Fisher Scientific, Inc.) and incubated overnight. The culture
medium was then replaced with complete α-MEM containing either
M-CSF alone (25 ng/ml; R&D Systems, Inc.) or M-CSF (25 ng/ml)
and RANKL (30 ng/ml; R&D Systems, Inc.) in the presence of
rSj-Cys (0, 0.01, 0.1, 0.3, 1 or 10 µM), and cells were cultured
for 5 days. The medium was replaced with fresh medium every 2
days.
TRAP staining
After induction for 5 days as aforementioned,
osteoclast formation was analyzed using a commercial TRAP staining
kit (Sigma-Aldrich; Merck KGaA), following the manufacturer's
instructions. In brief, the medium was removed and the cells were
fixed with 4% polyformaldehyde for 10 min at room temperature.
After the removal of stationary liquid and rinsing thoroughly with
distilled water, the cells were incubated for 1 h at 37°C with TRAP
staining solution. The number of TRAP-positive multinucleated cells
with three or more nuclei per well was visualized and counted under
an inverted fluorescence microscope (Olympus Corporation).
Additionally, the estimated half-maximal inhibitory concentration
(IC50) value of rSj-Cys was determined and used for
subsequent experiments.
Bone resorption assay
Bone resorptive ability was evaluated using the
Corning Osteo Assay Surface (COAS; Corning Inc.). RAW264.7 cells
were plated onto the COAS at a density of 3×103
cells/well and cultured in complete α-MEM containing M-CSF (25
ng/ml) and RANKL (30 ng/ml) with or without rSj-Cys
(IC50, 0.3 µM) for osteoclastogenesis. The medium was
replaced with fresh medium every 2 days. Following culture for 5
days, the medium was discarded, and the cells were lysed with 10%
sodium hypochlorite for 10 min before the wells were air-dried. The
surface images of pits were randomly captured using an inverted
fluorescence microscope, and the number and area of pit formation
were quantified using the ImageJ software (version 1.38X; National
Institutes of Health).
Cell viability assay
Cell viability in response to rSj-Cys was assessed
using the MTT colorimetric assay (Beijing Solarbio Science &
Technology Co., Ltd.). In short, the RAW264.7 cells were seeded in
96-well plates at a density of 3×103 cells/well and
cultured in complete DMEM. After 4 h, the cells were treated with
M-CSF (25 ng/ml) and rSj-Cys (concentration, 0 or IC50),
followed by further cultivation for 24, 48 or 72 h. The medium was
removed and MTT reagent was added to each well for an additional 4
h. The optical density was measured at a wavelength of 570 nm using
a microplate reader.
Reverse transcription-quantitative PCR
(RT-qPCR)
For the analysis of mRNA expression levels of
osteoclast-related genes, RAW264.7 cells were seeded in 6-well
plates at a density of 5×104 cells/well and
co-stimulated with M-CSF (25 ng/ml) and RANKL (30 ng/ml). The cells
were then treated with or without rSj-Cys (concentration,
IC50) and cultured until the indicated time points (4,
24, 48 or 72 h). Total RNA was isolated from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using a PrimeScript™ RT reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocol.
Amplification of the cDNA template was conducted using the Q6 Flex
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the SYBR® Premix Ex Taq II kit (Takara Bio,
Inc.) with specific oligonucleotide primers, as listed in Table I (Sangon Biotech Co., Ltd.). The
thermocycling conditions used for qPCR were as follows:
Denaturation at 95°C for 30 sec; followed by 40 cycles of 95°C for
5 sec and 60°C for 30 sec. The β-actin housekeeping gene was used
as an internal control. Relative fold changes in mRNA expression
were calculated using the formula 2−ΔΔCq (28).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Target gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Bax |
TGAAGACAGGGGCCTTTTTG |
AATTCGCCGGAGACACTCG |
| Bcl-2 |
GCTACCGTCGTGACTTCGC |
CCCCACCGAACTCAAAGAAGG |
| CTSK |
GAAGAAGACTCACCAGAAGCAG |
TCCAGGTTATGGGCAGAGATT |
| TRAP |
TGTCATCTGTGAAAAGGTGGTC |
ACTGGAGCAGCGGTGTTATG |
| ITGB3 |
GGCGTTGTTGTTGGAGAGTC |
CTTCAGGTTACATCGGGGTGA |
| RANK |
CCAGGAGAGGCATTATGAGCA |
ACTGTCGGAGGTAGGAGTGC |
| OSCAR |
CCGTGCTGACTTCACACCAA |
GGGGTGACAAGGCCACTTTT |
| TREM-2 |
CTGGAACCGTCACCATCACTC |
CGAAACTCGATGACTCCTCGG |
| NFATc1 |
GGAGAGTCCGAGAATCGAGAT |
TTGCAGCTAGGAAGTACGTCT |
| c-Fos |
CGGGTTTCAACGCCGACTA |
TTGGCACTAGAGACGGACAGA |
| IκBα |
TGAAGGACGAGGAGTACGAGC |
TTCGTGGATGATTGCCAAGTG |
| p65 |
AGGCTTCTGGGCCTTATGTG |
TGCTTCTCTCGCCAGGAATAC |
| β-actin |
AGAGGGAAATCGTGCGTGAC |
CCAAGAAGGAAGGCTGGAAA |
Western blot analysis
The culture conditions and interventions for
osteoclast differentiation were as aforementioned. After 24 or 72
h, total protein was collected using the RIPA lysis buffer
(Beyotime Institute of Biotechnology) and the protein concentration
was calculated using the BCA protein assay kit (Beyotime Institute
of Biotechnology). Aliquots (containing 30 µg protein) were
separated by 10% SDS-PAGE and electrophoretically transferred onto
polyvinylidene difluoride (Millipore) membranes. The membranes were
blocked with 5% skimmed milk for 1 h at room temperature prior to
incubation overnight at 4°C with primary antibodies against CTSK
(1:1,000; cat. no. DF6614), TRAP (1:1,000; cat. no. DF6989), ITGB3
(1:1,000; cat. no. AF6086), RANK (1:1,000; cat. no. DF12532),
triggering receptor expressed on myeloid cells 2 (TREM-2; 1:1,000;
cat. no. DF12529), NFATc1 (1:1,000; cat. no. DF6446), c-Fos
(1:1,000; cat. no. AF0132), NF-κB inhibitor α (IκBα; 1:1,000; cat.
no. AF5002), RELA proto-oncogene NF-κB subunit (p65; 1:1,000; cat.
no. AF5006), β-actin (1:2,000; cat. no. AF7018) (all from Affinity
Biosciences) and OSCAR (1:1,000; cat. no. AF1633; R&D Systems,
Inc.). Horseradish peroxidase (HRP)-conjugated secondary antibody
(1:5,000; cat. no. S0001; Affinity Biosciences) was then added for
90 min at room temperature; then, the immunoreactive proteins were
visualized using chemiluminescent HRP substrate (EMD Millipore).
Band intensity was semi-quantified using ImageJ software and
normalized against β-actin levels.
Statistical analysis
All experiments were conducted independently in
triplicates, and numerical data were presented as means ± standard
deviation. SPSS Statistics version 20.0 (IBM Corp.) and GraphPad
Prism 6.0 (GraphPad Software, Inc.) were used for statistical
analysis. Two-group comparisons were assessed using the Student's
t-test, whereas multiple comparisons were performed using one-way
analysis of variance followed by the Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
rSj-Cys suppresses M-CSF and
RANKL-induced osteoclastogenesis in vitro
After the RAW264.7 cells were incubated with either
M-CSF or M-CSF and RANKL in the absence or presence of rSj-Cys for
5 days, mature osteoclasts were fixed and subjected to TRAP
staining. The results demonstrated that the stimulation of RAW264.7
cells with M-CSF alone failed to induce the formation of
TRAP-positive multinucleated cells, whereas these cells were
frequently found upon treatment with both M-CSF and RANKL (Fig. 1A). However, the number of
TRAP-positive cells was gradually reduced in a dose-dependent
manner following exposure to rSj-Cys (Fig. 1A and B). Osteoclast formation was
restrained by ~30% with 0.1 µM rSj-Cys, and this was almost
completely inhibited with 10 µM rSj-Cys treatment (Fig. 1A and B). Based on the morphological
size and the number of TRAP-positive cells, the IC50
value of rSj-Cys was found to be 0.3 µM. Therefore, rSj-Cys exerted
an inhibitory effect on M-CSF and RANKL-induced osteoclastogenesis
in RAW264.7 cells.
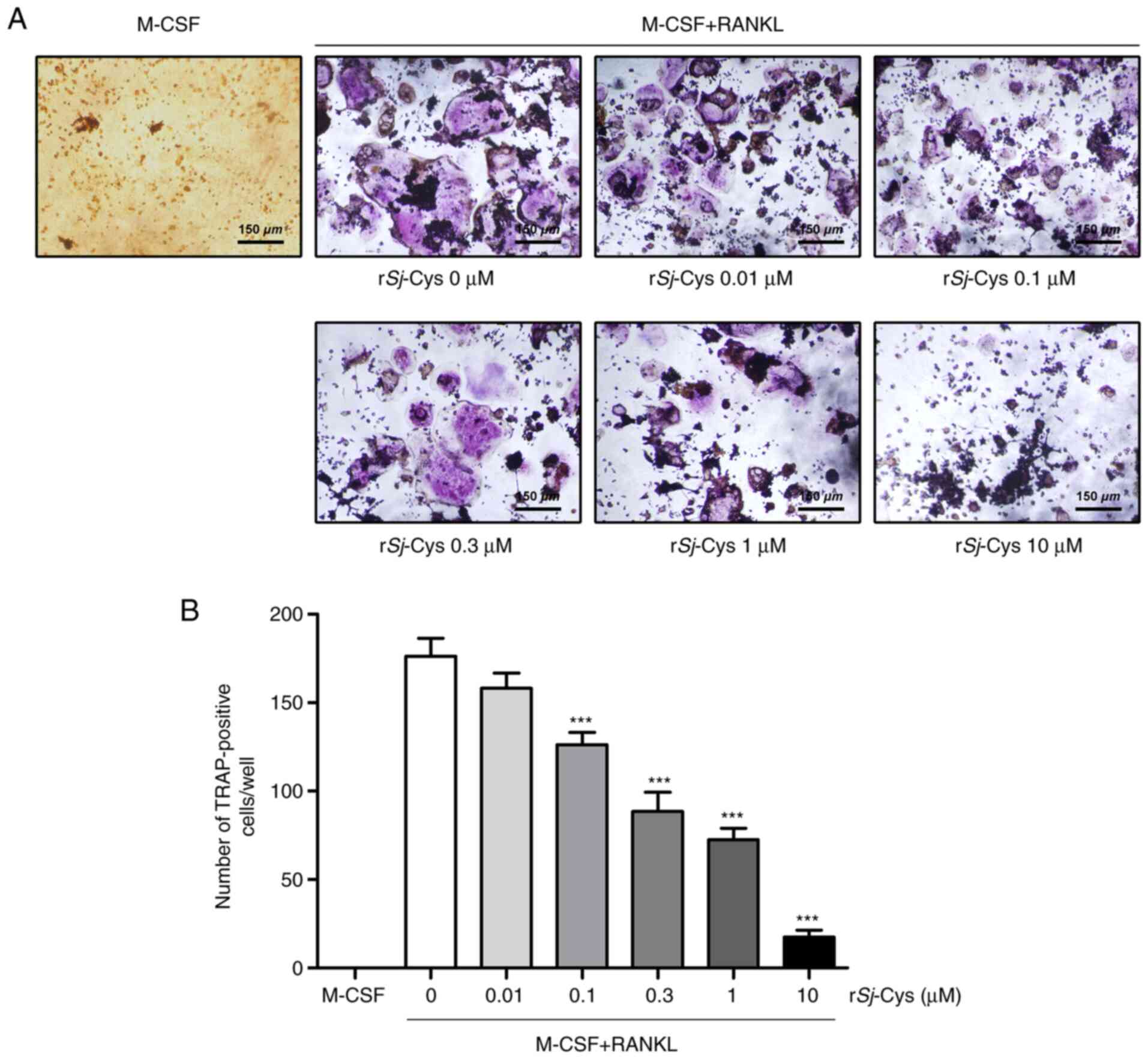 | Figure 1.rSj-Cys suppresses M-CSF and
RANKL-induced osteoclastogenesis in a dose-dependent manner in
vitro. (A) RAW264.7 cells were incubated with either M-CSF (25
ng/ml) alone, or with M-CSF and RANKL (30 ng/ml) in the absence or
presence of rSj-Cys (0, 0.01, 0.1, 0.3, 1 or 10 µM) for 5 days, and
then TRAP staining was performed. TRAP-positive multinucleated
cells were visualized under an inverted microscope. Original
magnification, ×100. (B) The numbers of TRAP-positive
multinucleated cells were counted and the IC50 of
rSj-Cys was found to be 0.3 µM. Data are presented as mean ± SD of
three independent experiments. ***P<0.001 vs. M-CSF + RANKL
group. rSj-Cys, recombinant Schistosoma japonicum cystatins;
M-CSF, macrophage colony-stimulating factor; RANKL, receptor
activator of NF-κB ligand; TRAP, tartrate resistant acid
phosphatase; IC50, half-maximal inhibition
concentration. |
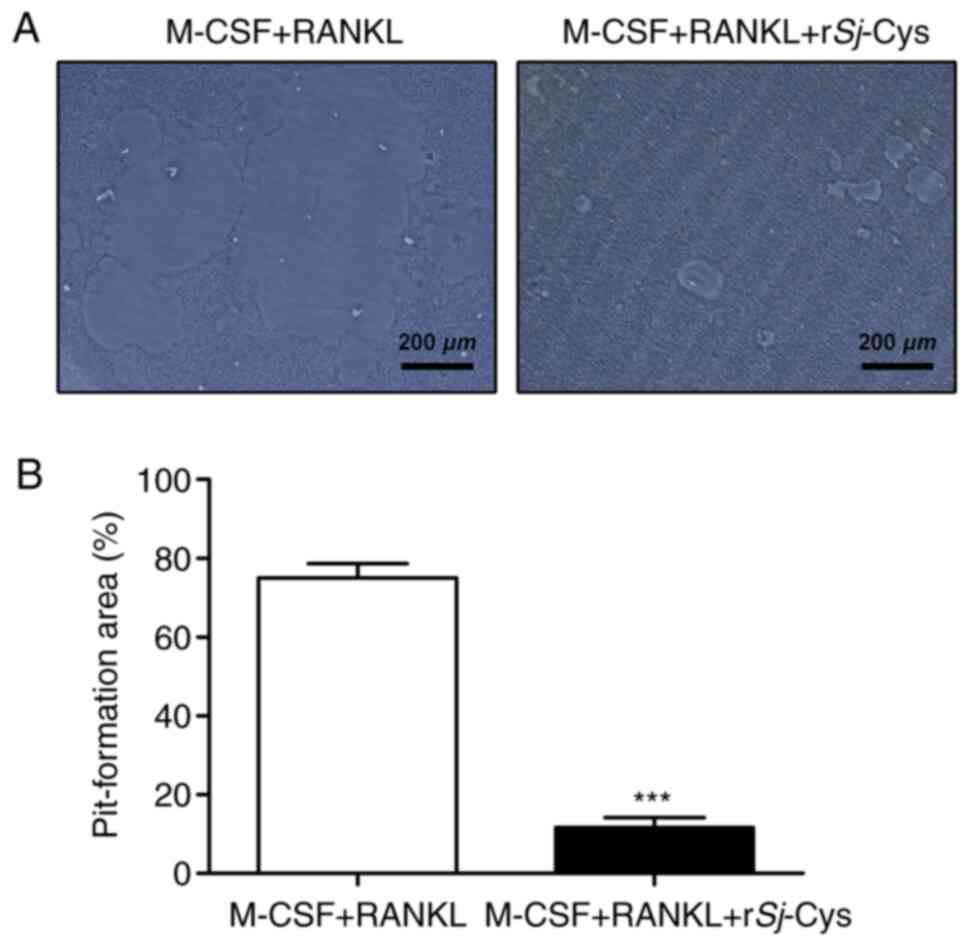
rSj-Cys decreases the bone resorptive
capability of osteoclasts
Since osteoclast formation is a necessary
presupposition for osteoclastic bone resorption, it was
hypothesized that the bone resorptive ability of osteoclasts would
also be markedly restricted by rSj-Cys. Thus, a bone resorption
assay was conducted to investigate the effects of rSj-Cys on M-CSF
and RANKL-induced bone resorption in RAW264.7 cells. As
anticipated, the addition of rSj-Cys significantly decreased the
area of resorption pits, even though the osteoclasts induced by
M-CSF and RANKL generated large resorption pits (Fig. 2A). Quantitative analysis of the
pit-formation area confirmed these results (Fig. 2B) and suggested that rSj-Cys
treatment attenuated the bone resorptive capability of
osteoclasts.
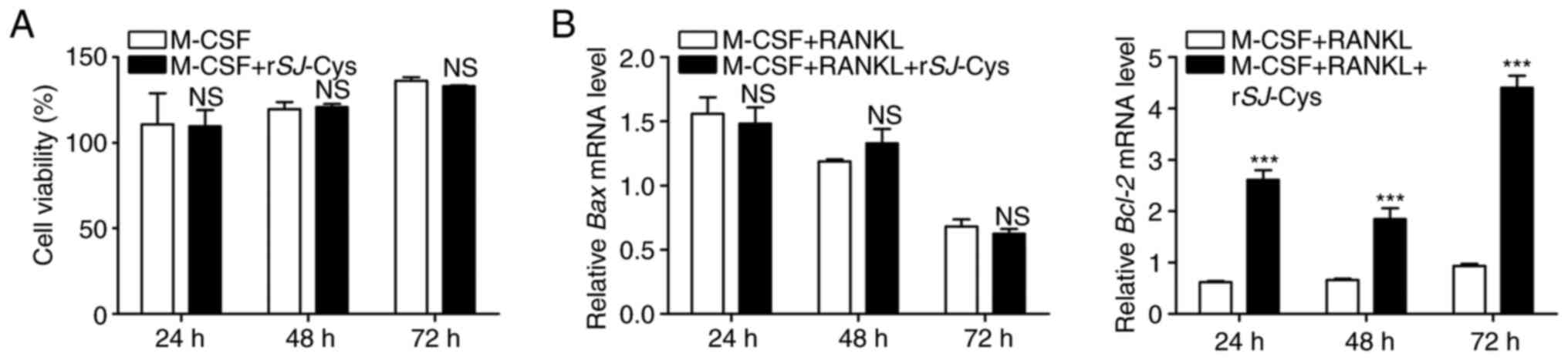
Effects of rSj-Cys on the survival of
RAW264.7 cells
The survival of osteoclast precursors is
indispensable for osteoclast differentiation and formation. To
investigate the survival of the osteoclast precursor cells used in
the present study, the potential cytotoxic effects of rSj-Cys on
the viability of RAW264.7 cells in the presence of M-CSF were
analyzed by performing an MTT colorimetric assay. The results
demonstrated that M-CSF-stimulated cell proliferation was not
evidently influenced by rSj-Cys at IC50 concentrations,
even after 72 h of exposure (Fig.
3A). Simultaneously, RT-qPCR was performed to assess expression
levels of the proapoptotic gene Bax and the antiapoptotic gene
Bcl-2 to evaluate the effects of rSj-Cys on M-CSF and RANKL-induced
apoptosis. As shown in Fig. 3B, the
mRNA expression levels of Bax were decreasing in a time-dependent
manner following stimulation with M-CSF and RANKL, and there was no
appreciable effect of rSj-Cys. By contrast, the mRNA expression
levels of Bcl-2 exhibited a reverse trend and were significantly
elevated following the addition of rSj-Cys (Fig. 3B). Collectively, the possibility
that rSj-Cys adversely affected the survival of RAW264.7 cells and
consequently inhibited osteoclast formation by promoting apoptosis
or by decreasing cell viability was excluded.
rSj-Cys inhibits the expression of
osteoclastogenesis-related genes and proteins
To further elucidate the effects of rSj-Cys on
osteoclastogenesis, the expression levels of genes and proteins
associated with the osteoclast phenotype (CTSK, TRAP and ITGB3) and
the cell surface receptors of osteoclast precursors (RANK, OSCAR
and TREM-2) were explored by RT-qPCR and western blot analysis,
respectively. The results demonstrated that treatment with M-CSF
and RANKL exerted a stimulative effect on the mRNA expression
levels of CTSK, TRAP, ITGB3, RANK and OSCAR in a time-dependent
manner (Fig. 4A). By contrast,
these enhancements were dramatically repressed following rSj-Cys
intervention (Fig. 4A). These
observations were identical to the findings from western blot
analysis at 72 h (Fig. 4B).
Notably, there were no evident variations in both mRNA and protein
expression levels of TREM-2 during the entire induction period
(Fig. 4A and B). The present
results indicated that rSj-Cys inhibited osteoclast differentiation
by regulating the expression of osteoclast phenotype markers and
cell surface receptors of osteoclast precursors, with the exception
of TREM-2.
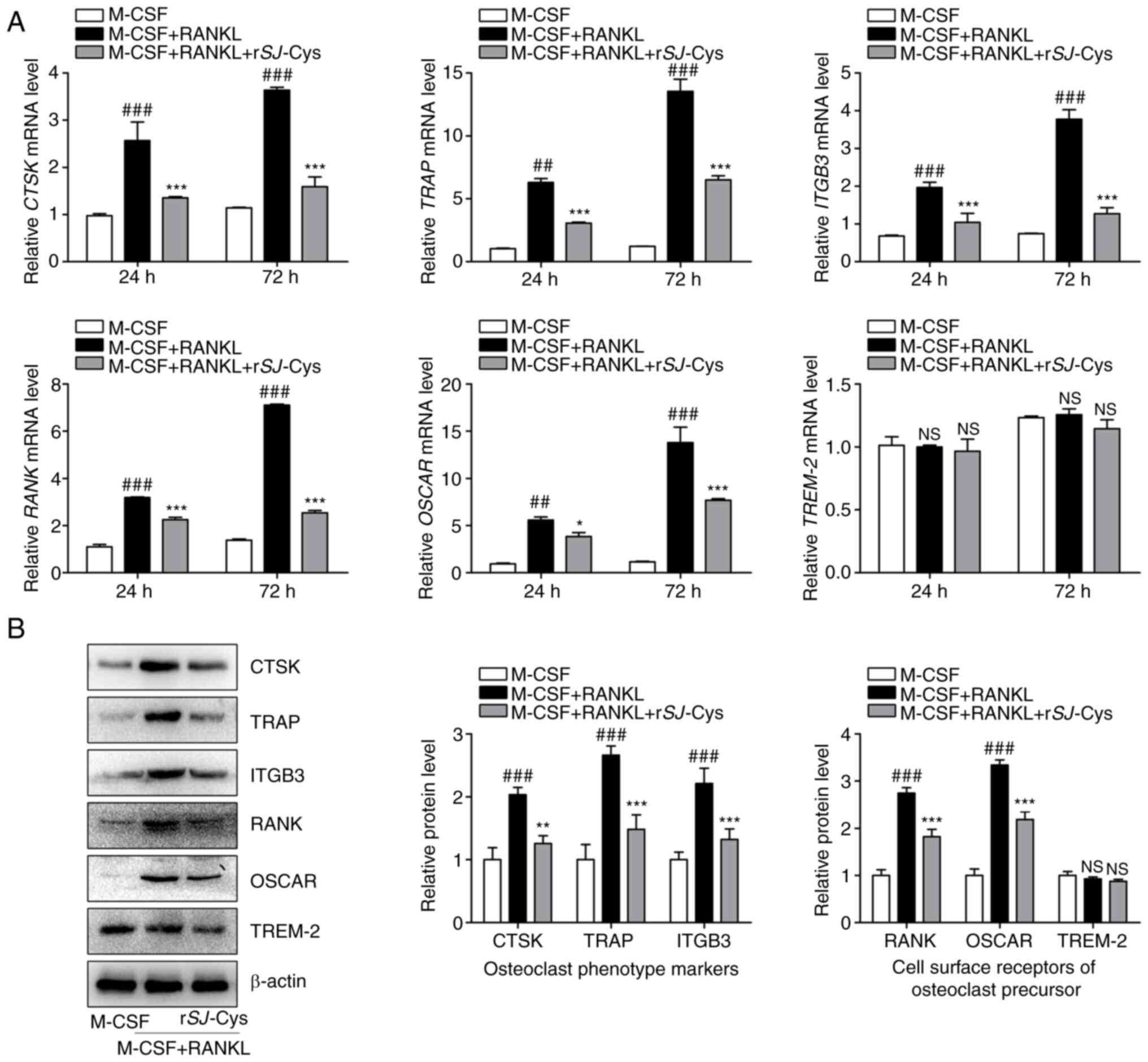 | Figure 4.rSj-Cys inhibits the expression of
osteoclastogenesis-related genes and proteins. RAW264.7 cells were
co-stimulated with M-CSF (25 ng/ml) and RANKL (30 ng/ml) and then
treated with or without rSj-Cys (0.3 µM) for different time periods
(24, 48 or 72 h). The expression levels of genes and proteins
associated with osteoclast phenotype markers (CTSK, TRAP, ITGB3)
and cell surface receptors of osteoclast precursors (RANK, OSCAR,
TREM-2) were determined by (A) reverse transcription-quantitative
PCR and (B) western blot analysis. Band intensity was
semi-quantified with ImageJ software and normalized against β-actin
levels. Data are presented as the mean ± SD of three independent
experiments. ##P<0.01, ###P<0.001 vs.
M-CSF alone group; *P<0.05, **P<0.01, ***P<0.001 vs. M-CSF
+ RANKL group. rSj-Cys, recombinant Schistosoma japonicum
cystatins; M-CSF, macrophage colony-stimulating factor; RANKL,
receptor activator of NF-κB ligand; CTSK, cathepsin K; TRAP,
tartrate resistant acid phosphatase; ITGB3, integrin β3; OSCAR,
osteoclast-associated receptor; TREM-2, triggering receptor
expressed on myeloid cells 2; NS, no significance. |
rSj-Cys represses the M-CSF and
RANKL-induced NF-κB signaling pathway during early-phase
osteoclastogenesis
The NF-κB signaling pathway serves a critical role
in the early phase of osteoclastogenesis and is responsible for the
activation of crucial downstream transcription factors, namely
NFATc1 and c-Fos, which are master regulators of osteoclast
differentiation. Hence, the mRNA and protein expression levels of
NF-κB-associated signaling molecules (IκBα and p65) and of NFATc1
and c-Fos were assessed by RT-qPCR and western blot analysis,
respectively, in order to explore the underlying molecular
mechanisms of the antiosteoclastogenic effects of rSj-Cys. As
presented in Fig. 5A, the mRNA
expression levels of IκBα, p65, NFATc1 and c-Fos were significantly
upregulated upon exposure to M-CSF and RANKL over time, whereas
these increases were significantly inhibited by rSj-Cys
intervention. These results suggested that rSj-Cys might suppress
the NF-κB signaling pathway by downregulating the mRNA expression
levels of IκBα and p65 and might further impact levels of NFATc1
and c-Fos. This was consistent with the findings from western blot
analysis at 24 h (Fig. 5B). The
present results indicated that rSj-Cys may neutralize early-phase
osteoclastogenesis primarily by regulating the NF-κB signaling
pathway.
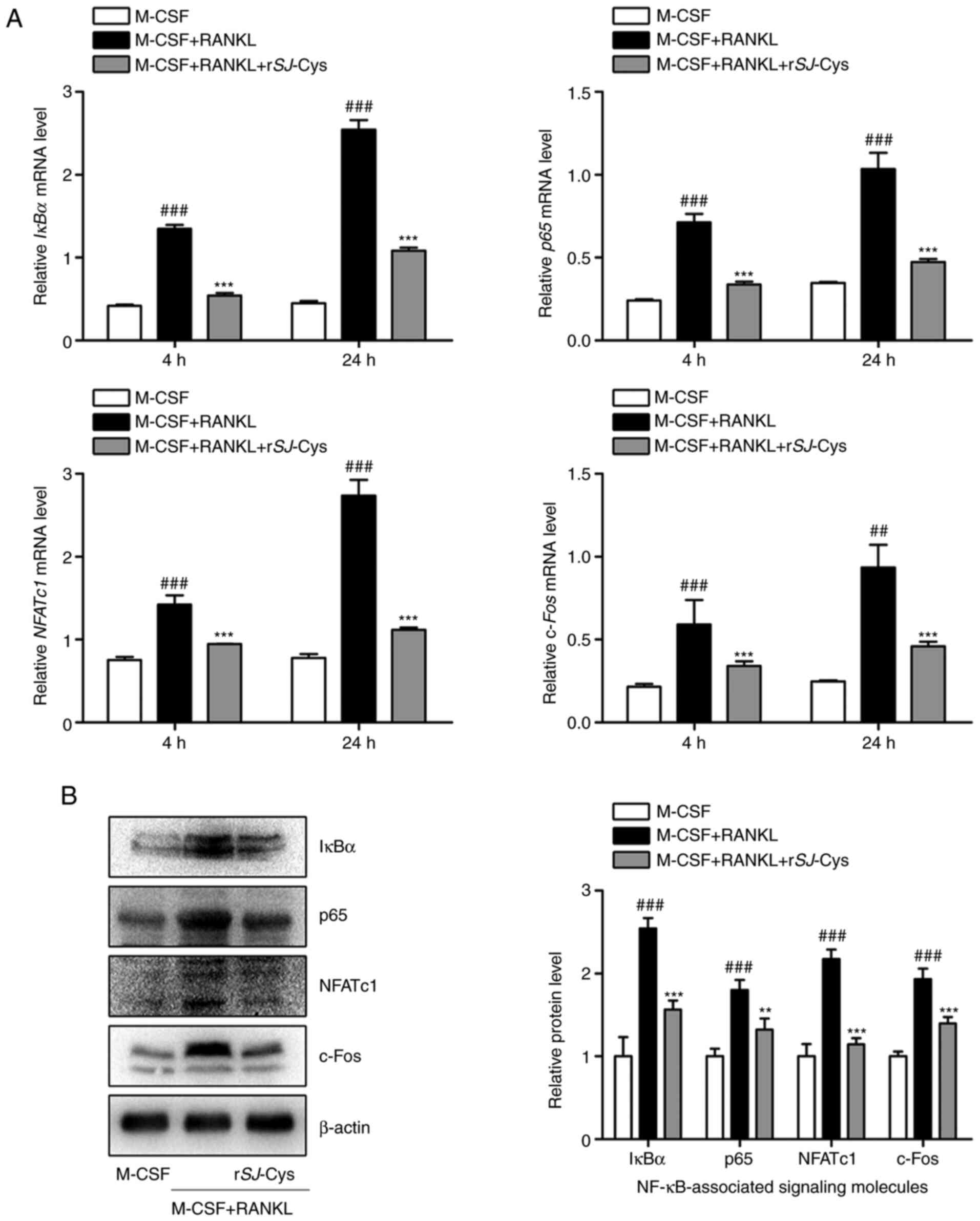 | Figure 5.rSj-Cys represses M-CSF and
RANKL-induced NF-κB signaling during early-phase
osteoclastogenesis. RAW264.7 cells were co-stimulated with M-CSF
(25 ng/ml) and RANKL (30 ng/ml) and then treated with or without
rSj-Cys (0.3 µM) for different time periods (4 or 24 h). The mRNA
and protein expression levels of NF-κB-associated signaling
molecules IκBα and p65, and of downstream targets NFATc1 and c-Fos,
were assessed by (A) reverse transcription-quantitative PCR and (B)
western blot analysis. Band intensity was semi-quantified using the
ImageJ software and normalized against β-actin levels. Data are
presented as mean ± SD of three independent experiments.
##P<0.01, ###P<0.001 vs. M-CSF alone
group; **P<0.01, ***P<0.001 vs. M-CSF + RANKL group. rSj-Cys,
recombinant Schistosoma japonicum cystatins; M-CSF,
macrophage colony-stimulating factor; RANKL, receptor activator of
NF-κB ligand; IκBα, NF-κB inhibitor α; p65, RELA proto-oncogene
NF-κB subunit; NFATc1, nuclear factor of activated T-cells
cytoplasmic 1; c-Fos, Fos proto-oncogene AP-1 transcription factor
subunit. |
Discussion
Excessive bone resorption due to the abnormal
osteoclastic activation and massive secretion of cysteine
proteinases is one of the characteristics of bone metabolic
disorders; and as such, reducing the number of osteoclasts and/or
constraining osteoclast function may be promising approaches for
treatment and prevention of osteolytic destruction. It is generally
known that cysteine proteinases, especially cathepsin K, act a
predominant role in osteoclastic bone matrix degradation (29). To this end, cystatins, as natural
antagonists of these enzymes, have garnered extensive attention.
Although multiple studies have indicated that several mammalian
cystatins, such as cystatin B, C and D, effectively inhibit
osteoclast differentiation and formation, the effects of parasite
cystatins on osteoclastogenesis have not been reported to date
(26,30). The present study explored the
potential role of rSj-Cys and its regulatory mechanism on M-CSF and
RANKL-induced osteoclast differentiation of RAW264.7 cells.
Currently, cystatins have been discovered in various
types of parasite species and mostly belong to the type II class
(31). Despite the numerous studies
on cystatins in a broad range of organisms, there have been few
studies on these molecules from S. japonicum, especially
type I class. Sj-Cys has an inhibitory effect on the proteolytic
activity of papain, primarily owing to the similarity between their
conserved domains and type I cystatins (23). However, the precise effect of Sj-Cys
on the formation and activation of osteoclasts remains unknown,
which motivated our team to conduct the presents study. Based on
previous research and data, it was hypothesized that Sj-Cys might
inhibit osteoclastogenesis and block the activity of cysteine
proteinases to protect against excessive bone resorption.
TRAP is generally utilized as an osteoclast
phenotypic marker because of its specific expression in osteoclasts
(32). The present study
established an osteoclast model using RAW264.7 cells that were
induced by M-CSF and RANKL stimulation, and then evaluated
osteoclast formation by TRAP staining, which is a standard method
to identify mature osteoclasts (TRAP-positive cells). Functionally,
osteoclasts possess the unique role of bone resorption; hence, a
bone resorption assay was conducted to measure pit-formation
ability. The results of these assays demonstrated that rSj-Cys
dose-dependently decreased the number of TRAP-positive cells, with
an IC50 value of 0.3 µM. In terms of the effect of
rSj-Cys on bone resorption, the results showed that rSj-Cys could
significantly reduce the area of resorption pits formed by
osteoclasts. Altogether, the present study suggested that rSj-Cys
inhibited osteoclast formation and impaired the bone resorptive
capability of osteoclasts.
During osteoclastogenesis, M-CSF is crucial for the
proliferation and survival of osteoclast precursors and exhibits an
antiapoptotic function by regulating the expression of Bcl-2
(33,34). In the present study, results of the
MTT colorimetric assay and RT-qPCR both demonstrated that rSj-Cys
did not show cytotoxicity accompanied by a decrease in cell
viability or the promotion of apoptosis in RAW264.7 cells.
Expression of RANK in osteoclast precursors is
induced by M-CSF, and the RANK/RANKL system is critical for
osteoclast differentiation and activation (6). The binding between RANK and RANKL
triggers the recruitment of c-Fos, and subsequent stimulation
directly activates the expression of NFATc1 (3). Previous studies have indicated that
NFATc1 and c-Fos are master transcription factors that regulate
osteoclast differentiation by initiating the transcription of
downstream targets of osteoclastogenesis-related genes (5). Osteoclast differentiation also
requires costimulatory signals induced by immunoglobulin-like
receptors, including TREM-2 and OSCAR. These cell surface receptors
that are expressed by osteoclast precursors are associated with
adaptor proteins, such as DNAX-activating protein 12 or Fc receptor
common γ-chain, and their activation leads to the amplification and
translocation of NFATc1 (10). In
the present study, the results demonstrated that rSj-Cys
dramatically repressed the expression of osteoclast phenotype
markers (CTSK, TRAP, and ITGB3) and cell surface receptors of
osteoclast precursor (RANK and OSCAR) at both the mRNA and protein
levels. These findings suggested that the inhibition of
osteoclastogenesis caused by rSj-Cys may be relevant to the
downregulation of osteoclastogenic genes and proteins.
Furthermore, the activation of c-Fos and NFATc1 is a
downstream event in the NF-κB signaling pathway, which acts an
essential role in the early phase of osteoclast development
(35). Following stimulation with
RANKL, phosphorylated IκBα is degraded and releases p65, which is
translocated into the nucleus and initiates the transcription of
related genes (36). In the present
study, the mRNA and protein expression levels of NF-κB-associated
signaling molecules (IκBα and p65), and of downstream targets
(c-Fos and NFATc1), were downregulated by rSj-Cys. These findings
suggested that rSj-Cys not only suppresses the NF-κB pathway, but
also impacts the expression of its downstream transcription factors
c-Fos and NFATc1 during early-phase osteoclastogenesis.
Taken together, the present study is the first to
shed light on the inhibitory effects of rSj-Cys on M-CSF and
RANKL-induced osteoclast differentiation using RAW264.7 cells.
rSj-Cys downregulated the expression of osteoclastogenesis-related
genes and proteins, thereby restraining osteoclast formation and
impairing the bone resorptive capability of osteoclasts.
Furthermore, the repression of rSj-Cys did not rely on effects on
cell viability or apoptosis, but rather functioned by interfering
with the M-CSF and RANKL-induced NF-κB signaling during early-phase
osteoclastogenesis. Nevertheless, there are limitations of the
present study. The main limitation is that the inhibitory effects
and regulatory mechanisms of rSj-Cys in osteoclastogenesis were
examined in only one cell line in vitro; thus, additional
in vivo investigations will be required to fully understand
its specific functions. Despite this, the present findings
suggested that rSj-Cys, as an inhibitor of osteoclast
differentiation, may serve as a prospective biotherapeutic
candidate for the treatment and prevention of bone metabolic
disorders.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Anhui Province (grant nos. 2008085MH260,
2008085QH362, 1908085MH276 and gxbjZD15), the Joint Science and
Technology Project of Bengbu City and Bengbu Medical College (grant
no. BYLK201830), the Translational Medicine Key Projects of Bengbu
Medical College (grant nos. BYTM2019006 and BYTM2019012), the
Scientific Research Innovation Team of Bengbu Medical College
(grant no. BYKC201910) and the 512 Talents Development Project of
Bengbu Medical College (grant no. by51201205).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YC, BW, PX, HT, LY and YW performed the experiments,
contributed to literature search, analyzed the data and wrote the
manuscript. XY, YF and YM contributed to the conception and design
of the study and reviewed the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ono T and Nakashima T: Recent advances in
osteoclast biology. Histochem Cell Biol. 149:325–341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soysa NS, Alles N, Aoki K and Ohya K:
Osteoclast formation and differentiation: An overview. J Med Dent
Sci. 59:65–74. 2012.PubMed/NCBI
|
|
4
|
Okamoto K, Nakashima T, Shinohara M,
Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T and
Takayanagi H: Osteoimmunology: The conceptual framework unifying
the immune and skeletal systems. Physiol Rev. 97:1295–1349. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI
|
|
7
|
Feng X: RANKing intracellular signaling in
osteoclasts. IUBMB Life. 57:389–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Q, Shao J, Chen W and Li YP:
Osteoclast differentiation and gene regulation. Front Biosci.
12:2519–2529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gruber R: Molecular and cellular basis of
bone resorption. Wien Med Wochenschr. 165:48–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delaissé JM, Andersen TL, Engsig MT,
Henriksen K, Troen T and Blavier L: Matrix metalloproteinases (MMP)
and cathepsin K contribute differently to osteoclastic activities.
Microsc Res Tech. 61:504–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Troen BR: The role of cathepsin K in
normal bone resorption. Drug News Perspect. 17:19–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arman A, Bereket A, Coker A, Kiper PÖ,
Güran T, Ozkan B, Atay Z, Akçay T, Haliloglu B, Boduroglu K, et al:
Cathepsin K analysis in a pycnodysostosis cohort: Demographic,
genotypic and phenotypic features. Orphanet J Rare Dis. 9:602014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lazner F, Gowen M and Kola I: An animal
model for pycnodysostosis: The role of cathepsin K in bone
remodelling. Mol Med Today. 5:413–414. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khatri V, Chauhan N and Kalyanasundaram R:
Parasite cystatin: Immunomodulatory molecule with therapeutic
activity against immune mediated disorders. Pathogens. 9:4312020.
View Article : Google Scholar
|
|
16
|
Ochieng J and Chaudhuri G: Cystatin
superfamily. J Health Care Poor Underserved. 21 (Suppl 1):S51–S70.
2010. View Article : Google Scholar
|
|
17
|
Abrahamson M, Alvarez-Fernandez M and
Nathanson CM: Cystatins. Biochem Soc Symp. 70:179–199. 2003.
View Article : Google Scholar
|
|
18
|
Leto G, Crescimanno M and Flandina C: On
the role of cystatin C in cancer progression. Life Sci.
202:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brage M, Abrahamson M, Lindström V, Grubb
A and Lerner UH: Different cysteine proteinases involved in bone
resorption and osteoclast formation. Calcif Tissue Int. 76:439–447.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Breznik B, Mitrović A, T Lah T and Kos J:
Cystatins in cancer progression: More than just cathepsin
inhibitors. Biochimie. 166:233–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Liu J, Yue Y, Chen W, Song M, Zhan
X and Wu Z: Cloning, expression and characterisation of a type II
cystatin from Schistosoma japonicum, which could regulate
macrophage activation. Parasitol Res. 113:3985–3992. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Wang S, Zhan B, He W, Chu L, Qiu D,
Li N, Wan Y, Zhang H, Chen X, et al: Therapeutic effect of
Schistosoma japonicum cystatin on bacterial sepsis in mice.
Parasit Vectors. 10:2222017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He B, Cai G, Ni Y, Li Y, Zong H and He L:
Characterization and expression of a novel cystatin gene from
Schistosoma japonicum. Mol Cell Probes. 25:186–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lehesjoki AE: Molecular background of
progressive myoclonus epilepsy. EMBO J. 22:3473–3478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lecaille F, Brömme D and Lalmanach G:
Biochemical properties and regulation of cathepsin K activity.
Biochimie. 90:208–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laitala-Leinonen T, Rinne R, Saukko P,
Väänänen HK and Rinne A: Cystatin B as an intracellular modulator
of bone resorption. Matrix Biol. 25:149–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao S, Li H, Xie H, Wu S, Yuan Y, Chu L,
Sun S, Yang H, Wu L, Bai Y, et al: Therapeutic efficacy of
Schistosoma japonicum cystatin on sepsis-induced
cardiomyopathy in a mouse model. Parasit Vectors. 13:2602020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson SR, Peters C, Saftig P and Brömme
D: Cathepsin K activity-dependent regulation of osteoclast actin
ring formation and bone resorption. J Biol Chem. 284:2584–2592.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goto T, Yamaza T and Tanaka T: Cathepsins
in the osteoclast. J Electron Microsc (Tokyo). 52:551–518. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klotz C, Ziegler T, Daniłowicz-Luebert E
and Hartmann S: Cystatins of parasitic organisms. Adv Exp Med Biol.
712:208–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayman AR: Tartrate-resistant acid
phosphatase (TRAP) and the osteoclast/immune cell dichotomy.
Autoimmunity. 41:218–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanaka S, Miyazaki T, Fukuda A, Akiyama T,
Kadono Y, Wakeyama H, Kono S, Hoshikawa S, Nakamura M, Ohshima Y,
et al: Molecular mechanism of the life and death of the osteoclast.
Ann N Y Acad Sci. 1068:180–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka S, Wakeyama H, Akiyama T, Takahashi
K, Amano H, Nakayama KI and Nakamura K: Regulation of osteoclast
apoptosis by Bcl-2 family protein Bim and Caspase-3. Adv Exp Med
Biol. 658:111–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boyce BF, Xiu Y, Li J, Xing L and Yao Z:
NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metab
(Seoul). 30:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|