Introduction
Alzheimer's disease (AD) is a common
neurodegenerative disease that occurs in the elderly population and
is characterized by progressive memory loss, mental decline and
behavioral abnormalities (1). Along
with an increased aging population, the global incidence of AD has
increased rapidly in recent years, affecting the health of patients
(2). Previous statistics have
indicated that the prevalence of AD in elderly patients >80
years old is 30%, and the number of patients with AD worldwide is
estimated to be ~26 million (3).
The regions of the brain most frequently involved in
the characteristic pathological alterations associated with AD
include those required for advanced cognitive functions, especially
the neocortex and hippocampus (4).
At present, senile plaque (SP) formation, neurofibrillary tangles,
neuronal loss (5), and the
formation, metabolism and toxicity of β-amyloid protein (Aβ) are
considered to be the core of AD pathogenesis (6). Recent data from cellular and animal
models proposed that Aβ deposition is preceded by intraneuronal
accumulation of the direct precursor of Aβ, C99 (7). Previous studies have suggested that Aβ
may lead to neuronal damage via neurotoxic excitotoxicity,
mitochondrial dysfunction, abnormal energy metabolism, calcium
homeostasis and apoptosis (8).
Moreover, it has been reported that oxidative stress serves a vital
role in the pathological processes of AD, causing damage to the
body when the generation of oxygen free radicals exceeds the limits
of the antioxidant system in the pathological environment (9). The available treatment strategies for
AD function via suppressing the formation of Aβ, preventing the
accumulation of Aβ to form SPs, reducing the amount of lysis in the
brain and depolymerizing SPs (10–12).
Transcription factor EB, which regulates C99 accumulation in AD
cellular models, serves as a potential strategy to prevent the
accumulation of this early neurotoxic catabolite (7). Furthermore, certain Aβ-associated
enzymes can regulate the metabolism of plaques, including
neprilysin (NEP) (13),
insulin-degrading enzyme (IDE) (14), receptor for advanced glycation end
products (RAGE) (15) and
low-density lipoprotein receptor-related protein 1 (LRP-1)
(16).
Cocaine and amphetamine regulated transcript (CART)
is a hypothalamic neuropeptide that is involved in feeding,
locomotor activity and conditioned place preference (17). CART is widely expressed in central
and peripheral neurons, as well as in endocrine cells (18). The known expression sites include
the brain, adrenal gland, pancreas and gastrointestinal tract. In
the brain, CART serves an important role in physiological and
pathological processes, including eating, stress and drug
dependence, and it has been used as an effective treatment for a
variety of central nervous system diseases (4,19). In
the pancreas, CART serves a physiological role in regulating both
endocrine and exocrine pancreatic secretions (20). CART also regulates islet hormone
secretion (21) and
gastrointestinal tract motility (22). A previous study demonstrated that
CART is positively associated with oxidative stress (23). However, the potential molecular
mechanism underlying the central role of CART is not completely
understood.
Our previous studies confirmed that CART improved
memory and synaptic structures, and modulated the expression of Aβ
metabolism-associated enzymes in an AD mouse model in APP/PS1 mice
(4,24). In the present study, whether CART
improved cognitive function by inhibiting oxidative stress in an AD
mouse model in APP/PS1 mice was investigated with the aim of
identifying a potential therapeutic strategy for AD.
Materials and methods
Animals and treatment
A total of 30 male APP/PS1 transgenic mice (25–30 g;
8 months old) and wild-type (WT) control mice (25–30 g; 8 months
old) were purchased and housed in the Model Animal Research Center
of Nanjing University. Mice were housed at 23–28°C with 30–60%,
12-h light/dark cycles, and free access to food and water.
All mice were of the C57BL/6J genetic background.
CART peptides were synthesized by Phoenix Pharmaceuticals Inc. Mice
were treated with CART peptides treated as previously described
(4,24) with a slight modification. Briefly,
APP/PS1 and age-matched B6 control mice were randomly divided into
CART-treated or normal saline-treated groups (10 mice per group).
CART was injected via the tail vein daily for 10 days at a dose of
0.5 µg/kg, and then injected daily intraperitoneally for 20 days.
All experimental procedures were approved by the Nanjing
University's Committee of Experimental Animal Administration
(Nanjing, China).
Cell culture
Primary cortical neurons were isolated and cultured
as previously described (25).
Dissociated cortical cells were seeded (2×105 cells/ml)
into 6-well plates. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) and exposed to CART peptide (0.4
nM; Phoenix Pharmaceuticals, Inc.) for 1 h at 37°C, followed by
incubation with Aβ1–42 (2 µM; MedChemExpress) for 24 h at 37°C.
Cell viability
Cell viability was assessed by performing the Cell
Counting Kit-8 (CCK-8) assay (cat. no. GB707; Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol.
Briefly, 10 µl CCK-8 solution was added to each well of a 96-well
plate. Following incubation for 4 h, absorbance was measured at a
wavelength of 450 nm using a microplate reader. The effect of CART
on cell viability was determined by calculating the cell viability
percentage of CART treated cells compared with saline-treated
cells.
Morris water maze (MWM)
The MWM test was performed to evaluate spatial
memory as previously described (26). On the 9th day of administration,
mice underwent the MWM directional navigation test, which lasted
for 5 days. Each day, mice were placed into the water from the
midpoint of the pool wall in the order of I–IV quadrants. The time
required for the mice to enter the platform from the water within
60 sec was recorded as the escape incubation period. If the mice
could not find the platform within 1 min, the experimenter led the
mice to the platform, recording an escape incubation period of 60
sec. Each training interval was 60 sec. For the cruise test, the
platform was removed and mice were placed in the pool in the order
of I–IV quadrants. Subsequently, the swimming time and the crossing
times in each quadrant were recorded within 60 sec. The
experimental data were recorded and analyzed using a camera and MWM
software (version 1.0; Shanghai Information Technology Co.,
Ltd.).
Intracellular reactive oxygen species
(ROS), 8-hydroxy-2′-deoxyguanosine (8-OhdG) and neurotrophin-3
(3-NT) analysis
Following sacrifice by cervical dislocation, the
cerebral cortex and hippocampus were isolated from each mouse and
were homogenized in 2% SDS containing a protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) and phosphatase inhibitors (Calbiochem;
Merck KGaA). The homogenized mixes were centrifuged at 100,000 × g
for 1 h at 4°C. The supernatant was stored as soluble fraction to
detect ROS (cat. no. E004-1-1; Nanjing Jiancheng Bioengineering
Institute), 8-OhdG (cat. no. H165; Nanjing Jiancheng Bioengineering
Institute) and 3-NT (cat. no. 267-N3; R&D Systems, Inc.)
according to the manufacturer's instructions (4).
Measurement of mitochondrial membrane
potential
Mitochondria were isolated from the hippocampus and
cortex sections using the Mitochondria Isolation kit (cat. no.
G006-1-1; Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's instructions. The mitochondrial membrane
potential was detected using a mitochondrial membrane potential
assay kit (cat. no. G009-1-3; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's instructions (9).
Histology experiments
Cerebral cortex and hippocampus samples were
isolated and frozen slices (40-µm thick; −20°C) were prepared.
Briefly, mice were anesthetized intra-peritoneally with 1%
pentobarbital at a dose of 50 mg/kg and fixed on their back. The
chest was opened to expose the heart and an infusion needle was
inserted into the left ventricle, then the right atrial appendage
was cut. Mice were perfused with 0.9% physiological saline for 10
min and then rapidly perfused with 4% precooled paraformaldehyde
(pH 7.4) for 30 min. The brain was exposed by performing a
craniotomy and fixed with 4% precooled paraformaldehyde at room
temperature overnight. After 24 h, the samples were rinsed with PBS
for 4–5 h and incubated overnight at 4°C. Samples were dehydrated
with a 20 and 30% sucrose gradient, then sliced with a cryostat.
Sections were stained using an anti-Aβ antibody (cat. no. 8243;
1:200; Cell Signaling Technology, Inc.) as previously described
(4). Briefly, tissue sections were
dewaxed and hydrated, and antigen retrieval was performed with
boiling citrate. Following washing three times with PBS for 5 min
each time, tissue sections were incubated with 3%
H2O2-PBS for 30 min and washed three times
with PBS for 5 min each time. Subsequently, tissue sections were
incubated with 0.5% BSA-PBS (Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h followed by incubation with the primary
antibody (1:200 in 0.5% BSA-PBS) overnight at 4°C. Tissue sections
were maintained at room temperature for 30–60 min, washed three
times with PBS for 5 min each time, then incubated with an
HRP-labeled sheep anti-mouse/rabbit IgG polymer (1:10,000; cat. no.
ab6795; Abcam) at room temperature for 1–2 h. Following washing
three times with PBS for 5 min each time, tissue sections were
stained with hematoxylin at room temperature for 3 min. Then, 1%
hydrochloric acid alcohol was extracted twice, rinsed with running
water for 3 min, incubated with gradient alcohol and xylene for 2
min. Finally, the slides were sealed and stained samples were
visualized using a fluorescent microscope (magnification, ×10).
Western blotting
Total protein was isolated from the cerebral cortex
and hippocampus samples using RIPA buffer. Subsequently, western
blotting was performed as previously described (27). Primary antibodies targeted against
the following were used: IDE (cat. no. ab133561; 1:2,000; Abcam),
NEP (cat. no. ab227195; 1:2,000; Abcam), LRP-1 (cat. no. sc-57352;
1:2,000; Santa Cruz Biotechnology Inc.), superoxide dismutase
(SOD)-1 (cat. no. 10269-1-AP; 1:2,000; ProteinTech Group, Inc.),
SOD-2 (cat. no. 66474-1-Ig; 1:2,000; ProteinTech Group, Inc.),
γ-H2A histone family member X (γ-H2A.X; cat. no. ab2893; 1:2,000;
Abcam), peroxiredoxin 1 (Prdx1; cat. no. 8499; 1:2,000; Cell
Signaling Technology, Inc.), polycomb complex protein (Bmi-1; cat.
no. 10832-1-AP; 1:2,000; ProteinTech Group, Inc.), p16 (cat. no.
80772; 1:2,000; Cell Signaling Technology, Inc.), TNFα (cat. no.
11948; 1:2,000; Cell Signaling Technology, Inc.), IL-1β (cat. no.
16806-1-AP; 1:2,000; ProteinTech Group, Inc.) and β-actin (cat. no.
BS6007M; 1:2,000; Bioworld Technology, Inc.). Briefly, gels were
placed into the electrophoresis tank, electrophoresis solution was
added, protein and marker were added, the lid was covered and the
gel was removed after electrophoresis. As for the transfer
solution, the sponge, filter paper, gel and PVDF were pressed on
the splint membrane, followed by filter paper and sponge. The
membrane was placed into the transfer film tank with an ice pack
and transfer solution, the lid was covered and the tank was
maintained at a constant pressure of 0.28A for 2 h. All experiments
were performed in at least triplicate.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells and tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
PrimeScript RT Reagent Kit (Takara Bio, Inc.) according to the
manufacturer's instructions. Subsequently, qPCR was performed as
previously described (28). The
sequences of the primers used for qPCR are listed in Table I.
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
| Gene | Sequence
(5′→3′) |
|---|
| IDE (mouse) | R:
CAAACACTGTTTATGGACTG |
|
| R:
TGCTGAATTGAATGTGTACC |
| Bmi-1 (mouse) | F:
ATCCCCACTTAATGTGTGTCCT |
|
| R:
CTTGCTGGTCTCCAAGTAACG |
| p16 (mouse) | F:
AACTCTTTCGGTCGTACCCC |
|
| R:
GCGTGCTTGAGCTGAAGCTA |
| IL-1α (mouse) | F:
CGAAGACTACAGTTCTGCCATT |
|
| R:
GACGTTTCAGAGGTTCTCAGAG |
| Sirt1 (mouse) | F:
GCTGACGACTTCGACGACG |
|
| R:
TCGGTCAACAGGAGGTTGTCT |
| NEP (mouse) | F:
GAAGACCGAAATGACCCA |
|
| R:
CGGATGTAGTCCCGTAAA |
| IL-6 (mouse) | F:
CCAAGAGGTGAGTGCTTCCC |
|
| R:
CTGTTGTTCAGACTCTCTCCCT |
| GAPDH (mouse) | F:
TGGATTTGGACGCATTGGTC |
|
| R:
TTTGCACTGGTACGTGTTGAT |
| RAGE (mouse) | F:
TCTTGGTGCCTTTTGTGTGAC |
|
| R:
CTCTTCCTCGTTTTTGCTCTC |
| Bmi-1 (human) | F:
CGTGTATTGTTCGTTACCTGGA |
|
| R:
TTCAGTAGTGGTCTGGTCTTGT |
| p53 (mouse) | F:
GCGTAAACGCTTCGAGATGTT |
|
| R:
TTTTTATGGCGGGAAGTAGACTG |
| LRP-1 (mouse) | F:
TCTTGGTGCCTTTTGTGTGAC |
|
| R:
CTCTTCCTCGTTTTTGCTCTC |
| SOD1 (mouse) | F:
AACCAGTTGTGTTGTCAGGAC |
|
| R:
CCACCATGTTTCTTAGAGTGAGG |
| SOD2 (mouse) | F:
CAGACCTGCCTTACGACTATGG |
|
| R:
CTCGGTGGCGTTGAGATTGTT |
| Nrf2 (mouse) | F:
TCTTGGAGTAAGTCGAGAAGTGT |
|
| R:
GTTGAAACTGAGCGAAAAAGGC |
Statistical analysis
All experiments were repeated at least three times
in a blinded manner. Data are presented as the mean ± SEM.
Comparisons between groups were analyzed using one-way ANOVA
followed by Bonferroni's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. All data were
analyzed using SPSS software, version 18.0 (SPSS, Inc.).
Results
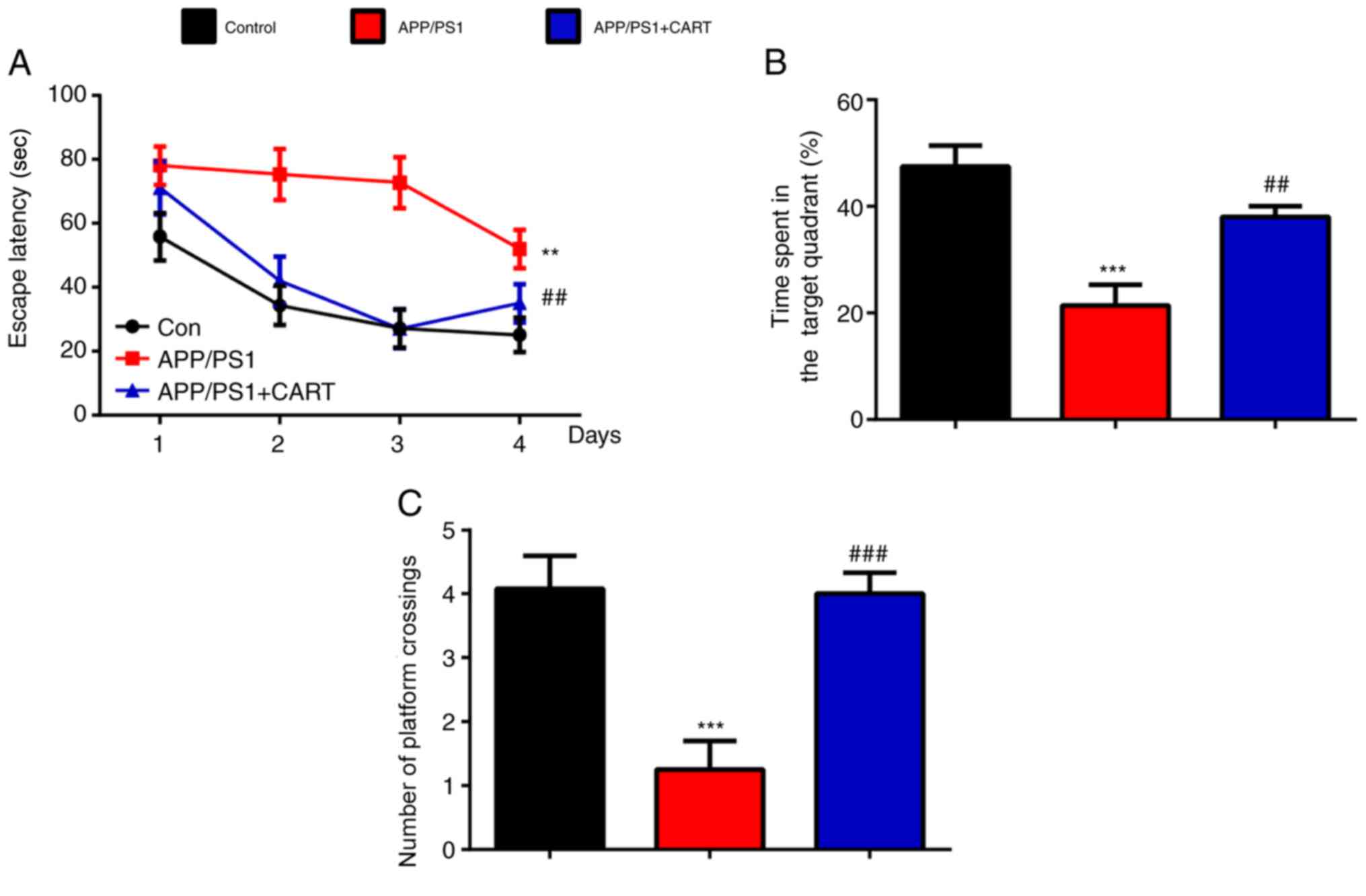
CART treatment attenuates spatial
memory impairment
MWM tests were performed to determine whether
treatment with CART improved the spatial memory abilities of AD
model (APP/PS1) mice. The escape latencies (Fig. 1A), time spent in the target quadrant
(Fig. 1B) and the number of
platform crossings (Fig. 1C) were
significantly decreased in APP/PS1 mice compared with WT control
mice, but were significantly increased in CART-treated APP/PS1 mice
compared with untreated APP/PS1 mice. The results suggested that
CART treatment significantly improved memory deficits in APP/PS1
mice.
CART modulates Aβ
metabolism-associated enzyme expression in the hippocampus and
cerebral cortex
To investigate the mechanism underlying
CART-mediated improvements in the spatial memory abilities of AD
model mice, immunohistochemistry staining for Aβ was performed. Aβ
expression was significantly increased in APP/PS1 mice compared
with WT control mice, but significantly decreased in CART-treated
APP/PS1 mice compared with untreated APP/PS1 mice (Fig. 2A and B). To investigate the
mechanism underlying the effects of CART on Aβ expression, the
expression levels of Aβ metabolism-associated enzymes were detected
via RT-qPCR and western blotting. The protein and mRNA expression
levels of IDE, NEP, and LRP-1 in the hippocampus (Fig. 2C-E) and cortex (Fig. 2F-H) were significantly decreased in
APP/PS1 mice compared with WT control mice, but were significantly
increased in CART-treated APP/PS1 mice compared with untreated
APP/PS1 mice. Compared with WT control mice, RAGE mRNA expression
was increased in untreated mice, and decreased in CART treated
mice.
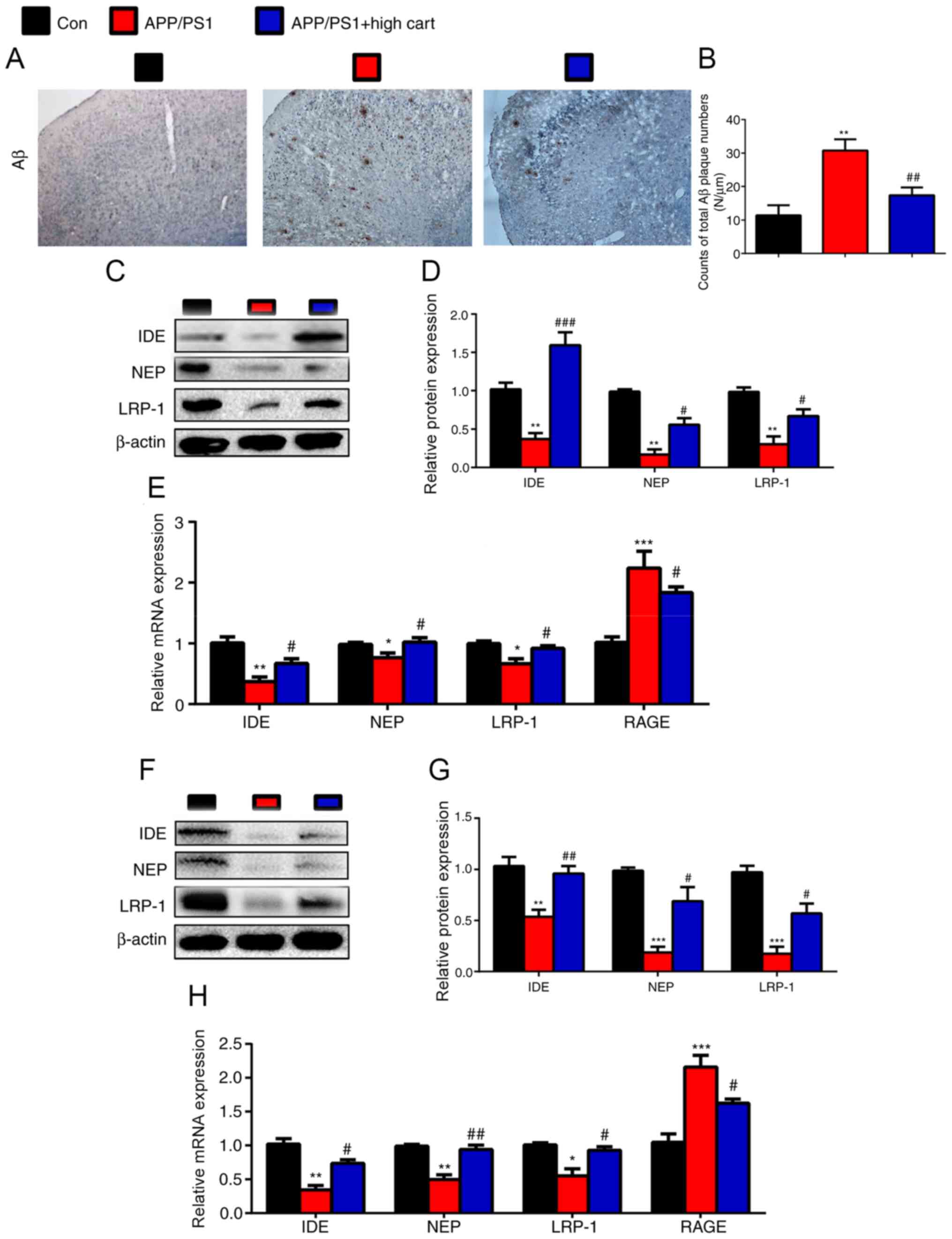 | Figure 2.Exogenous CART supplements reduce Aβ
protein accumulation by downregulating Aβ metabolism-associated
enzymes in APP/PS1 mice. WT, APP/PS1 and CART-treated APP/PS1 mice
(age, 8 months) were used. (A) Representative micrographs of the
brain with immunohistochemical staining for Aβ (scale bar, 200 µm).
(B) Counts of total Aβ plaque numbers (N/µm). Relative protein
expression levels of IDE, NEP and LRP-1 in the cerebral cortex were
(C) determined via western blotting and (D) semi-quantified. (E)
Relative mRNA expression levels of IDE, NEP, LRP-1 and RAGE in
cerebral cortex extracts were measured via RT-qPCR. GAPDH was used
as the internal control. Relative protein expression levels of IDE,
NEP and LRP-1 in the hippocampus were (F) determined via western
blotting and (G) semi-quantified. (H) Relative expression levels of
IDE, NEP, LRP-1 and RAGE in hippocampus extracts were measured via
RT-qPCR. GAPDH was used as the internal control. Data are presented
as the mean ± SEM (n=6 per group). *P<0.05, **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. APP/PS1.
CART, cocaine amphetamine regulated transcript; Aβ, β-amyloid
protein; WT, wild-type; IDE, insulin-degrading enzyme; NEP,
neprilysin; LRP-1, low-density lipoprotein receptor-related protein
1; RAGE, receptor for advanced glycation end products; RT-qPCR,
reverse transcription-quantitative PCR. |
CART attenuates mitochondrial
dysfunction by reducing oxidative stress and DNA damage in the
cortex
The roles of CART treatment on the functional status
of mitochondria, oxidative stress and DNA damage levels in WT,
APP/PS1 and CART-treated APP/PS1 were assessed. 8-OHdG (Fig. 3A), 3-NT (Fig. 3D) and ROS (Fig. 3C) levels in the cerebral cortex
tissues were significantly increased in APP/PS1 mice compared with
WT control mice, but were significantly decreased in CART-treated
APP/PS1 mice compared with untreated APP/PS1 mice. The
mitochondrial membrane potential of the cerebral cortex tissues was
significantly decreased in APP/PS1 mice compared with WT control
mice, but was significantly increased in CART-treated APP/PS1 mice
compared with untreated APP/PS1 mice (Fig. 3B).
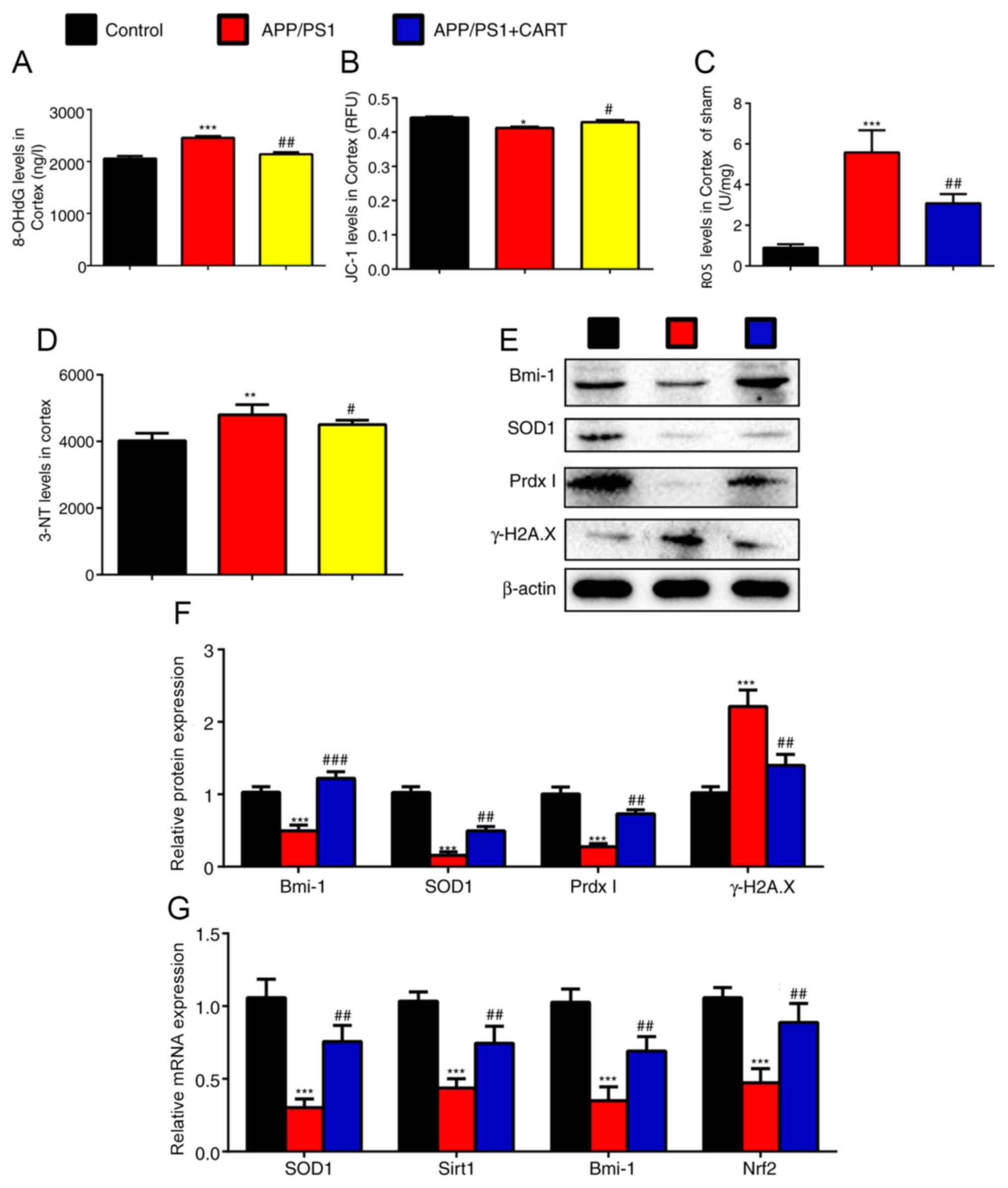 | Figure 3.Exogenous CART supplements reduce
oxidative stress and mitochondrial dysfunction in the cerebral
cortex of APP/PS1 mice. WT, APP/PS1 and CART-treated APP/PS1 mice
(age, 8 months) were used. (A) 8-OHdG level in 10% cerebral cortex
tissue homogenate. (B) The mitochondrial membrane potential (JC-1)
in the cerebral cortex tissue. (C) ROS levels in cerebral cortex
cells. (D) 3-NT levels in 10% cerebral cortex tissue homogenate.
Relative protein expression levels of Bmi-1, SOD1, Prdx1 and
γ-H2A.X in the cerebral cortex were (E) determined via western
blotting and (F) semi-quantified. (G) Relative mRNA expression
levels of SOD1, Sirt1, Bmi-1 and Nrf2 in cerebral cortex extracts
were measured via reverse transcription-quantitative PCR. GAPDH was
used as the internal control. Data are presented as the mean ± SEM
(n=6 per group). *P<0.05, **P<0.01 and ***P<0.001 vs.
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. APP/PS1. CART, cocaine amphetamine
regulated transcript; WT, wild-type; 8-OHdG,
8-hydroxy-2′-deoxyguanosine; 3-NT, neurotrophin-3; ROS, reactive
oxygen species; Bmi-1, polycomb complex protein; SOD1, superoxide
dismutase; Prdx1, peroxiredoxin 1; γ-H2A.X, γ-H2A histone family
member X; Sirt1, sirtuin 1; Nrf2, nuclear factor erythroid 2 like
2. |
In addition, the western blotting results indicated
that protein expression levels of Bmi-1, SOD1 and PrdxI in the
cortex were significantly decreased in APP/PS1 mice compared with
WT control mice, but were significantly increased in CART-treated
APP/PS1 mice compared with untreated APP/PS1 mice (Fig. 3E and F). Additionally, RT-qPCR
demonstrated that mRNA expression levels of Bmi-1, SOD1, Sirt1 and
nuclear factor erythroid 2 like 2 (Nrf2) in the cortex were
significantly decreased in APP/PS1 mice compared with WT control
mice, but were significantly increased in CART-treated APP/PS1 mice
compared with untreated APP/PS1 mice (Fig. 3G). γ-H2A.X protein expression levels
in the cortex were significantly increased in APP/PS1 mice compared
with WT control mice, but were significantly decreased in
CART-treated APP/PS1 mice compared with untreated APP/PS1 mice
(Fig. 3E and F).
CART attenuates mitochondrial
dysfunction by reducing oxidative stress and DNA damage in the
hippocampus
Subsequently, the effects of CART treatment on the
functional status of the mitochondria, oxidative stress and DNA
damage levels in WT, APP/PS1 and CART-treated APP/PS1 mice were
investigated. 8-OHdG (Fig. 4A),
3-NT (Fig. 4C) and ROS (Fig. 4D) levels in the hippocampus tissues
were significantly increased in APP/PS1 mice compared with WT
control mice, but significantly decreased in CART-treated APP/PS1
mice compared with untreated APP/PS1 mice. The mitochondrial
membrane potential of the hippocampus tissue was significantly
decreased in APP/PS1 mice compared with WT control mice, but was
significantly increased in CART-treated APP/PS1 mice compared with
untreated APP/PS1 mice (Fig.
4B).
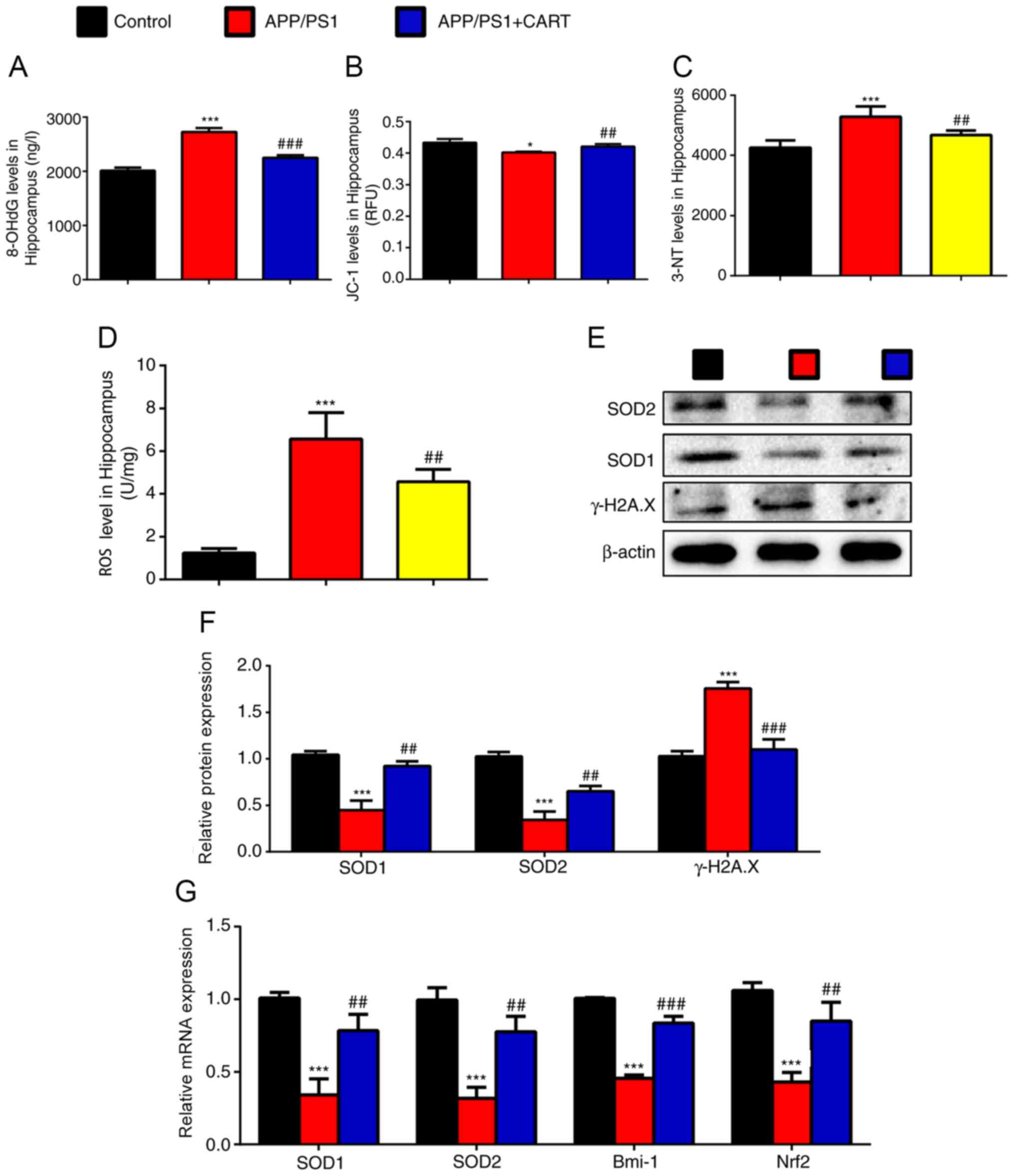 | Figure 4.CART supplements inhibit oxidative
stress and DNA damage in the hippocampus of APP/PS1 mice. WT,
APP/PS1 and CART-treated APP/PS1 mice (age, 8 months) were used.
(A) 8-OHdG level in 10% cerebral hippocampus homogenate. (B) The
mitochondrial membrane potential (JC-1) in the hippocampus tissue.
(C) 3-NT level in 10% hippocampus tissue homogenate. (D) ROS level
in the hippocampus cells. Relative protein expression levels of
SOD1, SOD2 and γ-H2A.X in the hippocampus were (E) determined via
western blotting and (F) semi-quantified. (G) Relative mRNA
expression levels of SOD1, SOD2, Bmi-1 and Nrf2 in hippocampus
extracts were measured via reverse transcription-quantitative PCR.
GAPDH was used as the internal control. Data are presented as the
mean ± SEM (n=6 per group). *P<0.05 and ***P<0.001 vs.
control; ##P<0.01 and ###P<0.001 vs.
APP/PS1. CART, cocaine amphetamine regulated transcript; WT,
wild-type; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ROS, reactive
oxygen species; SOD, superoxide dismutase; γ-H2A.X, γ-H2A histone
family member X; Bmi-1, polycomb complex protein; Nrf2, nuclear
factor, erythroid 2 like 2. |
In addition, the western blotting results indicated
that protein expression levels of SOD1 and SOD2 in the hippocampus
were significantly decreased in APP/PS1 mice compared with WT
control mice, but were significantly increased in CART-treated
APP/PS1 mice compared with untreated APP/PS1 mice (Fig. 4E and F). Furthermore, RT-qPCR
results demonstrated that SOD1, SOD2, Bmi-1 and Nrf2 mRNA
expression levels in the hippocampus were significantly decreased
in APP/PS1 mice compared with WT control mice, but were
significantly increased in CART-treated APP/PS1 mice compared with
untreated APP/PS1 mice (Fig. 4G).
γ-H2A.X protein expression levels in the hippocampus were
significantly increased in APP/PS1 mice compared with WT control
mice, but were significantly decreased in CART-treated APP/PS1 mice
compared with untreated APP/PS1 mice (Fig. 4E and F).
CART improves the expression of Aβ
metabolism-associated enzymes in Aβ1–42-exposed primary
cortical neurons
To validate the hypothesis, primary cortical neurons
were isolated and treated with Aβ1–42 and CART. CART
pretreatment significantly increased cell viability of cultured
primary cortical neurons exposed to Aβ1–42 compared with
the Aβ1–42-treated primary cortical neurons (Fig. 5A). The western blotting results
showed that IDE, NEP and LRP-1 protein expression levels were
significantly decreased in Aβ1–42-treated primary
cortical neurons, but were significantly increased following
pretreatment with CART (Fig. 5B and
C). Additionally, RT-qPCR results demonstrated that IDE, NEP,
LRP-1 and RAGE mRNA expression levels were significantly decreased
in Aβ1–42-treated primary cortical neurons, but were
significantly increased following pretreatment with CART (Fig. 5D).
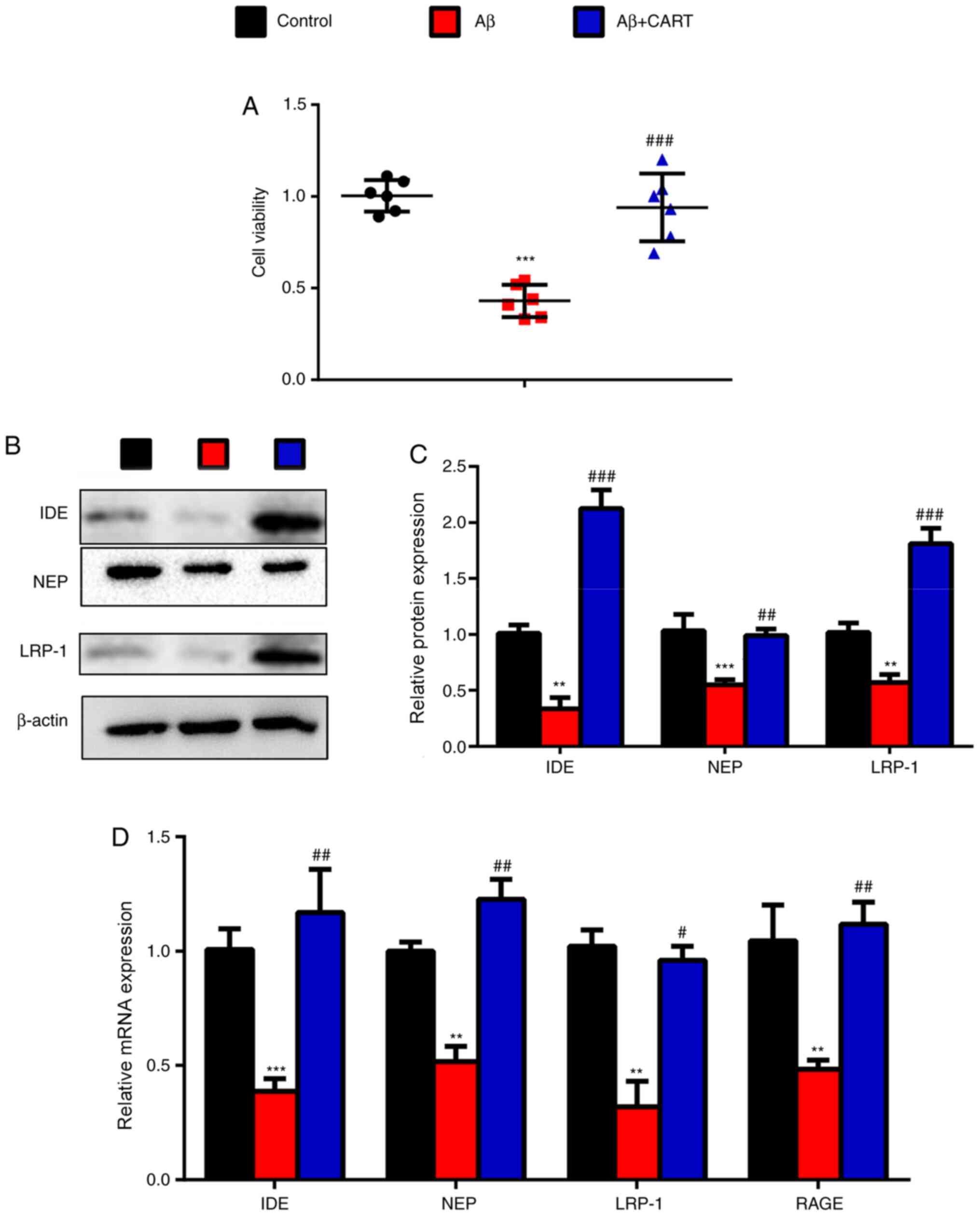 | Figure 5.Treatment with CART upregulates the
expression of Aβ metabolism-associated enzymes in
Aβ1–42-exposed primary cortical neurons. (A) CART
significantly increased cell viability in Aβ-exposed cultured
primary cortical neurons. Relative protein expression levels of
IDE, NEP and LRP-1 in cultured primary cortical neurons exposed to
Aβ with or without CART were (B) determined via western blotting
and (C) semi-quantified. (D) Relative mRNA expression levels of
IDE, NEP, LRP-1 and RAGE in cultured primary cortical neurons
exposed to Aβ with or without CART were measured via reverse
transcription-quantitative PCR. Data are presented as the mean ±
SEM (n=6 per group). **P<0.01 and ***P<0.001 vs. control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. APP/PS1. CART, cocaine amphetamine
regulated transcript; Aβ, β-amyloid protein; IDE, insulin-degrading
enzyme; NEP, neprilysin; LRP-1, low-density lipoprotein
receptor-related protein 1; RAGE, receptor for advanced glycation
end products; WT, wild-type. |
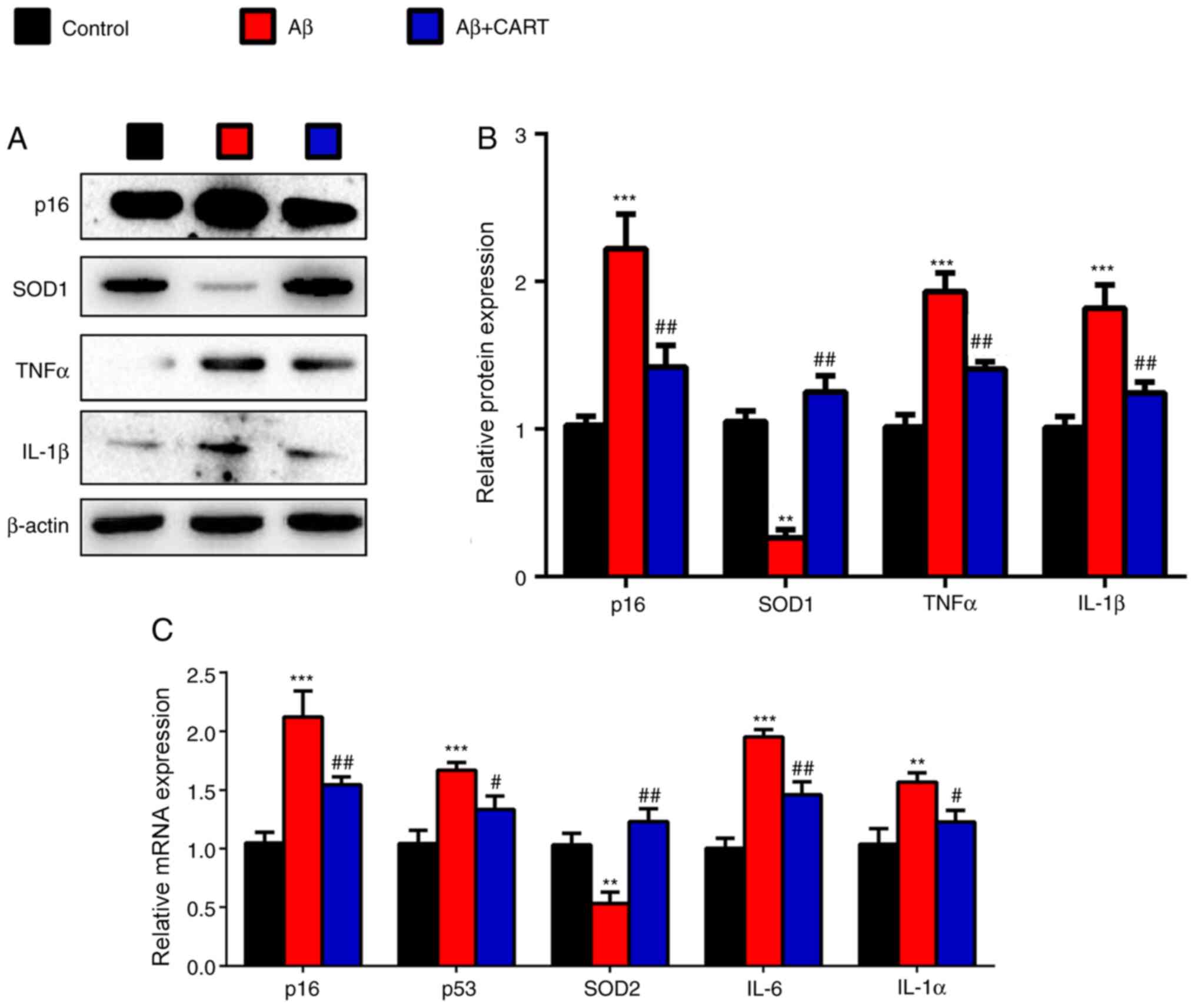
CART reduces cell senescence and
oxidative stress in Aβ1–42-exposed primary cortical
neurons
To further investigate whether the therapeutic
effects of CART on AD were mediated via oxidative stress and DNA
damage, western blotting and RT-qPCR were performed. The western
blotting results indicated that p16, TNFα and IL-1β protein
expression levels were significantly increased in
Aβ1–42-treated primary cortical neurons, but were
significantly decreased following pretreatment with CART (Fig. 6A and B). Furthermore, RT-qPCR
results demonstrated that p16, p53, IL-6 and IL-1β mRNA expression
levels were significantly increased in Aβ1–42-treated
primary cortical neurons, but were significantly decreased
following pretreatment with CART (Fig.
6C). SOD1 protein (Fig. 6A and
B) and SOD2 mRNA (Fig. 6C)
expression levels were significantly decreased in the
Aβ1–42-treated primary cortical neurons, but were
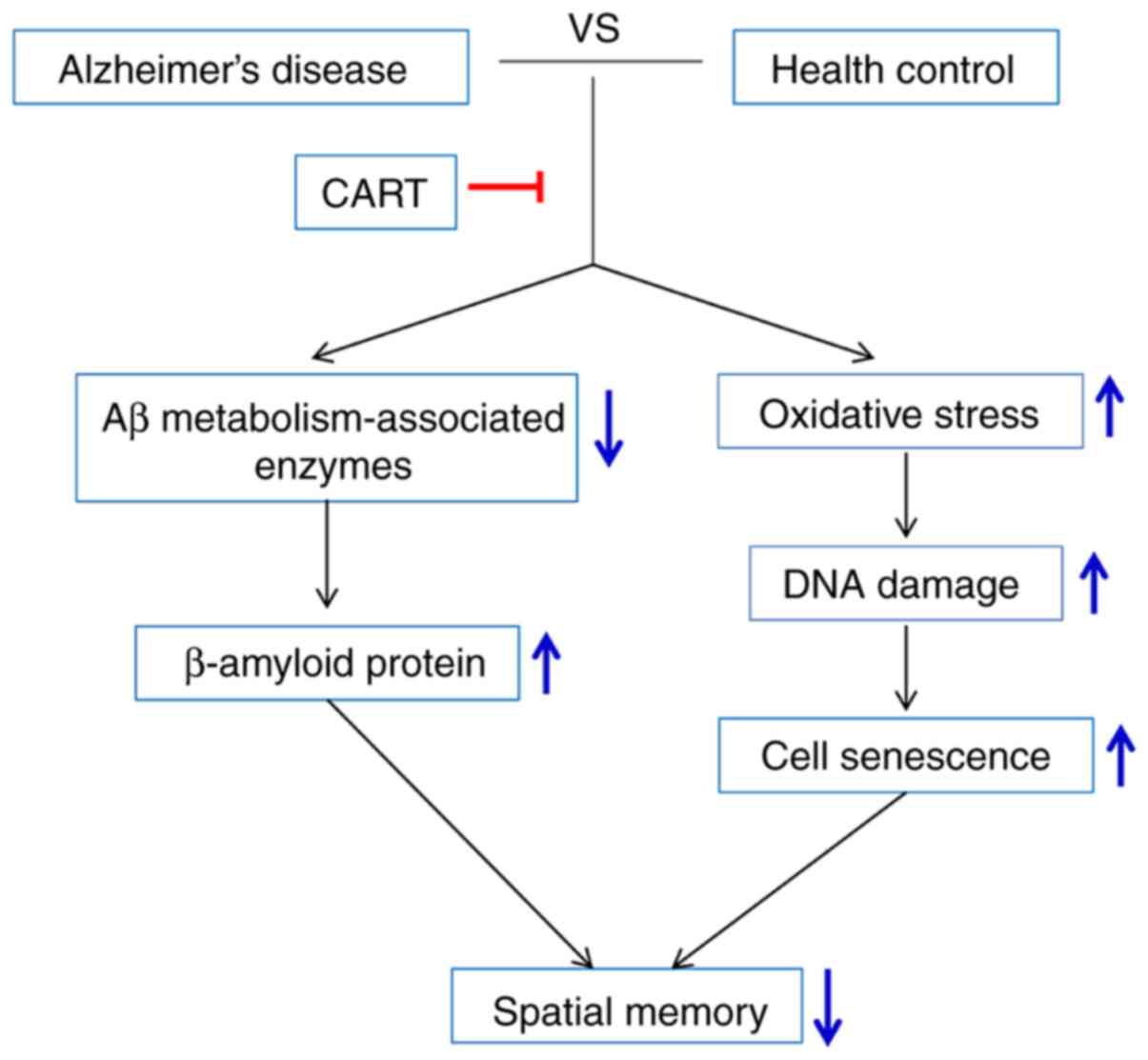
significantly increased following pretreatment with CART. The model
of the mechanism underlying CART-mediated improvements in memory
impairment in AD is presented in Fig.
7.
Discussion
In the present study, the impact of CART on spatial
memory impairment in AD was investigated. The results indicated
that the mechanism underlying the effects of exogenous CART
supplements may include: i) Reducing Aβ1–42 protein
accumulation by downregulating Aβ1–42
metabolism-associated enzymes in APP/PS1 mice; ii) mediating
oxidative stress, DNA damage and mitochondrial dysfunction in the
cerebral cortex and hippocampus of APP/PS1 mice; and iii) reducing
cell senescence and oxidative stress in Aβ1–42-exposed
primary cortical neurons. The results of the present study were
consistent with previous studies that reported that Aβ protein
accumulation, and cholinergic and neuroinflammatory oxidative
stress serve a vital role in the progress of AD (29,30).
AD is the most common cause of dementia and is
characterized by onset, progressive mental decline, and personality
and affective disorders (31). The
pathogenesis of AD is not completely understood. Aβ, as a toxic
protein, plays a detrimental role in the pathogenesis of AD
(32). Soluble Aβ oligomers affect
tau protein phosphorylation, affecting cytoskeletal changes
(33), and thereby inducing the
loss of synaptic plasticity and impairing memory function (34). Previous studies have reported that
exogenous CART treatment could ameliorate memory dysfunction
(4) and reduced levels of
Aβ1–40 and Aβ1–42 could account for the
neuroprotective effects of CART (24). The present study also indicated that
CART improved the learning and spatial memory capacity of APP/PS
mice, confirming a therapeutic effect for AD.
Age spots are the most important pathological
manifestation of AD (32). The AD
model used in the present study was established via an
intracerebroventricular injection of Aβ, which simulates the
pathological alterations of Aβ accumulation and deposition in the
brain. Several proteases have been found to be involved in the
generation and turnover of Aβ. IDE, NEP, LRP-1 and RAGE are capable
of regulating the Aβ levels in both experimental models and
patients with AD (35). Previous
reports demonstrated that CART modulated the levels of NEP
(13), IDE (14), RAGE (15) and LRP-1 (16) via inhibiting MAPK signaling pathways
and activating the AKT signaling pathway (24). Similarly, the results of the present
study suggested that CART regulated Aβ metabolism-associated
enzymes, including IDE, NEP, LRP-1 and RAGE, thereby promoting the
degradation of Aβ, which may serve a valuable role in the treatment
of AD.
A series of hypotheses have been proposed for AD, of
which oxidative stress being the most predominant (35–37).
When oxygen free radicals in the body are unbalanced, ROS or
related substances accumulate excessively in the body, which can
cause various toxic effects on the cells, resulting in reversible
or irreversible damage (38). The
peroxidation of lipids can inactivate ribonucleic acid, lead to DNA
damage and trigger DNA mutations. In addition, the peroxidation of
lipids can generate aldehydes that can combine with phosphoric acid
and proteins to form lipofuscin, which when deposited in the brain
leads to impaired mental ability (39,40).
Therefore, oxidative stress-induced damage in the brains of
patients with AD serves an important role in neuronal degeneration,
apoptosis, necrosis and pathogenesis. A previous study confirmed
that CART activated the Nrf2/heme oxygenase 1 antioxidant signaling
pathway and protected hippocampal neurons in AD (41). In accordance with a previous report
(24), the present study also
concluded that CART attenuated oxidative stress and maintained
mitochondrial integrity, contributing to improved cognitive
capacity. Moreover, CART alleviated DNA damage and cell senescence.
In addition, pathological staining of Aβ was performed, which
increased the reliability of the present study.
In conclusion, the present study suggested that CART
attenuated memory dysfunction in APP/PS1 mice by promoting Aβ
degradation and reducing oxidative stress and cell senescence.
Therefore, CART may serve as a potential therapeutic drug for the
treatment of AD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJ conceptualized the study, assisted with the
methodology and drafted the original manuscript. FN performed the
investigation. YZ acquired the data. YX made substantial
contributions to the conception and design of the study, and
provided project supervision. YX and HJ confirmed the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Ethics Committee of Drum Tower of Nanjing University
(Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnham KJ, Masters CL and Bush AI:
Neurodegenerative diseases and oxidative stress. Nat Rev Drug
Discov. 3:205–214. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalakas MC, Alexopoulos H and Spaeth PJ:
Complement in neurological disorders and emerging
complement-targeted therapeutics. Nat Rev Neurol. 16:601–617. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sastre M, Walter J and Gentleman SM:
Interactions between APP secretases and inflammatory mediators. J
Neuroinflammation. 5:252008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin JL, Liou AK, Shi Y, Yin KL, Chen L, Li
LL, Zhu XL, Qian L, Yang R, Chen J and Xu Y: CART treatment
improves memory and synaptic structure in APP/PS1 mice. Sci Rep.
5:102242015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hijazi S, Heistek TS, Scheltens P, Neumann
U, Shimshek DR, Mansvelder HD, Smit AB and van Kesteren RE: Early
restoration of parvalbumin interneuron activity prevents memory
loss and network hyperexcitability in a mouse model of Alzheimer's
disease. Mol Psychiatry. 25:3380–3398. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Javed I, Zhang Z, Adamcik J, Andrikopoulos
N, Li Y, Otzen DE, Lin S, Mezzenga R, Davis TP, Ding F and Ke PC:
Accelerated amyloid beta pathogenesis by bacterial amyloid FapC.
Adv Sci (Weinh). 7:20012992020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pulina MV, Hopkins M, Haroutunian V,
Greengard P and Bustos V: C99 selectively accumulates in vulnerable
neurons in Alzheimer's disease. Alzheimers Dement. 16:273–282.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswas SC, Shi Y, Vonsattel JP, Leung CL,
Troy CM and Greene LA: Bim is elevated in Alzheimer's disease
neurons and is required for beta-amyloid-induced neuronal
apoptosis. J Neurosci. 27:893–900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy PH and Oliver DM: Amyloid beta and
phosphorylated tau-induced defective autophagy and mitophagy in
Alzheimer's disease. Cells. 8:4882019. View Article : Google Scholar
|
|
10
|
Rafii MS and Aisen PS: Recent developments
in Alzheimer's disease therapeutics. BMC Med. 7:72009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
May-Zhang LS, Kirabo A, Huang J, Linton
MF, Davies SS and Murray KT: Scavenging reactive lipids to prevent
oxidative injury. Annu Rev Pharmacol Toxicol. Sep 30–2020.(Epub
ahead of print). doi: 10.1146/annurev-pharmtox-031620-035348.
PubMed/NCBI
|
|
12
|
Chen X, Ji B, Hao X, Li X, Eisele F,
Nyström T and Petranovic D: FMN reduces Amyloid-β toxicity in yeast
by regulating redox status and cellular metabolism. Nat Commun.
11:8672020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwata N, Tsubuki S, Takaki Y, Watanabe K,
Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E,
Sekine-Aizawa Y and Saido TC: Identification of the major
Abeta1-42-degrading catabolic pathway in brain parenchyma:
Suppression leads to biochemical and pathological deposition. Nat
Med. 6:143–150. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deprez-Poulain R, Hennuyer N, Bosc D,
Liang WG, Enée E, Marechal X, Charton J, Totobenazara J, Berte G,
Jahklal J, et al: Catalytic site inhibition of insulin-degrading
enzyme by a small molecule induces glucose intolerance in mice. Nat
Commun. 6:82502015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali T, Badshah H, Kim TH and Kim MO:
Melatonin attenuates D-galactose-induced memory impairment,
neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK
signaling pathway in aging mouse model. J Pineal Res. 58:71–85.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan FL, Zheng Y and Zhao FD: Effects of
Ginkgo biloba extract EGb761 on expression of RAGE and LRP-1
in cerebral microvascular endothelial cells under chronic hypoxia
and hypoglycemia. Acta Neuropathol. 116:529–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dallvechia-Adams S, Kuhar MJ and Smith Y:
Cocaine- and amphetamine-regulated transcript peptide projections
in the ventral midbrain: Colocalization with gamma-aminobutyric
acid, melanin-concentrating hormone, dynorphin, and synaptic
interactions with dopamine neurons. J Comp Neurol. 448:360–372.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Cao X, Bao X, Zhang Y, Xu Y and
Sha D: Cocaine- and amphetamine-regulated transcript protects
synaptic structures in neurons after ischemic cerebral injury.
Neuropeptides. 81:1020232020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunter RG and Kuhar MJ: CART peptides as
targets for CNS drug development. Current drug targets. Curr Drug
Targets CNS Neurol Disord. 2:201–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janiuk I and Mlynek K: Immunodetection of
cocaine- and amphetamine-regulated transcript in bovine pancreas.
Acta Histochem. 117:545–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gilon P: Cocaine- and
amphetamine-regulated transcript: A novel regulator of energy
homeostasis expressed in a subpopulation of pancreatic islet cells.
Diabetologia. 59:1855–1859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rząp D, Czajkowska M and Całka J:
Neurochemical plasticity of nNOS-, VIP- and CART-immunoreactive
neurons following prolonged acetylsalicylic acid supplementation in
the porcine jejunum. Int J Mol Sci. 21:21572020. View Article : Google Scholar
|
|
23
|
Hsieh YS, Chen PN, Yu CH, Chen CH, Tsai TT
and Kuo DY: Involvement of oxidative stress in the regulation of
NPY/CART-mediated appetite control in amphetamine-treated rats.
Neurotoxicology. 48:131–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin KL, Jin JL, Zhu XL, Yu L, Wang S, Qian
L, Han L and Xu Y: CART modulates beta-amyloid
metabolism-associated enzymes and attenuates memory deficits in
APP/PS1 mice. Neurol Res. 39:885–894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sha DJ, Li LL, Ye L, Li R and Xu Y:
Icariin inhibits neurotoxicity of beta-amyloid by upregulating
cocaine-regulated and amphetamine-regulated transcripts.
Neuroreport. 20:1564–1567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang GH, Jiang XY, Pu HJ, Zhang W, An C,
Hu X, Liou AK, Leak RK, Gao Y and Chen J: Scriptaid, a novel
histone deacetylase inhibitor, protects against traumatic brain
injury via modulation of PTEN and AKT pathway: Scriptaid protects
against TBI via AKT. Neurotherapeutics. 10:124–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Y, Zhang W, Klaus J, Young J, Koerner
I, Sheldahl LC, Hurn PD, Martínez-Murillo F and Alkayed NJ: Role of
cocaine- and amphetamine-regulated transcript in estradiol-mediated
neuroprotection. Proc Natl Acad Sci USA. 103:14489–14494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun W, Sun W, Liu J, Zhou X, Xiao Y,
Karaplis A, Pollak MR, Brown E, Goltzman D and Miao D: Alterations
in phosphorus, calcium and PTHrP contribute to defects in dental
and dental alveolar bone formation in calcium-sensing
receptor-deficient mice. Development. 137:985–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Müller S, Preische O, Sohrabi HR, Gräber
S, Jucker M, Ringman JM, Martins RN, McDade E, Schofield PR, Ghetti
B, et al: Relationship between physical activity, cognition, and
Alzheimer pathology in autosomal dominant Alzheimer's disease.
Alzheimers Dement. 14:1427–1437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vöglein J, Paumier K, Jucker M, Preische
O, McDade E, Hassenstab J, Benzinger TL, Noble JM, Berman SB,
Graff-Radford NR, et al: Clinical, pathophysiological and genetic
features of motor symptoms in autosomal dominant Alzheimer's
disease. Brain. 142:1429–1440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lane CA, Hardy J and Schott JM:
Alzheimer's disease. Eur J Neurol. 25:59–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haass C and Selkoe DJ: Soluble protein
oligomers in neurodegeneration: Lessons from the Alzheimer's
amyloid beta-peptide. Nat Rev Mol Cell Biol. 8:101–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin M, Shepardson N, Yang T, Chen G, Walsh
D and Selkoe DJ: Soluble amyloid beta-protein dimers isolated from
Alzheimer cortex directly induce Tau hyperphosphorylation and
neuritic degeneration. Proc Natl Acad Sci USA. 108:5819–5824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zempel H, Thies E, Mandelkow E and
Mandelkow EM: Abeta oligomers cause localized Ca(2+) elevation,
missorting of endogenous Tau into dendrites, Tau phosphorylation,
and destruction of microtubules and spines. J Neurosci.
30:11938–11950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barage SH and Sonawane KD: Amyloid cascade
hypothesis: Pathogenesis and therapeutic strategies in Alzheimer's
disease. Neuropeptides. 52:1–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tönnies E and Trushina E: Oxidative
stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers
Dis. 57:1105–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin F, Sancheti H, Patil I and Cadenas E:
Energy metabolism and inflammation in brain aging and Alzheimer's
disease. Free Radic Biol Med. 100:108–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen L, Yang R, Qiao W, Zhang W, Chen J,
Mao L, Goltzman D and Miao D: 1,25-Dihydroxyvitamin D exerts an
antiaging role by activation of Nrf2-antioxidant signaling and
inactivation of p16/p53-senescence signaling. Aging Cell.
18:e129512019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raefsky SM, Furman R, Milne G, Pollock E,
Axelsen P, Mattson MP and Shchepinov MS: Deuterated polyunsaturated
fatty acids reduce brain lipid peroxidation and hippocampal amyloid
β-peptide levels, without discernable behavioral effects in an
APP/PS1 mutant transgenic mouse model of Alzheimer's disease.
Neurobiol Aging. 66:165–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hardas SS, Sultana R, Clark AM, Beckett
TL, Szweda LI, Murphy MP and Butterfield DA: Oxidative modification
of lipoic acid by HNE in Alzheimer disease brain. Redox Biol.
1:80–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiao W, Wang Y, Kong L, Ou-Yang T, Meng Q,
Fu Q and Hu Z: CART peptide activates the Nrf2/HO-1 antioxidant
pathway and protects hippocampal neurons in a rat model of
Alzheimer's disease. Biochem Biophys Res Commun. 501:1016–1022.
2018. View Article : Google Scholar : PubMed/NCBI
|