Introduction
A number of conditions cause cardiomyocyte damage
due to lack of oxygen, such as coronary and cyanotic congenital
heart disease, high altitude and cardiopulmonary bypass. The heart
is one of the organs most sensitive to lack of oxygen (1). Following hypoxia, cardiomyocytes are
susceptible to necrosis and apoptosis via multiple signaling
pathways, thus leading to cardiac dysfunction and poor clinical
prognosis (2). Therefore, effective
strategies to protect cardiomyocytes against hypoxia-induced injury
are required.
Traditional Chinese medicine has attracted
increasing attention in cardiovascular medicine research due to its
potential ability to target a number of signaling pathways
(3,4). Quercetin-3-O-β-D-galactopyranoside,
also known as hyperoside (Hyp), is a flavonoid glycoside extracted
from Hypericum plants and may exert cytoprotective effects,
including antioxidant effects, decreasing calcium overload and
inhibiting apoptosis (5). In an
ischemia/reperfusion-mediated acute myocardial infarction model,
administration of Hyp improved heart contractility and decreased
the area of infarcted myocardium via activation of the
extracellular signal-regulated kinase 1/2 signaling pathway
(6), and has been identified to be
associated with high levels of ATP, as well as decreased oxidative
stress (7). In addition, Hyp has
been demonstrated to attenuate hypoxia/reoxygenation-induced
apoptosis in cardiomyocytes by suppressing Bcl-2 interacting
protein 3 expression (8). However,
the role of Hyp in the hypoxic heart and its potential underlying
regulatory mechanism have yet to be fully elucidated.
MicroRNAs (miRNAs/miRs) are a type of endogenous
small RNA 18–25 nucleotides in length that serve key roles in
numerous pathophysiological cell processes, such as apoptosis,
proliferation and inflammation (9).
miR-138 has been demonstrated to have notable cardioprotective
properties. Our previous study demonstrated that miR-138 is
upregulated in chronically hypoxic cardiomyocytes (10). By inhibiting the expression levels
of mixed lineage kinase 3 (MLK3) and the phosphorylation of its
downstream signaling targets, miR-138 effectively alleviates
apoptosis. Similar anti-apoptotic effects of miR-138 on hypoxic
cardiomyocytes are achieved via targeting of lipocalin-2 (Lcn2)
(11). Pyruvate dehydrogenase
kinase 1 (PDK1) has also been demonstrated to be a target protein
of miR-138 (11). By inhibiting
glycolysis and promoting mitochondrial respiration, increased
miR-138 levels improve myocardial viability under prolonged hypoxia
(12).
In the present study, the effects of Hyp on the
expression levels of miR-138 in hypoxic cardiomyocytes were
investigated. miR-138 antagomir transfection was performed to
assess the effect of Hyp on hypoxia-induced myocardial injury and
its underlying mechanism. The present study aimed to identify a
potential therapeutic strategy for chronic heart disease.
Materials and methods
Cell culture and treatment
The embryonic rat cardiomyoblast H9C2 cell line was
obtained from American Type Culture Collection and cultured in DMEM
with 10% FBS (both Gibco; Thermo Fisher Scientific, Inc.). In the
normoxia group, cells were incubated in an atmosphere of 5%
CO2 and 95% air at 37°C. In the hypoxia group, a Forma™
Series II 3131 incubator (Thermo Fisher Scientific, Inc.) was used
to maintain O2 at 1% by N2 displacement, and
cells were incubated at 37°C for 24 h. Hyp (molecular weight,
464.38; purity, ≥97%) was purchased from Sigma-Aldrich (Merck
KGaA). At the beginning of hypoxia exposure, Hyp was added at
various concentrations (1, 10, 50 and 100 µmol/l). Cells in the
control group were cultured without the administration of Hyp.
Animal treatment
Furthermore, a total of 100 6-week-old male C57BL/6
mice (weight, 20±2 g) were purchased from Vital River Laboratory
Animal Technology Corporation. Overall, a total of 90 mice were
included in the present study, as 10 mice were scarified after
improper establishment of the experimental model during the process
of tail vein injection. Mice were randomly divided into various
groups (n=9 per group): i) Control group; ii) 10 mg/kg group; iii)
50 mg/kg group; iv) 100 mg/kg group; v) 200 mg/kg group; vi)
normoxia group; vii) hypoxia control group; viii) hypoxia Hyp
group; ix) hypoxia Hyp + antagomir group; and x) hypoxia Hyp +
antagomir-NC group. All animals were fed with free access to
standard chow and tap water under specific pathogen-free conditions
with a 12-h light/dark cycle at 22±1°C and 45–55% relative
humidity. In the hypoxia group, mice were housed in a normobaric
hypoxic chamber at an oxygen concentration of 10% for 1 week. Hyp
(10 mg/ml in dimethyl sulfoxide) was administrated via oral gavage
at various doses (10, 50, 100 and 200 mg/kg) daily, while mice in
control group were housed without the addition of Hyp. In the
normoxia group, animals were maintained in a normobaric chamber at
an oxygen concentration of 21% for 1 week. All animal procedures
were approved by the Animal Care and Use Committee of the General
Hospital of Western Theater Command and Army Medical University
(Chengdu, China), and were performed in accordance with the Guide
for the Care and Use of Laboratory Animals (8th edition, 2011;
National Institutes of Health) (13).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA of myocardial tissues was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse transcribed to cDNA using the PrimeScript RT
reagent kit (Takara Bio, Inc.) according to the manufacturer's
instructions. Stem-loop primers of miR-138 (cat. no. MQPSCM001-1)
and internal control U6 (cat. no. MQPS0000002-1-100) were
synthesized by Guangzhou RiboBio Co., Ltd. PCR was performed using
the TB Green™ Premix Ex Taq™ GC kit (Takara Bio, Inc.) on the
Applied Biosystems® 7500 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. The thermocycling conditions were as
follows: Initial denaturation for 30 sec at 95°C, followed by 40
cycles of 5 sec at 95°C and 34 sec at 60°C. Each reaction was run
in triplicate. The relative expression levels were calculated using
the 2−ΔΔCq method (14).
miRNA antagomir transfection
miR-138 antagomir, a single-stranded RNA for
silencing of the expression of miR-138, was synthesized by
Genepharm, Inc. Antagomir and matched negative control (NC) were
dissolved in DEPC H2O at 20 µM. H9C2 cells were
transfected with miR-138 antagomir (5′-CGGCCCUGAUUCACAACACCAGCU−3′)
or NC (5′-CAGUACUUUUGUGUAGUACAA−3′) at a concentration of 50 nmol/l
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, and
incubated at 37°C for 48 h. C57BL/6 mice were injected with miR-138
antagomir or NC (10 mg/kg) via the tail vein once every 2 days for
2 weeks. Cells and myocardial tissues were collected to detect the
expression of miR-138 using RT-qPCR. Following successful
establishment of the miR-138 silencing model, cells and mice were
stimulated with Hyp and hypoxia as aforementioned.
Western blotting
Antibodies against cleaved caspase-3 (1:1,000; cat.
no. AF7022), cleaved poly(ADP)-ribose polymerase (PARP; 1:1,000;
cat. no. AF7023), Bcl-2 (1:1,000; cat. no. AF6139) and the internal
control GAPDH (1:2,000; cat. no. AF7021) were obtained from
Affinity Biosciences. Antibodies against Lcn2 (1:1,000; cat. no.
ab216462) and PDK1 (1:1,000; cat. no. ab202468) were obtained from
Abcam. The anti-MLK3 antibody (1:1,000; cat. no. sc-166639) was
obtained from Santa Cruz Biotechnology, Inc. H9C2 cells and heart
tissues were lysed in SDS lysis buffer (Beyotime Institute of
Biotechnology) containing protease inhibitor. Protein concentration
was detected using a BCA protein assay. Equivalent amounts of
proteins (30–100 µg per lane) were loaded and separated via
SDS-PAGE on 6–15% gels. After being transferred to PVDF membranes
(Roche Diagnostics), the samples were blocked with 5% non-fat milk
powder at room temperature for 2 h, and then incubated with primary
antibodies at 4°C overnight. Anti-rabbit (cat. no. SE134) or
anti-mouse (cat. no. SE131) secondary antibodies were purchased
from Beijing Solarbio Science & Technology Co., Ltd., and
diluted in TBS with 0.1% Tween-20 (1:5,000). Subsequently,
secondary antibodies were added at room temperature for 1 h and the
blots were detected using an ECL kit (Affinity Biosciences).
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) was used for
densitometry.
Cell survival assay
Cell viability was evaluated using a Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology). H9C2 cells were
seeded in a 96-well plate (4×104 cells per well) and
treated with CCK-8 reagent. After incubation at 37°C for 2 h, the
absorbance was measured at 450 nm. Additionally, a
5-ethynyl-2′-deoxyuridine (EdU) kit (Guangzhou RiboBio Co., Ltd.)
was used to assess the cell proliferation ability. EdU labeling
medium was added and nuclei were stained with Hoechst 33342 at room
temperature for 30 min, according to the manufacturer's protocols.
EdU-positive cells were observed under a fluorescence
microscope.
Cell apoptosis assay
The ratio of apoptotic H9C2 cells was determined
using Annexin V/FITC and PI (BD Biosciences). Cells were harvested
and suspended in binding buffer, and then stained with Annexin V
for 15 min and PI for 5 min at room temperature in dark conditions.
A flow cytometer (FACSCalibur; BD Biosciences) was used to analyze
cell apoptosis. Data was calculated using FlowJo software v10.5
(FlowJo LLC). For heart tissue samples from C57BL/6 mice, the
apoptosis ratio was determined via a TUNEL assay. The specimens
were first fixed with 4% paraformaldehyde at room temperature
overnight, and then sliced horizontally at a thickness of 5 µm.
Following embedding, deparaffinization and rehydration, the
sections were stained at 37°C for 1 h with the in situ Cell
Death Detection kit (cat. no. 11684817910; Roche Diagnostics)
according to the manufacturer's protocol. Subsequently, nuclei were
stained with hematoxylin at room temperature for 10 sec. Sections
were sealed with neutral resin. TUNEL-positive cells were
visualized using a light microscope in six randomly selected fields
(magnification, ×400) and are presented as the percentage of total
cells counted.
Measurement of myocardial injury
markers
After being fed under normoxic or hypoxic conditions
for 1 week, mice were sacrificed by cervical dislocation.
Immediately, blood samples from the right ventricle were collected
by cardiac puncture, and were centrifuged within 30 min to obtain
the supernatant serum. The serum was subsequently examined for
lactate dehydrogenase (LDH; cat. no. A020) and creatine kinase-MB
isoenzyme (CK-MB; cat. no. H197) using ELISA kits (Nanjing
Jiancheng Bioengineering Institute). Heart tissue was also quickly
obtained after sacrifice and stored in ice-cold PBS, and then
homogenized within 30 min. The supernatant was separated at a speed
of 1,000 × g for 15 min at 4°C for the detection of malondialdehyde
(MDA; cat. no. A003) and superoxide dismutase (SOD; cat. no. A001)
(both from Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's protocols.
Statistical analysis
All data are presented as the mean ± SD. SPSS v22.0
software (IBM Corp.) was used to perform statistical analysis.
Comparisons between two groups were analyzed using an unpaired
t-test. The differences among multiple groups were compared by
one-way ANOVA followed by Tukey's post hoc test. Each experiment
was repeated ≥3 times. All P-values were two-tailed. P<0.05 was
considered to indicate a statistically significant difference.
Results
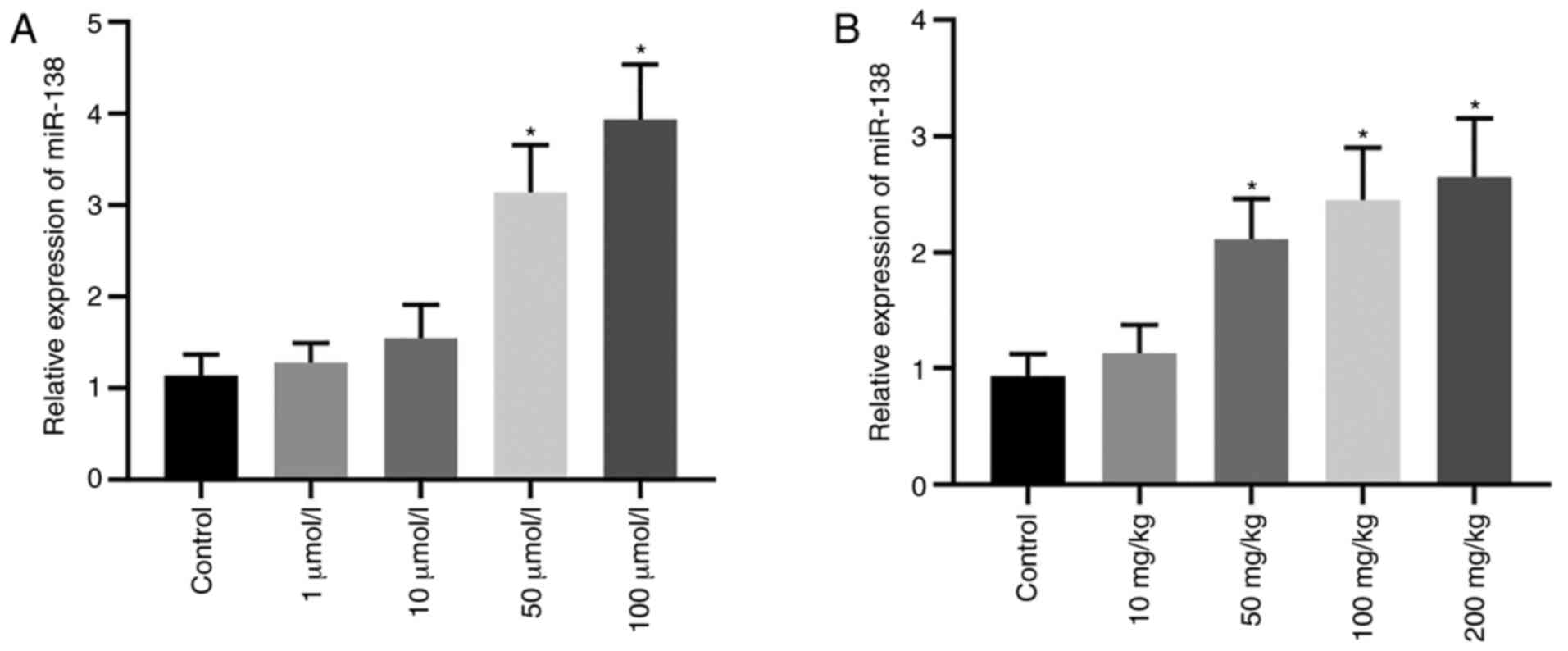
Effect of Hyp on the expression levels
of miR-138 in hypoxic cardiomyocytes
H9C2 cells were stimulated by Hyp at various
concentrations under hypoxic conditions. After 24 h, the expression
levels of miR-138 were significantly increased compared with the
control group when the concentration of Hyp was 50 and 100 µmol/l
(Fig. 1A). Additionally, in C57BL/6
mice fed under hypoxic conditions for 1 week, the expression levels
of miR-138 in heart tissues were significantly increased compared
with the control group when Hyp was administered at 50, 100 and 200
mg/kg (Fig. 1B). These data
demonstrated that Hyp treatment upregulated the expression levels
of miR-138.
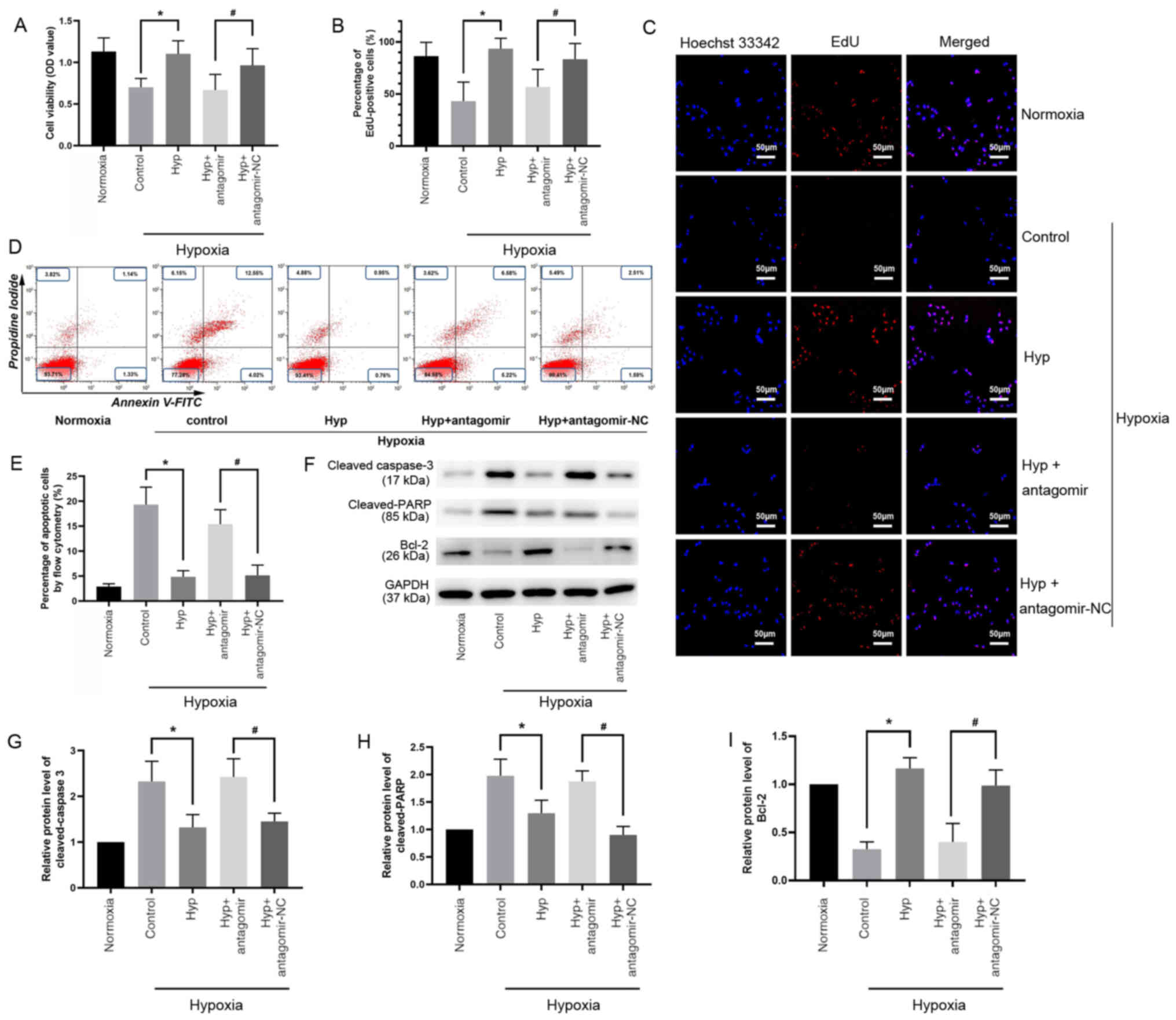
Hyp promotes H9C2 cell survival via
upregulation of miR-138 expression levels under hypoxic
conditions
Since miR-138 serves a notable cardioprotective role
(9), it was hypothesized that Hyp
promotes cardiomyocyte survival under hypoxic conditions. First, a
hypoxic model in H9C2 cells was established to investigate the role
of 100 µmol/l Hyp, which had the strongest effect on promoting the
expression of miR-138 among the different concentration gradients
that were selected. Antagomir was used to silence miR-138
expression. The transfection efficacy is shown in Fig. S1A. A CCK-8 assay demonstrated that
cell viability in the Hyp group was 1.5 times higher compared with
the control group (Fig. 2A).
miR-138 antagomir transfection significantly decreased cell
viability compared with antagomir-NC transfection in cells in
hypoxic conditions (Fig. 2A). Cells
were stained using an EdU assay (Fig.
2C), which revealed that Hyp significantly improved cell
proliferation, which had been impaired by hypoxia (Fig. 2B), and the percentage of
EdU-positive cells in the Hyp + antagomir group was significantly
lower compared with that in the Hyp + antagomir-NC group (Fig. 2B). Cell apoptosis was evaluated by
flow cytometry (Fig. 2D). Apoptosis
was induced under hypoxia, and this was significantly alleviated by
Hyp treatment (Fig. 2E). However,
Hyp did not decrease the number of apoptotic cells in the presence
of miR-138 antagomir (Fig. 2E). The
expression levels of apoptosis-associated proteins were detected by
western blotting (Fig. 2F).
Downregulated cleaved caspase-3 (Fig.
2G) and cleaved PARP (Fig. 2H),
and upregulated Bcl-2 (Fig. 2I)
expression was observed in the Hyp group. Compared with the Hyp +
antagomir-NC group, Hyp + antagomir significantly increased cleaved
caspase-3 (Fig. 2G) and cleaved
PARP (Fig. 2H) levels, and
decreased Bcl-2 expression levels (Fig.
2I). These results demonstrated that the protective role of Hyp
in vitro was mediated via upregulation of miR-138 under
hypoxic conditions.
Hyp attenuates hypoxia-induced
myocardial injury in mice via miR-138
Subsequently, the effect of Hyp was verified in mice
exposed to hypoxia. The dose of Hyp was set as 100 mg/kg, which had
the strongest effect on promoting the expression of miR-138 among
the different concentration gradients that were selected.
Transfection efficacy of miR-138 antagomir is presented in Fig. S1B. Detection of markers of
myocardial injury demonstrated that the expression levels of LDH
(Fig. 3A), CK-MB (Fig. 3B) and MDA (Fig. 3C) were significantly decreased in
the Hyp group compared with the control group, and increased in the
Hyp + antagomir group compared with the Hyp + antagomir-NC group.
In addition, the levels of SOD exhibited a significant increase in
the Hyp group, which was abated in the presence of miR-138
antagomir (Fig. 3D). The
morphological changes of the myocardium are shown in Fig. 3E, and apoptotic cells were labeled
in a TUNEL assay. Hypoxia-induced apoptosis was significantly
suppressed following administration of Hyp (Fig. 3F). This anti-apoptotic effect of Hyp
was reversed following inhibition of miR-138 by antagomir (Fig. 3F). The expression levels of
apoptosis-associated proteins were detected via western blotting
(Fig. 3G). Expression of cleaved
caspase-3 (Fig. 3H) and cleaved
PARP (Fig. 3I) in the Hyp group
were significantly decreased compared with those in the control
group, whereas the expression levels of Bcl-2 (Fig. 3J) were significantly upregulated. A
significant increase in the levels of cleaved caspase-3 and cleaved
PARP, and decreased expression levels of Bcl-2, were observed in
the presence of antagomir. Therefore, these findings indicated that
Hyp protected the myocardium from hypoxia-induced injury in
vivo via upregulation of miR-138.
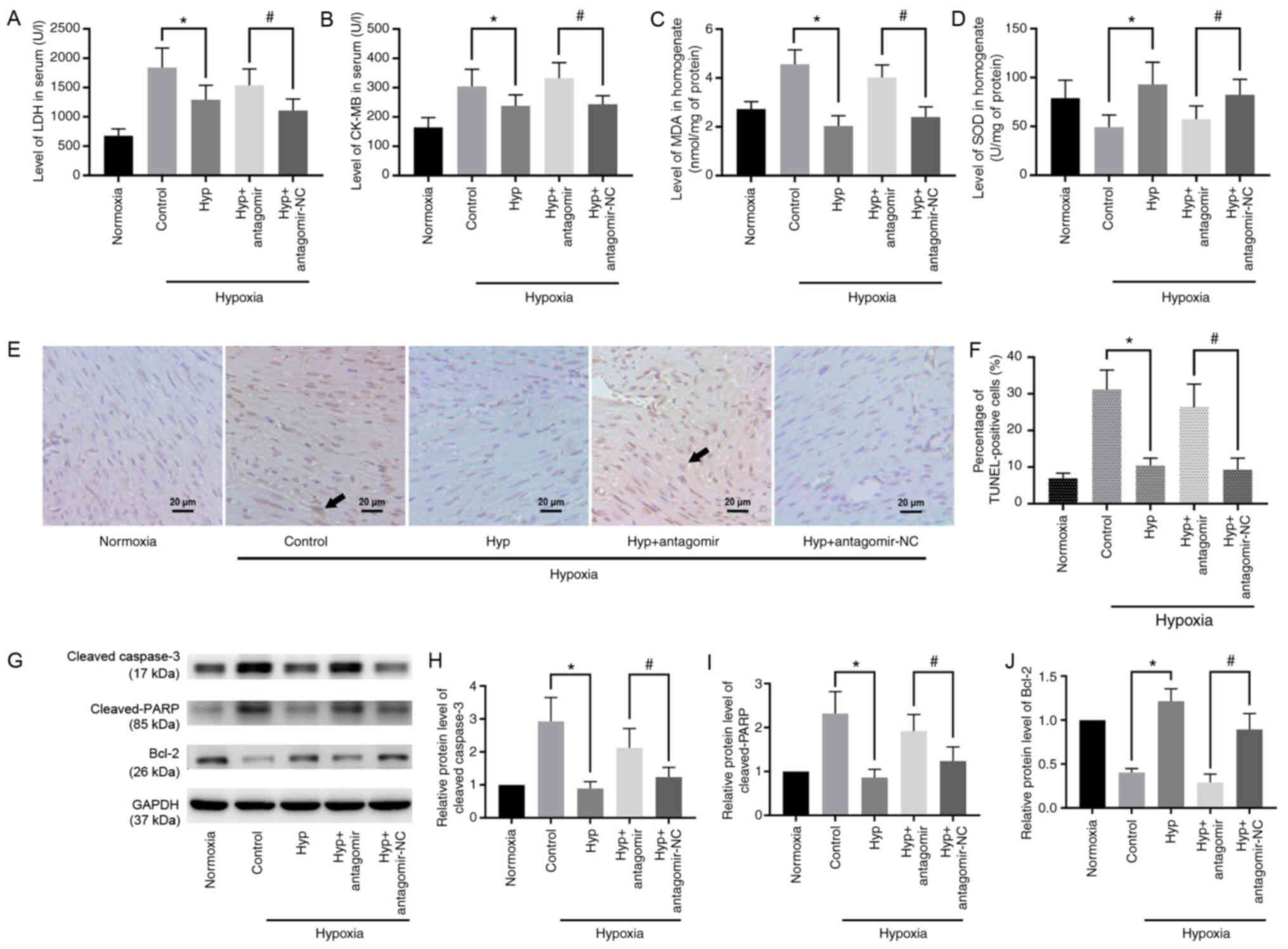 | Figure 3.Hyp protects mouse myocardium from
hypoxia-induced injury via miR-138. (A) LDH and (B) CK-MB levels in
serum, and (C) MDA and (D) SOD levels in myocardial homogenate were
detected. (E) Myocardial morphology was assessed using a light
microscope. Scale bar, 20 µm. Arrows indicate apoptotic cells. (F)
Cell apoptosis was examined using a TUNEL assay. (G) Western
blotting revealed the relative expression levels of (H) cleaved
caspase-3, (I) cleaved PARP and (J) Bcl-2. Data are presented as
the mean ± SD. *P<0.05. #P<0.05. Hyp, hyperoside;
miR-138, microRNA-138; LDH, lactate dehydrogenase; CK-MB, creatine
kinase-myocardial band; MDA, malondialdehyde; SOD, superoxide
dismutase; PARP, poly(ADP)-ribose polymerase; NC, negative
control. |
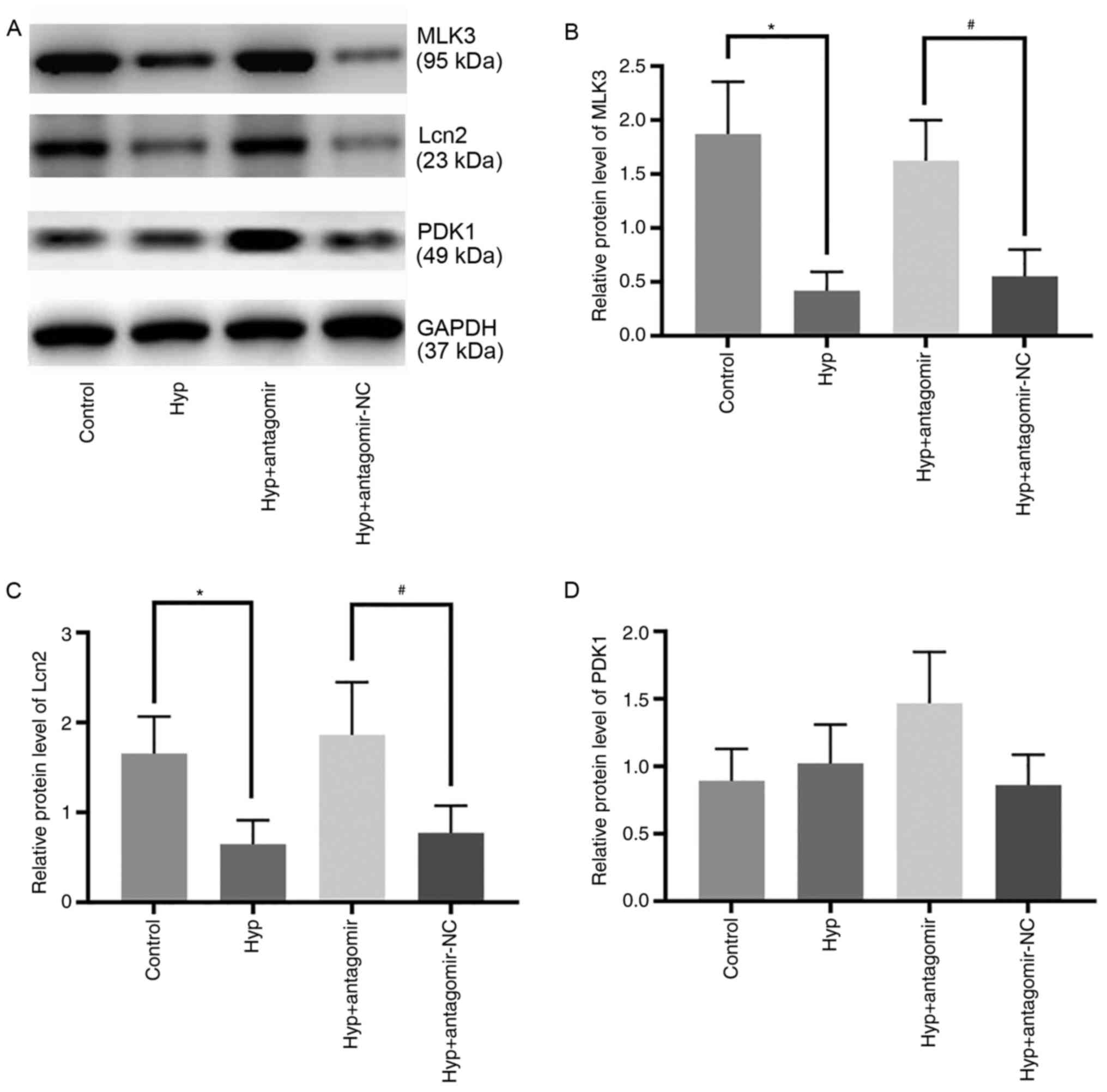
Effect of Hyp on the expression levels
of miR-138 target proteins
The expression levels of miR-138 target proteins
were detected to elucidate the potential mechanism of action of Hyp
(Fig. 4A). Following exposure of
H9C2 cells to hypoxia for 24 h, the expression levels of MLK3 and
Lcn2 were 22.3 and 38.9%, respectively, of those in the control
group (Fig. 4B and C). miR-138
antagomir upregulated the expression levels of these three target
proteins in the Hyp + antagomir group compared with the Hyp +
antagomir-NC group (Fig. 4B and C),
but there was no significant difference in the expression levels of
PDK1 between the Hyp and control groups. These data indicated that
Hyp exerted cardioprotective effects by regulating the expression
levels of MLK3 and Lcn2 but not PDK1.
Discussion
Hyp is a Chinese herbal medicine with multiple
biological properties that serve a key role in a number of diseases
(2,3). In an ischemia/reperfusion model, Hyp
has been identified to protect the heart (6), kidney (15) and liver (16) against injury by modulating
mitochondrial fission, oxidative stress and apoptosis. Hydrogen
peroxide induces oxidative stress in granulosa cells, which is
effectively suppressed by Hyp via the Sonic hedgehog signaling
pathway (17). In addition, Kwon
et al (18) observed that
Hyp decreased apoptosis of dopaminergic neurons via activation of
heme oxygenase-1 signaling. Hypoxia is a common condition in
numerous cardiovascular events, such as ischemic heart disease,
high altitude heart disease and cyanotic congenital heart disease
(1). When subjected to hypoxia,
cardiomyocytes develop disorders of mitochondria, membrane and
lysosomes, leading to the initiation of the caspase cascade and
activation of the Bcl-2 family (19). Oxidative stress and apoptosis are
key mechanisms underlying hypoxia-induced injury (20) and are primary targets of Hyp
(16). In the present study, Hyp
was applied to hypoxic cardiomyocytes and its cytoprotective role
was verified in vivo as well as in vitro. The
expression of SOD and MDA can partially reflect the level of
oxidative stress, which were examined in the present study, thus it
was found that hypoxia induced myocardial apoptosis and oxidative
stress, which were attenuated by Hyp.
A number of miRNAs serve as downstream factors of
Hyp, such as let-7a-5p (21) and
miR-27a (22). Our previous study
on diabetic nephropathy demonstrated that Hyp suppresses the
expression of miR-21 and improves renal function via targeting of
matrix metalloproteinase-9 (23).
The present study indicated that expression levels of miR-138 were
significantly increased in the hypoxic myocardium following
addition of Hyp at concentrations of >50 µmol/l in vivo
and >50 mg/kg in vitro. When miR-138 was inhibited by
antagomir, the protective role of Hyp was attenuated, suggesting
that the effects of Hyp were mediated via miR-138 signaling. Our
previous study (9) demonstrated
miR-138 is a key regulator of adaptation to chronic hypoxia in
cardiomyocytes. miR-138 serves an anti-apoptotic role by directly
affecting the expression levels of caspase and Bcl-2 family
members, as well as activation of c-Jun N-terminal kinase and p38
MAPK signaling (9,24,25).
Furthermore, miR-138 has been found to exert anti-inflammatory
effects by regulating the AKT and NF-κB signaling pathways
(26,27), and to maintain mitochondrial
homeostasis by targeting hypoxia-inducible factor 1-α (28).
miRNAs act by binding to and degrading target mRNA,
and suppress protein translation at the post-transcriptional level
(29). Previous data have
demonstrated that MLK3 (9), Lcn2
(10) and PDK1 (11) are target proteins of miR-138 in
hypoxic cardiomyocytes. In order to elucidate the mechanism of
action of Hyp, expression levels of these three targets were
assessed. Hyp caused significant downregulation of MLK3 and Lcn2,
but not PDK1. MLK3 is an important member of the MAPK family. MLK3
phosphorylates JNK and affects downstream c-Jun signaling, and also
serves a key role in cell apoptosis and response to stress
(30). Lcn2 is a secreted adipokine
of the lipocalin family that binds to and transports small
molecules, such as iron and fatty acids (10), and is considered to be a biomarker
of metabolic inflammation (31).
Absence of Lcn2 induces apoptosis in cardiomyocytes by increasing
intracellular iron levels (32).
Lcn2 also regulates the innate immune response in the pathogenesis
of heart failure (33). PDK1 is an
essential enzyme involved in glucose metabolism that promotes
gluconeogenesis from pyruvate, lactic acid and alanine (34), and prevents the incorporation of
pyruvate in the oxidative phosphorylation process, leading to
enhanced anaerobic glycolysis and decreased cellular respiration
(11,35). Based on the aforementioned evidence,
it was hypothesized that Hyp protects cardiomyocytes from
hypoxia-induced injury via regulation of apoptosis and stress
response rather than glucose metabolism.
In conclusion, the present study demonstrated that
Hyp upregulated miR-138 in hypoxic cardiomyocytes. Hyp treatment
promoted cardiomyocyte survival and alleviated hypoxia-induced
apoptosis by inhibiting the expression of downstream targets of
miR-138, namely MLK3 and Lcn2. These findings may provide promising
novel strategies for clinical cardioprotection.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81700277 and
81600635) and the Project of Youth Scientific and Technological
Innovation in General Hospital of Western Theater Command (grant
no. 41732C11K).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH wrote the manuscript. SH, XY and JC performed
cell experiments. FW, FG and MX performed animal experiments. SZ
and JW performed data management and analysis. LZ and JZ designed
the study and supervised the research. SH and JZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Animal
Care and Use Committee of General Hospital of Western Theater
Command and Army Medical University (approval no. 2020ky018;
Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Azzouzi HE, Leptidis S, Doevendans PA and
De Windt LJ: HypoxamiRs: Regulators of cardiac hypoxia and energy
metabolism. Trends Endocrinol Metab. 26:502–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu Q, Shen T, Wang Q, Yu X, Jia N and He
Q: Cardiac shock wave therapy protects cardiomyocytes from
hypoxia-induced injury by modulating miR-210. Mol Med Rep.
21:631–640. 2020.PubMed/NCBI
|
|
3
|
Zhang P: Advantages, disadvantages, and
trend of integrative medicine in the treatment of heart failure.
Cell Biochem Biophys. 72:363–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin F, Chen HW, Zhao GA, Li Y, He XH,
Liang WQ, Shi ZL, Sun SY, Tian PP, Huang MY and Liu C: Advances in
research on the circRNA-miRNA-mRNA network in coronary heart
disease treated with traditional chinese medicine. Evid Based
Complement Alternat Med. 2020:80486912020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Dai Q, Hu L, Yu H, Qiu J, Zhou J,
Long M, Zhou S and Zhang K: Hyperoside alleviates high
glucose-induced proliferation of mesangial cells through the
inhibition of the ERK/CREB/miRNA-34a signaling pathway. Int J
Endocrinol. 2020:13619242020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li ZL, Hu J, Li YL, Xue F, Zhang L, Xie
JQ, Liu ZH, Li H, Yi DH, Liu JC and Wang SW: The effect of
hyperoside on the functional recovery of the ischemic/reperfused
isolated rat heart: Potential involvement of the extracellular
signal-regulated kinase 1/2 signaling pathway. Free Radic Biol Med.
57:132–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou JY, Liu Y, Liu L and Li XM: Protective
effect of hyperoside on cardiac ischemia reperfusion injury through
inhibition of ER stress and activation of Nrf2 signaling. Asian Pac
J Trop Med. 9:76–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao R, Xiang AL, Pang HB and Liu KQ:
Hyperoside protects against hypoxia/reoxygenation induced injury in
cardiomyocytes by suppressing the Bnip3 expression. Gene.
629:86–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalayinia S, Arjmand F, Maleki M,
Malakootian M and Singh CP: MicroRNAs: Roles in cardiovascular
development and disease. Cardiovasc Pathol. 50:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z
and Xiao Y: miR-138 protects cardiomyocytes from hypoxia-induced
apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun.
441:763–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong H, Luo T, He W, Xi D, Lu H, Li M,
Liu J and Guo Z: Up-regulation of miR-138 inhibits hypoxia-induced
cardiomyocyte apoptosis via down-regulating lipocalin-2 expression.
Exp Biol Med (Maywood). 241:25–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Xue H, Jin QH, Guo J and Chen YD:
miR-138 protects cardiac cells against hypoxia through modulation
of glucose metabolism by targetting pyruvate dehydrogenase kinase
1. Biosci Rep. 37:BSR201702962017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Council N: Guide for the Care and Use of
Laboratory Animals: Eighth Edition. Publication. 327:963–965.
2011.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu L, Li Q, Liu S, An X, Huang Z, Zhang B,
Yuan Y and Xing C: Protective effect of hyperoside against renal
ischemia-reperfusion injury via modulating mitochondrial fission,
oxidative stress, and apoptosis. Free Radic Res. 53:727–736. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Qiu X, Dai M, Zhang X and Jin G:
Hyperoside attenuates hepatic ischemia-reperfusion injury by
suppressing oxidative stress and inhibiting apoptosis in rats.
Transplant Proc. 51:2051–2059. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Fan G, Wei F, Bu Y and Huang W:
Hyperoside protects rat ovarian granulosa cells against hydrogen
peroxide-induced injury by sonic hedgehog signaling pathway. Chem
Biol Interact. 310:1087592019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon SH, Lee SR, Park YJ, Ra M, Lee Y,
Pang C and Kim KH: Suppression of 6-hydroxydopamine-induced
oxidative stress by hyperoside via activation of Nrf2/HO-1
signaling in dopaminergic neurons. Int J Mol Sci. 20:58322019.
View Article : Google Scholar
|
|
19
|
Rodius S, de Klein N, Jeanty C,
Sánchez-Iranzo H, Crespo I, Ibberson M, Xenarios I, Dittmar G,
Mercader N, Niclou SP and Azuaje F: Fisetin protects against
cardiac cell death through reduction of ROS production and caspases
activity. Sci Rep. 10:28962020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loor G and Schumacker PT: Role of
hypoxia-inducible factor in cell survival during myocardial
ischemia-reperfusion. Cell Death Differ. 15:686–690. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li JP, Liao XH, Xiang Y, Yao A, Song RH,
Zhang ZJ, Huang F, Dai ZT and Zhang TC: Hyperoside and let-7a-5p
synergistically inhibits lung cancer cell proliferation via
inducing G1/S phase arrest. Gene. 679:232–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, He S, Yang F, Yu H, Xie W, Dai Q,
Zhang D, Liu X, Zhou S and Zhang K: Hyperoside ameliorates
glomerulosclerosis in diabetic nephropathy by downregulating
miR-21. Can J Physiol Pharmacol. 94:1249–1256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Qin Q, Dai H, Cai S, Zhou C and
Guan J: Emodin protects H9c2 cells from hypoxia-induced injury by
up-regulating miR-138 expression. Braz J Med Biol Res.
52:e79942019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Li J, Zhang P, Jiang X, Pan Z, Zheng
W and Lin H: lncRNA-LET relieves hypoxia-induced injury in H9c2
cells through regulation of miR-138. J Cell Biochem. 121:259–268.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JB, Wang HY, Yao Y, Sun QF, Liu ZH, Liu
SQ, Zhuang JL, Wang YP and Liu HY: Overexpression of microRNA-138
alleviates human coronary artery endothelial cell injury and
inflammatory response by inhibiting the PI3K/Akt/eNOS pathway. J
Cell Mol Med. 21:1482–1491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi S, Zhang S, Zhang H, Jin Q and Wu D:
Silencing circANKRD36 protects H9c2 cells against
lipopolysaccharide-induced injury via up-regulating miR-138. Exp
Mol Pathol. 111:1043002019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Zou J, Liu X and Zhang Q:
MicroRNA-138 attenuates myocardial ischemia reperfusion injury
through inhibiting mitochondria-mediated apoptosis by targeting
HIF1-α. Exp Ther Med. 18:3325–3332. 2019.PubMed/NCBI
|
|
29
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He S, Liu S, Wu X, Xin M, Ding S, Xin D,
Ouyang H and Zhang J: Protective role of downregulated MLK3 in
myocardial adaptation to chronic hypoxia. J Physiol Biochem.
73:371–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moschen AR, Adolph TE, Gerner RR, Wieser V
and Tilg H: Lipocalin-2: A master mediator of intestinal and
metabolic inflammation. Trends Endocrinol Metab. 28:388–397. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu G, Ahn J, Chang S, Eguchi M, Ogier A,
Han S, Park Y, Shim C, Jang Y, Yang B, et al: Lipocalin-2 induces
cardiomyocyte apoptosis by increasing intracellular iron
accumulation. J Biol Chem. 287:4808–4817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yndestad A, Landrø L, Ueland T, Dahl CP,
Flo TH, Vinge LE, Espevik T, Frøland SS, Husberg C, Christensen G,
et al: Increased systemic and myocardial expression of neutrophil
gelatinase-associated lipocalin in clinical and experimental heart
failure. Eur Heart J. 30:1229–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calleja V, Laguerre M, de Las
Heras-Martinez G, Parker PJ, Requejo-Isidro J and Larijani B: Acute
regulation of PDK1 by a complex interplay of molecular switches.
Biochem Soc Trans. 42:1435–1440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaplon J, Zheng L, Meissl K, Chaneton B,
Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M,
Shlomi T, et al: A key role for mitochondrial gatekeeper pyruvate
dehydrogenase in oncogene-induced senescence. Nature. 498:109–112.
2013. View Article : Google Scholar : PubMed/NCBI
|