Introduction
Pancreatic cancer is a fatal disease, representing
the fourth leading cause of cancer-related deaths worldwide. Only a
few patients demonstrate a sustained response to chemotherapy or
radiation therapy due to drug resistance or toxicity, and patient
prognosis is extremely poor, demonstrating a 5-year survival rate
of only 3% (1–3). The majority of patients with
pancreatic cancer are diagnosed with an advanced-stage disease
because of its poor prognosis; this excludes the possibility of
surgery. A chemotherapy regimen with gemcitabine is the most common
treatment prescribed for advanced pancreatic cancer (2). Although some progress has been made
with respect to novel targeted therapies and combination therapies
for patients, the overall survival rate has not been significantly
improved (3).
Cancer stem-like cells (CSCs) have been investigated
and used for the development of novel cancer therapies by serving
as biomarkers in lung, liver and pancreatic cancer (4–6). CSCs
exhibit properties that are similar to those of normal stem cells,
such as a long lifespan with relative quiescence, resistance to
drugs and toxins through the expression of several ATP-binding
cassette (ABC) transporters, an active DNA-repair capacity and
resistance to apoptosis (7).
Previous studies have indicated that CSCs may serve an important
role in cancer cell migration and the development of drug
resistance in cancer cells (8,9). The
presence of CSCs can be determined by analyzing the side population
(SP), a subpopulation of cells that express ABC superfamily G
member 2, and thus, are multidrug-resistant (10). Using dual wavelength flow cytometry,
SP cells can be identified by their ability to efflux Hoechst 33342
dye (11,12).
Several studies have reported that extracts of
herbal mixtures are excellent agents for the treatment of various
types of disease, including cardiovascular and Alzheimer's disease
and dermatitis (13–15). For example, extracts of herbal
mixtures have been shown to exhibit anticancer, anti-inflammatory,
antibacterial and antioxidative effects (16–19).
In particular, the observed anticancer effects of herbal mixture
extracts have included their ability to boost the efficacy of
standard chemotherapies and reduce side effects during chemotherapy
(16). In the current study, a
novel cocktail of 10 types of traditional Chinese medicine herbs
was made, based on the advice of a medical doctor who had
experience in treating cancer. The anticancer efficacy of the
combined treatment with the herbal mixture extract, C5E, and
gemcitabine was investigated. The C5E mixture consisted of several
herbal extracts (described in the Materials and methods section),
which are widely used to treat several types of disease, including
cancer (20–23). One of the ingredients, Panax
ginseng (Korean ginseng) is a well-known herbal medicine, which
has been reported to reduce the proliferative ability of prostate
and lung cancer cells (20,21). Another ingredient, Inonotus
obliquus, is a type of mushroom commonly known as chaga, which
was previously identified to have both antioxidative and anticancer
effects (22,23).
Materials and methods
Cell culture
The human pancreatic carcinoma cell line, PANC-1,
was obtained from the Korean Cell Line Bank; Korean Cell Line
Research Foundation (cat. no. 21469). PANC-1 cells were cultured in
DMEM (Welgene, Inc.) supplemented with 100 U/ml penicillin, 100
µg/ml streptomycin and 10% heat-inactivated FBS (Welgene, Inc.).
The culture was maintained at 37°С in a humidified atmosphere of 5%
CO2.
Preparation of the herbal mixture
extract, C5E
C5E consisted of ten traditional Chinese medicinal
herbs. The proportions, manufacturing process and analyses of the
major components were previously reported (8). C5E was prepared using 16.6% Panax
ginseng (w/w; supplied from Kyung-dong market, Seoul, Republic
of Korea), 16.6% Inonotus obliquus, 11.1% Pinellia
ternata, 5.6% rhizome of Sparganium stoloniferum
Buchanan-Hamilton, 5.6% Alpinia galanga, 5.6% Cinnamomum
cassia, 5.6% Astragalus membranaceus, 11.1% Psoralea
corylifolia L., 11.1% Tetradium ruticarpum and 11.1%
Melia azedarach L. The medicinal herbs were obtained from
the Oriental Medical Hospital, Dongguk University (Ilsan, Republic
of Korea). C5E was prepared as follows: The dried and pulverized
herbs were mixed and a 1 kg batch was soaked and slowly stirred
overnight at 25°C with 3 l ethanol (40%). The ethanol extract was
concentrated using a rotary evaporator to remove solvent,
lyophilized at −80°C using a freeze-dryer (FDU-2110; Eyela) and
reconstituted in Milli-Q water (18.2 MΩ; EMD Millipore) for in
vitro studies.
Treatment with gemcitabine and/or
C5E
PANC-1 cells were seeded at a density of
5×105 cells/ml and subjected to three different
treatments and control: i) 10 nM gemcitabine (Sigma-Aldrich; Merck
KGaA); ii) 30 µg/ml C5E; and iii) pretreatment with 15 µg/ml C5E
for 2 h before treatment with 5 nM gemcitabine; iv) untreated
control. After 72 h of treatment at 37°C, the cells were harvested
at the logarithmic growth stage by treatment with 0.05%
trypsin-EDTA and centrifugation (220 × g; 3 min; 25°C). Cells were
resuspended in an equal volume of DMEM (Welgene, Inc.), then a 10
µl sample was stained for 10 sec with 10 µl 0.4% trypan blue dye at
25°C. Viability was determined by cell counting using hemocytometer
and the half maximal inhibitory concentration (IC50)
values were calculated as previously described (24).
Analysis of cell cycle
distribution
Cell cycle progression was analyzed by flow
cytometry. Following 72 h incubation at 37°C, PANC-1 cells were
harvested using trypsin-EDTA treatment as described above and
washed twice with PBS. When cells were harvested for SP analysis,
the population was divided in half: One half was used for SP
analysis, and the other half was used to examine cell cycle arrest.
The cells were fixed in ice-cold 70% ethanol at −20°C for 24 h.
Prior to flow cytometric analysis, the ethanol was removed by
centrifugation (250 × g, 5 min, 25°C) and the cells were washed and
resuspended in 5 ml PBS. 100 µl propidium iodide (PI) staining
solution containing 50 µg/ml PI (Sigma-Aldrich; Merck KGaA) diluted
in 1X PBS, 1 mg/ml RNase (Sigma-Aldrich; Merck KGaA) diluted in 1X
PBS and 0.1% Triton X-100 was added to a FACS tube and incubated in
the dark at room temperature for 30 min. Cell cycle analysis was
performed using a FACSCalibur flow cytometer (BD Biosciences) at an
excitation wavelength of 488 nm and an emission wavelength of 610
nm by measuring the amount of PI-labeled DNA in the cells. Data
were analyzed using ModFit LT™ version 3.0 software (Verity
Software House, Inc.).
Flow cytometric analysis of early and
late apoptosis
Flow cytometric analysis was performed using an FITC
Annexin V Apoptosis Detection kit І (BD Biosciences). PANC-1 cells
were harvested via trypsin-EDTA treatment as described above,
washed twice with PBS and centrifuged (250 × g, 5 min, 25°C). The
pellets were resuspended in 100 µl Annexin V binding buffer, and
then incubated with 5 µl Annexin V-FITC and 5 µl PI for 15 min at
room temperature in the dark. For the analysis, 300 µl binding
buffer was added to each mixture and analyzed using a FACSCalibur
flow cytometer. The fluorescence intensity of Annexin V-FITC and PI
was analyzed at an excitation/emission wavelength of 488/530 nm and
488/617 nm, respectively. The data were analyzed using BD CellQuest
Pro software version 5.2.1 (Becton Dickinson, Inc.) and apoptotic
rate was calculated by the percentage of each of early and late
apoptotic cells.
Analysis of SP cells
PANC-1 cells were harvested via treatment with
trypsin-EDTA as described above and labeled with Hoechst 33342 dye
(Sigma-Aldrich; Merck KGaA), using the methods described by Goodell
et al (11). Briefly, the
cells were resuspended at a density of 1×106 cells/ml in
pre-warmed DMEM (phenol red-free) containing 2% FBS and 10 mM HEPES
buffer. Hoechst 33342 was added at a final concentration of 5 µg/ml
in the presence or absence of 50 µM verapamil (Sigma-Aldrich; Merck
KGaA) which inhibits ABC transporter to prevent generation of SP
cells, and the cells were incubated at 37°С for 90 min with
intermittent mixing. Following the incubation, the cells were
centrifuged (250 × g; 5 min; 25°C) and resuspended in ice-cold HBSS
(Welgene, Inc.) containing 2% FBS and 10 mM HEPES buffer. The cells
were then analyzed using a FACSAria™ flow cytometer (BD
Biosciences). Hoechst 33342 was excited at 357 nm, and its
fluorescence was analyzed using a dual-wavelength (blue, 402–446
nm; red, 650–670 nm). The data were analyzed using BD CellQuest Pro
software (version 5.2.1; Becton Dickinson, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using the RNeasy
Mini kit (Qiagen, Inc.), according to the manufacturer's
instructions, followed by processing with the QIAshredder
homogenizer (Qiagen, Inc.). The RNase-free DNase set (Qiagen, Inc.)
was used for further DNA removal. Oligo (dT)14
single-stranded cDNA was synthesized from the RNA using AMV Reverse
Transcriptase (Promega Corporation). The following conditions were
used for cDNA synthesis: Initial incubation at 65°C for 5 min;
followed by 42°C incubation for 60 min and termination at 70°C for
5 min. qPCR was subsequently performed on a CFX Connect Real-Time
PCR Detection system (Bio-Rad Laboratories, Inc.) using a TaqMan
PreAmp Master Mix kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Commercially available primers were obtained for
Sonic hedgehog (SHH) (cat. no. Hs00179843_m1; Applied Biosystems;
Thermo Fisher Scientific, Inc.) and GAPDH (cat. no. Hs02786624_g1;
Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: Initial denaturation
for 10 min at 95°C; followed by 45 cycles of denaturation for 15
sec at 95°C and annealing/elongation at 60°C for 60 sec. The
housekeeping gene, GAPDH, was used as an internal control. The
expression levels were measured using the 2−ΔΔCq method
(25). Data are expressed as the
fold change relative to the expression levels in the control
group.
Statistical analysis
Statistical analyses were performed using SPSS
version 25.0 software (IBM Corp.). Data are expressed as the mean ±
SD of ≥3 independent experiments. Significant differences were
analyzed using a one-way ANOVA followed by a Tukey's test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibitory effects of C5E and/or
gemcitabine
The viability of PANC-1 cells was evaluated using
the trypan blue exclusion assay 72 h after C5E treatment. The
results revealed that C5E treatment inhibited cell viability in a
dose-dependent manner (Fig. S1A).
The IC50 of C5E was calculated to be 30 µg/ml for PANC-1
cells. Gemcitabine also inhibited the cell viability of PANC-1
cells in a dose-dependent manner; the IC50 value of
gemcitabine was calculated to be 10 nM (Fig. S1B). Additionally, following the
co-treatment with C5E and gemcitabine, the cell viability was also
inhibited in a dose-dependent manner. The IC50 value of
co-treatment with C5E and gemcitabine decreased to 15 µg/ml for C5E
and 5 nM for gemcitabine (Fig.
S1C).
Analysis of cell cycle progression and
apoptotic cell death
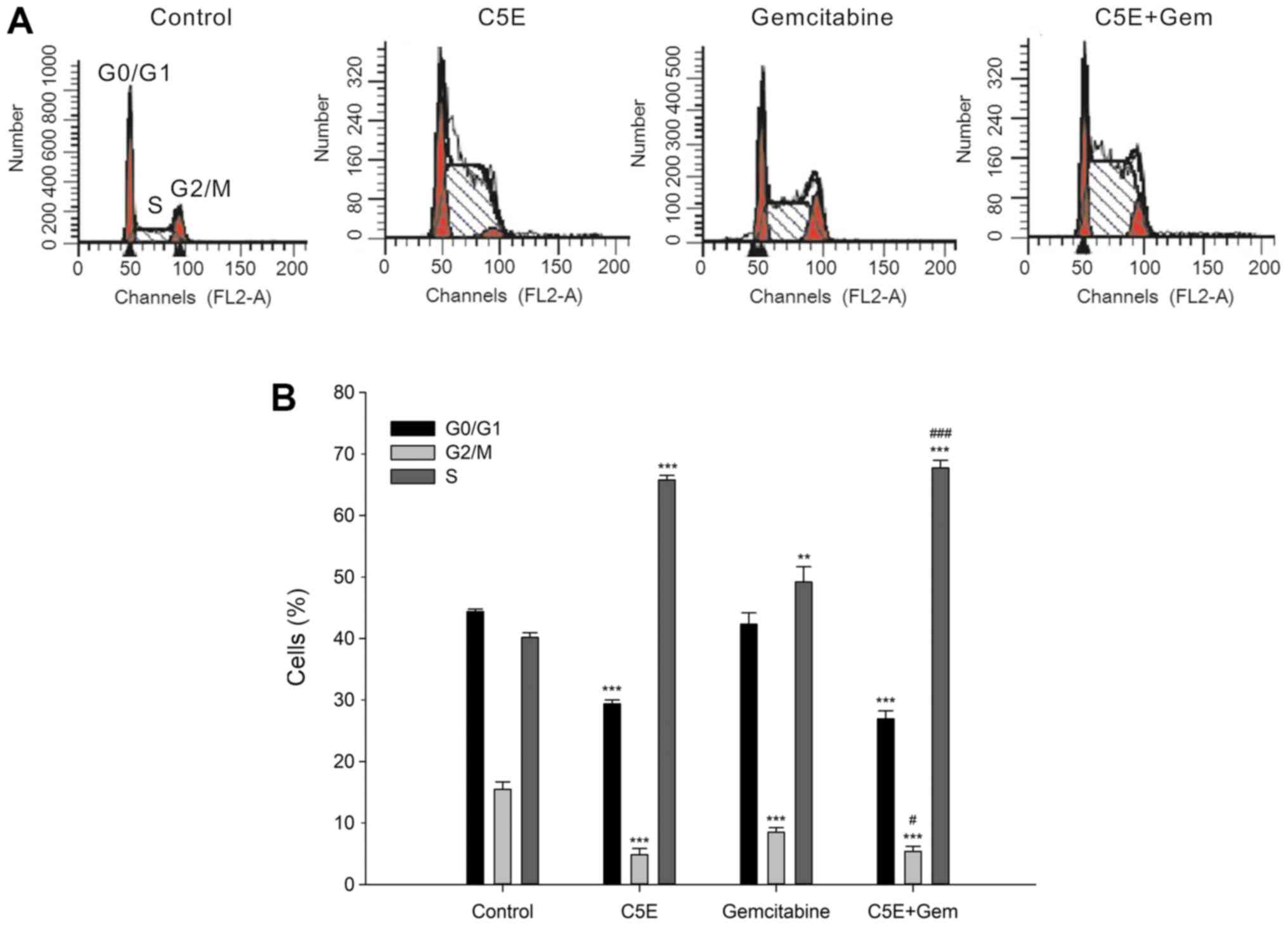
To determine the distribution of the cell cycle in
cells following C5E and gemcitabine treatment, cells were treated
with C5E and/or gemcitabine at their IC50 values. The
treatment of PANC-1 cells with 30 µg/ml C5E for 72 h significantly
increased the percentage of cells in the S phase (65.8±0.8%)
compared with the control group (40.2±0.8%), while concomitantly
significantly reducing the percentage of cells in the
G0/G1 and G2/M phases (Fig. 1A and B). The relative percentage
increase in S indicated that the cell cycle progress was blocked by
treatment (26,27). In addition, the increase in the
percentage of S phase cells following the treatment with
gemcitabine (49.2±2.5%) was significantly lower compared with the
C5E treatment. Notably, the percentage of S phase cells following
the co-treatment (67.8±1.2%) was significantly increased compared
with the treatment with gemcitabine-alone (49.2±2.5%). These
findings suggested that the co-treatment may have a synergistic
effect on cell cycle arrest compared with the treatment with C5E or
gemcitabine alone. In addition, the inhibition of cell viability
following the treatment with C5E and/or gemcitabine may be related
to S phase cell cycle arrest.
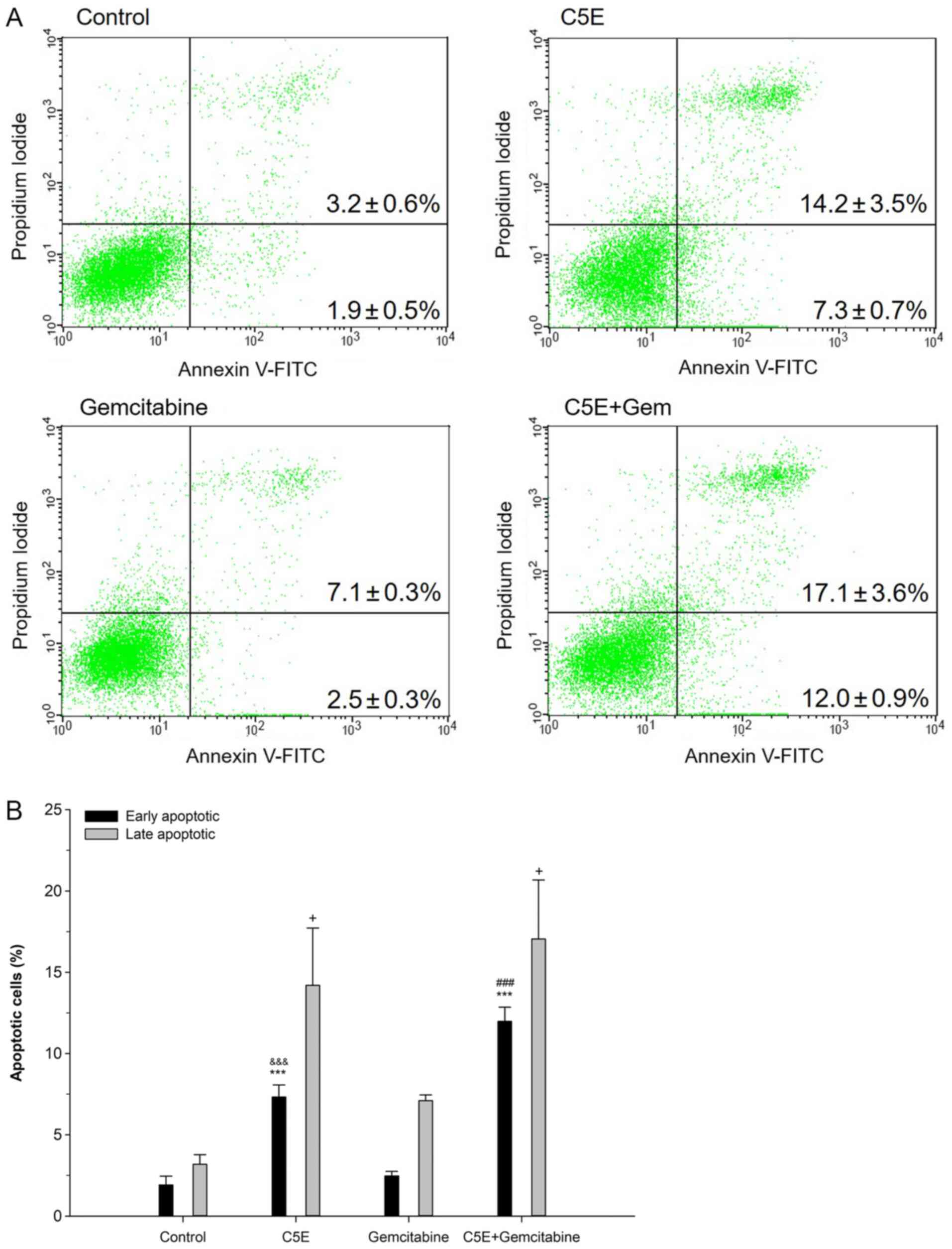
To analyze the effect of C5E on apoptosis, PANC-1
cells were treated with C5E and/or gemcitabine for 72 h, and the
number of apoptotic cells was analyzed using Annexin V/PI double
staining. The levels of early apoptosis in PANC-1 cells following
C5E treatment were significantly increased (7.3±0.7%) compared with
the control group (1.9±0.5%) (Fig. 2A
and B). In addition, the levels of early apoptosis following
C5E treatment (7.3±0.7%) were more than double that of the
gemcitabine-induced levels of early apoptosis (2.5±0.3%). Moreover,
the levels of early apoptosis following the co-treatment were
increased (12.0±0.9%) by 10.1% compared with the control and by
4.7% compared with the C5E treatment alone (Fig. 2A and B). Late apoptosis was also
assessed following the treatment with C5E and/or gemcitabine. The
levels of late apoptosis following the co-treatment were 17.1±1.6%,
which were increased compared with C5E treatment alone (14.2±3.5%)
and the control treatment (3.2±0.6%). The overall trend was very
similar to that observed for early apoptosis. Thus, these results
suggested that the co-treatment may have a synergistic effect on
apoptosis, as well as cell cycle progression.
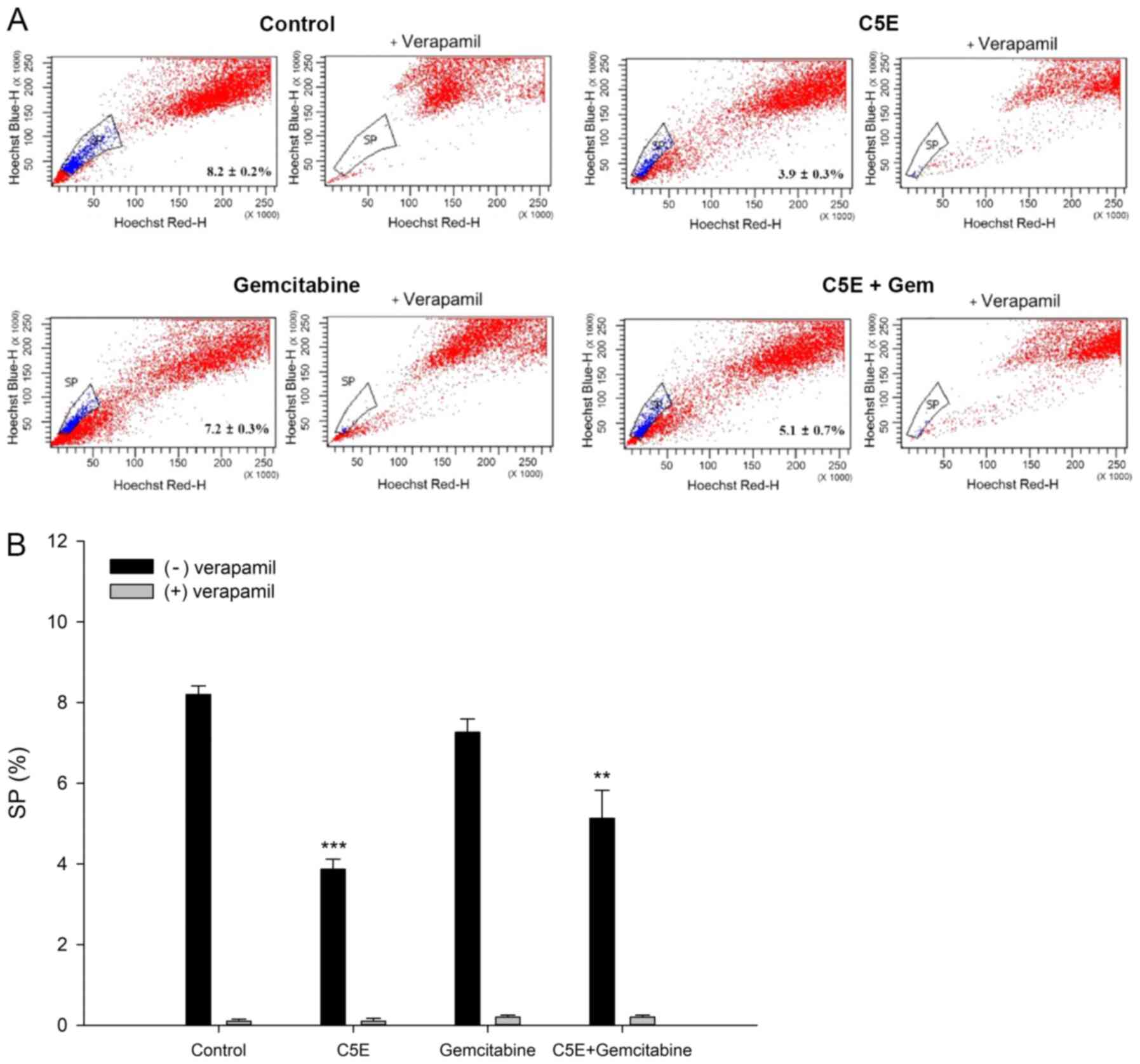
Analysis of the SP
Subsequently, the proportion of SP cells following
the treatment with C5E, gemcitabine or C5E plus gemcitabine was
analyzed by Hoechst 33342 staining. As expected, verapamil, which
is an inhibitor of ABC transporter, inhibited generation of SP
cells. Flow cytometry analysis showed 8.2±0.2% SP cells in the
control group without verapamil treatment (Fig. 3A and B). A decrease in the
percentage of SP cells in the PANC-1 cell culture was induced by
C5E and/or gemcitabine in the absence of verapamil treatment. The
percentage of SP cells significantly decreased to 3.9±0.3%
following treatment with C5E-alone and 7.2±0.3% following treatment
with gemcitabine-alone compared with the control group. Notably,
the percentage of SP cells was also significantly decreased to
5.1±0.7% following the co-treatment with C5E and gemcitabine
compared with the control group (Fig.
3). The percentage of SP cells with verapamil treatment were
<0.2% (Fig. 3B). Thus, the
percentage of SP cells following the co-treatment decreased by less
than C5E treatment alone. These results suggested that C5E may have
a higher influence than gemcitabine on the reduction of the SP cell
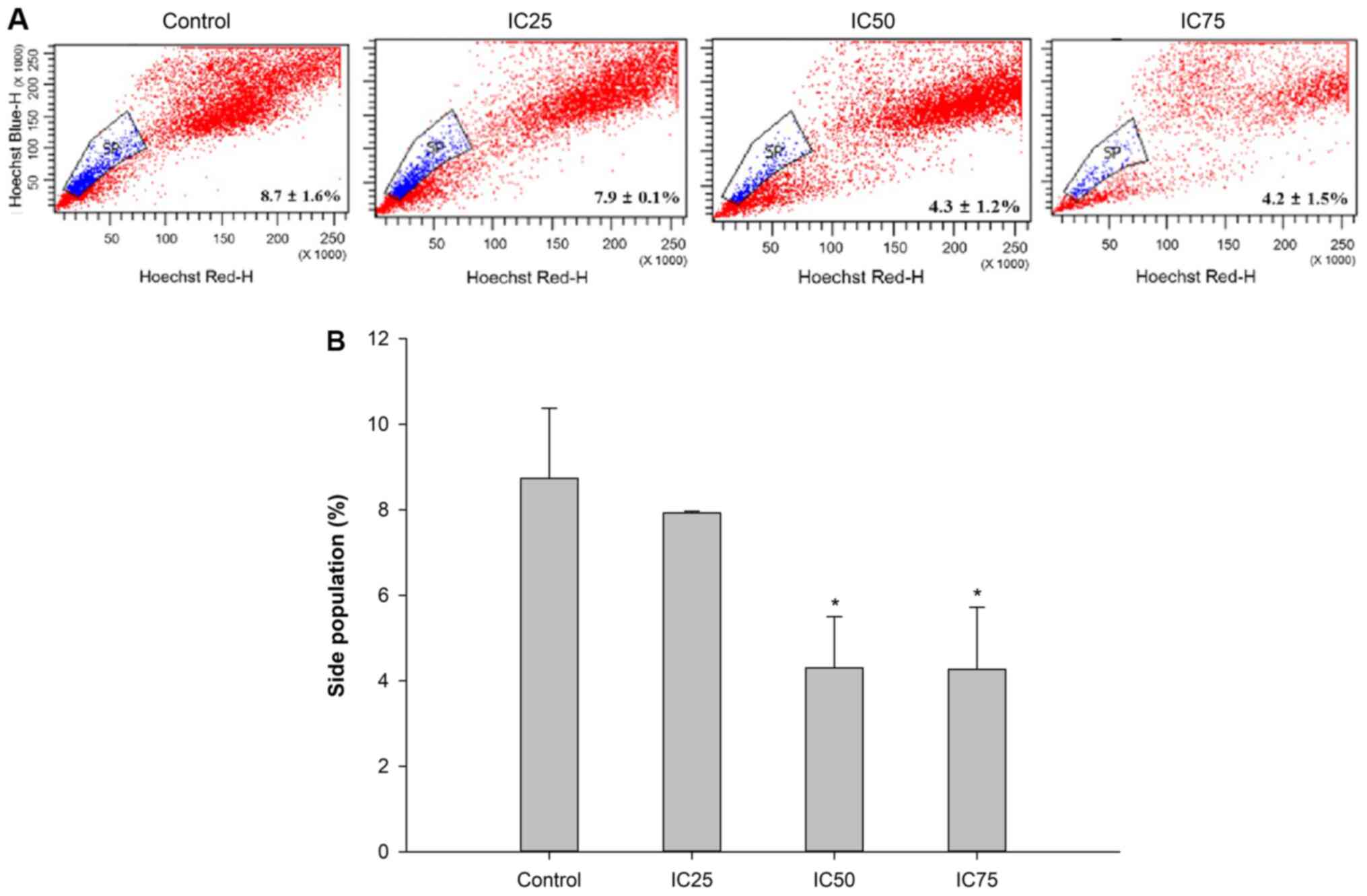
population. Subsequently, the changes in the percentage of SP cells
at varying concentrations of C5E were investigated. The percentage
of SP cells was 7.9±0.1, 4.3±1.2 and 4.2±1.5%, respectively, at the
IC25 (10 µg/ml), IC50 (30 µg/ml) and the
IC75 (50 µg/ml) doses, indicating that the proportion of
SP cells decreased with increasing concentration of C5E (Fig. 4A and B). Thus, the percentage of SP
cells was significantly decreased at IC50 and
IC75 compared with the control. However, the difference
in the percentages was much smaller between the IC50 and
IC75 doses compared with between the IC50 and
IC25 doses.
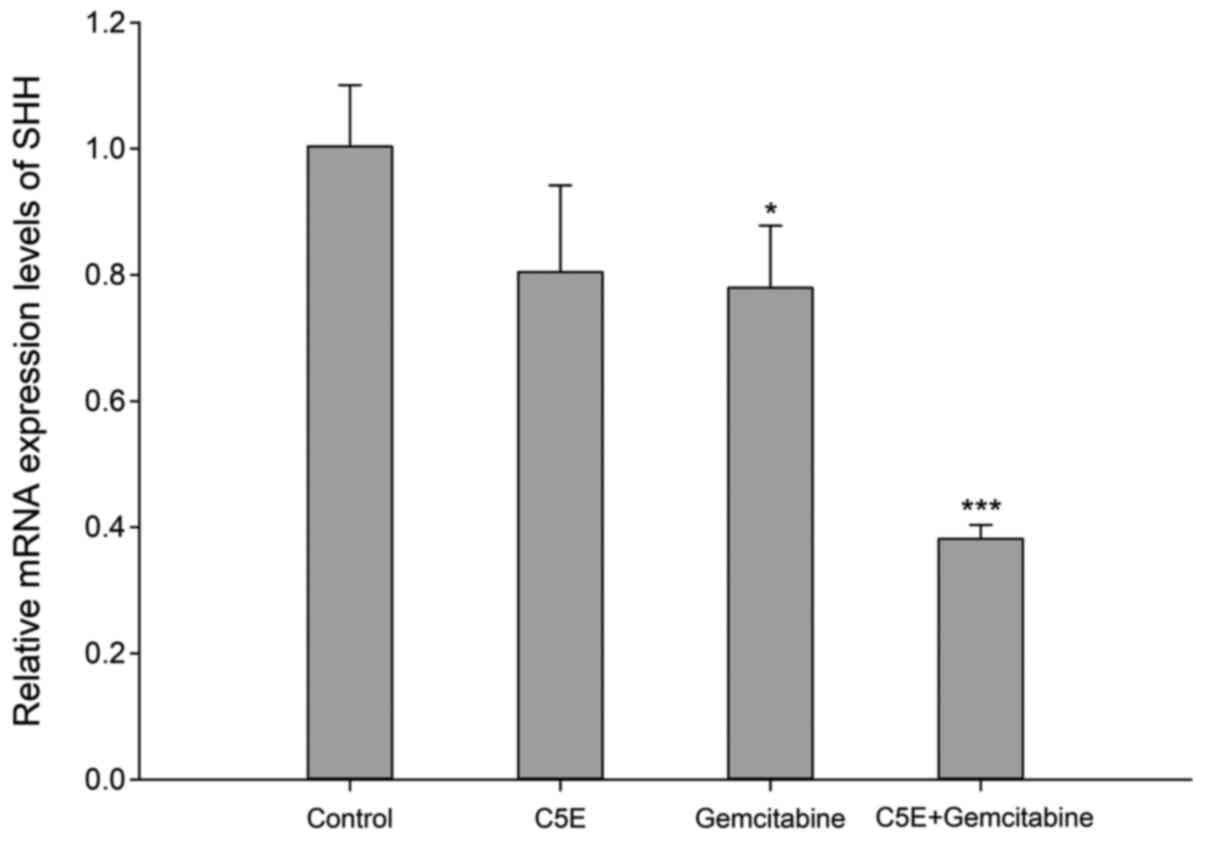
Sonic hedgehog (SHH) expression levels
following C5E and/or gemcitabine treatment
SHH is a stem cell-associated gene, which has been
implicated in the development of pancreatic cancer cells (28). The expression levels of SHH were
analyzed following the treatment with C5E and/or gemcitabine using
RT-qPCR. The results revealed that SHH expression levels
were downregulated by ~20% following the treatment with C5E or
gemcitabine alone compared with the control group (Fig. 5). However, the expression levels of
SHH were significantly downregulated by ~60% following the
co-treatment with C5E and gemcitabine compared with the control
group (Fig. 5). Thus, the
downregulation in SHH mRNA expression levels was more significant
upon the co-treatment with C5E and gemcitabine compared with the
treatment with each individual compound. These data indicated that
C5E and gemcitabine may exert a synergistic effect with respect to
the inhibition of SHH mRNA expression levels.
Discussion
The present study reported that C5E herbal mixture
extracts, which consists of ten traditional herbs, may be useful in
combination with chemotherapeutic drugs to increase the efficacy of
chemotherapy for pancreatic cancer cells, by decreasing the
necessary treatment volume of gemcitabine. Our previous study
demonstrated that herbal mixture extracts reduced the survival of
CSCs, which are associated with relapse and metastases (7). In the present study, an ethanol
extract of herbal mixture C5E containing Panax ginseng, Inonotus
obliquus and 8 other herbs was used. In a previous study, gas
chromatography-mass spectrometry analysis of the herbal extract C5E
identified that some of the components, including angelicin
(24.54%) and coumarin (2.65%), serve active anticancer roles
(16). Thus, several components of
C5E have already been shown to be involved in the suppression of
cancer cells. For example, a previous study identified that the
treatment with angelicin, the most dominant component of C5E,
induced apoptosis in neuroblastoma cells (29). Coumarin, a minor component of C5E,
has also received increasing attention because of its
antiangiogenic activity (30).
Another previous study revealed that the herbal extract C5E induced
apoptosis in human breast cancer cells, including MCF7 cells
(16). The present study
demonstrated the potential extended application of C5E to other
types of cancer cell, such as PANC-1 cells.
Firstly, the conditions for the treatment of PANC-1
cells with C5E in the absence or presence of gemcitabine were
optimized. C5E treatment was observed to inhibit the viability of
PANC-1 cells through the induction of S phase cell cycle arrest.
These findings indicated that C5E may exert its effect through a
mechanism similar to that of gemcitabine. However, the extent of S
phase cell cycle arrest was increased following C5E treatment
compared with gemcitabine treatment. Several anticancer drugs,
including gemcitabine and clofarabine, have been reported to exert
their effects through cell cycle arrest at the S phase (26,27).
It has been established that gemcitabine exerts its anticancer
effect by delaying the DNA replication forks during the S phase of
the cell cycle (31). In the
present study, C5E was discovered to induce cell death through
early apoptosis, as has been observed with gemcitabine in previous
studies (28–30) (Fig.
2). The majority of chemotherapy drugs induce apoptosis
(31,32). The results revealed that the
co-treatment with C5E and gemcitabine was markedly more effective
compared with the individual treatments alone at inducing S phase
cell cycle arrest and early apoptosis. These findings indicated
that C5E may be used alone or as an adjuvant drug for chemotherapy
with the standard drug gemcitabine for the treatment of pancreatic
cancer.
Subsequently, the present study investigated the
general effect of C5E on the human pancreatic carcinoma cell line
PANC-1 and its subpopulation of CSCs. Changes in SP cell
proportions were used to evaluate the effects on CSCs (33). In the initial stages of the study,
several pancreatic cancer cell lines (Capan-1 or 2, MIA-PaCa-2 and
PANC-1) were examined to identify cell lines that have a
substantial percentage of SP cells; however, only PANC-1 cells had
sufficient SP cells to study the effect on CSCs (data not shown).
The average percentage of SP cells present in among the whole
population of PANC-1 cells was revealed to be 5–10%, which is
consistent with the findings of previous reports (34,35).
Thus, PANC-1 cells were selected for all following experiments. The
current results discovered that the percentage of SP cells was
markedly decreased following the treatment with C5E alone. More
importantly, the extent of the decrease was higher following C5E
treatment compared with gemcitabine treatment. This suggested the
possibility of using C5E as a complementary drug to target the CSCs
of the PANC-1 cell population, thus obtaining a synergistic effect
alongside gemcitabine drugs for the treatment of pancreatic cancer.
However, additional research with several other pancreatic cell
lines is required to verify the effects of C5E. The present study
further investigated the molecular mechanism by which C5E may exert
its anticancer effects, in particular, its effects on the
expression levels of SHH. The mRNA expression levels of SHH serve
as a marker of CSCs, and numerous studies have reported that SHH
regulates the proliferation of stem cells and blocks SHH via the
Hedgehog (Hh) pathway; this may be a novel therapeutic strategy
(36–38). The SHH mRNA expression levels were
discovered to be downregulated to a similar extent following the
treatment with either C5E or gemcitabine; however, the co-treatment
with C5E and gemcitabine downregulated the expression levels of SHH
to a greater extent compared with the treatment with C5E or
gemcitabine alone. C5E in combination with gemcitabine may serve a
role as a Hh pathway antagonist (38). Nonetheless, despite the current
study demonstrating that C5E may be useful for the treatment of
pancreatic cancer, in vivo animal experiments should be
performed to ensure the safety and efficacy of the C5E herbal
extract for the treatment of pancreatic cancer.
In conclusion, the findings of the present study
suggested that the herbal mixture extract C5E may be used as a
complementary drug with gemcitabine against pancreatic cancer cells
and CSCs. Both combinatorial therapies and monotherapies should be
considered for the development of effective cancer therapies. C5E
with gemcitabine may serve as a potential candidate for
complementary use in the treatment of pancreatic cancer as it was
revealed to exert synergistic effects in pancreatic cancer
cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Korean Health Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (grant no. B110053).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PJP conceptualized the research and drafted the
manuscript. JHS and DGL analyzed the data. SHJ, TYH, SHP and NC
interpreted the data. NC supervised the research and revised the
manuscript All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnes AF, Yeo TP, Leiby B, Kay A and
Winter JM: Pancreatic cancer-associated depression: A case report
and review of the literature. Pancreas. 47:1065–1077. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skelton WP IV, Parekh H, Starr JS, Trevino
J, Cioffi J, Hughes S and George TJ Jr: Clinical factors as a
component of the personalized treatment approach to advanced
pancreatic cancer: A systematic literature review. J Gastrointest
Canc. 49:1–8. 2018. View Article : Google Scholar
|
|
4
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valle S, Martin-Hijano L, Alcalá S,
Alonso-Nocelo M and Sainz B Jr: The ever-evolving concept of the
cancer stem cell in pancreatic cancer. Cancers (Basel). 10:332018.
View Article : Google Scholar
|
|
6
|
Codony-Servat J, Codony-Servat C, Cardona
AF, Giménez-Capitán A, Drozdowskyj A, Berenguer J, Bracht JWP, Ito
M, Karachaliou N and Rosell R: Cancer stem cell biomarkers in
EGFR-mutation-positive non-small-cell lung cancer. Clin Lung
Cancer. 20:167–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Tiede B, Massagué J and Kang YB:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taylor WF and Jabbarzadeh E: The use of
natural products to target cancer stem cells. Am J Cancer Res.
7:1588–1605. 2017.PubMed/NCBI
|
|
10
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2-cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sung JH, Kim JB, Park SH, Park SY, Lee JK,
Lee HS and Chung N: Berberine decreases cell growth but increases
the side population fraction of h460 lung cancer cells. J Korean
Soc Appl Biol Chem. 55:491–495. 2012. View Article : Google Scholar
|
|
13
|
Shaito A, Thuan DTB, Phu HT, Nguyen THD,
Hasan H, Halabi S, Abdelhady S, Nasrallah GK, Eid AH and Pintus G:
Herbal medicine for cardiovascular diseases: Efficacy, mechanisms,
and safety. Front Pharmacol. 11:4222020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izzo AA and Capasso F: Herbal medicines to
treat Alzheimer's disease. Trends Pharmacol Sci. 28:47–48. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yun Y, Kim K, Choi I and Ko SG: Topical
herbal application in the management of atopic dermatitis: A review
of animal studies. Mediat Inflamm. 2014:7521042014. View Article : Google Scholar
|
|
16
|
Lee S, Han S, Park JS, Jeong AL, Jung SH,
Choi KD, Han TY, Han IY and Yang Y: Herb mixture C5E aggravates
doxorubicin-induced apoptosis of human breast cancer cell lines. J
Korean Soc Appl Biol Chem. 56:567–573. 2013. View Article : Google Scholar
|
|
17
|
Aung TN, Qu ZP, Kortschak RD and Adelson
DL: Understanding the effectiveness of natural compound mixtures in
cancer through their molecular mode of action. Int J Mol Sci.
18:6562017. View Article : Google Scholar
|
|
18
|
Lee DG, Go EB, Lee M, Pak PJ, Kim JS and
Chung N: Gold nanoparticles conjugated with resveratrol induce cell
cycle arrest in MCF-7 cell lines. Appl Biol Chem. 62:332019.
View Article : Google Scholar
|
|
19
|
Jeon HJ, Kim K, Kim YD and Lee SE:
Naturally occurring piper plant amides potential in agricultural
and pharmaceutical industries: Perspectives of piperine and
piperlongumine. Appl Biol Chem. 62:632019. View Article : Google Scholar
|
|
20
|
Park JY, Choi P, Kim HK, Kang KS and Ham
J: Increase in apoptotic effect of Panax ginseng by
microwave processing in human prostate cancer cells: in vitro and
in vivo studies. J Ginseng Res. 40:62–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo HS, Kim JM, Jo E, Cho CK, Lee SY, Kang
HS, Lee MG, Yang PY and Jang IS: Modified Panax ginseng
extract regulates autophagy by AMPK signaling in A549 human lung
cancer cells. Oncol Rep. 37:3287–3296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YO, Park HW, Kim JH, Lee JY, Moon SH
and Shin CS: Anti-cancer effect and structural characterization of
endo-polysaccharide from cultivated mycelia of Inonotus
obliquus. Life Sci. 79:72–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burmasova MA, Utebaeva AA, Sysoeva EV and
Sysoeva MA: Melanins of Inonotus obliquus: Bifidogenic and
antioxidant properties. Biomolecules. 9:2482019. View Article : Google Scholar
|
|
24
|
Voigt M, Bartels I, Nickisch-Hartfiel A
and Jaeger M: Determination of minimum inhibitory concentration and
half maximal inhibitory concentration of antibiotics and their
degradation products to assess the eco-toxicological potential.
Toxicol Environ Chem. 101:315–338. 2019. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo Y, Xu X, Qi W, Xie C, Wang G, Zhang A
and Ge Y: Synergistic antitumor interactions between gemcitabine
and clofarabine in human pancreatic cancer cell lines. Mol Med Rep.
5:734–738. 2012.PubMed/NCBI
|
|
27
|
Miao X, Koch G, Ait-Oudhia S, Straubinger
RM and Jusko WJ: Pharmacodynamic modeling of cell cycle effects for
gemcitabine and trabectedin combinations in pancreatic cancer
cells. Front Pharmacol. 7:4212016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahman MA, Kim NH, Yang H and Huh SO:
Angelicin induces apoptosis through intrinsic caspase-dependent
pathway in human SH-SY5Y neuroblastoma cells. Mol Cell Biochem.
369:95–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majnooni MB, Fakhri S, Smeriglio A,
Trombetta D, Croley CR, Bhattacharyya P, Sobarzo-Sanchez E, Farzaei
MH and Bishayee A: Antiangiogenic effects of coumarins against
cancer: From chemistry to medicine. Molecules. 24:42782019.
View Article : Google Scholar
|
|
31
|
Vogus DR, Evans MA, Pusuluri A, Barajas A,
Zhang M, Krishnan V, Nowak M, Menegatti S, Helgeson ME, Squires TM
and Mitragotri S: A hyaluronic acid conjugate engineered to
synergistically and sequentially deliver gemcitabine and
doxorubicin to treat triple negative breast cancer. J Control
Release. 267:191–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Utaipan T, Boonyanuphong P, Chuprajob T,
Suksamrarn A and Chunglok W: A trienone analog of curcumin,
1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and
caspase-mediated apoptosis in human oral squamous cell carcinoma
cells in vitro. Appl Biol Chem. 63:72020. View Article : Google Scholar
|
|
33
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhagwandin VJ, Bishop JM, Wright WE and
Shay JW: The metastatic potential and chemoresistance of human
pancreatic cancer stem cells. PLoS One. 11:e01488072016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao J, Cai HH, Wei JS, An Y, Ji ZL, Lu ZP,
Wu JL, Chen P, Jiang KR, Dai CC, et al: Side population in the
pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with
cancer stem-like cells. Oncol Rep. 23:1375–1382. 2010.PubMed/NCBI
|
|
36
|
Han L, Jiang J, Ma QY, Wu Z and Wang Z:
The inhibition of heme oxygenase-1 enhances the chemosensitivity
and suppresses the proliferation of pancreatic cancer cells through
the SHH signaling pathway. Int J Oncol. 52:2101–2109.
2018.PubMed/NCBI
|
|
37
|
Douard R, Moutereau S, Pernet P, Chimingqi
M, Allory Y, Manivet P, Conti M, Vaubourdolle M, Cugnenc PH and
Loric S: Sonic hedgehog-dependent proliferation in a series of
patients with colorectal cancer. Surgery. 139:665–670. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Sauvage FJ: Targeting the hedgehog
pathway in cancer. Ann Oncol. 20:182009.
|