Introduction
Colorectal cancer (CRC) is one of the commonest
malignant tumors worldwide, with high mortality and morbidity
(1,2). Due to a lack of early symptoms and
effective screening strategies, patients are commonly diagnosed
with advanced CRC (2). Although
there has been major progress in molecular target therapy and
immunotherapy (3,4), the overall survival time for patients
with CRC is still not satisfactory. Therefore, it is essential to
identify an effective bio-marker for CRC and explore its potential
regulatory mechanism.
Circular RNAs (circRNAs), a novel type of non-coding
RNA, are characterized as closed ring structure without 5′ caps or
3′ poly-A tails (5,6). Emerging studies have shown that
circRNAs may serve a function in the regulation of physiological
and pathological processes via serving as microRNA (miRNA) sponges
(7–9). A number of circRNAs are believed to
serve a function as a tumor promoter or a tumor inhibitor in
various cancer progression, including liver, ovarian and lung
cancer (10–12). For example, silencing of
circRNA-0060428 can inhibit cell growth in osteosarcoma by
regulating the miR-375/RPBJ axis (13). CircRNA-0003645 can sponge miR-1229
and then promote hepatocellular carcinoma progression (14). However, the biological role of
circRNAs in the development of CRC remains to be elucidated.
CircRNA-0074027 is a newly discovered competing
endogenous (ce)RNA, which is oriented from
chr5:134363423-134369964. The biological role of circRNA-0074027 in
malignant transfection has been explored (15,16).
Gao et al (16) reported
that circRNA-0074027 is upregulated in lung cancer and its
overexpression can promote cell growth and metastasis ability via
regulation of the miR-185-3p/bromodomain-containing protein
4/MAPK-activating death domain protein axis. In addition, Qian
et al (15) demonstrated
that the deletion of circRNA-0074027 can inhibit glioblastoma cell
proliferation and metastasis by regulating the miR-518a-5p/IL17RD
signaling pathway. However, it remains to be fully elucidated
whether circRNA-0074027 serves as a tumor promoter in CRC
progression.
The present study detected the expression of
circRNA-0074027 in CRC tissue samples and further investigated the
role of circRNA-0074027 in oncogenesis. In vitro experiments
were performed to identify the cell apoptosis rate, cell
proliferation and metastasis ability. In addition, miR-525-3p was
identified as a downstream target gene of circRNA-0074027 by
bioinformatics analysis and the relationship was further confirmed
by dual-luciferase reporter assays. Taken together, circRNA-0074027
is a promising bio-marker in CRC.
Materials and methods
Patient tissue samples
A total of 60 paired CRC tissue and normal tissue
samples were obtained from the patients with CRC who underwent
surgery at Dazhou Central hospital between January 2014 and January
2018. The inclusion criteria were: i) Age >18 and <80 years;
ii) written informed consent; and iii) primary CRC confirmed by
pathological examination. The exclusion criteria were: i) Patients
with other malignant diseases and ii) patients with previous
neoadjuvant chemotherapy or radiotherapy. All samples were
preserved in liquid nitrogen following histological confirmation by
experienced pathologists. Written informed consent was sought from
the participants before the samples were obtained. The present
study was approved by the Ethical Committees of Dazhou Central
Hospital (approval no. KY2020-086-01).
Cell culture
Normal epithelial cells (FHC) and CRC cancer cells
(Caco-2, LoVo, SW480, HCT-116 and HCT-8), as well as 293T cell
lines were acquired from American Type Culture Collection. All cell
lines were incubated in 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) supplemented with DMEM medium (Thermo Fisher
Scientific, Inc.) under standard culture conditions (5%
CO2 and 37°C).
RNA transfection
Small interfering (si) RNA against circRNA-0074027
(si-circRNA-0074027-1 and si-circRNA-0074027-2) and its paired
control (si-Control), as well as miR-525-3p inhibitors and its
negative control (miR-525-3p NC) were acquired from Shanghai
GenePharma Co., Ltd. Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for cell transfection. In
brief, Lipofectamine® 3000 was incubated with miRNA
inhibitor at a concentration of 100 nmol/l or siRNAs at a
concentration of 200 nmol/l for 20 min at room temperature and the
complex was then added to each well of a 6-well plate. Following
transfection for 24 h, the subsequent experiments were performed.
The sequences were: si-circRNA-0074027-1
(5′-GCGTGCTAAGCACCTGGCGCA-3′), circRNA-0074027-2
(5′-GTGCTAAGCACCTGGCGCAGG-3′) and miR-525-3p inhibitor
(5′-CGCUCUAAAGGGAAGCGCCUUC-3′).
Reverse transcription-quantitative
(RT-q) PCR
According to the manufacturer's protocols,
TRIzol® (Thermo Fisher Scientific, Inc.) was used for
total RNA extraction from tissue and cell samples. Subsequently,
total RNA (1 µg) was transcribed to cDNA via using PrimeScript RT
Master Mix (Takara Biotechnology Co., Ltd.). The temperature and
duration of RT were: 37°C for 30 sec, followed by 85°C for 5 sec
and 4°C for 10 min. The cDNA (10 ng) was subjected to qPCR (Bio-Rad
Laboratories, Inc.) using SYBR Premix Ex Taq II kit (Takara
Biotechnology Co., Ltd.), and the reaction volume was 10 µl. The
thermocycling conditions were: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 40 sec. GAPDH was used as
reference gene and relative mRNA expression changes were calculated
by 2−ΔΔCq method (17).
The primer sequences used are shown in Table I.
 | Table I.Sequences of oligomers and primers
used in the present study. |
Table I.
Sequences of oligomers and primers
used in the present study.
| Name | Sequence
(5′-3′) |
|---|
| CircRNA-0074027
forward |
GATTTCCCGACCCCGTACAA |
|
CircRNA-0074027 |
GGGGTGTTCTGAGATGGACC |
| reverse |
|
| miR-525-3p
forward |
GGAAGGCGCTTCCCTTT |
| miR-525-3p
reverse |
GTTGTGGTTGGTTGGTTTGT |
| GAPDH forward |
CCTTCCGTGTCCCCACT |
| GAPDH reverse |
GCCTGCTTCACCACCTTC |
Cell proliferation assay
A CCK-8 kit (Dojindo Molecular Technologies, Inc.)
was used to assess the cell viability at different time points. A
5-Ethynyl-2′-deoxyuridine (EdU) cell proliferation kit (Guangzhou
RiboBio Co., Ltd.) was used to identify the EdU positive rate of
CRC cells.
Flow cytometry
An Annexin V-FITC/PI apoptosis detection kit
(Nanjing KeyGen Biotech Co., Ltd.) was used for the detection of
the cell apoptosis rate. According to the manufacturer's protocols,
the transfected cells were collected and suspended with binding
buffer. Then, Annexin V-FITC and propidium iodide were added to the
buffer. Subsequently, the mixture was shielded from light and
incubated at room temperature for 15 min. Subsequently, the samples
were detected by flow cytometer (BD FACSCelesta™ Flow Cytometer; BD
Biosciences), and early plus late apoptotic cells were analyzed
using FlowJo software (FlowJo LLC).
Transwell assay
For the cell migration assay, the transfected cells
were suspended in DMEM without FBS, added into the upper Transwell
chambers (0.8 µm; Corning, Inc.) and incubated for 48 h. The lower
chamber was filled with DMEM supplemented with 20% FBS. The
migrated and invasive CRC cells were fixed with 4% methanol for 10
min, stained with crystal violet for 10 min at room temperature,
and finally captured with a light microscope. For the cell invasion
assay, the filter membranes were precoated with
Matrigel® (Corning, Inc.) at 37°C for 30 min, and the
other steps were performed as described in the migration assay.
Western blotting
The un-transfected and transfected cells were lysed
using RIPA buffer (Beyotime Institute of Biotechnology), followed
by homogenization at 4°C for 10 min. Protein concentration was
evaluated with BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Equal amounts (20 µg) of proteins were loaded and
subjected to 10% SDS-PAGE electrophoresis, followed by transferred
onto polyvinylidene fluoride membranes. The membranes were blocked
with 10% bovine serum albumin (Beijing Solarbio Science &
Technology Co., Ltd.) for 1 h at room temperature. Subsequently,
the membranes were incubated with primary antibodies: p-53 (cat.
no. ab32389), Bcl-2 (cat. no. ab32124), Bax (cat. no. ab32503),
E-cadherin (cat. no. ab11512), N-cadherin (cat. no. ab245117),
Vimentin (cat. no. ab16700) and GAPDH (cat. no. ab9485; all 1:1,000
dilution; Abcam) overnight at 4°C. Subsequently, proteins were
incubated with the goat anti-rabbit IgG H&L (HRP; cat. no.
ab6721; 1:2,000 dilution; Abcam) for 1 h at room temperature.
Finally, the protein bands were then analyzed with super sensitive
ECL luminescence reagent (Dalian Meilun Biology Technology Co.,
Ltd.). The protein expression levels were detected using a
chemi-luminescence detection system with Quantity One software
(v3.0; Sigma-Aldrich; Merck KGaA).
Prediction of downstream molecules
regulated by circRNA-0074027
A publicly available bioinformatics algorithm
(Starbase 2.0) was utilized to predict the downstream miRNAs of
circRNA-0074027 (9).
Dual-luciferase reporter assays
The wild-type (WT) and mutant (MUT) circRNA-0074027
fragments were constructed and inserted into the pmirGLO vector
(Ybscience). The WT and MUT plasmids, as well as miR-525-3p
inhibitor and miR-525-3p NC, were co-transfected into 293T cells
using Lipofectamine®3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 24 h, the firefly and Renilla
luciferase activities were measured using a dual-luciferase
reporter assay system (Promega Corporation) according to the
manufacturer's instructions.
Statistical analysis
All experiments were repeated at least three times.
The data are shown as the mean ± standard deviation. Comparisons
between two groups were performed using Student's t-test. In
addition, the samples were divided into a high and a low expression
group according to the median value of circRNA-0074027 expression
and χ2 test was performed to analyze the relationship
between the circRNA-0074027 expression and clinicopathological
characterization of patients with CRC. The data among three or more
groups were performed using one-way ANOVA followed by Fisher's
least significant difference post hoc test. The correlation between
circRNA-0074027 and miR-525-3p were analyzed by Pearson's
correlation. Statistical analyses were performed using SPSS 20.0
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
CircRNA-0074027 expression is
increased in CRC tissue samples
Results comparing circRNA-0074027 expression levels
in CRC tissues samples and samples obtained from adjacent normal
tissues revealed that there was an overexpression of
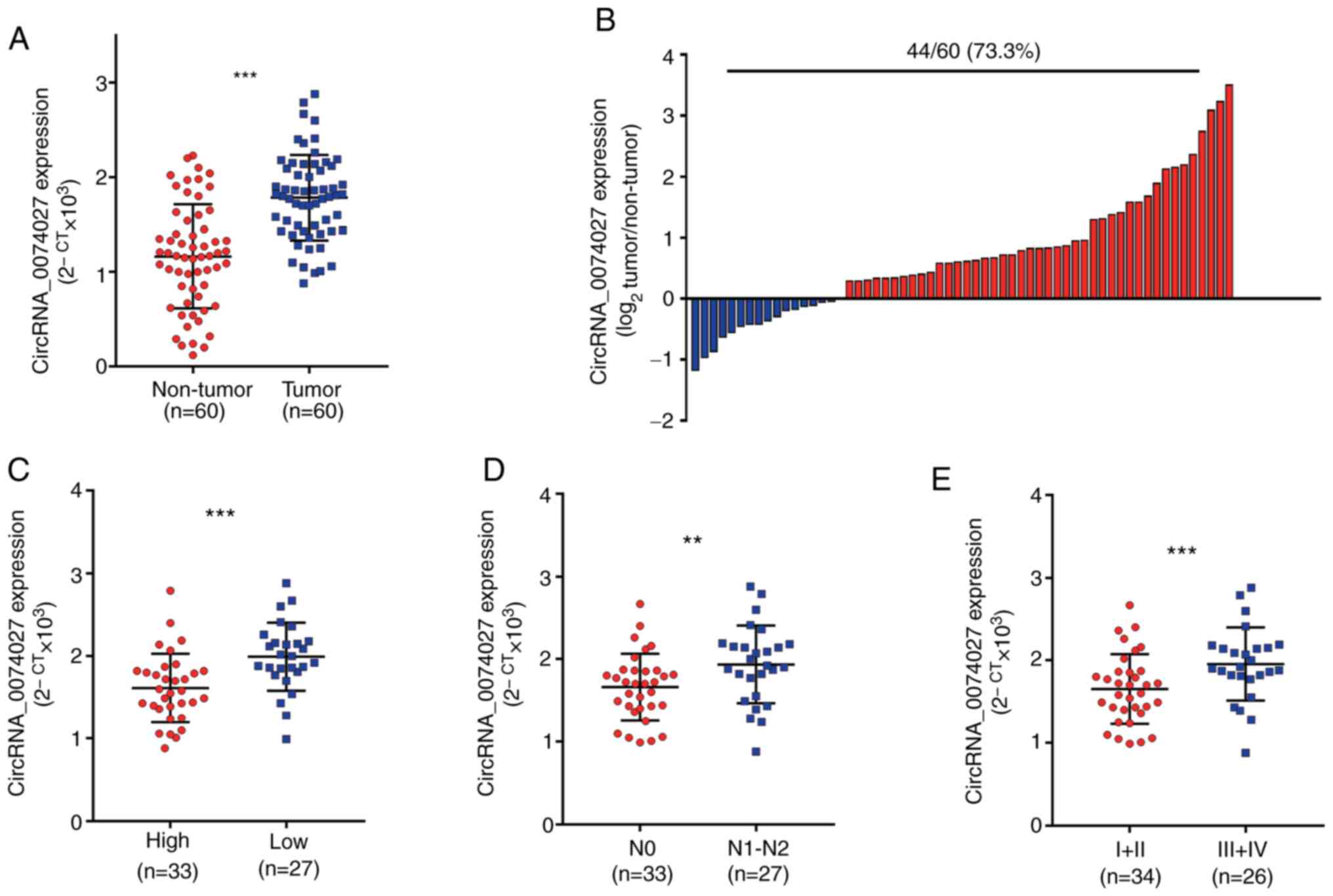
circRNA-0074027 in the CRC tissues (Fig. 1A). Subsequently, it was discovered
that among 60 paired tissue samples, almost 73.3% (44/60)
demonstrated higher circRNA-0074027 expression in CRC tissues
(Fig. 1B). Next, these 60 patients
with CRC were divided into two groups, including circRNA-0074027
high and low expression group, based on the median value of
circRNA-0074027 expression. According to the results of statistical
analysis, it was found that the circRNA-0074027 expression was
closely related to the differential status (P=0.001), N stage
(P=0.004), vascular invasion (P=0.016), tumor size (P<0.001) and
TNM stage (P=0.02; Table II).
Similarly, the data revealed that patients with CRC and
circRNA_0047027 overexpression were more likely to have low tumor
differentiation (Fig. 1C), lymph
node metastasis (Fig. 1D) and
advanced TMN stage (Fig. 1E). In
summary, circRNA-0074027 might have a role as a tumor inhibitor
role in CRC progression.
 | Table II.Correlation between the
clinicopathological data and circRNA-0074027 expression of in
colorectal cancer (n=60). |
Table II.
Correlation between the
clinicopathological data and circRNA-0074027 expression of in
colorectal cancer (n=60).
|
|
| circRNA-0074027
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | High | Low | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 27 | 12 | 15 | 0.586 |
|
Female | 33 | 17 | 16 |
|
| Age |
|
|
|
|
| <60
years | 25 | 12 | 13 | 0.879 |
| ≥60
years | 35 | 18 | 17 |
|
| Differential
status |
|
|
|
|
|
Moderate/well | 20 | 13 | 7 | 0.001 |
|
Undifferentiated/poorly | 40 | 9 | 31 |
|
| Vascular
invasion |
|
|
|
|
|
Negative | 22 | 14 | 8 | 0.016 |
|
Positive | 38 | 12 | 26 |
|
| Tumor size |
|
|
|
|
| ≤ 5
cm | 25 | 19 | 6 |
<0.001 |
| > 5
cm | 35 | 8 | 27 |
|
| N stage |
|
|
|
|
| N0 | 27 | 19 | 8 | 0.004 |
|
N1-N3 | 33 | 11 | 22 |
|
| TNM stage |
|
|
|
|
|
I–II | 24 | 16 | 8 | 0.02 |
|
III–IV | 36 | 13 | 23 |
|
| Tumor location |
|
|
|
|
| Left
colon cancer | 20 | 8 | 12 | 0.625 |
| Right
colon cancer | 22 | 10 | 12 |
|
| Rectal
cancer | 18 | 10 | 8 |
|
Knockdown of circRNA-0074027
suppresses CRC cell growth
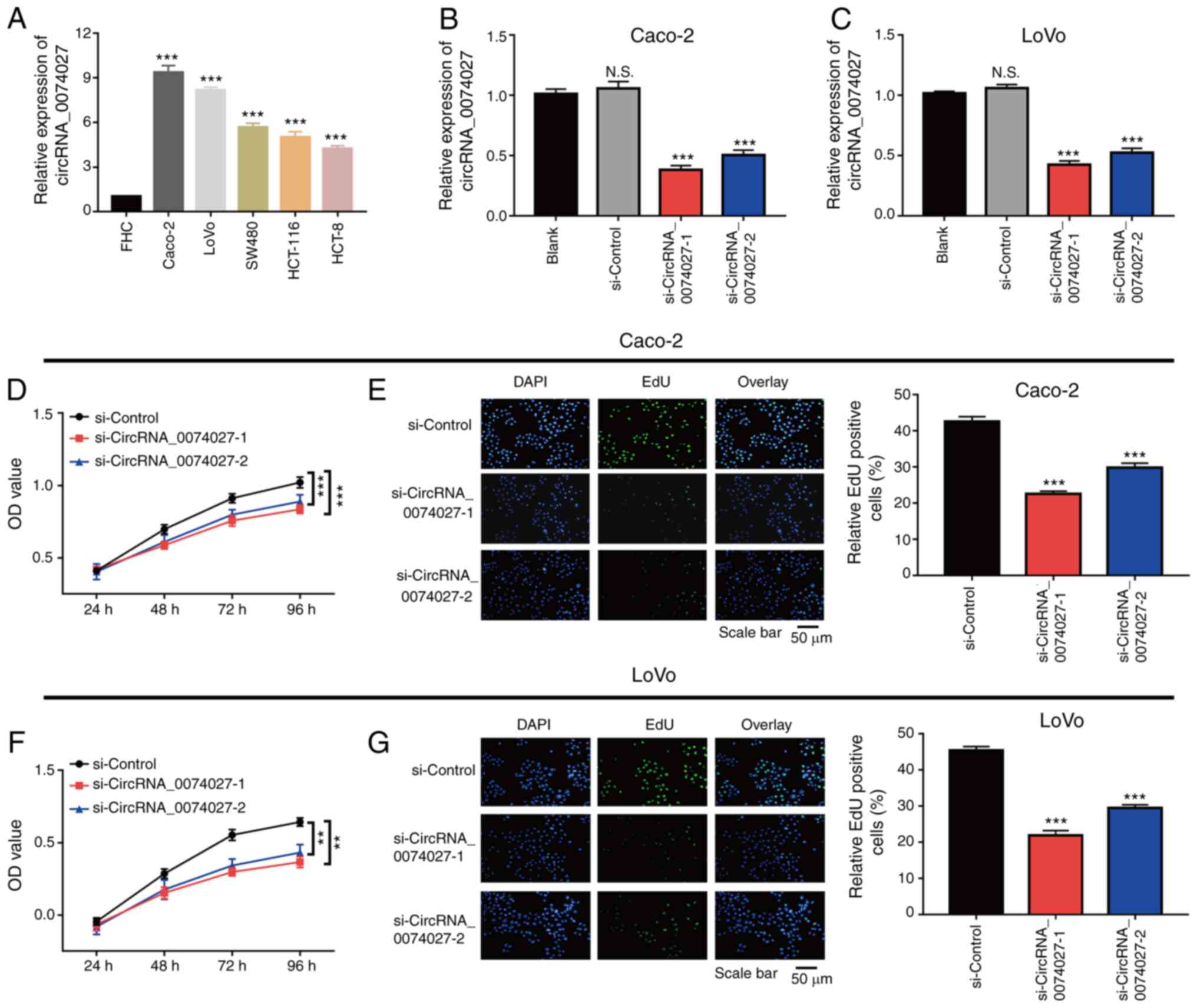
Since the expression of circRNA-0074027 was reduced
in CRC tissues, its expression was further detected in normal
epithelial cells and five different CRC cell lines. When compared
with normal epithelial cell lines (FHC), the expression of
circRNA-0074027 was higher in CRC cell lines, especially in Caco-2
and LoVo cells (Fig. 2A).
Therefore, the Caco-2 and LoVo cells were selected in the present
study for future in vitro experiments. The expression of
circRNA-0074027 in Caco-2 and LoVo cells was knocked down using RNA
transfection (Fig. 2B and C). The
results of CCK-8 assay displayed that silencing of circRNA-0074027
could markedly inhibit the cell growth rate in both of HGC-27 and
AGS cells (Fig. 2D and F).
Consistent with the previous results, the EdU assay also indicated
that there were fewer EdU-positive Caco-2 cells in
siRNA-circRNA-0074027-1 and siRNA-circRNA-0074027-2 group than that
in si-Control group (Fig. 2E).
Additionally, EdU-positive LoVo cells in siRNA-circRNA-0074027-1
and siRNA-circRNA-0074027-2 group were fewer compared with the
si-Control group (Fig. 2G). In
total, silencing of circRNA-0074027 could suppress cell growth rate
in CRC.
Knockdown of circRNA-0074027 increases
cell apoptosis rate via activating the p53 pathway
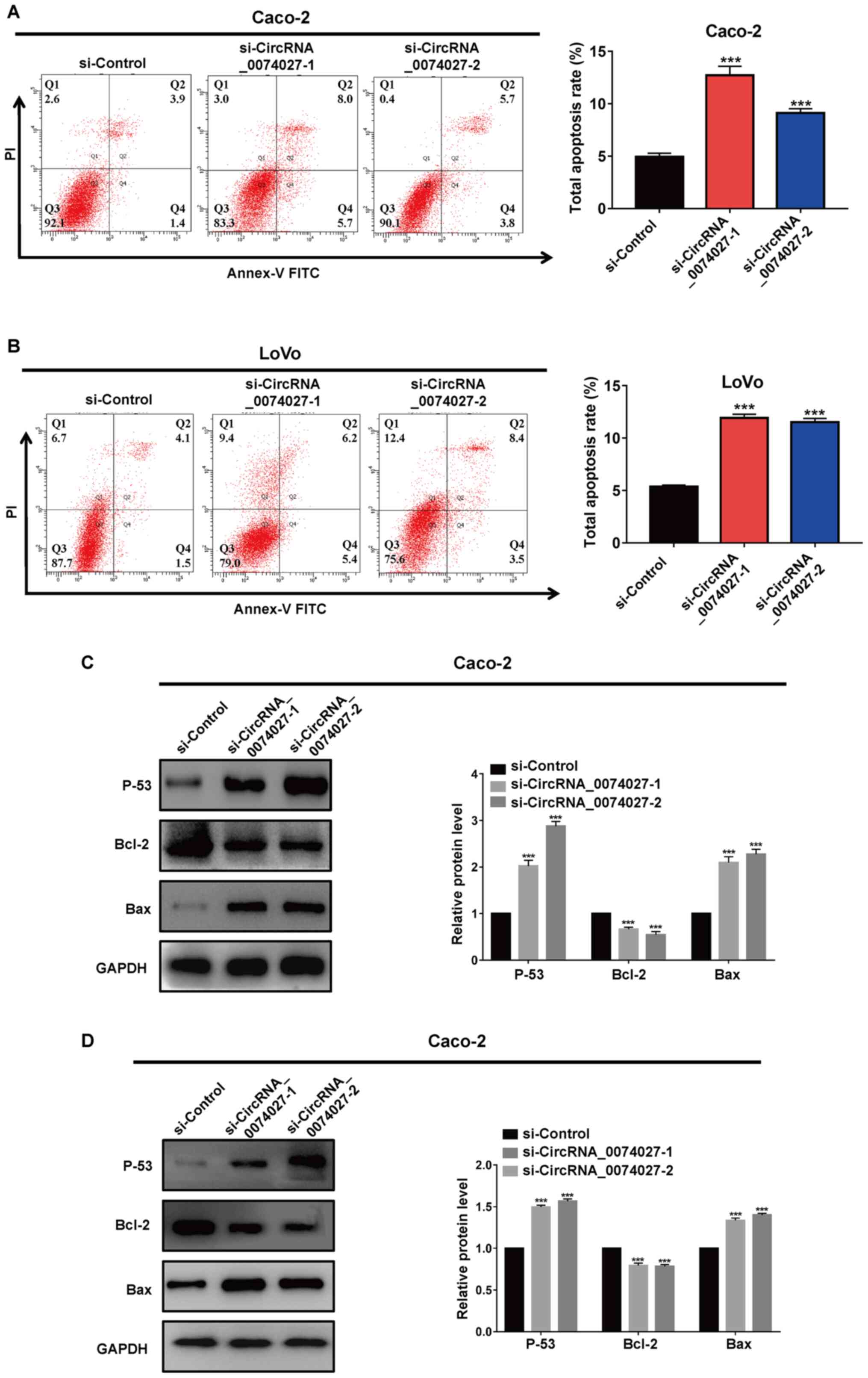
As circRNA-0074027 upregulation could suppress cell
proliferation ability in CRC, the changes of apoptosis level with
low expression of circRNA-0074027 required further research.
Therefore, in current study, the cell apoptosis rate was detected
by flow cytometry experiments. As shown in Fig. 3A, the total cell apoptosis rate of
Caco-2 cells in siRNA-circRNA-0074027-1 and siRNA-circRNA-0074027-2
group were higher compared with the si-Control group. Similarly,
silencing of circRNA-0074027 could induce more apoptosis in LoVo
cells compared with the si-Control group (Fig. 3B).
Western blotting was performed to identify the
potential mechanism of the downregulation of circRNA-0074027 on
cell apoptosis. The downregulation of circRNA-0074027 could
significantly increase p53 and Bax proteins expression, while
reducing the expression of anti-apoptosis protein Bcl-2 compared
with the si-Control group (Fig. 3C and
D). In all, these data consistently displayed that the
downregulation of circRNA-0074027 could significantly promote cell
apoptosis via the regulation of p53 signaling pathway in CRC.
Knockdown of circRNA-0074027 inhibits
cell metastasis via interfering with the epithelial to mesenchymal
transition (EMT) signaling pathway
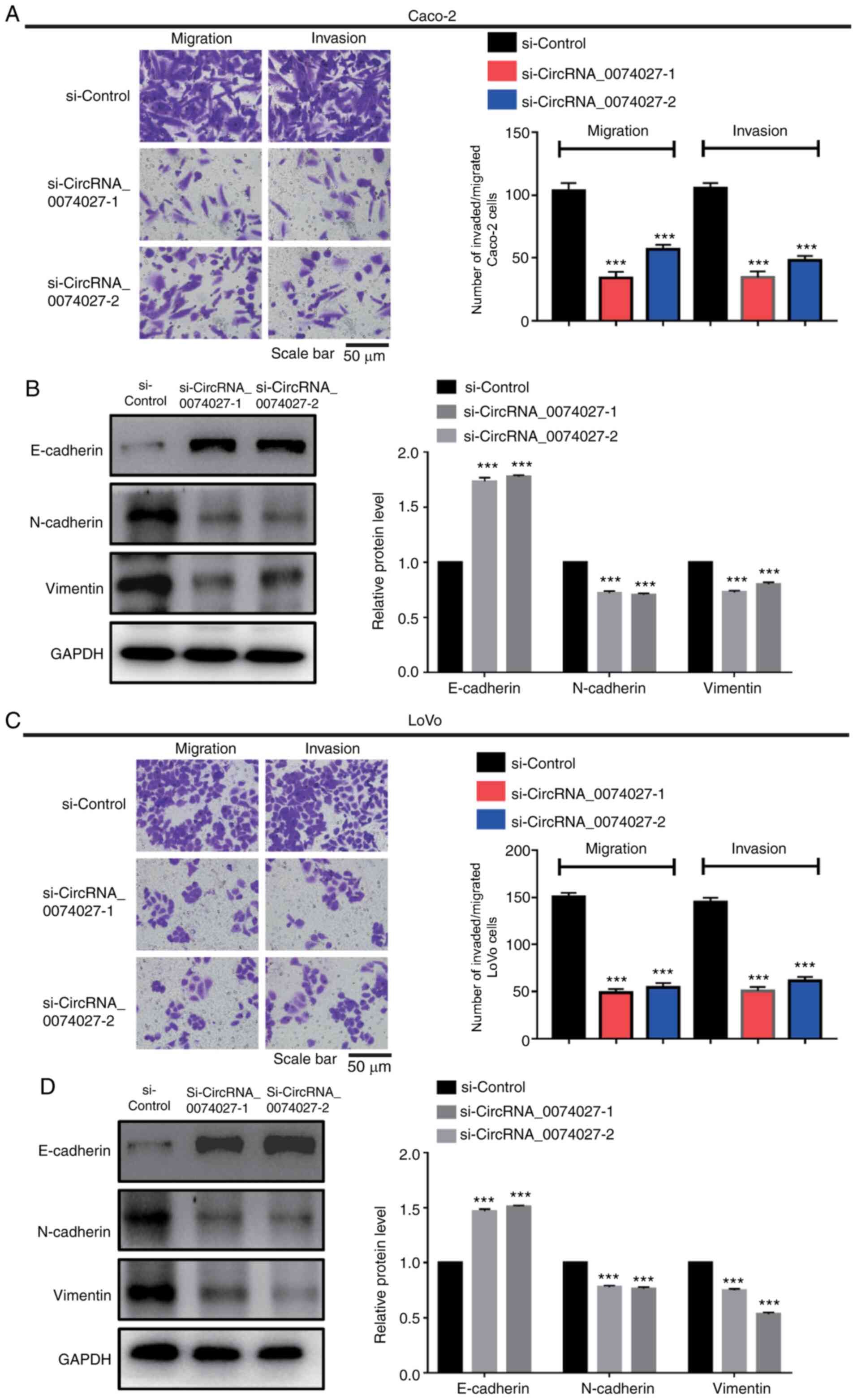
Statistical analysis of data revealed that
circRNA-0074027 expression was associated with lymph node
metastasis in CRC. Therefore, Transwell assay was performed to
determine the involvement of circRNA-0074027 in CRC cell
metastasis. As shown in Fig. 4A,
the number of cells migrating and invading decreased markedly
following downregulation of the expression of circRNA-0074027 in
Caco-2 cells compared with si-Control group. Fig. 4B demonstrates that the silencing of
circRNA-0074027 could significantly suppress cell migration and
invasion ability in LoVo cells compared with the control group. The
results of western blot analysis demonstrated that there was an
increase of epithelial-like phenotype protein (E-cadherin) and a
decrease of mesenchymal phenotype proteins (N-cadherin and
vimentin) when circRNA-0074027 was downregulated (Fig. 4C and D). Taken together, these data
suggested that knockdown of circRNA-0074027 suppresses CRC cell
metastasis ability by inhibiting the EMT pathway.
miR-525-3p is the target gene of
circRNA-0074027
Previous studies (18–20)
have revealed that circRNAs may act as miRNA sponges in regulation
of CRC progression. In the present study, miR-525-3p was proposed
as the downstream gene of circRNA-0074027 based on the results of
bioinformatics algorithms (Starbase.2). Subsequently, we discovered
that the low-expression of miR-525-3p accounted for 80.0% (48/60)
of the CRC tissue samples (Fig.
5A). The potential binding site between circRNA-0074027 and
miR-525-3p was predicted as shown in Fig. 5B. Similarly, the results of RT-qPCR
demonstrated that miR-525-3p was significantly downregulated in CRC
tissue samples (Fig. 5C). Pearson
correlation analysis revealed that the circRNA-0074027 expression
was negatively correlated with the expression of miR-525-3p in CRC
(Fig. 5D, r=−0.5483,
P<0.0001). To further explore their association, luciferase
reporter assay was performed and the results indicated that
miR-525-3p mimics could markedly decrease the luciferase activity
of wild-type circ_0074027, but with no effect on MUT circ_0074027
(Fig. 5E). The results of RT-qPCR
indicated that miR-525-3p inhibitor could significantly
downregulate the expression of miR-525-3p in Caco-2 cells, when
compared with the control group (Fig.
5F). In addition, RT-qPCR assay was performed to determine the
expression of miR-525-3p in Caco-2 cells. Notably, both
si-Circ_0074027-1 and si-Circ_0074027-2 groups demonstrated higher
expression of miR-525-3p in comparison with si-Control group, with
the si-Circ_0074027-1 group showing the highest expression
(Fig. 5G). Thus
si-circRNA-0074027-1 Caco-2 cells were suggested as ideal for
future rescue experiments. To elucidate the potential regulatory
role of miR-525-3p, the miR-525-3p inhibitor was used to decrease
the intra-cellular miR-525-3p expression in Circ_0074027
downregulated Caco-2 cells and this was verified with the RT-qPCR
assay (Fig. 5H). These data
consistently revealed that miR-525-3p would be direct target gene
of circRNA-0074027 in CRC.
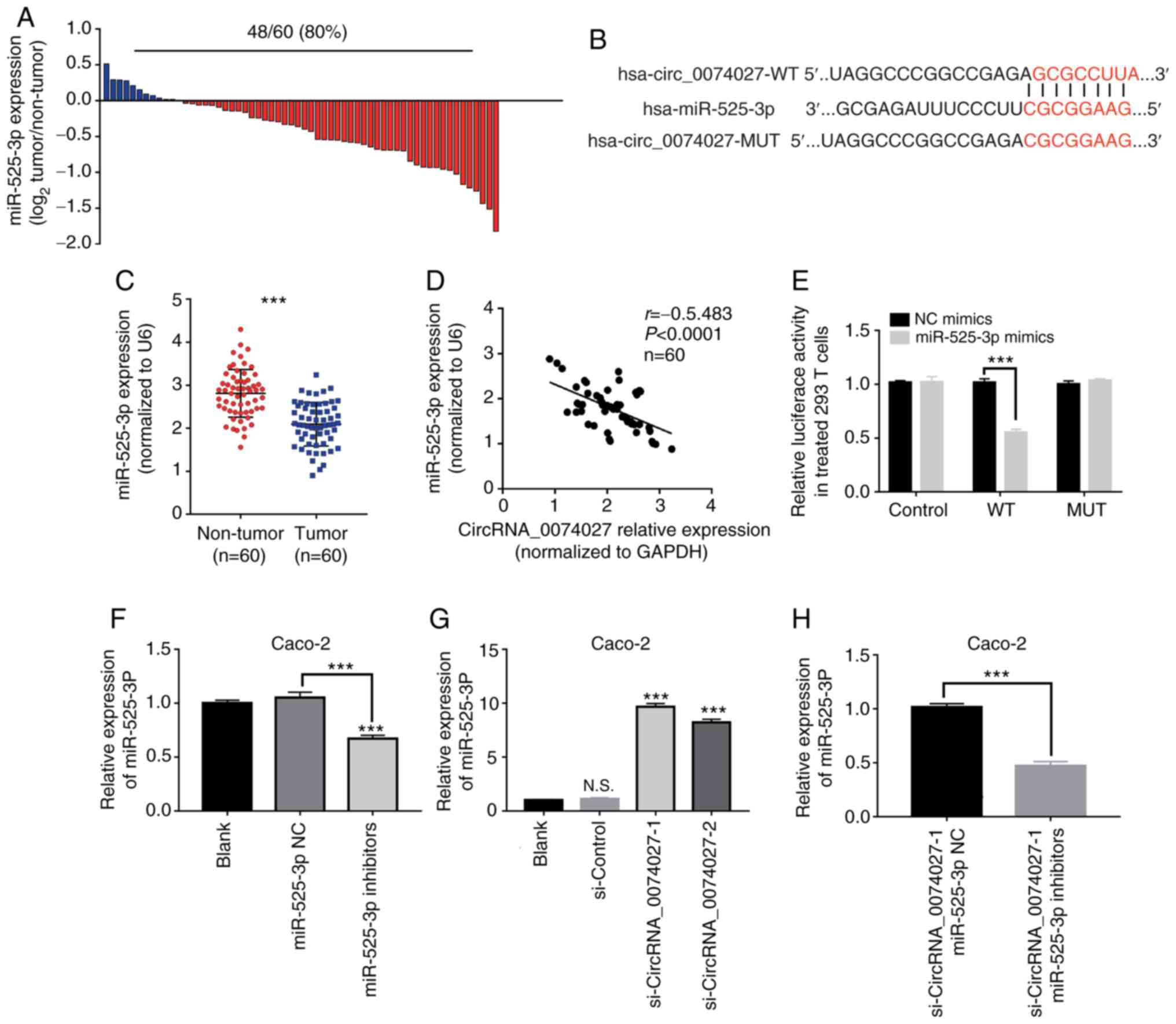 | Figure 5.miR-525-3p is a downstream target
gene of circRNA-0074027. (A) miR-525-3p is downregulated in CRC
tissues. (B) The predicted 3′UTR binding regions of circRNA-0074027
on miR-525-3p. (C) The expression of miR-525-3p in CRC tissues. (D)
The Pearson correlation analysis between the miR-525-3p and
circRNA-0074027 expression. (E) Relative luciferase activity in
293T cells following co-transfection. (F) The expression level of
miR-525-3p in Caco-2 cells following treatment with miR-525-3p
inhibitors, as detected by RT-qPCR. (G) The expression of
miR-525-3p in circRNA_007402-downregulated Caco-2 cells. (H) The
expression of miR-525-3p in circRNA_007402-downregulated Caco-2
cells following treatment with miR-525-3p inhibitors.
***P<0.001. miR, microRNA; circRNA, circular RNA; CRC,
colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR;
N.S., no significance; si, short interfering; WT, wild-type; MUT,
mutant; NC, negative control. |
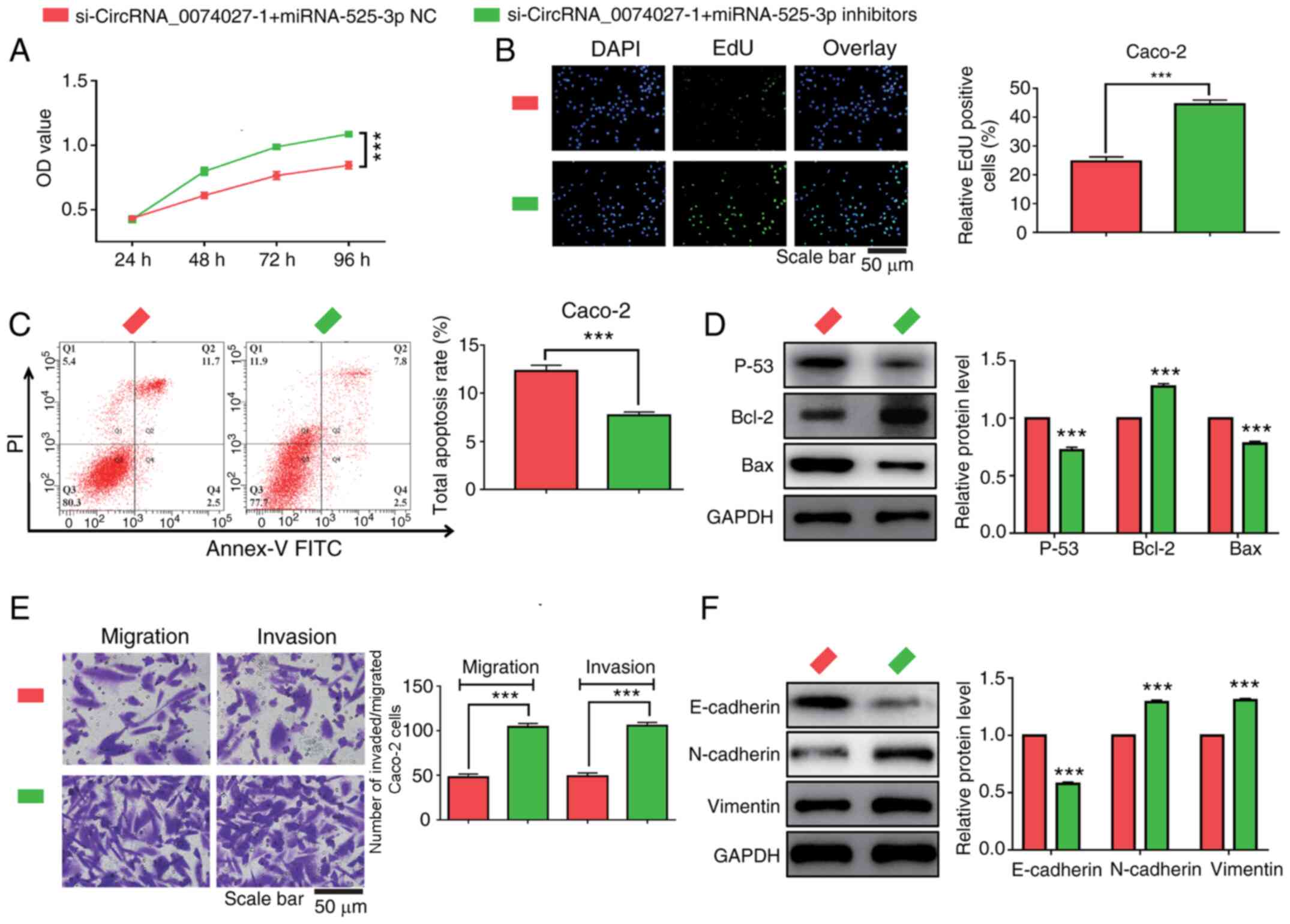
Knockdown of miR-525-3p expression
reverses the anti-tumor effects induced by the overexpression of
circRNA-0074027 in CRC
To explore the regulatory role of miR-525-3p in the
regulation of CRC progression, miR-525-3p inhibitors were added to
inhibit the expression of miR-525-3p in
circRNA-0074027-downregulated Caco-2 cells. As the results of CCK-8
and EdU assay demonstrated, the effects of circRNA-0074027
downregulation on suppression of cell proliferation could be
rescued by adding miR-525-3p inhibitors (Fig. 6A and B). Addition of miR-525-3p
inhibitors may also reduce the cell apoptosis rate in the
si-circRNA-0074027-1 Caco-2 cells (Fig.
6C). Similarly, the western blotting results also revealed that
when incubating with miR-525-3p inhibitors could lead to increased
expression of p53 and Bax proteins but decrease Bcl-2 protein
expression in si-circRNA-00074027-1 Caco-2 cells (Fig. 6D).
An increase in migrated and invasive Caco-2 cells
were was observed in si-circRNA-0074027-1 groups following the
addition of miR-525-3p inhibitors (Fig.
6E). Furthermore, the EMT signaling pathway-related markers in
CRC cells were also determined following adding miR-525-3p
inhibitor. The addition of miR-532-3p could lead to decreased
expression of E-cadherin proteins and an increased expression of
N-cadherin and Vimentin proteins in circRNA-0074027-low expression
CRC cells (Fig. 6F). Therefore,
these data revealed that downregulation of circRNA-0074027 could
exert anti-tumor effects through sponging miR-525-3p in CRC.
Discussion
CRC is one of the most prevalent malignancies in the
world (1,2). The main strategies for CRC treatment
include surgery, radiotherapy and chemotherapy, which have shown
positive therapeutic effects in early-stage patients. However, due
to the shortage of effective biomarkers, most patients are
diagnosed with advanced CRC and thus have short survival periods
(1). Therefore, the present study
was designed to identify effective diagnosis and therapeutic
bio-markers and determine their potential mechanism.
It is known that circRNAs are evolutionally
conserved and more stable than other non-coding RNAs, suggesting
that circRNAs might be the most suitable bio-markers for human
diseases (21–23). Several studies (15,16,24)
indicate that circRNA-0074027 is an important regulatory factor in
the progression of various types of cancer, including glioblastoma
and non-small-cell lung cancer. However, no study, to the best of
the authors' knowledge, has been performed on the regulatory role
of circRNA-0074027 in CRC progression. In the current study,
RT-qPCR was performed to detect the expression of circRNA-0074027
in CRC tissue and adjacent normal tissue samples. The results
indicated that circRNA-0074027 expression was upregulated in CRC
tissues compared with the normal tissues. According to the results
of statistical analysis, patients with CRC and circRNA-0074027
overexpression were more likely to have poor differential status,
larger tumor size, advanced TNM stage, advanced N stage and
vascular invasion. These results demonstrated that circRNA-0074027
might function as a tumor promoter in the development of CRC.
To clarify the biological role of circRNA-0074027 in
CRC progression, circRNA-0074027-downregulated CRC cells were
constructed. Results revealed that the deletion of circRNA-0074027
could markedly inhibit cell growth and promote cell apoptosis.
However, it remains unclear how circRNA-0074027 participated in the
regulation of cell proliferation and apoptosis in CRC. Previous
studies report that p53 is a pivotal anti-oncogene in cancer
progression and its dysfunction might contribute to the rapid cell
proliferation and decreased DNA repair capacity (25,26).
circRNA ZNF609 is shown to promote cell apoptosis rate via
upregulating p53 expression in CRC (27). Su et al (28) showed that circRNA_0055538 can exert
an anti-tumor effect by activating the p53/caspase signaling
pathway. The present study also demonstrated that knockdown of
circRNA-0074027 increased p53 and Bax proteins expression and
decreased Bcl-2 protein expression. Therefore, the silencing of
circRNA-0074027 could activate the p53-mediated pathway, which
contributes to the suppression of cell proliferation and promotion
of cell apoptosis in CRC.
The present study discovered that the patients with
CRC and circRNA-0074027 overexpression had advanced N stage, thus
it was hypothesized that circRNA-0074027 participated in the
regulation of cell migration and invasion ability in CRC. The data
of the present study demonstrated that the deletion of
circRNA-0074027 could markedly inhibit the cell metastasis ability
in CRC, but the potential regulatory mechanism remains to be
elucidated. EMT is a significant biological process, in which the
epithelial (E) cells transition to a mesenchymal (M) phenotype
(29–31). Previous studies indicate that EMT
signaling pathway serves an important role in the development of
malignant tumors (32–34). Wang et al (35) revealed that the circRNA circP4HB can
promote cell aggressiveness and metastasis of non-small cell lung
carcinoma via sponging miR-133a-5p. In addition, circRNA circPTPRA
can sponge miR-96-5p, thereby inhibiting cell metastasis ability in
non-small cell lung carcinomas cells via regulating the EMT pathway
(36). The results of the present
study were consistent with these previous studies and revealed that
the downregulation of circRNA-0074027 led to increased expression
of epithelial-like phenotype protein (E-cadherin) and decrease
expression of mesenchymal phenotype proteins (N-cadherin and
vimentin). Taken together, the present study hypothesized that
downregulation of circRNA-0074027 mediated inhibition of cell
migration and invasion in CRC and this might be caused by the
inactivation of EMT signaling pathway.
The primary function of circRNAs is miRNA sponging,
which contributes to the carcinogenesis and progression of various
types of cancer (8,37). For example, circRNA-MAN2B2 had been
identified as a tumor promotor in hepatocellular carcinoma
progression via sponging miRNA-217 (38). Another study indicates that
circFOXK2 can sponge miR-942, followed by enhanced cell
proliferation, migration and invasion ability in pancreatic ductal
adenocarcinoma (39). The present
study proposed miR-525-3p as the target gene of circRNA-0074027 in
CRC progression. The results of RT-qPCR revealed that miR-525-3p
was downregulated in CRC tissues in comparison with normal tissue
samples. The Pearson correlation analysis showed that
circRNA-0074027 expression was negatively correlated with the
expression of miR-525-3p, suggesting that miR-525-3p might be the
downstream target gene of circRNA-0074027. The association between
the circRNA-0074027 and miR-525-3p was confirmed by dual-luciferase
reporter assays. Previous studies identify that miR-525-3p may
function as a significant regulatory factor in the development of
various types of cancer, including liver cancer (40) and Hodgkin lymphoma (41). Pang et al (40) determined that miR-525-3p can enhance
the cell migration and invasion of liver cancer via modulating
ZNF395 expression. The results of the current study demonstrated
that the addition of miR-525-3p could reverse the anti-tumor
effects induced by the silencing of circRNA-0074027. Therefore,
these data suggested that circRNA-0074027 could sponge miR-525-3p,
thereby participating in the regulation of cell proliferation, cell
metastasis and cell apoptosis in CRC. However, the present study
has some limitations. First, the molecular targets downstream of
the circRNA-0074027/miR-525-3p axis were not fully elucidated.
Second, the present study was performed in vitro and there
is a need to carry it out in vivo to confirm the biological
function of circRNA-0074027. Third, the sample size and the
follow-up duration was insufficient. In the future, a large sample
size and a longer follow-up duration should be employed to validate
the diagnostic and prognostic significance of circRNA-0074027.
CircRNA-0074027 might function as a tumor promoter
in CRC, as the patients with CRC and overexpression were more
likely to have poor prognosis. In addition, the in vitro
experiments indicated that silencing of circRNA-0074027 could
directly regulate the function of miR-525-3p and then lead to the
suppression of cell proliferation and metastasis ability via
interfering with the p53/EMT signaling pathway. In conclusion,
circRNA-0074027 could act as a promising therapeutic bio-marker for
CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GX and YZ conceived and designed the study. GX, JZ
and YZ performed the experiments. GX, XP and BW were responsible
for the analysis and interpretation of data. XP, BW and YZ wrote
the manuscript. GX and YZ were responsible for confirming the
authenticity of the data. All authors read and approved the final
manuscript. This manuscript was revised by all authors.
Ethics approval and consent to
participate
Written informed consent was sought from the
participants before the samples were obtained. The present study
was approved by the Ethical Committees of Dazhou Central Hospital
(approval no. KY2020-086-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geng F, Wang Z, Yin H, Yu J and Cao B:
Molecular targeted drugs and treatment of colorectal cancer: Recent
progress and future perspectives. Cancer Biother Radiopharm.
32:149–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roelands J, Kuppen PJK, Vermeulen L,
Maccalli C, Decock J, Wang E, Marincola FM, Bedognetti D and
Hendrickx W: Immunogenomic Classification of Colorectal Cancer and
Therapeutic Implications. Int J Mol Sci. 18:22292017. View Article : Google Scholar
|
|
5
|
Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang
Y, Li X, Wu Z, Yang D, Zhou Y, et al: Circular RNAs in cancer:
Emerging functions in hallmarks, stemness, resistance and roles as
potential biomarkers. Mol Cancer. 18:902019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng
J, Hou J, Lin L and Cai J: Regulatory network of circRNA-miRNA-mRNA
contributes to the histological classification and disease
progression in gastric cancer. J Transl Med. 16:2162018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dori M and Bicciato S: Integration of
bioinformatic predictions and experimental data to identify
circRNA-miRNA associations. Genes (Basel). 10:6422019. View Article : Google Scholar
|
|
9
|
Wu J, Chen Z, Song Y, Zhu Y, Dou G, Shen
X, Zhou Y, Jiang H, Li J and Peng Y: CircRNA_0005075 suppresses
carcinogenesis via regulating miR-431/p53/epithelial-mesenchymal
transition axis in gastric cancer. Cell Biochem Funct. 38:932–942.
2020. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Q, Chen Z, Cao S, Guo B, Chen Y, Feng
Z, Wang J, Guo G, Chen X and Huang X: Role of CircRNAs_100395 in
proliferation and metastases of liver cancer. Med Sci Monit.
25:6181–6192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Yu P, Zhang P, Wu H, Chen Q, Li S
and Wang Y: Upregulation of hsa_circ_0007874 suppresses the
progression of ovarian cancer by regulating the miR-760/SOCS3
pathway. Cancer Med. 9:2491–2499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Li L, Wang Q, Han H, Zhan Q and Xu
M: CircRNA expression profile in early-stage lung adenocarcinoma
patients. Cell Physiol Biochem. 44:2138–2146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao J and Liu XS: Circular RNA 0060428
sponges miR-375 to promote osteosarcoma cell proliferation by
upregulating the expression of RPBJ. Gene. 740:1445202020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Q, Dai J and Shu M: Retraction:
Hsa_circ_0003645 shows an oncogenic role by sponging microRNA-1299
in hepatocellular carcinoma cells. J Clin Lab Anal. 34:e232492020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian L, Guan J, Wu Y and Wang Q:
Upregulated circular RNA circ_0074027 promotes glioblastoma cell
growth and invasion by regulating miR-518a-5p/IL17RD signaling
pathway. Biochem Biophys Res Commun. 510:515–519. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao P, Wang Z, Hu Z, Jiao X and Yao Y:
Circular RNA circ_0074027 indicates a poor prognosis for NSCLC
patients and modulates cell proliferation, apoptosis, and invasion
via miR-185-3p mediated BRD4/MADD activation. J Cell Biochem.
121:2632–2642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng X, Zhang L, Zhang K, Zhang G, Hu Y,
Sun X, Zhao C, Li H, Li YM and Zhao J: Circular RNA VMA21 protects
against intervertebral disc degeneration through targeting miR-200c
and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis.
77:770–779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu JZ, Shao CC, Wang XJ, Zhao X, Chen JQ,
Ouyang YX, Feng J, Zhang F, Huang WH, Ying Q, et al: circTADA2As
suppress breast cancer progression and metastasis via targeting
miR-203a-3p/SOCS3 axis. Cell Death Dis. 10:1752019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu W, Han Q, Zhao L and Wang L: Circular
RNA circRNA_15698 aggravates the extracellular matrix of diabetic
nephropathy mesangial cells via miR-185/TGF-β1. J Cell Physiol.
234:1469–1476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ng WL, Mohd Mohidin TB and Shukla K:
Functional role of circular RNAs in cancer development and
progression. RNA Biol. 15:995–1005. 2018.PubMed/NCBI
|
|
22
|
Bachmayr-Heyda A, Reiner AT, Auer K,
Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW,
Zeillinger R and Pils D: Correlation of circular RNA abundance with
proliferation-exemplified with colorectal and ovarian cancer,
idiopathic lung fibrosis, and normal human tissues. Sci Rep.
5:80572015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Letts. 365:141–148. 2015. View Article : Google Scholar
|
|
24
|
Yu C, Ying J, Yu K, Shen W and Jiang M:
Circ_0074027 contributes to nonsmall cell lung cancer progression
by upregulating CUL4B expression through miR-335-5p. Cancer Biother
Radiopharm. Jun 22–2020.(Epub ahead of print). doi:
10.1089/cbr.2020.3579. View Article : Google Scholar
|
|
25
|
Moch H, Sauter G, Gasser TC, Buchholz N,
Bubendorf L, Richter J, Jiang F, Dellas A and Mihatsch MJ: p53
protein expression but not mdm-2 protein expression is associated
with rapid tumor cell proliferation and prognosis in renal cell
carcinoma. Urol Res. 25 (Suppl 1):S25–S30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaber S, Toufektchan E, Lejour V, Bardot B
and Toledo F: p53 downregulates the Fanconi anaemia DNA repair
pathway. Nat Commun. 7:110912016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossi F, Legnini I, Megiorni F, Colantoni
A, Santini T, Morlando M, Di Timoteo G, Dattilo D, Dominici C and
Bozzoni I: Circ-ZNF609 regulates G1-S progression in
rhabdomyosarcoma. Oncogene. 38:3843–3854. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su W, Sun S, Wang F, Shen Y and Yang H:
Circular RNA hsa_circ_0055538 regulates the malignant biological
behavior of oral squamous cell carcinoma through the
p53/Bcl-2/caspase signaling pathway. J Transl Med. 17:762019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Derynck R and Weinberg RA: EMT and cancer:
More than meets the eye. Dev Cell. 49:313–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Li Y, Kong D and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu H, Hu S, Xu S, Gao Y, Zeng F and Shui
H: miR-29b regulates Ang II-induced EMT of rat renal tubular
epithelial cells via targeting PI3K/AKT signaling pathway. Int J
Mol Med. 42:453–460. 2018.PubMed/NCBI
|
|
35
|
Wang T and Wang X, Du Q, Wu N, Liu X, Chen
Y and Wang X: The circRNA circP4HB promotes NSCLC aggressiveness
and metastasis by sponging miR-133a-5p. Biochem Biophys Res Commun.
513:904–911. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei S, Zheng Y, Jiang Y, Li X, Geng J,
Shen Y, Li Q, Wang X, Zhao C, Chen Y, et al: The circRNA circPTPRA
suppresses epithelial-mesenchymal transitioning and metastasis of
NSCLC cells by sponging miR-96-5p. EBioMedicine. 44:182–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu X, Zhang J, He X, Yan X, Wei J, Huang
M, Liu Y, Lin J, Hu H and Liu L: Circular RNA MAN2B2 promotes cell
proliferation of hepatocellular carcinoma cells via the
miRNA-217/MAPK1 axis. J Cancer. 11:3318–3326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong CH, Lou UK, Li Y, Chan SL, Tong JHM,
To KF and Chen Y: CircFOXK2 promotes growth and metastasis of
pancreatic ductal adenocarcinoma by complexing with RNA binding
proteins and sponging MiR-942. Cancer Res. 80:2138–2149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pang F, Zha R, Zhao Y, Wang Q, Chen D,
Zhang Z, Chen T, Yao M, Gu J and He X: MiR-525-3p enhances the
migration and invasion of liver cancer cells by downregulating
ZNF395. PLoS One. 9:e908672014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paydas S, Acikalin A, Ergin M, Celik H,
Yavuz B and Tanriverdi K: Micro-RNA (miRNA) profile in Hodgkin
lymphoma: Association between clinical and pathological variables.
Med Oncol. 33:342016. View Article : Google Scholar : PubMed/NCBI
|