Introduction
Thyroid cancer (TC) is the most common malignant
tumor of the endocrine system, and is one of the top ten cancer
types threatening women's health. Serpin peptidase inhibitor clade
E member 2 (SERPINE2), also named as protease nexin-1 (PN-1), is a
single chain glycoprotein with a molecular weight of 45–50 kDa,
which acts as a secreted serine protease inhibitor. It is
overexpressed in various cancer types and is involved in tumor
formation (1–3). SERPINE2 has been demonstrated to play
vital roles in the progression of papillary TC (4). SERPINE2 expression is closely
associated with the poor survival of patients with gastric cancer,
and silencing SERPINE2 inhibits the migration and invasion of
gastric cancer cells (2).
SERPINE2 enables primary tumor cells to form a
vascular-like network in a variety of cancer tissues, including
lung, brain, head and neck and breast cancer (5–8).
Although increased expression of SERPINE2 has been demonstrated in
papillary thyroid carcinoma tissues (4), its role in the pathogenesis of TC
cells is still unknown. Epidermal growth factor receptor (EGFR),
the product of proto-oncogene CerB1, is a transmembrane
glycoprotein with tyrosine kinase activity that is commonly
expressed in human epithelial cells. EGF and EGFR are highly
expressed in TC (9), and EGF/EGFR
is closely associated with the migration and invasiveness of TC
cells (10).
Both EGF and its receptor partake in the
pathogenesis of multiple carcinomas, thus constituting attractive
targets for molecular therapy. Previous studies have demonstrated
that EGF and EGFR are implicated in the invasion and migration of
thyroid tumors, and that PN-1 can form a positive feedback with
EGF/EGFR signaling to promote the biological activity of breast
cancer cells (11–13). Therefore, the present study aimed to
explore whether SERPINE2 could form positive feedback signals with
EGF/EGFR, and participate in the proliferation, invasion and
migration of papillary thyroid carcinoma.
Materials and methods
Clinical samples
Clinical specimens were collected from 30 patients
diagnosed with papillary thyroid carcinoma at the Central Hospital
of Wuhan (Wuhan, China) between July 2018 and October 2019.
Informed consent was obtained from each patient, and the present
study was approved by the Human Ethics Committee of the Central
Hospital of Wuhan. Cancerous and adjacent normal tissues were
obtained after surgical resection, and the specimens were
immediately stored at −80°C. Adjacent normal tissue samples 2 cm
away from the cancerous tissue were collected. TC tissue specimens
were pathologically confirmed to be papillary TC.
Cell lines
Normal human thyroid epithelial cells (Nthy-ori
3-1), human anaplastic thyroid carcinoma cell lines (8505C, SW1736
and HTH83), and a human papillary thyroid carcinoma cell line
(TPC-1; all purchased from American Type Culture Collection) were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml) and streptomycin (100 g/ml). The medium was
changed once every 24 h. After adherent cell growth, 0.25% trypsin
was used for digestion and passaging. EGF (10 ng/ml; cat. no.
SRP3027; Sigma-Aldrich; Merck KGaA) or AG1478 (10 µM; cat. no.
T4182; Sigma-Aldrich; Merck KGaA) was used to treat the cells at
37°C for 24 or 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
In the first experiment, total RNA of Nthy-ori 3-1,
HTH83, 8505C, SW1736 or TPC-1 cells was respectively extracted with
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. In the following
experiments, TPC-1 cells were used. Following the experimental
treatments, the total RNA of TPC-1 cells was extracted with TRIzol.
Then, RNA was reverse transcribed into cDNA at 37°C for 15 min and
95°C for 5 min using PrimeScript™ RT Reagent Kit (Takara Bio,
Inc.). LightCycler® 480 software (Roche Diagnostics) was
employed to analyze cDNA in the LightCycler 480 RT-qPCR instrument
(Roche Diagnostics) with SYBR® Premix Ex Taq™ (Takara
Bio, Inc.).
The qPCR conditions included initial denaturation
for 10 min at 95°C and subsequently 40 cycles at 95°C for 10 min
and 60°C for 20 sec. GAPDH mRNA was used as the internal reference.
SERPINE2 forward, 5′-AATGAAACCAGGGATATGATTGAC-3′ and reverse,
5′-TTGCAAGATATGAGAAACATGGAG-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative levels of SERPINE2 mRNA
were calculated using 2−ΔΔCq method (14).
Western blotting
TPC-1 cells were seeded into a 6-well plate
(1×106/well). Following the experimental treatments, the
culture medium was discarded and the cells were washed twice with
pre-cooled PBS. The tissue or cells were lysed with lysis buffer
(Beyotime Institute of Biotechnology) on ice for 30 min. The
proteins were quantified using the BCA method (BCA Protein Assay
kit; cat. no. ab102536; Abcam). The proteins (50 µg) were separated
via 10% SDS-PAGE, then transferred to PVDF membranes and blocked
with 5% skimmed milk powder at room temperature for 2 h.
Subsequently, the following primary antibodies were added and
incubated at 4°C overnight: Anti-SERPINE2 (1:1,000; cat. no.
ab154591; Abcam), anti-Ki67 (1:500; cat. no. sc-23900; Santa Cruz
Biotechnology, Inc.), anti-proliferating cell nuclear antigen
(1:500; PCNA; cat. no. sc-56; Santa Cruz Biotechnology, Inc.),
anti-MMP2 (1:1,000; cat. no. ab92536; Abcam), anti-MMP9 (1:1,000;
cat. no. ab76003; Abcam), anti-Bcl-2 (1:1,000; cat. no. ab32124;
Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-cleaved
caspase-3 (1:500; cat. no. ab32042; Abcam), anti-caspase-3 (1:500;
cat. no. ab13847; Abcam), anti-EGF (1:1,000; cat. no. ab184265;
Abcam), anti-phosphorylated (p)-EGFR (1:1,000; cat. no. ab40815;
Abcam) and anti-EGFR (1:1,000; cat. no. ab52894; Abcam). Next, the
horseradish peroxidase-conjugated secondary antibody (10,000; cat.
no. ab97040; Abcam) was applied and incubated at 37°C for 1 h. The
bands were visualized using Super ECL Detection Reagent kit
(Shanghai Yeasen Biotech Co., Ltd.). The grey value of the protein
bands was analyzed using ImageJ 1.46r software (National Institutes
of Health).
Plasmid transfection
TPC-1 cells were seeded into a 24-well plate
(1×104 cells/well), when cell growth reached 65–75%, the
cells were transfected using TurboFect™ reagent (Thermo Fisher
Scientific, Inc.) as per the manufacturer's protocols.
Additionally, SERPINE2 overexpression plasmids or empty plasmids (1
µg), and SERPINE2 silencing plasmids or its negative control (1 µg
scramble shRNA) were constructed by Shanghai GenePharma Co., Ltd.,
and were dissolved in 25 µl serum-free medium and thoroughly mixed.
The mixture was then added to the culture medium, and after 6 h the
culture medium was replaced, and the culture was incubated at 37°C
for 24 h for further experiments. Next, the cells were treated with
AG1478 at 37°C for 48 h.
MTT assay
TPC-1 cell concentration was adjusted to
3×104 cells/ml and seeded into 96-well plates. A cell
suspension of 200 µl was added to each well for culture at 37°C
with 5% CO2 for 24, 48 and 72 h. Next, MTT reagent (10
µl; cat. no. C0009; Beyotime Institute of Biotechnology) was added
to the cells and incubated for 4 h. A 150-µl volume of DMSO was
added to each well to dissolve the crystals. After 10 min, the
absorbance value was detected at 490 nm and the cell proliferation
rate was calculated.
Colony formation assay
In total, 500 TPC-1 cells were seeded into a 6-well
culture plate, and fixed with 4% paraformaldehyde at room
temperature for 15 min and stained with crystal violet (Shanghai
Yuanye Bio-Technology Co., Ltd.) at room temperature for 15 min.
The number of clones containing >50 cells were counted under a
light microscope.
Wound healing and Transwell Matrigel™
assays
TPC-1 cells were grown in 6-well plates
(6×104 cells/ml). When the cells reached confluence,
scratches were made with a 10-µl tip pipette perpendicular to the
plate, and the width of each scratch was as close as possible. The
cell culture medium was removed, and the plates were rinsed with
PBS three times to remove cell fragments and serum-free medium was
added. The experiment was repeated three times.
The invasion of TPC-1 cells was detected using
Transwell chambers (EMD Millipore). Matrigel solution (50 µl) was
used to pre-coat upper chamber at 37°C for 5 h. TPC-1 cells
(2×106 cells/ml) were subsequently seeded into the upper
chamber of the Transwell invasion chamber (500 µl). DMEM medium
containing 5% FBS (600 µl) was added to lower chamber. After cells
were cultured for 24 h, the upper chamber was removed, and the
non-invasive cells were removed from the substrate membrane. After
fixing with 40% paraformaldehyde for 15 min at room temperature,
cells were stained with 0.1% crystal violet for 30 min at room
temperature, and then cells were washed with PBS three times before
images were observed under a fluorescence microscope.
TUNEL staining
TPC-1 cell concentration was adjusted to
2×106 cells/ml and then seeded cells into 6-well plates.
After experimental treatment, cells were washed using PBS three
times and then fixed with 4% paraformaldehyde for 1 h at room
temperature.
Cell sections were prepared and soaked with xylene
twice. Next, the sections were soaked and washed with a gradient of
ethanol solutions. The TUNEL reaction mixture (50 µl) was prepared
and added to the sections for incubation in dark for 1 h at 37°C.
The nucleus was stained with DAPI in the dark for 5 min. The excess
DAPI was washed with PBS four times. The slides were sealed with
anti-fluorescence quenching solution and observed under a
fluorescence microscope. A total of six fields of view was randomly
selected.
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used for data analysis. Data are expressed as the mean ±
standard deviation. One-way ANOVA was used for comparison between
multiple groups, followed by Tukey's test. All experiments were
repeated at least three times. P<0.05 was considered to indicate
a statistically significant difference.
Results
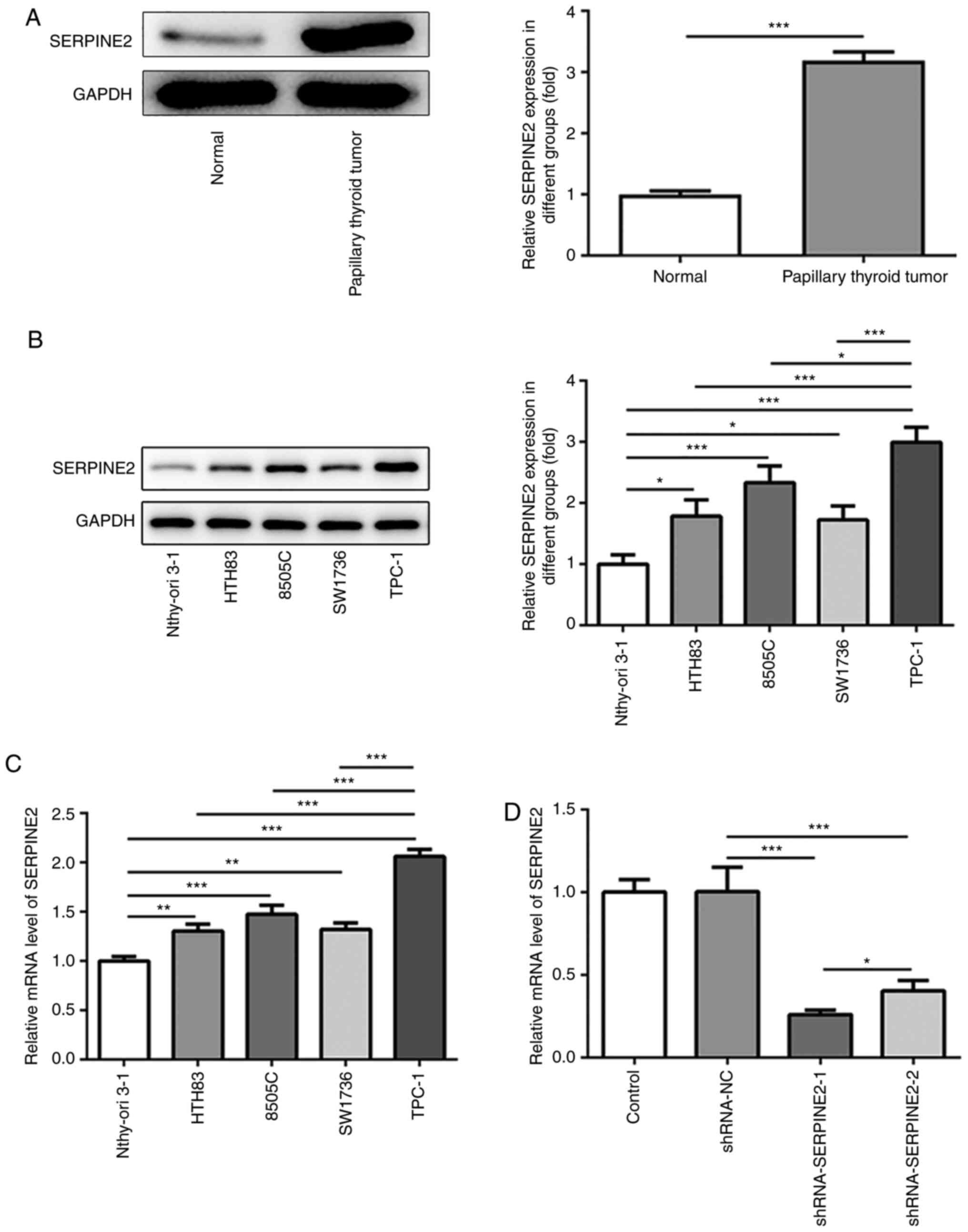
SERPINE2 levels are significantly
increased in TC cells
To investigate the role of SERPINE2 in TC, TC and
adjacent normal tissues were collected to detect the protein
expression of SERPINE2 via western blotting. Increased protein
expression of SERPINE2 was observed in TC tissue compared with
those in the normal group (Fig.
1A). Next, the expression of SERPINE2 in Nthy-ori 3-1, HTH83,
8505C, SW1736 and TPC-1 cell lines was evaluated by western
blotting and RT-qPCR (Fig. 1B and
C). The results demonstrated that, compared with that in normal
thyroid epithelial cells, there was a significant increase in the
expression of SERPINE2 in HTH83, 8505C, SW1736 and TPC-1 cells,
suggesting the possible oncogenic role of SERPINE2 in TC. Moreover,
SERPINE2 showed the highest expression in TPC-1 cells. Therefore,
TPC-1 cells were used in subsequent experiments (Fig. 1B and C). To investigate the loss of
function of SERPINE2 in TPC-1 cells, SERPINE2 expression was
silenced using shRNA. The knockdown effect of shRNA-SERPINE2-1 on
SERPINE2 expression was stronger than that of shRNA-SERPINE2-2
(Fig. 1D). Thus, shRNA-SERPINE2-1
was employed to silence SERPINE2 expression in subsequent
experiments.
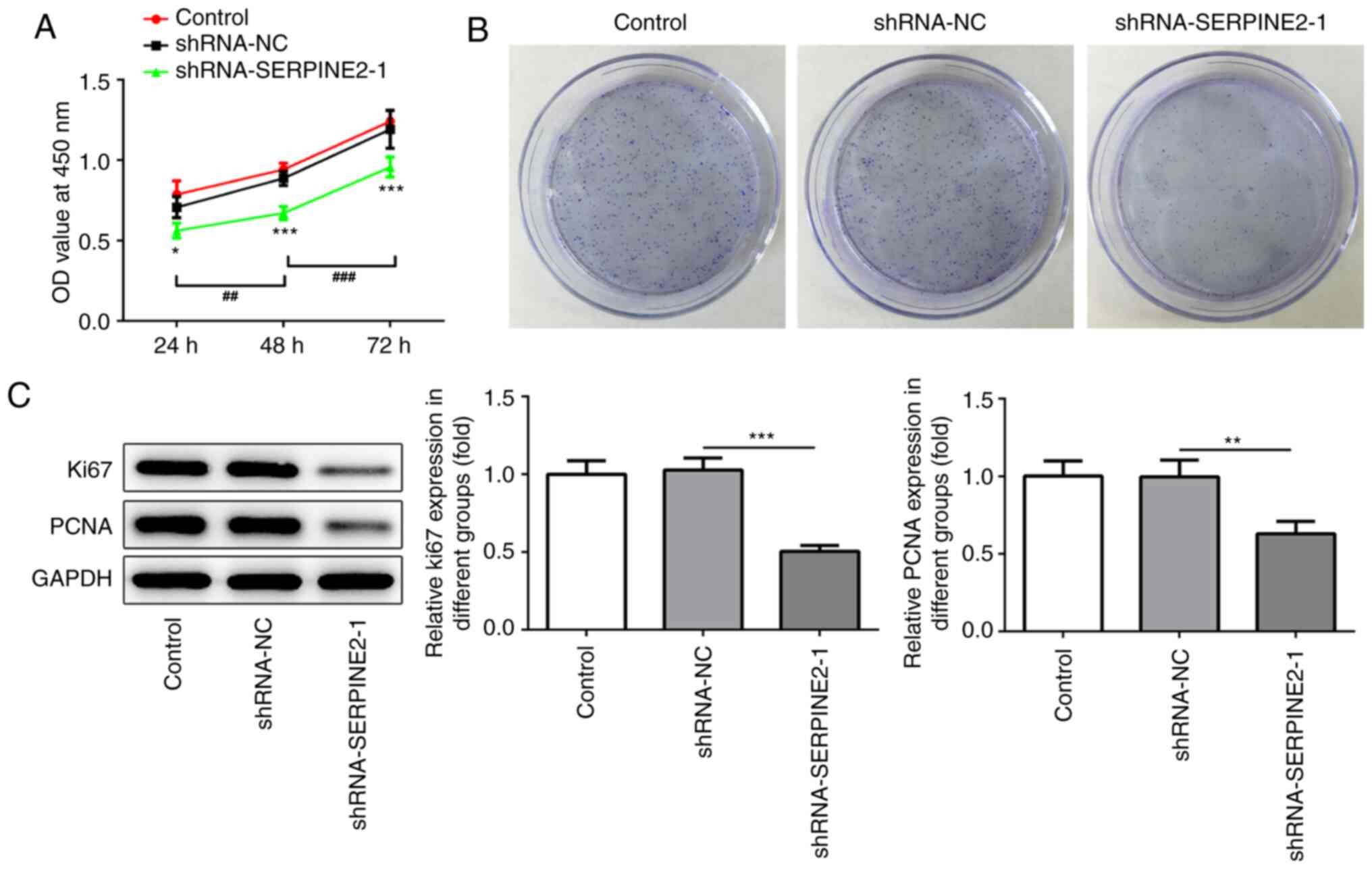
SERPINE2 silencing significantly
decreases cell proliferation and clone formation
The functions of SERPINE2 on TPC-1 cell
proliferation and clone formation were analyzed through silencing
SERPINE2 expression (Fig. 2A and
B). The MTT assay revealed that the silencing of endogenous
SERPINE2 suppressed the proliferation and clone formation of TPC-1
cells. In addition, western blotting revealed that SERPINE2
knockdown significantly reduced the expression of the proliferation
makers Ki67 and PCNA (Fig. 2C).
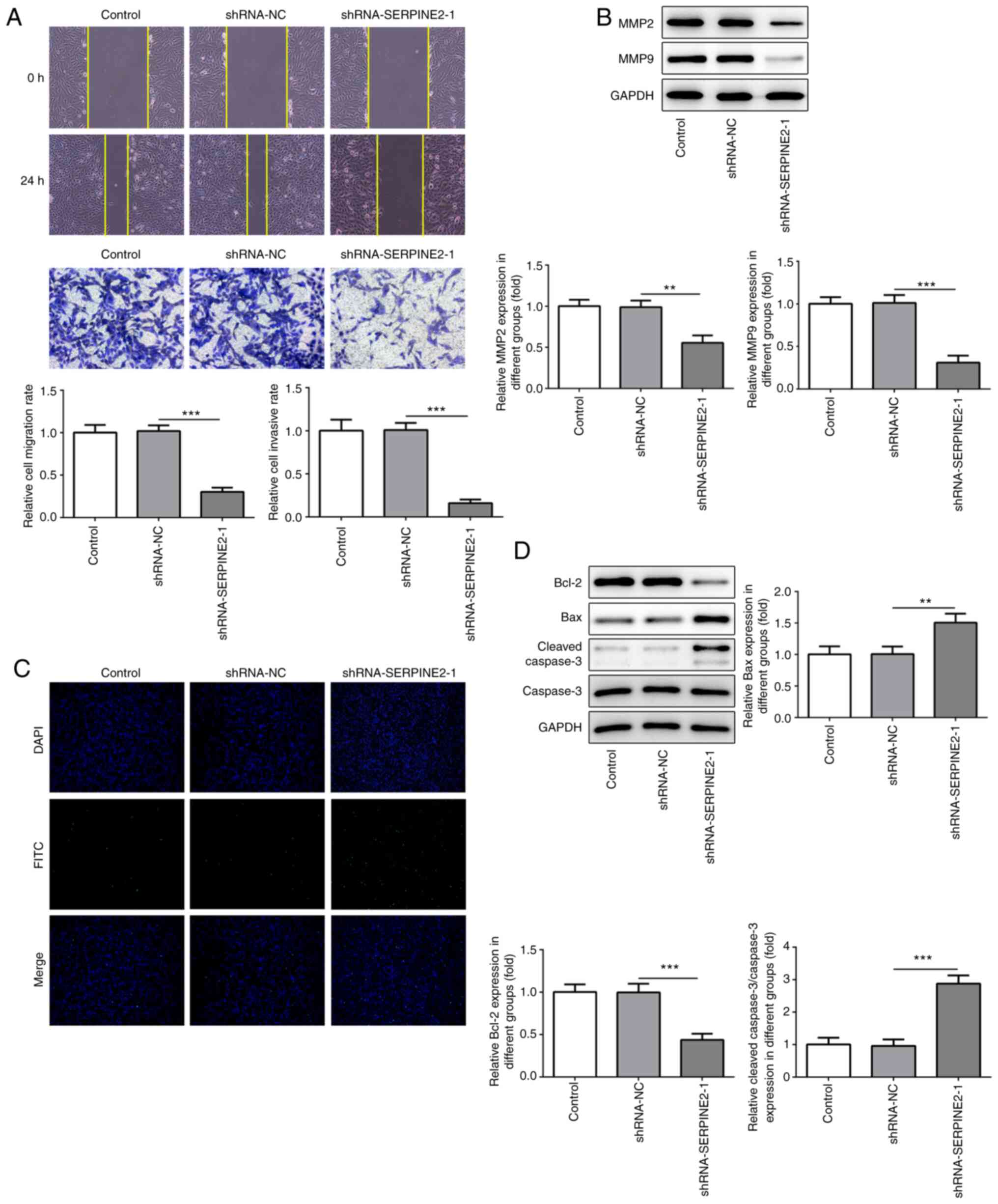
SERPINE2 knockdown suppresses the
metastatic potential of TPC-1 cells and promotes apoptosis
To investigate whether MBZ could inhibit the
metastatic potential of TC cells, TPC-1 cells transfected with
shRNA-SERPINE2-1 were used. The migration and invasion of TPC-1
cells were significantly suppressed when SERPINE2 was silenced
(Fig. 3A). Furthermore, MMP2 and
MMP9 expression levels were also significantly reduced by knockdown
of SERPINE2 (Fig. 3B). Next, the
present study explored whether SERPINE2 knockdown regulated cell
apoptosis. The results of TUNEL staining showed that cell apoptosis
was notably increased (Fig. 3C). In
addition, the expression of the anti-apoptotic protein Bcl-2 was
significantly decreased, whereas that of the pro-apoptotic proteins
Bax and cleaved caspase-3 was significantly increased (Fig. 3D).
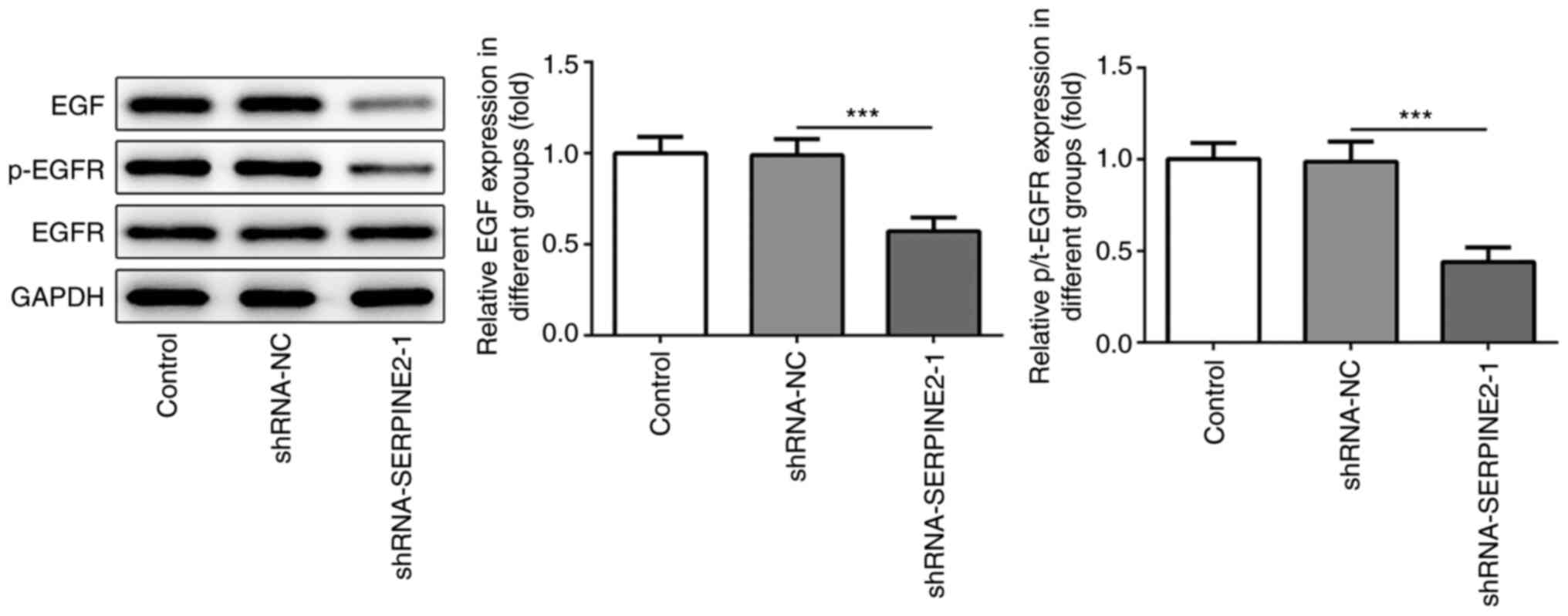
SERPINE2 forms a positive feedback
loop with EGF/EGFR in TPC-1 cells
According to a previous study, there is a feedback
association between SERPINE2 and EGF/EGFR (12). The expression levels of EGF and
p-EGFR were significantly reduced by SERPINE2 knockdown (Fig. 4), which suggested that SERPINE2
regulated EGF/EGFR signaling. Next, EGF (100 ng/ml) or the specific
tyrosine kinase inhibitor AG1478 (10 µM) were added to stimulate
the cells for 24 h or 48 h. EGF significantly increased the
expression of SERPINE2 in a time-dependent manner (Fig. 5A). The results showed that AG1478
significantly blocked the promoting effects of EGF on the
expression of SERPINE2, indicating that EGF/EGFR signaling could
also modulate SERPINE2 expression. Next, SERPINE2 was overexpressed
through transfection with OV-SERPINE2 plasmids into TPC-1 cells
(Fig. 5B). The EGFR inhibitor
significantly reversed the effects of SERPINE2 overexpression on
the upregulation of EGF and p-EGFR expression (Fig. 5C). The overexpression of SERPINE2 in
TPC-1 cells significantly increased cell proliferation, as revealed
by the MTT assay (Fig. 5D).
However, this effect could be significantly abolished by the EGFR
inhibitor AG1478. The same trend was observed in the clone
formation assay (Fig. 5E). The
aforementioned results implied that SERPINE2 regulated the
proliferative ability of TPC-1 cells via EGF/EGFR signaling. As a
cell proliferation marker, Ki67 plays a role in the occurrence and
development of tumors, and is closely associated with the
proliferation, infiltration, metastasis and prognosis of thyroid
carcinoma (15–17). As revealed by western blotting,
SERPINE2 overexpression enhanced the expression of Ki67 and PCNA,
which was reversed by the addition of AG1478 (Fig. 5F).
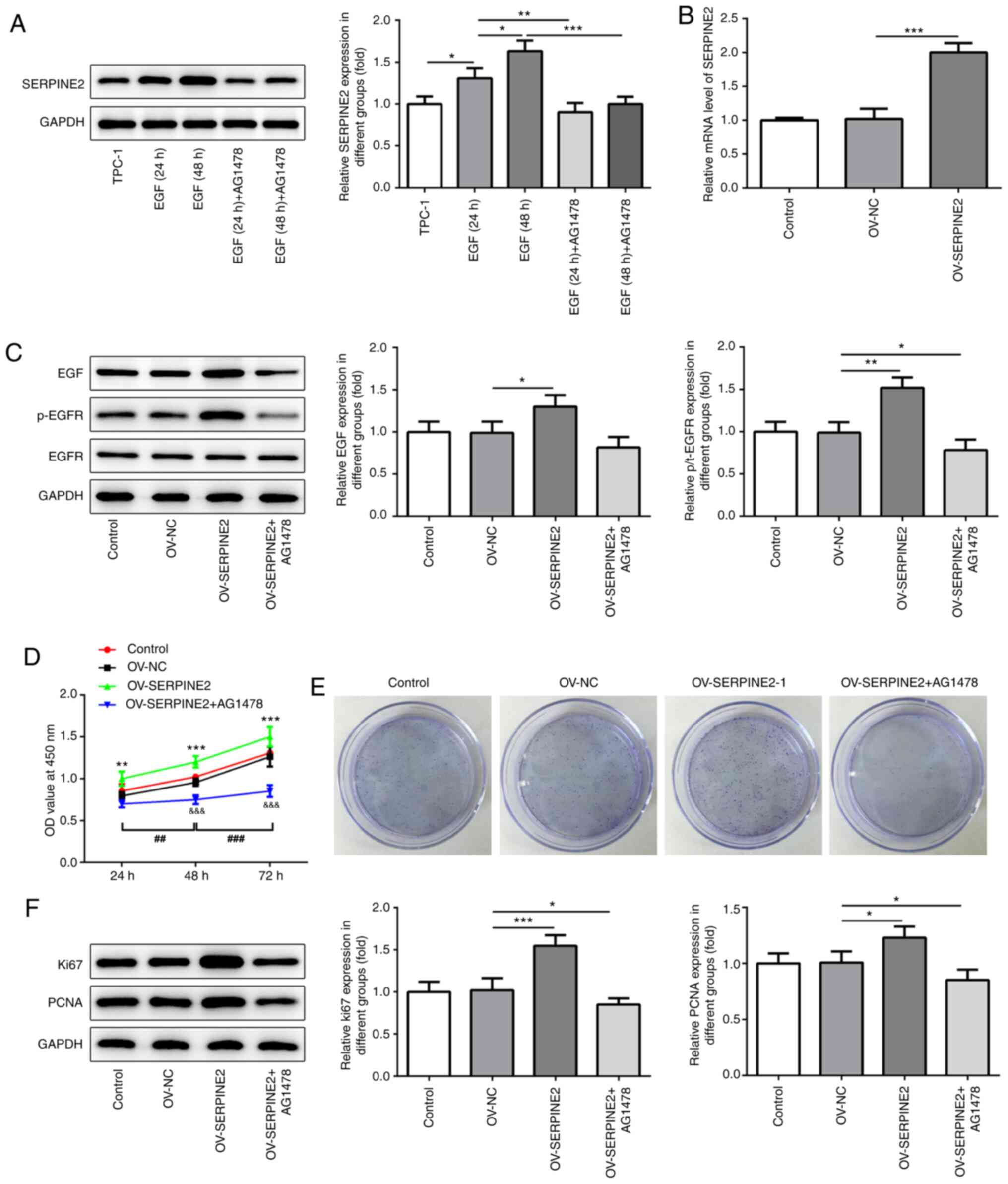 | Figure 5.AG1478 inhibits the effects of
SERPINE2 overexpression. (A) Western blotting and (B) reverse
transcription-quantitative PCR demonstrated that EGF/EGFR signaling
regulated SERPINE2 expression, as indicated by the addition of EGF
and the EGFR inhibitor AG1478. (C) AG1478 counteracted the effects
of SERPINE2 overexpression on inducing the expression of EGF and
p-EGFR. AG1478 blocked the effects of AERPINE2 overexpression on
the proliferation of TPC-1 cells, which was analyzed using (D) MTT
assay. **P<0.01, ***P<0.001 vs. OV-NC;
&&&P<0.001 vs. OV-SERPINE2;
##P<0.01, ###P<0.001 vs. 48 h. (E)
Clone formation assays. (F) SERPINE2 overexpression regulated the
expression of Ki67 and PCNA via EGF/EGFR signaling. *P<0.05,
**P<0.01, ***P<0.001. SERPINE2, serpin peptidase inhibitor
clade E member 2; OV, overexpression plasmid; NC, negative control;
EGF, epidermal growth factor; EGFR, epidermal growth factor
receptor; p-, phosphorylated; PCNA, proliferating cell nuclear
antigen. |
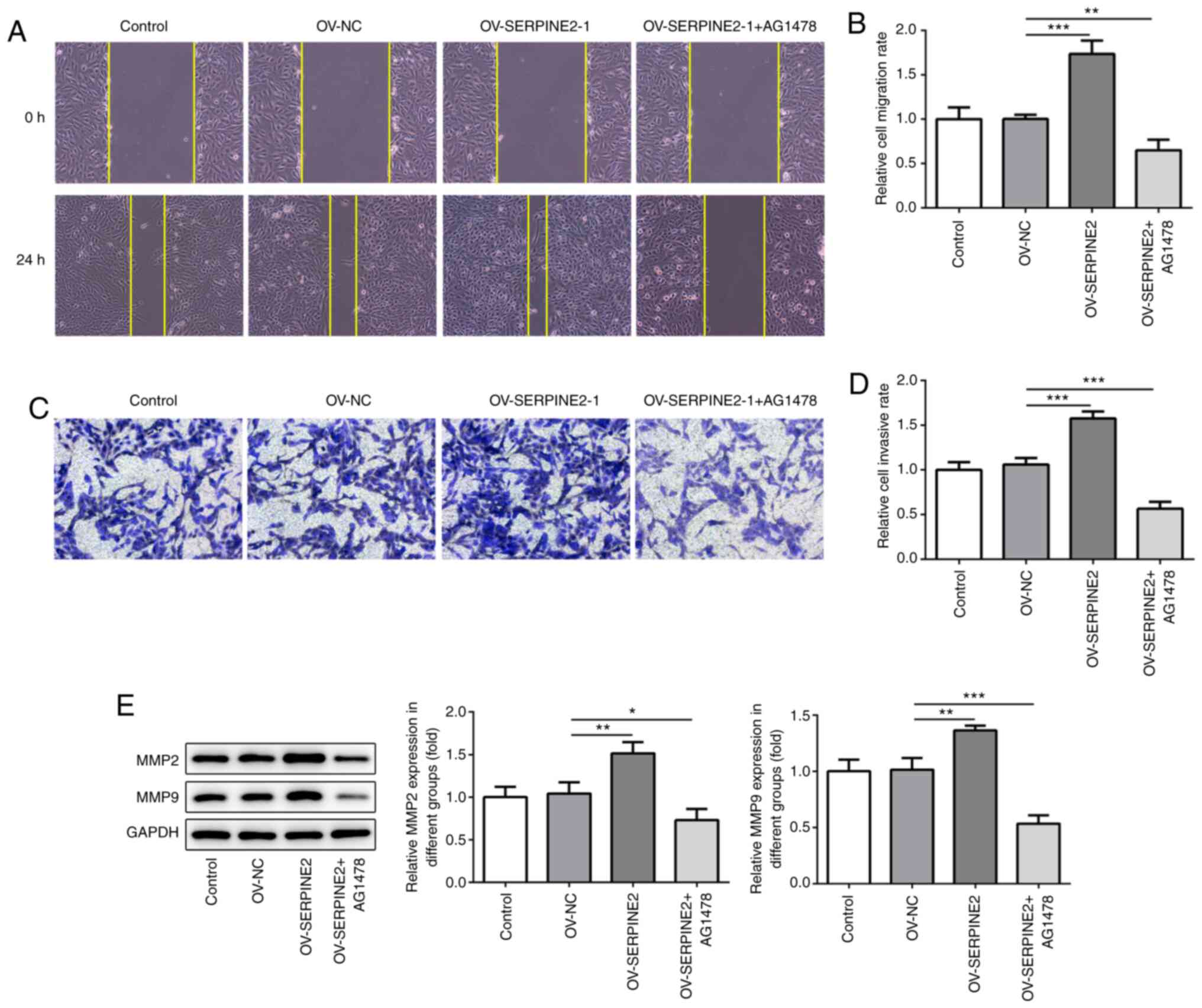
SERPINE2 modulates the migratory and
invasive abilities of TPC-1 cells via EGF/EGFR signaling
To investigate whether SERPINE2 regulated the
migratory and invasive abilities of TPC-1 cells wound healing and
Transwell assays were performed (Fig.
6A-D). SERPINE2 overexpression significantly increased the
migratory and invasive abilities of TPC-1 cells after transfection
for 72 h, which could be significantly abolished by inhibiting
EGFR. Moreover, MMP2 and MMP9 expression was significantly
increased by overexpression of SERPINE2 (Fig. 6E). Simultaneously, AG1478
significantly reversed the effect of SERPINE2 overexpression on
MMP2 and MMP9 expression.
Discussion
To the best of our knowledge, the present study was
the first to investigate the association between SERPINE2 and
EGF/EGFR in TPC-1 cells. A potential feedback loop between SERPINE2
and EGF/EGFR was demonstrated. This study found that SERPINE2
exhibited higher protein expression in TPC-1 cells compared with
Nthy-ori 3-1 or other TC cells, indicating that SERPINE2 had a
potential role in TPC-1 cells. It was further found that SERPINE2
could regulate TPC-1 cell proliferation, apoptosis, invasion and
migration via EGF/EGFR signaling. Regarding the association between
EGF and SERPINE2, a previous study demonstrated that EGF could
upregulate PN-1 levels through EGFR/protein kinase C δ type/MEK/ERK
(18). PN-1 upregulation could
further activate EGF signaling by blocking serine protease HTRA1
(8). In the present study, EGF and
p-EGFR levels were significantly decreased when TPC-1 cells were
treated with AG1478 following SERPINE2 overexpression. AG1478 was
previously used to block the phosphorylation of EGFR (19). Collectively, these results suggested
that there is a positive feedback loop between EGF and
SERPINE2.
The introduction of exogenous SERPINE2 significantly
promoted the proliferation, migration and invasion of TPC-1 cells.
In part, these results were similar to previously published data
that suggested that SERPINE2 facilitates cell migration and
invasion, but has no obvious effects on cell proliferation in
gastric cancer cells (2). As
observed in the present study, SERPINE2 regulated EGF/EGFR
signaling to accelerate cell proliferation in TPC-1 cells. The
activation of EGF/EGFR signaling is implicated in the proliferation
of TC cells (20). Overall, the
present study provided evidence for an underlying mechanism that
links SERPINE2 with EGF/EGFR signaling, revealing a positive
feedback loop between SERPINE2 and EGF/EGFR. In addition, SERPINE2
silencing notably activated TPC-1 cell apoptosis, and regulated
Bcl-2, Bax and cleaved caspase-3 expression. A previous study
demonstrated that SERPINE2 functions as an oncogene in endometrial
cancer cells and regulates cell apoptosis (21).
Ki67 frequently exhibits increased expression in
patients with anaplastic carcinoma and malignant nodule tumors
(22,23). In the present study, it was observed
that SERPINE2 knockdown reduced Ki67 and PCNA expression levels.
Ki67 is a well-known cell proliferation marker in anaplastic
thyroid carcinoma cells and is associated with the induction of
mitotic arrest (24). PCNA, which
is an auxiliary protein of DNA polymerase δ, plays an important
role in the initiation of cell proliferation (25–27).
It is a useful indicator of cell proliferation status, it is
closely associated with the clinical stage of TC, and its
expression is significantly higher in TC tissues from patients
(28). Furthermore, the expression
of PCNA is higher in thyroid nodules than in normal tissues
(29). In the present study, the
expression of Ki67 and PCNA was significantly reduced by the
silencing of SERPINE2. Therefore, SERPINE2 knockdown significantly
reduced the proliferative ability of TPC-1 cells. Besides, PN-1 has
been revealed to regulate cell invasion and migration through
MMP2/9, which degrades the extracellular matrix in C6 glioma cells
(30). The present our study also
identified a similar function of PN-1 in the regulation of MMP2/9
expression, and validated the involvement of EGF/EGFR in this
process. Furthermore, this study also found that an EGFR inhibitor
could block the effects of SERPINE2 overexpression on cell
proliferation.
Taken together, the present study confirmed that
SERPINE2 formed a positive feedback with EGF/EGFR to regulate the
proliferation, invasion and migration of TPC-1 cells, which may
provide potential new ideas for identifying novel therapeutic
targets of papillary thyroid tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC, BH and XS conceived and designed the study,
collected, analyzed and interpreted the data, and revised the
manuscript. HC wrote the manuscript. All authors read and approved
the final manuscript. HC and XS confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
Informed consent was obtained from each patient, and
the present study was approved by the Human Ethics Committee of the
Central Hospital of Wuhan (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergeron S, Lemieux E, Durand V, Cagnol S,
Carrier JC, Lussier JG, Boucher MJ and Rivard N: The serine
protease inhibitor serpinE2 is a novel target of ERK signaling
involved in human colorectal tumorigenesis. Mol Cancer. 9:2712010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang K, Wang B, Xing AY, Xu KS, Li GX and
Yu ZH: Prognostic significance of SERPINE2 in gastric cancer and
its biological function in SGC7901 cells. J Cancer Res Clin Oncol.
141:805–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng D, Gui B, Gray KP, Tinay I, Rafiei
S, Huang Q, Sweeney CJ, Kibel AS and Jia L: Secretory leukocyte
protease inhibitor is a survival and proliferation factor for
castration-resistant prostate cancer. Oncogene. 35:4807–4815. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stępień T, Brożyna M, Kuzdak K, Motylewska
E, Komorowski J, Stępień H and Ławnicka H: Elevated concentrations
of SERPINE2/protease nexin-1 and secretory leukocyte protease
inhibitor in the serum of patients with papillary thyroid cancer.
Dis Markers. 2017:49621372017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monard D: SERPINE2/Protease Nexin-1 in
vivo multiple functions: Does the puzzle make sense? Semin Cell Dev
Biol. 62:160–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nukiwa T, Suzuki T, Fukuhara T and Kikuchi
T: Secretory leukocyte peptidase inhibitor and lung cancer. Cancer
Sci. 99:849–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valiente M, Obenauf AC, Jin X, Chen Q,
Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E and Massagué
J: Serpins promote cancer cell survival and vascular co-option in
brain metastasis. Cell. 156:1002–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wagenblast E, Soto M, Gutiérrez-Ángel S,
Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY,
Dickopf S, et al: A model of breast cancer heterogeneity reveals
vascular mimicry as a driver of metastasis. Nature. 520:358–362.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Konturek A, Barczyński M, Cichoń S,
Pituch-Noworolska A, Jonkisz J and Cichoń W: Significance of
vascular endothelial growth factor and epidermal growth factor in
development of papillary thyroid cancer. Langenbecks Arch Surg.
390:216–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue L, Su D, Li D, Gao W, Yuan R and Pang
W: MiR-200 regulates epithelial-mesenchymal transition in
anaplastic thyroid cancer via EGF/EGFR signaling. Cell Biochem
Biophys. 72:185–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang T, Zhu Q, Li X, Zhu G, Deng S, Wang
Y, Ni L, Chen X, Zhang Y, Xia T, et al: Correction: Protease Nexin
I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast
cancer cells metastasis and stemness. Cell Death Dis. 11:132020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Liu ZB, Ren WM, Ye XG and Zhang
YY: The miR-200 family regulates the epithelial-mesenchymal
transition induced by EGF/EGFR in anaplastic thyroid cancer cells.
Int J Mol Med. 30:856–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Zhang X, Wang H, Ge S, Gao T, Song
L, Wang X, Li H, Qin Y and Zhang Z: HCRP1 downregulation promotes
hepatocellular carcinoma cell migration and invasion through the
induction of EGFR activation and epithelial-mesenchymal transition.
Biomed Pharmacother. 88:421–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-DDct method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Zheng X and Li M: Correlation
analysis between the pre-operative contrast-enhanced ultrasound
parameters and biological characteristics of papillary thyroid
carcinoma and associated risk factors for prognosis after
radiofrequency ablation. Exp Ther Med. 20:1575–1581. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta S, Patel A, Folstad A, Fenton C,
Dinauer CA, Tuttle RM, Conran R and Francis GL: Infiltration of
differentiated thyroid carcinoma by proliferating lymphocytes is
associated with improved disease-free survival for children and
young adults. J Clin Endocrinol Metab. 86:1346–1354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barabadze E, Munjishvili V and Burkadze G:
Braf antibody expression in different types of thyroid nodular
lesions. Georgian Med News. 271:107–113. 2017.
|
|
18
|
Tang T, Zhu Q, Li X, Zhu G, Deng S, Wang
Y, Ni L, Chen X, Zhang Y, Xia T, et al: Protease Nexin I is a
feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer
cells metastasis and stemness. Cell Death Dis. 10:6492019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Anneo A, Carlisi D, Emanuele S, Buttitta
G, Di Fiore R, Vento R, Tesoriere G and Lauricella M: Parthenolide
induces superoxide anion production by stimulating EGF receptor in
MDA-MB-231 breast cancer cells. Int J Oncol. 43:1895–1900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su X, Shen Z, Yang Q, Sui F, Pu J, Ma J,
Ma S, Yao D, Ji M and Hou P: Vitamin C kills thyroid cancer cells
through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways
via distinct mechanisms. Theranostics. 9:4461–4473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen Y, Wang X, Xu J and Lu L: SerpinE2, a
poor biomarker of endometrial cancer, promotes the proliferation
and mobility of EC cells. Cancer Biomark. 19:271–278. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akaishi J, Kondo T, Sugino K, Ogimi Y,
Masaki C, Hames KY, Yabuta T, Tomoda C, Suzuki A, Matsuzu K, et al:
Prognostic impact of the turin criteria in poorly differentiated
thyroid carcinoma. World J Surg. 43:2235–2244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su JJ, Hui LZ, Xi CJ and Su GQ:
Correlation analysis of ultrasonic characteristics, pathological
type, and molecular markers of thyroid nodules. Genet Mol Res.
14:9–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williamson T, Mendes TB, Joe N, Cerutti JM
and Riggins GJ: Mebendazole inhibits tumor growth and prevents lung
metastasis in models of advanced thyroid cancer. Endocr Relat
Cancer. 27:123–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bravo R, Frank R, Blundell PA and
Macdonald-Bravo H: Cyclin/PCNA is the auxiliary protein of DNA
polymerase-delta. Nature. 326:515–517. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JY, Kim KH, Lee WR, An HJ, Lee SJ, Han
SM, Lee KG, Park YY, Kim KS, Lee YS and Park KK: Apamin inhibits
PDGF-BB-induced vascular smooth muscle cell proliferation and
migration through suppressions of activated Akt and Erk signaling
pathway. Vascul Pharmacol. 70:8–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sajeevan TP, Saraswathi TR, Ranganathan K,
Joshua E and Rao UD: Immunohistochemical study of p53 and
proliferating cell nuclear antigen expression in odontogenic
keratocyst and periapical cyst. J Pharm Bioallied Sci. 6 (Suppl
1):S52–S57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang D, Yao X, Zhou J, Zhou H, Lu G and
Wang Y: Correlations of PCNA expression with thyroid cancer
ultrasound and histopathologic features. Int J Clin Exp Pathol.
12:1378–1384. 2019.PubMed/NCBI
|
|
29
|
Cornianu M, Stan V, Lazăr E, Dema A, Golu
I, Tăban S, Vlad M, Faur A, Vărcuş F and Babău F: Evaluation of
proliferation potential in thyroid normo-/hypofunctioning and
hyperfunctioning nodules. Rom J Morphol Embryol. 52:545–553.
2011.PubMed/NCBI
|
|
30
|
Pagliara V, Adornetto A, Mammì M, Masullo
M, Sarnataro D, Pietropaolo C and Arcone R: Protease Nexin-1
affects the migration and invasion of C6 glioma cells through the
regulation of urokinase plasminogen activator and matrix
metalloproteinase-9/2. Biochim Biophys Acta. 1843:2631–2644. 2014.
View Article : Google Scholar : PubMed/NCBI
|