Introduction
At present, gastric cancer (GC) is still one of the
most common malignant tumors (1)
and the second leading cause of cancer-related deaths worldwide
(2). Although the incidence rates
in Western countries have begun to decline, rates remain high in
Asian countries (3). The incidence
of GC in China accounts for >40% of the global incidence rate,
and >1.6 million individuals succumb to GC each year (4). Immune dysfunction is considered to be
involved in the occurrence and progression of tumors (5). A number of pro-inflammatory factors,
such as IL-6, IL-10 and IL-8, have been found to be significantly
elevated in tumor tissues and serum, and are usually associated
with poor prognosis (6–8).
As a member of the IL-1 family, IL-33 is originally
produced as a 30 kDa precursor protein, which is then cleaved by
caspase-1 into a 18 kDa mature and secreted form (9). IL-33 has been reported to be related
to inflammation, infectious diseases and tumor progression
(10). Elevated serum levels of
IL-33 have been demonstrated in numerous types of cancer, including
breast (11) and hepatocellular
cancer (12). However, it has been
reported that in patients with multiple myeloma, decreased plasma
levels of IL-33 are associated with more advanced stages of the
disease (13). Serum IL-33 levels
are significantly elevated in patients with GC, and elevated serum
IL-33 levels are related to infiltration, distant metastasis and
advanced stages (14,15). In GC tissues, IL-33 expression is
also significantly increased and associated with the infiltration
depth, but it does not seem to be significantly associated with the
overall survival rate of patients with GC (16). ST2 is a recognized IL-33 receptor.
Compared with IL-33, the role of ST2 in cancer has rarely been
investigated.
In the present study, the effects of the IL-33/ST2
axis on the biological functions of GC cells were examined. It was
found that the IL-33/ST2 axis contributed to GC cell proliferation,
cell cycle progression, apoptosis inhibition, invasion and
migration by inducing the activation of ERK1/2, JNK and p38.
Materials and methods
Patients and tissue samples
A total of 75 patients who were diagnosed and
underwent surgical resection of gastric carcinoma at the Affiliated
Hospital of Shandong University of Traditional Chinese Medicine
(Jinan, China) were enrolled, and GC tissue specimens and adjacent
normal tissue specimens (<3 cm) were collected. The tissue
samples were immediately frozen in liquid nitrogen and stored in a
refrigerator at −80°C. None of the patients included in this study
had received chemotherapy or radiotherapy before surgery, and all
patients signed written informed consent. Patients diagnosed with
rheumatic diseases, acute infections or other types of cancer were
also excluded. The study was approved by the Ethics Committee of
the Affiliated Hospital of Shandong University of Traditional
Chinese Medicine.
Immunohistochemical analysis
The tissues were fixed in 10% formalin at 4°C for 24
h, and then embedded in paraffin. The tissue samples were cut into
4 µm slices, and the paraffin-embedded slices were deparaffinized
in detergent and rehydrated in alcohol. Sodium citrate buffer (pH
6.0) was used for antigen retrieval. After blocking with 5% goat
serum (Thermo Fisher Scientific Inc.) at room temperature for 1 h,
the slides were incubated with anti-ST2 (1:100; cat. no. ab233433,
Abcam) for 1 h at room temperature, followed by incubation with the
horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit IgG
polymer (1:5,000; cat. no. 160101405L; Fuzhou Maixin Biotech Co.,
Ltd.) for 1 h at room temperature. The enhanced DAB chromogenic kit
(cat. no. 1705252031; Fuzhou Maixin Biotech Co., Ltd.) was used for
color development. After counterstaining with hematoxylin at room
temperature for 5 min, it was dehydrated in gradient ethanol and
fixed with neutral resin. Then, slides were observed and imaged
under a light microscope. The ST2 immunostaining score was the sum
of the staining intensity score and the positive staining cell rate
score. The staining intensity was scored as follows: i) 0, no
staining; ii) 1, weak staining; iii) 2, moderate staining; and iv)
3, strong staining. The positive staining cell rate was scored as
follows: i) 0, 0–5%; ii) 1, 5–25%; iii) 2, 26–50%; iv) 3, 51–75%;
and v) 4, >75%. A score <2 points was considered as ST2 low
expression, >3 points as ST2 high expression.
Cell culture and transfection
Human GC cell lines, AGS and MKN45, were obtained
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences and 1×105 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Hyclone; Cytiva)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 0.1 mg/ml streptomycin (Sigma-Aldrich; Merck
KGaA) in 6-well plates. Cells were maintained in a humidified
incubator at 37°C with 5% CO2. AGS and MKN45 cells were
incubated with IL-33 at a final concentration of 10 ng/ml.
Cells in the logarithmic growth phase were
transfected with control small interfering (si)RNA (NC;
5′-AAUUCUCCGAACGUGUCACGU-3′) or ST2 siRNA (siST2;
5′-CCAGAAAGGCCUCUAGUUUUU-3′) (6 µg) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C. After 6
h of transfection, the medium was changed and the cells were
cultured for 24 h. Then, the subsequent experiments were performed.
The ST2 siRNA and negative control (NC) siRNA were designed and
obtained from Shanghai GenePharma Co., Ltd.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from the cells was extracted using the
Ultrapure RNA extraction kit (CoWin Biosciences), and reverse
transcribed into cDNA using the HiFiScript cDNA Synthesis kit
(CoWin Biosciences) at 37°C for 30 min. RT-qPCR was performed with
the Magic SYBR mixture (CoWin Biosciences) and a StepOne™ Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The cycling conditions were: Denaturation at 95°C for 5 min,
followed by 40 cycles of 95°C for 30 sec, 60°C for 45 sec and 72°C
for 30 sec. The ST2 siRNA was designed and obtained from Invitrogen
(Thermo Fisher Scientific, Inc.) with the sequence
5′-CCAGAAAGGCCUCUAGUUUUU-3′. The relative expression of ST2 was
quantified using the 2−ΔΔCq method (17) and normalized to the expression of
β-actin.
Western blotting
Total protein in cells was extracted using RIPA
buffer containing protease cocktail inhibitor I (Calbiochem; Merck
KGaA). Protein (15 µg) was separated via SDS-PAGE on a 10% gel, and
subsequently electroblotted onto a PVDF membrane. After blocking
with 5% non-fat milk for 1 h at room temperature, the PVDF membrane
was incubated with the primary antibodies against ST2 (1:1,000;
cat. no. ab233433, Abcam), CDK4 (1:2,000; cat. no. ab108357;
Abcam), CDK6 (1:2,000; cat. no. ab124821; Abcam), cyclin D1
(1:2,000; cat. no. ab134175; Abcam), Bcl-2 (1:2,000; 60178–1-Ig;
ProteinTech Group Inc.), Bax (1:1,000; 50599-2-Ig; ProteinTech
Group Inc.), caspase-3 (1:1,000; 19677-1-AP; ProteinTech Group
Inc.); ERK (1:1,000; cat. no. ab32537; Abcam), p-ERK (1:1,000; cat.
no. ab192591; Abcam), JNK (1:1,000; cat. no. ab179461; Abcam),
p-JNK (1:1,000; cat. no. ab124956; Abcam), p38 (1:1,000; cat. no.
ab170099; Abcam), p-p38 (1:1,000; cat. no. ab178867; Abcam), and
GAPDH (1:5,000; cat. no. ab8245; Abcam) at 4°C overnight, and then
incubated with the anti-rabbit IgG (1:2,000; cat. no. GTX300119;
GeneTex, Inc.) or anti-mouse IgG (1:2,000; cat. no. GTX300120;
GeneTex, Inc.) secondary antibodies for 1 h at room temperature.
The protein bands were visualized using a Pierce™ ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). Bands were
semi-quantified using ImageJ software v1.8.0 (National Institutes
of Health). GAPDH was used as the internal reference.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was assessed using a CCK-8 assay.
A total of 2,000 cells were seeded into each well of a 96-well
plate. CCK-8 solution (10 µl) (Beijing Solarbio Science and
Technology Co., Ltd.) was added into each well at different times
points (0, 24, 48 and 72 h) and cells were incubated for 2 h at
37°C. The absorbance at 450 nm was measured using a microplate
reader.
Cell cycle analysis using flow
cytometry
After 48 h of treatment, the cells were trypsinized
with EDTA-free trypsin and resuspended in cold PBS. Then, the cells
were fixed in cold 70% ethanol at −20°C for 24 h. The fixed cells
were incubated with RNase A at room temperature for 30 min, and
stained with propidium iodide for another 30 min at room
temperature in the dark using Cell Cycle Analysis Kit (Beyotime
Institute of Biotechnology). Cell cycle progression was immediately
measured using a FACSCalibur Flow Cytometer (BD Biosciences), and
analyzed using FCS Express 4 software (De Novo Software).
Apoptosis analysis using flow
cytometry
Cells were stained with 5 µl Annexin V-FITC (BD
Biosciences) at room temperature for 5 min. Then 10 µl propidium
iodide (PI) and 400 µl PBS buffer were added to the cell
suspension. The early and late apoptosis was detected using a BD
FACSCanto™ II (BD Biosciences) flow cytometer. Data were collected
and analyzed using FlowJo software v.4.5 (FlowJo LLC).
Invasion assay
The Transwell chambers (BD Biosciences) coated with
Matrigel at 37°C for 4 h were used to measure cell invasion. Cells
transfected with control siRNA or ST2 siRNA for 12 h were digested
and resuspended into cell suspension at a density of
1×106 cells/ml. Cell suspension (200 µl) with serum-free
medium was loaded into the upper chamber. IL-33 was added or not
added to the upper chamber with a final concentration of 10 ng/ml
at 37°C for 24 h. The lower chambers were filled with 600 µl DMEM
containing 10% FBS to induce cell invasion. After incubation at
37°C for 24 h, cells on the upper surface of the chambers were
removed with a cotton swap. The invading cells on the lower surface
of the chambers were fixed with 4% paraformaldehyde for 30 min at
room temperature and stained with 0.1% crystal violet at room
temperature for 10 min. The number of cells was counted under a
light microscope (magnification, ×200, Nikon TE2000; Nikon
Corporation).
Migration assays
A wound healing assay was performed to evaluate the
migratory ability of GC cells. AGS and MKN45 cells were grown to
95% confluence in 6-well plates. A sterile plastic tip was used to
create the wound. After washing with PBS, the cells were cultured
for 48 h in serum-free medium. Images were taken using a light
microscope (magnification, ×100). The average of five random widths
of each wound was measured for quantitation.
Statistical analysis
All data analysis was performed as the mean ± SD
using GraphPad software Prism 8 (GraphPad Software, Inc.) and SPSS
13.0 (SPSS, Inc.). All experiments were repeated at least three
times. The differences between two groups were analyzed using a
Student's t-test, and the statistical differences among multiple
groups were assessed by one-way analysis of variance followed by
Tukey's post hoc test. The data in Table I were analyzed by the χ2
test and those in Table II were
analyzed by the Fishers test. P<0.05 was considered to indicate
a statistically significant difference.
 | Table I.ST2 expression in GC compared with
para-carcinoma tissues. |
Table I.
ST2 expression in GC compared with
para-carcinoma tissues.
|
|
| ST2 expression |
|
|---|
|
|
|
|
|
|---|
| Group | n | Low, n (%) | High, n (%) | P-value |
|---|
| GC | 75 | 31 (41.3) | 44 (58.6) | 0.001a |
| Para-carcinoma | 75 | 52 (69) | 23 (30.6) |
|
 | Table II.Association between ST2 expression and
the clinicopathological parameters of GC. |
Table II.
Association between ST2 expression and
the clinicopathological parameters of GC.
|
|
| ST2 expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | Low, n (%) | High, n (%) | P-value |
|---|
| Sex |
|
|
| 0.2471 |
|
Male | 56 | 21 (37.5) | 35 (62.5) |
|
|
Female | 19 | 10 (52.6) | 9
(47.4) |
|
| Age, years |
|
|
| 0.9144 |
|
<60 | 32 | 13 (40.7) | 19 (59.3) |
|
|
≥60 | 43 | 18 (42.9) | 25 (58.1) |
|
| Tumor diameter,
cm |
|
|
| 0.698 |
|
<5 | 31 | 12 (38.7) | 19 (61.3) |
|
| ≥5 | 44 | 19 (43.2) | 25 (56.8) |
|
| Pathological
grading |
|
|
| 0.3496 |
|
I–III | 49 | 19 (38.8) | 30 (61.2) |
|
|
III–IV | 26 | 13 (50) | 13 (50) |
|
Results
ST2 is highly expressed in GC
tissues
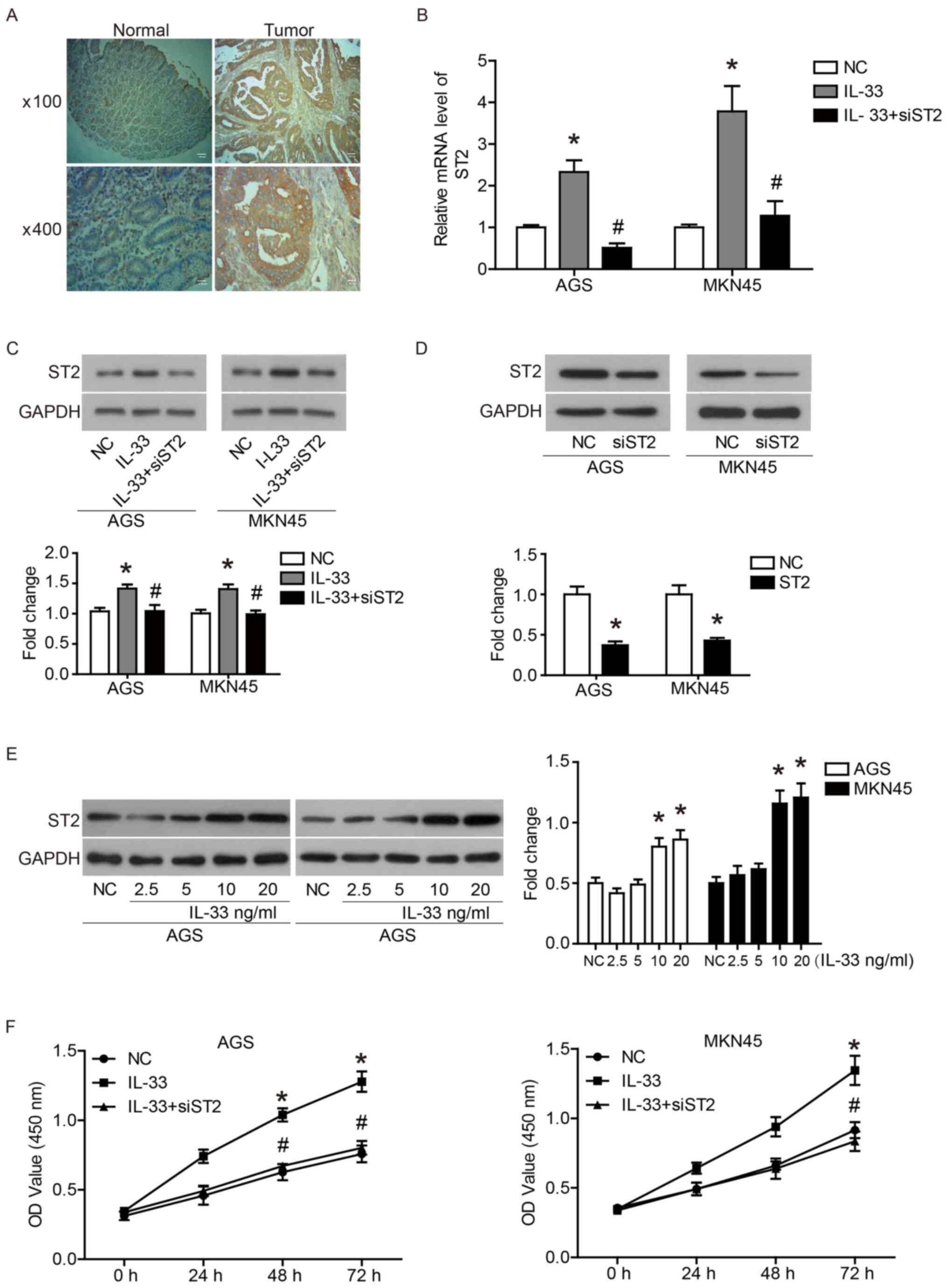
First, the expression of ST2 in GC tissues and
adjacent normal tissue was examined by immunohistochemical
analysis. As shown in Fig. 1A and
Table I, ST2 was expression was
significantly higher in GC tissues compared with normal tissues. It
was found that the expression of ST2 had no association with the
sex, age, tumor size and pathological grade of patients with GC
(Table II).
IL-33 induces ST2 expression in GC
cells
The human GC cell lines, AGS and MKN45, were
selected for subsequent experiments. As shown in Fig. 1B and C, IL-33 (10 ng/ml) induced ST2
mRNA and protein expression in AGS and MKN45 cells. Under IL-33
stimulation, transfection of siST2 significantly inhibited ST2 mRNA
and protein expression in AGS and MKN45 cells (Fig. 1B and C). Without the addition of
IL-33, transfection of siST2 also significantly reduced the
expression of ST2 (Fig. 1D). In
addition, IL-33 stimulated the expression of ST2 in a
dose-dependent manner (Fig.
1E).
IL-33/ST2 axis promotes GC cell
proliferation by regulating the cell cycle
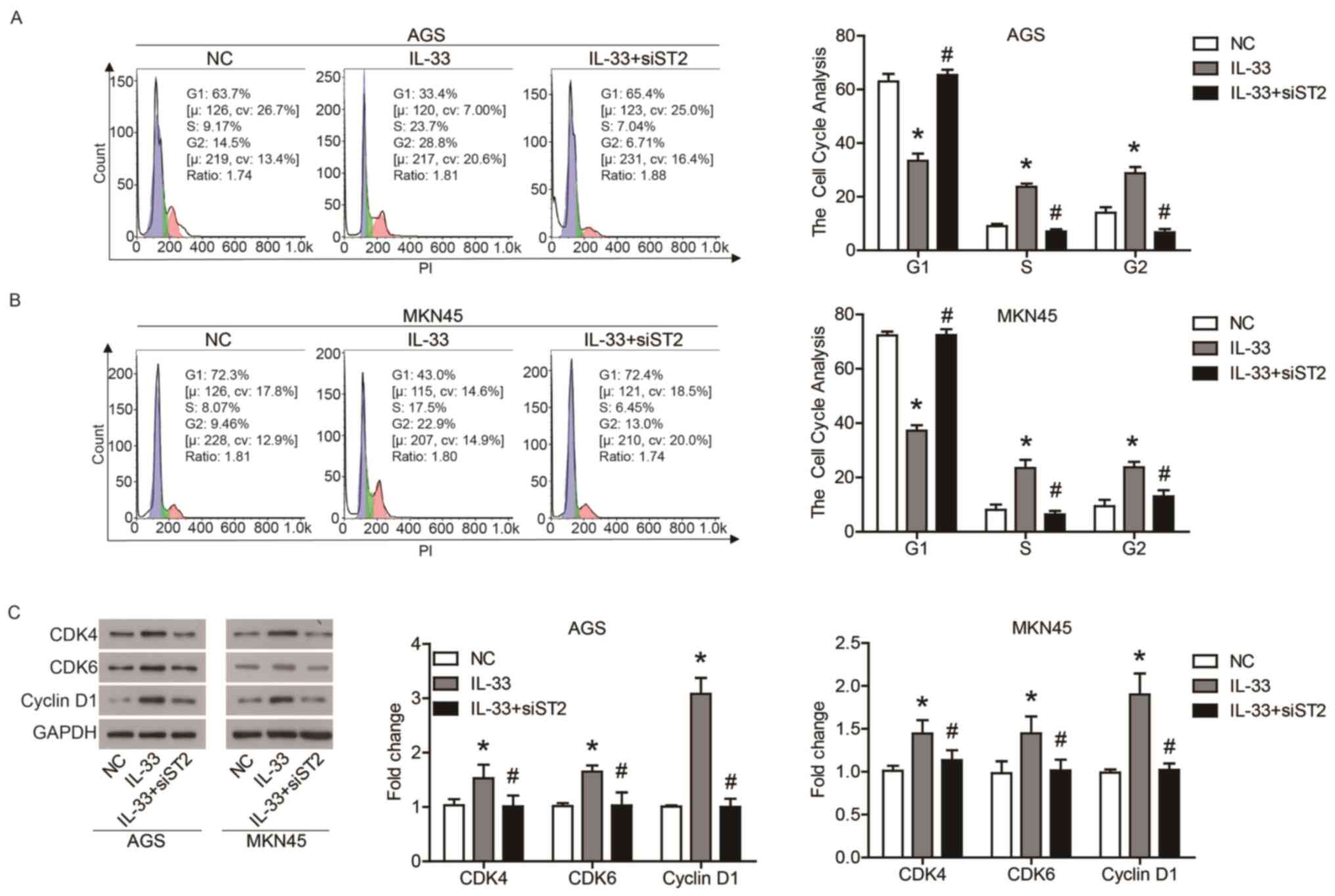
In order to investigate the role of IL-33 and ST2 in
the biological function of GC cells, the proliferation and cell
cycle of AGS and MKN45 cells were examined. As shown in Fig. 1F, IL-33 promoted the proliferation
of AGS and MKN45 cells compared with the NC cells, and the
promotion of IL-33 on cell proliferation was reduced by the
transfection of siST2. Furthermore, IL-33 stimulation increased the
ratio of S phase and G2 phase cells, and decreased the ratio of G1
phase cells, which was offset by the transfection of siST2
(Fig. 2A and B). In addition, IL-33
stimulation upregulated the expression of CDK4, CDK6 and cyclin D1,
which drive cell cycle progression (Fig. 2C). Transfection of siST2 reduced the
regulation of IL-33 on cell cycle-associated proteins (Fig. 2C). These results indicated that the
IL-33/ST2 axis promoted the proliferation of GC cells by regulating
the cell cycle.
IL-33 inhibits GC cell apoptosis via
the ST2 receptor
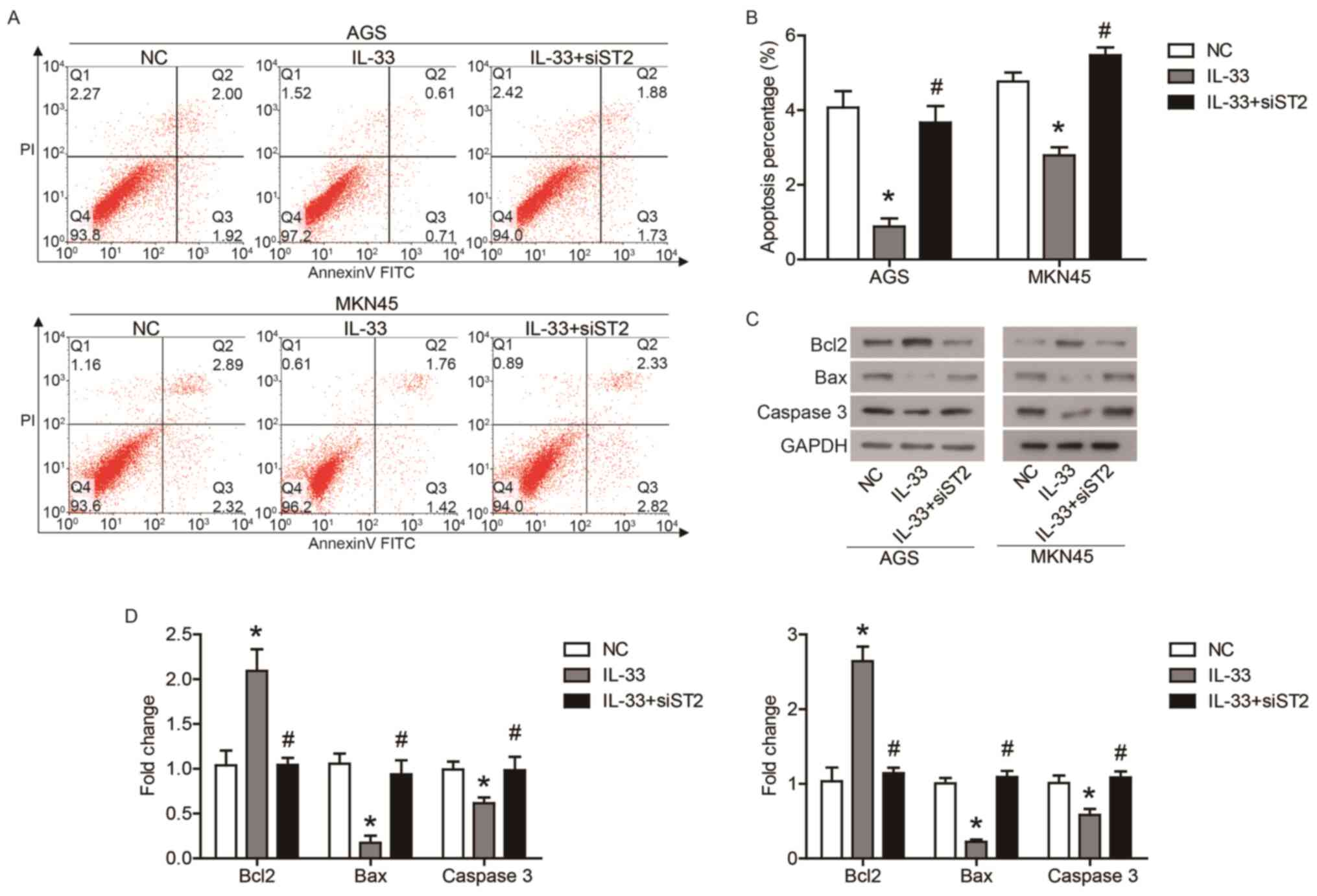
Furthermore, the effects of IL-33 and ST2 on GC cell
apoptosis were determined using flow cytometry. As shown in
Fig. 3A and B, IL-33 inhibited the
apoptosis of AGS and MKN45 cells, while the transfection of siST2
counteracted the effect of IL-33 on apoptosis. In addition, IL-33
stimulation increased protein expression of Bcl2, and decreased the
protein expression of Bax and caspase-3, which was also affected by
transfection of siST2 (Fig. 3C and
D). These data demonstrated that IL-33 inhibited GC cells
apoptosis through the ST2 receptor.
ST2 receptor contributes to
IL-33-induced GC cell invasion and migration
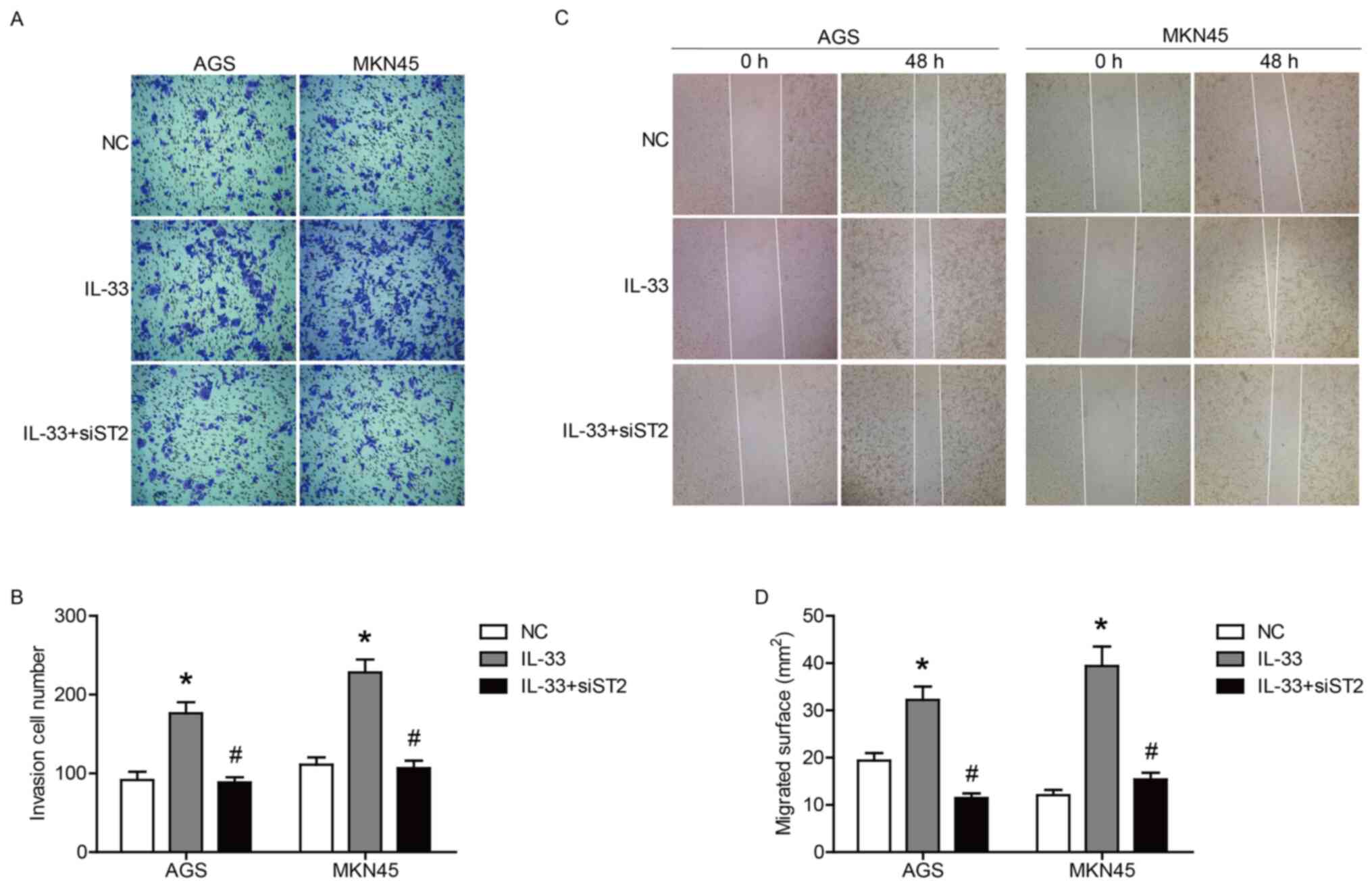
To clarify the role of IL-33 and ST2 in the invasion
and migration of GC cells, Transwell and wound healing assays were
performed. The results of the Transwell assay showed that IL-33
stimulated the invasion of AGS and MKN45 cells (Fig. 4A and B), whereas the invasive
ability of both cells induced by IL-33 was significantly inhibited
by the transfection of siST2 (Fig. 4A
and B). In addition, the transfection of siST2 also reduced the
migration of both cell lines stimulated by IL-33 (Fig. 4C and D). Taken together, these data
indicated that IL-33 promoted the invasion and migration of GC
cells through the ST2 receptor.
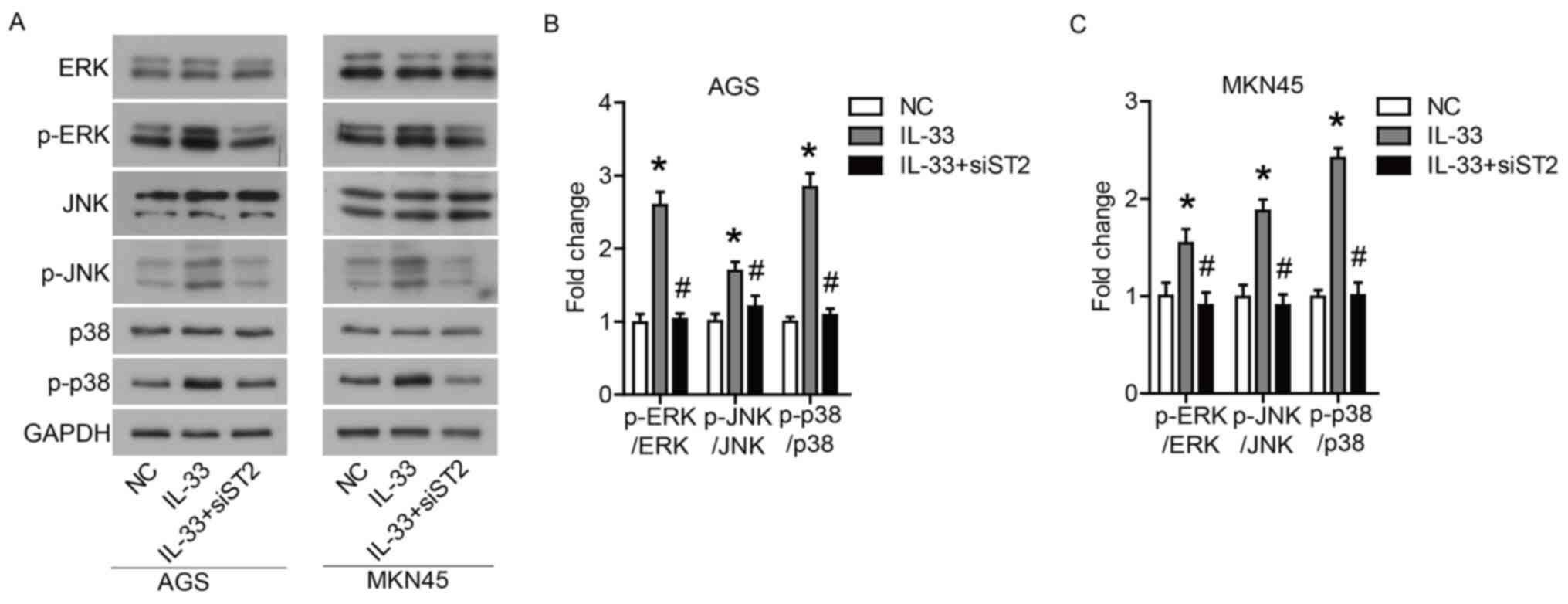
IL-33 induces the activation of
ERK1/2, JNK and p38 via the ST2 receptor
Finally, the signaling pathways affected by IL-33
stimulation in GC cells were investigated. Western blotting showed
that IL-33 stimulation increased the phosphorylation levels of
ERK1/2, JNK and p38. Furthermore, it was found that the
transfection of siST2 significantly inhibited the IL-33-induced
activation of ERK1/2, JNK and p38 (Fig.
5A-C). These data suggested that IL-33 could induce the
activation of ERK1/2, JNK and p38 through the ST2 receptor.
Discussion
In the present study, it was found that ST2 was
expressed significantly higher in GC tissues, and IL-33 could
induce ST2 expression in GC cells. The IL-33/ST2 axis promoted GC
cell proliferation, cell cycle, apoptosis inhibition, and invasion
and migration by inducing the activation of ERK1/2, JNK and
p38.
The pro-inflammatory cytokine IL-33 is related to
the occurrence and development of GC (4,5).
Overexpression of IL-33 has been observed in a number of types of
tumors, including lung cancer and uterine leiomyoma (18,19).
However, little is known concerning the expression pattern of ST2
in tumor tissues. Although Bergis et al (3) demonstrated that serum ST2 levels in
patients with GC are significantly higher than those in patients
with gastritis or healthy volunteers. Moreover, ST2 serum levels
may be significantly related to advanced or metastatic disease, as
well as disease duration (3). The
present study found that ST2 was significantly upregulated in GC
tissues, although its expression level did not have a significant
association with tumor size or pathological stage. Bai et al
(20) suggested that the expression
of ST2 protein in primary GC tissues is markedly lower than that in
adjacent non-cancerous tissues, and may act as a tumor suppressor
gene in GC, which is inconsistent with the present research. The
controls in the studies of Bergis et al (3) and Bai et al (20) were adjacent tissues rather than
normal tissues. Based on the complexity of the tumor
microenvironment and the role of IL-33/ST2 axis in the
para-cancerous mucosa, there may be individual differences in the
ST2 expression in the para-cancerous tissues. The ST2 gene is
widely expressed in different cell types, including immune and
non-immune cells (21). Therefore,
the source of soluble ST2 in serum is still uncertain. ST2 present
in GC cells includes a full-length transmembrane form, a soluble
secreted form, and other variants. In addition, the present study
also found that IL-33 stimulation could induce ST2 expression.
In the present study, it was discovered that the
IL-33/ST2 axis regulated the expression of cell cycle-associated
proteins (CDK4, CDK6 and cyclin D1), thereby promoting cell cycle
progression and ultimately affecting GC cell proliferation. In
addition, it was also confirmed that the IL-33/ST2 axis could
inhibit the apoptosis of GC cells. This seems to be consistent with
research by Ye et al (4),
which reported that IL-33 can protect GC cells from apoptosis
induced by chemotherapy drugs. These data indicated that IL-33/ST2
is critical for the survival of GC cells.
The IL-33/ST2 axis is involved in the interaction
between cancer cells and the tumor microenvironment. Retrospective
studies of human GC have reported that tumor-adjacent, submucosal
mast cells promote the growth of GC and participate in the
formation of advanced disease and metastasis (22). Furthermore, a previous study
suggested that mast cells recruit tumor-associated macrophages via
the gastric cancer cell-derived IL-33/ST2 axis to promote tumor
cell proliferation and angiogenesis (23). In fact, IL-33 is released by damaged
epithelial cells as an endogenous danger signal that activates the
innate immune response (9). IL-33
is reported to bind to a heterodimeric receptor complex composed of
ST2 and IL-1R accessory proteins (24), and activate NK-κB (25), PI3K/AKT (26) and mitogen-activated proteins kinases
(MAPKs) (27). Activated MAPK can
transmit extracellular signals, and regulate cell growth,
proliferation, differentiation, migration and apoptosis (27). The MAPK pathway is involved in
extracellular signal-regulated kinases: ERK1/2, JNK and p38 kinase
(27). In the present study, it was
found that IL-33 could induce the activation of ERK1/2, JNK and p38
via the ST2 receptors in GC cells. Ye et al (4) demonstrated that JNK may be a key
factor underlying the effect of IL-33 on GC cell
chemotherapy-induced apoptosis. Furthermore, it was also reported
that ERK1/2 activation is required for IL-33/ST2 axis-induced
invasion and migration of GC cells (5).
In view of the role of the IL-33/ST2 axis in
promoting the progression of GC, therapeutic inhibition of
signaling is a future direction for research. In addition, in order
to clarify the molecular and cellular immune functions of IL-33 and
ST2 in GC, further studies are required in the local tumor
environment.
In conclusion, it was found that IL-33/ST2
contributed to the malignant behaviors of GC cells by inducing the
activation of ERK1/2, JNK and p38. This axis could become a
potential effective target for the treatment of GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Program
of National Natural Science Foundation of China (grant no.
81202839).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NH, XC, WL, CZ, LL and JL generated the hypothesis,
designed and performed the experiments, analyzed the data, provided
conceptual advice and technical expertise and edited the
manuscript. NH conceived and supervised the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Shandong University of
Traditional Chinese Medicine (Jinan, China). All patients provided
written informed consent prior to enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergis D, Kassis V and Radeke HH: High
plasma sST2 levels in gastric cancer and their association with
metastatic disease. Cancer Biomark. 16:117–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye XL, Zhao YR, Weng GB, Chen YC, Wei XN,
Shao JP and Ji H: IL-33-induced JNK pathway activation confers
gastric cancer chemotherapy resistance. Oncol Rep. 33:2746–2752.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ and
Hu WH: IL-33 promotes gastric cancer cell invasion and migration
via ST2-ERK1/2 pathway. Dig Dis Sci. 60:1265–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikeguchi M, Hatada T, Yamamoto M, Miyake
T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H and Tatebe
S: Serum interleukin-6 and −10 levels in patients with gastric
cancer. Gastric Cancer. 12:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang JS, Bae SY, Kim HR, Kim YS, Kim DJ,
Cho BJ, Yang HK, Hwang YI, Kim KJ, Park HS, et al: Interleukin-18
increases metastasis and immune escape of stomach cancer via the
downregulation of CD70 and maintenance of CD44. Carcinogenesis.
30:1987–1996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haghshenas MR, Hosseini SV, Mahmoudi M,
Saberi-Firozi M, Farjadian S and Ghaderi A: IL-18 serum level and
IL-18 promoter gene polymorphism in Iranian patients with
gastrointestinal cancers. J Gastroenterol Hepatol. 24:1119–1122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mirchandani AS, Salmond RJ and Liew FY:
Interleukin-33 and the function of innate lymphoid cells. Trends
Immunol. 33:389–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Shen JX, Hu JL, Huang WH and Zhang
GJ: Significance of interleukin-33 and its related cytokines in
patients with breast cancers. Front Immunol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohran ZY, Ali-Eldin FA and Abdel Aal HA:
Serum interleukin-18: Does it have a role in the diagnosis of
hepatitis C virus related hepatocellular carcinoma? Arab J
Gastroenterol. 12:29–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musolino C, Allegra A, Profita M, Alonci
A, Saitta S, Russo S, Bonanno A, Innao V and Gangemi S: Reduced
IL-33 plasma levels in multiple myeloma patients are associated
with more advanced stage of disease. Br J Haematol. 160:709–710.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serum interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu W and Wu C, Li X, Zheng Z, Xie Q, Deng
X, Jiang J and Wu C: Serum IL-33 level is a predictor of
progression-free survival after chemotherapy. Oncotarget.
8:35116–35123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu W, Li X, Li Q, Tan Y, Xu B, Xie Q, Deng
X, Lu B, Jiang J and Wu C: Interleukin-33 expression does not
correlate with survival of gastric cancer patients. Pathol Oncol
Res. 23:615–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santulli P, Even M, Chouzenoux S,
Millischer AE, Borghese B, de Ziegler D, Batteux F and Chapron C:
Profibrotic interleukin-33 is correlated with uterine leiomyoma
tumour burden. Hum Reprod. 28:2126–2133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Liu XK, Chu Z, Ye JC, Li KL,
Zhuang WL, Yang DJ and Jiang YF: Detection of interleukin-33 in
serum and carcinoma tissue from patients with hepatocellular
carcinoma and its clinical implications. J Int Med Res.
40:1654–1661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai F, Ba F, You Y, Feng Y, Tao W, Wu C,
Jiu M and Nie Y: Decreased ST2 expression is associated with
gastric cancer progression and pathogenesis. Oncol Lett.
17:5761–5767. 2019.PubMed/NCBI
|
|
21
|
Trajkovic V, Sweet MJ and Xu D: T1/ST2-an
IL-1 receptor-like modulator of immune responses. Cytokine Growth
Factor Rev. 15:87–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y, Wu K, Cai K, Zhai R, Tao K, Wang G
and Wang J: Increased numbers of gastric-infiltrating mast cells
and regulatory T cells are associated with tumor stage in gastric
adenocarcinoma patients. Oncol Lett. 4:755–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eissmann MF, Dijkstra C, Jarnicki A,
Phesse T, Brunnberg J, Poh AR, Etemadi N, Tsantikos E, Thiem S,
Huntington ND, et al: IL-33-mediated mast cell activation promotes
gastric cancer through macrophage mobilization. Nat Commun.
10:27352019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chackerian AA, Oldham ER, Murphy EE,
Schmitz J, Pflanz S and Kastelein RA: IL-1 receptor accessory
protein and ST2 comprise the IL-33 receptor complex. J Immunol.
179:2551–2555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Wang F, Fan L, Zhang W, Wang T, Du Y
and Bai X: Baicalin alleviates atherosclerosis by relieving
oxidative stress and inflammatory responses via inactivating the
NF-κB and p38 MAPK signaling pathways. Biomed Pharmacother.
97:1673–1679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Arsenijevic NN and Lukic ML: IL-33/ST2 axis in innate and
acquired immunity to tumors. Oncoimmunology. 1:229–231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|