Introduction
Acute lung injury (ALI) is a pulmonary inflammatory
response produced by inflammatory mediators and effector cells. It
is characterized by decreased lung capacity and an imbalance in
ventilatory flow (1). The
inflammatory response in ALI results from a signaling cascade with
amplified secondary damage, which may be accompanied by systemic
immune inflammatory disorders. The onset and progression of ALI are
rapid, the prognosis is extremely poor, and the mortality rate may
be >30% (2).
The primary pathological features of ALI include the
destruction of microvascular endothelial cells and alveolar
epithelial cells, increased permeability of the alveolar-capillary
barrier and infusion of protein-rich exudate into the alveoli,
leading to pulmonary edema and hyaline membrane formation. The loss
of control of the intrapulmonary inflammatory response mediated by
inflammatory cells, such as polymorphonuclear (PMN) leukocytes and
macrophages, is one of the primary pathogenic processes of ALI.
When stimulated by non-cardiac factors, such as sepsis, trauma,
shock and disseminated intravascular coagulation, the
monocyte/macrophage system can become activated and release large
numbers of inflammatory cytokines, including IL-1, IL-8, IL-6,
IL-10 and TNF-α; the majority of which are pro-inflammatory
cytokines. Pro-inflammatory cytokines further activate PMN
leukocytes and macrophages, resulting in a severe imbalance between
pro- and anti-inflammatory cytokines. Studies have reported that
the upregulation of IL-6, IL-8 and TNF-α is positively correlated
with mortality in ALI (3,4). In addition, the expression levels of
cell adhesion molecules, such as cell adhesion molecule-1,
P-selectin and E-selectin, on the surface of PMN leukocytes is
upregulated, which may further aggravate pulmonary tissue
inflammatory responses (5–7). The pathophysiological mechanisms of
ALI are not only associated with inflammatory responses, but also
with the activation of lung epithelial cells and inflammatory cell
apoptosis (8,9). Albertine et al (10) demonstrated that the expression of
Fas and FasL was positively associated with clinical prognosis in
patients with ALI/acute respiratory distress syndrome. Compared
with autologous serum, the expression levels of Fas and FasL in
bronchoalveolar lavage fluid (BALF) were increased, indicating that
apoptosis primarily occurs in lung tissues.
The immune system is not isolated and it interacts
with the nervous system. When the central nervous system is
stimulated by certain forms of immune stimulation, it can activate
efferent vagus nerve fibers, leading to the release of
acetylcholine (Ach) from peripheral nerve endings and the
inhibition of the release of pro-inflammatory factors through
intracellular signal transduction, thereby mitigating an excessive
systemic inflammatory response (11–13).
In sepsis, stimulation of the vagus nerve inhibits PMN activation
and recruitment, decreases inflammation and improves survival rate
(14). Guarini et al
(15) demonstrated that stimulating
the vagus nerve could attenuate the activity of NF-κB and inhibit
the expression levels of MIP-2 and TNF-α, while simultaneously
inhibiting the inflammatory cascade triggered by these factors and
alleviating lung tissue damage. This protective effect was reversed
following the administration of nicotinic receptor antagonists.
Other studies have demonstrated that the cholinergic
anti-inflammatory pathway associated with the suppression of
inflammation is activated by the binding of acetylcholine released
from vagus nerve endings to α-7 nicotinic acetylcholine receptors
(α-7nACHR), which are found primarily on the surface of alveolar
macrophages and alveolar epithelial cells (16,17).
In a model of high tidal volume-induced lung injury (18), electrical stimulation of the vagus
nerve was shown to decrease pulmonary edema and central granulocyte
infiltration, thereby inhibiting lung tissue inflammation.
In summary, vagus nerve stimulation can decrease the
release of inflammatory factors and affect the balance of pro- and
anti-inflammatory cytokines, thus affecting the progress of ALI.
However, the specific mechanism of these effects remains to be
clarified. The present study investigated the role of the vagus
nerve in ALI by establishing a model of LPS-induced lung injury,
electrically stimulating the vagus nerve, measuring the levels of
key inflammatory factors and determining the number of apoptotic
cells in BALF. The present study aimed to provide a new theoretical
basis for the diagnosis and treatment of ALI.
Materials and methods
Experimental animals
A total of 60 healthy and clean adult male (n=30)
and female (n=30) Sprague-Dawley rats weighing 250±20 g were
provided by the Animal Science Research Department of Nanchang
University (Nanchang, China). Prior to the experiment, the animals
were caged under normal conditions [normal atmospheric pressure,
circadian rhythm (12-h light/dark cycle) and 40% humidity] and the
room temperature was maintained at 22–24°C. Stimulation with strong
light and loud noises was avoided and normal circadian rhythm was
maintained. Food and water were provided ad libitum. The present
study was approved by the Medical Research Ethics Committee of The
Second Affiliated Hospital of Nanchang University (http://www.chinalaw.gov.cn/news/node_search.html?q=%E5%8C%BB%E5%AD%A6%E4%BC%A6%E7%90%86&order=releasetime).
Animal treatment and experimental
grouping
The rats were anesthetized with intraperitoneal
injections of 40 mg/kg pentobarbital (Sinopharm Shanghai Shyndec
Pharmaceutical Co., Ltd.; batch no. F20151216). The skin was shaved
around the windpipe, the trachea opened and rats were mechanically
ventilated. Ventilator parameters were: Tidal volume 2 ml/100 g;
respiratory rate 60 beats/min; breathing ratio 1:1. The rats were
randomly divided into four groups: Normal saline group (NS group),
lipopolysaccharide group (LPS group), vagotomy group (LPS+VNB
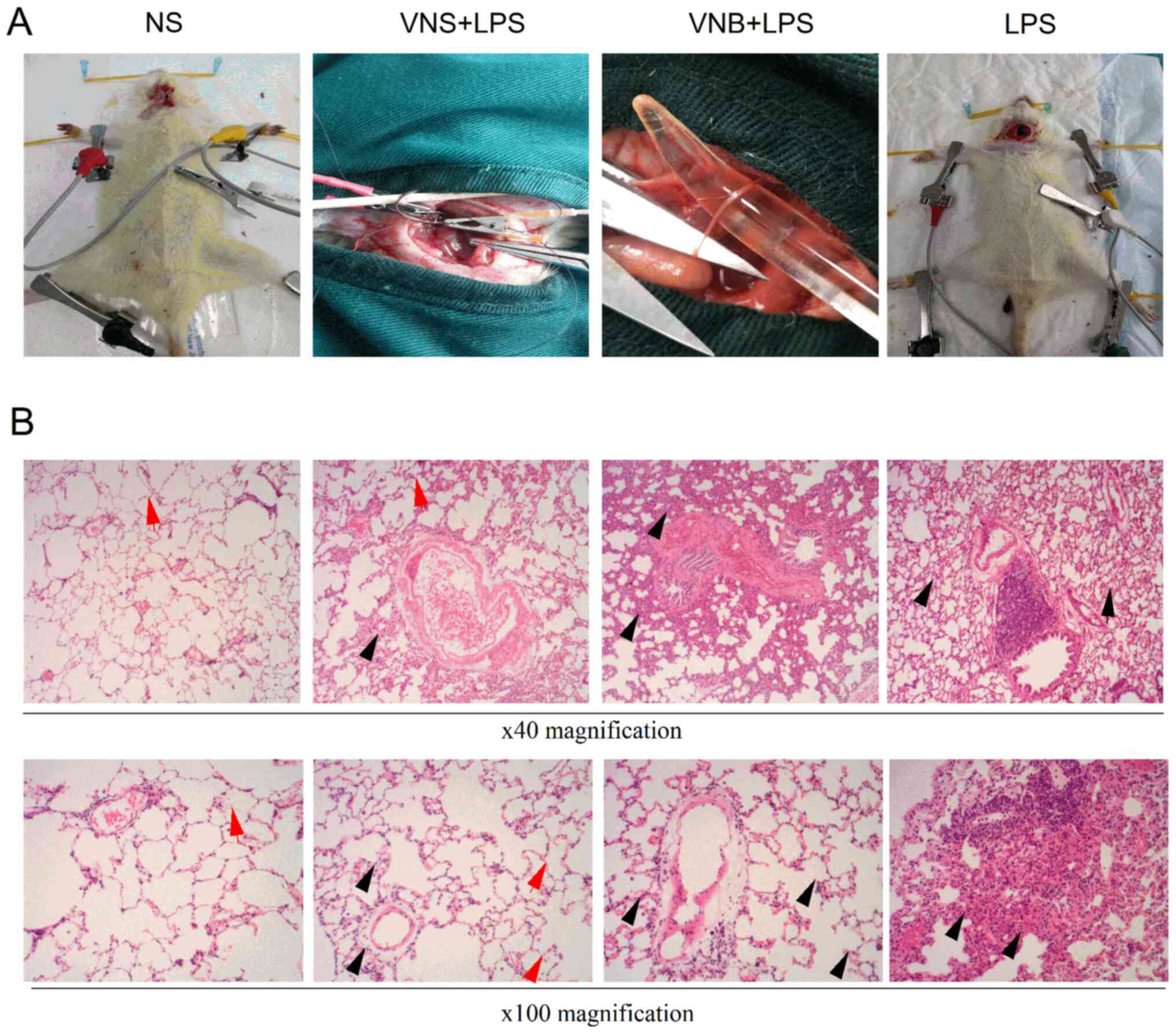
group) and vagus nerve stimulation group (LPS+VNS group; Fig. 1A). Each group included 15 rats. In
the NS group, 5 mg/kg normal saline (Beijing Solarbio Science &
Technology Co., Ltd.) was injected into the tail vein, the
bilateral carotid sheaths were incised, and the bilateral vagus
nerves were separated. In the LPS group, 5 mg/kg of 1% LPS (cat.
no. L2880; Sigma-Aldrich; Merck KGaA) was injected into the tail
vein, the bilateral carotid sheaths were incised, and the bilateral
vagus nerves were separated. In the LPS+VNB group, 5 mg/kg of 1%
LPS was injected into the tail vein, the bilateral carotid sheaths
were opened, the bilateral vagus nerve was separated, the bilateral
vagus nerve was cut and the stimulation electrode was connected to
the distal end of the left vagus nerve proximal to the heart for
continuous electrical stimulation (1 mA; 1 msec; 10 Hz). In the
LPS+VNS group, 5 mg/kg of 1% LPS was injected into the tail vein,
the bilateral carotid sheaths were opened, the bilateral vagus
nerves were separated, stimulating electrodes were connected to the
distal end of the left vagus nerve and continuous electrical
stimulation (1 mA; 1 msec; 10 Hz) was applied. After 120 min, the
ventilator was disconnected and the rats were euthanized by
cervical dislocation under 40 mg/kg pentobarbital anesthesia.
HE staining
Rat lung tissue was taken for hematoxylin and eosin
(HE) staining. Stains, Harris hematoxylin (Anatech Ltd. cat. no.
842), 1X stock for 2 min at 25°C. Eosin (Anatech cat. no. 837), 1X
stock for 15 sec at 25°C. The stained sections were observed under
an inverted light microscope (magnification ×100 and ×40; Olympus
Corporation) and images were captured.
Flow cytometry analysis of the
inflammatory cell apoptosis rate
BALF from the rats was collected in a 1.5 ml
centrifuge tube 120 min after the ventilator was disconnected and
the rats were euthanized by cervical dislocation under 40 mg/kg
pentobarbital (Sigma-Aldrich; Merck KGaA, cat. no. P0900000-1EA)
anesthesia, centrifuged at 1,006.2 × g for 20 min at 4°C
(Eppendorf), and the cell supernatant was discarded. 5 µl
FITC-conjugated Annexin V and 10 µl propidium iodide (PI) was added
to the cell pellet, incubated at 4°C for 20 min, and the
inflammatory cell apoptosis rate was measured by a flow cytometer
(BD Biosciences, US6510007B1). A minimum of 10,000 events were
acquired for each sample. The cytometer's inbuilt EXPO32 ADC
software (Beckman Coulter, Inc.) was used for analysis.
RNA extraction
Total RNA was extracted from lung tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA quality was ensured
by distinct 18S, 28S and total RNA bands separated by
electrophoresis in 1% agarose gel, The quantity of RNA was
determined by A260 measurement (Carestream Gel Logic 2200 Pro
imaging system, Carestream Molecular Imaging).
Protein extraction
Total proteins were extracted from the lung tissues
by radio immunoprecipitation assay cell lysis reagent (C3702-120
ml; Beyotime Institute of Technology) containing proteinase and
phosphatase inhibitors (Beijing Solarbio Science & Technology
Co., Ltd.) at 4°C for 30 min. The cell extracts were centrifuged at
12,000 × g for 20 min at 4°C. The supernatants containing total
proteins were mixed with an equal volume of 5× sodium dodecyl
sulfate (SDS) loading buffer (P0015L; Beyotime Institute of
Technology) and the samples were heated to 95°C for 5 min. All
procedures were performed according to the manufacturers'
instructions.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA (1 µg) extracted from lung tissues was
used for RT-qPCR using the Reverse Transcription system (Takara
Bio, Inc.) according to the manufacturer's protocol. RT-qPCR was
performed using SYBR® Premix Ex Taq II (TliRNase H Plus;
Takara Bio, Inc.) in the ABI PRISM® 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
β-actin as an internal control. Thermocycling was performed in a
final volume of 20 µl consisting of 40 cycles at 95°C for 5 sec
then 55°C for 30 sec, following an initial denaturation step at
95°C for 10 sec. The sequences of the PCR primers of IL-1, IL-6,
IL-10, TNF-α and IL-8 are listed in Table I. The results were analyzed by the
2−ΔΔCq method (19).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Sequence |
|---|
| IL-1 | (F)
5′-AGAAGCTTCCACCAATACTC-3′ |
|
| (R)
5′-AGAAGCTTCCACCAATACTC-3′ |
| IL-8 | (F)
5′-ATTTCAGCAGCTCTGTGTGAA-3′ |
|
| (R)
5′-TGAATTCTCAGCCCTCTTCAA-3′ |
| IL-10 | (F)
5′-AGGGCACCCAGTCTGAGAACA-3′ |
|
| (R)
5′-CGGCCTTGCTCTTGTTTTCAC-3′ |
| IL-6 | (F)
5′-AACTCCTTCTCCACAAGCG-3′ |
|
| (R)
5′-TGGACTGCAGGAACTCCTT-3′ |
| β-actin | (F)
5′-CATGTACGTTGCTATCCAGGC-3′ |
|
| (R)
5′-CTCCTTAATGTCACGCACGAT-3′ |
| TNF-α | (F)
5′-ATGAGCACTGAAAGCATGATC-3′ |
|
| (R)
5′-TCACAGGGCAATGATCCCAAAGTAGACCTGCCC-3′ |
Western blot analysis
Protein samples were denatured in SDS sample buffer
(125 mmol/l Tris-HCl; pH 6.8; 50% glycerol; 2% SDS; 5%
mercaptoethanol; 0.01% bromophenol blue) and subjected to SDS-PAGE
(5% concentration and 12% separation) and blotted onto Immobilon-FL
transfer membranes (EMD Millipore). The blotted membranes were
blocked at 4°C with 5% skimmed milk in Tris-buffered saline
containing 0.1% Tween-20 (Beijing Solarbio Science & Technology
Co., Ltd.) for 2 h and were subsequently incubated with primary
antibodies against IL-1 (1:500, cat. no. 50794), IL-6 (1:400, cat.
no. 12912), IL-10 (1:400, cat. no. 12163), IL-8 (1:100, ab110727
Abcam) and TNF-α (1:100, cat. no. 8184) proteins (all from Cell
Signaling Technology, Inc. unless indicated otherwise) overnight at
4°C. After three washes in Tris-buffered saline containing 0.1%
Tween-20, the PVDF membranes were incubated with anti-mouse IgG
(dilution 1:5,000, cat. no. SW1030, Beijing Solarbio Science &
Technology Co., Ltd.) for 2 h at 25°C. Then, the PVDF membranes
were soaked in chemiluminescence reagents (cat. no. SW2030, Beijing
Solarbio Science & Technology Co., Ltd.). Quantification of the
western blotting was performed using a Bio-Rad imaging system
(ChemiDoc MP v721BR06186; Bio-Rad Laboratories, Inc.).
Statistical analysis
The data were analyzed using SPSS 21.0 statistical
software (IBM Corp.). Each experiment was performed in triplicate.
The measurement data are expressed as the mean ± standard
deviation. Statistical analysis was performed to determine the
significance of the difference between groups using ANOVA with
Tukey's honestly significant difference test used as the post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HE staining to observe the extent of
lung tissue damage in each group
In the LPS group, the normal structure of the
alveoli disappeared and notable amounts of inflammatory cell
infiltration, pulmonary tissue congestion and edema were observed.
In the LPS+VNB group, the alveolar structure was destroyed,
inflammatory cell infiltration was observed, the lung tissue was
congested and the alveolar wall and the interstitial space
exhibited edema and increased thickness. In the LPS+VNS group, the
alveolar structure was clearly distinguishable by different degrees
of inflammatory cell infiltration and alveolar structure damage), a
small amount of inflammatory cell infiltration was observed, there
was slight thickening of the alveolar wall and lung interstitial
space and a small amount of congestion in the lung tissue (Fig. 1B).
Detection of inflammatory cell
apoptosis rates in BALF by flow cytometry
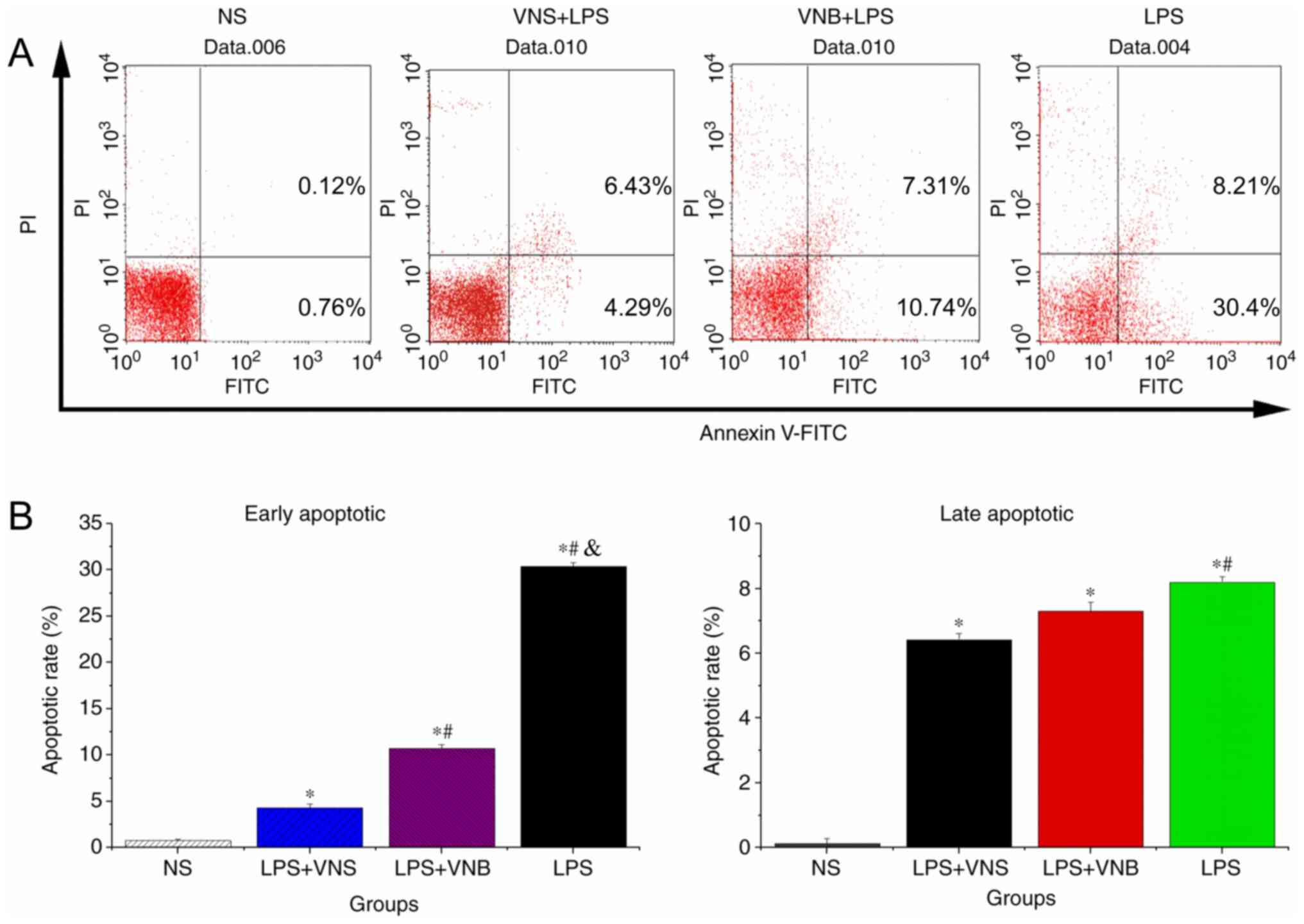
Compared with the NS group, the early and late
inflammatory cell apoptosis rates in BALF from the LPS, LPS+VNB and
LPS+VNS groups were significantly increased (P<0.05). The early
and late inflammatory cell apoptosis rates in BALF from the LPS+VNS
group were significantly lower than those of the LPS group
(P<0.05). Compared with the LPS group, the early apoptosis rate
in BALF from the LPS+VNB group was significantly lower (P<0.05)
and the late apoptosis rate was also lower, but the difference was
not statistically significant (P>0.05; Fig. 2).
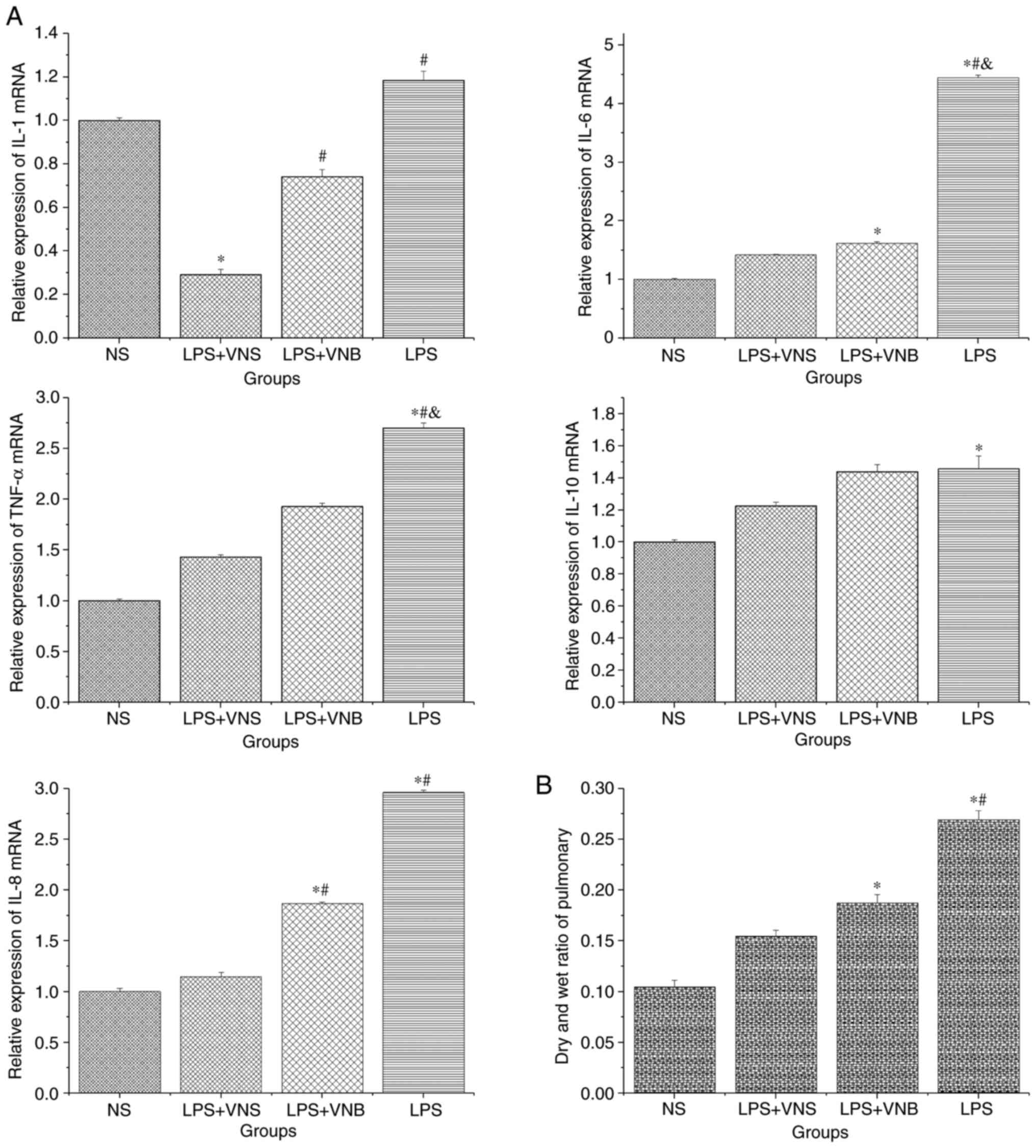
Detection of changes in IL-1, IL-6,
IL-10, TNF-α and IL-8 in lung tissue mRNA levels by RT-qPCR
The expression levels of IL-6, IL-10, IL-8 and TNF-α
mRNA in lung tissue from the LPS group were significantly higher
than that of the NS group (P<0.05). In the LPS+VNS group, the
expression levels of IL-1, IL-6, IL-8 and TNF-α mRNA were
significantly lower than those of the LPS group (P<0.05) and the
mRNA expression level of IL-10 was decreased in the LPS+VNS group
compared with the LPS group, although the difference was not
statistically significant (P>0.05). The expression levels of
IL-6 and TNF-α mRNA in the LPS+VNB group were significantly lower
than those in the LPS group (P<0.05) and the mRNA expression
levels of IL-1, IL-8 and IL-10 in the LPS+VNS group compared with
were lower than those in the LPS group, although the difference was
not statistically significant (P>0.05). The expression levels of
IL-1 and IL-8 mRNA were significantly higher in the LPS+VNB group
compared with the LPS+VNS group (P<0.05). The expression levels
of IL-6, IL-10 and TNF-α mRNA were comparatively higher in the
LPS+VNB group compared with the LPS+VNS group, but the difference
was not statistically significant (P>0.05; Fig. 3A).
Western blot analysis of protein
expression levels of IL-1, IL-6, IL-10, IL-8 and TNF-α in lung
tissue
The expression levels of IL-1, IL-6, IL-10, IL-8 and
TNF-α in lung tissue from the LPS group were significantly
increased compared with those of the NS group (P<0.05). In
contrast, in the LPS+VNS group, the expression levels of IL-1,
IL-6, IL-10, IL-8 and TNF-α protein in lung tissue were
significantly lower than those in the LPS group (P<0.05). In the
LPS+VNB group, the expression levels of IL-1, IL-6, IL-10, IL-8 and
TNF-α in lung tissue were significantly increased compared with
those of the LPS+VNS group (P<0.05), whereas the expression
levels of IL-1 and IL-10 protein were significantly decreased
compared with those of the LPS group (P<0.05). The expression
levels of TNF-α and IL-8 protein in the LPS+VNB group were not
significantly different from those of the LPS group (P>0.05),
whereas the expression level of IL-6 protein was significantly
increased compared with the LPS group (P<0.05; Fig. 4).
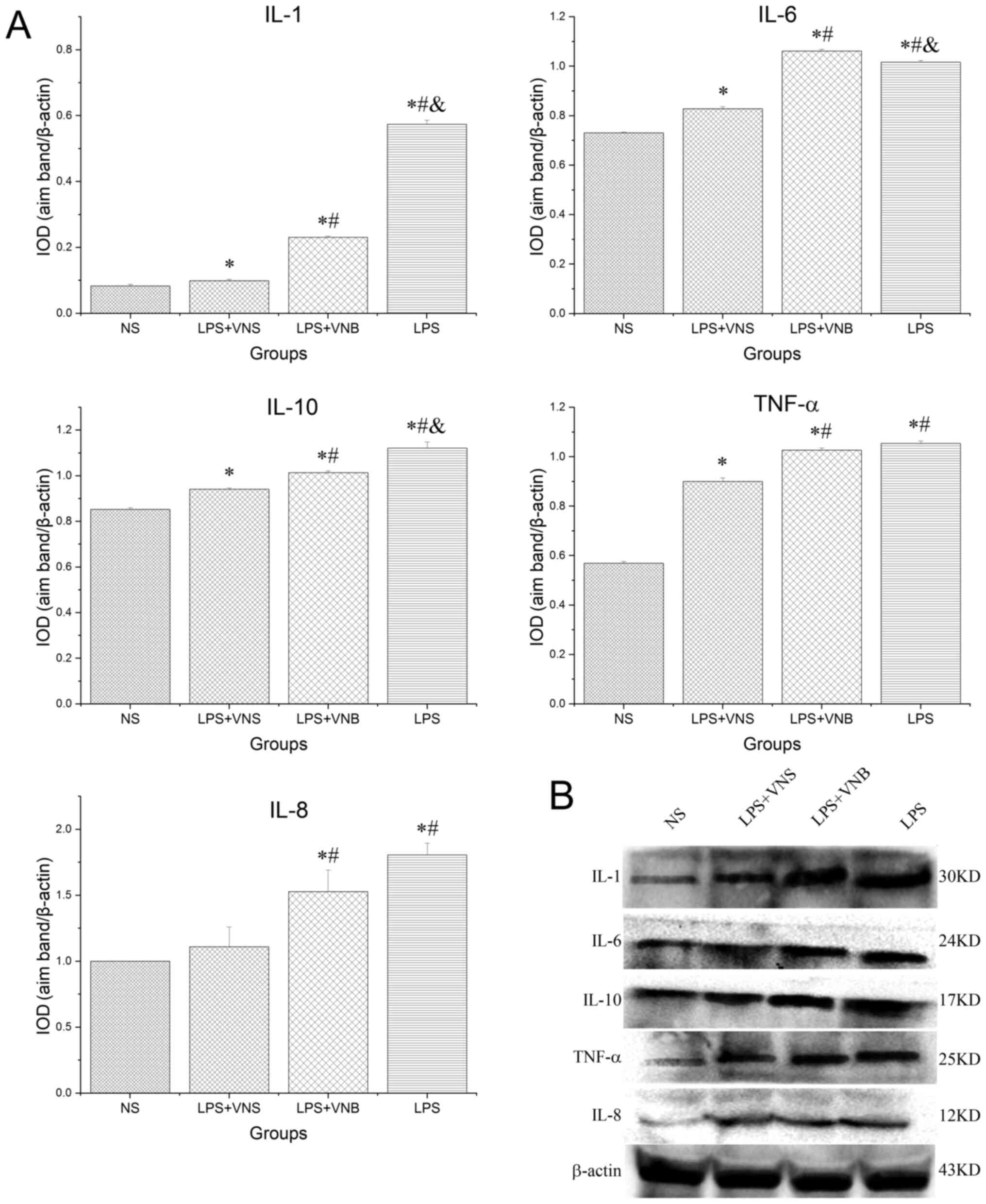 | Figure 4.(A) Detection of IL-1, IL-6, IL-10,
TNF-α and IL-8 protein expression levels in lung tissue by (B)
western blot. *P<0.05 vs. NS group, #P<0.05 vs.
LPS+VNS group, &P<0.05 vs. LPS+VNB group. NS,
normal saline; LPS, lipopolysaccharide; VNS, vagus nerve
stimulation; VNB, vagotomy; IOD, integral optical density. |
Wet/dry lung weight ratio
determination
The wet/dry lung weight ratio of the LPS group was
significantly higher than that of the NS group (P<0.05), whereas
the wet/dry lung ratio of the LPS+VNS group was significantly lower
than that of the LPS group (P<0.05). The wet/dry lung ratio of
the LPS+VNB group was compared with that of the LPS group and
showed a decrease, but the difference was not statistically
significant (P>0.05; Fig.
3B).
Discussion
Since Borovikova et al (20) proposed the concept of a cholinergic
anti-inflammatory pathway (CAP) in 2000, the role of CAP in
regulating inflammation has become a topic of interest. Situated
between the immune system and the nervous system, CAP consists of
the vagus nerve and releases Ach, which suggests a new approach to
the treatment of critical illnesses caused by excessive
inflammation. The molecular basis of the close association between
the immune system and the nervous system is α7nACHR, which is
expressed on the surface of alveolar macrophages and alveolar
epithelial cells. After receiving immune stimulation, the central
nervous system activates the vagus nerve to send out nerve fibers,
releasing Ach and α7nACHR, and activating the anti-inflammatory
pathway, decreasing the recruitment and infiltration of
inflammatory cells and decreasing the inflammatory response in lung
tissue, thereby exerting a pulmonary protective effect (21–23).
A number of studies have demonstrated that CAP may
inhibit lung tissue inflammation caused by LPS and decrease damage
to microvascular endothelial cells and alveolar epithelial cells,
thus contributing to lung protection (24–26).
Reports have confirmed that stimulation of the vagus nerve (via
electrical stimulation or drug agonist) could significantly improve
the prognosis of ALI caused by burns, multiple injuries,
hemorrhagic shock and severe infection (27–30).
These studies demonstrate that vagus nerve stimulation may be an
important breakthrough in treatment strategies for ALI.
Su et al (17) reported that α7nACHR agonists
inhibited NF-κB activity and the production of pro-inflammatory
factors (such as MIP-2 and TNF-α) in alveolar macrophages, and
decreased the migration and accumulation of neutrophils and lung
edema. dos Santos et al (18) used the model of ventilator-induced
lung injury caused by high tidal volume to demonstrate that vagus
nerve separation could aggravate pulmonary edema and neutrophil
infiltration, accelerate epithelial cell apoptosis and increase
IL-6 expression levels, but that electrical stimulation of the
vagus nerve decreased inflammation in the lung tissue, apoptosis of
epithelial cells and the expression levels of IL-6, and alleviated
the symptoms of lung injury. In vivo experiments with septic shock
caused by endotoxins demonstrated that vagal nerve stimulation with
drugs (such as CNI-1943) or electrophysiological methods inhibited
the release of TNF-α and decreased the systemic inflammatory
response (18). However, in the
vagus nerve separation group, treatment of inflammation was far
less effective than in the vagus nerve stimulation group (31).
TNF-α is the first cytokine to be released during
the inflammatory response. It can directly stimulate alveolar
endothelial cells or indirectly stimulate macrophages and
granulocytes to promote their migration, recruitment and
infiltration, further stimulating the release of IL-1 and IL-6, and
induce lung injury (6). IL-1 and
IL-6 are cytokines with roles in cell proliferation and
differentiation in inflammatory and immune responses and can
promote the migration and recruitment of inflammatory cells
(32,33). IL-10 originates from Th2 cells and
certain regulatory T-cells and inhibits the antigen presentation
and cytokine synthesis functions of macrophages. It has a dual
pro-inflammatory and anti-inflammatory role and its expression
level is significantly increased in the early stage of ALI, in
which the pro-inflammatory effect is predominant (34).
The present study demonstrated that LPS+VNS
significantly decreased the inflammatory response of ALI, the
damage to lung tissues, the infiltration of inflammatory cells,
edema in the alveolar wall, alveolar expansion and the apoptosis
rate in BALF cells. Elevated IL-1, IL-6, IL-10 and TNF-α protein
expression levels were inhibited and lung protection was achieved.
After the vagus nerve was separated in the present study, it was
electrically stimulated, and it was demonstrated that the
inflammatory reaction of the lung tissues was inhibited. IL-1 and
IL-10 protein expression levels were significantly decreased and
the expression level of IL-6 protein was significantly increased,
whereas the RT-qPCR assay showed that IL-6 mRNA expression levels
were significantly lower in the LPS+VNS group than in the LPS
group. IL-6 is a B-cell growth factor produced by activated
macrophages and is considered to be a cytokine produced early in
the inflammatory response (35). A
previous study revealed that elevated levels of IL-6 were closely
associated with the severity of lung injury caused by acute
pancreatitis (36). The results of
the present study suggest that it is possible that protein
expression is affected by the regulation of a certain step in the
mRNA translation pathway.
Compared with the LPS+VNB group, the VNS+LPS group
had a more pronounced effect on preventing the inflammatory
response. This statistically significant difference suggests that
when the integrity of the vagus nerve was destroyed, electrical
stimulation of the vagus nerve could not achieve pulmonary
protection, and that intactness of the vagus nerve was an important
factor in ensuring that electrical stimulation of the vagus nerve
could regulate inflammation in the pulmonary protection
mechanism.
Compared with the LPS groups, levels of inflammatory
cytokines in the LPS+VNB group were significantly decreased. To the
best of our knowledge, this phenomenon has not previously been
reported. Studies suggest that following vagotomy, inhibition of
vagal nerve activity on the heart is decreased, resulting in the
acceleration of heart rate (37),
the acceleration of cardiopulmonary blood flow (38), the shortening of the stay time of
inflammatory cells in the pulmonary capillaries and the relative
decrease in the exudation of inflammatory cells.
That the death rate was not compared with longevity
and the lack of an explanation for why the inflammation cytokines
in the LPS+VNB group were significantly decreased compared with to
LPS group were limitations of the present study; the BALF may have
also contained non-inflammatory cells, such as alveolar epithelial
cells. This will be considered in future research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Project of
Jiangxi Natural Science Foundation (grant nos. 20171BAB205027,
20151BBG70186 and 20161BAB205269); Jiangxi Youth Science Foundation
(grant no. 20161BAB215250); Youth Foundation of Second Affiliated
Hospital of Nanchang University (grant no. 2016YNQN12029); and
Jiangxi Health and Family Planning Commission Foundation (grant no.
20195216)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and LW made substantial contributions to the
conception or design of the work. WH and YW analyzed or interpreted
data. WH, drafted the manuscript or revised it critically for
important intellectual content. YW, BC and LX interpreted data and
agreed to be accountable for the work and ensuring that questions
related to the integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The use of animals in this study was approved by the
animal research committee in the Second Affiliated Hospital of
Nanchang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang L, Yuan R, Yao C, Wu Q, Christelle M,
Xie W, Zhang X, Sun W, Wang H and Yao S: Effects of resolvin D1 on
inflammatory responses and oxidative stress of
lipopolysaccharide-induced acute lung injury in mice. Chin Med J
(Engl). 127:803–809. 2014.PubMed/NCBI
|
|
2
|
Tang BM, Craig JC, Eslick GD, Seppelt I
and McLean AS: Use of corticosteroids in acute lung injury and
acute respiratory distress syndrome: A systematic review and
meta-analysis. Crit Care Med. 37:1594–1603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson ER and Matthay MA: Acute lung
injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butt Y, Kurdowska A and Allen TC: Acute
Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tasaka S: Acute lung injury/acute
respiratory distress syndrome: progress in diagnosis and treatment.
topics: I. Pathogenesis and pathophysiology; 3. Pathogenesis and
pathophysiology of ALI/ARDS. Nippon Naika Gakkai Zasshi.
100:1529–1535. 2011.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SH, Jang AS, Kim YE, Cha JY, Kim TH,
Jung S, Park SK, Lee YK, Won JH, Kim YH, et al: Modulation of
cytokine and nitric oxide by mesenchymal stem cell transfer in lung
injury/fibrosis. Respir Res. 11:162010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liou CJ, Lai YR, Chen YL, Chang YH, Li ZY
and Huang WC: Matrine attenuates COX-2 and ICAM-1 expressions in
human lung epithelial cells and prevents acute lung injury in
LPS-induced mice. Mediators Inflamm. 2016:36304852016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cox G, Crossley J and Xing Z: Macrophage
engulfment of apoptotic neutrophils contributes to the resolution
of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol.
12:232–237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galani V, Tatsaki E, Bai M, Kitsoulis P,
Lekka M, Nakos G and Kanavaros P: The role of apoptosis in the
pathophysiology of Acute Respiratory Distress Syndrome (ARDS): An
up-to-date cell-specific review. Pathol Res Pract. 206:145–150.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albertine KH, Soulier MF, Wang Z, Ishizaka
A, Hashimoto S, Zimmerman GA, Matthay MA and Ware LB: Fas and fas
ligand are up-regulated in pulmonary edema fluid and lung tissue of
patients with acute lung injury and the acute respiratory distress
syndrome. Am J Pathol. 161:1783–1796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kenney MJ and Ganta CK: Autonomic nervous
system and immune system interactions. Compr Physiol. 4:1177–1200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Selmi C, Barin JG and Rose NR: Current
trends in autoimmunity and the nervous system. J Autoimmun.
75:20–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reardon C, Murray K and Lomax AE:
Neuroimmune Communication in Health and Disease. Physiol Rev.
98:2287–2316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boland C, Collet V, Laterre E, Lecuivre C,
Wittebole X and Laterre PF: Electrical vagus nerve stimulation and
nicotine effects in peritonitis-induced acute lung injury in rats.
Inflammation. 34:29–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guarini S, Altavilla D, Cainazzo MM,
Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini
A, Marini R, et al: Efferent vagal fibre stimulation blunts nuclear
factor-kappaB activation and protects against hypovolemic
hemorrhagic shock. Circulation. 107:1189–1194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Yu M, Ochani M, Amella CA, Tanovic
M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su X, Lee JW, Matthay ZA, Mednick G,
Uchida T, Fang X, Gupta N and Matthay MA: Activation of the alpha7
nAChR reduces acid-induced acute lung injury in mice and rats. Am J
Respir Cell Mol Biol. 37:186–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
dos Santos CC, Shan Y, Akram A, Slutsky AS
and Haitsma JJ: Neuroimmune regulation of ventilator-induced lung
injury. Am J Respir Crit Care Med. 183:471–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonaz B, Sinniger V and Pellissier S:
Vagal tone: Effects on sensitivity, motility, and inflammation.
Neurogastroenterol Motil. 28:455–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonaz B: The vagus nerve and the
sympathetic nervous system act in concert to modulate immunity.
Brain Behav Immun. 84:6–7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Browning KN, Verheijden S and Boeckxstaens
GE: The Vagus Nerve in Appetite Regulation, Mood, and Intestinal
Inflammation. Gastroenterology. 152:730–744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Li L and Su X: Vagus nerve through
α7 nAChR modulates lung infection and inflammation: Models, cells,
and signals. BioMed Res Int. 2014:2835252014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma P, Yu K, Yu J, Wang W, Ding Y, Chen C,
Chen X, Zhao K, Zuo T, He X, et al: Effects of nicotine and vagus
nerve in severe acute pancreatitis-associated lung injury in rats.
Pancreas. 45:552–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
26. Liu JS, Wei XD, Lu ZB, Xie P, Zhou HL,
Chen YY, Ma JM and Yu LZ: Liang-Ge-San, a classic traditional
Chinese medicine formula, protects against
lipopolysaccharide-induced inflammation through cholinergic
anti-inflammatory pathway. Oncotarget. 7:21222–21234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reys LG, Ortiz-Pomales YT, Lopez N,
Cheadle G, de Oliveira PG, Eliceiri B, Bansal V, Costantini TW and
Coimbra R: Uncovering the neuroenteric-pulmonary axis: Vagal nerve
stimulation prevents acute lung injury following hemorrhagic shock.
Life Sci. 92:783–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krzyzaniak MJ, Peterson CY, Cheadle G,
Loomis W, Wolf P, Kennedy V, Putnam JG, Bansal V, Eliceiri B, Baird
A, et al: Efferent vagal nerve stimulation attenuates acute lung
injury following burn: The importance of the gut-lung axis.
Surgery. 150:379–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Langness S, Costantini TW, Morishita K,
Eliceiri BP and Coimbra R: Modulating the biologic activity of
mesenteric lymph after traumatic shock decreases systemic
inflammation and end organ injury. PLoS One. 11:e01683222016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Li JG, Jia BH, Wu YB, Zhou Q and
Du ZH: Protective effects of electric stimulation of vagus nerve on
acute lung injury in rat with sepsis. Zhongguo Wei Zhong Bing Ji
Jiu Yi Xue. 19:593–595. 2007.(In Chinese). PubMed/NCBI
|
|
31
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang M, Zhang P, Chang XL, Gu XA, Wu Q
and Zhou ZJ: Changes of cytokine and nuclear factor-kappa B in
acute paraquat poisoned rats. Zhonghua Lao Dong Wei Sheng Zhi Ye
Bing Za Zhi. 27:463–467. 2009.(In Chinese). PubMed/NCBI
|
|
33
|
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L,
Billiar TR, Wilson MA, Shi X and Fan J: TLR4-upregulated IL-1β and
IL-1RI promote alveolar macrophage pyroptosis and lung inflammation
through an autocrine mechanism. Sci Rep. 6:316632016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li HX, Zhang JC, Zhao YL and Hao HJ:
Effects of interleukin-10 on expression of inflammatory mediators
and anti-inflammatory mediators during acute lung injury in rats.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 17:338–341. 2005.(In
Chinese). PubMed/NCBI
|
|
35
|
Swaroopa D, Bhaskar K, Mahathi T, Katkam
S, Raju YS, Chandra N and Kutala VK: Association of serum
interleukin-6, interleukin-8, and Acute Physiology and Chronic
Health Evaluation II score with clinical outcome in patients with
acute respiratory distress syndrome. Indian J Crit Care Med.
20:518–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Neuhöfer P, Song L, Rabe B,
Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius
H, et al: IL-6 trans-signaling promotes pancreatitis-associated
lung injury and lethality. J Clin Invest. 123:1019–1031. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alcayaga J, Del Rio R, Moya EA, Freire M
and Iturriaga R: Effects of vagotomy on cardiovascular and heart
rate variability alterations following chronic normobaric hypoxia
in adult rabbits. Biol Res. 51:572018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schertel ER, Brourman JD, Kling SM,
Schmall LM, Tobias TA and Myerowitz PD: Vagal innervation
influences the whole body oxygen consumption-delivery relationship
in the dog. Shock. 2:127–132. 1994. View Article : Google Scholar : PubMed/NCBI
|