Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial
carcinoma arising from the nasopharynx epithelium (1) and is characterized by a high
prevalence, especially in south China, southeastern Asia and Africa
(2), affecting ~130,000 patients
worldwide in 2018 (3). A study
identified environmental factors, Epstein-Barr virus infection and
genetic susceptibility as risk factors for NPC (4). At present, the application of
radiotherapy and optimization of chemotherapy strategies have
greatly contributed to improvements in survival rates in patients
with NPC (3). However, these
treatments have some limitations, including complications and
potential adverse effects (5).
Therefore, there is an urgent need to explore novel drugs to treat
NPC in clinic settings.
Tanshinone IIA is a major component of Salvia
miltiorrhiz and exhibits various physiological activities,
including anti-tumor, anti-inflammatory and antioxidant effects. In
a hypoxic microenvironment, tanshinone IIA was shown to decrease
hypoxia-inducible factor α expression and suppress the secretion of
vascular endothelial growth factor and basic fibroblast growth
factor by regulating the b-catenin/TCF3/LEF1 signaling pathway,
leading to the inhibition of proliferation, tube formation and
metastasis of human umbilical vein endothelial cells (6). In addition, tanshinone IIA inhibited
PI3K/Akt/FoxO1 signaling and alleviated blast-induced inflammation
and oxidative stress, leading to improvement in lung blast injuries
(7). Importantly, treatment with
tanshinone IIA significantly suppressed proliferation and induced
apoptosis in various cancer cell types, including ovarian cancer
(8), cervical cancer (9) and gastric cancer (10). For instance, Tong et al
(11) demonstrated that tanshinone
IIA could promote the expression of microRNA (miR)-145 and enhance
pyroptosis of HeLa cells, resulting in decreased proliferation of
these cells. Notably, tanshinone IIA could also downregulate the
expression of miR30b and initiate apoptosis in HepG2 cells
(12). These findings reveal a
critical role of miRs in mediating the effects of tanshinone IIA;
this prompted further exploration of their association.
Recent research has focused on programmed cell death
in the treatment of tumors. A lytic and inflammatory type of
regulated cell death, pyroptosis requires membrane-damaging
gasdermin proteins and is characterized by swelling and lysis of
cells and release of several pro-inflammatory factors, including
interleukin (IL)-18, IL-1β and caspase-3 (13). Caspase-3 is one of the decisive
markers of apoptosis, and is positively associated with some
inflammatory factors, such as CD68, tumor necrosis factor α and
IL-1b. Additionally, Gasdermin D (GSDMD) is the pyroptotic
substrate of caspase-1 and belongs to the conserved gasdermin
family. The gasdermin-N domains in GSDMD bind to membrane lipids
and perforate the membrane, leading to the disruption of osmotic
potential and further causing cell swelling, with large bubbles
blowing from the plasma membrane (13). It has been demonstrated that p53
directly enhances lipopolysaccharide (LPS)-induced pyroptosis to
markedly decrease tumor growth in A549 cells, suggesting a critical
role of p53 in inhibiting tumor growth in patients (14). Notably, a series of studies have
focused on the anticancer effects of natural molecules, including
tanshinone IIA (11), omega-3
docosahexaenoic acid (15) and
paclitaxel (16). However, how
tanshinone IIA regulates pyroptosis in NPC cells remains
elusive.
In the present study, the effects of tanshinone IIA
on HK1 cells were detected and their apoptotic levels, including
the activity of intracellular caspase-3 and caspase-9, was
assessed. The expression of GSDMD and caspase-1 was also evaluated.
Furthermore, the role of miR-125b/foxp3 signaling in the activity
of tanshinone IIA was explored by transfecting cells with a
miR-125b agomir and small interfering RNA (si)-foxp3 plasmid.
Materials and methods
Cell culture and chemicals
Human NPC cell line HK1 was obtained from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
HK1 cells were seeded in DMEM (cat. no. C11995500BT; Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum (cat.
no. P30-3301; PAN-Biotech) and 1% penicillin-streptomycin (cat. no.
15140-122; Gibco; Thermo Fisher Scientific, Inc.) in a
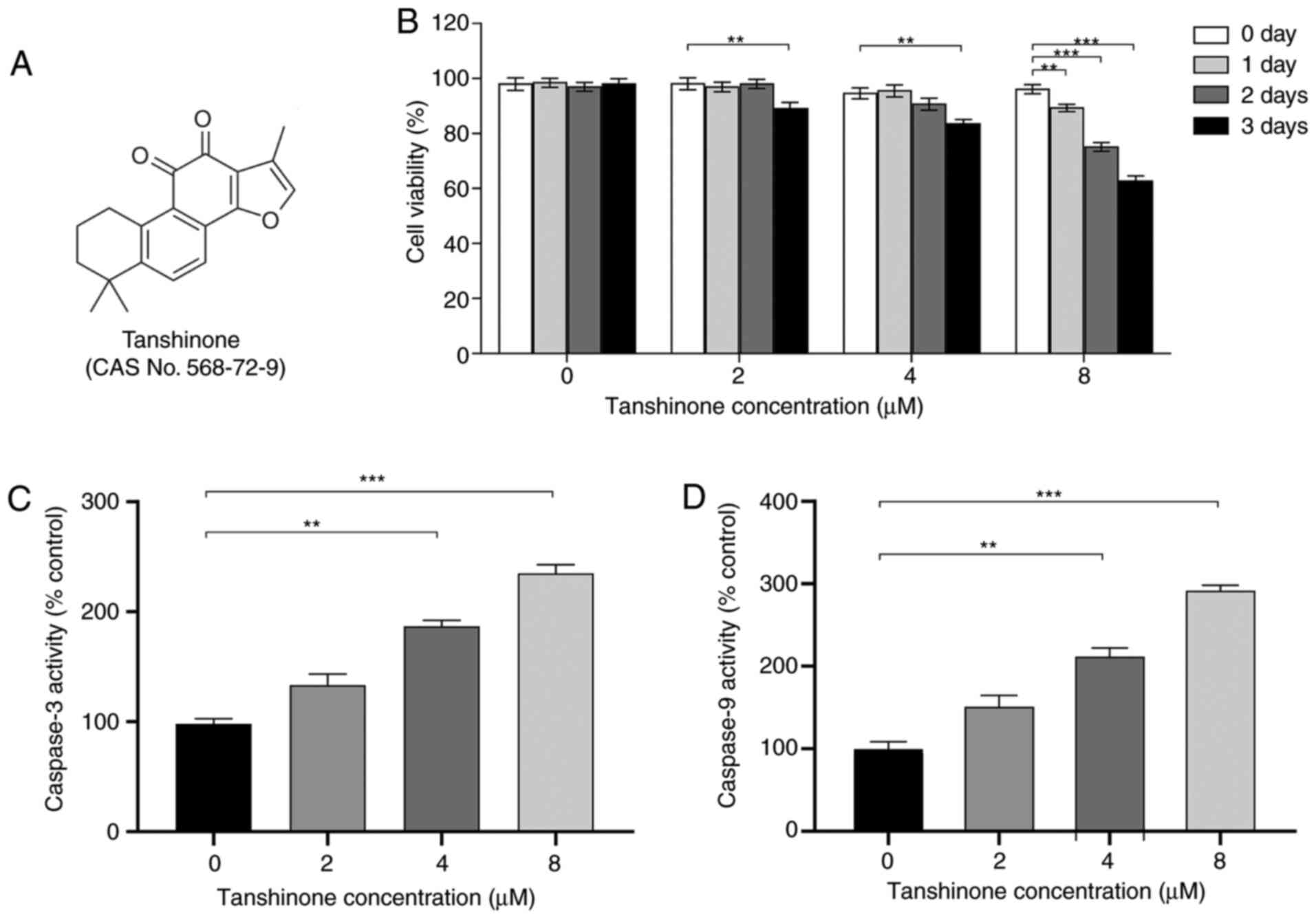
water-saturated atmosphere at 37°C. Tanshinone IIA (Fig. 1A) was procured from Selleck
Chemicals (cat. no. S2365). Dimethyl sulfoxide (cat. no. 276855;
Sigma-Aldrich; Merck KGaA) was used as a negative control.
Cell transfection
The miR-125b agomir, si-foxp3 plasmid (constructed
in pEGFP-N2), and their negative controls (pEGFP-N2) were obtained
from Guangzhou RiboBio Co., Ltd. The plasmid was resuspended to a
final concentration of 1 µg/µl. HK1 cells (1×106
cells/ml) were cultured in six-well plates for 24 h. According to
the manufacturer's instructions, the plasmid and agomir were
transfected using Lipofectamine® 2000 reagent (cat. no.
11668-027; Invitrogen; Thermo Fisher Scientific, Inc.). After
transfection for 72 h, cells were harvested for further
research.
MTT assay
HK1 cells (1×106 cells/well) were seeded
and cultured in six-well plates for 12 h. Then, the cells were
treated with different concentrations of tanshinone IIA for 0, 24,
48 and 72 h. MTT assays were performed using a commercial kit
according to the manufacturer's instructions (cat. no. M1020;
Beijing Solarbio Science & Technology Co., Ltd.). An
enzyme-linked immunosorbent assay (ELISA) plate reader was used to
determinate the optical density at 490 nm.
Caspase-3 and caspase-9 activity
assay
HK1 cells (1×106 cells/well) were seeded
in six-well plates. Then, total protein was extracted from cells
using a cell lysis buffer (cat. no. R0020; Beijing Solarbio Science
& Technology Co., Ltd.). The concentration of protein was
detected with a BCA protein assay kit (cat. no. P1511-1, Applygen
Technologies, Inc.). According to the manufacturer's instructions,
the activity of caspase-3 and caspase-9 was assessed with a
Caspase3 Activity lit (cat. no. BC3830; Beijing Solarbio Science
& Technology Co., Ltd.) and Caspase9 Activity kit (cat. no.
BC3890, Beijing Solarbio Science & Technology Co., Ltd.). A
microplate reader was used to determine the absorbance values at
405 nm. The activity levels were expressed relative to the control
group.
Western blot analysis
This analysis was performed as described in a
previous study (16). Total protein
of HK1 cells was extracted using RIPA lysis buffer (cat. no. R0020,
Beijing Solarbio Science & Technology Co., Ltd.) containing 1%
protease inhibitor cocktail and phosphatase inhibitor. The protein
concentration of the lysate was detected with a BCA Assay kit (cat.
no. P1511-1, Applygen Technologies, Inc.). Protein lysates were
resolved by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and then transferred to polyvinylidene fluoride
membranes (cat. no. IPVH00010; EMD Millipore). The membranes were
blocked with 5% skimmed milk for 2 h, incubated with primary
antibodies, and then with horseradish peroxidase-conjugated
secondary antibodies. Proteins were visualized with an ECL western
blotting substrate (cat. no. 170-5060; Bio-Rad Laboratories, Inc.)
using a Bio-Rad System (Bio-Rad Laboratories, Inc.). Actin served
as a loading control. The primary antibodies were diluted at
1:2,000 and the secondary antibodies were diluted at 1:5,000.
Antibodies against GSDMD (cat nos. ab210070 and ab215203) and foxp3
(cat. no. ab215206) were obtained from Abcam. The caspase-1 (cat.
no. 3866T) antibody was obtained from Cell Signaling Technology,
Inc. The cleaved caspase-1 (cat. no. A0964) and actin (cat. no.
AC026) antibodies were purchased from ABclonal Biotech Co., Ltd.
The secondary antibodies were obtained from Biodragon.
Reverse transcription-quantitative PCR
(RT-qPCR)
This assay was performed according to the
manufacturer's instructions. Briefly, total RNA was isolated from
control and treated cells using TRIzol reagent (cat. no. 15596-026;
Invitrogen; Thermo Fisher Scientific, Inc.). Then, a cDNA synthesis
kit (cat. no. 04896866001; Roche) and SYBR green (TransGen Biotech
Co., Ltd.) were used to determine the relative expression of target
genes with the ABI-Quant Studio 5 system (Thermo Fisher Scientific,
Inc.). The mRNA expression levels of the target genes were
normalized to β-actin expression. The relative mRNA
expression was calculated using 2−∆∆Cq (17,18).
The primer pairs used in the present study are listed in Table SI.
ELISA assays
HK1 cells (1×105 cells/well) were
cultured in 24-well plates and treated with tanshinone IIA,
miR-125b agomir, or si-foxp3 plasmid. Levels of IL-1b and IL-18
were measured using commercially available ELISA kits following the
manufacturer's instructions (cat. nos. ab214025 and ab215539;
Abcam). Experiments were repeated three times.
Statistical analysis
For statistical analyses, the results were expressed
as mean ± SEM. Statistical significance was determined by
two-tailed student's t-test or one-way analysis of variance
followed by Bonferroni analysis (for data meeting homogeneity of
variance) or Tamhane's T2 analysis (for data demonstrating
heteroscedasticity). P<0.05 was considered to indicate a
statistically significant difference. Statistical calculations were
performed using the SPSS software (version 24.0; IBM Corp.).
Results
Tanshinone IIA inhibits cell
proliferation and enhances apoptosis of HK1 cells
Previous studies have demonstrated the anticancer
effects of tanshinone IIA in cervical cancer (9,11). In
the present study, the effects of tanshinone IIA on HK1 cells were
assessed (Fig. 1A). The results
showed that the proliferation of HK1 cells was significantly
inhibited following tanshinone IIA treatment, in a dose- and
time-dependent manner. Compared with controls, HK1 cells treated
with tanshinone IIA for 72 h showed markedly decreased viability.
In addition, the inhibitory effect on HK1 cells was gradually
enhanced with increasing concentration of tanshinone IIA (Fig. 1B). Moreover, the apoptotic levels of
HK1 cells treated with tanshinone IIA were detected. Caspase-3 and
caspase-9 activity was notably increased compared with that of
control cells after treatment for 72 h (Fig. 1C and D), suggesting enhanced
apoptosis of HK1 cells. These results demonstrate that tanshinone
IIA upregulated the apoptotic levels of NPC cells, leading to a
decrease in cell proliferation.
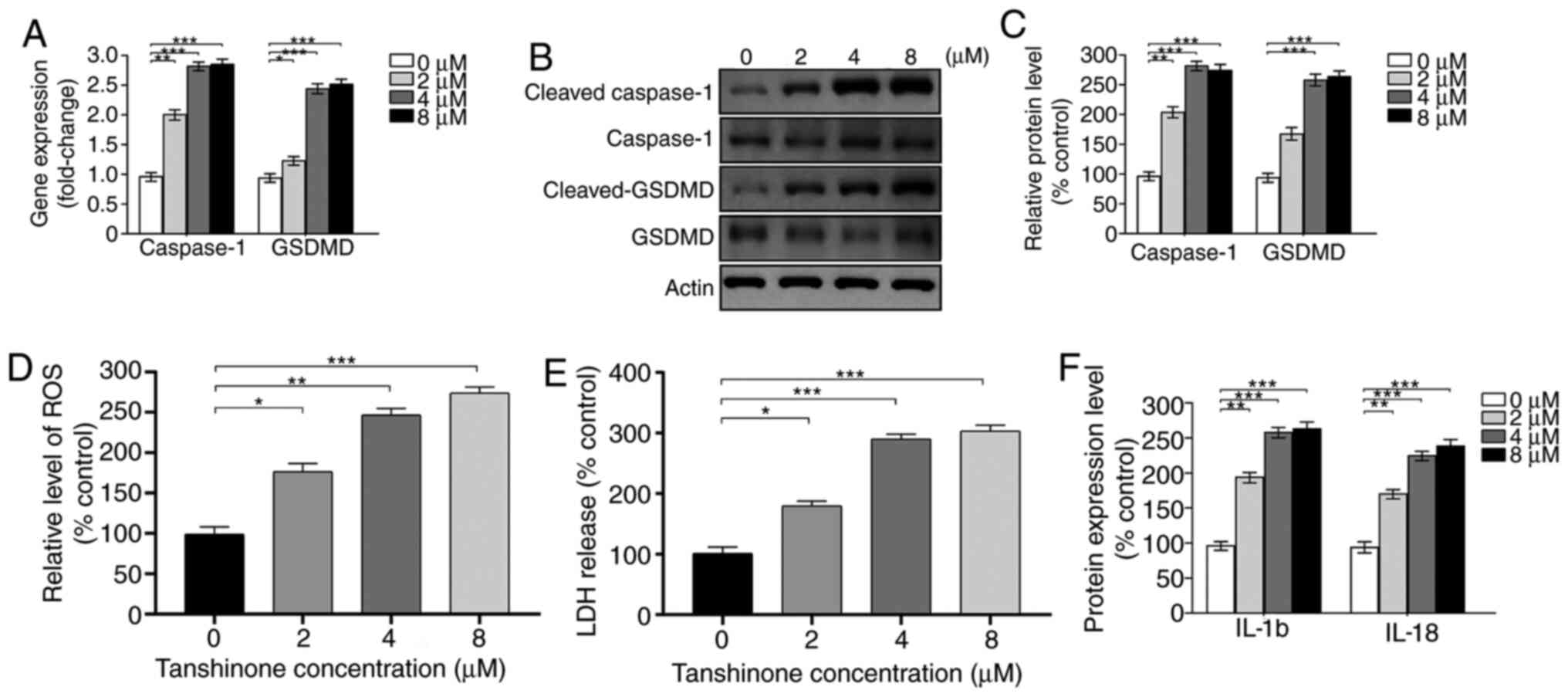
Tanshinone IIA enhances pyroptotic
level of HK1 cells
According to a previous study, HK1 was treated with
8 µM tanshinone IIA for 72 h (11).
Considering the potential effects of tanshinone IIA on pyroptosis,
it was assessed whether administration of tanshinone IIA regulated
pyroptosis in HK1 cells. As expected, the expression of caspase-1
and GSDMD was significantly upregulated by treatment with
tanshinone IIA, according to the results of the RT-qPCR assay
(Fig. 2A). Protein levels of
cleaved GSDMD and caspase-1 were also increased (Fig. 2B and C), indicating enhanced
pyroptosis of HK1 cells after tanshinone IIA treatment. Moreover,
intracellular ROS levels and LDH release were assessed. Compared
with control cells, administration of tanshinone IIA elevated ROS
levels and promoted LDH release (Fig.
2D and E). Pyroptosis enhances the secretion of inflammatory
cytokines such as IL-18 and IL-1β. Therefore, the levels of cleaved
IL-18 and IL-1β were further detected in HK1 cells treated with
tanshinone IIA by ELISA. The results showed that the expression
levels of IL-18 and IL-1β were markedly increased (Fig. 2F), consistent with the results of a
previous study (11). Notably,
tanshinone IIA exhibits the opposite effect on normal cells and
tumor cells, which may attribute to the different microenvironment.
For example, tanshinone IIA attenuates atherosclerosis by
inhibiting NLR family pyrin domain containing 3 (NLRP3)
inflammasome activation in macrophages (19), while the expression of IL-1β and
IL18 was inhibited in Hela cells after tanshinone II A treatment
(11). Briefly, tanshinone IIA
enhanced pyroptosis in HK1 cells.
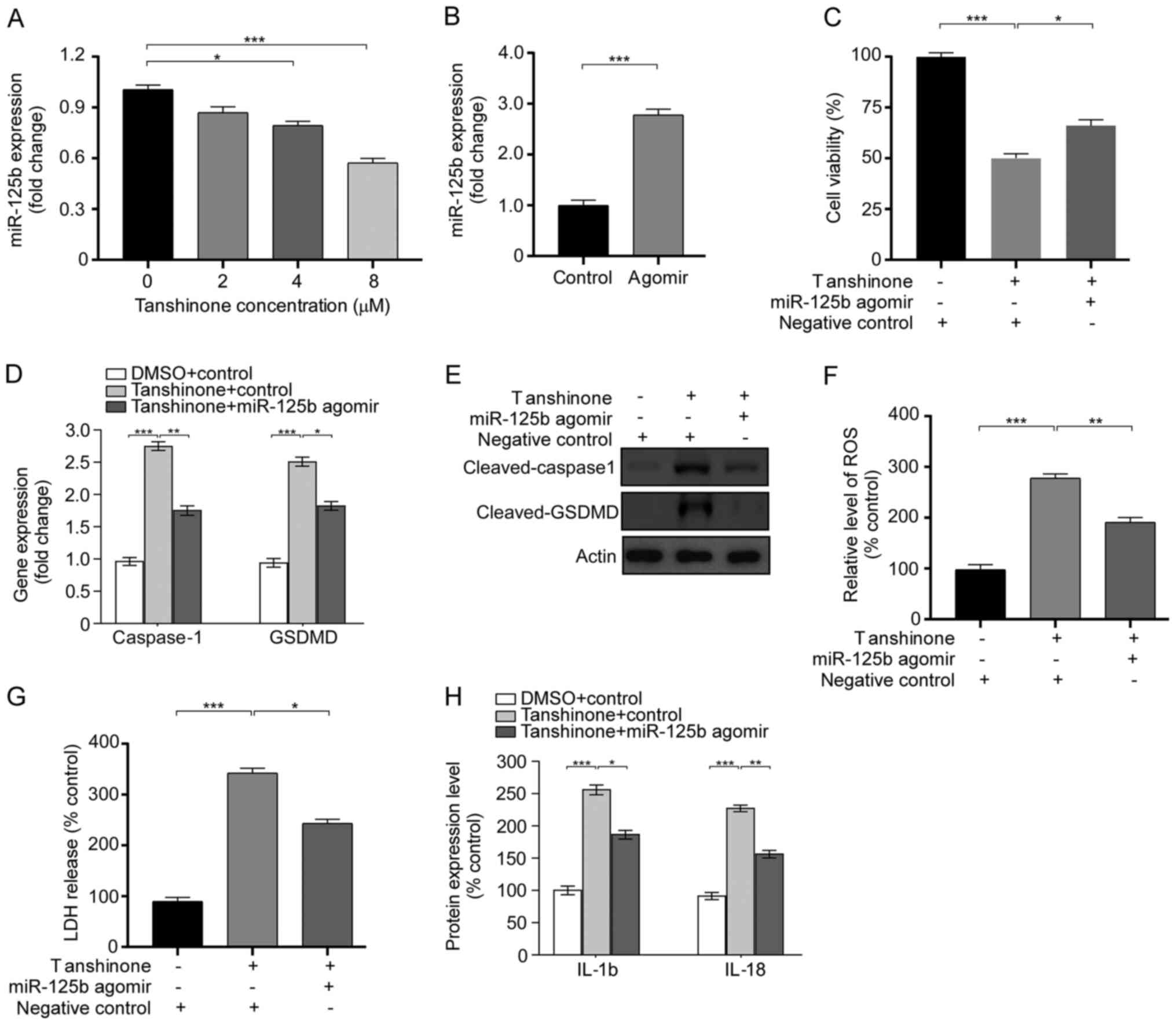
Tanshinone IIA enhances pyroptosis by
regulating miR-125b
In a previous study, Batool et al (17) demonstrated that miR-125b acts as a
tumor suppressor by regulating intrinsic properties of testicular
germ cell tumors. In the present study, the association between
miR-125b and the anticancer effects of tanshinone IIA were
explored. It was found that tanshinone IIA decreased miR-125b
levels in a dose-dependent manner. Compared with cells without
treatment, administration of tanshinone IIA suppressed the
expression level of miR-125b (Fig.
3A). Subsequently, miR-125b agomir was transfected into HK1
cells to explore the role of miR-125b in the activity of tanshinone
IIA. The expression of miR-125b was significantly increased after
agomir transfection (Fig. 3B). The
miR-125b agomir reversed the inhibitory effect of tanshinone IIA on
the proliferation of HK1 cells (Fig.
3C). Furthermore, the effect of miR-125b on pyroptosis was
assessed after tanshinone IIA treatment. It was found that compared
with the control groups, the expression levels of cleaved GSDMD and
caspase-1 were significantly decreased after transfection with the
miR-125b agomir, according to the results of RT-qPCR and western
blotting (Fig. 3D and E). Moreover,
the results showed that overexpression of miR-125b decreased the
increase in reactive oxygen species (ROS) levels and LDH release
(Fig. 3F and G). Given that
pyroptosis induces the secretion of IL-18 and IL-1β, the levels of
cleaved IL-1β and IL-18 in HK1 cells were detected after
transfection with the miR-125b agomir. As expected, overexpressing
mir-125b inhibited the increase in protein levels of IL-1β and
IL-18 induced by tanshinone IIA treatment (Fig. 3H). These results indicate that
tanshinone IIA promotes pyroptosis in HK1 cells by regulating
miR-125b.
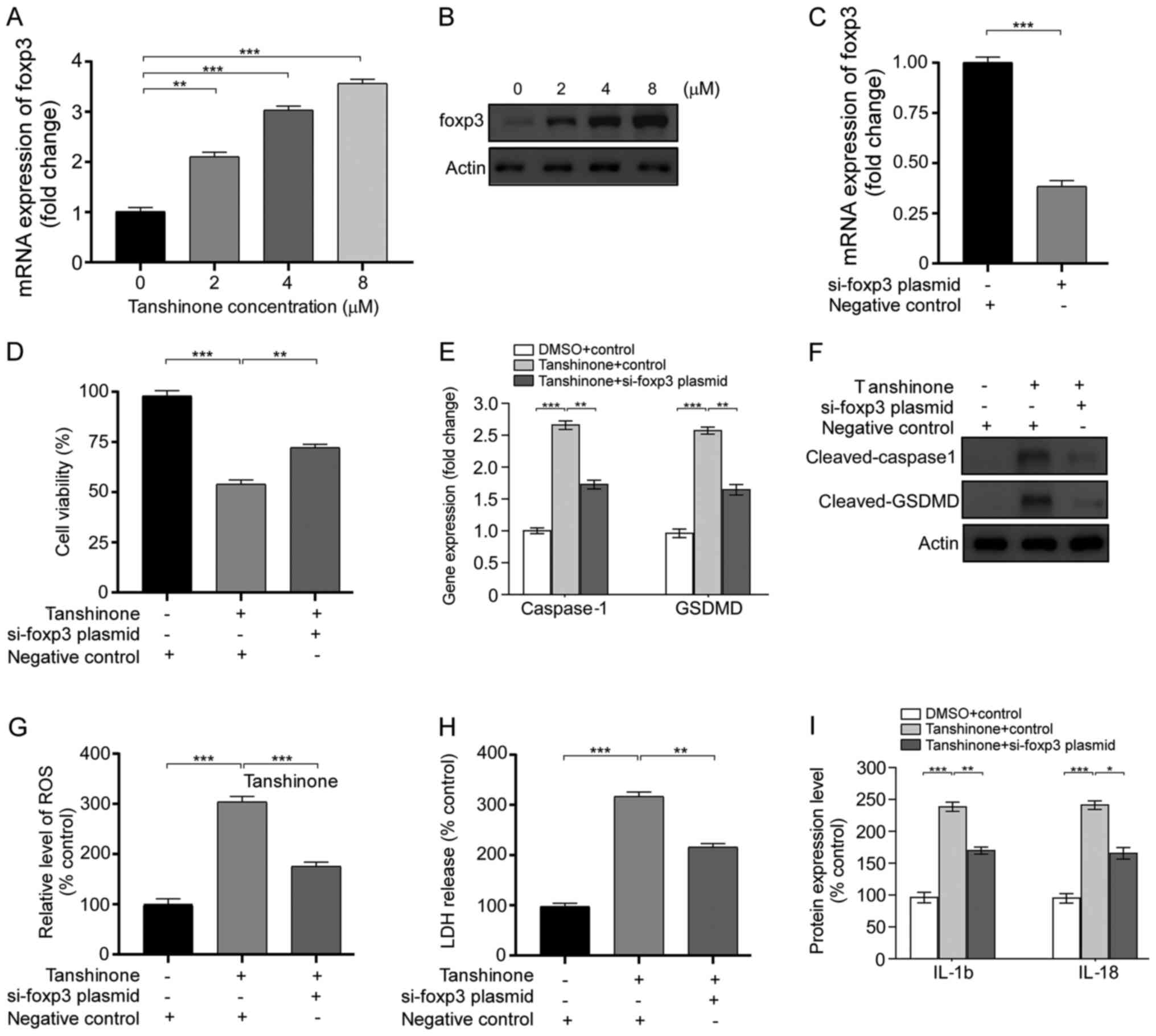
Tanshinone IIA facilitates pyroptosis
by regulating the miR-125b/foxp3 signaling pathway
It is well known that miR-125b negatively regulates
foxp3 and promotes autophagy in thyroid cancer, thereby enhancing
the efficacy of cisplatin (20). In
the present study, the expression of foxp3 in HK1 cells was
detected after tanshinone IIA treatment. Compared with controls,
HK1 cells treated with 8 µM tanshinone IIA exhibited elevated foxp3
expression (Fig. 4A and B). Then,
HK1 cells were transfected with the si-foxp3 plasmid. The
expression of foxp3 was remarkably downregulated after plasmid
transfection (Fig. 4C). The results
showed that the anti-foxp3 plasmid was able to reverse the
inhibition of proliferation of HK1 cells after tanshinone IIA
treatment (Fig. 4D). Similarly,
plasmid transfection induced a decrease in cleaved GSDMD and
caspase-1 expression compared with cells treated with tanshinone
IIA (Fig. 4E and F). Moreover,
transfection with the si-foxp3 plasmid led to decrease in
intracellular ROS levels and LDH release (Fig. 4G and H). Notably, the expression of
cleaved IL-18 and IL-1β was downregulated after treatment of
tanshinone IIA and si-foxp3 plasmid transfection (Fig. 4I). Collectively, these results
indicate that tanshinone IIA enhances pyroptosis by regulating
miR-125b/foxp3 signaling and inhibits the proliferation of HK1
cells.
Discussion
NPC is characterized by a specific geographic
distribution, affecting >130,000 patients worldwide (3). At present, a combination of concurrent
chemoradiotherapy and platinum-based agents is the main clinical
approach used to treat NPC (21);
however, this leads to side effects including rhinosinusitis
(22), dysphagia (23) and transient granulocytopenia
(24). Therefore, researchers aim
to identify novel natural molecules to inhibit cell proliferation
and tumor growth in NPC. In the present study, tanshinone IIA was
identified as a potential drug that suppresses cell proliferation
of HK1 cells and explored its underlying mechanism. The results
showed that the modulation of miR-125b/foxp3/caspase-1 signaling by
tanshinone IIA enhances pyroptotic levels in HK1 cells. Tanshinone
IIA was also shown to enhance the inflammatory level in HK1 cells,
which is opposite to the effect on normal cells. This phenomenon
may attribute to the different microenvironment in cells. For
example, tanshinone IIA attenuates atherosclerosis by inhibiting
NLRP3 inflammasome activation in macrophages (19), while the expression of IL-1β and
IL18 was inhibited in HeLa cells after tanshinone II A treatment
(11). Briefly, the present study
provides evidence to support tanshinone IIA as a potent drug to
treat NPC.
Foxp3 is a transcription factor belonging to the
forkhead-winged-helix family and is centrally involved in the
establishment and maintenance of the Treg cell phenotype (25). In general, foxp3+ Treg
cells can secrete a series of anti-inflammatory cytokines, express
co-inhibitory molecules, and modulate the activity of
antigen-presenting cells (26).
Various studies have clarified a critical role of foxp3 in the
treatment of tumors including non-small-cell lung cancer (NSCLC)
(27), thyroid cancer (20) and breast cancer (28). For instance, foxp3 overexpression
significantly induced cell proliferation, migration and invasion by
regulating the Wnt/b-catenin signaling pathway in NSCLC cells,
which led to the identification of foxp3 as a co-activator that
could facilitate tumor growth and metastasis in NSCLC. However, a
study showed that foxp3 acted as a critical suppressor of breast
cancer by interacting with Gal-1 (29). Therefore, the precise role of foxp3
in the progress of tumor is still elusive. Notably, studies have
shown a positive association of foxp3 with IL-1β (30) and IL-18 (31), which prompted us to explore whether
foxp3 was involved in the regulation of pyroptosis. In the present
study, it was found that tanshinone IIA upregulated foxp3
expression in HK1 cells. Furthermore, si-foxp3 was transfected into
HK1 cells to precisely assess the role of foxp3 on the effect of
tanshinone IIA. As expected, plasmid transfection of si-foxp3
reversed the inhibitory effects of tanshinone IIA on HK1 cells,
including an increase in cell proliferation, a decrease in
intracellular ROS levels, and a decrease in LDH release. Notably,
si-foxp3 inhibited pyroptosis of HK1 cells following treatment with
tanshinone IIA, indicating an important role of foxp3 in mediating
the protective effects of tanshinone IIA. In future, the role of
foxp3 in the progress of NPC will be further explored from the
aspect of epigenetics, in order to identify other novel molecules
to treat NPC in the clinic.
miRs are a class of non-coding RNA that have been
shown to have key roles in cell physiology, including in cell
differentiation, proliferation and survival (32). miRs can bind to complementary target
mRNAs and induce inhibition or degradation of the mRNA (33). It has been well demonstrated that
miRs are potential targets in the treatment of cancer. In several
types of cancer, the expression of certain miRs is markedly
associated with poor patient outcomes (34). For example, Jin et al
(35) reported that miR15a/16
targeted and inhibited the components of the transforming growth
factor-β signaling pathway in the LNCaP cell line; this finding
provided potential drug targets for the treatment of prostate
cancer. In addition, Hong et al (36) found abnormally high levels of
miR-663b in colorectal cancer, and a miR-663b inhibitor exerted an
inhibitory effect on the proliferation of colorectal cancer cells
by regulating the TNK1/Ras/Raf signaling pathway. In addition to
the finding that foxp3 overexpression suppresses the proliferation
of tumor cells, it has been shown that miR-125b can directly
interact with foxp3 by binding to its 3′-untranlsated region and
inhibit its expression, leading to increased autophagy and enhanced
efficacy of cisplatin in thyroid cancer (20). Moreover, researchers have identified
a series of natural molecules that can depress tumor growth by
downregulating the expression of miR-125b, including ganoderma
lucidum polysaccharides (37),
camptothecin (38) and silibinin
(39). Thus, the role of miR-125b
on the effect of tanshinone IIA in HK1 cells was explored. Firstly,
it was found that tanshinone IIA downregulated the expression of
miR-125b in HK1 cells. Furthermore, transfection with the miR-125b
agomir reversed the increase in pyroptosis of HK1 cells induced by
tanshinone IIA, with increased expression of GSDMD and caspase-1,
elevation of ROS levels and LDH release, and upregulation of IL-18
and IL-1β, suggesting that miR-125b/foxp3 signaling was closely
involved in mediating the effect of tanshinone IIA. In addition,
the role of the miR-125b/foxp3 signaling pathway in apoptosis was
investigated after tanshinone IIA administration. The results
showed that transfection with the miR-125b agomir or si-foxp3
plasmid reversed the effects of tanshinone IIA on HK1 cells (data
not shown), demonstrating miR-125b/foxp3 signaling as a potential
novel drug target for the treatment of NPC.
In conclusion, tanshinone IIA was identified as a
novel molecule that could inhibit cell proliferation of HK1 cells
by regulating miR-125b/foxp3/caspase-1/GSDMD signaling. Tanshinone
IIA treatment elevated the pyroptotic levels in HK1 cells. However,
transfection withs miR-125b agomir and si-foxp3 plasmids reversed
the protective effects of tanshinone IIA. To our knowledge, this is
the first study to assess the effects of tanshinone IIA on the
expression of miR-125b and to explore the critical role of
miR-125b/foxp3 signaling in the regulation of pyroptosis in HK1
cells. In future, the effects of tanshinone IIA on NPC will be
explored in vivo, providing a potential approach to treat
NPC in the clinic.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YW designed and performed the study, analyzed the
data, and wrote the manuscript. WJ was involved in conducting the
experiments, analyzing the data, and writing the manuscript. JW
contributed to the writing of the manuscript and data analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD,
Yang KY, Jin F, Shi M, Chen YP, Hu WH, et al: Gemcitabine and
cisplatin induction chemotherapy in nasopharyngeal carcinoma. N
Engl J Med. 381:1124–1135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang LQ, Chen DP, Guo L, Mo HY, Huang Y,
Guo SS, Qi B, Tang QN, Wang P, Li XY, et al: Concurrent
chemoradiotherapy with nedaplatin versus cisplatin in stage II–IVB
nasopharyngeal carcinoma: An open-label, non-inferiority,
randomised phase 3 trial. Lancet Oncol. 19:461–473. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sui H, Zhao J, Zhou L, Wen H, Deng W, Li
C, Ji Q, Liu X, Feng Y, Chai N, et al: Tanshinone IIA inhibits
β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in
normoxic and HIF-1α in hypoxic microenvironments in human
colorectal cancer. Cancer Lett. 403:86–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Tong C, Tang Y, Cong P, Liu Y, Shi
X, Shi L, Zhao Y, Jin H, Li J and Hou M: Tanshinone IIA alleviates
blast-induced inflammation, oxidative stress and apoptosis in mice
partly by inhibiting the PI3K/Akt/FoxO1 signaling pathway. Free
Radic Biol Med. 152:52–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Yang L, Zhang B and Chen S:
Tanshinone IIA effects on ovarian cancer cell line. J Pharm
Pharmacol. 70:1369–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Zhu W, Kong X, Chen X, Sun X, Zhang
W and Zhang R: Tanshinone IIA inhibits glucose metabolism leading
to apoptosis in cervical cancer. Oncol Rep. 42:1893–1903.
2019.PubMed/NCBI
|
|
10
|
Xu Z, Chen L, Xiao Z, Zhu Y, Jiang H, Jin
Y, Gu C, Wu Y, Wang L, Zhang W, et al: Potentiation of the
anticancer effect of doxorubicinin drug-resistant gastric cancer
cells by tanshinone IIA. Phytomedicine. 51:58–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong W, Guo J and Yang C: Tanshinone II A
enhances pyroptosis and represses cell proliferation of HeLa cells
by regulating miR-145/GSDMD signaling pathway. Biosci Rep.
40:BSR202002592020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren X, Wang C, Xie B, Hu L, Chai H, Ding
L, Tang L, Xia Y and Dou X: Tanshinone IIA induced cell death via
miR30b-p53-PTPN11/SHP2 signaling pathway in human hepatocellular
carcinoma cells. Eur J Pharmacol. 796:233–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Li Y, Zhu R, Song P, Wei Y, Liang
T and Xu G: Transcription factor p53 suppresses tumor growth by
prompting pyroptosis in non-small-cell lung cancer. Oxid Med Cell
Longev. 2019:87468952019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pizato N, Luzete BC, Kiffer LFMV, Corrêa
LH, de Oliveira Santos I, Assumpção JAF, Ito MK and Magalhães KG:
Omega-3 docosahexaenoic acid induces pyroptosis cell death in
triple-negative breast cancer cells. Sci Rep. 8:19522018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng QZ, Yang F, Li CG, Xu LH, He XH, Mai
FY, Zeng CY, Zhang CC, Zha QB and Ouyang DY: Paclitaxel enhances
the innate immunity by promoting NLRP3 inflammasome activation in
macrophages. Front Immunol. 10:722019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Batool A, Wang YQ, Hao XX, Chen SR and Liu
YX: A miR-125b/CSF1-CX3CL1/tumor-associated macrophage recruitment
axis controls testicular germ cell tumor growth. Cell Death Dis.
9:9622018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen J, Chang Y, Huo S, Li W, Huang H, Gao
Y, Lin H, Zhang J, Zhang Y, Zuo Y, et al: Tanshinone IIA attenuates
atherosclerosis via inhibiting NLRP3 inflammasome activation. Aging
(Albany NY). 13:910–932. 2020.PubMed/NCBI
|
|
20
|
Wang S, Wu J, Ren J, Vlantis AC, Li MY,
Liu SYW, Ng EKW, Chan ABW, Luo DC, Liu Z, et al: MicroRNA-125b
interacts with Foxp3 to induce autophagy in thyroid cancer. Mol
Ther. 26:2295–2303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamel R, Al-Badawy S, Khairy A, Kandil T
and Sabry A: Nasal and paranasal sinus changes after radiotherapy
for nasopharyngeal carcinoma. Acta Otolaryngol. 124:532–535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang L, Huang C, Gan Y, Wu T, Tang X,
Wang Y, Wang R and Zhang Y: Radiation-induced late dysphagia after
intensity- modulated radiotherapy in nasopharyngeal carcinoma
patients: A dose-volume effect analysis. Sci Rep. 8:163962018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujii H, Kamada T, Tsuji H, Takamura A,
Matsuoka Y, Usubuchi H and Irie G: Improved results in the
treatment of nasopharyngeal carcinoma using combined radiotherapy
and chemotherapy. Cancer. 63:1668–1672. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vignali DA, Collison LW and Workman CJ:
How regulatory T cells work. Nat Rev Immunol. 8:523–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer. 16:1242017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang G, Zhang W, Li B, Stringer-Reasor E,
Chu C, Sun L, Bae S, Chen D, Wei S, Jiao K, et al: MicroRNA-200c
and microRNA-141 are regulated by a FOXP3-KAT2B axis and associated
with tumor metastasis in breast cancer. Breast Cancer Res.
19:732017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Li X, Shu Z, Zhang K, Xue X, Li W,
Hao Q, Wang Z, Zhang W, Wang S, et al: Nuclear galectin-1-FOXP3
interaction dampens the tumor-suppressive properties of FOXP3 in
breast cancer. Cell Death Dis. 9:4162018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Germanidis G, Argentou N, Hytiroglou P,
Vassiliadis T, Patsiaoura K, Germenis AE and Speletas M: Liver
FOXP3 and PD1/PDL1 expression is down-regulated in chronic HBV
hepatitis on maintained remission related to the degree of
inflammation. Front Immunol. 4:2072013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang C, Huang XR, Fung E, Liu HF and Lan
HY: The regulatory T-cell transcription factor Foxp3 protects
against crescentic glomerulonephritis. Sci Rep. 7:14812017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang L, Chen Y, Xiang Q, Xiang J, Tang Y
and Li J: The association between IL18, FOXP3 and IL13 genes
polymorphisms and risk of allergic rhinitis: A meta-analysis.
Inflamm Res. 69:911–923. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin W, Chen F, Wang K, Song Y, Fei X and
Wu B: miR-15a/miR-16 cluster inhibits invasion of prostate cancer
cells by suppressing TGF-β signaling pathway. Biomed Pharmacother.
104:637–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong S, Yan Z, Wang H, Ding L, Song Y and
Bi M: miR-663b promotes colorectal cancer progression by activating
Ras/Raf signaling through downregulation of TNK1. Hum Cell.
33:104–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li A, Shuai X, Jia Z, Li H, Liang X, Su D
and Guo W: Ganoderma lucidum polysaccharide extract inhibits
hepatocellular carcinoma growth by downregulating regulatory T
cells accumulation and function by inducing microRNA-125b. J Transl
Med. 13:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY,
Zhang H and Chen YQ: Camptothecin induces apoptosis in cancer cells
via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol.
81:578–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hossainzadeh S, Ranji N, Naderi Sohi A and
Najafi F: Silibinin encapsulation in polymersome: A promising
anticancer nanoparticle for inducing apoptosis and decreasing the
expression level of miR-125b/miR-182 in human breast cancer cells.
J Cell Physiol. 234:22285–22298. 2019. View Article : Google Scholar : PubMed/NCBI
|