Introduction
Retinoblastoma (RB) is an intraocular malignancy
affecting young children, with the majority of reported cases
occurring before the age of 6 years old (1). The global incidence rate of RB is ~1
in 16,000-18,000 live births per year (2). Common clinical treatment strategies
for RB include chemical volume reduction, intra-arterial and
intravitreal chemotherapy, transpupillary thermotherapy, laser
photocoagulation and scleral application radiotherapy (3). The development of precision medicine
and tumor biological therapy, drugs or specific molecules that
target key regulatory proteases in cells have shown effective
application prospects (4,5). Due to the high rate of intracranial
and distant metastasis of RB, which often endangers the lives of
children, there is an urgent requirement to investigate the
potential biological regulation mechanism of RB progression,
thereby improving the cure rate and reducing the mortality
rate.
Most cases of RB develop from a mutation or the
inactivation of the RB1 tumor suppressor gene; however, the loss of
RB1 function may directly or indirectly lead to the disruption of
the intracellular signaling pathways, which ultimately leads to
tumor progression (2,6). As intracellular signaling pathways
have been frequently associated with abnormal expression of
microRNAs (miRNA/miR), this led to the hypothesis that miRNAs could
be involved in RB development. As reported by previous studies,
miRNAs are endogenous, conserved non-coding RNAs that are 19–22
nucleotides in length (7). The most
well-known function of miRNA is to inhibit gene translation or
induce subsequent mRNA degradation by binding to the
3′-untranslated region (3′-UTR) of their target mRNAs (8,9).
miRNAs are differentially expressed in tumor cells and have been
associated with the occurrence and development of tumors (10,11);
therefore, differentially expressed miRNAs in RB (compared with
those in healthy tissues or cells) in the Gene Expression Omnibus
(GEO) database were investigated in the present study. The GEO
database contains microarray, next-generation sequencing and other
high-throughput sequencing data (12). From the GEO database, the GSE7072
dataset was used in the present study and it was found that
miR-338-3p expression was significantly decreased in RB compared
with that in healthy tissues. Previous studies revealed that
miR-338-3p was a tumor suppressor gene, and was significantly
decreased in non-small cell lung cancer (NSCLC), nasopharyngeal
carcinoma and gastric cancer (13–15).
However, to the best of our knowledge, the role of miR-338-3p in RB
remains unknown. Thus, miR-338-3p and its potential downstream
targets were investigated in the present study.
Among the results from bioinformatics target
prediction, neuro-oncological ventral antigen 1 (NOVA1) was a gene
of interest. NOVA1 is an RNA binding protein, which was found to
affect cellular signal transduction and ligand-binding, as well as
possesses ion channel electrophysiological properties (16). It has been reported that NOVA1
serves an important role in the occurrence and development of
various diseases, including neurological diseases and tumors
(17,18). However, the mechanism of NOVA1 in RB
remains to be determined. Thus, the present study aimed to identify
the molecular mechanism of NOVA1 and the signaling pathway involved
in RB.
Materials and methods
Data collection and screening
The GSE7072 dataset from the GEO database was used
to screen target miRNAs for the present research.
Cell culture
The human retinoblastoma (HXO-RB44, SO-RB50, Y79 and
WERI-Rb-1) and the human retinal epithelial (ARPE-19) cell lines
were purchased from the Chinese Academy of Sciences Cell Bank, and
were cultured in DMEM, supplemented with 10% FBS (all from Hyclone;
Cytiva) at 37°C in a humidified incubator with 5% CO2.
The human retinal epithelial (ARPE-19) cell line, which was the
cell line closest to the source of the human retinoblastoma cell
lines (HXO-RB44, SO-RB50, Y79 and WERI-Rb-1), was used as the
control. The reported literatures also used ARPE cells as the
control of the Y79 cell line (19–21).
Tissue samples
The human retinoblastoma tissue samples were
obtained from The First Affiliated Hospital of Harbin Medical
University between April 2015 and September 2018. The RB tumor and
healthy adjacent tissues were matched. The distance of healthy
tissues from RB tissues was 2 cm. All patients (age, 35–58 years;
seven female patients and five male patients) were informed and
agreed to the use of tissue samples. Ethics approval was obtained
for the use of human tissues from the Ethics Committee of The First
Affiliated Hospital of Harbin Medical University. The data were
evaluated using a paired t-test.
Cell transfection
miR-338-3p mimics (agomir-338-3p) and the
corresponding negative control (agomir-NC) (cat. no.
miR40000763-4-5 for agomir-338-3p and cat. no. miR4N0000001-4-5 for
agomir-NC) were purchased from Guangzhou RiboBio Co., Ltd. The
pcDNA-NOVA1 and pcDNA-3.1 plasmids (cat. no. 4857 for pcDNA-NOVA1
and pcDNA-3.1 plasmids; Shanghai GeneChem Co., Ltd.) were
constructed and purchased from Shanghai GeneChem Co., Ltd.
Agomir-338-3p, agomir-NC, and the pcDNA-NOVA1 and pcDNA-3.1
plasmids were transfected into the Y79 and WERI-Rb-1 cells using
Lipofectamine® 2000 (cat. no. 11668027; Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions at 37°C for 48 h. For the agomir-338-3p, agomir-NC and
small interfering RNA (si)-NOVA1 or si-NC (Shanghai GeneChem Co.,
Ltd.) the amount used for transfection was 100 pmol. The sequences
of si-NOVA1 were: Sense 5′-AGACAGAACCAGUCAGCAUTT-3′ and antisense
5′-AUGCUGACUGGUUCUGUCUTT-3′. The sequences of si-NC were: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. For the pcDNA-NOVA1 and pcDNA-3.1
plasmids, the amount used for transfection was 3 µg. The
transfection was performed when the cell density reached ~40%.
After the transfection was completed, the cells were cultured in a
37°C incubator for 48 h. Transfection efficiency was determined
using reverse transcription-quantitative PCR (RT-qPCR). The cells
were collected for further experimentation at 48 h following
transfection.
RT-qPCR
The extraction of total RNA from cells or tissues
was performed using TRIzol® (cat. no. 15596-026;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. First-strand cDNA was reverse
transcribed from RNA (37°C for 15 min, 98°C for 5 min) using the
cDNA Synthesis kit (cat. no. 04897030001; Roche Diagnostics). For
RT-qPCR analysis, the SYBR Green master mix (ROX) (cat. no.
04913914001; Roche Diagnostics) was used with a 7500 Fast Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) at
the following thermocycling conditions: Initial denaturation (95°C
for 10 min; 40 cycles of denaturation (95°C for 15 sec), annealing
(60°C for 30 sec) and elongation (72°C for 30 sec). GAPDH and U6
were used as the controls for mRNA and miRNA, respectively. All the
primer sequences were designed and synthesized by Sangon Biotech
Co., Ltd. The primer sequence used for qPCR was as follows: GAPDH
forward, 5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; miR-338-3p forward,
5′-TCCCCTAACTCCCAGTGTCT-3′ and reverse, 5′-CTTGCCTTGGAGATTTGGG-3′;
and NOVA1 forward, 5′-GGGTTCCCATAGACCTGGAC-3′ and reverse,
5′-CGCTCAGTAGTACCTGGGTAA-3′. Relative gene differential expression
was determined using the 2−ΔΔCq method (22). All experiments were repeated in
triplicate.
Cell Counting Kit (CCK)-8 assay
The cells at a confluence of 60% in the logarithmic
growth phase were cultured in 96-well plates at 37°C in a
humidified incubator for 24 h. Then, the cells in each well were
incubated with 10 µl CCK-8 solution (cat. no. C0037; Beyotime
Institute of Biotechnology) for 1 h on a shaker according to the
manufacturer's instructions. Following which, the absorbance value
was measured at 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.). The experiments were performed in
triplicate.
Colony formation assay
The cells (~400/well) in the different treatment
groups were seeded in 6-well plates and cultured for 2 weeks.
Subsequently, each well of the cells was washed with PBS and fixed
with 4% paraformaldehyde on ice for 15 min. Then, 0.1% crystal
violet was used to stain the cells at room temperature for 10 min.
Finally, images of the cells were captured using an optical camera
(Olympus Corporation).
TUNEL assay
The TUNEL assay was performed using the one-step
TUNEL cell apoptosis detection kit (cat. no. C1088; Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. In brief, the cells were fixed with 4%
paraformaldehyde at room temperature for 30 min and washed with
PBS. Then, the cells were incubated with 0.3% Triton X-100 in PBS
for 5 min at room temperature. Following which, the cells were
stained with the staining mixture from the one-step TUNEL cell
apoptosis detection kit at room temperature for 30 min. The cells
in the sections were observed using a fluorescence microscope
(Olympus Corporation) in six fields of view randomly selected under
×200 magnification. The nuclei in the apoptotic cells were stained
with DAPI (10 µg/ml, cat. no. C1002; Beyotime Institute of
Biotechnology) at room temperature for 8 min. The number of
positive cells was calculated using ImageJ software (version 1.8.0;
National Institutes of Health).
Western blot analysis
Total protein was extracted from the cells or
tissues using RIPA buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology), which was mixed with protease inhibitors (Thermo
Fisher Scientific, Inc.). The concentration of the total protein
was quantified using a BCA assay (cat. no. PC0020-500; Beyotime
Institute of Biotechnology). Then, 10% SDS-PAGE was used to
separate the proteins (100 µg), which were subsequently transferred
onto nitrocellulose membranes (cat. no. 88025; Thermo Fisher
Scientific, Inc.) for 2 h, at a current of 300 mA. The membranes
were blocked with 5% skimmed milk for 2 h at room temperature, then
incubated with the following primary antibodies (all from Abcam)
overnight at 4°C: NOVA1 (cat. no. ab183024; 1:1,000), Bax (cat. no.
ab32503; 1:1,000), Bcl-2 (cat. no. ab182858; 1:1,000),
cleaved-caspase-3 (cat. no. ab32042; 1:1,000), tubulin (cat. no.
ab7291; 1:1,000), PI3K (cat. no. ab140307; 1:1,000), phosphorylated
(p)-PI3K (cat. no. ab278545; 1:1,000), AKT (cat. no. ab8805;
1:1,000), p-AKT (cat. ab38449; 1:1,000). On the second day, the
nitrocellulose membranes were incubated at room temperature for 45
min with the IRDye-labeled fluorescent secondary antibody
(IRDye-conjugated Goat anti-Mouse IgG, cat. no. 926-32210;
IRDye-conjugated Goat anti-Rabbit IgG, cat. no. 926-32211; LI-COR
Biosciences; 1:8,000) according to the source of the primary
antibody (rabbit or mouse). Subsequently, the bands on the
membranes were visualized using an Odyssey infrared fluorescence
scanning instrument (LI-COR Biosciences) and the detection of band
gray value was performed using Image studio software (version 4.0;
LI-COR Biosciences).
Cell migration and invasion
assays
For the cell invasion assay, the upper chambers (BD
Biosciences) were precoated with Matrigel (cat. no. 356234; BD
Biosciences) at 37°C for 2 h, then 5×104 cells were
seeded in the upper chambers of the culture plate with Matrigel and
cultured in serum-free medium. The lower chambers contained medium
supplemented with 15% FBS. After incubation at 37°C for 24 h, the
cells that invaded to the lower surface of the chamber were fixed
with 4% paraformaldehyde at room temperature for 20 min, stained
with 0.1% crystal violet at room temperature for 15 min and
subsequently observed using an optical microscope (Olympus
Corporation). For the cell migration assay, the cells
(5×104 cells per well) were seeded in the upper chambers
of the Transwell plate without Matrigel and the same protocol as
the invasion assay was used. The cells were randomly selected from
six fields of view using a confocal microscope (Olympus CX23;
Olympus Corporation) at ×200 magnification, then ImageJ software
(version 1.8.0; National Institutes of Health) was used to
calculate the number of invasive and migrated cells.
Dual-luciferase reporter assay
The Starbasev3.0 (http://starbase.sysu.edu.cn/) and the TargetScanv7.2
(http://www.targetscan.org/vert_72/)
predictive databases were used to predict the potential targets of
miR-338-3p in humans. Following bioinformatics prediction and
screening, potential binding sites were identified between
miR-338-3p and NOVA1. Therefore, a dual-luciferase reporter assay
was performed to verify that miR-338-3p directly binds to NOVA1.
Briefly, wild-type (wt) and mutant (mut) 3′-UTR of NOVA1 were
cloned into the pmirGLO luciferase reporter vector (Guangzhou
RiboBio Co., Ltd.). Subsequently, the Y79 and WERI-Rb-1 cells were
co-transfected with wt-NOVA1 or mut-NOVA1 and 100 nM agomir-338-3p
(Guangzhou RiboBio Co., Ltd.) or 100 nM agomir-NC Shanghai GeneChem
Co., Ltd. using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 48 h. Finally, luciferase
activity was determined using the dual-luciferase reporter assay
system (Promega Corporation) and normalized to Renilla
luciferase activity.
Tumor xenograft mouse model
The animal study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Research Council), and was approved by the Institutional Animal
Care and Use Committee of Harbin Medical University (Heilongjiang,
China). The mouse were kept under controlled conditions
(temperature, 21°C; humidity, 50–55%) with a 12-h light/dark cycle,
and free access to food and water. A mouse xenograft model was
randomly established by subcutaneously injecting 1×106
Y79 cells into 4-week-old female or male BALB/c nude mice (weight,
18–20 g) purchased from the Shanghai Laboratory Animal Center, CAS.
A total of 9 mice were used as the NCs and 9 mice were assigned to
the agomir-338-3p group. Then, the mice were injected intravenously
(tail vein) with either agomir-338-3p or agomir-NC (10 nM). The
tumor volumes were measured every 5 days following observation and
were calculated using the following formula: Volume = (length ×
width2)/2. The maximum diameter of the tumor was 1.85 cm
and the maximum volume was 1.85×1.72×1.5 cm. A month later the mice
were euthanized using isoflurane (induction, 3% and maintenance,
2%). The duration of isoflurane exposure was within 5 min. Death
was confirmed by the observing breathing rate, the heartbeat, the
pupils and the nerve reflex of the mouse. Finally, the tumor was
removed for further experiments.
Immunohistochemistry
The tumor tissues were fixed with 4%
paraformaldehyde at room temperature for 30 min. After the
transplanted tumor was paraffin-embedded and sliced into 0.5-µm
sections, immunohistochemistry analysis was performed. Briefly, the
paraffin sections were dewaxed in xylene and rehydrated in a graded
alcohol series (100, 95 and 80%). Then, the sections were blocked
with 5% BSA (cat. no. ST025; Beyotime Institute of Biotechnology)
at 37°C for 1 h, and heated in a microwave oven in sodium citrate
buffer (0.1 mM, pH 6.0) for 5 min for antigen retrieval.
Subsequently, the sections were incubated overnight at 4°C with a
primary antibody against Ki67 (cat. no. ab15580; 1:1,000; Abcam).
Following which, the sections were incubated with a secondary
antibody (cat. no. ab205718; 1:2,000; Abcam) at 37°C for 1 h. The
sections were then stained with diaminobenzidine at room
temperature for 3–15 min and counterstained with hematoxylin at
room temperature for 5 min. Finally, the images were captured using
an Olympus light microscope (Olympus Corporation) under ×200
magnification.
Statistical analysis
All the data are presented as the mean ± SEM from
three independent experiments. The software used for statistical
analysis was GraphPad Prism 5 (version 5.01; GraphPad Software,
Inc.). Statistical analysis between two groups was performed using
an unpaired Student's t-test, while in the analysis of miR-338-3p
expression levels in RB tumor and adjacent tissues, the data were
analyzed with a paired t-test. One-way ANOVA followed by Tukey's
post hoc test was performed when comparing >2 groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression levels of miR-338-3p and
possible roles in the RB cell lines
First, the GEO database was used to identify
differentially expressed miRNAs in RB cases compared with those in
normal tissues or retinal epithelial cells. Notably, in the GSE7072
dataset, it was found that miR-338-3p expression was significantly
decreased in RB compared with that in normal tissues (Fig. 1A). However, the role of miR-338-3p
in RB remains unknown. To further confirm the expression of
miR-338-3p in RB tissues and cell lines, RT-qPCR was performed. It
was found that the expression level of miR-338-3p in human RB
tissues was lower compared with that in normal tissues (Fig. 1B). Similarly, the expression levels
of miR-338-3p in the human RB cell lines (HXO-RB44, SO-RB50, Y79
and WERI-Rb-1) were downregulated (Fig.
1C), compared with those in the normal human retinal epithelial
cells (ARPE-19). The Y79 and WERI-Rb-1RB cell lines were selected
for further experiments, as there was a notably lower expression
level of miR-338-3p compared with the other cell lines.
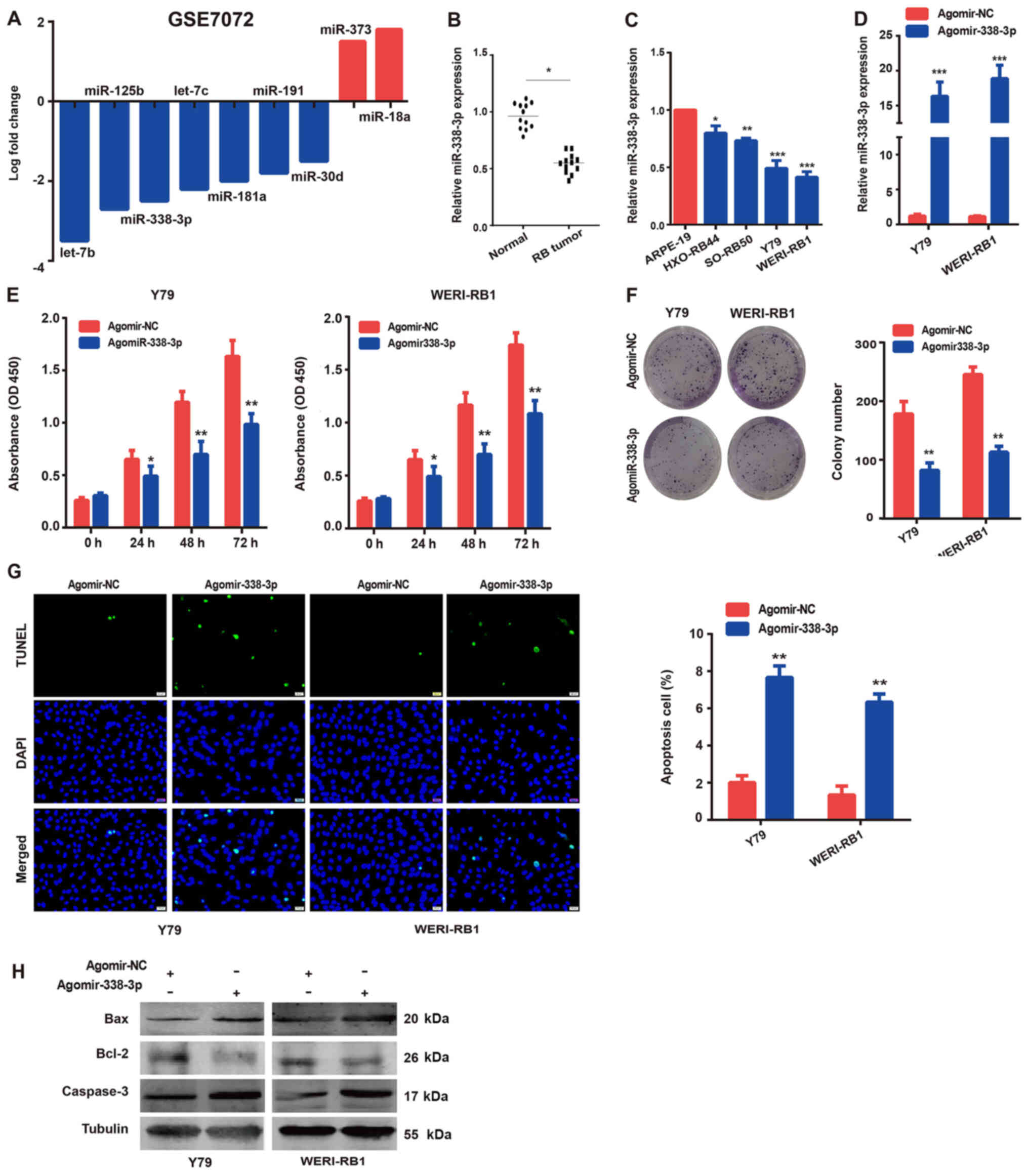 | Figure 1.Roles of miR-338-3p in the RB cell
lines. The expression levels of (A) miRNAs in the RB tissues from
the GSE7072 dataset. miR-338-3p expression levels in (B) RB tissues
compared with normal tissues, and (C) in the human RB (HXO-RB44,
SO-RB50, Y79 and WERI-Rb-1) cells compared with the normal human
retinal epithelial (ARPE-19) cell lines. (D) Transfection
efficiency of miR-338-3p mimics was confirmed using reverse
transcription-quantitative PCR. Overexpression of miR-338-3p
inhibited the (E) proliferation and (F) colony formation ability of
the Y79 and WERI-Rb-1 cells, as determined using Cell Counting
Kit-8 and colony formation assays, respectively. (G) Overexpression
of miR-338-3p increased the rate of apoptosis in the Y79 and
WERI-Rb-1 cells, as determined using a TUNEL assay (×200
magnification). (H) Western blot analysis was used to analyze the
protein expression levels of the apoptosis-related proteins. The
data are presented as the mean ± SEM from ≥3 independent
experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. ARPE-19
cells or agomir-NC. RB, retinoblastoma; miRNA/miR, microRNA; NC,
negative control; OD, optical density. |
To determine the role of miR-338-3p in RB cells,
miR-338-3p was overexpressed in the Y79 and WERI-Rb-1 cells by
transfecting them with agomir-338-3p. The transfection efficiency
was confirmed using RT-qPCR (Fig.
1D). After the overexpression of miR-338-3p, cell proliferation
and apoptosis were investigated, as well as the expression levels
of related protein markers. As shown in Fig. 1E and F, overexpression of miR-338-3p
significantly inhibited Y79 and WERI-Rb-1 cell proliferation,
compared with that observed in cells transfected with agomir-NC, as
detected using CCK-8 and colony formation assays. Conversely, the
apoptotic ratio of the Y79 and WERI-Rb-1 cells was significantly
elevated in the agomir-338-3p group (Fig. 1G). In addition, the expression
levels of several key regulatory proteins in the apoptosis
signaling pathway were determined using western blot analysis, and
the results (Fig. 1H) were
consistent with cell apoptosis analysis. The aforementioned results
suggested that miR-338-3p may act as a tumor suppressor in the RB
cells.
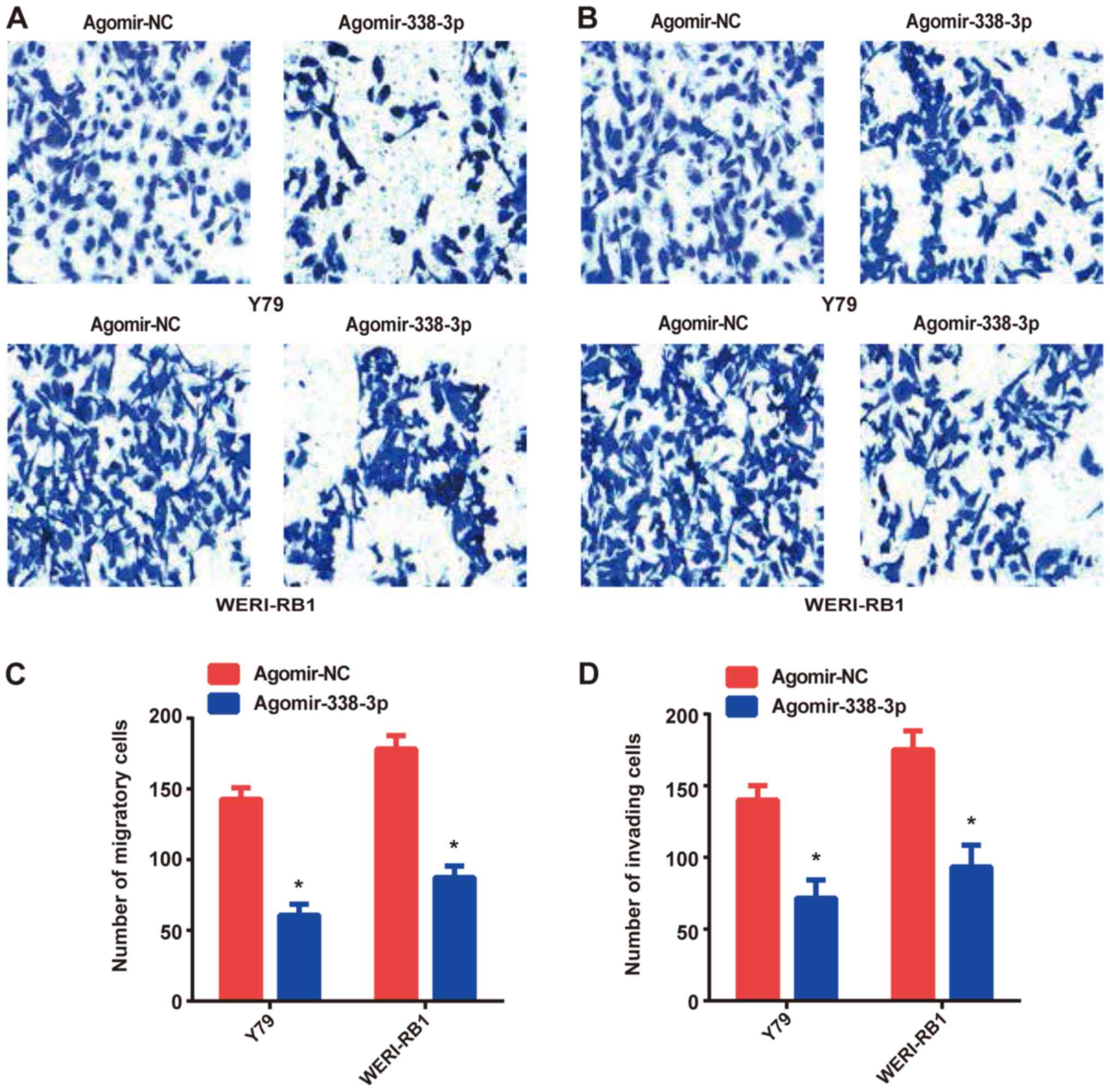
Overexpression of miR-338-3p inhibits
the migration and invasion of RB cells
Transwell and Matrigel assays were performed to
investigate the migratory and invasive abilities of the RB cells,
respectively. Compared with the agomir-NC group, overexpression of
miR-338-3p (agomir-338-3p group) significantly suppressed the
migratory and invasive abilities of Y79 and WERI-Rb-1 cells
(Fig. 2).
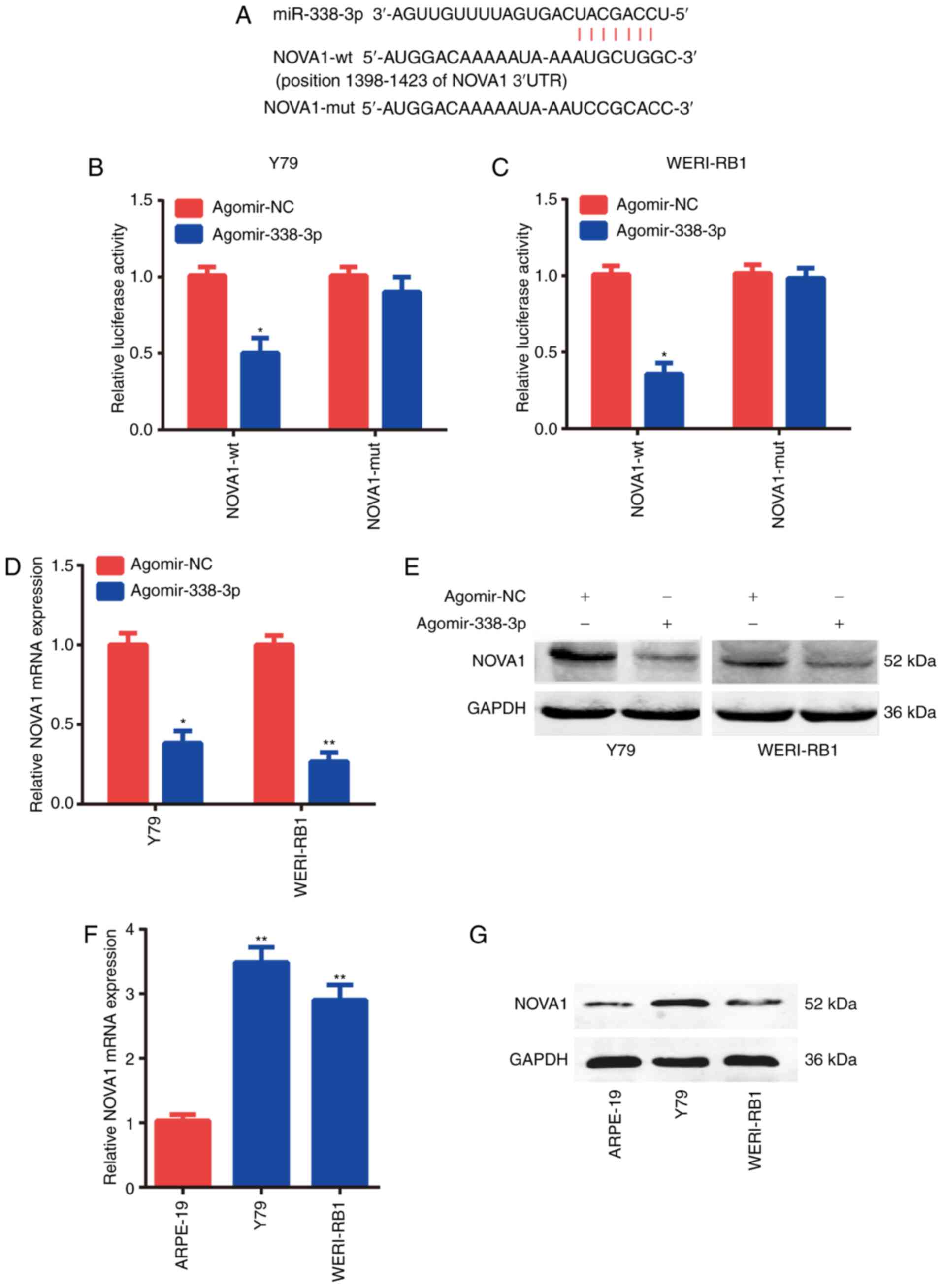
Target gene of miR-338-3p
The binding sites between miR-338-3p and the 3′-UTR
of NOVA1 mRNA were predicted using the Starbasev3.0 and TargetScan
online databases (Fig. 3A), which
indicated that NOVA1 was a potential target gene of miR-338-3p. To
confirm the binding sequences between miR-338-3p and NOVA1, a
dual-luciferase reporter assay was performed. The binding sites of
miR-338-3p with the position 1,398-1,423 of NOVA1 3′-UTR were used.
The results demonstrated that overexpression of miR-338-3p
suppressed luciferase activity in the Y79 and WERI-Rb-1 cells
co-transfected with the reporter plasmid containing the wt 3′-UTR
of NOVA1 and agomir-338-3p (Fig. 3B and
C). However, agomir-338-3p had no effect on luciferase activity
when the cells were transfected with the reporter plasmid
containing the MUT 3′-UTR of NOVA1.
It was found that overexpression of miR-338-3p could
notably decrease the mRNA (Fig. 3D)
and protein (Fig. 3E) expression
levels of NOVA1. As the expression levels of miR-338-3p were lower
in the Y79 and WERI-Rb-1 cells compared with those in the ARPE-19
cells, it was hypothesized that the expression levels of NOVA1
would be increased. Subsequently, it was found that the mRNA
(Fig. 3F) and protein (Fig. 3G) expression levels of NOVA1 were
significantly higher in the Y79 and WERI-Rb-1 cells compared with
those in the ARPE-19 cells. These data suggested that NOVA1 was a
direct target of miR-338-3p.
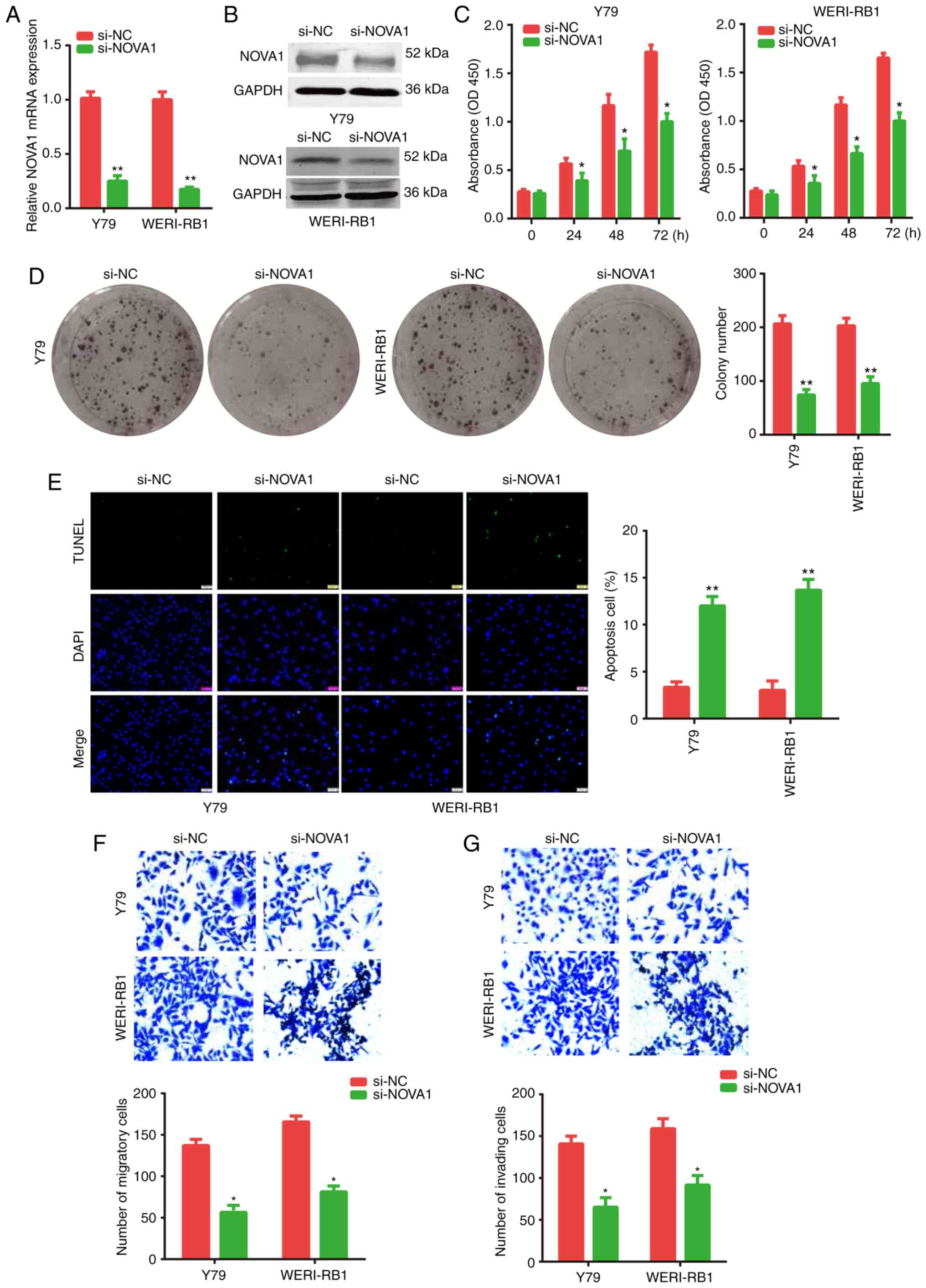
Knockdown of NOVA1 on the biological
activities of the RB cells
As the expression level of endogenous NOVA1 was
increased in the RB cells, NOVA1 expression was knocked down by
transfecting the Y79 or WERI-Rb-1 cells with si-NOVA1.
Subsequently, various biological activities of the cells were
investigated. The knockdown efficiency of NOVA1 was determined
using RT-qPCR (Fig. 4A) and western
blotting (Fig. 4B). Further
analysis revealed that knockdown of NOVA1 significantly inhibited
the proliferation of the Y79 and WERI-Rb-1 cells, which was
determined using CCK-8 (Fig. 4C)
and colony formation assays (Fig.
4D). As shown in Fig. 4E,
knockdown of NOVA1 significantly increased the apoptotic rate of
both the Y79 and WERI-Rb-1 cells. The Transwell assay also
confirmed that the NOVA1 gene may be an oncogene, as the migratory
and invasive abilities of the Y79 and WERI-Rb-1 cells were
significantly suppressed (Fig. 4F and
G) following knockdown of NOVA1. These findings indicated that
NOVA1 may act as an oncogene and serve a pivotal role in the
progression of RB cells.
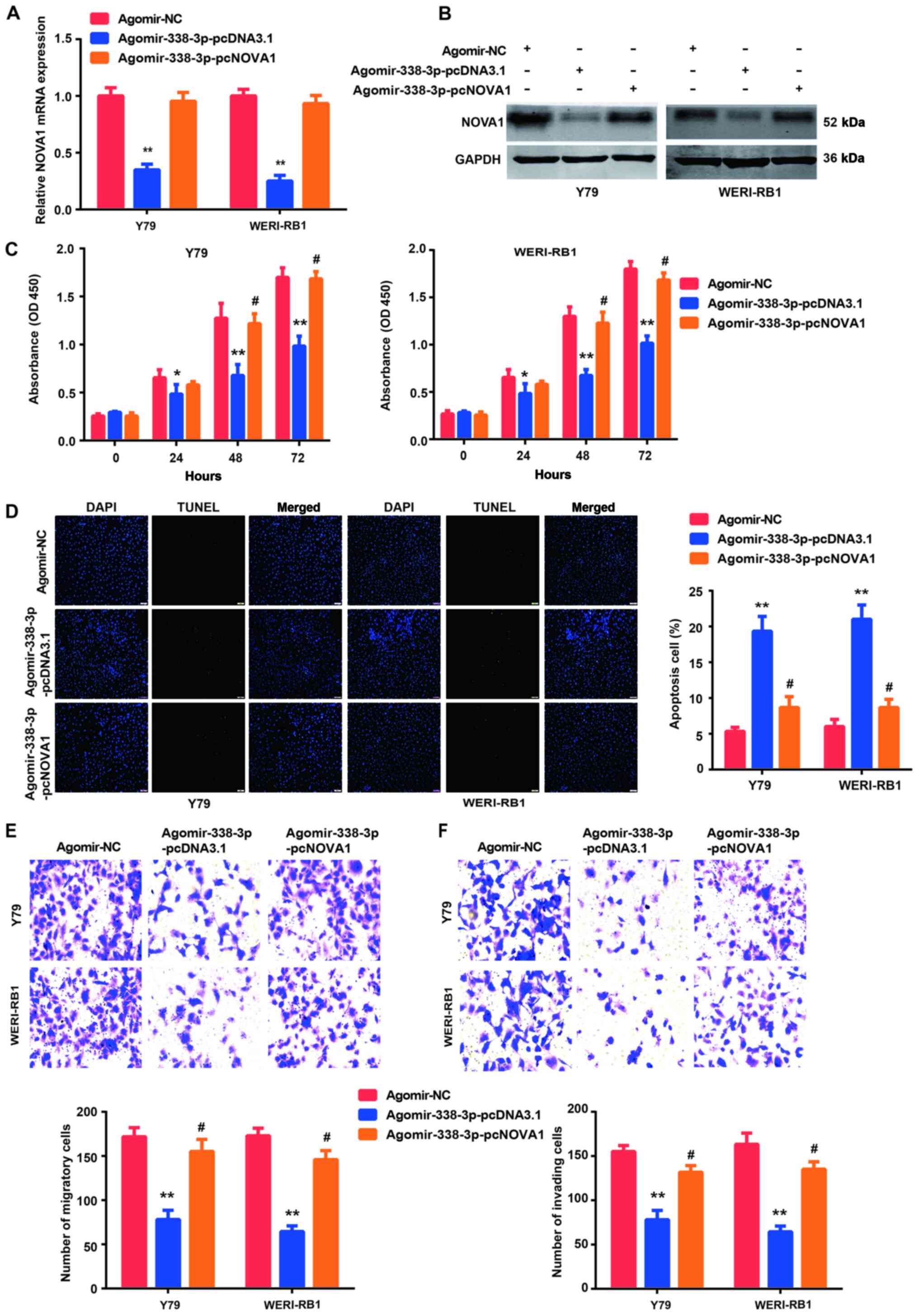
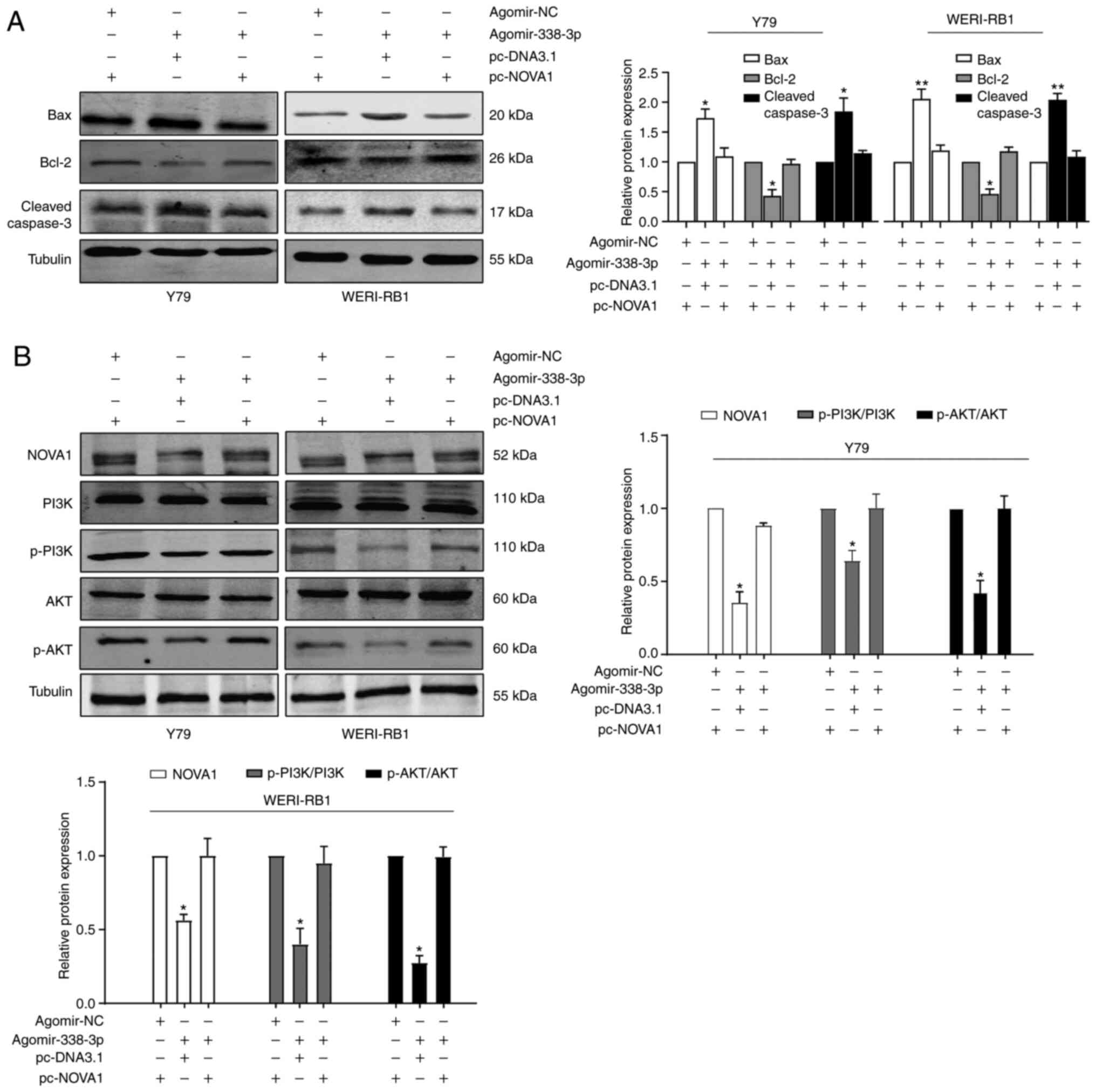
Co-transfection of agomir-338-3p and
pcNOVA1 plasmid counteract each other
According to the aforementioned results, it was
suggested that the miR-338-3p/NOVA1 axis may serve an important
role in the progression of RB. Thus, rescue experiments were
performed by co-transfecting agomir-338-3p and pcNOVA1 plasmid into
the Y79 and WERI-Rb-1 cells. The efficiency of overexpression of
NOVA1 was verified via RT-qPCR and western blot analysis (Fig. S1). The results demonstrated that
the decrease in NOVA1 mRNA (Fig.
5A) and protein (Fig. 5B)
expression levels, following overexpression of agomir-338-3p, were
rescued when the NOVA1 overexpression plasmid (pcNOVA1) was
co-transfected into Y79 and WERI-Rb-1 cells. Similarly, the induced
changes in cell proliferation (Fig.
5C), apoptosis (Fig. 5D),
migration (Fig. 5E) and invasion
(Fig. 5F), following overexpression
of agomir-338-3p, were all partially reversed by the
co-transfection of the pcNOVA1 plasmid.
Subsequently, the expression levels of several key
regulatory proteins in the apoptosis signaling pathway were
determined using western blot analysis, and the results were
consistent with the results from the cell apoptosis experiments
(Fig. 6A). Overexpression of
miR-338-3p increased the rate of cell apoptosis. With regards to
protein expression levels, there was a decrease in the expression
levels of Bcl-2, and an increase in the expression levels of Bax
and cleaved caspase-3 in the agomir-338-3p group compared with
those in the agomir-NC group. In addition, as PI3K and AKT are
known as anti-apoptotic protein markers (23), the expression levels of PI3K and AKT
were also detected. The results demonstrated that the expression
levels of PI3K and AKT were decreased in the agomir-338-3p group
(Fig. 6B), which was consistent
with the increased level of apoptosis. Furthermore, all of the
miR-338-3p-induced protein alterations could be partially reversed
by the overexpression of NOVA1. Taken together, it was indicated
that the miR-338-3/NOVA1 axis may serve an oncogenic role in the
progression of the RB cells.
Overexpression of miR-338-3p inhibits
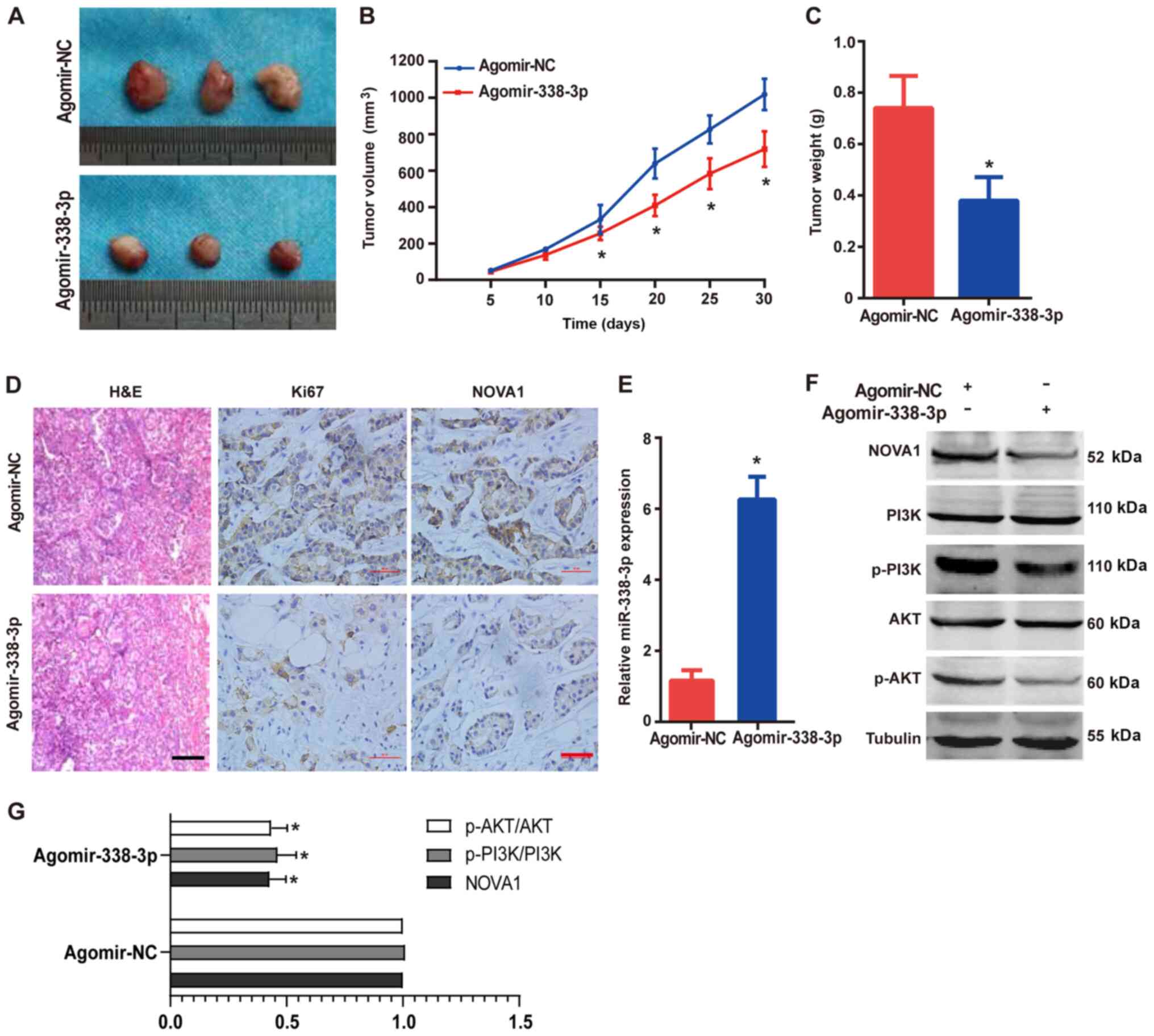
RB tumor progression in vivo
To validate the aforementioned results in
vivo, nude mice were injected with the Y79 cells to establish
xenograft RB tumors. Then, the mice were injected intravenously
with either agomir-338-3p or agomir-NC. The tumor volumes were
measured every 5 days after the outline of the tumors were
observed. A month later, all the mice were sacrificed under
anesthesia and the tumor was removed for further analysis. As
presented in Fig. 7A, compared with
that in the agomir-NC group, overexpression of miR-338-3p
significantly inhibited the growth of the RB tumor, as determined
by visual tumor size (Fig. 7A),
tumor volume (Fig. 7B) and weight
(Fig. 7C). In addition, the results
of immunohistochemistry identified that overexpression of
miR-338-3p inhibited the expression level of Ki67 (Fig. 7D), which indicated that the
proliferation of the tumor cells was decreased. The expression
levels of NOVA1 in the xenograft tumors were also downregulated
following overexpression of miR-338-3p (Fig. 7D), which was consistent with the
results of the in vitro experiments. Furthermore, the
expression levels of miR-338-3p in the xenograft tumors after
agomir-338-3p transfection were confirmed using RT-qPCR (Fig. 7E). The expression levels of PI3K and
AKT were detected using western blot analysis, and the results
(Fig. 7F and G) indicated that
overexpression of miR-338-3p inhibited the expression levels of
PI3K and AKT (in the phosphorylation state), suggesting that the
growth of the tumor was inhibited. Consistent with the results
obtained from the RB cell lines, overexpression of miR-338-3p in
the xenograft tumors inhibited the expression levels of NOVA1. The
in vivo results further demonstrated the tumor suppressor
role of miR-338-3p in the RB cells.
Discussion
It is well-known that RB is a common infant retinal
cancer, which develops when both RB1 alleles are mutated or have a
loss of function (6). Moreover, the
occurrence and further progression of RB requires additional
epigenetic dysregulation, following the inactivation of the RB1
protein, which manifests as the disruption of multiple signaling
pathways in the tumor cells (24).
The epigenetic dysregulation in RB includes abnormal histone
modifications, DNA methylation or acetylation and non-coding RNAs
(25). With regards to non-coding
RNAs, aberrant non-coding RNA expression in RB tumorigenesis and
progression has been reported (21,26,27).
While aberrant non-coding RNAs may not be the initial cause of RB,
the progression of RB has been associated with these molecular
alterations.
In recent years, a large number of miRNAs have been
reported to serve as tumor suppressors or oncogenes in tumor cells.
For instance, miR-338-3p has been discovered to be a tumor
suppressor in numerous types of cancer, such as NSCLC,
nasopharyngeal carcinoma and gastric cancer (13–15).
As the expression level of miR-338-3p in RB was downregulated in
the GEO database, which was consistent with different types of
cancer examined in previous studies (28,29),
it was suggested that miR-338-3p could be a tumor suppressor. Thus,
we hypothesized that miR-338-3p may also be a tumor suppressor in
RB. Combined with the prediction from bioinformatics analysis, it
was found that the oncogene, NOVA1, was the downstream target gene
of miR-338-3p. Thus, the present study aimed to further examine the
miR-338-3p/NOVA1 axis and its role in RB progression. It was
identified that the expression level of miR-338-3p was increased in
both RB tissues (compared with that in normal tissues) and cell
lines. Overexpression of miR-338-3p notably inhibited the viability
of the RB cells, induced cell apoptosis and suppressed the
migratory and invasive abilities. In addition, the RB cells with
decreased NOVA1 expression level exhibited a similar phenotype to
cells with miR-338-3p overexpression. The effects of agomir-338-3p
on the RB cells were partially reversed by NOVA1 overexpression
plasmid. Mechanistically, the downstream target gene of miR-338-3p,
NOVA1, was demonstrated to be responsible for the regulation of the
aforementioned cellular activities. In addition, the expression
levels of several proteins associated with cell proliferation and
apoptosis were determined in vivo and in vitro;
however, there was no direct evidence that the miR-338-3p/NOVA1
axis could directly regulate the function or expression level of
these proteins, although the present study provided evidence that
overexpression of miR-338-3p or NOVA1 could cause changes in the
expression of these proteins.
Accumulating evidence has reported the function of
RB1 in RB (24,30,31).
The main research goal of the present study was to investigate the
role of the miR-338-3p/NOVA1 axis in RB. Whether the alteration of
the miR-338-3p/NOVA1 axis was associated with RB1 required further
examination. In the current study, the relevance of the
miR-338-3/NOVA1 axis to the RB1 gene was not determined. One
possibility is that NOVA1 is a protein that can alternatively
splice the mRNA of certain genes, including oncogenes (32,33).
After the expression level of NOVA1 is increased (for example, in
an environment of low miR-338-3p expression), the variable splicing
mode of the genes regulated by NOVA1 changes, which eventually
leads to the disorder of the cell signaling pathway (34).
A limitation of the present was that there were no
experiments to examine all the upstream and downstream proteins
associated with cell apoptosis, as well as the signal pathways
associated with cell proliferation, migration and invasion.
Furthermore, in the in vivo xenograft tumor experiment, only
the effect of miR-338-3p on RB tumors was confirmed. Due to the
technical difficulty of knocking down NOVA1 in the mice, there was
a lack of NOVA1 knockdown experiments in vivo. Moreover, due
to the limitations of experimental equipment, conditions and
technical level, the present study was currently unable to
establish orthotopic mouse models. Thus, the establishment of
xenotransplantation mouse model (Y-79 cells subcutaneously injected
into mice) is permitted and reasonable (35,36).
These limitations in the present study require further
investigation.
Although the previous published literature has
proved the role of NOVA1 in RB (37), the underlying molecular mechanism
remains unknown and needs to be verified by in vivo animal
experiments. The present study provided evidence that the
miR-338-3p/NOVA1 axis serves a key regulatory role in various
activities of RB through in vivo and in vitro
experiments. To the best of our knowledge, this was the first time
that it has been confirmed in RB that miR-338-3p is a tumor
suppressor gene. Due to the complexity of the regulatory pathways
inside the cell, other proteases or molecules are also reported to
regulate the progress of RB, including long non-coding RNA
(38) and circular RNA (39). These factors ultimately led to
changes in miRNAs. Therefore, miRNAs serve important roles in the
process of regulating cellular activities.
In conclusion, the results of the present study
suggested that the miR-338-3p/NOVA1 axis could be a key signaling
pathway in regulating the progression of the RB tumor.
Overexpression of miR-338-3p inhibited cell proliferation,
migration and invasion, as well as promoted the apoptosis of the RB
cells by targeting NOVA1. Understanding the aberrant molecular
signaling pathways in RB could be beneficial to develop improved
treatments and prevention strategies in a clinical setting. The
present study, to determine the molecular characteristics of RB,
may theoretically contribute to the potential therapeutic
strategies for RB.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the China Postdoctoral
Science Foundation (grant no. 2019M651310), the National Natural
Science Foundation of China (grant no. 81671741) and the Excellent
Youth Project of The Fourth Affiliated Hospital of Harbin Medical
University (grant no. HYDSYYXQN202021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS designed the project, wrote the manuscript and
performed the immunohistochemistry experiments. RW performed the
western blot analysis and PCR. SY and SL performed the
immunohistochemistry, cell proliferation, apoptosis, invasion and
migration experiments. LW performed the colony formation experiment
and the in vivo experiments. JW was the project leader, was
responsible for the design of the project, the revision of the
manuscript and performed some of the experiments. SS and JW are
responsible for confirming the authenticity of the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Research Council), and was approved by the Institutional Animal
Care and Use Committee of Harbin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interets.
References
|
1
|
Lin FY and Chintagumpala MM: Neonatal
retinoblastoma. Clin Perinatol. 48:53–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee C and Kim JK: Chromatin regulators in
retinoblastoma: Biological roles and therapeutic applications. J
Cell Physiol. 236:2318–2332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fabian ID, Johnson KP, Stacey AW, Sagoo MS
and Reddy MA: Focal laser treatment in addition to chemotherapy for
retinoblastoma. Cochrane Database Syst Rev.
6:CD0123662017.PubMed/NCBI
|
|
4
|
Evangelatos G, Fragoulis GE, Koulouri V
and Lambrou GI: MicroRNAs in rheumatoid arthritis: From
pathogenesis to clinical impact. Autoimmun Rev. 18:1023912019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimaras H, Corson TW, Cobrinik D, White A,
Zhao J, Munier FL, Abramson DH, Shields CL, Chantada GL, Njuguna F,
et al: Retinoblastoma. Nat Rev Dis Primers. 1:150212015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng R, Huang J, Sun Y and Luo J: Cell
proliferation is induced in renal cell carcinoma through miR-92a-3p
upregulation by targeting FBXW7. Oncol Lett. 19:3258–3268.
2020.PubMed/NCBI
|
|
9
|
Wang H, Li X, Li T, Wang L, Wu X, Liu J,
Xu Y and Wei W: Multiple roles of microRNA-146a in immune responses
and hepatocellular carcinoma. Oncol Lett. 18:5033–5042.
2019.PubMed/NCBI
|
|
10
|
Virga F, Quirico L, Cucinelli S, Mazzone
M, Taverna D and Orso F: MicroRNA-mediated metabolic shaping of the
tumor microenvironment. Cancers (Basel). 13:1272021. View Article : Google Scholar
|
|
11
|
Annese T, Tamma R, De Giorgis M and
Ribatti D: microRNAs biogenesis, functions and role in tumor
angiogenesis. Front Oncol. 10:5810072020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu L, Wu M, Lu Y, Zhao Z, Liu T, Fu W and
Li W: MicroRNA-424 regulates cisplatin resistance of gastric cancer
by targeting SMURF1 based on GEO database and primary validation in
human gastric cancer tissues. OncoTargets Ther. 12:7623–7636. 2019.
View Article : Google Scholar
|
|
13
|
Chen Q, Guo SM, Huang HQ, Huang GP, Li Y,
Li ZH, Huang R, Xiao L, Fan CR, Yuan Q, et al: Long noncoding RNA
SBF2-AS1 contributes to the growth and metastatic phenotypes of
NSCLC via regulating miR-338-3p/ADAM17 axis. Aging (Albany NY).
12:17902–17920. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Liu F, Wang F, Yang X and Guo W:
CircZNF609 promotes cell proliferation, migration, invasion, and
glycolysis in nasopharyngeal carcinoma through regulating HRAS via
miR-338-3p. Mol Cell Biochem. 476:175–186. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu H, Zhang Q, Sun Y, Wu D and Liu L:
LINC00689 induces gastric cancer progression via modulating the
miR-338-3p/HOXA3 axis. J Gene Med. 22:e32752020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buckanovich RJ, Posner JB and Darnell RB:
Nova, the paraneoplastic Ri antigen, is homologous to an
RNA-binding protein and is specifically expressed in the developing
motor system. Neuron. 11:657–672. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gumina V, Colombrita C, Fallini C,
Bossolasco P, Maraschi AM, Landers JE, Silani V and Ratti A: TDP-43
and NOVA-1 RNA-binding proteins as competitive splicing regulators
of the schizophrenia-associated TNIK gene. Biochim Biophys Acta
Gene Regul Mech. 1862:1944132019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang YA, Liu HN, Zhu JM, Zhang DY, Shen
XZ and Liu TT: RNA binding protein Nova1 promotes tumor growth in
vivo and its potential mechanism as an oncogene may due to its
interaction with GABAA Receptor-γ2. J Biomed Sci. 23:712016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai S, Tian B, Li A, Yao Q, Zhang G and Li
F: MicroRNA-125b promotes tumor growth and suppresses apoptosis by
targeting DRAM2 in retinoblastoma. Eye (Lond). 30:1630–1638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jwala J, Vadlapatla RK, Vadlapudi AD,
Boddu SH, Pal D and Mitra AK: Differential expression of folate
receptor-alpha, sodium-dependent multivitamin transporter, and
amino acid transporter (B (0, +)) in human retinoblastoma (Y-79)
and retinal pigment epithelial (ARPE-19) cell lines. J Ocul
Pharmacol Ther. 28:237–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Zhu Y, Zuo G, Chen X, Cheng J and
Zhang S: LINC00858 promotes retinoblastoma cell proliferation,
migration and invasion by inhibiting miR-3182. Exp Ther Med.
19:999–1005. 2020.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu ACY, Chern YJ, Zhang P, Pasiliao CC,
Rahman M, Chang G, Ren J and Tai IT: Inhibition of nucleophosmin 1
suppresses colorectal cancer tumor growth of patient-derived
xenografts via activation of p53 and inhibition of AKT. Cancer Biol
Ther. 22:112–123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Francis JH, Richards AL, Mandelker DL,
Berger MF, Walsh MF, Dunkel IJ, Donoghue MT and Abramson DH:
Molecular changes in retinoblastoma beyond RB1: Findings from
next-generation sequencing. Cancers (Basel). 13:1492021. View Article : Google Scholar
|
|
25
|
Guzman F, Fazeli Y, Khuu M, Salcido K,
Singh S and Benavente CA: Retinoblastoma tumor suppressor protein
roles in epigenetic regulation. Cancers (Basel). 12:28072020.
View Article : Google Scholar
|
|
26
|
Zhang C and Wu S: microRNA-378a-3p
restrains the proliferation of retinoblastoma cells but promotes
apoptosis of retinoblastoma cells via inhibition of FOXG1. Invest
Ophthalmol Vis Sci. 61:312020. View Article : Google Scholar
|
|
27
|
Kashiwa A, Aiba T, Makimoto H, Shimamoto
K, Yamagata K, Kamakura T, Wada M, Miyamoto K, Inoue-Yamada Y,
Ishibashi K, et al: Systematic evaluation of KCNQ1 variant using
ACMG/AMP guidelines and risk stratification in long QT syndrome
type 1. Circ Genom Precis Med. Sep 16–2020.(Epub ahead of print).
doi: 10.1161/CIRCGEN.120.002926. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y and Qin H: miR-338-3p targets RAB23
and suppresses tumorigenicity of prostate cancer cells. Am J Cancer
Res. 8:2564–2574. 2018.PubMed/NCBI
|
|
29
|
Wang L, Peng X, Lu X, Wei Q, Chen M and
Liu L: Inhibition of hsa_circ_0001313 (circCCDC66) induction
enhances the radio-sensitivity of colon cancer cells via tumor
suppressor miR-338-3p: Effects of cicr_0001313 on colon cancer
radio-sensitivity. Pathol Res Pract. 215:689–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raizis AM, Racher HM, Foucal A, Dimaras H,
Gallie BL and George PM: DNA hypermethylation/boundary control loss
identified in retinoblastomas associated with genetic and
epigenetic inactivation of the RB1 gene promoter. Epigenetics. Dec
1–2020.(Epub ahead of print). doi: 10.1080/15592294.2020.1834911.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehyar M, Mosallam M, Tbakhi A, Saab A,
Sultan I, Deebajah R, Jaradat I, AlJabari R, Mohammad M, AlNawaiseh
I, et al: Impact of RB1 gene mutation type in retinoblastoma
patients on clinical presentation and management outcome. Hematol
Oncol Stem Cell Ther. 13:152–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sayed ME, Yuan L, Robin JD, Tedone E,
Batten K, Dahlson N, Wright WE, Shay JW and Ludlow AT: NOVA1
directs PTBP1 to hTERT pre-mRNA and promotes telomerase activity in
cancer cells. Oncogene. 38:2937–2952. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villate O, Turatsinze JV, Mascali LG,
Grieco FA, Nogueira TC, Cunha DA, Nardelli TR, Sammeth M, Salunkhe
VA, Esguerra JL, et al: Nova1 is a master regulator of alternative
splicing in pancreatic beta cells. Nucleic Acids Res.
42:11818–11830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trujillo CA, Rice ES, Schaefer NK, Chaim
IA, Wheeler EC, Madrigal AA, Buchanan J, Preissl S, Wang A, Negraes
PD, et al: Reintroduction of the archaic variant of NOVA1 in
cortical organoids alters neurodevelopment. Science.
371:eaax25372021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim DY, Choi JA, Koh JY and Yoon YH:
Efficacy and safety of aflibercept in in vitro and in vivo models
of retinoblastoma. J Exp Clin Cancer Res. 35:1712016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kulkarni AD, van Ginkel PR, Darjatmoko SR,
Lindstrom MJ and Albert DM: Use of combination therapy with
cisplatin and calcitriol in the treatment of Y-79 human
retinoblastoma xenograft model. Br J Ophthalmol. 93:1105–1108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu XM, Li XF and Li JC: MiR-146a
functions as a potential tumor suppressor in retinoblastoma by
negatively regulate neuro-oncological ventral antigen-1. Kaohsiung
J Med Sci. Dec 19–2020.(Epub ahead of print). doi:
10.1002/kjm2.12337. View Article : Google Scholar
|
|
38
|
Xu L, Zhu S, Tang A and Liu W: LncRNA
MBLN1-AS1 inhibits the progression of retinoblastoma through
targeting miR-338-5p-Wnt/β-catenin signaling pathway. Inflamm Res.
70:217–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Yuan HF, Xu D, Chen KJ, Tan N and
Zheng QJ: Circular RNA circ_0000034 upregulates STX17 level to
promote human retinoblastoma development via inhibiting miR-361-3p.
Eur Rev Med Pharmacol Sci. 24:12080–12092. 2020.PubMed/NCBI
|