Introduction
Parkinson's disease (PD), second only to Alzheimer's
disease in prevalence, is diagnosed in ~1% of individuals over the
age of 65, and is a progressive multi-system neurodegenerative
disease closely associated with age (1). A meta-analysis of worldwide data has
revealed that the incidence and prevalence of PD increase with age
(2). Notably, PD is characterized
by the progressive death of dopaminergic neurons in the substantia
nigra pars compacta, as well as the widespread presence of the
intracellular protein, α-Synuclein (A-SYN) (3). The clinical symptoms of PD mainly
include tremors, stiffness, posture instability, bradykinesia,
autonomic nerve dysfunction and mental disorders (4). Currently, dopamine-based drugs, such
as levodopa, are being utilized in PD therapy to correct dyspraxia
(5). However, the long-term use of
these drugs can result in a variety of serious side effects,
including cardiovascular disease and pneumonia (6). Therefore, it is crucial to
comprehensively understand the pathogenesis of PD and develop novel
biological therapeutic targets against PD.
Studies have shown that the occurrence and
development of PD are related to the environment, genetics,
metabolic deficiency, oxidative stress and neuroinflammatory
response (2,7,8).
Reportedly, inhibition of tyrosine hydroxylase (TH) activity was
found to be closely related to the occurrence of PD, and
immunohistochemical determination of TH expression has provided an
essential tool for visualization and quantification of the damage
and loss to dopaminergic neurons in PD models (9,10).
Additionally, an in vitro study by Salemme et al
(11) indicated that
dihydroasparagusic acid can alleviate neurodegenerative diseases by
inhibiting inflammatory and oxidative processes. Another study has
shown that compared with healthy subjects, the production of
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α was
significantly enhanced in the peripheral blood of patients with PD
(12). Nuclear factor-κB (NF-κB),
an important transcription factor, plays an essential role in cell
growth and proliferation, regulating the inflammatory response by
affecting the expression of its downstream genes, including TNF-α,
IL-6 and IL-1β (13). Furthermore,
the NF-κB signaling pathway plays a key physiological role in the
central nervous system. Ghosh et al (4) reported that the NF-κB essential
modifier-binding domain enters the central nervous system, blocks
the activation of NF-κB and inhibits the expression of
pro-inflammatory factors, thereby improving behavioral functions in
PD mice.
A previous study has suggested the interaction
between the nuclear receptor subfamily 4 group A member 2 (Nurr1)
and NF-κB (14). Nurr1 is a
transcription factor belonging to the nuclear steroid hormone
receptor superfamily and is involved in numerous biological
processes, including cell proliferation, apoptosis and migration
(15). McEvoy et al
(16) elucidated that NF-κB binds
to the Nurr1 promoter and stimulates Nurr1 expression in rheumatoid
arthritis (RA) synovial cells, thus participating in RA by
mediating multiple inflammatory signals. However, the roles of
Nurr1 and NF-κB in neuroinflammation associated with PD remain
unclear.
Typically, the pheochromocytoma (PC12) cell line is
employed as a cell model for neurobiological and neurochemical
investigations (17,18). Therefore, in the present study,
NF-κB-knockdown PC12 cells were first constructed; alternatively,
PC12 cells were first treated with the NF-κB inhibitor, quinazoline
(QNZ). Next, the cells were utilized to build an inflammatory cell
model using lipopolysaccharide (LPS). Then, mechanisms underlying
the role of NF-κB in the inflammatory progression of PD were
explored. These findings may help improve our understanding of the
inflammatory progression during PD and provide novel therapeutic
targets for the amelioration and treatment of PD.
Materials and methods
Cell culture
Highly differentiated rat PC12 cells were obtained
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. PC12 cells were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.), 100 kU/l penicillin
(Thermo Fisher Scientific, Inc.) and 100 mg/l streptomycin (Thermo
Fisher Scientific, Inc.), and incubated in an incubator with 5%
carbon dioxide at 37°C.
Cell transfection
NF-κB-knockdown PC12 cells were constructed
using small interfering (si)-NF-κB (Guangzhou RiboBio Co., Ltd.).
Cell transfection was performed as previously described (19). The PC12 cells were seeded into
6-well plates (5×105 cells/well), and then transfected
with 50 nM si-negative control (si-NC, forward:
UUCUCCGAACGUGUCACGUTT, reverse: ACGUGACACGUUCGGAGAATT) and 50 nM
si-NF-κB [si-NF-κB-1 (forward: GCUUUGACUCACUCCAUAUTT, reverse:
AUAUGGAGUGAGUCAAAGCTT), si-NF-κB-2 (forward: GCACCAAGACCGAAGCAAUTT,
reverse: AUUGCUUCGGUCUUGGUGCTT), si-NF-κB-3 (forward:
GCAGGUAUUUGACAUACUATT, reverse: UAGUAUGUCAAAUACCUGCTT)] using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The cells in the
control group were cultured in the medium. After culturing for
another 24 h, the total RNA of cells was extracted, and the
transfection efficiency was evaluated by determining the expression
level of NF-κB using reverse transcription-quantitative PCR
(RT-qPCR) and western blotting.
RT-qPCR
After transfection, total RNA was extracted from the
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. Then, total RNA
was reverse transcribed into cDNA using the PrimeScript™ II 1st
Strand cDNA Synthesis kit (Takara Bio, Inc.), and the temperature
protocol used for reverse transcription was 37°C for 60 min, and
85°C for 5 sec. SYBR Premix Ex Taq (2X, Thermo Fisher Scientific,
Inc.) was used for qPCR, and the thermocycling conditions were as
follows: Initial denaturation at 95°C for 2 min; followed by 40
cycles of denaturation at 95°C for 15 sec, and 60°C for 60 sec, and
annealing and extension at 95°C for 15 sec, 60°C for 60 sec and
95°C for 15 sec. The relative expression of NF-κB was normalized to
the internal reference gene GAPDH (forward:
5′-AGACAGCCGCATCTTCTTGT-3′, reverse: 5′-CTTGCCGTGGGTAGAGTCAT-3′),
and calculated using the 2−ΔΔCq method (20). The primer sequence of NF-κB
was as follows: Forward, 5′-ACTATGAGGTCTCTGGGGGGA−3′ and reverse,
5′-GAAGCTGAGTTTGCGGAAGG-3′.
Cell viability assay
For all treated PC12 cells, cell viability was
measured using the Cell Counting Kit-8 (CCK-8; Biosharp Life
Sciences). Highly differentiated PC12 cells were seeded into
96-well plates (1×104 cells/well), and the cells were
randomly divided into the following groups: i) Control group; ii)
QNZ groups with different concentrations (3, 5, 7, 9 and 11 nM);
iii) LPS group; iv) LPS + QNZ group; v) LPS + si-NC group; and vi)
LPS + si-NF-κB group. Except for the control and LPS groups, cells
in other groups were first treated with QNZ (Selleck Chemicals) or
transfected for 6 h, and then LPS was added at a final
concentration of 600 ng/ml. Cells in the control group were treated
with phosphate-buffered saline (PBS), whereas cells in the LPS
group were first treated with PBS for 6 h, and then with LPS. After
24 h of cell culture, 20 µl CCK-8 reagent was added to cells and
incubated at 37°C for 4 h. Finally, absorbance was measured at 450
nm on a microplate reader.
Enzyme-linked immunosorbent assay
(ELISA)
In PC12 cells (5×105 cells/well) that
underwent different treatments, the levels of IL-1β, IL-6 and TNF-α
were determined using rat IL-1β, IL-6 and TNF-α ELISA assay kits
(all purchased from Elabscience, Inc.), respectively, according to
the manufacturer's instructions.
Immunofluorescent microscopy of TH,
A-SYN and Nurr1 expression
Briefly, PC12 cells were divided into six groups: i)
Control; ii) QNZ; iii) LPS; iv) LPS + QNZ; v) LPS + si-NC; and vi)
LPS + si-NF-κB. The differentiated PC12 cells (5×105
cells/well) were plated on glass coverslips, washed with PBS three
times, and then fixed with 4% paraformaldehyde for 15 min at room
temperature. After washing, fixed cells were permeabilized with
0.5% Triton X-100 (Beyotime Institute of Biotechnology) at room
temperature for 20 min, and blocked with 3% bovine serum albumin
(Thermo Fisher Scientific, Inc.) in PBS for 10 min at room
temperature. Then, anti-TH antibody (1:100; cat. no. 25859-1-AP;
ProteinTech Group, Inc.), anti-A-SYN antibody (1:100; cat. no.
10842-1-AP; ProteinTech Group, Inc.) and anti-Nurr1 antibody
(1:100; cat. no. ab41917, Abcam) were added to the coverslips at
4°C and incubated overnight. After washing, cells were incubated
with a Cy3-labeled sheep anti-rabbit IgG secondary antibody (1:100;
cat. no. BA1032, Wuhan Boster Biological Technology, Ltd.) at 25°C
for 1 h. After washing, DAPI (two drops) was added to the
coverslips and incubated in the dark for 5 min. After washing with
PBS, coverslips were mounted in a mounting medium containing DAPI
(SouthernBiotech), and then observed under a fluorescence
microscope.
Western blotting
In brief, total protein was isolated from different
PC12 cell groups using RIPA protein lysis buffer (Beyotime
Institute of Biotechnology), and the concentrations were determined
using a BCA protein assay kit (Wuhan Boster Biological Technology,
Ltd.) in accordance with the manufacturer's protocols. Western
blotting was performed as described in a previous study (21). Proteins (20 µg) were separated via
SDS-PAGE on a 10% gel, and separated proteins were subsequently
transferred to PVDF membranes. After blocking with 5% skimmed milk
for 2 h at 37°C, the membranes were incubated with anti-p65
antibody (1:100; cat. no. 10745-1-AP; ProteinTech Group, Inc.),
anti-TH antibody (1:100; cat. no. 25859-1-AP; ProteinTech Group,
Inc.), anti-Nurr1 antibody (1:100; cat. no. ab41917, Abcam),
anti-A-SYN antibody (1:100; cat. no. 10842-1-AP; ProteinTech Group,
Inc.) and anti-β-actin antibody (1:200; cat. no. ab8226, Abcam) at
4°C overnight. After washing with PBS with 0.05% Tween-20, the
membranes were incubated with HRP-labeled goat anti-rabbit IgG
(1:500; ProteinTech Group, Inc.) at 37°C for 2 h. After washing
three times, protein bands were visualized with a ECL assay kit
(Beyotime Institute of Biotechnology) and chemiluminescence system
(Tanon Science and Technology Co., Ltd.). The protein bands were
analyzed using ImageJ software (version 6.0; National Institutes of
Health).
Statistical analysis
Each experiment was performed in triplicate. Data
are presented as the mean ± standard deviation of three independent
experiments. GraphPad Prism 5.0 (GraphPad Software, Inc.) was used
for statistical analyses. ANOVA followed by Tukey's post hoc test
was used to compare the differences among >two groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
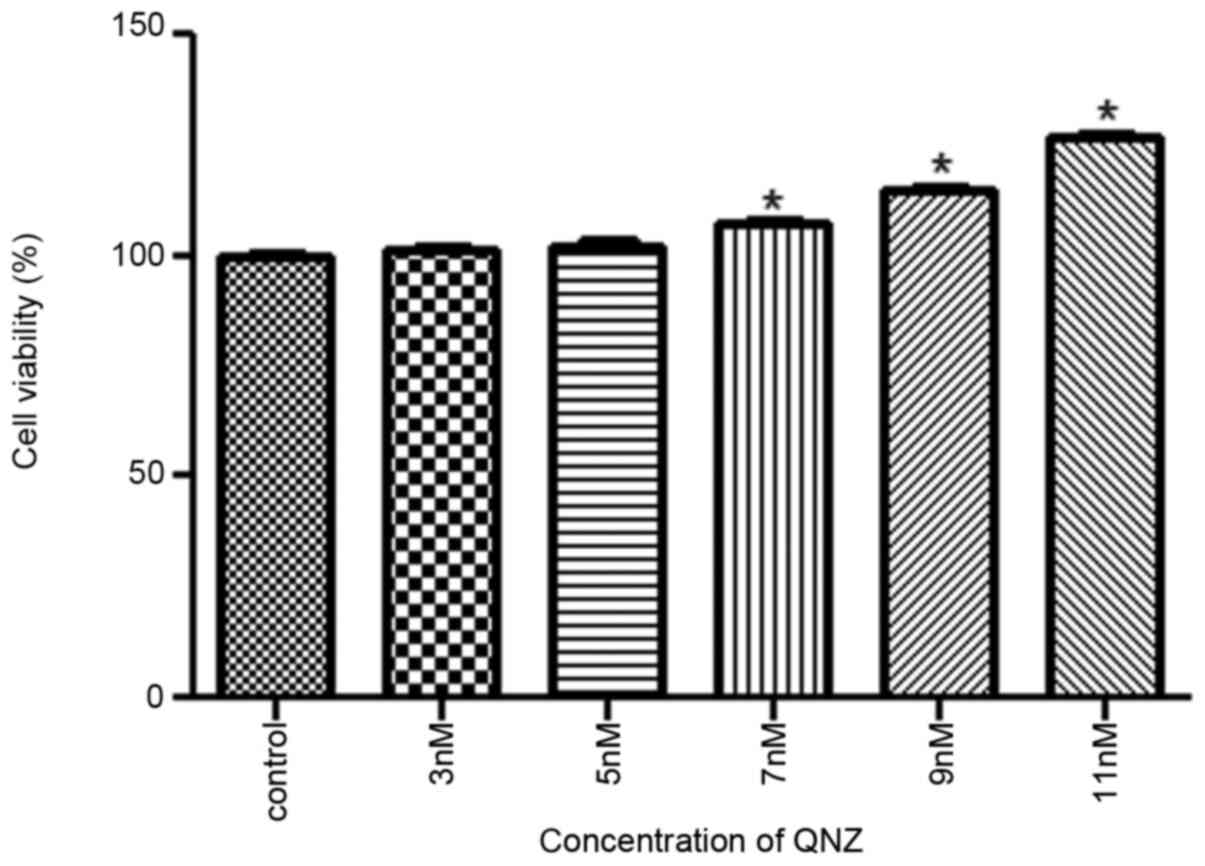
Optimum concentration of QNZ
To determine the optimum concentration of the NF-κB
inhibitor QNZ, different concentrations of QNZ were used to treat
PC12 cells. The results revealed that cell viability gradually
increased with increasing QNZ concentrations. When the QNZ
concentration was 11 nM, the PC12 cell viability was significantly
increased by ~28% when compared with the control group (P<0.05;
Fig. 1). As cell viability was
highest in the 11 nM QNZ group, this concentration was used in
subsequent experiments.
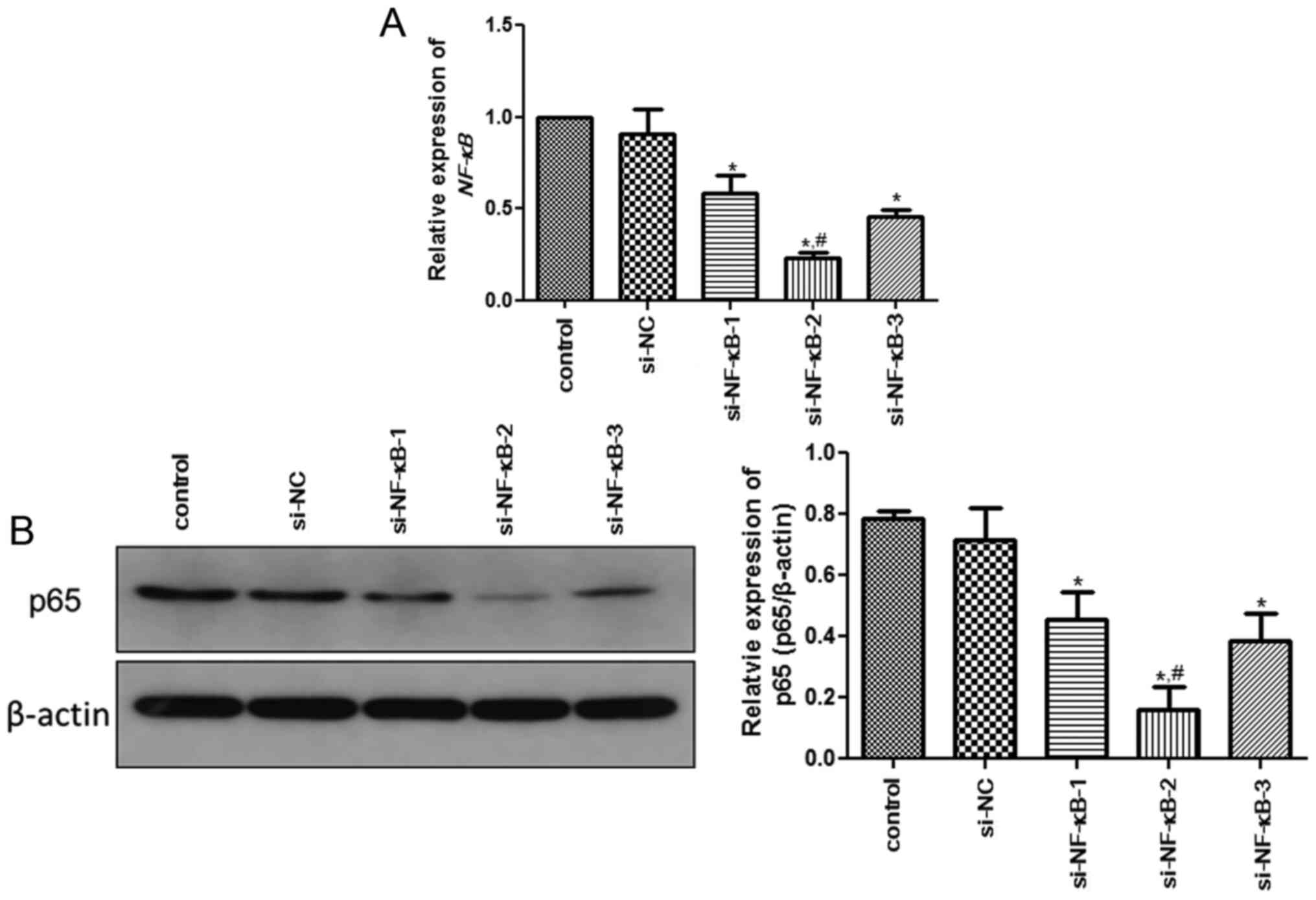
Analysis of transfection
efficiency
In PC12 cells, the expression of NF-κB was
determined by RT-qPCR and western blotting to evaluate the cell
transfection efficiency. No significant difference in the
expression of NF-κB was observed between the control and
si-NC groups (P>0.05; Fig. 2A).
Additionally, the expression of NF-κB in the si-NF-κB-1,
si-NF-κB-2 and si-NF-κB-3 groups was significantly decreased when
compared with that in the control group (P<0.05; Fig. 2A). However, the expression of
NF-κB in the si-NF-κB-2 group was significantly lower than
that in the si-NF-κB-1 and si-NF-κB-3 groups (P<0.05; Fig. 2A). In addition, the expression trend
of NF-κB determined by western blotting was similar with that
measured using RT-qPCR (Fig. 2B).
Based on these findings, si-NF-κB-2 was selected to construct
NF-κB-knockdown PC12 cells for subsequent experiments.
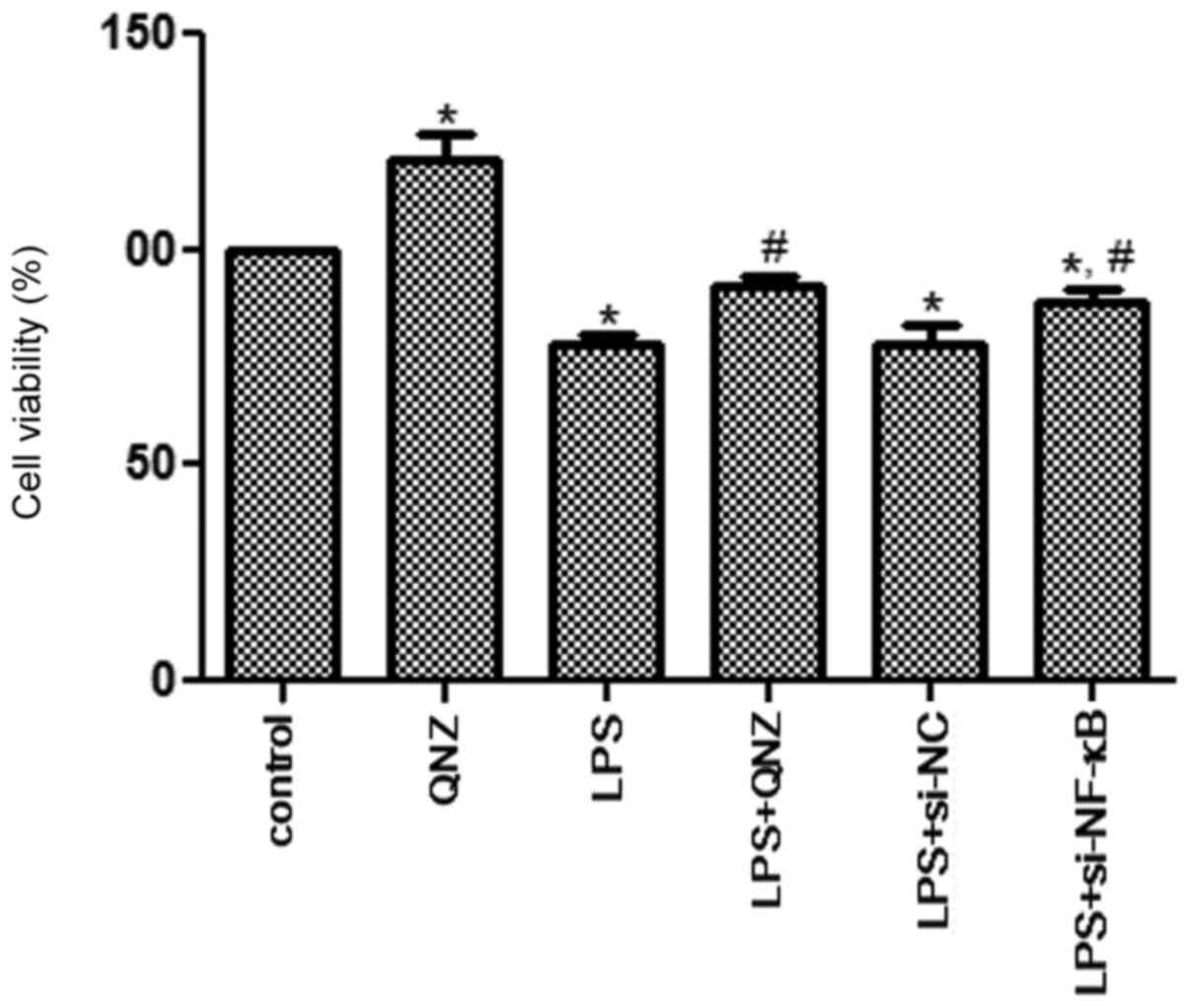
Cell viability analysis
To investigate the effects of NF-κB on
inflammatory PC12 cells, LPS was used to induce cellular
inflammation, and the CCK-8 assay was used to measure cell
viability. Compared with the control group, the cell viability of
the QNZ group was significantly increased (P<0.05; Fig. 3), indicating that QNZ could enhance
the viability of PC12 cells. After LPS treatment, cell viability
was significantly inhibited when compared with the control group
(P<0.05; Fig. 3). No significant
difference in cell viability was observed between the LPS and LPS +
si-NC groups. However, cell viabilities in the LPS + QNZ and LPS +
si-NF-κB groups were significantly increased when compared with
that in the LPS group (P<0.05; Fig.
3), and the effects of QNZ and NF-κB knockdown
were similar (P>0.05). These results suggested that
NF-κB could affect the cell viability of LPS-induced
PC12 cells.
Levels of IL-1β, IL-6 and TNF-α in the
PC12 cells
To further clarify the effects of NF-κB on
inflammatory factors, the expression levels of IL-1β, IL-6 and
TNF-α were measured in PC12 cells. After LPS induction, IL-1β
expression was significantly upregulated when compared with that in
the control group (P<0.05; Fig.
4A). No significant difference was observed in IL-1β levels
between the LPS and LPS + si-NC groups (P>0.05; Fig. 4A). However, IL-1β expression in the
LPS + QNZ and LPS + si-NF-κB groups were 88.39±8.86 and 101.75±4.65
pg/ml, respectively, which were significantly reduced when compared
with those in the LPS group (151.67±3.02 pg/ml, P<0.05; Fig. 4A). For IL-6 and TNF-α, the
expression trends were similar to those of IL-1β (Fig. 4B and C). Of note, TNF-α expression
in the QNZ group was significantly lower than that in the control
group (P<0.05; Fig. 4C),
indicating that QNZ may inhibit the generation of TNF-α.
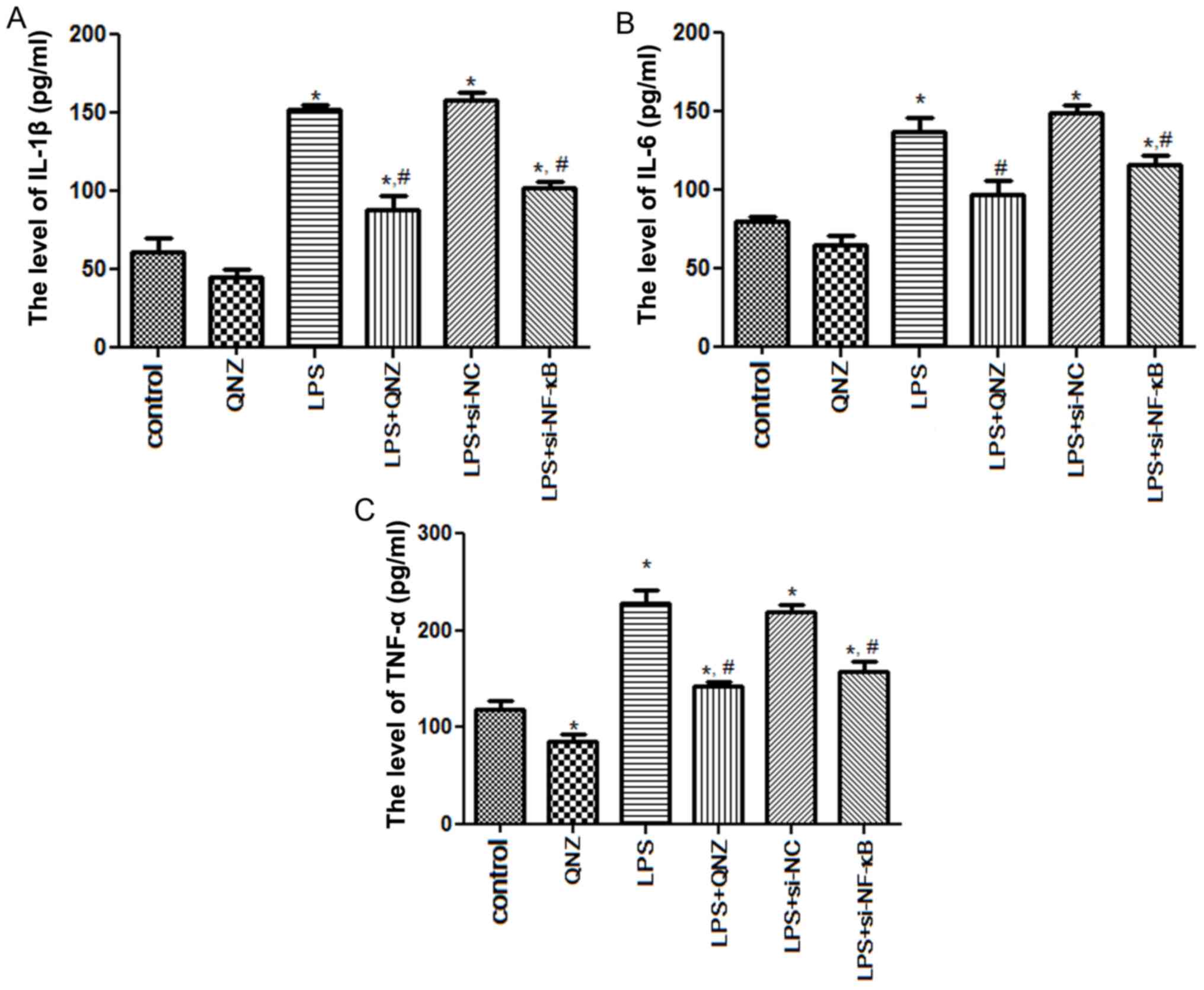 | Figure 4.Effects of NF-κB on the related
inflammatory cytokines in PC12 cells. Expression levels of (A)
IL-1β, (B) IL-6 and (C) TNF-α in PC12 cells following different
treatments, as determined via enzyme-linked immunosorbent assay
kits. *P<0.05 vs. Control group; #P<0.05 vs. LPS
group. IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor
necrosis factor-α; NF-κB, nuclear factor-κB; si-, small interfering
RNA; NC, negative control; LPS, lipopolysaccharide; QNZ,
quinazoline. |
Immunofluorescence analysis
Immunofluorescence was used to examine the relative
expression levels of TH, A-SYN and Nurr1. The expression levels of
TH and Nurr1 were significantly increased in the QNZ group when
compared with the control group (P<0.05), but were significantly
decreased in the LPS group (P<0.05; Fig. 5A and C). On pretreating PC12 cells
with QNZ and si-NF-κB, the expression levels of TH and Nurr1 in LPS
+ QNZ and LPS + si-NF-κB groups were significantly increased
compared with the LPS group (P<0.05), and QNZ and si-NF-κB
restored TH and Nurr1 expression levels to a level similar to that
in the control group (P<0.05; Fig.
5A and C). However, the trend of A-SYN expression was the
opposite to that of TH and Nurr1 expression levels (Fig. 5B). Compared with the control group,
A-SYN expression was significantly reduced in the QNZ group
(P<0.05), but significantly elevated in the LPS and LPS + si-NC
groups (P<0.05; Fig. 5B). In the
LPS + QNZ and LPS + si-NF-κB groups, A-SYN expression levels were
significantly lower than that in the LPS group (P<0.05), and
were restored to a level similar to that in the control group
(P<0.05; Fig. 5B). These results
suggested that NF-κB could alleviate LPS-induced
inflammation in PC12 cells by regulating the expression of TH,
A-SYN and Nurr1.
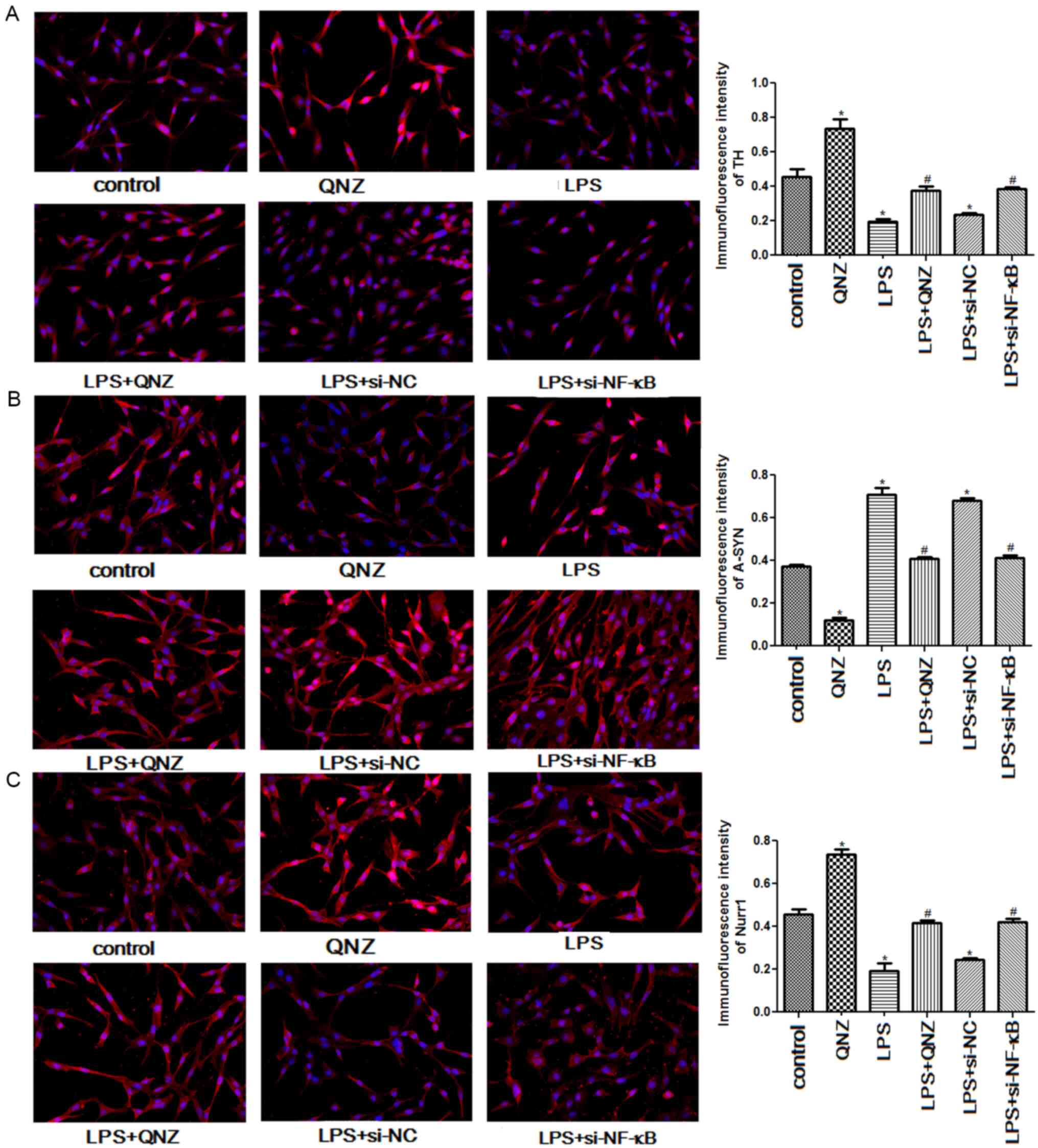 | Figure 5.Effects of NF-κB on the expressions
of TH, A-SYN, and Nurr1 examined using immunofluorescence at ×400
magnification. Relative expression levels of (A) TH, (B) A-SYN and
(C) Nurr1. *P<0.05 vs. Control group; #P<0.05 vs.
LPS group. TH, tyrosine hydroxylase; A-SYN, α-Synuclein; Nurr1,
nuclear receptor subfamily 4 group A member 2; NF-κB, nuclear
factor-κB; si-, small interfering RNA; NC, negative control; LPS,
lipopolysaccharide; QNZ, quinazoline. |
Western blot analysis
The protein expression levels of p65, TH, A-SYN and
Nurr1 were detected by western blotting. Protein expression levels
of p65 and A-SYN were significantly increased in the LPS group when
compared with the control group (P<0.05), whereas p65 and A-SYN
expression levels in the LPS + QNZ and LPS + si-NF-κB were
significantly reduced (P<0.05; Fig.
6A, B and D). The protein expression levels of TH and Nurr1
were significantly downregulated in the LPS group when compared
with the control group; however, these expression levels were
upregulated following treatment with QNZ and si-NF-κB (P<0.05;
Fig. 6A, C and E).
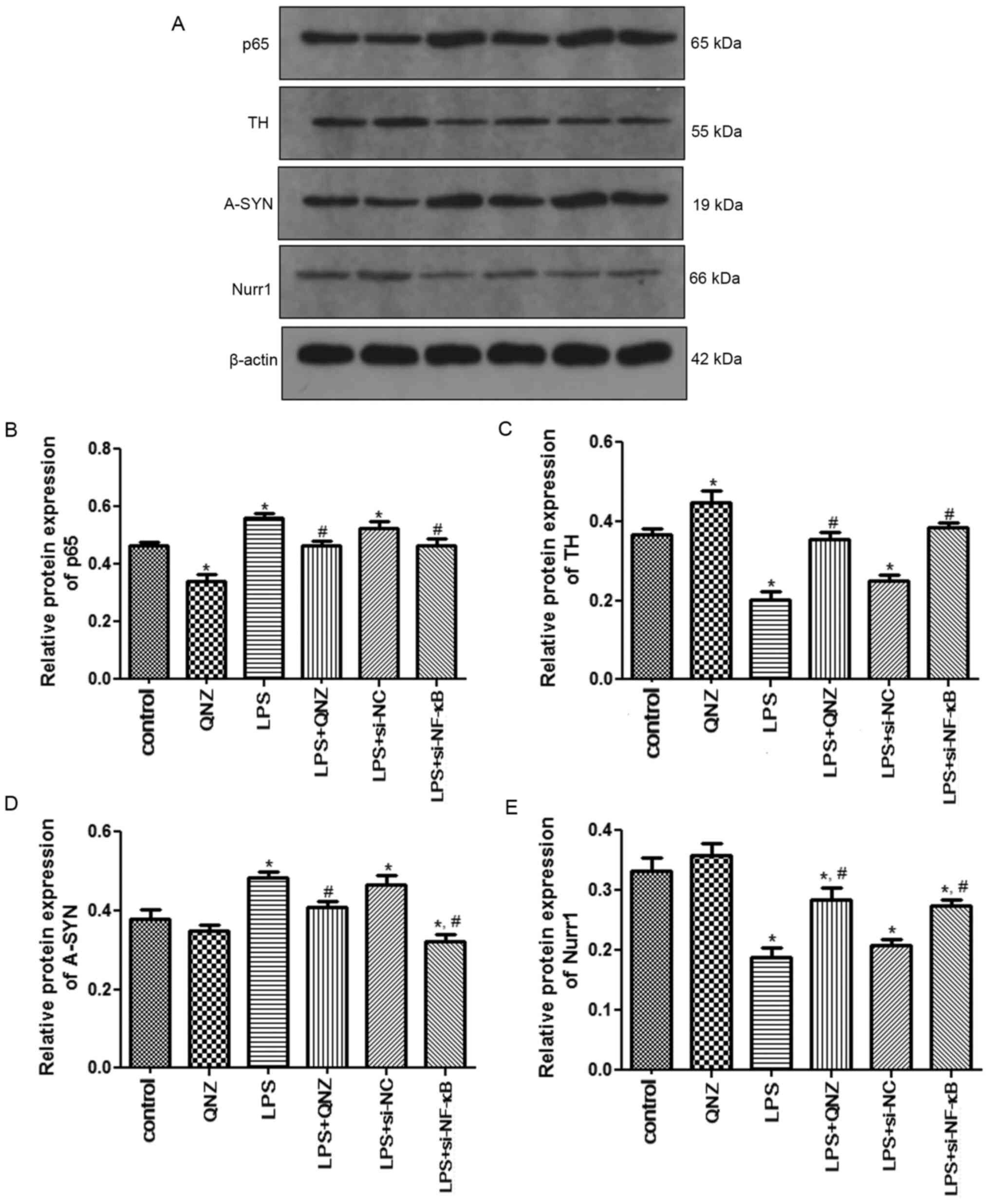 | Figure 6.Protein expression levels of p65, TH,
A-SYN and Nurr1 determined by western blotting. (A) Representative
western blotting images of p65, TH, A-SYN and Nurr1 expression.
Semi-quantification of (B) p65, (C) TH, (D) A-SYN and (E) Nurr1
expression. *P<0.05 vs. Control group; #P<0.05 vs.
LPS group. TH, tyrosine hydroxylase; A-SYN, α-Synuclein; Nurr1,
nuclear receptor subfamily 4 group A member 2; NF-κB, nuclear
factor-κB; si-, small interfering RNA; NC, negative control; LPS,
lipopolysaccharide; QNZ, quinazoline. |
Discussion
PD is one of the most disabling diseases of the
central nervous system, seriously impacting health and day-to-day
living in the elderly. A previous study reported the interaction
between NF-κB and Nurr1 (14). In the present study, LPS was
administered to PC12 cells to construct a PD cellular model,
followed by the pretreatment of these cells with an NF-κB
inhibitor, QNZ, and si-NF-κB. Based on the CCK-8 assay results, the
cell viability of PC12 cells was significantly reduced after LPS
induction, whereas pretreatment with QNZ and si-NF-κB significantly
enhanced their viability following LPS induction. Based on ELISA,
the levels of IL-1β, IL-6 and TNF-α were significantly higher in
the inflammatory PC12 cells, and QNZ treatment and NF-κB
interference restored these levels, relieving cellular
inflammation. Furthermore, immunofluorescence and western blotting
revealed that in the LPS group, p65 expression was higher, while
Nurr1 expression was lower. On pretreating cells with QNZ and
si-NF-κB, p65 expression was downregulated, and Nurr1 expression
was upregulated, indicating that p65 may be negatively associated
with Nurr1. In terms of TH and A-SYN expression levels, LPS
enhanced the expression of A-SYN and suppressed the expression of
TH. However, QNZ and NF-κB interference reversed the LPS-induced
expression levels of TH and A-SYN.
Previous studies have revealed that
neuroinflammation is the primary pathological mechanism of PD and
the main target for PD treatment (22,23).
It has been reported that NF-κB, a transcription factor that
regulates the production of pro-inflammatory cytokines, promotes an
enhanced inflammatory response, participating in the physiological
and pathological processes of several diseases (24,25).
IL-1β, IL-6 and TNF-α are pro-inflammatory factors associated with
inflammation. In the current study, the levels of IL-1β, IL-6 and
TNF-α were increased in LPS-induced PC12 cells, whereas their
levels were decreased following NF-κB interference and QNZ
treatment. Wei and Shao (26)
revealed that nobiletin significantly inhibited the levels of IL-6,
IL-1β, TNF-α, matrix metalloproteinase-1 (MMP-1) and MMP-3 in the
ectopic endometrium by downregulating NF-κB activity, thus
increasing protection against endometriosis. Additionally, another
study has shown that pannexin 3 inhibits the inflammatory response
induced by TNF-α by suppressing the NF-κB signaling pathway in
dental pulp inflammation (27).
Based on the aforementioned findings, the present study speculated
that activation of NF-κB may be related to the inflammatory
response observed in PD, and NF-κB interference may restrict the
generation of pro-inflammatory cytokines, including IL-1β, IL-6 and
TNF-α, thus downregulating the neuroinflammatory response in
PD.
To further elucidate the potential molecular
mechanisms of NF-κB in PD, immunofluorescence and western
blotting were performed. In the present study, LPS enhanced the
expression of A-SYN and suppressed the expression of TH, whereas
QNZ and NF-κB interference recovered their expression. TH, an
enzyme known to catalyze the formation of L-dihydroxyphenylalanine,
is a key enzyme of immune reactivity in PD models and is also used
to evaluate the efficacy of novel therapeutic agents (28). A-SYN, a rich neuronal protein, is
highly abundant in presynaptic nerve terminals and is deemed a
neuropathological feature of PD (29). A previous study by Ma et al
(30) demonstrated that
electroacupuncture intervention improved motor function in a PD rat
model by upregulating the expression of TH and downregulating the
expression of A-SYN. Mani et al (31) reported that naringenin exerts a
protective role in PD by significantly inhibiting the expression of
A-SYN and enhancing TH expression, as well as by mediating the
inflammatory response and oxidative stress. Combined with the
present results, we assumed that downregulation of NF-κB may have
the same effects as those of QNZ and may alleviate the symptoms of
PD by regulating the expression of TH and A-SYN.
Moreover, a recent study demonstrated that Nurr1 can
inhibit TNF-α production by interacting with NF-κB/p65 and
inhibiting its nuclear translocation (32). In the present study, the results
showed that in the LPS group, the expression of p65 was higher,
whereas the expression of Nurr1 was lower; however, following
pretreatment with QNZ and si-NF-κB, p65 expression was
downregulated and Nurr1 expression was upregulated. Nurr1
reportedly plays a crucial role in the development, differentiation
and maintenance of dopaminergic neurons (33). Saijo et al (34) reported that Nurr1 interacts with
NF-κB/p65 promoters and subsequently recruits the REST corepressor
complex, thereby resulting in the clearance of NF-κB/p65 and
transcriptional repression. Based on the present findings, it was
speculated that NF-κB may be negatively associated with Nurr1, and
inhibition of NF-κB activation and enhancement of Nurr1 expression
may contribute to reduced inflammation in PD.
In conclusion, NF-κB may be negatively associated
with Nurr1. Additionally, inhibition of NF-κB reduced the
production of inflammatory factors (IL-1β, IL-6 and TNF-α) by
upregulating the expression of Nurr1 and TH and downregulating
A-SYN expression, thus ameliorating the inflammatory status of PD.
These findings may contribute to our understanding of the
progression of PD and provide therapeutic targets for the
prevention and amelioration of PD.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. U1503222).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XY, HG and DW conceived and designed the research.
HG and JM was responsible for data acquisition. HG and DW analyzed
and interpreted data. SJ performed statistical analysis. HG wrote
the original draft preparation. XY, HG and DW reviewed and edited
the manuscript. XY supervised the study. HG, DW and XY confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sveinbjornsdottir S: The clinical symptoms
of Parkinson's disease. J Neurochem. 139 (Suppl 1):S318–S324. 2016.
View Article : Google Scholar
|
|
2
|
Cacabelos R: Parkinson's disease: From
pathogenesis to pharmacogenomics. Int J Mol Sci. 18:5512017.
View Article : Google Scholar
|
|
3
|
Radhakrishnan DM and Goyal V: Parkinson's
disease: A review. Neurol India. 66 (Suppl):S26–S35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghosh A, Roy A, Liu X, Kordower JH, Mufson
EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE and Pahan K:
Selective inhibition of NF-kappaB activation prevents dopaminergic
neuronal loss in a mouse model of Parkinson's disease. Proc Natl
Acad Sci USA. 104:18754–18759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katzenschlager R and Lees AJ: Treatment of
Parkinson's disease: Levodopa as the first choice. J Neurol. 249
(Suppl 2):II19–II24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Csoti I, Jost WH and Reichmann H:
Parkinson's disease between internal medicine and neurology. J
Neural Transm (Vienna). 123:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Irwin DJ, Grossman M, Weintraub D, Hurtig
HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK,
et al: Neuropathological and genetic correlates of survival and
dementia onset in synucleinopathies: A retrospective analysis.
Lancet Neurol. 16:55–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen KX, Miliç J, El-Khodor B, Dhana K,
Nano J, Pulido T, Kraja B, Zaciragic A, Bramer WM, Troup J, et al:
The role of DNA methylation and histone modifications in
neurodegenerative diseases: A systematic review. PLoS One.
11:e01672012016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roostalu U, Salinas CBG, Thorbek DD,
Skytte JL, Fabricius K, Barkholt P, John LM, Jurtz VI, Knudsen LB,
Jelsing J, et al: Quantitative whole-brain 3D imaging of tyrosine
hydroxylase-labeled neuron architecture in the mouse MPTP model of
Parkinson's disease. Dis Model Mech. 12:dmm0422002019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Yang HA, Wang XN and Du YF: Effect
of siRNA-induced silencing of cellular prion protein on tyrosine
hydroxylase expression in the substantia nigra of a rat model of
Parkinson's disease. Genet Mol Res. 15:2016.
|
|
11
|
Salemme A, Togna AR, Mastrofrancesco A,
Cammisotto V, Ottaviani M, Bianco A and Venditti A:
Anti-inflammatory effects and antioxidant activity of
dihydroasparagusic acid in lipopolysaccharide-activated microglial
cells. Brain Res Bull. 120:151–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bessler H, Djaldetti R, Salman H, Bergman
M and Djaldetti M: IL-1 beta, IL-2, IL-6 and TNF-alpha production
by peripheral blood mononuclear cells from patients with
Parkinson's disease. Biomed Pharmacother. 53:141–145. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jing H, Wang S, Wang M, Fu W, Zhang C and
Xu D: Isobavachalcone attenuates MPTP-induced Parkinson's disease
in mice by inhibition of microglial activation through NF-κB
pathway. PLoS One. 12:e01695602017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Yang ZH, Chen H, Li HH, Chen LY,
Zhu Z, Zou Y, Ding CC, Yang J and He ZW: Nemo-like kinase as a
negative regulator of nuclear receptor Nurr1 gene transcription in
prostate cancer. BMC Cancer. 16:2572016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonta PI, Pols TW, van Tiel CM, Vos M,
Arkenbout EK, Rohlena J, Koch KT, de Maat MP, Tanck MW, de Winter
RJ, et al: Nuclear receptor Nurr1 is expressed in and is associated
with human restenosis and inhibits vascular lesion formation in
mice involving inhibition of smooth muscle cell proliferation and
inflammation. Circulation. 121:2023–2032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McEvoy AN, Murphy EA, Ponnio T, Conneely
OM, Bresnihan B, FitzGerald O and Murphy EP: Activation of nuclear
orphan receptor NURR1 transcription by NF-kappa B and cyclic
adenosine 5′-monophosphate response element-binding protein in
rheumatoid arthritis synovial tissue. J Immunol. 168:2979–2987.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasban-Aliabadi H, Esmaeili-Mahani S,
Sheibani V, Abbasnejad M, Mehdizadeh A and Yaghoobi MM: Inhibition
of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea
europaea L.) leaf extract is performed by its main component
oleuropein. Rejuvenation Res. 16:134–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu C, Zhao W, Yu J, Li S, Lin L and Chen
X: Induction of ferroptosis and mitochondrial dysfunction by
oxidative stress in PC12 cells. Sci Rep. 8:5742018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahoo S, Meijles DN, Al Ghouleh I, Tandon
M, Cifuentes-Pagano E, Sembrat J, Rojas M, Goncharova E and Pagano
PJ: MEF2C-MYOCD and leiomodin1 suppression by miRNA-214 promotes
smooth muscle cell phenotype switching in pulmonary arterial
hypertension. PLoS One. 11:e01537802016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu G, Ao R, Zhi Z, Jia J and Yu B: miR-21
and miR-19b delivered by hMSC-derived EVs regulate the apoptosis
and differentiation of neurons in patients with spinal cord injury.
J Cell Physiol. 234:10205–10217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng S, Jiang X, Ding C, Du C,
Owusu-Ansah KG, Weng X, Hu W, Peng C, Lv Z, Tong R, et al:
Expression and critical role of interleukin enhancer binding factor
2 in hepatocellular carcinoma. Int J Mol Sci. 17:13732016.
View Article : Google Scholar
|
|
22
|
Rizzo F, Riboldi G, Salani S, Nizzardo M,
Simone C, Corti S and Hedlund E: Cellular therapy to target
neuroinflammation in amyotrophic lateral sclerosis. Cell Mol Life
Sci. 71:999–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Russo I, Bubacco L and Greggio E: LRRK2
and neuroinflammation: Partners in crime in Parkinson's disease? J
Neuroinflamm. 11:522014. View Article : Google Scholar
|
|
24
|
Aloor R, Zhang C, Bandyopadhyay M and
Dasgupta S: Impact of nuclear factor-κB on restoration of neuron
growth and differentiation in hippocampus of degenerative brain. J
Neurosci Res. 93:1471–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Negi G, Kumar A and Sharma SS: Melatonin
modulates neuroinflammation and oxidative stress in experimental
diabetic neuropathy: Effects on NF-κB and Nrf2 cascades. J Pineal
Res. 50:124–131. 2011.PubMed/NCBI
|
|
26
|
Wei X and Shao X: Nobiletin alleviates
endometriosis via down-regulating NF-κB activity in endometriosis
mouse model. Biosci Rep. 38:BSR201804702018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song F, Sun H, Wang Y, Yang H, Huang L, Fu
D, Gan J and Huang C: Pannexin3 inhibits TNF-α-induced inflammatory
response by suppressing NF-κB signalling pathway in human dental
pulp cells. J Cell Mol Med. 21:444–455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santos CM: New agents promote
neuroprotection in Parkinson's disease models. CNS Neurol Disord
Drug Targets. 11:410–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burre J, Sharma M and Sudhof TC: Cell
biology and pathophysiology of α-synuclein. Cold Spring Harb
Perspect Med. 8:a0240912018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Yuan L, Wang SJ, Lei J, Wang Y, Li
YN and Yu BL: Electroacupuncture improved locomotor function by
regulating expression of tyrosine hydroxylase and α-synuclein
proteins and transcription activating factor 6 and transcription
factor X box binding protein 1 mRNAs in substantia nigra of rats
with Parkinson's disease. Zhen Ci Yan Jiu. 44:805–809. 2019.(In
Chinese). PubMed/NCBI
|
|
31
|
Mani S, Sekar S, Barathidasan R,
Manivasagam T, Thenmozhi AJ, Sevanan M, Chidambaram SB, Essa MM,
Guillemin GJ and Sakharkar MK: Naringenin decreases α-synuclein
expression and neuroinflammation in MPTP-Induced Parkinson's
disease model in mice. Neurotox Res. 33:656–670. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shao QH, Yan WF, Zhang Z, Ma KL, Peng SY,
Cao YL, Yuan YH and Chen NH: Nurr1: A vital participant in the
TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2cells.
Neuropharmacology. 144:388–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Zhuang W, Fu W and Wang X, Lv E,
Li F, Zhou S, Rausch WD and Wang X: The lentiviral-mediated Nurr1
genetic engineering mesenchymal stem cells protect dopaminergic
neurons in a rat model of Parkinson's disease. Am J Transl Res.
10:1583–1599. 2018.PubMed/NCBI
|
|
34
|
Saijo K, Winner B, Carson CT, Collier JG,
Boyer L, Rosenfeld MG, Gage FH and Glass CK: A Nurr1/CoREST pathway
in microglia and astrocytes protects dopaminergic neurons from
inflammation-induced death. Cell. 137:47–59. 2009. View Article : Google Scholar : PubMed/NCBI
|