Introduction
Autism spectrum disorder (ASD) is a
neurodevelopmental disorder that includes a social interaction
deficit and restrictive and repetitive behavioural patterns
(DSM-5). Besides the key symptoms, cognitive deficits are
frequently present in patients with ASD (1). ASD affects approximately 1 in 54
people in the United States of America (CDC, 2020). Despite the
fact that a significant proportion of children are diagnosed with
ASD, no effective treatment exists for core ASD symptoms.
Mouse models with face validity to the core
symptoms, such as a social interaction deficit and restrictive and
repetitive behavioural patterns, have offered experimental
approaches to evaluate potential treatments for ASD. Inbred BTBR
T+ Itpr3tf/J (BTBR) mice exhibited reduced sociability
and increased self-grooming behaviour, which mimics the core
symptoms of social interaction deficits and repetitive behaviours
seen in ASD patients (2,3). In addition, BTBR mice also exhibited
object-based attention deficits (4), and complete absence of corpus
callosum, which is comparable to ASD patients with reduced volume
of the corpus callosum (5,6). Alterations in glia, neurons, and
synapses and reduction in neurogenesis in the brain of BTBR mice
have also been reported (7).
Microglia are well-known immune cells of the central
nervous system. Maternal immune activation and increased
pro-inflammatory cytokines lead to a higher risk of ASD (8). A growing body of evidence indicates
that microglial dysfunction is a potential target for ASD (9). In human post-mortem studies, increased
gliosis and glial cell proliferation have been reported in patients
with ASD (10). Immunomodulatory
treatments such as vitamin D, suramin, minocycline, and gut
microbiota effectively improved autistic-like behaviours and
decreased pro-inflammatory cytokines (11). In addition, neuroinflammation has
been linked to synaptic loss, and microglia play critical roles in
activity-dependent synapse remodelling (12).
Humulus japonicus (HJ) is a perennial herb
distributed in Asian countries. HJ has traditionally been used in
patients with hypertension, pulmonary disease, and skin disease in
Korea. Recently, the protective effects of HJ on neurodegenerative
diseases involving Alzheimer's disease (AD) and Parkinson's disease
(PD) have been reported in in vivo animal studies (13,14).
HJ has demonstrated anti-inflammatory effects on paw oedema, AD,
and PD animal models (13–15). HJ has also been reported to
significantly improve the cognitive function of APP/PS1 transgenic
mice for AD (13). However, the
effects of HJ on neurodevelopmental disorders and ASD have not yet
been elucidated. In this study, we employed BTBR mice to
investigate the hypothesis that autism-like behaviours could be
ameliorated by HJ treatment. We also examined the effects of HJ on
microglia activation, inflammatory cytokines, and the excitatory
signalling pathway in the brain of BTBR mice.
Materials and methods
Animals
Male C57LB/6J (B6 mice) and BTBR T+
Itpr3tf/J inbred strains (BTBR mice) were obtained from
the Korea Research Institute of Bioscience and Biotechnology and
the Jackson Laboratory (Bar Harbor, ME, USA). BTBR mice were
maintained with BTBR × BTBR mice and their progenies were used in
the study. Mice were housed in plastic cages (25×20×12.5
cm3) in a humidity-(50%-60%) and temperature-controlled
(21–22°C) environment under specific pathogen-free conditions on a
12-h light/dark cycle (lights on at 07:00) with free access to
autoclaved food and water. The microbiological status of the mice
was monitored once every three months and serological
investigations for viral infection were performed every month.
Animal care and use were in accordance with the National Institutes
of Health Guide for the Care and Use of Laboratory Animals and was
approved by the Institutional Animal Use and Care Committee of the
Korea Research Institute of Bioscience and Biotechnology. Mice were
randomised into vehicle and HJ-treated groups at the age of 3 weeks
to the following: B6 group treated with vehicle alone (n=12), B6
group treated with 400 mg/kg HJ (n=6), BTBR mice treated with
vehicle alone (n=13), BTBR mice treated with 200 mg/kg HJ (n=13),
and BTBR mice treated with 400 mg/kg HJ (n=13). HJ and 0.5%
carboxymethyl cellulose (CMC, vehicle) were administered by oral
gavage for 6 weeks daily.
Preparation of HJ
HJ was purchased from Gangwon Herbs, Gangwon
Province, Republic of Korea, in July 2014. The voucher specimen was
identified by Professor WK. Oh, and a voucher specimen
(SNU-2014-0004) was deposited at the College of Pharmacy, Seoul
National University, Korea. Then, the HJ extract was prepared and
supplied by the Korea Bioactive Natural Material Bank (Seoul,
Korea). The dried aerial parts of HJ were soaked in 20% ethanol in
an extraction container for 2 days at room temperature. The
ethanol-soluble extracts of HJ were filtered through cheesecloth,
concentrated exhaustively, and dried to produce an ethanolic
extract under reduced pressure. The 20% ethanol extract of HJ was
used in this study. The HJ extract was suspended in 0.5% CMC at a
concentration of 50 mg/ml as a stock solution, and the working
solution of HJ was adjusted to the intended concentrations for use
in in vivo experiments.
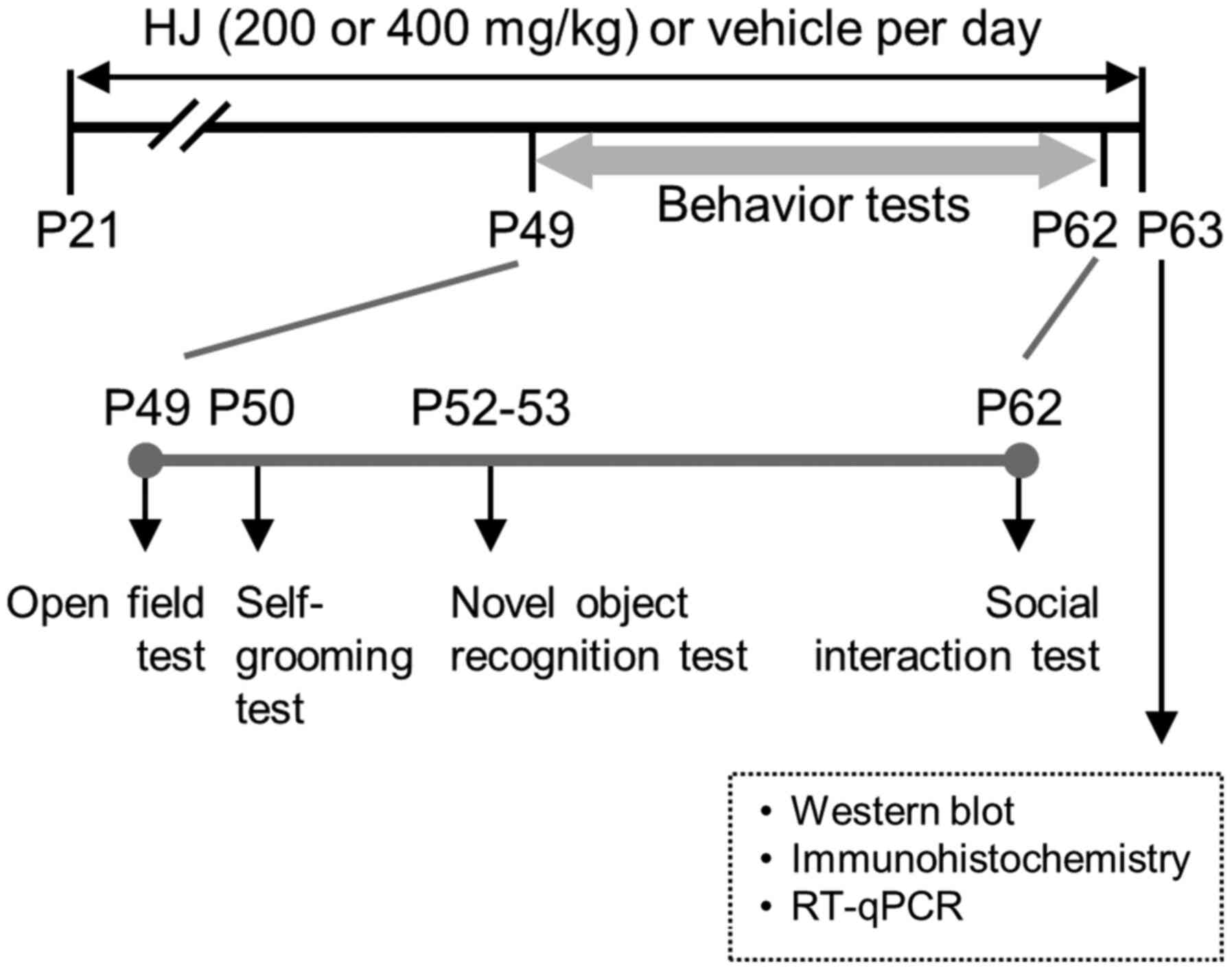
Behavioural tests
Behavioural tests were performed between 4 and 6
weeks of HJ and vehicle treatment. Vehicle or drugs were
administered 30 min before all behavioural tests. The mice were
acclimatised to the test room for 30 min prior to each test. The
following tests were sequentially performed: Open field test →
self-grooming test → novel object recognition test → social
interaction test). No more than one test was performed on any given
day (Fig. 1).
Open field test
General exploration of mice was performed using an
open field test, which has been described in previous studies
(16). Each mouse was gently
introduced into a white plexiglass chamber (45×45×40 cm), and the
horizontal locomotor activity was monitored simultaneously for 5
min using SMART video tracking software (Panlab, Barcelona,
Spain).
Self-grooming test
Spontaneous self-grooming behaviour has been
described in previous studies (17,18).
Each mouse was gently introduced into a transparent acrylic
cylinder (diameter, 20 cm). Repetitive self-grooming behaviours
were recorded for 30 min using a video camera (Samsung, Korea).
Grooming behaviour included head washing, body grooming,
genital/tail grooming, and paw and leg licking. The cylinders were
cleaned with 70% ethanol between each subject's test session.
Novel object recognition test
The novel-object recognition test has been described
in previous studies (16). Mice
were individually habituated in the testing chamber (40×20×20 cm)
for 5 min. After placing 2 identical objects (familiar objects,
cylindrical wooden blocks, 10 cm high ×2 cm diameter), mice were
allowed to move freely for 10 min. The mice were then returned to
their cages. Twenty-four hours later, mice were placed back into
the testing chamber in the presence of one of the familiar objects
and one novel object (rectangular wooden block, 10×2.5×2 cm) for 10
min. The sessions were video-recorded, and the time spent exploring
the objects was scored. The objects and chambers were cleaned with
70% ethanol between each session. Sniffing and touching the object
with the nose and/or forepaws were defined as exploration. Sitting
on the object was not considered an exploratory behaviour.
Social interaction test
The social interaction test has been described in
previous studies (19). The social
interaction chamber consisted of a white acryl wall box (40×20×20
cm). Individual mice were placed in the chamber for 3 min for
habituation. Then, an age-matched novel C57BL/6J male mouse was
introduced into the test chamber and allowed to explore freely for
3 min. The behaviours of the mice were video-recorded, and social
interaction, such as body sniffing, anogenital sniffing, and direct
contact were analysed for 3 min.
Reverse-transcription quantitative
PCR
Thirty minutes after the 6-week HJ (400 mg/kg)
treatment schedule, mice were euthanized by quick cervical
dislocation approved by the IACUC and their brains are removed.
Total RNA was extracted from the hippocampus using TRI reagent
(Sigma-Aldrich; Merck KGaA). cDNA synthesis was performed using the
Reverse Transcription System (Promega) according to the
instructions of the supplier. RT-PCR analysis used in Fig. 5 was carried out using the following
primer sets: IL-1b (5′-CTACAGGCTCCGAGATGAACAAC-3′ and
5′-TCCATTGAGGTGGAGAGCTTTC-3′), IL6 (5′-TTCCATCCAGTTGCCTTCTTG-3′ and
5′-GGGAGTGGTATCCTCTGTGAAGTC-3′), CCL2 (5′-TTAAAACCTGGATCGGAACCAA-3′
and 5′-GCATTAGCTTCAGATTTACGGGT-3′), CXCL10
(5′-CCAAGTGCTGCCGTCATTTTC-3′ and 5′-GGCTCGCAGGGATGATTTCAA-3′), and
18s ribosomal RNA (18s, 5′-GACACGGACAGGATTGACAGATTGATAG-3′ and
5′-GTTAGCATGCCAGAGTCTCGTTCGTT-3′). Comparative qPCR was performed
using an SYBR Green Master Mix (Applied Biosystems). The expression
level of target genes was normalized to the expression 18s
(20) and calculated based on the
comparative cycle threshold Ct method
(2-∆∆Cq) (21).
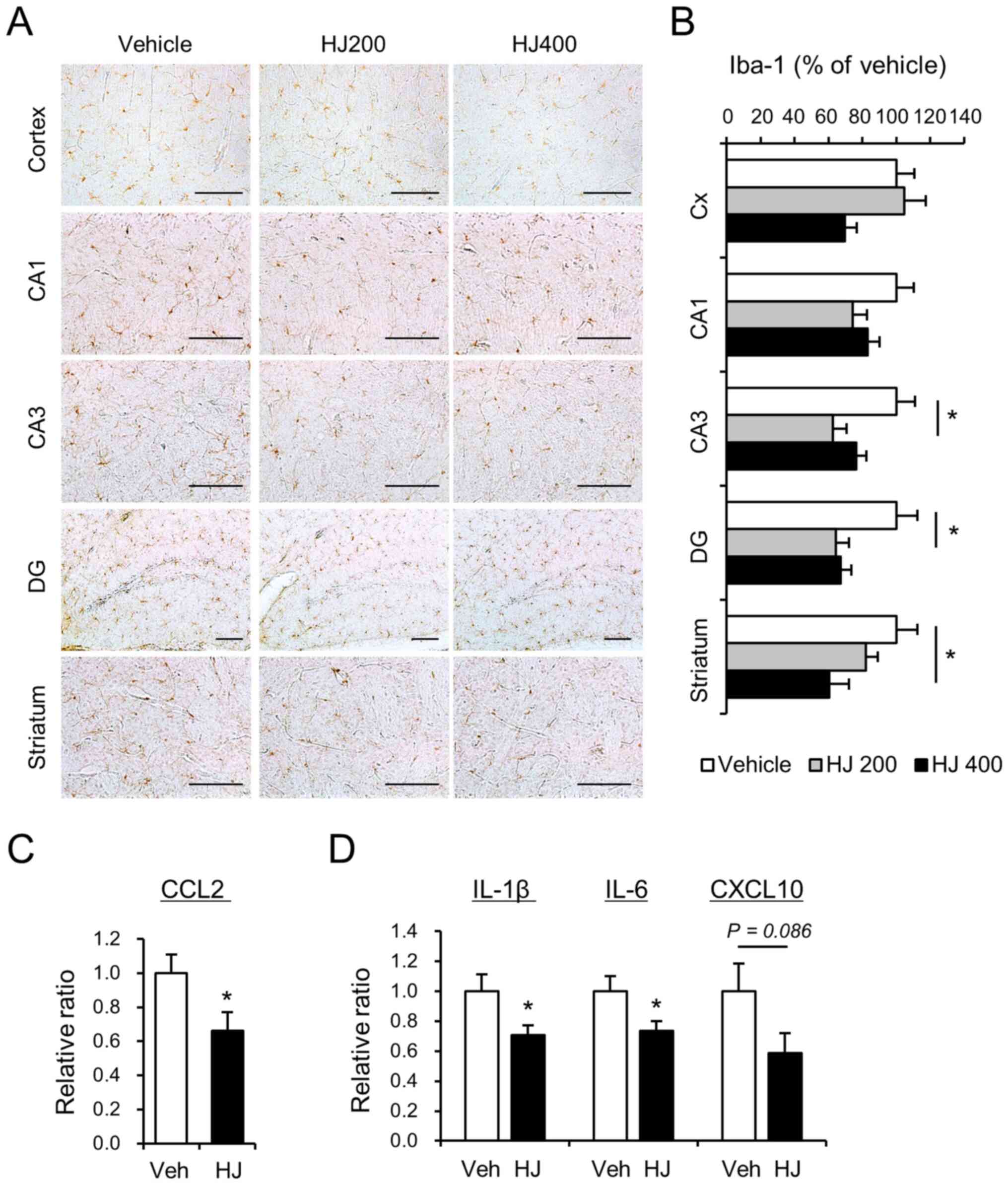 | Figure 5.Effects of HJ treatment on the
activation of microglia and the expression level of
pro-inflammatory cytokines in BTBR mice. (A) Micrograph
representation of the cerebral cortex, hippocampus (CA1, CA3 and
DG), and striatum stained for Iba-1 in vehicle-treated (vehicle),
200 mg/kg HJ-treated (HJ200) and 400 mg/kg HJ-treated (HJ400) BTBR
mice. Quantification of positive area stained is represented for
the cerebral cortex (Cx), hippocampus (CA1, CA3 and DG), and (B)
striatum for Iba-1. Scale bar represents 200 µm in all images. (C
and D) mRNA expression levels of cytokines following HJ treatment
in the hippocampus of BTBR mice. Relative mRNA levels of (C) CCL2,
(D) IL-1β, IL-6 and CXCL10 were measured using
reverse-transcription quantitative PCR. *P<0.05 vs. indicated
group. Data are presented as mean ± SEM. HJ, Humulus
japonicus; CCL2, chemokine CC motif ligand 2; IL,
interleukin. |
Western blotting
Western blotting was conducted as previously
described (16). Thirty minutes
after the 6-week HJ (400 mg/kg) treatment schedule, mice were
euthanized by quick cervical dislocation and their brains were
removed. Left hippocampus was homogenised in homogenisation buffer
(1X RIPA buffer, Cat# 02-188; Millipore) containing a cocktail of
protease inhibitors (Roche). The homogenates were centrifuged at
600 × g at 4°C for 10 min and the supernatants were centrifuged at
17,000 × g at 4°C for 10 min to obtain the supernatant containing
the cytosolic fraction. Protein samples were resolved by SDS-PAGE
and then transferred onto a polyvinylidene fluoride membrane
(Bio-Rad). Blots were incubated overnight at 4°C with the following
primary antibodies: N-methyl-D-aspartate receptor subtype 2B (NR2B,
Cat# 06-600; Millipore), pNR2B (Tyr1472, Cat# 4212; Cell Signaling
Technology), calcium/calmodulin-dependent protein kinase type II
subunit alpha (CaMKIIα, Cat# sc-13141; Santa Cruz Biotechnology),
pCaMKIIα (Thr286, Cat# sc-12886; Santa Cruz Biotechnology),
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)
receptor subunit GluR1 (GluR1, Cat# 31232; Millipore), pGluR1
(Ser831, Cat# 04-823; Millipore), pGluR1 (Ser 845, Cat# 04-1073;
Millipore), gamma-aminobutyric acid (GABA) receptor subunits: α1
(Cat# ab33299, Abcam), β2/3 (Cat# 05-474, Millipore), and γ2 (Cat#
ab240445; Abcam), and actin (Cat# MAB1501; Millipore).
Immunohistochemistry
Immunohistochemistry was performed as previously
described (22). Mice were
transcardially perfused with saline followed by 4% paraformaldehyde
in phosphate-buffered saline. The perfused brains were dissected,
post-fixed overnight, and then cut into 40 µm coronal sections on a
vibratome (Leica). Free-floating sections were blocked with serum
for 1 h and incubated overnight at 4°C with the primary rabbit
polyclonal antibody for Iba-1 (ionised calcium-binding adaptor
molecule 1, Cat# 019-19741; Wako). Immunohistochemistry was then
performed using biotinylated secondary anti-rabbit IgG (Vector
Laboratory), avidin-biotinylated peroxidase complex (Vector
Laboratory), and 3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA).
The occupied areas of Iba-1 positive cells were measured in the
cerebral cortex, hippocampus, and dorsal striatum. Images were
taken at one or two optical planes per section at ×200
magnification (Olympus Corporation; 10× ocular and 20× objective).
Qualitative evaluations of immunoreactivity were performed in a
blinded manner.
Statistical analysis
GraphPad Prism software (GraphPad Software, Inc.)
was used to perform all statistical analyses. Two-sample
comparisons were conducted with Student's t-tests, while multiple
comparisons were performed with a one-way ANOVA followed by
Tukey-Kramer post hoc tests. All data are presented as the mean ±
standard error of the mean (SEM). Differences with a P-value
<0.05 were considered statistically significant.
Results
Self-grooming behaviour is decreased
by HJ treatment in BTBR mice
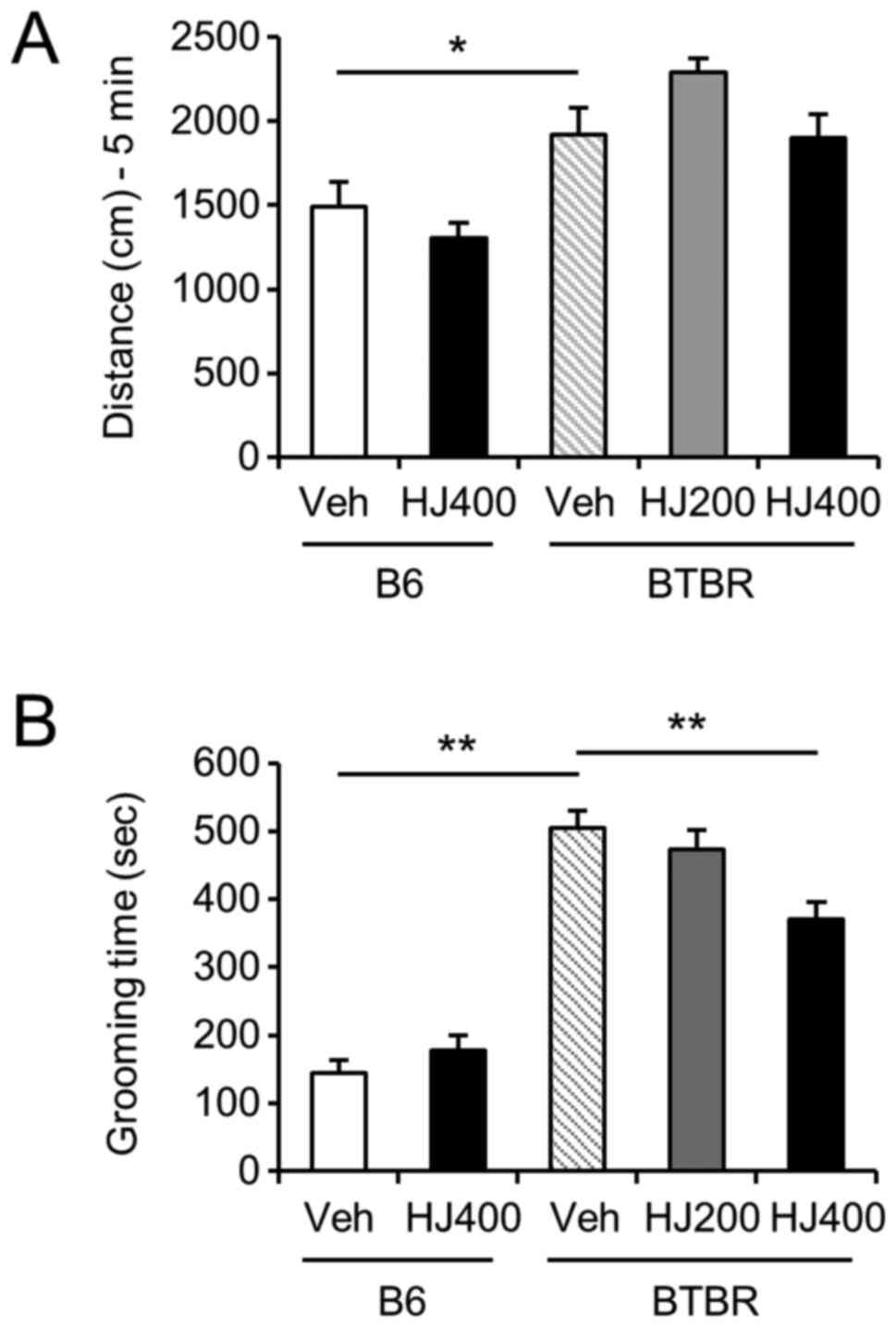
To investigate the effect of HJ on locomotor
activity and repetitive behaviours, we performed the open field
test and cylinder test in the vehicle-treated or HJ-treated B6 and
BTBR mice. One-way ANOVA analysis of distance travelled revealed
that the BTBR mice moved more than the B6 control mice in the open
field test (Fig. 2A, P<0.05).
However, HJ treatment did not alter locomotor activity in either B6
or BTBR mice. In the cylinder test, total self-grooming behaviours
were significantly increased in vehicle-treated BTBR mice compared
to vehicle-treated B6 mice (Fig.
2B, P<0.01). The increased self-grooming in the BTBR mice
was significantly decreased in the 400 mg/kg HJ-treated group
compared to the vehicle-treated group (Fig. 2B, P<0.01), but not in B6 mice.
The results suggested that the repetitive behaviour was ameliorated
by HJ treatment in BTBR mice.
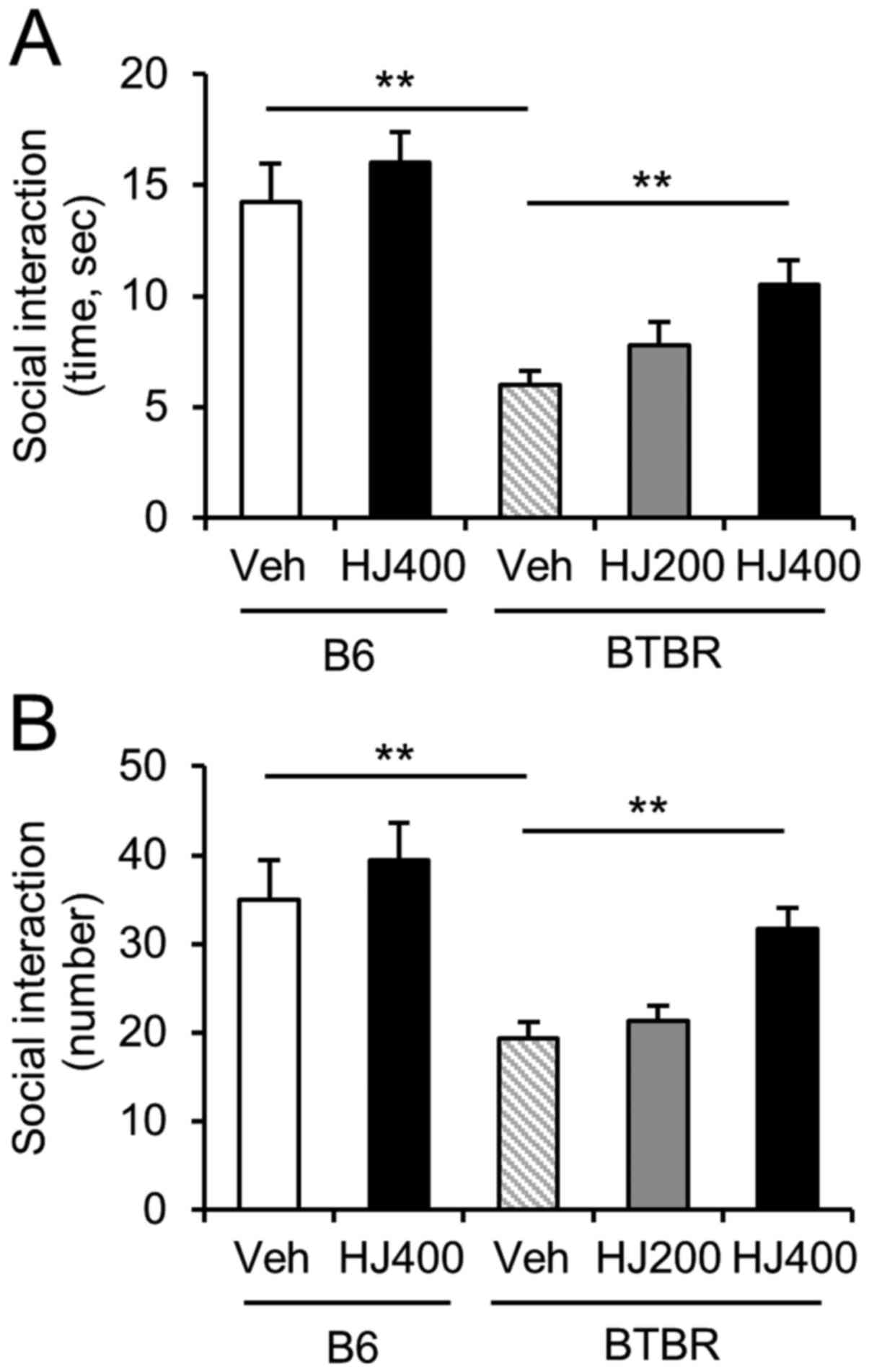
Social interaction is increased by HJ
treatment in BTBR mice
To determine the effect of HJ on social deficits, we
performed a social interaction test in the vehicle-treated or
HJ-treated B6 and BTBR mice. The total duration and number of
sniffings in the sociability session were significantly decreased
in the vehicle-treated BTBR mice compared to vehicle-treated B6
mice (Fig. 3A and B, P<0.01). We
found significant effects of 400 mg/kg HJ treatment in BTBR mice,
but not B6 mice (Fig. 3A and B,
P<0.01). BTBR mice treated with 400 mg/kg HJ exhibited
significantly more time and number of sniffings in the social
interaction test than the vehicle-treated BTBR mice (Fig. 3A; F(4,47)=0.9223,
P=0.0004; Fig. 3B,
F(4,47)=1.444, P<0.0001). This finding indicates that
the administration of HJ markedly improved the sociability of BTBR
mice.
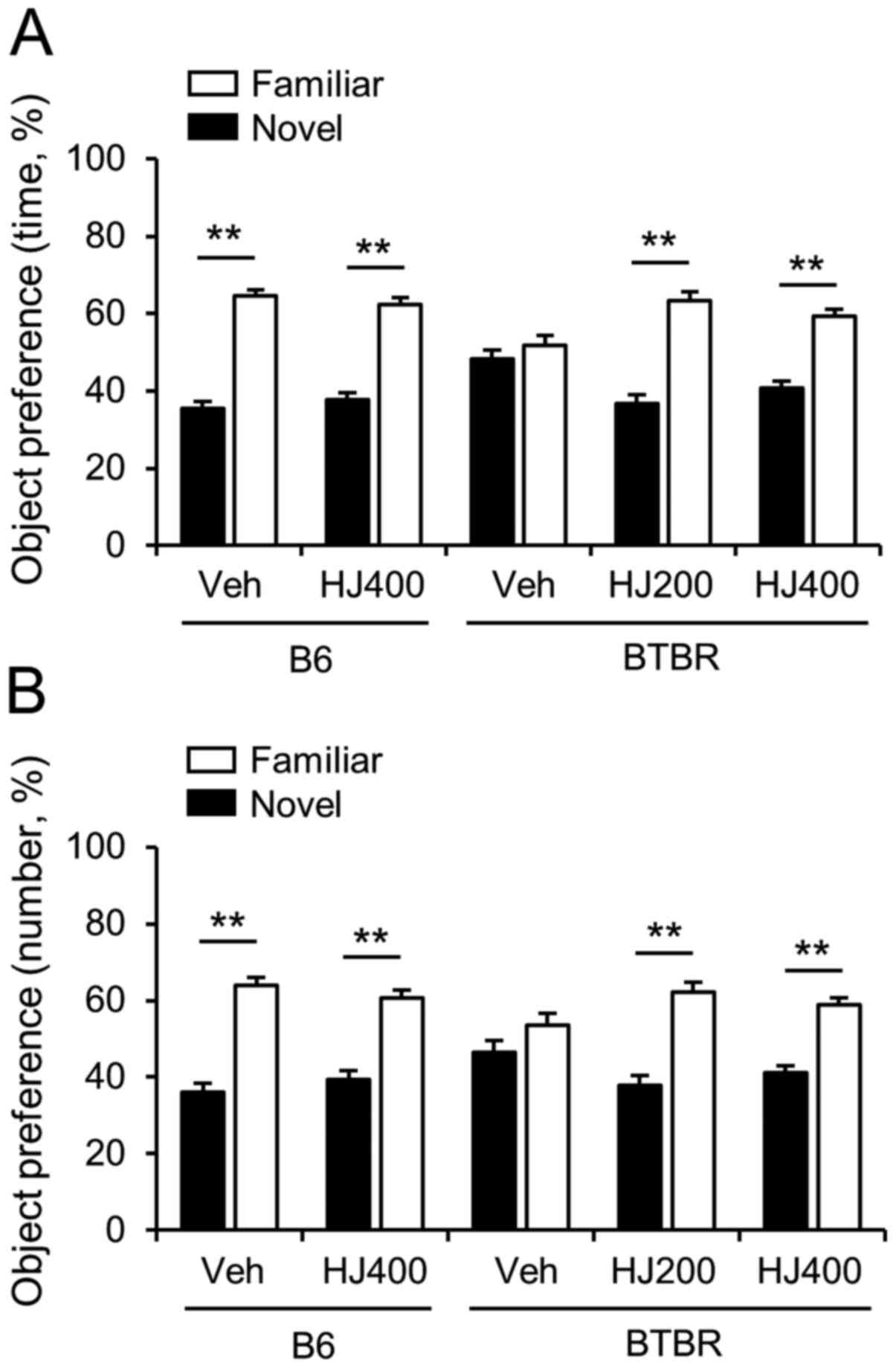
Cognitive deficit is improved by HJ
treatment in BTBR mice
To investigate whether impaired cognitive function
in BTBR mice is improved by HJ treatment, we performed a novel
object recognition test. We measured the total time spent sniffing
and the total number of contacts with the novel object in each
group (Fig. 3A and B). B6 mice
spent significantly more time and had a significantly higher number
of contacts with novel objects compared to the familiar object. B6
mice treated with HJ showed no significant difference in the total
time spent sniffing and in contact with the novel object (Fig. 4A and B). Vehicle-treated BTBR mice
did not show a preference for the novel object. However, 200 mg/kg
or 400 mg/kg HJ treatment rescued the preference for the novel
object (Fig. 4A and B, P<0.01).
These results suggest that HJ can improve cognitive function in
BTBR mice.
Microglial activation is reduced in
the cortex, hippocampus, and dorsal striatum
Since the anti-inflammatory effects of HJ on
neurodegenerative disorders such as AD and PD have been reported
(13,14), we also investigated the effects of
HJ on microglial activation in the brain of BTBR mice. The
percentage area occupied by Iba-1 immunoreactive cells were
analysed in cerebral cortex, hippocampus, and striatum. HJ
treatment (200 mg/kg) significantly decreased the percentage of
occupied Iba-1 immunoreactive cells in the CA1, CA3, and DG of the
hippocampus (Fig. 5A and B). HJ
treatment (400 mg/kg) markedly decreased the percentage of occupied
Iba-1 immunoreactive cells in the cerebral cortex, DG, and striatum
(Fig. 5A and B). These results
suggest that HJ can influence microglial activation in the cerebral
cortex, hippocampus, and striatum.
The levels of cytokines and chemokines in patients
with ASD have been shown to be altered compared to those in
typically developing children (23). In particular, chemokine CC motif
ligand 2 (CCL2) is significantly increased in patients with ASD
(23). To determine the effect of
HJ on the expression of CCL2 in the hippocampus, we performed
quantitative RT-PCR analysis. The administration of HJ
significantly decreased the mRNA expression of CCL2 in the
hippocampus of BTBR mice compared to vehicle-treated group
(Fig. 5C, P<0.05). Upregulation
of chemokine CCL2 and activation of microglia can induce
pro-inflammatory cytokines, such as IL-1β, IL-6, and CXCL10
(11,24). To analyse the effect of HJ on the
mRNA expression of pro-inflammatory cytokines, the mRNA expression
levels of IL-1β, IL-6, and CXCL10 were measured in the hippocampi
of the mice. Interestingly, the mRNA expression of IL-1β and IL-6
were significantly decreased by HJ treatment (Fig. 5D, P<0.05). The mRNA expression of
CXCL10 also showed a decreasing trend in the HJ-treated group,
although it did not reach statistical significance (Fig. 5D, P=0.086).
NR2B and CaMKIIα signalling is
decreased by HJ treatment in the hippocampus of BTBR mice
To investigate the effects of HJ on the NR2B subunit
of NMDA receptors and CaMKIIα signalling, western blot analysis was
performed in the hippocampus of vehicle-treated and HJ-treated
mice. In the hippocampus of BTBR mice, the phosphorylation of NR2B
and CaMKIIα was significantly increased compared to their
hippocampal expression in B6 mice (Fig.
6A and B). The administration of HJ significantly decreased
phosphorylation of NR2B and CaMKIIα in the hippocampus of BTBR mice
(Fig. 6A and B), but not in B6
mice. Taken together, these data indicate that HJ attenuates the
NR2B and CaMKIIα-mediated signalling pathways in the hippocampus of
BTBR mice. However, the protein expression and phosphorylation (at
Ser831 and Ser845) of the GluR1 subunit of AMPA receptor were not
altered by HJ treatment (Fig. 6C and
D).
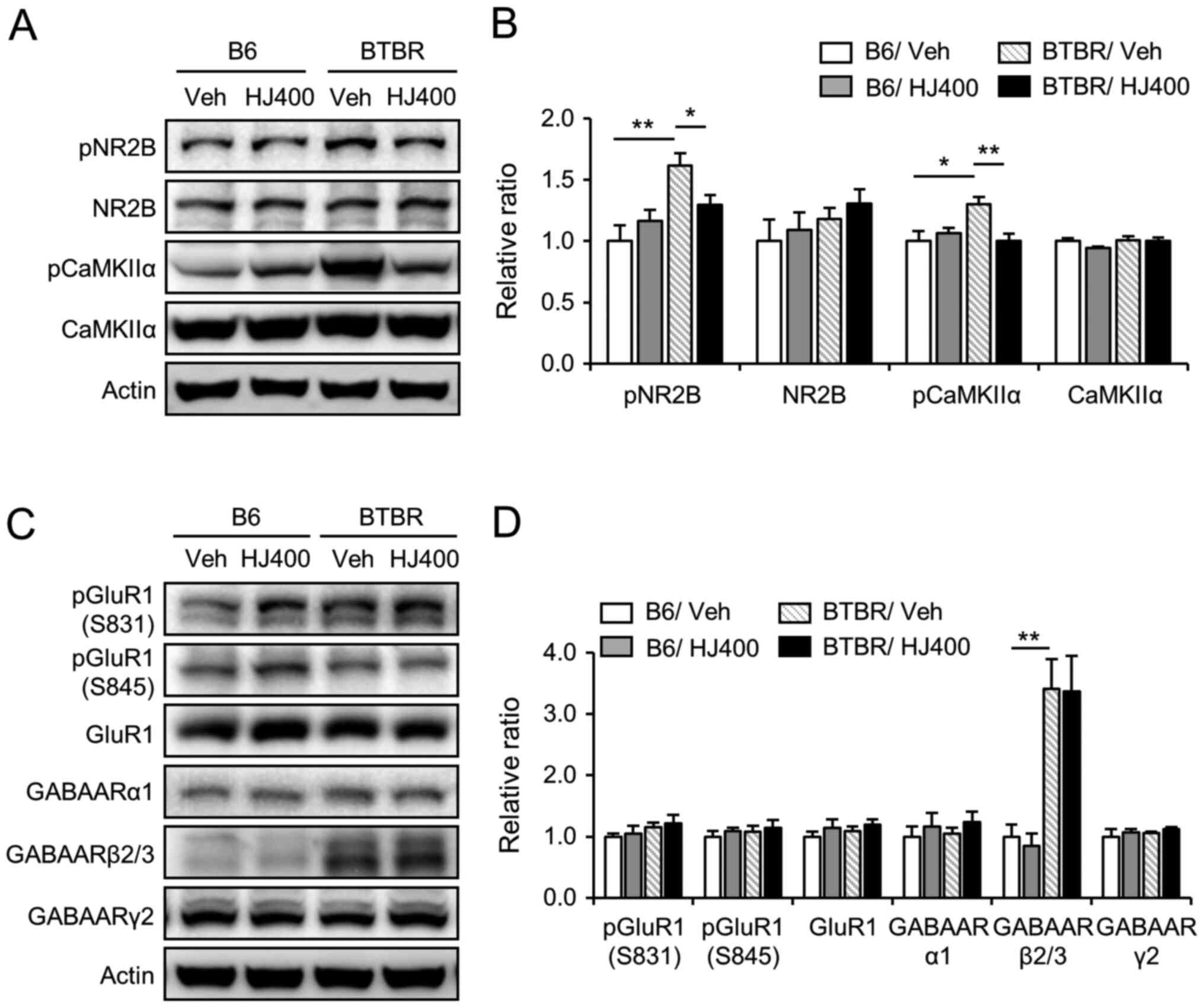 | Figure 6.Effects of HJ treatment on the
phosphorylation of NR2B and CaMKIIα in the hippocampus of C57BL/6J
and BTBR mice. Western blot analysis of NMDA receptor subunit NR2B,
pNR2B (Tyr1472), CaMKIIα, pCaMKIIα, AMPA receptor subunit GluR1,
pGluR1 (Ser831), pGluR1 (Ser845), and GABAA receptor subunits α1,
β2/3, and γ2 in the hippocampus of the vehicle-treated (vehicle)
and 400 mg/kg HJ-treated (HJ400) B6 or BTBR mice. Band densitometry
values normalised to actin levels. (A) Representative image and (B)
quantitative analysis of NMDA receptor subunit NR2B, pNR2B
(Tyr1472), CaMKIIα, pCaMKIIα (Thr286) in the hippocampus of the
vehicle-treated (vehicle) and 400 mg/kg HJ-treated (HJ400) B6 or
BTBR mice. (C) Representative image and (D) quantitative analysis
of AMPA receptor subunit GluR1, pGluR1 (Ser831), pGluR1 (Ser845),
and GABAA receptor subunits α1, β2/3, and γ2 in the hippocampus of
the vehicle-treated (vehicle) and 400 mg/kg HJ-treated (HJ400) B6
or BTBR mice. *P<0.05 and **P<0.01 vs. indicated group. Data
are presented as mean ± SEM. HJ, Humulus japonicus; NR2B,
N-methyl-D-aspartate receptor subtype 2B; CaMKIIα,
calcium/calmodulin-dependent protein kinase type II subunit α; p,
phosphorylated. |
In addition, we used western blotting to test
whether HJ treatment affects the protein expression of GABA
receptors. The expression of GABAA receptor subunits α1, β2/3, and
γ2 was analysed in the hippocampus of vehicle-treated and
HJ-treated B6 or BTBR mice. The protein expression of GABAA
receptor subunit β2/3 was enhanced in the hippocampus of BTBR mice
compared to B6 mice (Fig. 6C and D,
P<0.01). However, the protein expression of GABAA receptor
subunits α1, β2/3, and γ2 was not regulated by HJ treatment in BTBR
and B6 mice.
Discussion
ASD is a neurodevelopmental disorder characterised
by deficits in social interaction and restrictive, repetitive, and
stereotypical patterns of behaviour. However, there is no
pharmacological drug currently used to target these core ASD
symptoms. In this study, we demonstrated the protective effects of
HJ on autistic-like behaviours in BTBR mice. BTBR mice showed
hyperlocomotion activity, more repetitive behaviours, lower
sociability, and impaired cognitive function compared to B6 control
mice. The administration of HJ in BTBR mice markedly decreased core
autistic symptoms, such as self-grooming behaviour and lower
sociability. Self-grooming behaviour represents a stereotypical
pattern of behaviour in rodents (25). Self-grooming behaviour was
significantly reduced by 400 mg/kg HJ treatment in BTBR mice, but
not in B6 control mice. Moreover, we also found that sniffing and
direct contact between the two mice were markedly increased by 400
mg/kg HJ treatment in BTBR mice, but not in control B6 mice.
Although intellectual disability is not a DSM-5-specified
criterion, BTBR mice showed significant impairments in novel object
recognition, which markedly improved following HJ administration in
BTBR mice.
Previous evidence has indicated the involvement of
CCL2 in various neurological disorders, such as AD, epilepsy, and
stroke (26). In addition, elevated
CCL2 levels in cerebrospinal fluid and serum have a positive
correlation with higher cognitive impairment (23). In an in vitro assay, CCL2
injured neuronic dendrites in the CA1 region of hippocampal slices
and induced primary hippocampal neuronic death (24,27).
Treatment with 400 mg/kg HJ significantly decreased expression of
CCL2 in the hippocampus. In order to explore the effect of HJ on
neuroinflammation, we examined the mRNA expression of
pro-inflammatory cytokines. Pro-inflammatory cytokines including
IL-1β, IL-6, and CXCL-10 were downregulated by HJ administration in
the hippocampus. CCL2 can significantly promote the expression of
cytokines IL-1β, IL-6, and CXCL-10 (24). In this study, the negative
regulatory effect of HJ on CCL2 expression may influence the
expression of these cytokines in the hippocampus.
Neuronic excitotoxicity is one of the major
pathological mechanisms in various neurological disorders,
including AD, depression, and ASD (28–30).
Glutamate is the main excitatory neurotransmitter in the brain and
plays a crucial role in synaptic transmission (30). However, excessive accumulation of
glutamate results in neuronic excitotoxicity (31,32).
Since CCL2 could enhance NMDAR-mediated excitatory postsynaptic
currents and lead to hippocampal neuron death (27,33),
we investigated the signalling pathway alteration mediated by NMDA
receptors. Intriguingly, the phosphorylation levels of NR2B and
CaMKIIα were significantly reduced in the hippocampus of the
HJ-treated group. In our study, although excessive accumulation of
glutamate in BTBR mice has not been studied, pNR2B and pCaMKIIα
were markedly increased in the hippocampus of BTBR mice. Increased
NR2B signalling was decreased by HJ treatment in BTBR mice. Thus,
the effects of HJ on NR2B signalling may be beneficial in the
hippocampus of BTBR mice.
Alterations of GABAergic signalling common in ASD
patients have been detected in animal models of syndromic forms of
autism and in BTBR mice (34). BTBR
mice showed a reduced level of inhibitory neurotransmission
mediated by GABAA receptors in the hippocampus and
enhancement of their inhibitory neurotransmission with positive
allosteric modulators of GABAA receptors ameliorated
autism-like behaviours (34). In
our study, the protein expression levels of GABAA
receptor subunits α1, β2/3, and γ2 were analysed in the hippocampus
of vehicle-treated and HJ-treated B6 or BTBR mice. Intriguingly, in
the hippocampus, GABAAR subunit β2/3 was highly enhanced
in the BTBR mice compared to B6 mice. However, there was no
statistical difference in the expression of the
GABAARβ2/3 proteins between vehicle- and HJ-treated
groups. These data indicate that the protective effects of HJ on
autistic-like behaviours are not caused by the modulation of the
expression of GABAA receptor subunits α1, β2/3 and
γ2.
In conclusion, this study showed that HJ treatment
from postnatal 3-weeks to 9-weeks rescued repetitive behaviour and
social interaction ability in BTBR mice. Further, we also explored
the underlying mechanisms, including the influence of microglia
activation, pro-inflammatory cytokines, and glutamate signalling.
Overall, these data suggest that HJ treatment may provide a
promising strategy to treat the core symptoms of ASD. The negative
regulatory effects of HJ on NR2B signaling pathway and CCL2
expression may have a good influence in brain. The anti-autistic
effects of HJ may last relatively long. The treatment-period
studies are needed to evaluate the treatment strategy. In addition,
our previous study on chemical profiling of HJ using HPLC-qTOF-MS
and NMR found biologically active substances from HJ against PD
(35). Further studies are needed
to evaluate the effects of these compounds on ASD.
Acknowledgements
Not applicable.
Funding
This study was supported by the KRIBB Research
Initiative Program of the Republic of Korea and by the Development
of Platform Technology for Innovative Medical Measurements Program
(grant no. KRISS-2020-GP2020-0004) from the Korea Research
Institute of Standards and Science.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HYP, CHL and KSK designed the study; HYP, JG, YKR,
DHC, JRN and JPA performed the experiments; HYP, JG, YKR, JRN, WKO,
PLH and KSK analysed the data; and HYP, CHL and KSK interpreted
data and wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal care and use were in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals and was approved by the Institutional Animal Use
and Care Committee of the Korea Research Institute of Bioscience
and Biotechnology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rommelse N, Langerak I, van der Meer J, de
Bruijn Y, Staal W, Oerlemans A and Buitelaar J: Intelligence may
moderate the cognitive profile of patients with ASD. PLoS One.
10:e01386982015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McFarlane HG, Kusek GK, Yang M, Phoenix
JL, Bolivar VJ and Crawley JN: Autism-like behavioral phenotypes in
BTBR T+tf/J mice. Genes Brain Behav. 7:152–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverman JL, Tolu SS, Barkan CL and
Crawley JN: Repetitive self-grooming behavior in the BTBR mouse
model of autism is blocked by the mGluR5 antagonist MPEP.
Neuropsychopharmacology. 35:976–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chao OY, Yunger R and Yang YM: Behavioral
assessments of BTBR T+Itpr3tf/J mice by tests of object attention
and elevated open platform: Implications for an animal model of
psychiatric comorbidity in autism. Behav Brain Res. 347:140–147.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hardan AY, Minshew NJ and Keshavan MS:
Corpus callosum size in autism. Neurology. 55:1033–1036. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frazier TW and Hardan AY: A meta-analysis
of the corpus callosum in autism. Biol Psychiatry. 66:935–941.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stephenson DT, O'Neill SM, Narayan S,
Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher
M, et al: Histopathologic characterization of the BTBR mouse model
of autistic-like behavior reveals selective changes in
neurodevelopmental proteins and adult hippocampal neurogenesis. Mol
Autism. 2:72011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solek CM, Farooqi N, Verly M, Lim TK and
Ruthazer ES: Maternal immune activation in neurodevelopmental
disorders. Dev Dyn. 247:588–619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrelli F, Pucci L and Bezzi P:
Astrocytes and microglia and their potential link with autism
spectrum disorders. Front Cell Neurosci. 10:212016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vargas DL, Nascimbene C, Krishnan C,
Zimmerman AW and Pardo CA: Neuroglial activation and
neuroinflammation in the brain of patients with autism. Ann Neurol.
57:67–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JW, Hong JY and Bae SM: Microglia and
autism spectrum disorder: Overview of current evidence and novel
immunomodulatory treatment options. Clin Psychopharmacol Neurosci.
16:246–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henstridge CM, Tzioras M and Paolicelli
RC: Glial contribution to excitatory and inhibitory synapse loss in
neurodegeneration. Front Cell Neurosci. 13:632019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park TS, Ryu YK, Park HY, Kim JY, Go J,
Noh JR, Kim YH, Hwang JH, Choi DH, Oh WK, et al: Humulus
japonicus inhibits the progression of Alzheimer's disease in a
APP/PS1 transgenic mouse model. Int J Mol Med. 39:21–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryu YK, Kang Y, Go J, Park HY, Noh JR, Kim
YH, Hwang JH, Choi DH, Han SS, Oh WK, et al: Humulus
japonicus prevents dopaminergic neuron death in
6-hydroxydopamine-induced models of Parkinson's disease. J Med
Food. 20:116–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang SY, Jo MJ, Kim SC and Jee SY:
Anti-inflammaory effects of the MeOH extract of Humulus
japonicus in vivo. J Korean Orient Med Ophthalmol Otolaryngol
Dermato. 22:92–103. 2009.
|
|
16
|
Go J, Park TS, Han GH, Park HY, Ryu YK,
Kim YH, Hwang JH, Choi DH, Noh JR, Hwang DY, et al: Piperlongumine
decreases cognitive impairment and improves hippocampal function in
aged mice. Int J Mol Med. 42:1875–1884. 2018.PubMed/NCBI
|
|
17
|
Edfawy M, Guedes JR, Pereira MI, Laranjo
M, Carvalho MJ, Gao X, Ferreira PA, Caldeira G, Franco LO, Wang D,
et al: Abnormal mGluR-mediated synaptic plasticity and autism-like
behaviours in Gprasp2 mutant mice. Nat Commun. 10:14312019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Taylor AMW, Nagai J, Golshani P,
Evans CJ, Coppola G and Khakh BS: Reducing astrocyte calcium
signaling in vivo alters striatal microcircuits and causes
repetitive behavior. Neuron. 99:1170–1187 e1179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Felix-Ortiz AC and Tye KM: Amygdala inputs
to the ventral hippocampus bidirectionally modulate social
behavior. J Neurosci. 34:586–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kozera B and Rapacz M: Reference genes in
real-time PCR. J Appl Genet. 54:391–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryu YK, Park HY, Go J, Choi DH, Kim YH,
Hwang JH, Noh JR, Lee TG, Lee CH and Kim KS: Metformin inhibits the
development of L-DOPA-induced dyskinesia in a murine model of
Parkinson's disease. Mol Neurobiol. 55:5715–5726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han YM, Cheung WK, Wong CK, Sze SL, Cheng
TWS, Yeung MK and Chan AS: Distinct cytokine and chemokine profiles
in autism spectrum disorders. Front Immunol. 8:112017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Tan L, Liao Y, Long J and Zhou Y,
Wei J and Zhou Y: Chemokine CCL2 impairs spatial memory and
cognition in rats via influencing inflammation, glutamate
metabolism and apoptosis-associated genes expression-A potential
mechanism for HIV-associated neurocognitive disorder. Life Sci.
255:1178282020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silverman JL, Pride MC, Hayes JE, Puhger
KR, Butler-Struben HM, Baker S and Crawley JN: GABAB receptor
agonist R-baclofen reverses social deficits and reduces repetitive
behavior in two mouse models of autism. Neuropsychopharmacology.
40:2228–2239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thames AD, Briones MS, Magpantay LI,
Martinez-Maza O, Singer EJ, Hinkin CH, Morgello S, Gelman BB, Moore
DJ, Heizerling K and Levine AJ: The role of chemokine C-C motif
ligand 2 genotype and cerebrospinal fluid chemokine C-C motif
ligand 2 in neurocognition among HIV-infected patients. AIDS.
29:1483–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Tang H and Xiong H: Chemokine CCL2
enhances NMDA receptor-mediated excitatory postsynaptic current in
rat hippocampal slices-a potential mechanism for HIV-1-associated
neuropathy? J Neuroimmune Pharmacol. 11:306–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang R and Reddy PH: Role of glutamate and
NMDA receptors in Alzheimer's disease. J Alzheimers Dis.
57:1041–1048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hermens DF, Chitty KM, Lee RS, Tickell A,
Haber PS, Naismith SL, Hickie IB and Lagopoulos J: Hippocampal
glutamate is increased and associated with risky drinking in young
adults with major depression. J Affect Disord. 186:95–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Otaish H, Al-Ayadhi L, Bjorklund G,
Chirumbolo S, Urbina MA and El-Ansary A: Relationship between
absolute and relative ratios of glutamate, glutamine and GABA and
severity of autism spectrum disorder. Metab Brain Dis. 33:843–854.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Groc L and Choquet D: Linking glutamate
receptor movements and synapse function. Science. 368:eaay46312020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huo TG, Li WK, Zhang YH, Yuan J, Gao LY,
Yuan Y, Yang HL, Jiang H and Sun GF: Excitotoxicity induced by
realgar in the rat hippocampus: The involvement of learning memory
injury, dysfunction of glutamate metabolism and NMDA receptors. Mol
Neurobiol. 51:980–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou Y, Tang H, Liu J, Dong J and Xiong H:
Chemokine CCL2 modulation of neuronal excitability and synaptic
transmission in rat hippocampal slices. J Neurochem. 116:406–414.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han S, Tai C, Jones CJ, Scheuer T and
Catterall WA: Enhancement of inhibitory neurotransmission by GABAA
receptors having α2,3-subunits ameliorates behavioral deficits in a
mouse model of autism. Neuron. 81:1282–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee HJ, Dhodary B, Lee JY, An JP, Ryu YK,
Kim KS, Lee CH and Oh WK: Dereplication of components coupled with
HPLC-qTOF-MS in the active fraction of Humulus japonicus and
it's protective effects against parkinson's disease mouse model.
Molecules. 24:14352019. View Article : Google Scholar : PubMed/NCBI
|