Introduction
Acute lung injury (ALI) is a serious pathological
condition characterised by neutrophil-derived inflammation,
surfactant dysfunction, diffuse alveolar injury and lung oedema
formation (1). Clinically, ALI is
characterised by bilateral pulmonary infiltrates, decreased lung
compliance and severe hypoxemia (2). ALI is a common, costly, and
potentially lethal disease (3). It
is estimated that ALI accounts for ~79,000 deaths per year in the
United States, and was one of the top 8 causes of death in 2018
(4). Despite extensive large-scale
clinical studies and trials, no effective treatments for ALI have
been identified (5). Thus, it is
essential to investigate novel approaches for the treatment of
ALI.
Fat emulsions are typically regarded as a caloric
source that is required to alleviate essential fatty acid (FA)
deficiency in critically ill patients, including those with ALI
(6). Certain fat emulsions
attenuate injury and inflammation in different organs. For
instance, fish oil-based fat emulsion suppresses injury and
inflammation in mice with acute kidney injury (7). Fish oil-based fat emulsion decreases
lipopolysaccharide (LPS)-induced alveolar leukocyte transmigration
and pro-inflammatory cytokine expression in ALI mice (8). However, certain fat emulsions may
induce side effects in patients with lung injury due to the
presence of medium-chain triacylglycerols (MCTs) (9,10). To
improve the tolerance and effectiveness of fat emulsions, a
structured triglyceride (STG), which is a combination of MCTs and
long-chain triglycerides (LCTs) and is also known as a structured
fat emulsion or structured lipid, has been proposed (11). Treatment with STG ameliorates the
inflammation in rats undergoing a total gastrectomy (12). Furthermore, STG alleviates renal
injury in diabetic rats (13).
However, the specific regulatory role of STG in ALI remains
unclear.
G protein-coupled receptors (GPRs), including
GPR120, are pivotal signalling molecules in multiple aspects of
cellular function (14). GPR120
exerts anti-inflammatory effects in various diseases. Activation of
GPR120 protects against focal cerebral ischaemic injury in mice by
attenuating apoptosis and inflammation (15). Flaxseed oil has been found to
increase GPR120 expression, thereby restraining the inflammatory
response in the livers of obese mice (16). STG represents an innovative way to
supply a mixture of FAs with different chain lengths (17). GPR120 serves as an omega-3 (ω-3) FA
receptor that exerts anti-inflammatory effects (18). Ginsenoside Rb2 promotes the
anti-inflammatory effects of ω-3 FA in LPS-induced RAW264.7
macrophages by enhancing GPR120 (19). However, whether the effects of STG
are mediated via GPR120 in ALI remains unknown.
An LPS-induced ALI mouse model was constructed in
the present study and the regulatory effects of STG on lung injury,
lung inflammation, and transforming growth factor-α-activated
kinase 1 (TAK1)/NF-κB pathway activity in ALI was evaluated.
Moreover, the associations between STG and GPR120 were identified.
Thus, the present study suggests a potential compound for treating
ALI.
Materials and methods
Animals and drugs
In total, 40 male C57BL/6 mice (age, 8 weeks; body
weight, 22–26 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. Mice were fed standard chow and water
and maintained at 22–25°C and 55–65% relative humidity with a 12-h
light/dark cycle. The animal experiments were approved by the
Ethics Committee of Heze Municipal Hospital (approval no.
KYLL-hzsl2020008). STG is an innovative mixture of MCTs and LCTs
containing combinations different from chain length FAs. The STG
used in the present study was purchased from FRESENIUS
(C6-24).
ALI mouse model
After 1 week of adjustment, 20 mice were randomly
allocated into four groups (n=5): Sham, ALI, ALI + STG 2.5 mg/kg
and ALI + STG 7.5 mg/kg. To establish the LPS-induced ALI mouse
model, mice were intranasally administered 50 µl LPS. The Sham
group was intranasally administered 50 µl PBS. Then, 1 day
postperfusion, mice in the ALI + STG 2.5 mg/kg and ALI + STG 7.5
mg/kg groups were intravenously injected via the tail with 2.5 or
7.5 mg/kg STG three times at 6, 12 and 18 h. Mice in the ALI group
received only LPS administration. Moreover, 20 additional mice were
randomly divided into four groups (n=5): Sham, ALI, ALI + STG and
ALI + STG + AH7614. The protocol of the Sham, ALI and ALI + STG
groups was consistent with the previously described Sham, ALI and
ALI + STG 7.5 mg/kg groups, respectively. Additionally, AH7614
(GPR120 inhibitor) was used to further investigate the association
between STG and the GPR120. In the ALI + STG + AH7614 group,
LPS-induced ALI mice were intravenously injected via the tail with
7.5 mg/kg STG three times at 6, 12 and 18 h prior to receiving 50
µg/mg AH7614 by intravenous tail injection.
Sample collection
A total of 1 h after the last treatment, all mice
were anaesthetised by intraperitoneal injection of sodium
pentobarbital (50 mg/kg). Blood was extracted through the hepatic
portal vein under aseptic conditions and centrifuged at 3,000 × g
for 15 min at 4°C. Both the STG-containing serum and other serum
were filtered through a 0.22-µm filter membrane and stored at −80°C
for cell culture. Then, 1 day later, all mice were anaesthetised
and sacrificed by decapitation. Bronchoalveolar lavage fluid (BALF)
was acquired by lavaging the left lung with 4 ml PBS through the
tracheal cannula. The left lung was collected for measurement of
the lung wet/dry weight (W/D) ratio assay. The right lung was
separated, and a part of the lung tissue was fixed (24 h, 37°C) in
4% paraformaldehyde for haematoxylin and eosin (H&E) staining
(0.5% hematoxylin for 2 min at 37°C, followed by 0.5% eosin for 2
min at 37°C). Another part of the right lung tissue was stored at
−80°C for reverse transcription-quantitative PCR (RT-qPCR), western
blotting and measurement of myeloperoxidase (MPO) activity.
Analysis of lung W/D ratio
The left lung was carefully rinsed, blotted and
weighed (wet weight). Subsequently, the left lung was dried in an
oven for 24 h at 80°C and weighed (dry weight). The lung W/D ratio
was calculated as follows: (Wet weight/dry weight) ×100%.
MPO activity
The lung homogenates were centrifuged at 1,000 × g
for 10 min at 4°C. The MPO in the supernatant was assayed using
commercial kits (Nanjing Jiancheng Bioengineering Institute).
H&E staining
Lung tissues were fixed in 4% paraformaldehyde for
24 h at 37°C, embedded in paraffin, cut into 5-µm-thick sections
and stained with 0.5% hematoxylin for 2 min at 37°C, followed by
0.5% eosin for 2 min at 37°C. By means of light microscopy
(magnification, ×400; BX51; Olympus Corporation), the lung
pathological changes were evaluated. Lung injury was scored using a
5-point system according to a previous study (20). The scoring criteria were as follows:
0, normal tissue; 1, tiny inflammatory change; 2, mild to moderate
inflammatory change (no marked damage to the lung architecture); 3,
moderate inflammatory injury (thickening of the alveolar septa); 4,
moderate to severe inflammatory injury (formation of nodules or
areas of pneumonitis); and 5, severe inflammatory injury (total
obliteration of the field).
ELISA
BALF was collected. After being centrifuged at 3,000
× g for 10 min at 4°C, the supernatant was collected for cytokine
detection. The levels of interleukin (IL)-1β, IL-6 and tumour
necrosis factor-α (TNF-α) in BALF were assessed using mouse IL-1β
ELISA kit (cat. no. RAB0275MSDS; Sigma-Aldrich; Merck KGaA), mouse
IL-6 ELISA kit (cat. no. RAB0309MSDS; Sigma-Aldrich; Merck KGaA),
and mouse TNF-α ELISA kit (cat. no. RAB0477MSDS; Sigma-Aldrich;
Merck KGaA), respectively.
Cell culture and treatment
RAW264.7 cells (murine macrophage cell line) were
cultured in DMEM/F12 medium (American Type Culture Collection) with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C and in
5% CO2. RAW264.7 cells were divided into the negative
control (RAW264.7 cells pre-treated with serum from mice in the
Sham group for 1 h at 37°C), LPS (RAW264.7 cells pre-treated with
serum from mice in the ALI group for 1 h and then stimulated with
0.5 µg/ml LPS for 6 h at 37°C), and LPS + STG serum (RAW264.7 cells
pre-treated with serum from mice in the ALI + STG group for 1 h and
then stimulated with 0.5 µg/ml LPS for 6 h at 37°C) groups.
Additionally, the LPS + STG serum + AH7614 group comprised RAW264.7
cells pre-treated with serum from mice in the ALI + STG + AH7614
group for 1 h, stimulated with 0.5 µg/ml LPS for 6 h, and treated
with 100 µM AH7614 for another 4 h at 37°C.
RT-qPCR
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Thereafter, complementary DNA samples were attained through
RT using PrimeScript RT Reagent kit (Takara Bio, Inc.). The
reaction mixtures were incubated at 37°C for 60 min, 95°C for 5 min
and then held at 4°C. RT-qPCR analysis was detected using
SYBR-Green PCR Master Mix (Takara Bio, Inc.). RT-qPCR was conducted
on the 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), and the reaction conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles at
95°C for 10 sec, 60°C for 20 sec and 72°C for 34 sec. The relative
protein expression level was calculated using the 2−ΔΔCq
method (21). The primer sequences
used are shown in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR.
| Name of primer | Sequence 5′-3′ |
|---|
| IL-1β-F |
GGTCAAAGGTTTGGAAGCAG |
| IL-1β-R |
TGTGAAATGCCACCTTTTGA |
| IL-6-F |
TAGTCCTTCCTACCCCAATTTCC |
| IL-6-R |
TTGGTCCTTAGCCACTCCTTC |
| TNF-α-F |
ATGGGAAGGGAATGAATCCACC |
| TNF-α-R |
GTCCACATCCTGTAGGGCGTCT |
| β-actin-F |
GGGAAATCGTGCGTGACATTAAG |
| β-actin-R |
TGTGTTGGCGTACAGGTCTTTG |
Western blotting
Total proteins were extracted from tissues and cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology). The
concentration of total protein was detected using the bicinchoninic
acid method. The proteins (20 µg per lane) were then separated by
10–12% sodium dodecyl sulphate gel electrophoresis gels. Separated
protein was transferred onto polyvinylidene fluoride membranes,
blocked with 5% skim milk for 1 h at 37°C, and incubated at 4°C
overnight with primary antibodies, including anti-GPR120 (1:1,000;
cat. no. SAB4501490MSDS; Sigma-Aldrich; Merck KGaA), anti-p65
(1:1,000; cat. no. SAB4301496MSDS; Sigma-Aldrich; Merck KGaA),
anti-phosphorylated (p)-p65 (1:500; cat. no. MAB3026MSDS;
Sigma-Aldrich; Merck KGaA), anti-TAK1 (1:1,000, cat. no.
SAB4502922MSDS; Sigma-Aldrich; Merck KGaA), anti-p-TAK1 (1:500;
cat. no WH0006885M2MSDS; Sigma-Aldrich; Merck KGaA), anti-Toll-like
receptor 4 (TLR4; 1:500; cat. no. PRS3141MSDS; Sigma-Aldrich; Merck
KGaA), and anti-family pyrin domain-containing 3 (NLRP3; 1:500;
cat. no. HPA012878MSDS; Sigma-Aldrich) antibodies. Thereafter, the
membranes were subjected to the horseradish peroxidase-labelled
goat anti-rabbit IgG (1:5,000; cat. no. 12-348MSDS; Sigma-Aldrich;
Merck KGaA) secondary antibody at 25°C for 1 h. The immunoblots
were analysed via enhanced chemiluminescence (Invitrogen; Thermo
Fisher Scientific, Inc.) and semi-quantified using the ImageLab
software (version 3.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis of the data was performed using
GraphPad Prism 7.0 (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation. The differences among multiple
groups were assessed using one-way ANOVA followed by Tukey's
post-hoc test. In vivo, the experiments were repeated three
times for each mouse. In vitro, the experiments were
performed in triplicate and repeated three times. P<0.05 was
considered to indicate statistically significant differences.
Results
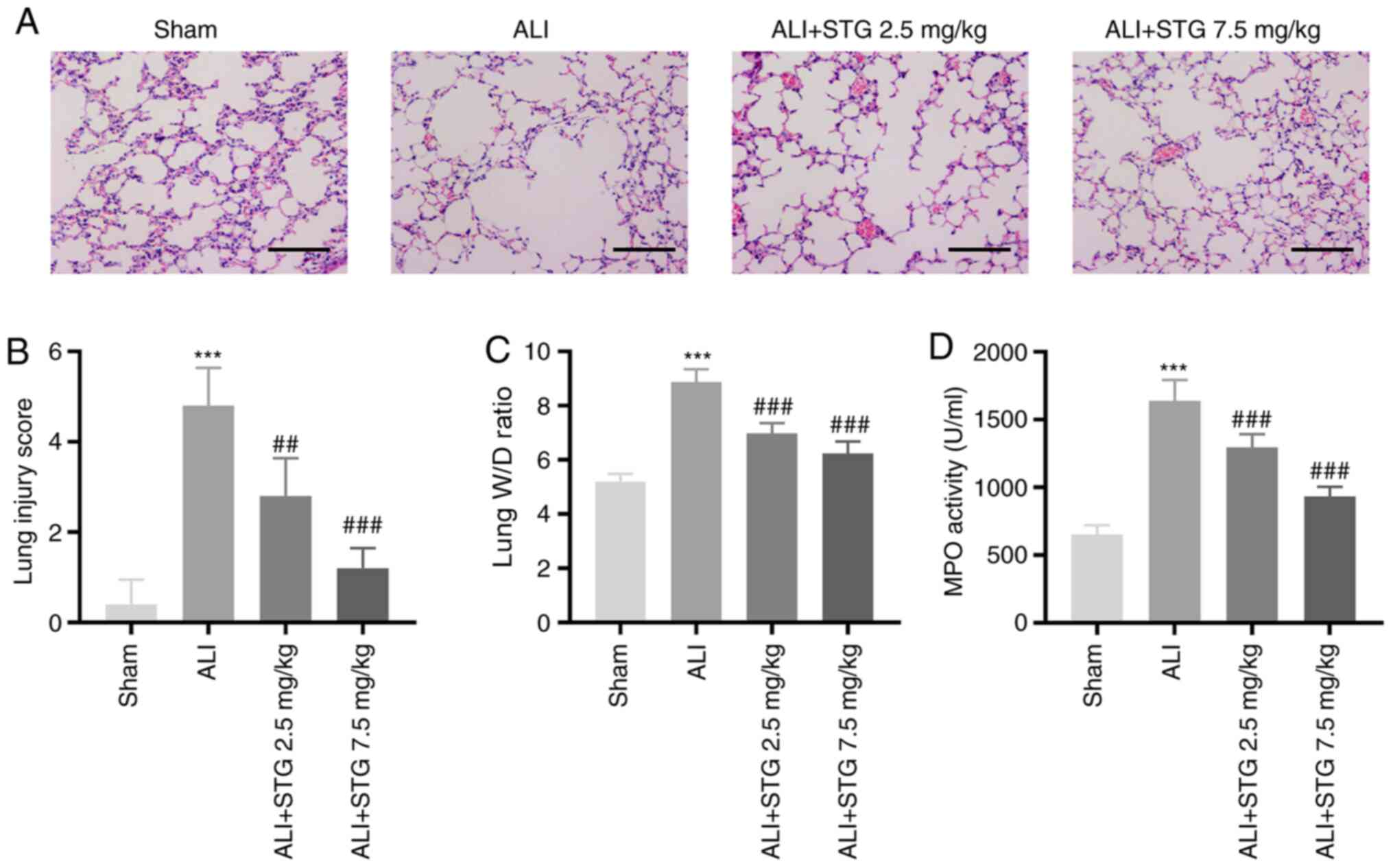
STG alleviates lung injury in the
LPS-induced ALI mouse model
To investigate the roles of STG in the pathogenesis
of ALI, an LPS-induced ALI mouse model was established. LPS
administration caused considerable neutrophil infiltration,
haemorrhage, interstitial oedema and thickening of the alveolar
septum. Treatment with STG markedly alleviated pathological changes
at both concentrations, although 7.5 mg/kg STG displayed more
favourable results (Fig. 1A).
Furthermore, the lung injury score was clearly elevated by LPS
administration (P<0.001). Low STG concentration markedly
decreased the lung injury score (P<0.01), and a high
concentration of STG exhibited a more favourable result
(P<0.001; Fig. 1B). Following
LPS administration, the lung W/D ratio was markedly increased
(P<0.001). The LPS-induced ALI mouse model treated with STG
exhibited a dose-dependent reduction in the lung W/D ratio
(P<0.001; Fig. 1C).
Determination of MPO activity was used to assess lung neutrophil
infiltration. As depicted in Fig.
1D, MPO activity was elevated in ALI lung tissues (P<0.001).
STG decreased lung MPO activity in a concentration-dependent manner
(P<0.001).
STG attenuates lung inflammation in
the LPS-induced ALI mouse model
Inflammation was evaluated by examining the
pro-inflammatory cytokine expression levels in lung tissues and
BALF. As illustrated in Fig. 2A and
B, the expression levels of IL-1β, IL-6 and TNF-α in lung
tissues and BALF were significantly elevated following LPS
administration (P<0.001). The LPS-induced ALI mouse model
treated with STG displayed a dose-dependent reduction in the
expression levels of IL-1β, IL-6 and TNF-α (P<0.001). Moreover,
GPR120 protein expression was clearly inhibited in the LPS-induced
ALI mouse model (P<0.001). STG markedly enhanced GPR120 protein
expression (P<0.001). Notably, AH7614 clearly weakened the
promotive effect of STG on GPR120 protein expression (P<0.001;
Fig. 2C). As displayed in Fig. 2D, GPR120 expression in the ALI group
was lower compared with that in the sham group (P<0.001). STG
increased GPR120 expression in ALI mice (P<0.001), while AH7614
markedly reversed the promotive effect of STG on GPR120 expression
(P<0.001).
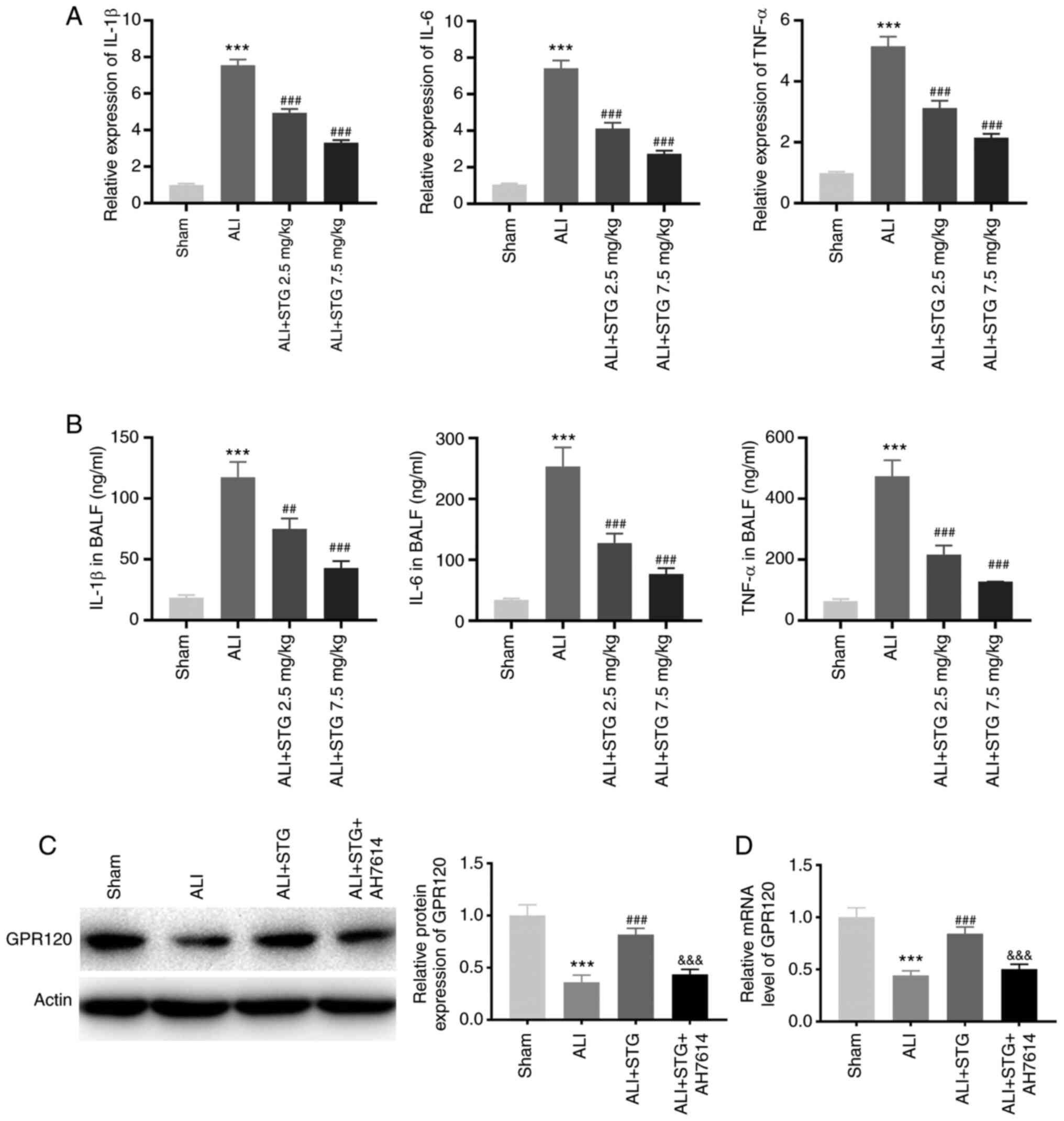 | Figure 2.STG attenuates lung inflammation in
the LPS-induced ALI mouse model. (A) RT-qPCR was performed to
confirm the expression of IL-1β, IL-6, and TNF-α in lung tissues.
***P<0.001 vs. Sham; ###P<0.001 vs. ALI. (B) The
levels of IL-1β, IL-6 and TNF-α in BALF were measured by
enzyme-linked immunosorbent assay. ***P<0.001 vs. Sham;
##P<0.01; and ###P<0.001 vs. ALI. (C)
The GPR120 protein expression in lung tissues was detected by
western blotting. ***P<0.001 vs. Sham; ###P<0.001
vs. ALI; &&&P<0.001 vs. ALI + STG; (D)
RT-qPCR was performed to measure the GPR120 mRNA expression level
in lung tissues. ***P<0.001 vs. Sham; ###P<0.001
vs. ALI; &&&P<0.001 vs. ALI + STG. Each
experiment was performed in triplicate for each mouse (n=5 in each
group). STG, structured triglyceride; LPS, lipopolysaccharide; ALI,
acute lung injury; IL, interleukin; TNF-α, tumor necrosis factor-α;
RT-qPCR, reverse transcription-quantitative PCR; BALF,
bronchoalveolar lavage fluid. |
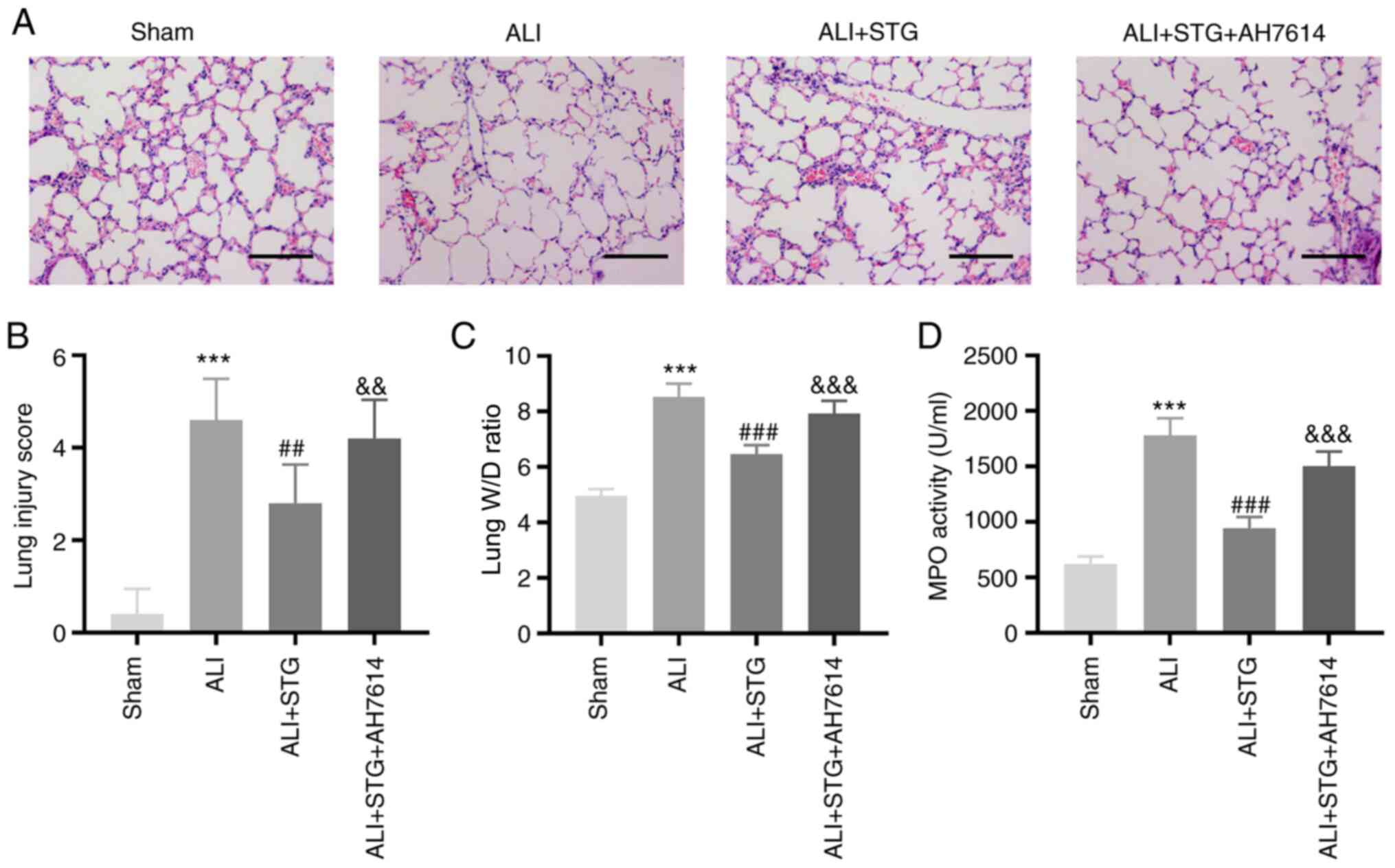
STG alleviates lung injury in the
LPS-induced ALI mouse model by enhancing GPR120
AH7614 was intravenously injected into LPS-induced
ALI mice to verify whether STG regulates GPR120 in ALI. Following
LPS administration, the lung tissues isolated from mice treated
with STG exhibited markedly decreased neutrophil infiltration,
haemorrhage, interstitial oedema and thickening of the alveolar
septum (Fig. 3A). Moreover, STG
could decrease the lung injury score in ALI mice (P<0.01;
Fig. 3B). AH7614 reversed the
inhibitory effect of STG on the degree of lung tissue injury
(Fig. 3A). Additionally, AH7614
simultaneously weakened the lowering effect of STG on the lung
injury score (P<0.01; Fig. 3B).
As shown in Fig. 3C and D, STG
treatment visibly decreased the lung W/D ratio (P<0.001) and MPO
activity (P<0.001). Furthermore, AH7614 rescued the decreased
lung W/D ratio and MPO activity caused by STG in the LPS-induced
ALI mouse model (P<0.001).
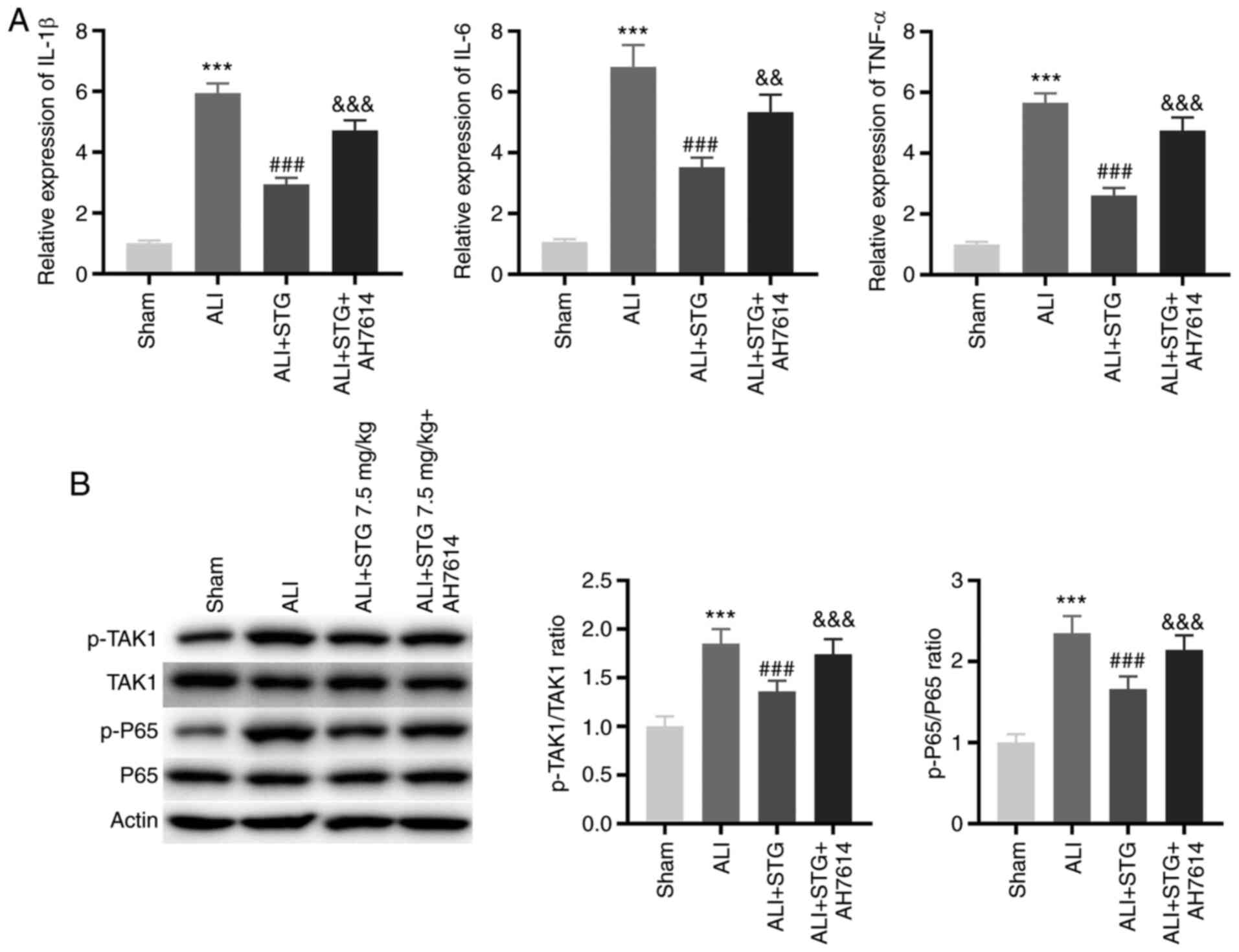
STG attenuates lung inflammation and
TAK1/NF-κB pathway activity in the LPS-induced ALI mouse model by
enhancing GPR120
The GPR120 inhibitor, AH7614, was intravenously
injected into ALI mice, and the expression levels of
pro-inflammatory cytokines and the phosphorylation of p65 and TAK1
in lung tissues were assessed. As displayed in Fig. 4A, the expression levels of IL-1β,
IL-6 and TNF-α in lung tissues in the ALI + STG group were lower
compared with those in the ALI group (P<0.001). Interestingly,
AH7614 treatment clearly mitigated the suppressive effects of STG
on the expression levels of IL-1β, IL-6 and TNF-α in lung tissues
in the LPS-induced ALI mouse model (P<0.01). p65 is a major
subunit of NF-κB. The p-TAK1/TAK1 and p-p65/p65 ratios were used to
evaluate the activity of the TAK1/NF-κB pathway. The p-TAK1/TAK1
and p-p65/p65 ratios were clearly decreased by STG treatment, and
AH7614 reversed the inhibitory effects of STG on the p-TAK1/TAK1
and p-p65/p65 ratios in the LPS-induced ALI mouse model
(P<0.001; Fig. 4B).
STG ameliorates lung inflammation and
TAK1/NF-κB pathway activity in LPS-induced RAW264.7 cells by
enhancing GPR120
To evaluate the effect of STG on ALI in
vitro, LPS-induced RAW264.7 cells acted as the LPS-induced ALI
model at the cellular level. As shown in Fig. 5A, treatment with STG-containing
serum visibly decreased the expression levels of pro-inflammatory
cytokines in LPS-induced RAW264.7 cells (P<0.001), and AH7614
reversed the inhibitory effects of STG on the expression levels of
pro-inflammatory cytokines (P<0.001). Furthermore, treatment
with STG-containing serum significantly repressed the TAK1/NF-κB
pathway activity in LPS-induced RAW264.7 cells (P<0.001), and
AH7614 rescued the suppressed TAK1/NF-κB pathway activity caused by
STG-containing serum treatment (P<0.01; Fig. 5B). As displayed in Fig. 5C, the protein expression levels of
TLR4 and NLRP3 in the LPS + STG serum group were lower compared
with those in the LPS group (P<0.001), and AH7614 reversed the
lowering effects of STG-containing serum treatment on the protein
expression levels of TLR4 and NLRP3 in LPS-induced RAW264.7 cells
(P<0.05).
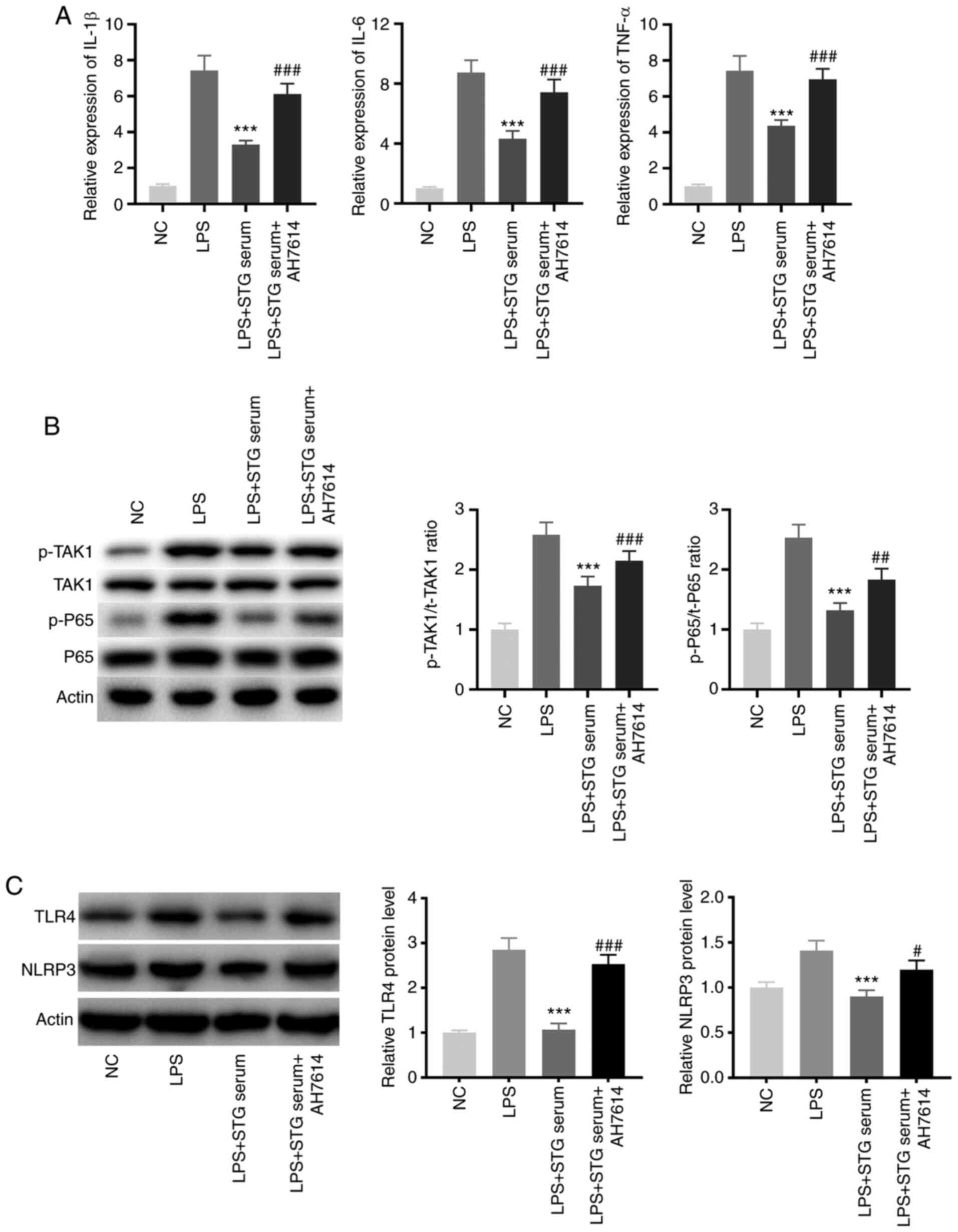 | Figure 5.STG ameliorates lung inflammation and
TAK1/nuclear factor-κB pathway activity in LPS-induced RAW264.7
cells by enhancing GPR120. (A) The levels of IL-1β, IL-6 and TNF-α
in LPS-induced RAW264.7 cells were measured by reverse
transcription-quantitative PCR. ***P<0.001 vs. LPS;
###P<0.001 vs. LPS + STG serum. (B) The activity of
TAK1 and NF-κB pathway was evaluated by western blotting.
***P<0.001 vs. LPS; ##P<0.01 and
###P<0.001 vs. LPS + STG serum. (C) Western blotting
was performed to measure the protein expression of TLR4 and NLRP3.
***P<0.001 vs. LPS; #P<0.05 and
###P<0.001 vs. LPS + STG serum. Each experiment was
performed in triplicate and repeated three times. STG, structured
triglyceride; LPS, lipopolysaccharide; ALI, acute lung injury; NC,
negative control; TAK, transforming growth factor-α-activated
kinase; IL, interleukin, TNF-α, tumor necrosis factor-α; p-,
phosphorylated; TLR, Toll-like receptor; NLRP3, family pyrin
domain-containing 3. |
Discussion
LPS facilitates the release of inflammatory
cytokines and leads to lung injury, which is a well-known
experimental model of ALI (22,23).
In the present study, the lung pathological changes, lung W/D
ratio, MPO activity, expression levels of pro-inflammatory
cytokines and TAK1/NF-κB pathway activity were clearly increased in
LPS-induced ALI mouse model. Previous studies have verified that
the degree of lung injury and inflammatory response are both
increased in LPS-induced ALI (24,25).
All the aforementioned descriptions suggest that the LPS-induced
ALI mouse model was established successfully.
In the present study, STG decreased the lung
pathological changes, lung W/D ratio and MPO activity in the
LPS-induced ALI mouse model. The functions of STG were similar to
the previously described emulsions. For instance, perfluorocarbon
emulsion decreases the lung W/D ratio and suppresses MPO activity
in LPS-induced ALI (26).
Pre-treatment with fish oil-based emulsion decreases macrophage
infiltration and improves the lung endothelial barrier in
intestinal ischaemia-reperfusion-induced ALI (27). Interestingly, STG retains the
hepatic integrity in patients undergoing abdominal surgery
(28). Among these options, it is
suggested that treatment with STG may attenuate lung injury in ALI.
Some lipids exert protective functions in different diseases by
alleviating inflammation. For example, certain dietary oils (borage
oil or fish oil) suppress pulmonary inflammation in patients with
acute respiratory distress syndrome (29). Fish oil-based lipid emulsion
decreases the generation of pro-inflammatory cytokines in
LPS-induced ALI (30). Importantly,
STG represses the expression levels of TNF-α, IL-6 and IFN-γ in the
renal tissue of diabetic rats (13). In the present study, STG decreased
the expression levels of pro-inflammatory cytokines in the
LPS-induced ALI mouse model, indicating that STG hampers lung
inflammation in ALI. To further verify the regulation of
inflammation by STG in ALI, LPS-induced RAW264.7 cells were
utilised. STG attenuated the inflammation in LPS-induced RAW264.7
cells. In summary, STG may protect against ALI by suppressing lung
injury and inflammation. Nowadays, the roles of STG have been
identified in different patients, such as moderately catabolic
patients (31), as well as those
receiving liver resection (32),
and parenteral nutrition (33).
However, the anti-inflammatory effect of STG on lung injury in
humans remains unclear. In addition, the advantages of this drug in
comparison with other conventional drugs have not been determined.
Further research on these topics is still needed.
It has been documented that the protein expression
of certain GPRs is inhibited in different diseases, such as GPR40
in ureteral obstructed kidneys (34), GPR55 in Crohn's disease (35) and GPR120 in osteoarthritis (36). Similarly, GPR120 protein expression
was decreased in the LPS-induced ALI mouse model in the present
study. The GPR family exerts protective functions in various lung
diseases. For instance, activation of GPR kinase (GRK)-2 decreases
the lung injury score, MPO activity and lung inflammation in
endotoxin-induced ALI (37). GRK-6
deficiency increases lung pathological changes and inflammatory
cytokine expression levels in an Escherichia coli lung
infection mouse model (38).
Importantly, GPR120 has protective effects against different
diseases. GPR120 alleviates acute kidney injury by suppressing
apoptosis and ER stress (39). In
addition, GPR120 mediates anti-inflammatory actions in immortalised
hypothalamic neurons (40). Here,
STG enhanced GPR120 protein expression in the LPS-induced ALI mouse
model. Above all, it was suggested that STG protects against
LPS-induced ALI by enhancing GPR120 expression. Encouragingly,
feedback experiments demonstrated that AH7614 reversed the lowering
effects of STG on the pathological changes in the lung, lung W/D
ratio, MPO activity and lung inflammation in the LPS-induced ALI
mouse model. In vitro, AH7614 reversed the suppressive
effect of STG on inflammation in LPS-induced RAW264.7 cells. Taken
together, these results suggest that STG alleviates the lung injury
and inflammation in ALI by enhancing GPR120 expression.
TAK1, a mitogen-activated protein kinase kinase
kinase family member, has been characterised as a critical mediator
in the inflammatory signalling pathway (41). Notably, NF-κB is the downstream
signalling pathway of TAK1 (42),
and their combination promotes the inflammatory response (43,44).
In the present study, STG attenuated TAK1/NF-κB pathway activity in
the LPS-induced ALI mouse model. Inhibition of TAK1/NF-κB pathway
activity has a suppressive effect on different diseases. For
instance, tubulointerstitial inflammation is ameliorated by
TAK1/NF-κB pathway suppression in proteinuric kidney disease
(45) and inhibition of the
TAK1/NF-κB pathway attenuates LPS-induced ALI (46). Thus, STG may protect against
LPS-induced ALI by limiting the TAK1/NF-κB pathway. Previous
studies have confirmed that there is a negative association between
GPR120 and the TAK1 pathway, as well as between GPR120 and the
NF-κB pathway. For example, GPR120 specifically decreases TAK1
phosphorylation and activation in human embryonic kidney 293 cells
(47). GPR120 protects the liver
from hepatic ischaemia-reperfusion injury by attenuating the
NF-κB-mediated inflammatory response (48). Notably, GPR120 protein expression is
decreased and TAK1/NF-κB protein expression is increased in bladder
inflammation (49). In the present
study, considering the interaction between STG and GPR120, it was
assumed that STG may inhibit TAK1/NF-κB pathway activity by
enhancing GPR120 expression in ALI. Interestingly, feedback
experiments displayed that AH7614 weakened the inhibitory effect of
STG on TAK1/NF-κB pathway activity in the LPS-induced ALI mouse
model. The effect of STG on TAK1/NF-κB pathway activity was further
evaluated in vitro. STG not only decreased the p-TAK1/TAK1
and p-p65/p65 ratios but also inhibited the protein expression
levels of TLR4 (an upstream target of TAK1) (50,51)
and NLRP3 (a downstream target of the NF-κB pathway) (52,53) in
LPS-induced RAW264.7 cells, whereas AH7614 reversed the inhibitory
effects exerted by STG. The present results suggest that the
GPR120-TAK1/NF-κB pathway axis participated in the regulation of
ALI. In summary, STG may attenuate TAK1/NF-κB pathway activity by
enhancing GPR120 expression, thereby protecting against ALI.
In conclusion, a LPS-induced ALI mouse model was
established to evaluate the regulatory effects of STG on lung
injury and inflammation in ALI. The regulatory mechanism of STG
associated with GPR120 and the TAK1/NF-κB pathway was further
analysed. The present results show that STG may attenuate the lung
injury and inflammation in ALI by regulating the GPR120-TAK1/NF-κB
pathway axis. Thus, STG may be a valuable compound for treating
ALI, and the GPR120-TAK1/NF-κB pathway axis may serve as a novel
therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by the project that
Effect of structural triglycerides on early inflammatory response
and respiratory function in patients with acute lung injury (grant
no. 2017WSA17003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization and performing the experiments: HS
and YP. Formal analysis and investigation: WL. Writing-original
draft preparation: HS. Writing-review and editing: DZ. Funding
acquisition: HS and WL. Resources: YP and DZ. Supervision: WL. Data
analysis and interpretation: HS, DZ and WL. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were permitted by the Ethics
Committee of Heze Municipal Hospital (approval no.
KYLL-hzsl2020008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butt Y, Kurdowska AK and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elicker BM, Jones KT, Naeger DM and Frank
JA: Imaging of acute lung injury. Radiol Clin North Am.
54:1119–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mowery NT, Terzian WTH and Nelson AC:
Acute lung injury. Curr Probl Surg. 57:1007772020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janz DR and Ware LB: Biomarkers of
ALI/ARDS: Pathogenesis, discovery, and relevance to clinical
trials. Semin Respir Crit Care Med. 34:537–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lekka ME, Liokatis S, Nathanail C, Galani
V and Nakos G: The impact of IV fat emulsion administration in
acute lung injury. Nutr Clin Pract. 19:531–532. 2004. View Article : Google Scholar
|
|
7
|
Shih JM, Shih YM, Pai MH, Hou YC, Yeh CL
and Yeh SL: Fish oil-based fat emulsion reduces acute kidney injury
and inflammatory response in antibiotic-treated polymicrobial
septic mice. Nutrients. 8:1652016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaefer MB, Ott J, Mohr A, Bi MH, Grosz
A, Weissmann N, Ishii S, Grimminger F, Seeger W and Mayer K:
Immunomodulation by n-3-versus n-6-rich lipid emulsions in murine
acute lung injury-role of platelet-activating factor receptor. Crit
Care Med. 35:544–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lekka ME, Liokatis S, Nathanail C, Galani
V and Nakos G: The impact of intravenous fat emulsion
administration in acute lung injury. Am J Respir Crit Care Med.
169:638–644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishitsuka Y, Moriuchi H, Yang C, Golbidi
S, Irikura M and Irie T: Effects of bolus injection of
soybean-based fat emulsion and fatty acids on pulmonary gas
exchange function. Biol Pharm Bull. 32:500–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu GH, Zaniolo O, Schuster H, Schlotzer E
and Pradelli L: Structured triglycerides versus physical mixtures
of medium- and long-chain triglycerides for parenteral nutrition in
surgical or critically ill adult patients: Systematic review and
meta-analysis. Clin Nutr. 36:150–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin M, Yeh SL, Tsou SS, Wang MY and Chen
WJ: Effects of parenteral structured lipid emulsion on modulating
the inflammatory response in rats undergoing a total gastrectomy.
Nutrition. 25:115–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das K and Ghosh M: Structured DAG oil
ameliorates renal injury in streptozotocin-induced diabetic rats
through inhibition of NF-κB and activation of Nrf2 pathway. Food
Chem Toxicol. 100:225–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marinissen MJ and Gutkind JS:
G-protein-coupled receptors and signaling networks: Emerging
paradigms. Trends Pharmacol Sci. 22:368–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Z, Chen L, Wang Y, Wei X, Zeng S,
Zheng Y, Gao C and Liu H: Activation of the omega-3 fatty acid
receptor GPR120 protects against focal cerebral ischemic injury by
preventing inflammation and apoptosis in mice. J Immunol.
202:747–759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaspar RC, Veiga CB, Bessi MP, Dátilo MN,
Sant'Ana MR, Rodrigues PB, de Moura LP, da Silva ASR, Santos GA,
Catharino RR, et al: Unsaturated fatty acids from flaxseed oil and
exercise modulate GPR120 but not GPR40 in the liver of obese mice:
A new anti-inflammatory approach. J Nutr Biochem. 66:52–62. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wanten GJ and Calder PC: Immune modulation
by parenteral lipid emulsions. Am J Clin Nutr. 85:1171–1184. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh DY, Talukdar S, Bae EJ, Imamura T,
Morinaga H, Fan W, Li P, Lu WJ, Watkins SM and Olefsky JM: GPR120
is an omega-3 fatty acid receptor mediating potent
anti-inflammatory and insulin-sensitizing effects. Cell.
142:687–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Q, Wang T and Wang HY: Ginsenoside
Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in
LPS-stimulated RAW264.7 macrophages by upregulating GPR120
expression. Acta Pharmacol Sin. 38:192–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li
SY, Zhu XY and Jiang L: Salidroside attenuates ventilation induced
lung injury via SIRT1-dependent inhibition of NLRP3 inflammasome.
Cell Physiol Biochem. 42:34–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv H, Liu Q, Wen Z, Feng H, Deng X and Ci
X: Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute
lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox
Biol. 12:311–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei J, Wei Y, Song P, Li Y, Zhang T, Feng
Q and Xu G: Cordycepin inhibits LPS-induced acute lung injury by
inhibiting inflammation and oxidative stress. Eur J Pharmacol.
818:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X,
Zhu XY, Liu YJ and Wang CN: H2S attenuates LPS-induced acute lung
injury by reducing oxidative/nitrative stress and inflammation.
Cell Physiol Biochem. 40:1603–1612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou S, Ding H, Lv Q, Yin X, Song J, Landén
NX and Fan H: Therapeutic effect of intravenous infusion of
perfluorocarbon emulsion on LPS-induced acute lung injury in rats.
PLoS One. 9:e878262014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jing H, Yao J, Liu X, Fan H, Zhang F, Li
Z, Tian X and Zhou Y: Fish-oil emulsion (omega-3 polyunsaturated
fatty acids) attenuates acute lung injury induced by intestinal
ischemia-reperfusion through adenosine 5′-monophosphate-activated
protein kinase-sirtuin1 pathway. J Surg Res. 187:252–261. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piper SN, Röhm KD, Boldt J, Odermatt B,
Maleck WH and Suttner SW: Hepatocellular integrity in patients
requiring parenteral nutrition: Comparison of structured MCT/LCT vs
a standard MCT/LCT emulsion and a LCT emulsion. Eur J Anaesthesiol.
25:557–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mizock BA and DeMichele SJ: The acute
respiratory distress syndrome: Role of nutritional modulation of
inflammation through dietary lipids. Nutr Clin Pract. 19:563–574.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hecker M, Linder T, Ott J, Walmrath HD,
Lohmeyer J, Vadász I, Marsh LM, Herold S, Reichert M, Buchbinder A,
et al: Immunomodulation by lipid emulsions in pulmonary
inflammation: A randomized controlled trial. Crit Care. 19:2262015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y and Wang C: Meta-analysis of
structured triglyceride versus physical mixture medium- and
long-chain triglycerides for PN in liver resection patients. Biomed
Res Int. 2017:49201342017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kruimel JW, Naber TH, van der Vliet JA,
Carneheim C, Katan MB and Jansen JB: Parenteral structured
triglyceride emulsion improves nitrogen balance and is cleared
faster from the blood in moderately catabolic patients. JPEN J
Parenter Enteral Nutr. 25:237–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Ni Q, Pei Y, Ren Y and Feng Y:
Meta-analysis of the efficacy and safety of structured triglyceride
lipid emulsions in parenteral nutrition therapy in China. Clin
Nutr. 38:1524–1535. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim CS, Joo SY, Kim IJ, Choi HI, Bae EH,
Kim SW and Ma SK: Anti-apoptotic effect of G-protein-coupled
receptor 40 activation on tumor necrosis factor-α-induced injury of
rat proximal tubular cells. Int J Mol Sci. 20:33862019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wlodarczyk M, Sobolewska-Włodarczyk A,
Cygankiewicz AI, Jacenik D, Krajewska WM, Stec-Michalska K,
Piechota-Polańczyk A, Wiśniewska-Jarosińska M and Fichna J: G
protein-coupled receptor 55 (GPR55) expresses differently in
patients with Crohn's disease and ulcerative colitis. Scand J
Gastroenterol. 52:711–715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu M and Zhou E: Long noncoding RNA
LINC00662-miR-15b-5p mediated GPR120 dysregulation contributes to
osteoarthritis. Pathol Int. 70:155–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su VY, Chiou SH, Lin CS, Chen WC, Yu WK,
Chen YW, Chen CY and Yang KY: Induced pluripotent stem cells reduce
neutrophil chemotaxis via activating GRK2 in endotoxin-induced
acute lung injury. Respirology. 22:1156–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee T, Packiriswamy N, Lee E, Lucas PC,
Mccabe LR and Parameswaran N: Role of G protein-coupled receptor
kinase-6 in Escherichia coli lung infection model in mice.
Physiol Genomics. 49:682–689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Z, Guo F, Xia Z, Liang Y, Lei S, Tan
Z, Ma L and Fu P: Activation of GPR120 by TUG891 ameliorated
cisplatin-induced acute kidney injury via repressing ER stress and
apoptosis. Biomed Pharmacother. 126:1100562020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wellhauser L and Belsham DD: Activation of
the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory
actions in immortalized hypothalamic neurons. J Neuroinflammation.
11:602014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao D, Wang R, Li B, Yang Y, Zhai Z and
Chen DY: WDR34 is a novel TAK1-associated suppressor of the
IL-1R/TLR3/TLR4-induced NF-kappaB activation pathway. Cell Mol Life
Sci. 66:2573–2584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Xia T and Yu X: Wogonin suppresses
inflammatory response and maintains intestinal barrier function via
TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res.
64:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Tu Q, Yan W, Xiao D, Zeng Z,
Ouyang Y, Huang L, Cai J, Zeng X, Chen YJ and Liu A: CXC195
suppresses proliferation and inflammatory response in LPS-induced
human hepatocellular carcinoma cells via regulating
TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem Biophys
Res Commun. 456:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang X, Kume S, Tanaka Y, Isshiki K, Araki
S, Chin-Kanasaki M, Sugimoto T, Koya D, Haneda M, Sugaya T, et al:
GW501516, a PPARδ agonist, ameliorates tubulointerstitial
inflammation in proteinuric kidney disease via inhibition of
TAK1-NFκB pathway in mice. PLoS One. 6:e252712011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang S, Yu Z, Yuan T, Wang L, Wang X, Yang
H, Sun L, Wang Y and Du G: Therapeutic effect of methyl salicylate
2-O-β-d-lactoside on LPS-induced acute lung injury by inhibiting
TAK1/NF-kappaB phosphorylation and NLRP3 expression. Int
Immunopharmacol. 40:219–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hara T, Hirasawa A, Ichimura A, Kimura I
and Tsujimoto G: Free fatty acid receptors FFAR1 and GPR120 as
novel therapeutic targets for metabolic disorders. J Pharm Sci.
100:3594–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baker MA, Nandivada P, Mitchell PD, Fell
GL, Pan A, Cho BS, De La Flor DJ, Anez-Bustillos L, Dao DT, Nosé V
and Puder M: Omega-3 fatty acids are protective in hepatic ischemia
reperfusion injury in the absence of GPR120 signaling. J Pediatr
Surg. 54:2392–2397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen YL, Lin YP, Sun CK, Huang TH, Yip HK
and Chen YT: Extracorporeal shockwave against inflammation mediated
by GPR120 receptor in cyclophosphamide-induced rat cystitis model.
Mol Med. 24:602018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meng Z, Si CY, Teng S, Yu XH and Li HY:
Tanshinone IIA inhibits lipopolysaccharide-induced inflammatory
responses through the TLR4/TAK1/NF-κB signaling pathway in vascular
smooth muscle cells. Int J Mol Med. 43:1847–1858. 2019.PubMed/NCBI
|
|
51
|
Sun P, Song SZ, Jiang S, Li X, Yao YL, Wu
YL, Lian LH and Nan JX: Salidroside regulates inflammatory response
in raw 264.7 macrophages via TLR4/TAK1 and ameliorates inflammation
in alcohol binge drinking-induced liver injury. Molecules.
21:14902016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yi H, Peng R, Zhang LY, Sun Y, Peng HM,
Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH and Zhang Z:
LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3
inflammasome-mediated inflammation in diabetic nephropathy. Cell
Death Dis. 8:e25832017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bai Y, Li Z, Liu W, Gao D, Liu M and Zhang
P: Biochanin A attenuates myocardial ischemia/reperfusion injury
through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir Bras.
34:e2019011042019. View Article : Google Scholar : PubMed/NCBI
|