Introduction
Breast cancer (BC) is one of the most commonly
diagnosed cancer types among females worldwide (1). Although great efforts have been made
in the treatment of BC, the overall survival of patients with BC
remains poor (2). Local tumor
invasion and distant metastasis are key reasons that account for
the poor survival of patients with BC in the advanced stages
(3). Therefore, it is necessary to
reveal the underlying mechanisms of progression of BC to develop
more effective therapeutic targets.
The fibronectin type III domain-containing protein 1
(FNDC1), also known as AGS8, contains the conserved fibronectin
type III domain of fibronectin (FN1) (4). FN1 was documented as an essential
player in tumorigenesis and has been shown to affect various
physiological processes, including proliferation, migration,
metabolism and apoptosis (5).
It has been reported that the upregulation of
intracellular FN1 is associated with distant metastasis of BC
(6). Numerous studies have
demonstrated that FNDC1 also serves critical roles in different
diseases. For instance, the upregulation of FNDC1 has been
associated with skin tumor progression and increases in tumor
thickness (7). In prostate cancer
cells, the silencing of FNDC1 inhibited proliferation and migration
while increasing apoptosis (8).
FNDC1 was also found to be highly expressed in gastric cancer, and
the upregulation of FNDC1 was associated with a poor prognosis in
patients with gastric cancer. However, the role of FNDC1 in BC has
not been studied yet.
Therefore, in the present study, The Cancer Genome
Atlas (TCGA) database was used to compare the mRNA expression
levels of FNDC1 in BC and normal breast tissues. The Kaplan-Meier
curves were used to evaluate the prognostic value of levels of
FNDC1 in BC. Furthermore, the biological functions of FNDC1 were
investigated in two breast cancer cell lines. The present study
assessed the effect of silencing FNDC1 on the proliferation,
migration, invasion and epithelial-to-mesenchymal (EMT) transition
of breast cancer cells.
Materials and methods
Cell culture and chemicals
Human BC cell lines (MCF-7, MDA-MB-468, LCC9 and
T-47D) and the normal human breast MCF-10A cell line were purchased
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences. All cells were maintained in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin and streptomycin
(Sigma-Aldrich; Merck KGaA) in a humidified atmosphere of 5%
CO2 at 37°C. All chemicals were purchased from
Sigma-Aldrich (Merck KGaA).
Cell transfection
The lentivirus plasmids containing the short hairpin
RNA (shRNA) against FNDC1 (shFNDC1) or a negative control (shNC)
were synthesized by Suzhou GenePharma Co., Ltd. shRNAs were
subcloned into pRNA-H1.1 (Thermo Fisher Scientific, Inc.). The
plasmids were transfected into 293T cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's guide. Briefly, 24 h prior to the
transfection, 2.5×106 cells were seeded into a 10 cm
dish until confluency of 50–70% the next day. A total of 4 µg
lentiviral plasmid, 4 µg each 3rd generation viral packaging
vectors (pMDL, pRSV and pVSV-G) and 20 µl Lipofectamine 2000 in 600
µl serum-free DMEM was incubated for 15–20 min at room temperature
and added into the cells. A total of 72 h later, the supernatant
was collected. Prior to the viral transduction, cells were
harvested upon reaching 70–80% confluence. Lentiviral vectors were
added at an MOI of 50 into the plate. The medium was replaced with
fresh medium 24 h after transfection. Then, cells were treated with
puromycin (2 µg/ml) for 3 days and the transfection efficiency was
evaluated by western blotting.
Cell viability assay
Cell viability was assayed by the Cell Counting
Kit-8 (CCK-8) (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol. In brief, the cells were seeded onto
96-well plates at the density of 5×103 cells/well. After
infections for different times (24, 48, 72 and 96 h), 10 µl CCK-8
solution was added to each well and incubated for another 2 h. The
absorbance was measured at 450 nm by a microplate reader Elx808
(BioTek Instruments, Inc.).
Colony formation assay
Following transfection for 24 h, the cells were
seeded into the 6-well plates at a density of 500 cells/well in
triplicate. After culturing for two weeks, the cells were washed
with PBS, fixed with methanol for 0.5 h at room temperature and
stained with crystal violet for 2 h at room temperature (0.1%;
Beyotime Institute of Biotechnology). The colonies were visualized
and counted under a CKX31 inverted light microscope at ×100
magnification (Olympus Corporation).
Wound healing assay
A wound healing assay was performed with minor
modifications to a previously reported method (9). As serum-free medium caused excessive
apoptosis and cell detachment, 10% FBS-supplemented medium was used
(9,10). In brief, cells were seeded onto
6-well plates in 10% FBS medium until they reached 80–90%
confluency. The monolayers were scratched using 200 µl sterile
pipette tips, and the cells were washed with PBS three times to
remove the debris and 10% FBS-supplemented fresh medium was added.
A total of 24 h later, images were captured under a CKX31 inverted
light microscope at ×100 magnification (Olympus Corporation).
Invasion assay
For the cell invasion assay, 3×104 cells
were seeded into the upper chamber of Transwell assay inserts (8
µm; Corning Inc.). The Transwell chamber was precoated with
Matrigel (Thermo Fisher Scientific, Inc.) before cell seeding. The
chambers were inserted into a 24-well plate. The upper chambers
were filled with 200 µl serum-free medium (RPMI-1640; Gibco; Thermo
Fisher Scientific, Inc.), while the bottom chambers were filled
with 500 µl complete medium (RPMI-1640; Gibco; Thermo Fisher
Scientific, Inc.). After incubation at 37°C for 24 h, the cells
were fixed at room temperature in 4% paraformaldehyde (Beyotime
Institute of Biotechnology) for 10 min and stained at room
temperature with 0.1% crystal violet (Beyotime Institute of
Biotechnology) for 10 min. Nonmigrating cells in the upper chambers
were wiped off. The migrated cells were counted in three randomly
selected fields and photographed at a ×100 magnification with a
CKX31 inverted light microscope at ×100 magnification (Olympus
Corporation).
Western blot analysis
The total cellular proteins were obtained using RIPA
lysis buffer (Beyotime Institute of Biotechnology), supplemented
with a proteinase and phosphatase inhibitor cocktail
(Sigma-Aldrich; Merck KGaA). The protein concentration was assayed
by Bradford kit (Beyotime Institute of Biotechnology) according to
the manufacturer's guide. Twenty micrograms of total protein were
loaded onto a 12% SDS-PAGE and then transferred onto a PVDF
membrane (EMD Millipore). The membranes were blocked with skimmed
milk for 1 h at room temperature, after which the membrane was
incubated with primary antibodies against the following overnight
at 4°C: FNDC1 (cat. no. PA5-56603; dilution, 1:1,000; Thermo Fisher
Scientific, Inc.), GAPDH (cat. no. G9545; dilution, 1:10,000,
Sigma-Aldrich; Merck KGaA), MMP-2 (cat. no. 40994; dilution,
1:1,000; Cell Signaling Technology, Inc.), MMP-9 (cat. no. 13667;
dilution, 1:1,000; Cell Signaling Technology, Inc.), vimentin (cat.
no. 5741; dilution, 1:1,000; Cell Signaling Technology, Inc.),
N-cadherin (cat. no. 13116; dilution, 1:1,000; Cell Signaling
Technology, Inc.), E-cadherin (cat. no. 14472; dilution, 1:1,000;
Cell Signaling Technology, Inc.), p-ERK (cat. no. 4370; dilution,
1:1,000; Cell Signaling Technology, Inc.), ERK (cat. no. 4696;
dilution, 1:1,000; Cell Signaling Technology, Inc.), p-JNK (cat.
no. 9255; dilution, 1:1,000; Cell Signaling Technology, Inc.), JNK
(cat. no. 9252; dilution, 1:1,000; Cell Signaling Technology,
Inc.), p-p38 (cat. no. 4511; dilution, 1:1,000; Cell Signaling
Technology, Inc.), p38 (cat. no. 8690; dilution, 1:1,000; Cell
Signaling Technology, Inc.), p-Akt (cat. no. 4060; dilution,
1:1,000; Cell Signaling Technology, Inc.) and Akt (cat. no. 4685;
dilution, 1:1,000; Cell Signaling Technology, Inc.). Subsequently,
the membrane was washed three times with PBS and incubated with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (both 1:2,000; anti-mouse secondary antibody; cat. no.
7076; and anti-rabbit secondary antibody; cat. no., 7074), which
were obtained from Cell Signaling Technology, Inc. at room
temperature for 1 h. The membrane was visualized using an ECL Prime
Western Blotting kit (cat. no. 21342; Beyotime Institute of
Biotechnology). All western blots were repeated three times. Band
intensities were quantified using NIH ImageJ (version 2.0;
imgaej.niv.gov/).
Data mining
The bioinformatics data for FNDC1 expression in BC
were retrieved from The Cancer Genome Atlas (TCGA)
(portal.gdc.cancer.gov) and the Genotype-Tissue Expression (GETx)
database (gtexportal.org/home/) using the Gene Expression Profiling
Interactive Analysis (GEPIA) tool ver.2002 (gepia.cancer-pku.cn).
The associations of FNDC1 expression with overall survival (OS) and
disease-free survival (DFS) were analyzed by data mining in the
Kaplan-Meier plotter (kmplot.com). The median FNDC1 expression was
applied as the cut-off. Hazard ratios with a 95% confidence
interval (CI) and log-rank P-values were calculated.
Statistical analysis
Statistical analyses were performed with SPSS 12.0
(SPSS, Inc.). The data are expressed as the mean ± standard
deviation. The paired Student's t-test was used for comparisons
between BC and normal tissues. In in vitro experiments,
unpaired Student's t-test was used to compare the difference
between two groups, and differences between multiple groups were
analyzed using one-way analysis of variance followed by Tukey's
post hoc test. Survival probability was calculated using the
Kaplan-Meier method and the log-rank test was used to compare
survival curves. P<0.05 (two-tailed) was considered to indicate
a statistically significant difference.
Results
Expression of FNDC1 in BC tissues
According to the data extracted from TCGA database,
the expression of mRNA levels of FNDC1 was significantly
upregulated in BC tissues when compared with normal tissues that
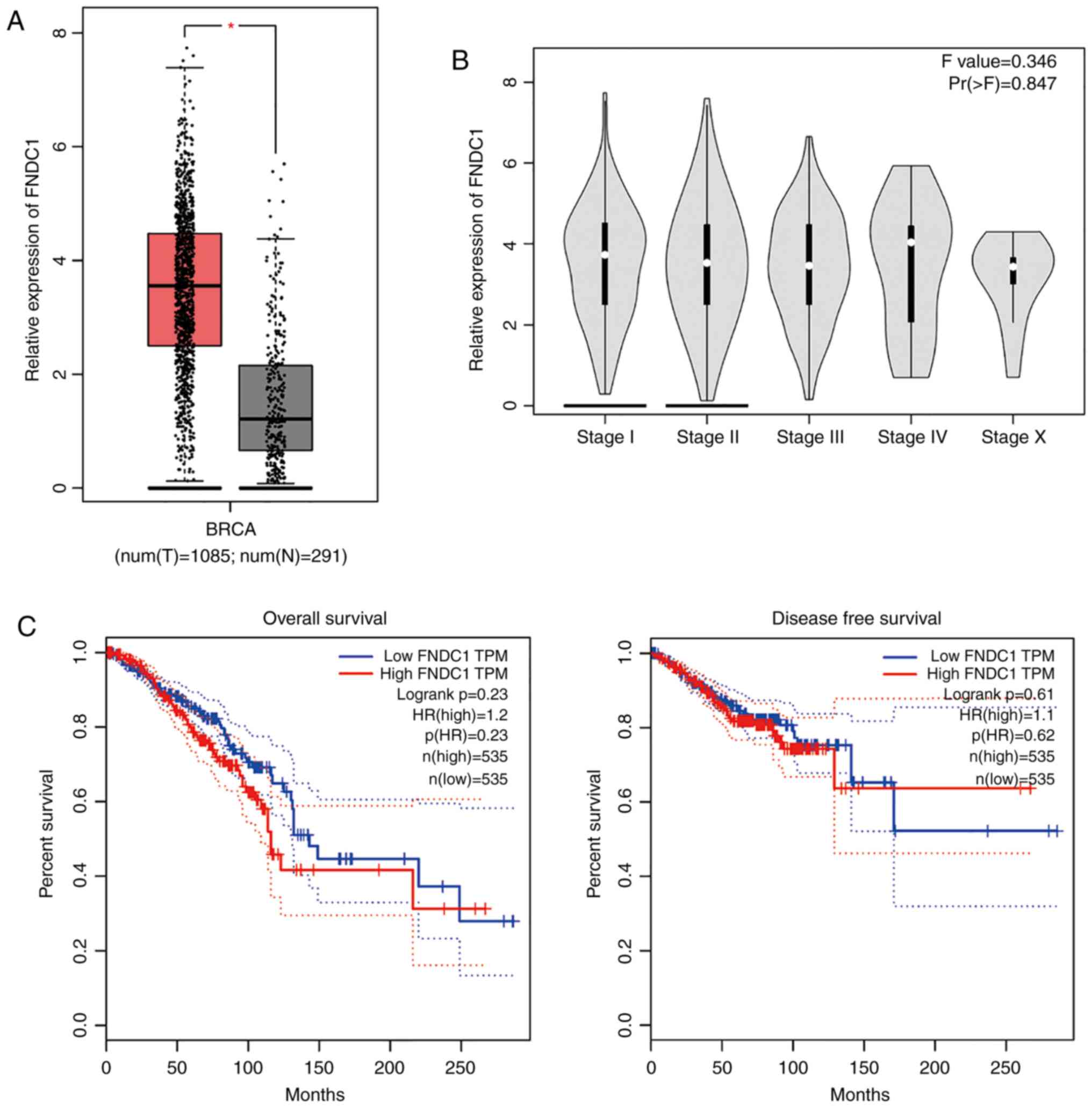
were collected from the same patients (Student's t-test; Fig. 1A), although there was no significant
difference in expression of FNDC1 among the different BC stages
(Fig. 1B). The OS curve, which was
constructed using the Kaplan-Meier method from TCGA database,
demonstrated that there is no significant difference between the BC
patients with high or low expression of FNDC1 (after dividing the
patients' survival times by the median time, with a follow-up
threshold of 250 months; P=0.23; log-rank test; Fig. 1C). Furthermore, DFS curves did not
show an association between the survival of patients with BC and
the expression of FNDC1 (after dividing the patients' survival time
by the median time, with a follow-up threshold of 250 months;
P=0.61; log-rank test; Fig. 1C).
These findings unveil the complexity of FNDC1 in the tumorigenesis
of BC.
Downregulation of FNDC1 inhibits the
proliferation, colony formation, migration, invasion and EMT
process of BC cells
The biological functions of FNDC1 in BC cells were
investigated. The protein levels of FNDC1 in normal human breast
MCF-10A cells and four breast cancer LCC9, MCF-7, MDA-468 and T-47D
cell lines were assayed. As shown in Fig. 2A, the expression of FNDC1 was
highest in MCF-7 and MDA-468 cells; therefore, these two cell lines
were selected for the subsequent studies. Subsequently, the
expression of FNDC1 was successfully silenced in two BC cell lines
(MCF-7 and MDA-MB-468; Fig. 2B). A
CCK-8 assay demonstrated that the silencing of FNDC1 significantly
inhibited the viability of BC cells, compared with to the control
group (Fig. 2C). Colony formation
assays indicated that the colony numbers of BC cells were markedly
decreased following knockdown of FNDC1 (Fig. 2D). Wound healing and Transwell
invasion assays demonstrated that the silencing of FNDC1
significantly repressed the migration and invasion of BC cells
(Fig. 2E and F). To reveal the
molecular mechanisms, western blot analysis was performed. It was
revealed that the silencing of FNDC1 led to the downregulation of
MMP-2/9, vimentin and N-cadherin and the upregulation of E-cadherin
in BC cells (Fig. 2G). Taken
together, these results suggested that the silencing of FNDC1
inhibits the tumorigenesis of BC cells.
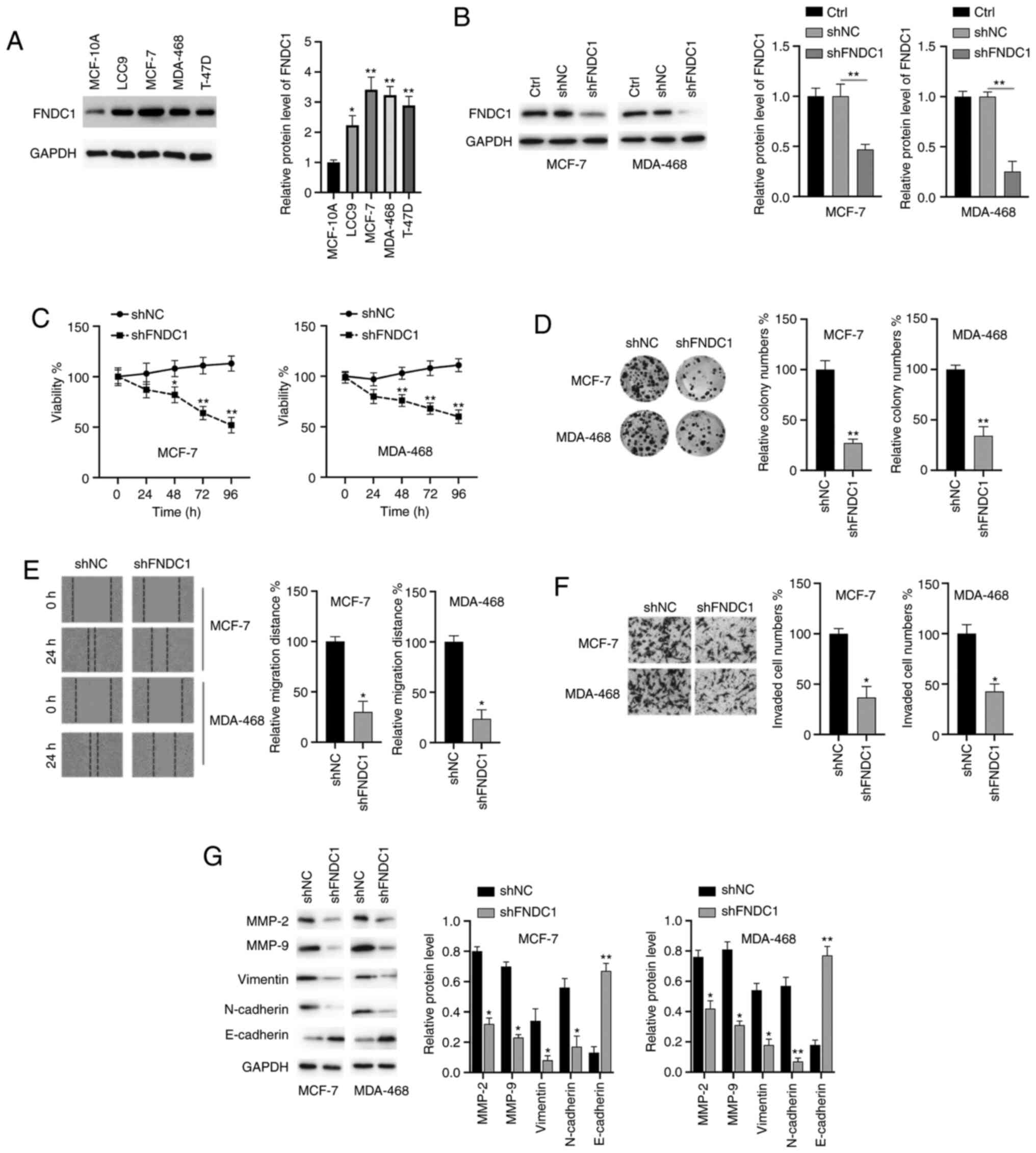 | Figure 2.The silencing of FNDC1 inhibits the
tumorigenesis of BC. (A) Expression of FNDC1 in normal breast cell
and BC cells were assayed by western blotting. (B) BC cells were
transfected as indicated for 24 h, after which the protein levels
of FNDC1 were measured by western blotting. (C) BC cells were
transfected as indicated, after which the cell viability was
measured by a Cell Counting kit-8 assay at different time points.
(D) BC cells were transfected as indicated, after which a colony
formation assay was performed. (E) BC cells were transfected as
indicated, after which a wound healing assay was conducted to
measure the migration ability. (F) BC cells were transfected as
indicated, after which a Transwell assay was performed to measure
the invasive ability. (G) BC cells were transfected as indicated
for 24 h, after which the expression levels of the indicated
proteins were measured by western blotting. The data are presented
as the mean ± standard deviation. The experiments were performed at
least three times. *P<0.05; **P<0.01. FNDC1, fibronectin type
III domain-containing protein 1; BC, breast cancer; sh, short
hairpin RNA; NC, negative control; Ctrl, control. |
Knockdown of FNDC1 led to the
inhibition of the PI3K/Akt signaling pathway
Whether FNDC1 has any effects on signaling pathways
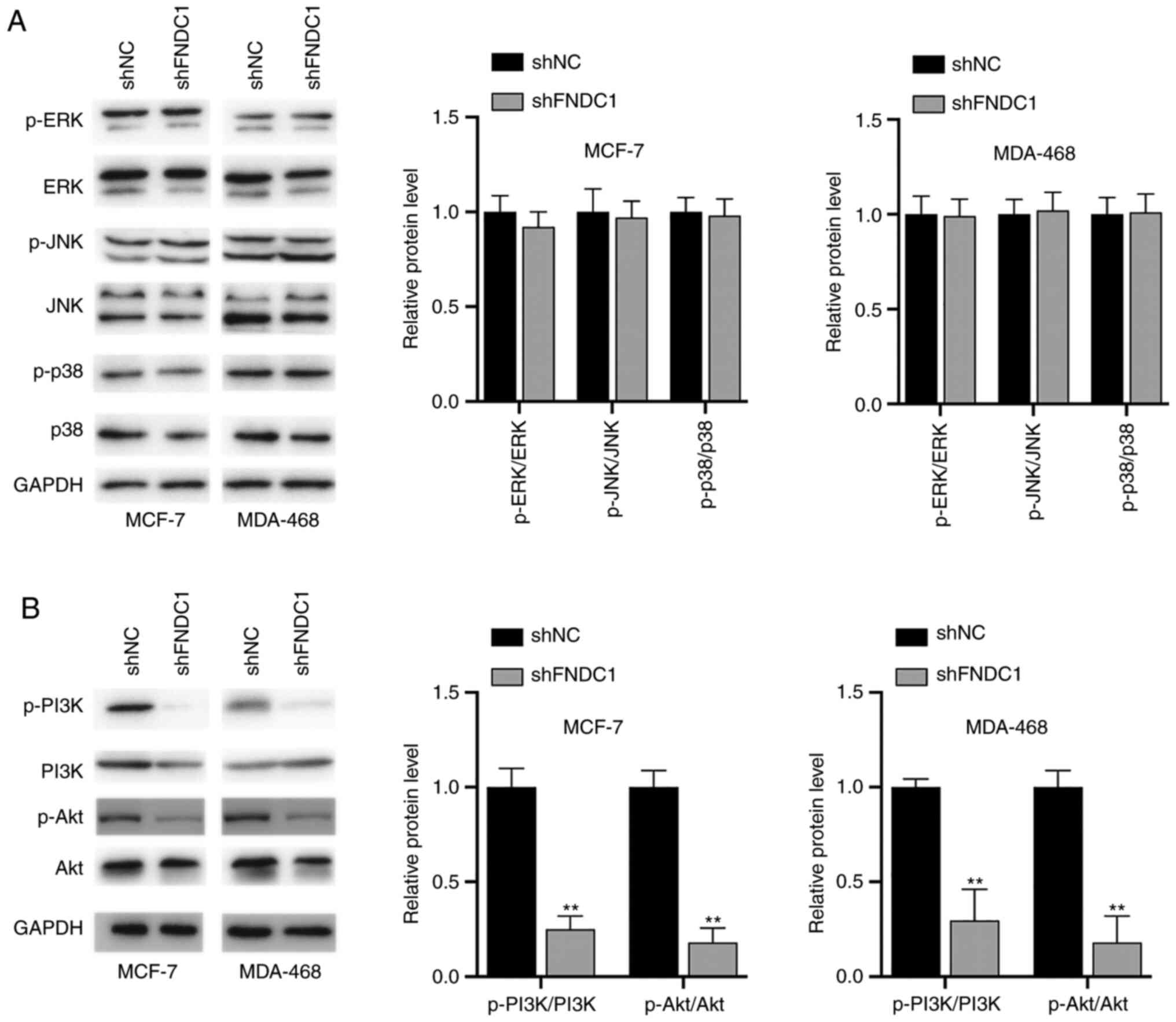
was investigated. To begin with, the status of the MAPK signaling
pathways was investigated. It was demonstrated that ERK, JNK and
p38 were not affected following the silencing of FNDC1 in BC cells
(Fig. 3A). Subsequently, the
PI3K/Akt signaling pathway was investigated. It was found that the
phosphorylation levels of PI3K and Akt were decreased following the
silencing of FNDC1 (Fig. 3B).
Therefore, the silencing of FNDC1 led to the inhibition of
PI3K.
Inactivation of the PI3K/Akt signaling
pathway is essential for the effects of the silencing of FNDC1 on
BC cells
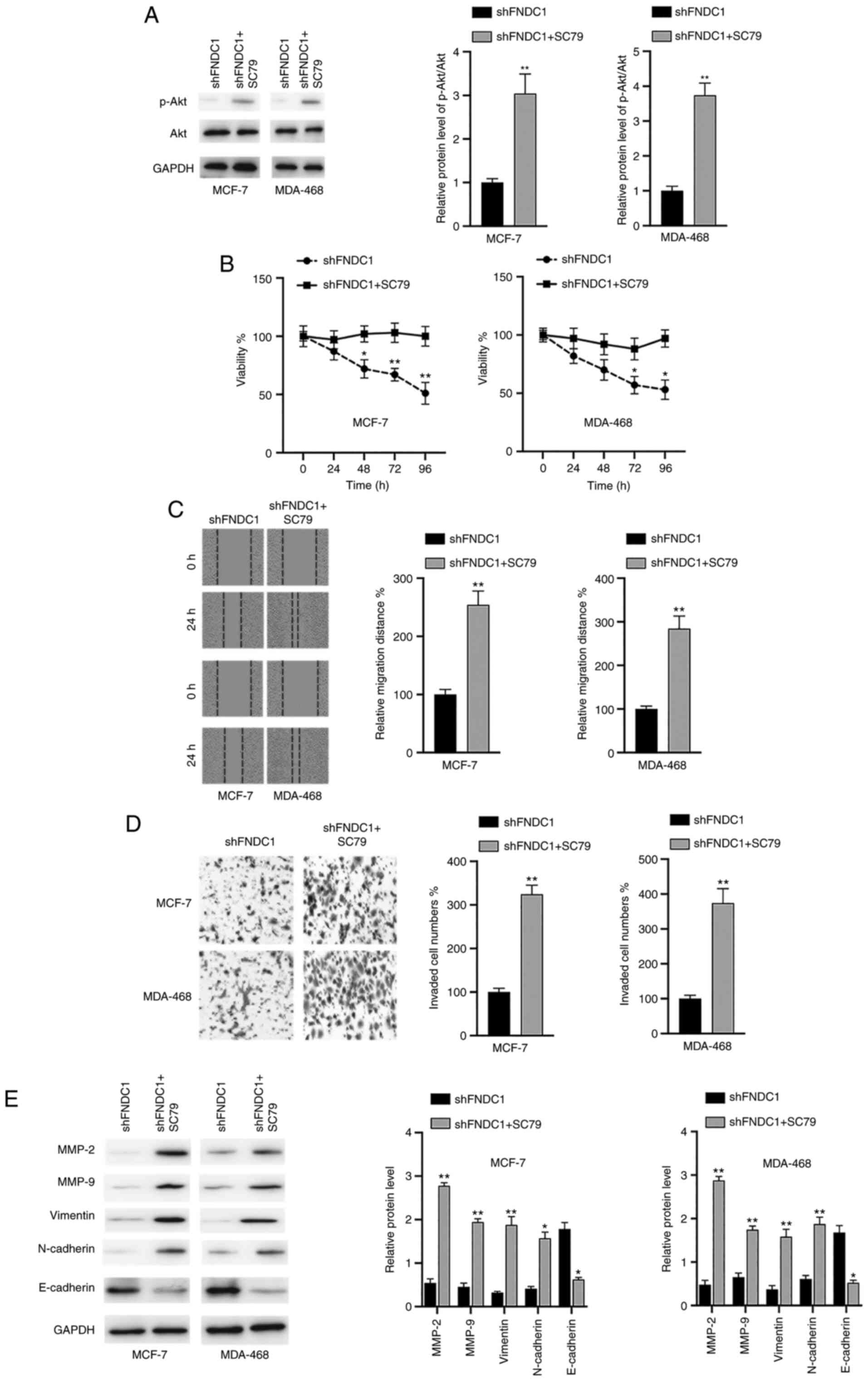
To verify whether the PI3K/Akt signaling pathway is
required for the oncogenic effects of FNDC1, BC cells were
transfected with shFNDC1 and then the cells were incubated with the
Akt activator SC79 24 h after transfection. It was revealed that
SC79 reactivated the Akt signaling pathway following the silencing
of FNDC1 in the two BC cell lines, as indicated by increased
p-Akt/Akt ratios (Fig. 4A). In
addition, it was found that SC79 reversed the effects of the
silencing of FNDC1 on cell viability (Fig. 4B), migration (Fig. 4C) and invasion (Fig. 4D). Furthermore, the effects of the
silencing of FNDC1 on MMPs and EMT markers were also reversed by
the treatment of SC97 in BC cells (Fig.
4E). Taken together, these results suggested that the effects
of the silencing of FNDC1 on the tumorigenesis of BC cells relied
on the inactivation of the PI3K/Akt signaling pathway.
Discussion
The role of FNDC1 has been investigated in various
cancer types, which demonstrated that it may be an oncogene. For
instance, the overexpression of FNDC1 was found to be correlated
with a poor prognosis in patients with gastric cancer (11). An upregulation of FNDC1 has also
been revealed in head and neck squamous cell carcinoma and
colorectal cancer (12,13). However, there have been few studies
regarding the roles of FNDC1 in the progression of BC. Therefore,
the aim of the present study was to investigate the roles of FNDC1
in the tumorigenesis of BC cells via loss-of-function experiments.
The underlying molecular mechanisms were also investigated.
In line with the results of previous studies, TCGA
database analyses demonstrated that FNDC1 was upregulated in BC
tissues. However, Kaplan-Meier analysis showed that no significant
difference in OS time between the BC patients with high or low
expression of FNDC1. Furthermore, the expression of FNDC1 was not
associated with DFS in patients with BC. By contrast, previous
studies have suggested that the expression of FNDC1 was associated
with prognosis in gastric cancer (11,14).
These results suggested that the role of FNDC1 in tumors is
complicated. Dysregulated proliferation is one of the key hallmarks
of cancer (15). In the present
study, it was revealed that the knockdown of FNDC1 repressed the
viability of BC cells. This finding is in line with that of a
previous study, which reported that the downregulation of FNDC1
decreased the viability of prostate cancer cells (8). Additionally, the knockdown of FNDC1
may suppress the growth of gastric cancer cells (11). However, the roles of FNDC1 in the
growth of BC cells remains unclear.
As another hallmark of cancer, metastasis is a
leading cause of cancer-associated mortality (15). During metastasis, the surrounding
tissues will be invaded by tumor cells, which will further
intravasate into the lymph nodes and eventually migrate to distant
organs (16). It is well documented
that the extracellular matrix (ECM) serves essential roles in the
process of metastasis (17). FN1 is
an important ECM protein that has been revealed to enhance the
invasiveness of various cancer cells, including gastric cancer
cells, oral squamous cell carcinoma and ovarian cancer (18–20).
Since FNDC1 contains a major component of the structural domain of
FN1, this indicates that FNDC1 may also serve a critical role in
metastasis (5). In the present
study, it was demonstrated found that the silencing of FNDC1
inhibited the migration and invasion of BC cells, which is in line
with the results of previous studies (11,21).
Investigation of mechanisms revealed that the silencing of FNDC1
decreased the expression of MMPs, which are a group of
zinc-dependent endopeptidases (22). MMP-2 and MMP-9 participate in the
process of cell invasion and metastasis via regulation of the
degradation of the ECM (23). A
decrease in MMP-2/9 may repress the migration and metastasis of
tumor cells (24). It was found
that the silencing of FNDC1 led to the downregulation of MMP-2/9,
which revealed the molecular mechanisms underlying the repression
of migration and invasion caused by the silencing of FNDC1. It has
been well documented that the EMT serves an essential role in the
progression of various cancer types, including BC (25). EMT accounts for the change in
various cell phenotypes that are involved in the progression of
tumors (26). The results of the
present study demonstrated that the silencing of FNDC1 led to the
downregulation of proteins, including vimentin and N-cadherin and
to the upregulation of E-cadherin. These data suggested that the
EMT process was inhibited by the downregulation of FNDC1. The
findings of the present study are in accordance with those of a
previous study, which also found that the silencing of FNDC1
blocked the EMT process in gastric cancer cells (11).
The PI3K/Akt signaling pathway has been reported to
participate in cancer cell activities, including metabolism,
proliferation, invasion and metastasis (27). The constitutive activation of Akt
has been found to contribute toward the initiation and development
of different cancer types, including BC (28). Targeting PI3K/Akt may be a potential
strategy for treatment and for overcoming drug resistance in BC
(29). Until now, whether FNDC1 may
modulate the PI3K/Akt signaling pathway has remained elusive. The
present study found that the downregulation of FNDC1 led to the
inactivation of the PI3K/Akt pathway. In addition, SC79 reversed
the inhibitory effects of the silencing of FNDC1 on tumorigenesis
in BC cells. To the best of our knowledge, the present study was
the first to indicate that the downregulation of FNDC1 leads to the
inhibition of the PI3K/Akt pathway. Notably, a previous study
demonstrated that the Akt signaling pathway may regulate the
expression of FNDC1 in bone marrow mesenchymal stem cells (30). Therefore, there may be a correlation
between the PI3K/Akt pathway and FNDC1; therefore, more studies are
required. There are certain limitations to the present study. To
begin with, the expression of FNDC1 was not assayed in patients
with BC and the levels of FNDC1 in BC tissues should be measured in
the future. Additionally, the effects of FNDC1 on the tumorigenesis
of BC were not studied in a xenograft mouse model, which may be
interesting if performed in the future.
In summary, the present study revealed that FNDC1
expression is upregulated in BC. The silencing of FNDC1 inhibited
the proliferation, migration, invasion and EMT of BC cells by
modulating the PI3K/Akt signaling pathway. Therefore, FNDC1 may be
a potential therapeutic target for the treatment of BC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Scientific Research Foundation of Zhejiang Province, China (grant
no. 2019KY596).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY and GS performed the experiments. CY and GS
confirm the authenticity of all the raw data. GS performed the
statistical analysis. BZ and CS performed the bioinformatics
analysis. YH designed the study and drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khosravi-Shahi P, Cabezon-Gutierrez L and
Custodio-Cabello S: Metastatic triple negative breast cancer:
Optimizing treatment options, new and emerging targeted therapies.
Asia Pac J Clin Oncol. 14:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albrecht M, Renneberg H, Wennemuth G,
Möschler O, Janssen M, Aumüller G and Konrad L: Fibronectin in
human prostatic cells in vivo and in vitro: Expression,
distribution, and pathological significance. Histochem Cell Biol.
112:51–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez-Garcia B, Eiro N, Marin L,
González-Reyes S, González LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
64:512–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderegg U, Breitschwerdt K, Kohler MJ,
Sticherling M, Haustein UF, Simon JC and Saalbach A: MEL4B3, a
novel mRNA is induced in skin tumors and regulated by TGF-beta and
pro-inflammatory cytokines. Exp Dermatol. 14:709–718. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das DK, Naidoo M, Ilboudo A, Park JY, Ali
T, Krampis K, Robinson BD, Osborne JR and Ogunwobi OO: miR-1207-3p
regulates the androgen receptor in prostate cancer via
FNDC1/fibronectin. Exp Cell Res. 348:190–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valster A, Tran NL, Nakada M, Berens ME,
Chan AY and Symons M: Cell migration and invasion assays. Methods.
37:208–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pijuan J, Barcelo C, Moreno DF, Maiques O,
Sisó P, Marti RM, Macià A and Panosa A: In vitro cell migration,
invasion, and adhesion assays: From cell imaging to data analysis.
Front Cell Dev Biol. 7:1072019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu YP, Chen WD, Li WN and Zhang M:
Overexpression of FNDC1 relates to poor prognosis and its knockdown
impairs cell invasion and migration in gastric cancer. Technol
Cancer Res Treat. Jan 1–2019.(Epub ahead of print). doi:
10.1177/1533033819869928. View Article : Google Scholar
|
|
12
|
Wuensch T, Wizenty J, Quint J, Spitz W,
Bosma M, Becker O, Adler A, Veltzke-Schlieker W, Stockmann M, Weiss
S, et al: Expression analysis of fibronectin type III
domain-containing (FNDC) genes in inflammatory bowel disease and
colorectal cancer. Gastroenterol Res Pract. 2019:37841722019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddy RB, Khora SS and Suresh A: Molecular
prognosticators in clinically and pathologically distinct cohorts
of head and neck squamous cell carcinoma-A meta-analysis approach.
PLoS One. 14:e02189892019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong M and Zhang Y, Yuan F, Peng Y, Wu J,
Yuan J, Zhu W and Zhang Y: High FNDC1 expression correlates with
poor prognosis in gastric cancer. Exp Ther Med. 16:3847–3854.
2018.PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clark AG and Vignjevic DM: Modes of cancer
cell invasion and the role of the microenvironment. Curr Opin Cell
Biol. 36:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eble JA and Niland S: The extracellular
matrix in tumor progression and metastasis. Clin Exp Metastasis.
36:171–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao S, Lv J, Yang Q, Wang A, Li Z, Guo Y
and Zhang G: Identification of key genes and circular RNAs in human
gastric cancer. Med Sci Monit. 25:2488–2504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Tao Q, Qiao B and Zhang L:
Silencing of LINC01116 suppresses the development of oral squamous
cell carcinoma by up-regulating microRNA-136 to inhibit FN1. Cancer
Manag Res. 11:6043–6059. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang H, Yu M, Yang R, Zhang L, Zhang L,
Zhu D, Luo H, Hong Y, Yu T, Sun J, et al: A PTAL-miR-101-FN1 axis
promotes EMT and invasion-metastasis in serous ovarian cancer. Mol
Ther Oncolytics. 16:53–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren J, Niu G, Wang X, Song T, Hu Z and Ke
C: Overexpression of FNDC1 in gastric cancer and its prognostic
significance. J Cancer. 9:4586–4595. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein T and Bischoff R: Physiology and
pathophysiology of matrix metalloproteases. Amino Acids.
41:271–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nemeth JA, Yousif R, Herzog M, Che M,
Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R and Cher
ML: Matrix metalloproteinase activity, bone matrix turnover, and
tumor cell proliferation in prostate cancer bone metastasis. J Natl
Cancer Inst. 94:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotiyal S and Bhattacharya S: Breast
cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res
Commun. 453:112–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vincent CT and Fuxe J: EMT, inflammation
and metastasis. Semin Cancer Biol. 47:168–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aoki M and Fujishita T: Oncogenic roles of
the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 407:153–189.
2017.PubMed/NCBI
|
|
28
|
Sharma VR, Gupta GK and Sharma AK, Batra
N, Sharma DK, Joshi A and Sharma AK: PI3K/Akt/mTOR intracellular
pathway and breast cancer: Factors, mechanism and regulation. Curr
Pharm Des. 23:1633–1638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao Y, Wei R, Yuan Z, Lan X, Kuang J, Hu
D, Song Y and Luo J: Rutin suppresses FNDC1 expression in bone
marrow mesenchymal stem cells to inhibit postmenopausal
osteoporosis. Am J Transl Res. 11:6680–6690. 2019.PubMed/NCBI
|