Introduction
Postoperative pain is characterized by acute pain
that occurs immediately after surgery. The duration is usually no
more than 7 days, but when poorly controlled, can develop into
chronic pain syndrome, which not only causes physical and mental
distress to the patient, but also increases economic burden on the
family and society in general (1).
In previous years, the role of glial cells in the transformation
from acute to chronic pain, as well as the maintenance of slow
pain, has received increasing attention (2,3).
Gap junction (GJ)-mediated coupling occurs among
satellite glial cells and is facilitated by GJ proteins composed of
two hemichannels (HC), including connexins (Cx), pannexin (Px) and
innexin (Inx) (4,5). Previous studies have indicated that
Px1 affects the activation of astrocytes by regulating the release
of ATP and the flow of calcium (6,7). At
the neuronal membrane, ATP binds to the ATP receptor to induce the
production of glutamic acid. ATP and glutamic acid have been
identified as important molecules for the transmission of pain
signals in the spinal dorsal horn. Px1 is expressed in a variety of
tissues, and mediates the propagation of the calcium wave, which
may influence the maintenance of pain (8). As such, Px1 plays a vital role in the
transmission and maintenance of pain. Previous studies reported
that Px1 activated inflammasome signaling, and that its inhibition
reduced chronic pain and hypersensitivity in glial fibrillary
acidic protein (GFAP)-positive glia cells (9–11),
while its upregulation promoted the development of chronic pain
(9).
Intrathecal administration of carbenoxolone (CBX), a
gap junction blocker, can suppress central sensitization of
existing neuropathic pain (12).
CBX has been reported to suppress Cx expression, including that of
Cx43 (13). Cxs are involved in
inducing and maintaining chronic pain (14); Cx43 forms a hemichannel, resulting
in the release of mediators, such as ATP and glutamate, into the
extracellular space, which activate nociceptive neurons to induce
pain. Moreover, non-neuronal cells also initiate pain through the
release of cytokines triggered by extracellular ATP binding to its
receptor (15). However, another
study revealed that a reduction in the expression of one GJ protein
could cause an increase in that of another (16). Therefore, the present study aimed to
determine whether Px1 and Cx43 are involved in the formation of
acute pain, and whether this can be relieved by the intrathecal
injection of CBX.
Materials and methods
Animals
Sprague-Dawley rats of clean grade (n=102; weight,
200–250 g, male) were provided by the Laboratory Animal Center of
Lanzhou Veterinary Research Institute, Chinese Academy of
Agricultural Sciences. All experiments were performed in accordance
with the guidance of The International Association for the Study of
Pain (17), and approved by the
Ethics Committee of The Second Hospital of Lanzhou University
(approval no. D2019-064; Lanzhou, China). The rats were kept at
22–26°C with a relative humidity of 45–60% and a 12 h light/dark
cycle. They had free access to food and water. The rats were
randomly divided into the following five groups: i) Control group
(C, n=6); ii) incision pain model group (IP, n=24); iii) normal
saline group (NS, 10 µl, n=24); iv) CBX treatment group (CBX, 100
µM, 10 µl, n=24); and v) pannexin-1 mimetic inhibitory peptide
treatment group (10panx, 100 µM, 10 µl, n=24). CBX and 10panx were
purchased from APExBIO Technology LLC. With the exception of the
normal group, the rats in each group were further divided into four
groups: i) 2 h post-surgery (n=6); ii) 4 h post-surgery (n=6); iii)
6 h post-surgery (n=6); and iv) 24 h post-surgery (n=6). The
experimental process is shown in Fig.
1. The rationale for choosing the time points was based on
previous literature (5,12,18).
In the present study, rats were injected with 50 µM (0.3 µg/10 µl)
or 100 µM (0.6 µg/10 µl) CBX intragastric, and the pain relief of
acute IP was observed. Finally, it was verified that 100 µM (0.6
µg/10 µl) CBX could significantly relieve acute IP, so this
concentration of CBX was selected. The rats in the control group
received no treatment. The baseline mechanical withdrawal threshold
(MWT) was measured for each rat. After MWT and thermal withdrawal
latency (TWL) were measured at different time points, the rats were
sacrificed by cervical dislocation under anesthesia with 1%
pentobarbital sodium (intraperitoneal) and the lumbar enlargement
of the spinal cord was removed.
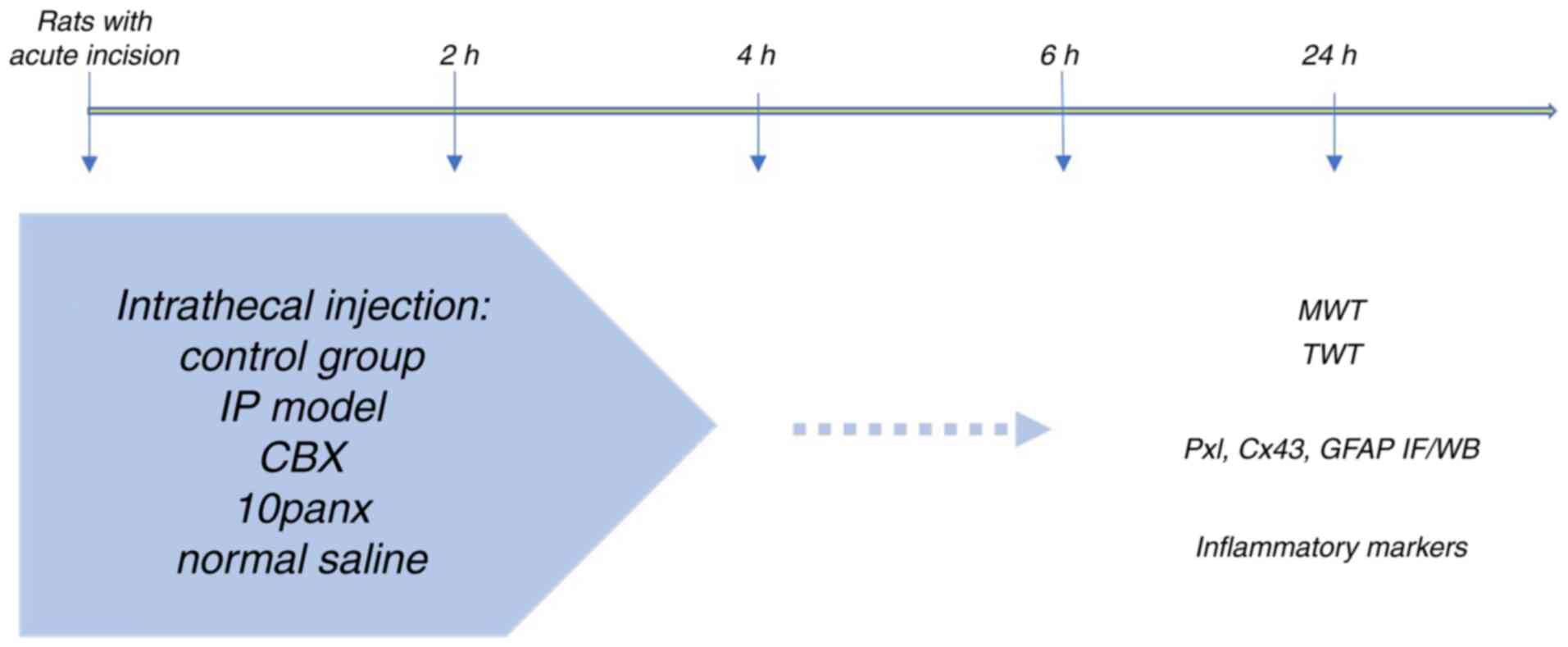 | Figure 1.Flow chart of the experimental
process. The rat model of acute incision was established and CBX,
10panx or normal saline administrated. IP, incision pain; CBX,
carbenoxolone; 10panx, pannexin-1 mimetic inhibitory peptide; MWT,
mechanical withdrawal threshold; TWL, thermal withdrawal latency;
Px1, pannexin 1; Cx43, connexin 43; GFAP, glial fibrillary acidic
protein; IF, immunofluorescence; WB, western blotting. |
Animal model establishment
A rat model of acute IP was established using the
Brennan method (19). The rats were
anesthetized in a transparent anesthesia box with 2% sevoflurane
inhalation in oxygen and then fixed on the operating table. A
longitudinal incision of ~1 cm was made 0.5 cm from the proximal
heel of the foot to the toe. After cutting the skin and fascia, the
muscle was located with forceps and cut longitudinally to avoid
injury and comprising muscle integrity during the procedure. After
hemostasis, the skin was sutured with two stitches of 3-0 fine
thread, keeping an even distance between the two stitches.
Behavioral examination
MWT detection
The rats were placed in a plexiglass box with a
metal mesh bottom for 30 min. Once the rats had acclimated to these
conditions (remaining relatively quiet), a series of standardized
von Frey filaments were inserted through the metal grid, and used
to vertically stimulate the skin near the incision of the left
posterior claw, so that the filaments were continuously bent and
maintained for 6–8 sec. The time interval between each stimulation
was >30 sec, and the initial stimulation intensity was 2.0 g. If
three of the five stimuli instigated a rapid foot retraction or
licking response, pain ‘X’ was recorded and a lower level of
stimulation intensity was administered. On the contrary, if
<three stimuli instigated this response, it was denoted as ‘O’,
and a stimulus intensity of a higher level was used. When a
different reaction occurred, the MWT was measured another four
times in sequence. The last stimulus was recorded after six
measurements. If the stimulus intensity was 15 or 0.4 g, the MWT
value was directly denoted as 15 or 0.4 g. The investigators were
blinded to the experimental grouping of the animals.
TWL
TWL was determined in accordance with the Hargreaves
method (13). The rats were first
allowed to adapt to the organic glass box for 10–15 min.
Thereafter, as a heat source, a thermal radiation stimulator was
placed under the glass plate next to the incision of the rear claw.
The shrinkage leg incubation period was determined as the time from
the initial heat source stimulation to the retraction of the leg.
The duration time of each stimulation was 30 sec, and the test was
performed three times using the same stimulus intensity
(measurement interval, 5 min). The investigators were blinded to
the experimental grouping of the animals.
Western blot analysis
After behavioral examination at 2, 4, 6 or 24 h, the
rats were sacrificed by cervical dislocation under anesthesia
intraperitoneally with 1% pentobarbital sodium (50 mg/kg body
weight), and the lumbar enlargement of the spinal cord was
retrieved. The lumbar enlargement was lysed on ice with RIPA Lysis
Buffer (cat. no. R0020, Solarbio) for 15 min and centrifuged at
4,025 × g at 4°C for 30 min. Part of the supernatant was taken and
the protein concentration was measured by BCA method; 4X loading
buffer was added into the rest of the supernatant for western
blotting. The supernatant was incubated in boiling water for 5 min.
The mass of protein loaded per lane was 40 µg. The proteins were
then subjected to 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and then transferred onto a PVDF membrane, which
was subsequently blocked for 2 h at room temperature using 5%
skimmed milk powder dissolved into TBST with 0.05% Tween-20 (TBST)
solution. The membrane was incubated at 4°C overnight with the
following antibodies: Anti-Px1 antibody (1:800; cat. no. ab139715;
Abcam), anti-Cx43 antibody (1:1,000; cat. no. ab11369; Abcam) and
anti-GADPH (1:5,000; cat. no. sc-365062; Santa Cruz Biotechnology,
Inc.). Then, the membrane was rinsed three times with TBST for 10
min each, and incubated with HRP-conjugated goat anti-mouse IgG
(cat. no. 15014) and goat anti-rabbit IgG (cat. no. 15015)
secondary antibodies (1:8,000; ProteinTech Group, Inc.). The
protein bands were visualized using ECL luminescent solution
(Biosharp Life Sciences), and the gray value was analyzed using
ImageJ software v1.52r (National Institutes of Health).
Immunofluorescence (IF) assay
An IF assay was performed 2 h post-surgery. After
the behavioral test, the heart was exposed under deep anesthesia
and the aorta was catheterized by apical puncture, followed by
sequential infusion of 250 ml normal saline and 500 ml
paraformaldehyde (4%). The lumbago was exposed and removed,
immersed in 4% paraformaldehyde overnight, and then sequentially
immersed in 20% and 30% sucrose solution for frozen sectioning.
After drying at room temperature, the sections (3–5 µm) were fixed
with 4% paraformaldehyde for 15 min at room temperature, and then
washed with PBS three times for 5 min each. Then, the sections were
permeabilized with 0.5% TritonX-100 for 10 min at room temperature
and washed with PBS three times for 5 min each. The sections were
then immersed in citrate antigen retrieval solution, heated for 15
min at 95–100°C, washed with PBS and blocked with 10% bovine serum
albumin for 1 h at room temperature.
Px1 and GFAP double staining, along with Cx43 and
GFAP double staining (Px1, Cx43, GFAP, 1:100. GFAP, Affinity
Biosciences), were performed. The sections were incubated at 4°C
overnight with the primary antibodies (anti-GFAP, 1:100; cat. no.
BF0345; Affinity Biosciences), followed by incubation for 1 h at
37°C with FITC-conjugated goat anti-mouse IgG (H+L) (cat. no.
S0007; 1:100; Affinity Biosciences) and goat anti-rabbit IgG (H+L)
secondary antibodies (cat. no. S0015; 1:100; Affinity Biosciences).
The sections were stained with DAPI solution at room temperature
for 8 min and sealed with immunofluorescence quencher at room
temperature (Beijing Solarbio Science & Technology Co., Ltd.)
and images were captured under a fluorescence microscope
(magnification, ×200; Olympus Corporation).
ELISA
The levels of TNF-α (cat. no. D731168), 1L-1β (cat.
no. D731007) and P substance (SP; cat. no. D751030) were analyzed
using ELISA kits (all purchased from Sangon Biotech Co., Ltd.),
according to the manufacturer's instructions. Blank, standard and
sample wells were set. For each sample, 50 µl standard substance
was added to each standard well, and the sample was diluted five
times with sample diluent prior to its addition to the sample well.
After 30 min at 37°C, each well was washed five times. Color
developing agents A (50 µl) and B (50 µl) were then sequentially
added to each well. Finally, the reaction was terminated by adding
50 µl termination reagent to each well. The absorption value of
each well was measured using a microplate reader at a wavelength of
450 nm (Tecan Group, Ltd.).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
software (IBM Corp), and the results are expressed as the mean ±
SD. Figures were created using GraphPad Prism 7.0 software
(GraphPad Software, Inc.). The experimental data of MWT and TWL
among different groups were analyzed using mixed ANOVA, followed by
Bonferroni's post hoc test. Comparisons among multiple groups were
performed using one-way ANOVA, followed by Tukey's post hoc test.
If the data had marked deviations from normality, Friedman's test
was performed, followed by Bonferroni-Dunn's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of CBX on MWT and TWL in rats
with IP
There were no significant differences between MWT
and TWL among the different groups. The MWT was significantly
decreased in the IP and NS groups compared with the control group
(P<0.001; Table I), although
there was no significance change between the IP and NS groups at
any time point. Compared with the IP group, the MWT and TWL were
significantly increased by CBX or 10panx 2 and 4 h after surgery
(P<0.001; Tables I and II). In addition, TWL was increased by CBX
and 10panx 6 h after surgery compared with the IP group
(P<0.001; Table II). The MWT in
the 10panx group was similar to that in the CBX group. However, the
TWL in the 10panx group was higher than that in the CBX group 2 and
24 h post-surgery (P<0.05; Table
II). Therefore, the results suggested that CBX significantly
increased the pain threshold of rats experiencing acute IP, and
that CBX enhanced the heat pain threshold to a higher degree than
the MWT.
 | Table I.MWT at different time points of each
group. |
Table I.
MWT at different time points of each
group.
|
|
| MWT |
|---|
|
|
|
|
|---|
| Group | Baseline, g | 2 h | 4 h | 6 h | 8 h |
|---|
| C | 12.10±3.47 | 12.78±3.44 | 12.00±3.31 | 12.11±2.22 | 12.14±2.73 |
| IP | 12.10±3.47 |
2.03±0.97a |
0.97±0.77a |
1.73±1.13a | 8.05±3.59 |
| NS | 12.78±3.44 |
1.92±1.09a |
0.63±0.35a |
2.31±0.63a | 5.55±1.96 |
| CBX | 12.00±3.31 |
6.02±1.06b |
7.95±1.48b | 3.39±1.72 | 6.34±1.16 |
| 10panx | 10.39±3.59 |
7.00±1.63b |
5.94±1.73b | 2.43±0.93 | 7.95±1.58 |
 | Table II.TWL at different time points of each
group. |
Table II.
TWL at different time points of each
group.
|
|
| TWL |
|---|
|
|
|
|
|---|
| Group | Baseline, g | 2 h | 4 h | 6 h | 8 h |
|---|
| C | 25.13±0.69 | 24.73±0.81 | 25.01±0.98 | 25.14±0.53 | 24.64±0.68 |
| IP | 25.13±0.69 |
5.54±1.15a |
3.32±0.36a |
5.98±0.55a |
9.17±1.99a |
| NS | 24.73±0.81 |
6.07±0.58a |
3.32±0.57a |
6.39±1.32a |
9.12±0.46a |
| CBX | 25.27±0.69 |
13.76±3.33b |
17.08±2.73b |
13.53±3.73b | 18.74±0.90 |
| 10panx | 24.68±0.65 |
18.57±2.64b,c |
15.82±2.42b |
13.77±2.70b |
20.96±2.06c |
CBX significantly decreases Px1 and
Cx43 expression
After surgery, Px1 was significantly upregulated in
the spinal cords of the IP rats compared with the control group
(P<0.001; Fig. 2). Moreover, Px1
levels in the IP rats showed a greater increase at 6 and 24 h
post-surgery than at 2 and 4 h. Thus, to a certain extent, Px1 was
significantly increased in a time-dependent manner. Compared with
NS treatment, CBX significantly reduced Px1 levels at 4 and 24 h
(P<0.05). However, the decrease in Px1 was most significant at 4
h post-surgery (P<0.001). 10panx administration significantly
reduced Px1 levels at 2, 4, 6 and 24 h compared with the NS group
(P<0.05), which was most significant at 24 h post-surgery
(P<0.05). This could indicate that CBX and 10panx reduce Px1
levels via different molecular mechanisms. Furthermore, toe
incision postoperatively increased Cx43 expression levels at 2, 6
and 24 h compared with the control group (Fig. 2). CBX significantly reduced the
levels of Cx43 at 2 h post-surgery, but led to an increase in these
levels at 4, 6 and 24 h after surgery. 10panx significantly
decreased Cx43 levels at 4, 6 and 24 h post-surgery compared with
the NS group, suggesting that CBX pretreatment decreased Cx43
expression shortly after surgery.
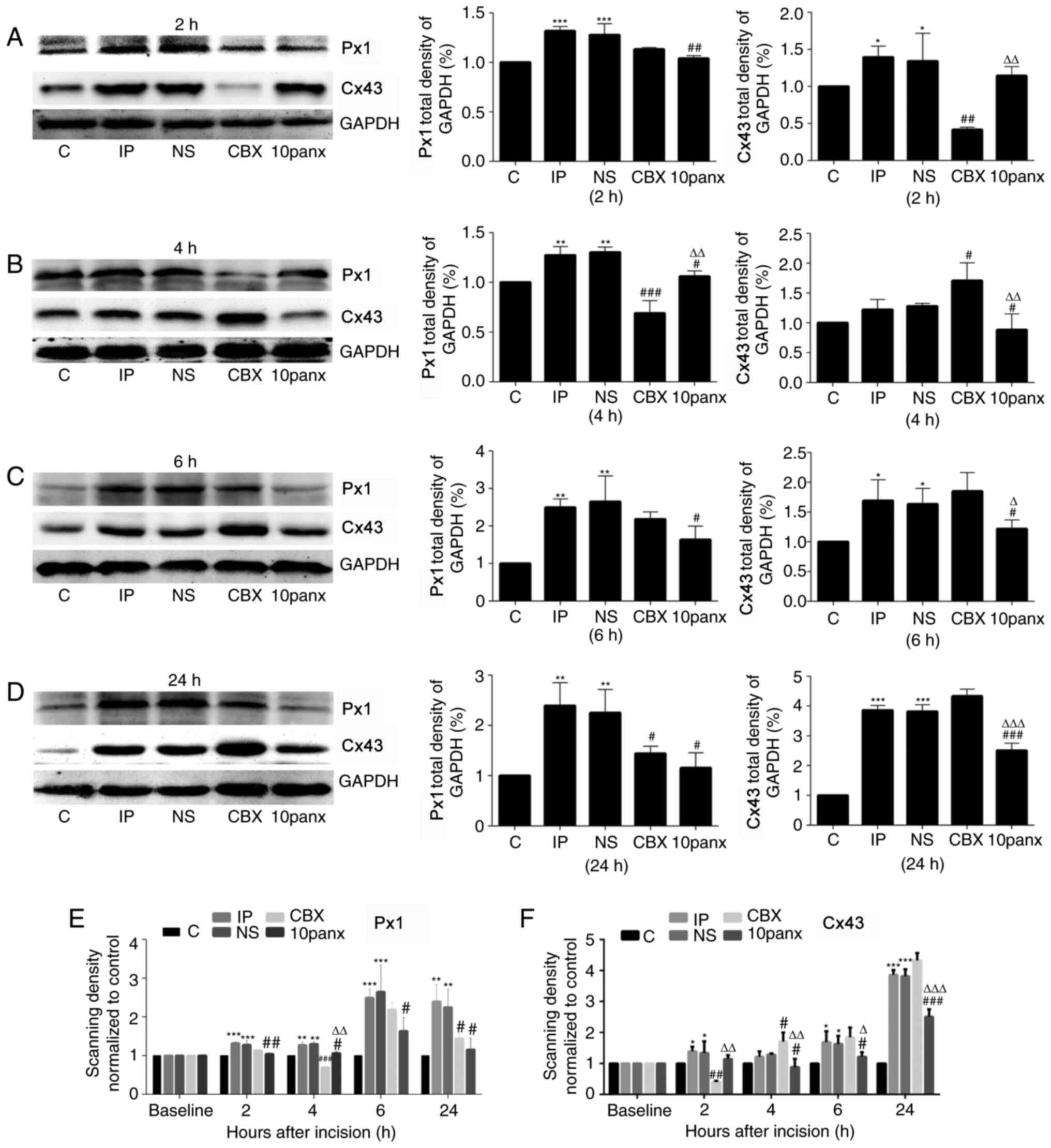 | Figure 2.Px1 and Cx43 expression in the spinal
cords of IP model rats. Expression of Px1 and Cx42 in rats was
detected via western blotting at (A) 2, (B) 4, (C) 6 and (D) 24 h
post-surgery. The scanning density of (E) Px1 and (F) Cx43
expression normalized to control. *P<0.05, **P<0.01 and
***P<0.001 vs. C group; #P<0.05,
##P<0.01 and ###P<0.001 vs. NS group;
ΔP<0.05, ΔΔP<0.01 and
ΔΔΔP<0.001 vs. CBX group. Data are presented as the
mean ± SD. C, control group; IP, incision pain; NS, normal saline;
CBX, carbenoxolone; 10panx, pannexin-1 mimetic inhibitory peptide;
Px1, pannexin 1; Cx43, connexin 43. |
Co-localization of Px1/Cx43 and
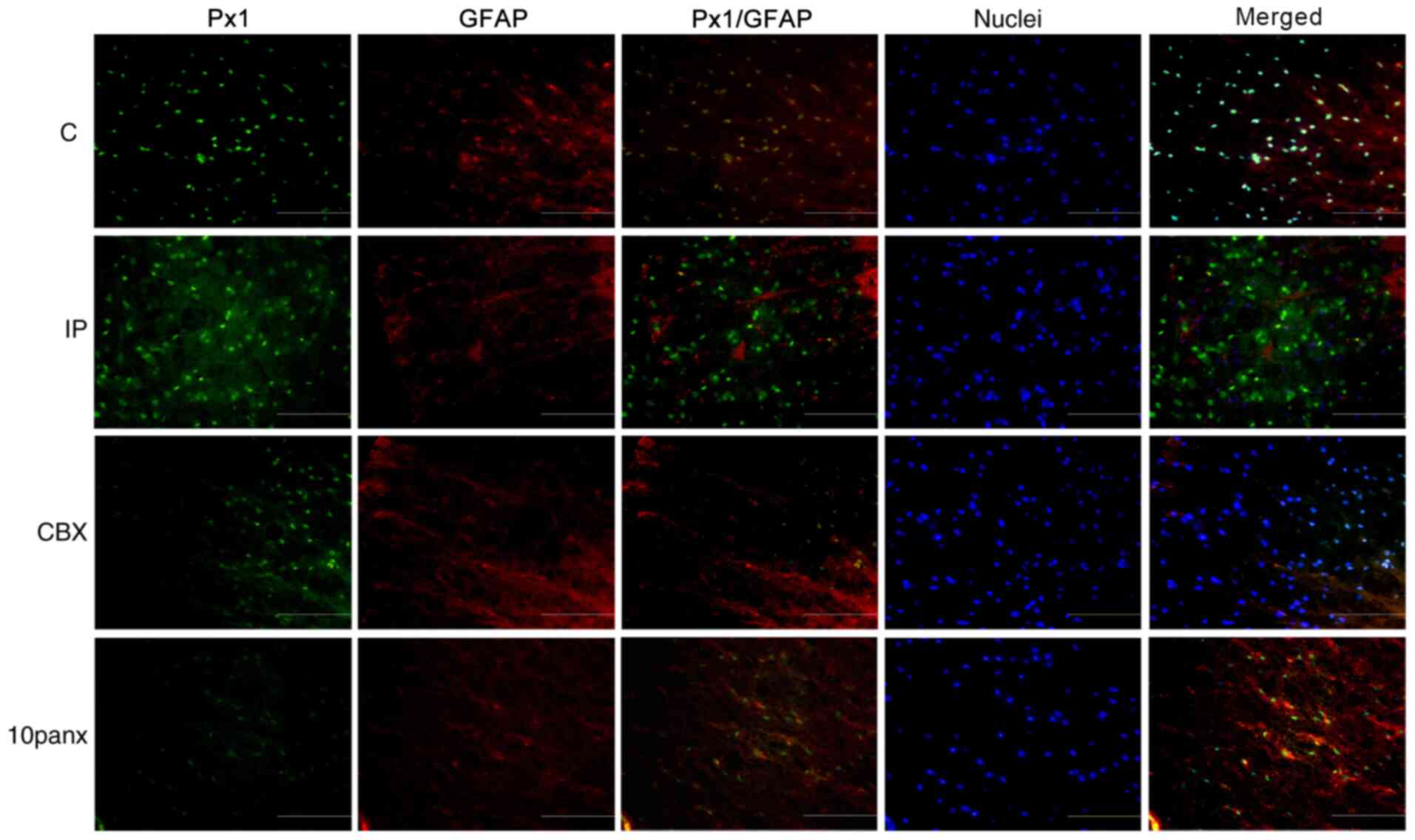
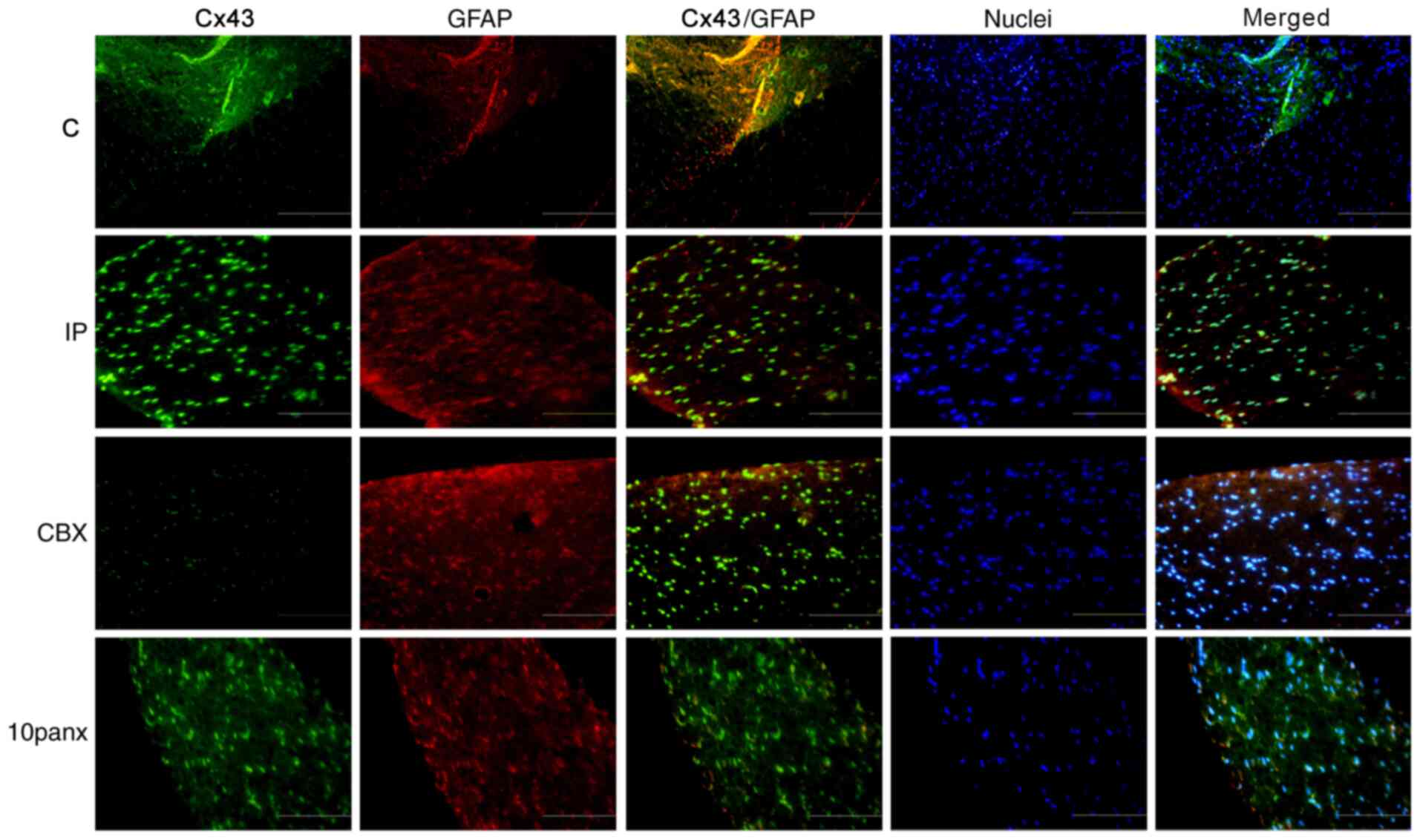
GFAP
An IF assay was performed to analyze the
co-localization of Px1 or Cx43 with GFAP 2 h after incision. Green
indicates Px1 expression in Fig. 3
and Cx43 expression in Fig. 4, red
indicates astrocytes, while yellow indicates the co-localization of
Px1 or Cx43 with GFAP. As shown in Fig.
3, the co-expression of Px1 and GFAP was most apparent in the
IP group. Simultaneously, CBX markedly reduced the co-expression of
Px1 and GFAP in the intumescentia lumbalis compared with the
IP group, which was higher than that in the 10panx group. It was
also found that CBX treatment led to a slight decrease in the
co-localization of Cx43 and GFAP compared with the IP group
(Fig. 4). Additionally, 10panx
treatment appeared to lead to a more pronounced reduction in the
co-localization of Cx43 and GFAP compared with CBX group.
Therefore, the results suggested that CBX induced astrocytes to
express low levels of Cx43 and Px1 in rats with toe incision.
CBX reduces the expression levels of
inflammatory markers at different time points after toe
incision
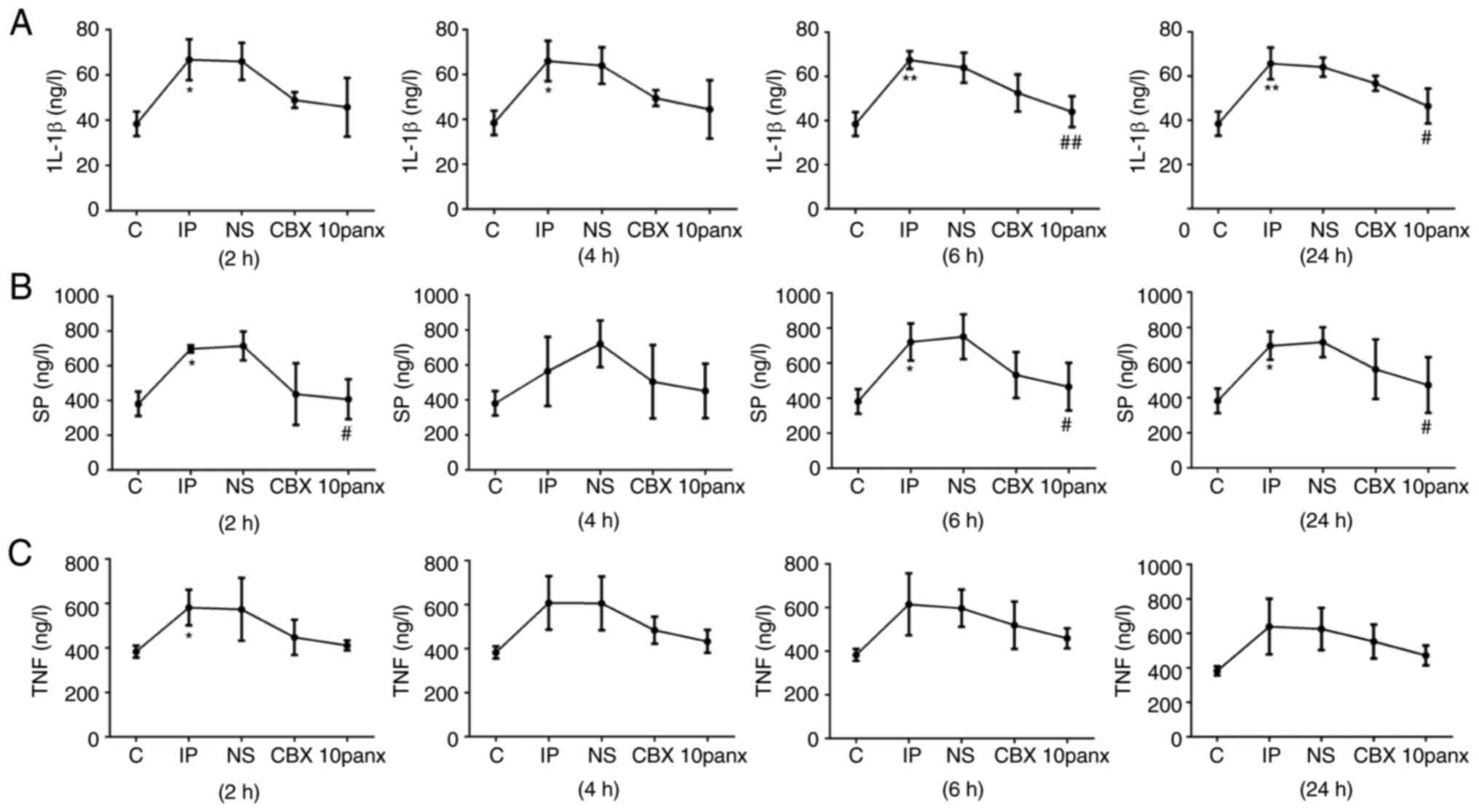
Glia cells contribute to pain effects via the
synthesis and release of a variety of inflammatory mediators,
including IL-1β and TNF-α (20,21).
In the present study it was observed that IL-1β expression levels
were markedly increased at 2, 4, 6 and 24 h post-surgery in the IP
group compared with the control group (P<0.05), while the
protein levels of SP were increased 2, 6 and 24 h after surgery in
the IP group (P<0.05). However, incision only induced a
significant increase in TNF-α at the 2 h time point (P<0.05).
These results implied that toe incision can induce an inflammatory
response 2 h after surgery. Although a decline in the levels of
inflammatory-related markers was observed following CBX
pretreatment (Fig. 5), this effect
was not significant. Notably, 10panx treatment led to a decrease in
IL-1β levels at 6 h (P<0.01) and 24 h (P<0.05) compared with
the IP group, as well as a decrease in SP levels at 2 and 6 h
(P<0.05), demonstrating that the inhibition of Panx1 could
effectively reduce the levels of pro-inflammatory factors increased
by incision surgery.
Discussion
The preferred method of treatment for relieving
postoperative pain currently involves the use of opioids, which are
associated with adverse side effects (22). The present study showed that CBX
could relieve acute surgical pain with a higher specificity and
reduced adverse effects. Thus, CBX may have potential to be used as
an analgesic in the future.
As a GJ inhibitor, CBX has significant effects in
pain reduction (23), ameliorating
Px1 channel activity by regulating the first extracellular loop
(16). In addition, CBX has been
found to have potential effects on potentially suppressing
neuropathic antitumor drug-induced pain by blocking astrocyte GJs
and inhibiting the increase of GFAP levels in the spinal cord
(18). Collectively, the formation
of astrocyte GJs could play a vital role in the transition of acute
pain to chronic pain (24–26). In the present study, it was found
that inducing an IP model of pain led to an increase in Px1 levels
2, 4, 6 and 24 h after incision, as well as an increase in Cx43
levels at 2, 6 and 24 h. There are close correlations between the
increased expression of PX channels and mechanical pain
sensitization (10). In the present
study, Px1 and Cx43 expression levels increased in the
intumescentia lumbalis of rats post-surgery. Additionally,
the IF assay results revealed that Px1 and Cx43 expression was
increased in astrocytes in the intumescentia lumbalis of IP
rats, indicating that their expression in astrocytes was tightly
associated with the production of acute pain. CBX and 10panx
pretreatment led to an increase in the pain threshold to a similar
degree at both 2 and 4 h post-surgery, suggesting that CBX relieved
acute pain possibly by inhibiting Px1 expression. At 6 or 24 h
post-surgery, the expression levels of Px1 remained high compared
with 2 h or 4 h post-surgery. Simultaneously, Cx43 was
significantly increased 24 h after incision compared with the
control group. However, the pain threshold value had returned to
within normal levels, implying that Px1 and Cx43 play essential
roles in the transition of acute to chronic pain.
A previous review indicated that astrocytes both
cause and maintain chronic pain (27). GJ regulation, inflammatory factor
releases and the activation of specific receptors in astrocytes
have been reported to be involved in the development of pain
(28). Px1 and Cx43 have been
demonstrated to regulate the release of ATP and glutamate (29). Specifically, CBX-induced ATP release
is probably due to the involvement of Panx1 channels in umbilical
vein endothelial cells. Cx43 is not involved in the process of ATP
release (30). It was found in the
present study that compared with the control group, IL-1β, SP and
TNF-α exhibited relatively high expression levels in the
intumescentia lumbalis of IP rats at 2, 4, 6 and 24 h
post-surgery.
Cx43 is specifically expressed in the astrocytes of
mammals (26,31). In the present study, the protein
expression levels of Cx43 in the CBX treatment group were
significantly lower than those in the IP group at 2 h post-surgery.
Compared with inhibitors, CBX has high specificity, relatively few
side effects, and it is easy to manufacture at a low price, thus it
has the potential to become a new generation of targeted analgesics
(32). 10panx is a mimic peptide of
pannexin 1, which suppresses the formation of Px1 channels
(33). A previous study suggested
that 10panx inhibits neuronal death and the inflammatory response
(34), and astrocytic Cx43 is
reportedly involved in the development of neuropathic and chronic
pain (35–38).
Astrocytes also play important roles in inducing
pain in acute pain models. A previous study indicated that
astrocytes could cause pain via the release of TNF-α and stromal
cell-derived factor 1 (39).
Furthermore, IL-1β serves important roles in the production of
acute pain, contributing to increased calcium and glutamatergic
activities (40). Blocking
astrocyte activation has been reported to suppress and ameliorate
pain sensitivity (41).
Collectively, these findings indicate that CBX decreases acute pain
by regulating Px1 and Cx43 hemichannels, as well as the
inflammatory response, which the present study also demonstrated.
The mechanism of action of CBX in astrocytes in acute pain, as well
as the efficacy of CBX on acute pain caused by other factors, still
requires further exploration. In the present study, the drug was
administrated through intrathecal administration. Intrathecal
administration is an important means of investigating drug
mechanisms at the spinal cord level, which increases local drug
concentrations (42). In addition,
compared with an intrathecal catheter, intrathecal injection is not
only simple and convenient with a high success rate, but also
rarely results in spinal cord injury or secondary infection
(42). In addition, poorly managed
acute pain has the potential to progress to chronic pain (43). Taken together, Px1 and Cx43 in
astrocytes could be implicated in pain behaviors improved by CBX
and CBX has the potential to reduce acute pain by decreasing Px1
and Cx43 levels. Px1 and Cx43 from spinal astrocytes may serve
important roles in the early stages and maintenance of acute pain,
while preoperative injection of CBX has the potential to relieve
hyperalgesia.
Acknowledgements
Not applicable.
Funding
The present study was supported 2016 Master
Supervisor Scientific Research Fund of The Second Hospital of
Lanzhou University entitled ‘Effect of propentofylline on MAPKs
signal transduction pathway expression in rats with acute incision
pain’ (grant no. sdkyjj-15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SD and YS made substantial contributions to
conception and design, acquisition of data, analysis interpretation
of data, acquisition of funding, collection of data and general
supervision of the research group. SD, KZ and YS made substantial
contributions to acquisition of data and analysis interpretation of
data. SD and KZ confirmed the authenticity of all the raw data. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the guidance of The International Association for the Study of
Pain, and approved by the Ethics Committee of The Second Hospital
of Lanzhou University (approval no. D2019-064; Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kehlet H: Postoperative pain, analgesia,
and recovery-bedfellows that cannot be ignored. Pain. 159 (Suppl
1):S11–S16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji RR, Chamessian A and Zhang YQ: Pain
regulation by non-neuronal cells and inflammation. Science.
354:572–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng J, Gu N, Zhou L, B Eyo U, Murugan M,
Gan WB and Wu LJ: Microglia and monocytes synergistically promote
the transition from acute to chronic pain after nerve injury. Nat
Commun. 7:120292016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramani M, Mylvaganam S, Krawczyk M, Wang
L, Zoidl C, Brien J, Reynolds JN, Kapur B, Poulter MO, Zoidl G and
Carlen PL: Differential expression of astrocytic connexins in a
mouse model of prenatal alcohol exposure. Neurobiol Dis. 91:83–93.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dahl G and Muller KJ: Innexin and pannexin
channels and their signaling. FEBS Lett. 588:1396–1402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garre JM, Yang G, Bukauskas FF and Bennett
MV: FGF-1 triggers pannexin-1 hemichannel opening in spinal
astrocytes of rodents and promotes inflammatory responses in acute
spinal cord slices. J Neurosci. 36:4785–4801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bargiotas P, Krenz A, Hormuzdi SG, Ridder
DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H and
Schwaninger M: Pannexins in ischemia-induced neurodegeneration.
Proc Natl Acad Sci USA. 108:20772–20777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reyes EP, Cerpa V, Corvalán L and Retamal
MA: Cxs and Panx-hemichannels in peripheral and central
chemosensing in mammals. Front Cell Neurosci. 8:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Laumet G, Chen SR, Hittelman WN
and Pan HL: Pannexin-1 Up-regulation in the dorsal root ganglion
contributes to neuropathic pain development. J Biol Chem.
290:14647–14655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burma NE, Bonin RP, Leduc-Pessah H, Baimel
C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL,
Baimoukhametova D, Bains JS, et al: Blocking microglial pannexin-1
channels alleviates morphine withdrawal in rodents. Nat Med.
23:355–360. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanstein R, Hanani M, Scemes E and Spray
DC: Glial pannexin1 contributes to tactile hypersensitivity in a
mouse model of orofacial pain. Sci Rep. 6:382662016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bravo D, Ibarra P, Retamal J, Pelissier T,
Laurido C, Hernandez A and Constandil L: Pannexin 1: A novel
participant in neuropathic pain signaling in the rat spinal cord.
Pain. 155:2108–2115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michalski K and Kawate T: Carbenoxolone
inhibits Pannexin1 channels through interactions in the first
extracellular loop. J Gen Physiol. 147:165–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morioka N, Nakamura Y, Zhang FF,
Hisaoka-Nakashima K and Nakata Y: Role of connexins in chronic pain
and their potential as therapeutic targets for next-generation
analgesics. Biol Pharm Bull. 42:857–866. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Hondt C, Iyyathurai J, Himpens B,
Leybaert L and Bultynck G: Cx43-hemichannel function and regulation
in physiology and pathophysiology: Insights from the bovine corneal
endothelial cell system and beyond. Front Physiol. 5:3482014.
View Article : Google Scholar
|
|
16
|
Dierks A, Bader A, Lehrich T and Ngezahayo
A: Stimulation of the A2B adenosine receptor subtype
enhances connexin26 hemichannel activity in small airway epithelial
cells. Cell Physiol Biochem. 53:606–622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Treede RD: The international association
for the study of pain definition of pain: As valid in 2018 as in
1979, but in need of regularly updated footnotes. Pain Rep.
3:e6432018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ,
Beitz AJ and Lee JH: Intrathecal injection of carbenoxolone, a gap
junction decoupler, attenuates the induction of below-level
neuropathic pain after spinal cord injury in rats. Exp Neurol.
224:123–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–502. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao H, Alam A, Chen Q, A Eusman M, Pal A,
Eguchi S, Wu L and Ma D: The role of microglia in the pathobiology
of neuropathic pain development: What do we know? Br J Anaesth.
118:504–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu LW, Cheng KI, Chen JY, Cheng YC, Chang
YC, Yeh JL, Hsu JH, Dai ZK and Wu BN: Loganin prevents chronic
constriction injury-provoked neuropathic pain by reducing
TNF-α/IL-1β-mediated NF-κB activation and Schwann cell
demyelination. Phytomedicine. 67:1531662020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Boer HD, Detriche O and Forget P:
Opioid-related side effects: Postoperative ileus, urinary
retention, nausea and vomiting, and shivering. A review of the
literature. Best Pract Res Clin Anaesthesiol. 31:499–504. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng GC, Lu M, Zhao YY, Yuan Y and Chen G:
Activated spinal astrocytes contribute to the later phase of
carrageenan-induced prostatitis pain. J Neuroinflammation.
16:1892019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson CR and Dougherty PM: Spinal
astrocyte gap junction and glutamate transporter expression
contributes to a rat model of bortezomib-induced peripheral
neuropathy. Neuroscience. 285:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wieseler-Frank J, Maier SF and Watkins LR:
Glial activation and pathological pain. Neurochem Int. 45:389–395.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MJ, Kress B, Han X, Moll K, Peng W,
Ji RR and Nedergaard M: Astrocytic CX43 hemichannels and gap
junctions play a crucial role in development of chronic neuropathic
pain following spinal cord injury. Glia. 60:1660–1670. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Callaghan JP and Miller DB: Spinal glia
and chronic pain. Metabolism. 59 (Suppl 1):S21–S26. 2010.
View Article : Google Scholar
|
|
28
|
Xing L, Yang T, Cui S and Chen G: Connexin
hemichannels in astrocytes: Role in CNS disorders. Front Mol
Neurosci. 12:232019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei H, Deng F, Chen Y, Qin Y, Hao Y and
Guo X: Ultrafine carbon black induces glutamate and ATP release by
activating connexin and pannexin hemichannels in cultured
astrocytes. Toxicology. 323:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gdecke S, Roderigo C, Rose CR, Rauch BH,
Gödecke A and Schrader J: Thrombin-induced ATP release from human
umbilical vein endothelial cells. Am J Physiol Cell Physiol.
302:C915–C923. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon SY, Robinson CR, Zhang H and
Dougherty PM: Spinal astrocyte gap junctions contribute to
oxaliplatin-induced mechanical hypersensitivity. J Pain.
14:205–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Cesare Mannelli L, Marcoli M, Micheli
L, Zanardelli M, Maura G, Ghelardini C and Cervetto C: Oxaliplatin
evokes P2X7-dependent glutamate release in the cerebral cortex: A
pain mechanism mediated by Pannexin 1. Neuropharmacology.
97:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basova LV, Tang X, Umasume T, Gromova A,
Zyrianova T, Shmushkovich T, Wolfson A, Hawley D, Zoukhri D,
Shestopalov VI and Makarenkova HP: Manipulation of panx1 activity
increases the engraftment of transplanted lacrimal gland epithelial
progenitor cells. Invest Ophthalmol Vis Sci. 58:5654–5665. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei R, Bao W, He F, Meng F, Liang H and
Luo B: Pannexin1 channel inhibitor (10panx) protects
against transient focal cerebral ischemic injury by inhibiting RIP3
expression and inflammatory response in rats. Neuroscience.
437:23–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang A and Xu C: The role of connexin43 in
neuropathic pain induced by spinal cord injury. Acta Biochim
Biophys Sin (Shanghai). 51:555–561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Ao L, Yan Y, Li C, Li W, Ye A, Liu
J, Hu Y, Fang W and Li Y: Levo-corydalmine attenuates
vincristine-induced neuropathic pain in mice by upregulating the
Nrf2/HO-1/CO pathway to inhibit connexin 43 expression.
Neurotherapeutics. 17:340–355. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vicario N, Pasquinucci L, Spitale FM,
Chiechio S, Turnaturi R, Caraci F, Tibullo D, Avola R, Gulino R,
Parenti R and Parenti C: Simultaneous activation of Mu and Delta
opioid receptors reduces allodynia and astrocytic connexin 43 in an
animal model of neuropathic pain. Mol Neurobiol. 56:7338–7354.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin YZ, Zhang P, Hao T, Wang LM, Guo MD
and Gan YH: Connexin 43 contributes to temporomandibular joint
inflammation induced-hypernociception via sodium channel 1.7 in
trigeminal ganglion. Neurosci Lett. 707:1343012019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Tonello R, Ling Y, Gao YJ and Berta
T: Paclitaxel-activated astrocytes produce mechanical allodynia in
mice by releasing tumor necrosis factor-α and stromal-derived cell
factor 1. J Neuroinflammation. 16:2092019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan X, Li F, Maixner DW, Yadav R, Gao M,
Ali MW, Hooks SB and Weng HR: Interleukin-1beta released by
microglia initiates the enhanced glutamatergic activity in the
spinal dorsal horn during paclitaxel-associated acute pain
syndrome. Glia. 67:482–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Obata H, Sakurazawa S, Kimura M and Saito
S: Activation of astrocytes in the spinal cord contributes to the
development of bilateral allodynia after peripheral nerve injury in
rats. Brain Res. 1363:72–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Sun S, Wang Q and Zhao Z: Population
pharmacokinetics of combined intravenous and local intrathecal
administration of meropenem in aneurysm patients with suspected
intracranial infections after craniotomy. Eur J Drug Metab
Pharmacokinet. 43:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ossipov MH, Morimura K and Porreca F:
Descending pain modulation and chronification of pain. Curr Opin
Support Palliat Care. 8:143–151. 2014. View Article : Google Scholar : PubMed/NCBI
|