Introduction
Acute lung injury (ALI) is a type of severe acute
lung inflammation that causes diffuse lung injury induced by
various direct or indirect factors (1). The main manifestations of ALI are
increased permeability of the alveolar wall and pulmonary
capillaries, pulmonary interstitial and alveolar edema, eventually
leading to acute respiratory insufficiency, and even acute
respiratory distress syndrome (2).
The etiology and mechanism of ALI are complex.
During the past half-century, there has been considerable progress
in the field of ALI research, although the exact pathogenesis has
not been fully clarified. Previous studies have suggested that
excessive activation of inflammatory cells, release of excessive
inflammatory mediators, mutual activation and the interactions of
numerous inflammatory factors and effector cells, combined with
uncontrolled inflammatory responses are the main pathophysiological
changes (3).
A variety of inflammatory cells are recruited and
activated in the lung in response to various direct and indirect
harmful stimuli, leading to the release of a large number of
pro-inflammatory mediators. This ‘inflammatory cascade’ of
cytokines results in the induction of the inflammatory response
(4). Therefore, strategies for the
prevention and treatment of ALI should be focused on inhibiting the
overexpression and hyperactivation of inflammatory mediators.
Regulating the occurrence and development of the inflammatory
response has important clinical significance in preventing the
occurrence of ALI and decreasing mortality (5).
Although there are numerous strategies for ALI
treatment in the clinic, including cytokine therapy, stem cell
therapy and hormone therapy, the incidence rate and mortality rate
of ALI remains high (6).
Comprehensive elucidation of the molecular pathological mechanism
of ALI is required to improve the clinical efficacy of these
therapeutic strategies.
MicroRNAs (miRNAs) are small (22–25 nucleotide),
non-coding RNAs that regulate gene expression by inhibiting
translation or promoting the degradation of target mRNAs and
regulating a variety of important cell activities by specifically
pairing with the 3′-untranslated region (3′-UTR) of the target gene
(7). miRNAs are also associated
with the occurrence and development of lung diseases, including
pneumonia, lung cancer and pulmonary fibrosis (8,9). For
example, Shen et al (10)
demonstrated that miR-200b may attenuate cellular senescence and
inflammatory responses by targeting ZEB2 in pulmonary emphysema.
Furthermore, Liang et al (11) demonstrated that miR-187 may present
a novel therapeutic target in non-small-cell lung cancer based on
its ability to regulate cyclins-related protein expression
(11). miR-140 was also shown to
suppress interstitial lung disease (ILD) development by
downregulating osteoglycin (OGN) via the Wnt signaling pathway
(12).
Recent studies have demonstrated the involvement of
miRNAs in the regulation of the occurrence and development of the
immune response that leads to ALI. For example, Huang et al
(13) demonstrated that
downregulation of miR-27b enhanced the expression of Nrf2 and HO-1,
inhibited NF-κB signaling pathway, and protected against
LPS-induced ALI in mice. In addition, Ju et al (14) reported that miR-27a attenuates
LPS-induced ALI in mice by blocking activation of the
TLR4/MyD88/NF-κB axis. Another study indicated that miR-326
activates the NF-κB signaling pathway by targeting the
BCL2A1 gene, leading to an enhanced inflammatory response
and the lung injury associated with septic shock in ALI induced in
mice (15).
Recent studies have demonstrated that miR-421
regulates inflammatory responses. Zheng et al (16) reported that miR-421 inhibited p65
mRNA translation by targeting YTHDF1 to prevent inflammation in
cerebral ischemia reperfusion injury. In addition, Jiang et
al (17) demonstrated that
miR-421 promotes the inflammatory response of fibroblast-like
synoviocytes in rheumatoid arthritis by downregulating the
expression of SPRY1. Furthermore, inhibition of miR-421 promotes
the development of bronchopulmonary dysplasia by upregulating Fgf10
(18). However, the effect of
miR-421 on ALI is not clear. In this study, we used the
lipopolysaccharide-induced model of ALI to investigate the effect
of miR-421 on ALI in vitro and the underlying mechanism.
Materials and methods
RAW 264.7 macrophages cells
culture
RAW 264.7 macrophage cells were purchased from the
American Type Culture Collection (ATCC). RAW 264.7 cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% (volume/volume)
fetal bovine serum (FBS; HyClone), 100 U penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C under
5% CO2 in a humidified incubator. For the LPS-induced
model of ALI, RAW 264.7 cells were plated into 12-well plates
(1×106 cells/well) and incubated for 24 h before
treatment with 0, 50, 100 or 200 ng/ml LPS (Beijing Solarbio
Science & Technology Co., Ltd.) for a further 24 h, as
previously described (19).
Transfection
miR-421 mimic, miR-421 mimic control, programmed
cell death 4 (PDCD4) siRNA and PDCD4 siRNA control were designed
and synthesized by GenePharma (Shanghai GenePharma Co., Ltd.). RAW
264.7 cells in the logarithmic growth phase were seeded into 6-well
plates and incubated for 12 h at 37°C under 5% CO2 in a
humidified incubator prior to transfection with miR-421 mimic
(5′-AUCAACAGACAUUAAUUGGGCGC-3′, 20 µM), miR-421 mimic control
(5′-UUCUCCGAACGUGUCACGUTT-3′, 20 µM), PDCD4 siRNA
(5′-GAGGCUAUGAGAGAAUUUATT-3′, 20 µM) and PDCD4 siRNA control
(5′-UAGCCUAGUCCAAAGCAGCAT-3′, 20 µM) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h.
After 48 h, RNA and protein were extracted for further
experiments.
Cell Counting kit-8 (CCK-8)
Cell viability was measured using the CCK-8 (Dojindo
Molecular Technologies, Inc.) method. In brief, RAW 264.7
macrophages were seeded into 96-well plates (5×103
cells/well) and incubated for 12 h at 37°C under 5% CO2
in a humidified incubator. Subsequently, 10 µl CCK-8 reagent was
added to each well and the RAW 264.7 cells were incubated for 1 h.
The optical density in each well was measured at 450 nm using an
iMark Microplate Absorbance Reader (Bio-Rad Laboratories,
Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW 264.7 cells were harvested and total RNA was
extracted with RNAiso (Takara Bio, Inc.) according to
manufacturer's protocols. The concentration and purity of the
isolated RNA was determined using a NanoDrop spectrophotometer
(Thermo Fisher Scientific, Inc.). RNA was reverse-transcribed into
cDNA using PrimeScript™ RT reagent kit (Takara Bio, Inc.) and Mir-X
miRNA RT-qPCR TB Green® Kit (Takara Bio, Inc.). RT was
performed at 37°C for 15 min, then 85°C for 5 sec. qPCR was
performed on an ABI Prism 7500 (Thermo Fisher Scientific, Inc.)
using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus;
Takara Bio, Inc.) according to manufacturer's protocols. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 31 sec.
The sequences of the primers as follows: miR-21 forward,
5′-TAGCTTATCAGACTGATGTTGA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; PDCD4 forward,
5′-AGGCCGAGGTGGGCGGATCACTTGA-3′ and reverse,
5′-GCCACCATGCCTGGCTACT-3′; and GAPDH forward,
5′-CCTCTGACTTCAACAGCGACAC-3′ and reverse,
5′-TGGTCCAGGGGTCTTACTCC-3′. The relative expression level was then
calculated using 2−ΔΔCq method (20).
Western blotting
Total proteins were extracted from RAW 264.7 cells
using a RIPA kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocols. Nuclear proteins were extracted using
the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime
Institute of Biotechnology). The protein concentration was measured
with BCA kits (Beyotime Institute of Biotechnology) according to
the manufacturer's protocols. The proteins were separated by 10%
SDS-PAGE for 1.5 h. Next, the protein was transfected into PVDF
(Millipore) membrane (40 µg/lane). The PVDF membrane was blocked
with 5% skimmed milk at room temperature for 1 h before incubation
overnight at 4°C with primary antibodies for the detection of
p-NF-κB (p65) (cat. no. ab76302; dilution, 1:1,000; Abcam), NF-κB
(p65; cat. no. ab76311; dilution, 1:1,000; Abcam) and β-actin (cat.
no. ab8227; dilution, 1:2,000; Abcam). Membranes were then
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibodies (cat. no. ab6721; 1:5,000; Abcam)
for 1 h at room temperature. Immunoreactive bands were visualized
using the ECL Plus Western Blotting Substrate (Pierce; Thermo
Fisher Scientific, Inc.) kit and the FluorChem FC3 imaging system
(ProteinSimple).
ELISA
RAW 264.7 cells in the logarithmic growth phase were
seeded into 6-well plates and cultured at 37°C under 5%
CO2 in a humidified incubator for 24 h. The culture
supernatant was collected and the concentrations of IL-1β (cat. no.
MLB00C) and TNF-α (cat. no. MTA00B) were detected using ELISA kits
(R&D Systems, Inc.) according to the manufacturer's protocols.
The optical density at 450 nm was measured using an iMark
Microplate Absorbance Reader (Bio-Rad Laboratories, Inc.).
Dual-luciferase reporter assay
StarBase 3.0 (starbase.sysu.edu.cn/) was used to predict that
miR-421 binds to the 3′-UTR of PDCD4 mRNA. PDCD4 reporter plasmids
(pmirGLO) with the wild-type miR-421 binding site
(pmirGLO-PDCD4-WT) and a mutant-type miR-421 binding site
(pmirGLO-PDCD4-MUT) in the 3′-UTR of PDCD4 mRNA were synthesized by
Shanghai GenePharma Co., Ltd. using a site-directed mutagenesis kit
(cat. no. 200518; Agilent Technologies Inc.). Next,
pmirGLO-PDCD4-WT and pmirGLO-PDCD4-MUT were co-transfected with
synthesized reporter vectors (0.8 µg) and miR-421 mimic (0.8 µg) or
miR-421 mimic control (0.8 µg) using Lipofectamine 2000 reagent at
37°C for 48 h. Renilla luciferase activity was measured
using a Dual-Luciferase Reporter Assay system (Promega
Corporation), according to the manufacturer's protocol.
Statistical analysis
SPSS 19.0 (IBM Corp.) was used for statistical
analysis, and the measurement data are expressed as the mean ±
standard deviation. Differences between two groups were compared
using t-tests. Differences between multiple groups were compared
using one-way analysis of variance, followed by Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
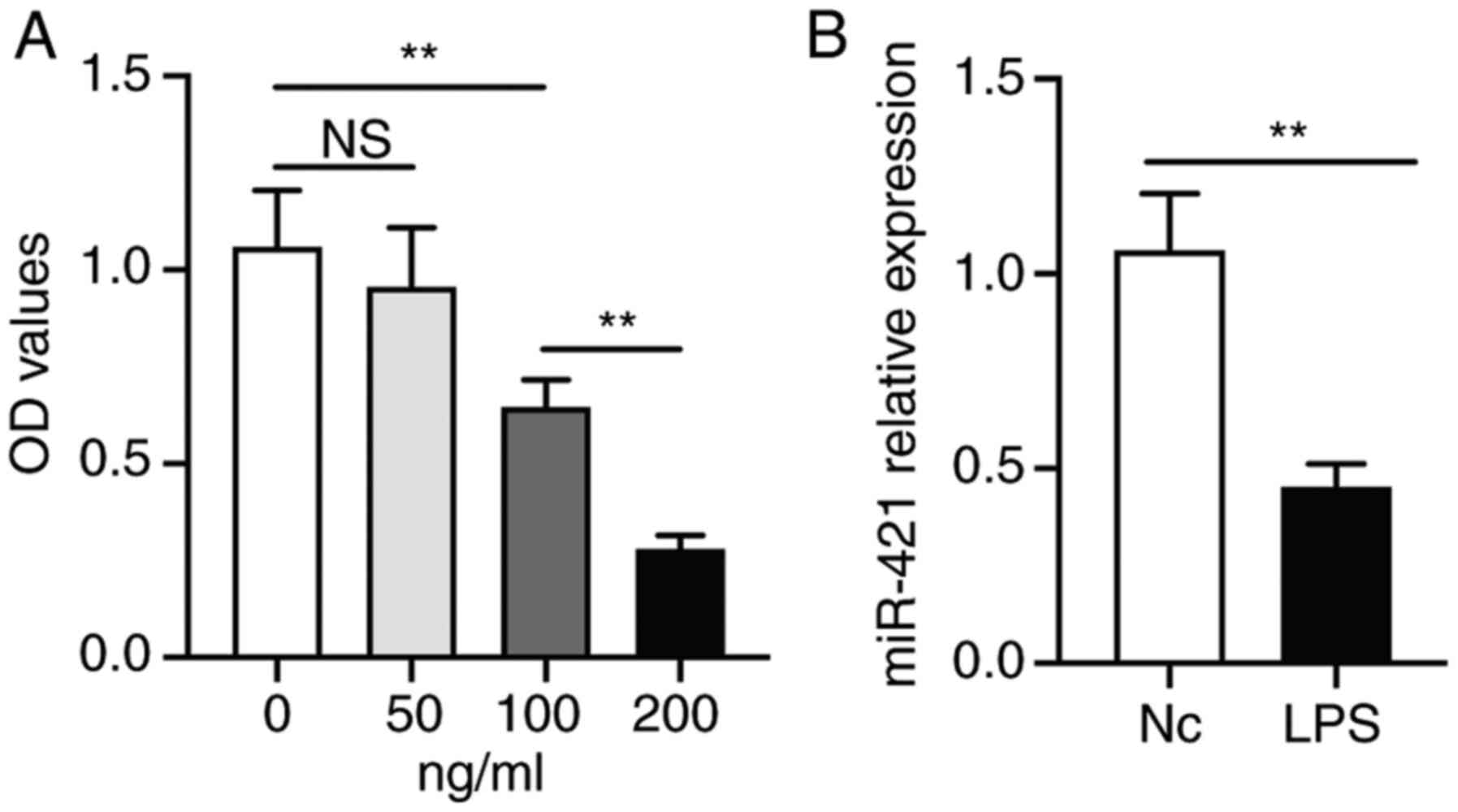
LPS inhibits RAW 264.7 macrophage cell
viability and miR-421 expression
RAW 264.7 macrophages were treated with different
concentrations of LPS for 24 h. At 100 ng/ml, LPS significantly
inhibited the viability of RAW 264.7 cells. Furthermore, the
inhibitory effect of LPS at 200 ng/ml was greater than that at 100
ng/ml (Fig. 1A). Therefore, LPS at
100 ng/ml was selected as the suboptimal inhibitory dose and LPS
was added at 100 ng/ml in the subsequent experiments. Furthermore,
RT-qPCR demonstrated that LPS (100 ng/ml) significantly inhibited
miR-421 expression in RAW 264.7 cells (Fig. 1B).
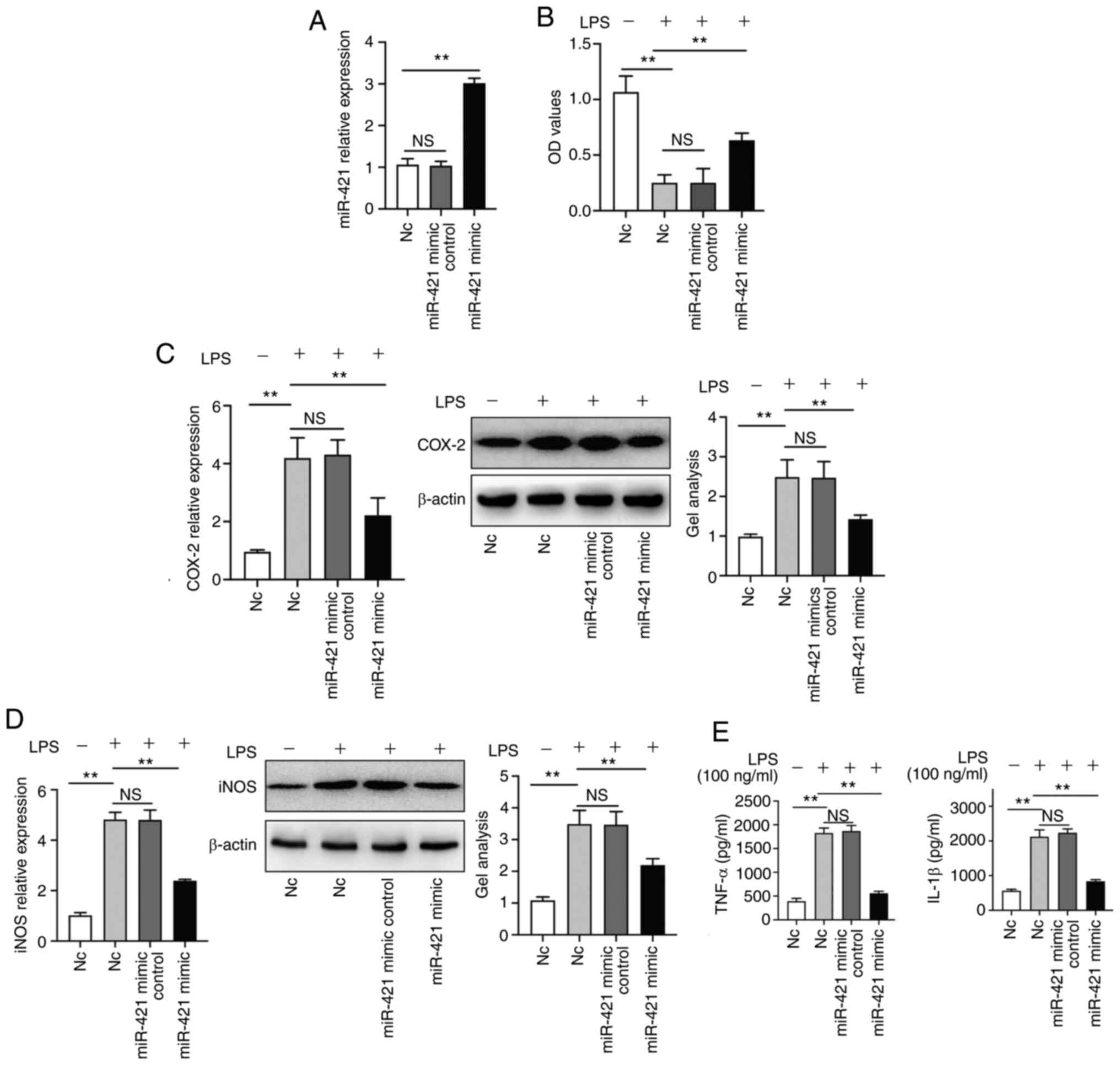
miR-421 upregulation inhibits the
release of inflammatory factors by RAW 264.7 macrophages
The miR-421 mimic significantly increased miR-421
expression in RAW 264.7 macrophages (Fig. 2A). LPS (100 ng/ml) treatment
inhibited RAW 264.7 cell viability. In addition, the miR-421 mimic
reversed the inhibitory effect of LPS (100 ng/ml) on RAW 264.7 cell
viability (Fig. 2B). The miR-421
mimic inhibited the LPS-induced expression of COX-2 and iNOS in RAW
264.7 cells (Fig. 2C and D). LPS
(100 ng/ml) treatment promoted the release of IL-1β and TNF-α by
RAW 264.7 cells and this effect was inhibited by the miR-421 mimic
(Fig. 2E).
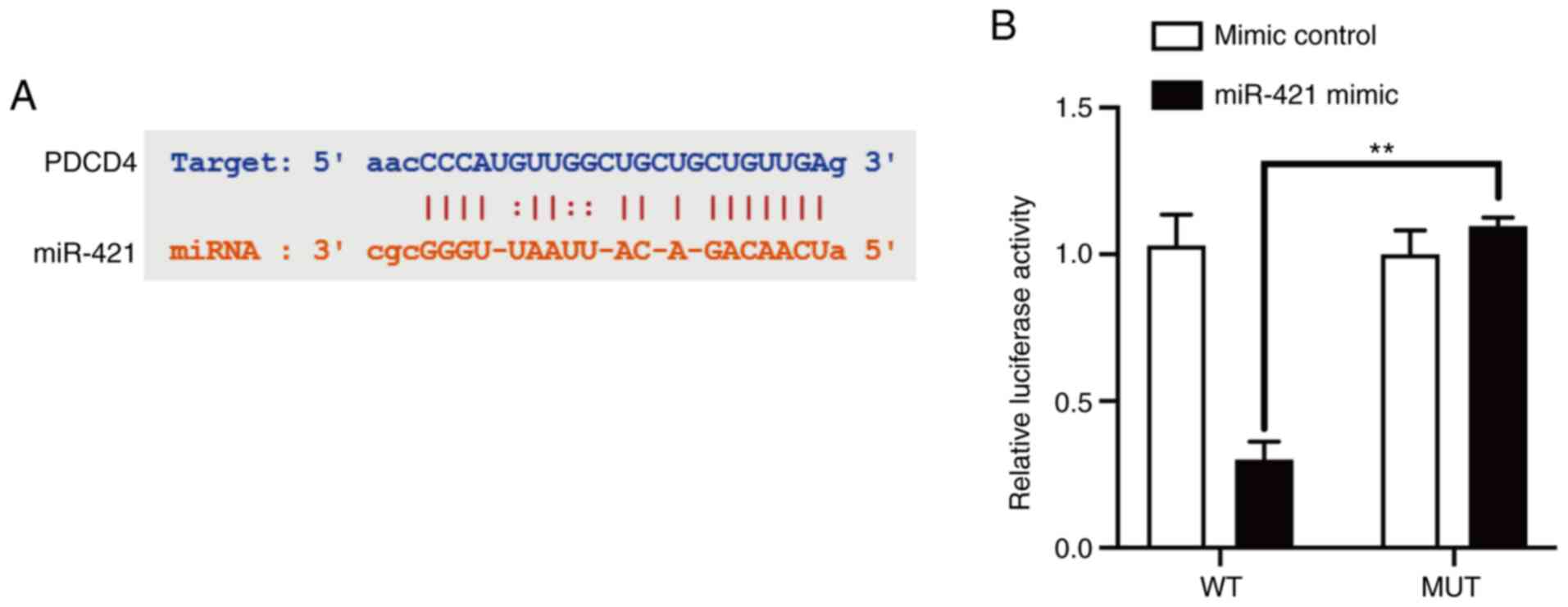
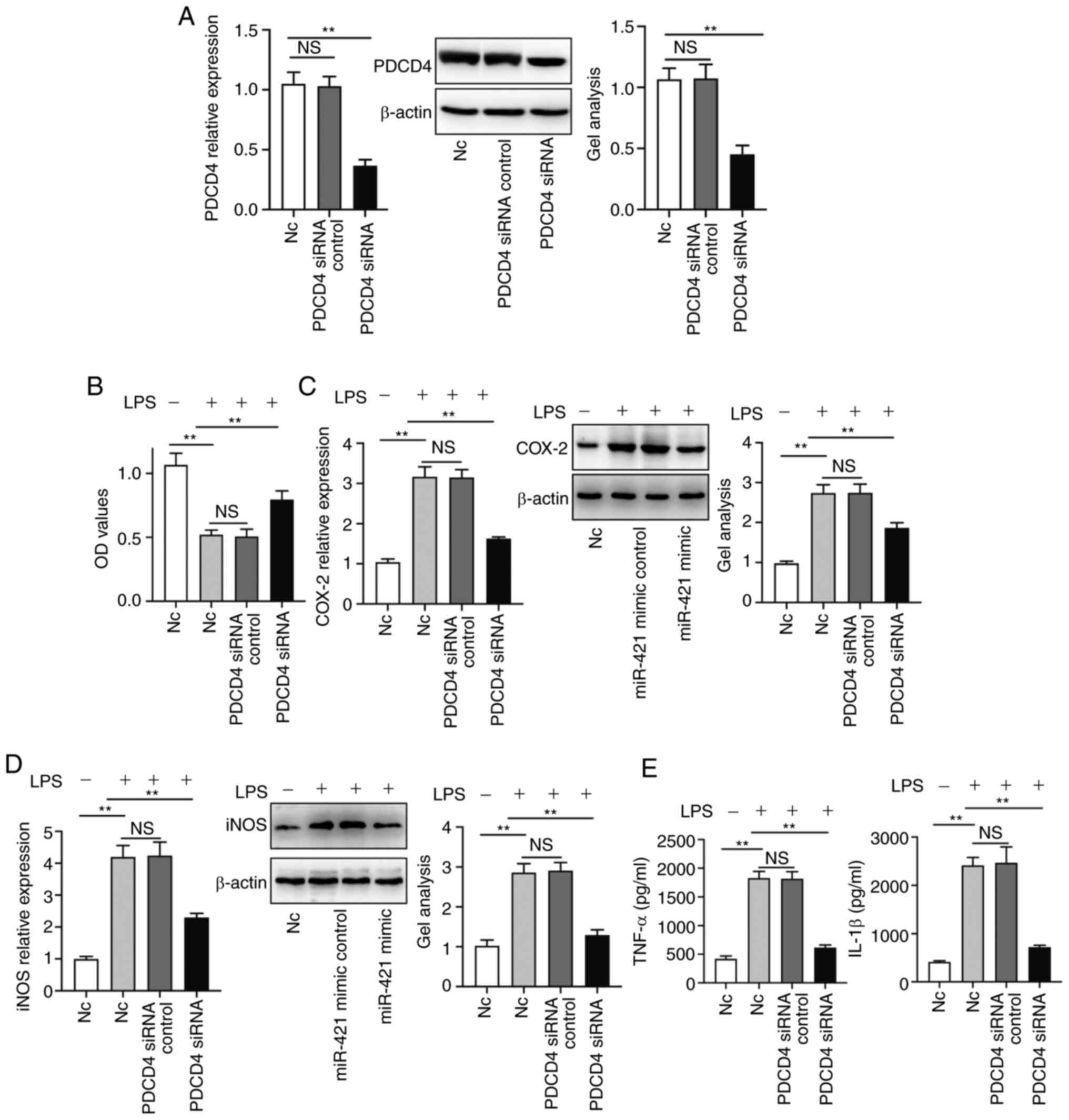
miR-421 directly inhibits PDCD4
expression and PDCD4 downregulation inhibits RAW 264.7 cell
production of inflammatory factors
Using StarBase 3.0, it was predicted that miR-421
binds to a specific site in the 3′-UTR of PDCD4 mRNA (Fig. 3A). Dual-luciferase reporter assay
confirmed that miR-421 directly targeted the 3′-UTR of PDCD4 mRNA
to inhibit its expression (Fig.
3B). Further studies on the function of the PDCD4 gene
demonstrated that PDCD4 siRNA blocked the inhibitory effect of LPS
on RAW 264.7 cell viability (Fig. 4A
and B). PDCD4 siRNA also inhibited LPS-induced expression of
COX-2 and iNOS in RAW 264.7 cells (Fig.
4C and D). In addition, PDCD4 siRNA inhibited the LPS-induced
increase of IL-1β and TNF-α production by RAW 264.7 cells (Fig. 4E).
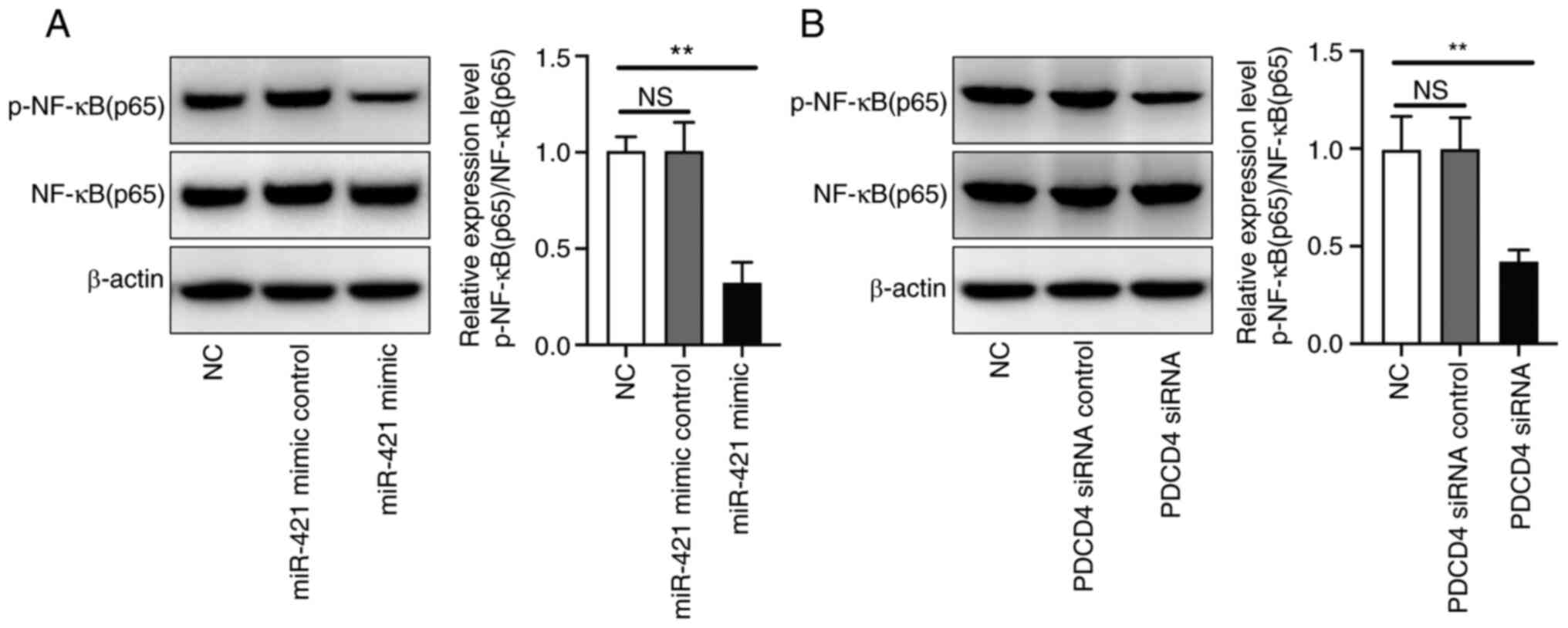
miR-421 mimic and PDCD4 siRNA inhibit
p-NF-κB (p65) expression in RAW 264.7 cells
Western blot analysis demonstrated that the miR-421
mimic inhibited p-NF-κB (p65) expression in RAW 264.7 cells
(Fig. 5A). Furthermore, PDCD4 siRNA
also inhibited p-NF-κB (p65) expression in RAW 264.7 cells
(Fig. 5B).
Discussion
LPS stimulates monocytes and macrophages to release
pro-inflammatory factors that mediate inflammatory reactions.
Excessive inflammatory reactions may cause injury to tissues such
as the lung (21). LPS may also
damage pulmonary vascular endothelial cells, increase vascular
permeability, cause pulmonary edema and lead to progressive
disorder of gas exchange. These changes are consistent with the
histopathological and physiological changes of ALI. Therefore, LPS
is generally considered as an ideal stimulant to induce ALI
(21).
Tang et al demonstrated that increased iNOS
expression serves a key role in the pathogenesis of LPS-induced ALI
(22). LPS stimulates iNOS
expression in a variety of cell types found in the lung, including
macrophages, neutrophils and endothelial cells (23). The present study demonstrated that
miR-421 alleviated the increase in iNOS expression induced in RAW
264.7 macrophages by treatment with LPS. Therefore, we hypothesized
that the protective effect of miR-421 on ALI may be associated with
its inhibitory effect on iNOS.
Cyclooxygenase (COX), which converts arachidonic
acid into prostaglandins, is considered to serve an important role
in the occurrence and development of numerous inflammatory
diseases, including ALI (24). As
an inducible COX subtype, COX-2 may be induced by cytokines, growth
factors, viruses, LPS and other inflammatory stimuli associated
with ALI (25). In the present
study, COX-2 expression was significantly increased in RAW 264.7
cells following LPS stimulation, while miR-421 attenuated this
change, suggesting that the protective effect of miR-421 on ALI is
associated with its inhibitory effect on COX-2.
Inflammatory mediators, particularly cytokines,
serve key roles in the development and prognosis of ALI (26). In the early stage of ALI, numerous
inflammatory factors, including TNF-α and IL-1β, serve a decisive
role in the initiation of the early inflammatory response, and may
maintain the continuous development and expansion of inflammation
through a variety of mechanisms (26). While inflammatory factors may remove
harmful microorganisms, excessive production of inflammatory
factors will cause damage to lung tissue (27).
High levels of TNF-α may also cause severe systemic
inflammatory reactions, shock, vascular leakage and pulmonary edema
(28,29). In the present study, cells were
stimulated with 100 ng/ml LPS for 24 h, and it was found that LPS
(100 ng/ml) treatment for 24 h induced a significant increase in
the production of TNF-α in RAW 264.7 cells and this effect was
significantly inhibited by the miR-421 mimic.
IL-1β may also activate neutrophils, which are
considered to serve a very important initiating role in the
initiation of the inflammatory cascade (30,31).
In the present study, it was demonstrated that LPS (100 ng/ml)
treatment for 24 h induced a significant increase in the production
of IL-1 β in RAW 264.7 cells and this effect was significantly
inhibited by the miR-421 mimic.
Using StarBase 3.0, it was predicted that miR-421
directly targets a site in the 3′-UTR of PDCD4 mRNA. As reported
previously, Yang et al (32)
reported that miR-421 promotes the proliferation and invasion of
non-small cell lung cancer cells through targeting PDCD4. This was
also confirmed in the present study using dual-luciferase reporter
assays. Furthermore, Western blot analysis demonstrated that the
miR-421 mimic inhibited PDCD4 in RAW 264.7 cells. Li et al
(33) demonstrated that
PDCD4-overexpression significantly attenuated the anti-apoptotic
effect of MSC-Exo in lung cells.
In ALI, NF-κB activation enhances the transcription
of numerous pro-inflammatory factors, including adhesion molecules,
cytokines and chemokines, and induces the expression of enzymes,
including COX-2 and iNOS (34). It
was demonstrated that PDCD4 siRNA inhibited the phosphorylation of
NF-κB, indicating that NF-κB serves a role in the protective effect
of miR-421 on ALI.
The results of the present study demonstrated the
protective effect of miR-421 on ALI. Furthermore, it was
demonstrated that miR-421 may attenuate LPS-induced ALI by
inhibiting PDCD4 and NF-κB. These results provided a theoretical
basis for the development of strategies for the prevention and
treatment of ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Inner Mongolia Autonomous Region (grant no.
2018LH08039).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW designed the experiments. HL, JS, LG, LF and DC
performed the experiments. HL collected and analyzed the data. All
authors confirmed the authenticity of all the raw data. HL and LW
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szabo C, Martins V and Liaudet L:
Poly(ADP-Ribose) polymerase inhibition in acute lung injury. A
reemerging concept. Am J Respir Cell Mol Biol. 63:571–590. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Needham DM, Colantuoni E, Mendez-Tellez
PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, Desai SV,
Shanholtz C, Brower RG and Pronovost PJ: Lung protective mechanical
ventilation and two year survival in patients with acute lung
injury: Prospective cohort study. BMJ. 344:e21242012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reagan-Steiner S, Gary J, Matkovic E,
Ritter JM, Shieh WJ, Martines RB, Werner AK, Lynfield R, Holzbauer
S, Bullock H, et al: Pathological findings in suspected cases of
e-cigarette, or vaping, product use-associated lung injury (EVALI):
A case series. Lancet Respir Med. 8:1219–1232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Do-Umehara H, Chen C, Urich D, Zhou L, Qiu
J, Jang S, Zander A, Baker MA, Eilers M, Sporn PH, et al:
Suppression of inflammation and acute lung injury by Miz1 via
repression of C/EBP-δ. Nat Immunol. 14:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vlaar AP and Juffermans NP:
Transfusion-related acute lung injury: A clinical review. Lancet.
382:984–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Needham DM, Dinglas VD, Morris PE, Jackson
JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW,
Rice TW, et al: Physical and cognitive performance of patients with
acute lung injury 1 year after initial trophic versus full enteral
feeding. EDEN trial follow-up. Am J Respir Crit Care Med.
188:567–576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Witwer KW and Halushka MK: Toward the
promise of microRNAs-enhancing reproducibility and rigor in
microRNA research. RNA Biol. 13:1103–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rezaei S, Mahjoubin-Tehran M,
Aghaee-Bakhtiari SH, Jalili A, Movahedpour A, Khan H, Moghoofei M,
Shojaei Z R, Hamblin M and Mirzaei H: Autophagy-related MicroRNAs
in chronic lung diseases and lung cancer. Crit Rev Oncol Hematol.
153:1030632020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cushing L, Jiang Z, Kuang P and Lü J: The
roles of microRNAs and protein components of the microRNA pathway
in lung development and diseases. Am J Respir Cell Mol Biol.
52:397–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Z, Xuan W, Wang H, Sun F, Zhang C,
Gong Q and Ge S: miR-200b regulates cellular senescence and
inflammatory responses by targeting ZEB2 in pulmonary emphysema.
Artif Cells Nanomed Biotechnol. 48:656–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang Z, Xu J, Ma Z, Li G and Zhu W:
miR-187 suppresses non-small-cell lung cancer cell proliferation by
targeting FGF9. Bioengineered. 11:70–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi S and Li H: Overexpressed microRNA-140
inhibits pulmonary fibrosis in interstitial lung disease via the
Wnt signaling pathway by downregulating osteoglycin. Am J Physiol
Cell Physiol. 319:C895–C905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Huang L, Zhu G, Pei Z and Zhang
W: Downregulated microRNA-27b attenuates lipopolysaccharide-induced
acute lung injury via activation of NF-E2-related factor 2 and
inhibition of nuclear factor κB signaling pathway. J Cell Physiol.
234:6023–6032. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CT, Huang Y, Pei ZY, Xi X and Zhu GF:
MicroRNA-326 aggravates acute lung injury in septic shock by
mediating the NF-κB signaling pathway. Int J Biochem Cell Biol.
101:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng L, Tang X, Lu M, Sun S, Xie S, Cai J
and Zan J: microRNA-421-3p prevents inflammatory response in
cerebral ischemia/reperfusion injury through targeting m6A reader
YTHDF1 to inhibit p65 mRNA translation. Int Immunopharmacol.
88:1069372020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang F, Zhou HY, Zhou LF, Wen YH, Gai HH
and Wu GM: MicroRNA-421 promotes inflammatory response of
fibroblast-like synoviocytes in rheumatoid arthritis by targeting
SPRY1. Eur Rev Med Pharmacol Sci. 23:8186–8193. 2019.PubMed/NCBI
|
|
18
|
Yuan HS, Xiong DQ, Huang F, Cui J and Luo
H: MicroRNA-421 inhibition alleviates bronchopulmonary dysplasia in
a mouse model via targeting Fgf10. J Cell Biochem. 120:16876–16887.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee MR, Kim JE, Park JJ, Choi JY, Song BR,
Choi YW, Kim DS, Kim KM, Song HK and Hwang DY: Protective role of
fermented mulberry leave extract in LPS-induced inflammation and
autophagy of RAW264.7 macrophage cells. Mol Med Rep. 22:4685–4695.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu D, Zhang H, Wu Q, Li F, Wang Y, Liu S
and Wang J: Sestrin 2 protects against LPS-induced acute lung
injury by inducing mitophagy in alveolar macrophages. Life Sci.
267:1189412020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang L, Gao XH, Zhao B, Luo JR, Shi XY, Ge
R, Ban SR and Li QS: Design and synthesis of new disubstituted
benzoxazolone derivatives that act as iNOS inhibitors with potent
anti-inflammatory activity against LPS-induced acute lung injury
(ALI). Bioorg Med Chem. 28:1157332020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li G, Dai Y, Tan J, Zou J, Nie X, Yang Z,
Zhao J, Yang X and Chen J: SB203580 protects against inflammatory
response and lung injury in a mouse model of
lipopolysaccharide-induced acute lung injury. Mol Med Rep.
22:1656–1662. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shaikh SB and Prabhakar Bhandary Y: Effect
of curcumin on IL-17A mediated pulmonary AMPK
kinase/cyclooxygenase-2 expressions via activation of NFκB in
bleomycin-induced acute lung injury in vivo. Int Immunopharmacol.
85:1066762020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang HH, Duan JX, Liu SK, Xiong JB, Guan
XX, Zhong WJ, Sun CC, Zhang CY, Luo XQ, Zhang YF, et al: A
COX-2/sEH dual inhibitor PTUPB alleviates
lipopolysaccharide-induced acute lung injury in mice by inhibiting
NLRP3 inflammasome activation. Theranostics. 10:4749–4761. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Butt Y, Kurdowska A and Allen T: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng JC and Standiford TJ: Growth factors
and cytokines in acute lung injury. Compr Physiol. 1:81–104.
2011.PubMed/NCBI
|
|
28
|
Li X, Ye C, Mulati M, Sun L and Qian F:
Ellipticine blocks synergistic effects of IL-17A and TNF-α in
epithelial cells and alleviates severe acute
pancreatitis-associated acute lung injury. Biochem Pharmacol.
177:1139922020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Yan J, Xu X, Duan C, Xie Z, Su Z,
Ma H, Ma H, Wei X and Du X: Puerarin prevents LPS-induced acute
lung injury via inhibiting inflammatory response. Microb Pathog.
118:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Y, Luo J, Yang N, Wang S, Ye M and
Pan G: Activation of the IL-1β/KLF2/HSPH1 pathway promotes STAT3
phosphorylation in alveolar macrophages during LPS-induced acute
lung injury. Biosci Rep. 40:BSR201935722020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nosaka N, Martinon D, Moreira D, Crother
TR, Arditi M and Shimada K: Autophagy protects against developing
increased lung permeability and hypoxemia by down regulating
inflammasome activity and IL-1β in LPS plus mechanical
ventilation-induced acute lung injury. Front Immunol. 11:2072020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang YN, Bian LQ, Ling XD, Fang CY and
Jiang SL: MicroRNA-421 promotes proliferation and invasion of
non-small cell lung cancer cells through targeting PDCD4. Pathol
Res Pract. 215:1525552019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JW, Wei L, Han Z and Chen Z:
Mesenchymal stromal cells-derived exosomes alleviate
ischemia/reperfusion injury in mouse lung by transporting
anti-apoptotic miR-21-5p. Eur J Pharmacol. 852:68–76. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Harbi NO, Imam F, Al-Harbi MM, Ansari
MA, Zoheir KM, Korashy HM, Sayed-Ahmed MM, Attia SM, Shabanah OA
and Ahmad SF: Dexamethasone attenuates LPS-induced acute lung
injury through inhibition of NF-κB, COX-2, and pro-inflammatory
mediators. Immunol Invest. 45:349–369. 2016. View Article : Google Scholar : PubMed/NCBI
|