Introduction
Parkinson's disease (PD) is one of the most frequent
degenerative disorders of the nervous system, and is characterized
by the large-scale loss of dopaminergic neurons of the substantia
nigra pars compacta, as well as the gradual accumulation of
intracellular α-synuclein (1). In
general, PD occurs frequently amongst the elderly; however,
previous studies have shown that PD is occurring earlier compared
with during past decades (2–4). It
has been determined that ~18 new PD cases will occur per year in a
population of 1,010,000 (5).
Abnormal nerve signals caused by PD lead to a range of movement
impairments and cognitive deficits, thereby greatly reducing the
quality of life of patients with PD and significantly increasing
the medical and economic burden on society (6). Although current treatments have made
progress in relieving PD symptoms, the effects on inhibiting PD
development remain disappointing (7). It is therefore essential to conduct
studies aimed at understanding the etiology of PD and developing
more effective therapeutic options for patients with PD.
Circular RNAs (circRNAs/circs) comprise one of the
most common subtypes of non-coding RNAs, which are ubiquitously
present in mammal cells and characterized by loop structures
(8). Unlike classic linear RNA
molecules, circRNAs have no 5′-cap and 3′-poly A tail, which makes
circRNAs resistant to endonucleases (9). circRNAs can therefore exist in a
cellular environment for a relatively long time and may be used as
promising diagnostic markers for multiple human diseases including
breast cancer, colorectal cancer and hepatocellular carcinoma
(10,11). circRNAs play a key role in numerous
cellular processes, including apoptosis, autophagy, proliferation
and differentiation (10).
Moreover, aberrant circRNA expression profiles have been associated
with the initiation and progression of various human diseases,
especially cancer (12). In recent
years, circRNAs were reported to be responsible for the
pathogenesis of multiple acute neurodegenerative disorders,
including cerebral ischemia, stroke and epilepsy (13–15).
Its role in Alzheimer's disease (AD) has also been demonstrated by
accumulating evidence (16,17). However, the role of circRNAs has
rarely been studied in PD.
circ-sterile α motif domain containing 4A
(circSAMD4A; ID, hsa_circ_0004846) was originally reported by Zhao
and Jing (18) to facilitate
osteosarcoma cell proliferation by regulating the microRNA
(miRNA/miR)-1244/MDM2 proto-oncogene axis. The results from our
preliminary experiments revealed that circSAMD4A was markedly
increased in 1-methyl-4-phenylpyridinium (MPP+)-induced
SH-SY5Y cells, indicating that circSAMD4A may serve a role in the
pathogenesis of PD (data not shown). The present study therefore
aimed to characterize the role and mechanism of circSAMD4A in PD,
to further identify the pathogenesis of PD and to develop more
effective therapeutic targets for patients with PD.
Materials and methods
PD animal model
All animal experiments were conducted following
stated protocols from the Research Committee of Ningbo No. 6
Hospital and permission was obtained from the Animal Experiment
Center of the Institute of Radiation Medicine of the Chinese
Academy of Medical Sciences [permit no. SCXK(JING)2019-0010].
A total of 36 male C57BL/6J mice (10 weeks, 22±2 g)
were obtained from the Animal Experiment Center of the Institute of
Radiation Medicine of the Chinese Academy of Medical Sciences.
Animals were kept in separate cages at 23±2°C under controlled
humidity (45–65%) with free access to food and water under a 12-h
light/dark cycle, and were randomly divided into PD (n=30) and sham
groups (n=6). A PD animal model was established using an
intraperitoneal injection of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride
(MPTP-HCl; 30 mg/kg/day; Sigma-Aldrich; Merck KGaA) for 5
consecutive days as previously described (19). Sham animals were given an equivalent
volume of sterile saline. Mice were decapitated at 0, 1, 3, 5 and 7
days after the last injection, and the midbrain tissue was isolated
and stored at −80°C. The experiment was completed between January
2019 and December 2019.
For the exogenous delivery of circSAMD4A in PD
animals, all mice were divided into six groups (n=6) at random:
Negative control (NC) group (saline); MPTP-HCl group (MPTP group);
MPTP + NC short hairpin (sh)RNAs (shNC) group; MPTP + circSAMD4A
shRNAs (sh-circ) group; MPTP + sh-circ + NC miR-29c-3p inhibitor
(NC inhibitor) group; and the MPTP + sh-circ + miR-29c-3p inhibitor
group. The sh-circ and shNC were purchased from Promega
Corporation. The mice were anesthetized using 2% isoflurane
(20), then fixed in a stereotaxic
frame (Stoelting, Co.), which was followed by disinfecting the skin
near the brain with alcohol, cutting open the skull surface,
ascertaining the stereotaxic coordinates of dorsal hippocampus
(AP=−5.4 mm; ML=+1.7 mm; DV=−7.5 mm) and drilling a hole. Then
sh-circ, miR-29c-3p inhibitor or a matched control was
intraperitoneally injected into the hippocampus (0.3 µl per site)
via a tip needle (33 G) with a syringe (10 µl; Hamilton Company).
At 3 days after vector injection, the injections of MPTP-HCl or
sterile saline were conducted in the same manner, as
aforementioned.
During the course of the experiment, the mice were
monitored daily for their health and specific behaviors, including
reduced locomotor activity and abnormal postures and squeals.
Losing 20% of body weight was considered the humane end point for
euthanasia. At the end of the experiment, mice were put into the
euthanasia chamber, which was then filled with CO2 with
a displacement rate of 20% chamber volume/min. When the mice were
unconscious and stopped breathing, the CO2 flow was
maintained for 1 min. The death of the mice was confirmed by
cardiac arrest and no reaction to the toe pinch reflex (21). Then, cervical dislocation was
adopted to determine death.
Cell lines and establishment of a PD
cell model
SH-SY5Y and 293 cell lines were obtained from the
American Type Culture Collection, and were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% FBS and 100
U/ml penicillin/streptomycin in a humidified atmosphere with 5%
CO2 at 37°C. To establish the PD cell model, SH-SY5Y
cells were incubated with MPP+ (0.25, 0.5 or 1 mM) for
24 h at 37°C.
Cell transfection and luciferase
reporter (DLR) assay
miR-29c-3p mimics (including miR control, cat. no.
HMI0439, concentration: ≥106 TU/ml), miR-29c-3p
inhibitor (including miR-29c-3p inhibitor NC, cat. no. HSTUD0440,
concentration: ≥106 TU/ml) were purchased from
Sigma-Aldrich (Shanghai) Trading Co., Ltd., shNC (cat. no. C01001,
concentration: 108 TU/ml), sh-circ (cat. no. C03002,
concentration: 108 TU/ml), pcDNA empty vector (cat. no.
G01001, concentration: 108 TU/ml), and pcDNA-circSAMD4A
(cat. no. G04002, concentration: 108 TU/ml) were
purchased from Shanghai GenePharma Co., Ltd. The sequences of
miR-29c-3p mimics were: (5′-3′) sense UAGCACCAUUUGAAAUCGGUUA, and
antisense ACCGAUUUCAAAUGGUGCUAUU; mir-29c-3p inhibitor: (5′-3′)
UAACCGAUUUCAAAUGGUGCUA; sh-circ: (5′-3′) AGCACAAGTACAAGAATCATT;
miRNA mimic NC: (5′-3′) sense UUUGUACUACACAAAAGUACUG and (5′-3′)
antisense CAGUACUUUUGUGUAGUACAAA; miRNA inhibitor NC: (5′-3′)
CAGUACUUUUGUGUAGUACAAA. The cell transfection was performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's instructions for 48 h
at 37°C. Then, cells were treated with 1 mM MPP+
solution for 24 h at 37°C.
The binding site seed regions of miR-29c-3p and
3′-UTR of circSAMD4 were determined using TargetScan (release 7.2,
http://www.targetscan.org). A pmirGLO
vector (Guangzhou RiboBio Co., Ltd.) containing wild-type (WT) and
mutant (MUT) 3′-UTR of circSAMD4A was constructed according to the
predicted binding sites. Then, cells were co-transfected with the
circSAMD4A 3′-UTR-WT reporter vector or 3′-UTR-MUT reporter vector
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. Subsequently, a dual-reporter
luciferase assay system in a Tecan SpectraFluorPlus plate-reader
(Tecan Group, Ltd.) was employed to determine luciferase
activity.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNAs of midbrain tissues and SH-SY5Y cells
were isolated using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), followed by the evaluation of its quality and
concentration using a SMA 400 UV-vis spectrophotometer (Merinton
Instrument, Ltd.). RNA was subsequently transcribed into cDNA using
a RevertAid First Strand cDNA Synthesis kit (cat. no. K1622,
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. A SYBR Green PCR kit was used for
RT-qPCR on a CFX96 real-time PCR system (Bio-Rad Laboratories,
Inc.) with the thermocycling conditions: Initial denaturation at
95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec,
annealing at 61°C for 40 sec and elongation at 72°C for 40 sec.
Final extension step was at 72°C for 5 min. U6 was used as the
internal control of miR-29c-3p, and GAPDH was applied as the
internal control of mRNAs. U6 was used as the internal control of
miR-29c-3p, and GAPDH was applied as the internal control of mRNAs.
The expression levels of GAPDH or U6 were used to calculate the Cq
value based on the 2−ΔΔCq method (22). The primers sequences used in this
study appear in Table 1.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR assay. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR assay.
| Gene ID | Sequence
(5′-3′) |
|---|
| hsa-GAPDH | Forward:
TGTTCGTCATGGGTGTGAAC |
|
| Reverse:
ATGGCATGGACTGTGGTCAT |
| mmu-GAPDH | Forward:
AGGTCGGTGTGAACGGATTTG |
|
| Reverse:
GGGGTCGTTGATGGCAACA |
| mmu_circSAMD4A | Forward:
TATGTTGTGGATCCTGTTCGGCAAC |
|
| Reverse:
TGGTGGTAGACCAAGACTTGTGAT |
| hsa_circSAM4A | Forward:
GCTCCTGATGGTCACCTTGT |
|
| Reverse:
TCACCACTCCTGGTTCTTCC |
|
mmu/hsa-miR-29c-3p | RT:
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCAC |
|
|
CAGAGCCAACTAACCG |
|
| Forward:
TAGCACCATTTGAAATCGGTTA |
|
| Reverse:
GTGCAGGGTCCGAGGTATTC |
| mmu-U6 | RT:
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCAC |
|
|
CAGAGCCAACAAAAATATGG |
|
| Forward:
CGCAAGGATGACACGCAAAT |
|
| Reverse:
GTGCAGGGTCCGAGGTATTC |
| hsa-U6 | RT:
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAG |
|
|
AGCCAACAAAATATGG |
|
| Forward:
GCTTCGGCAGCACATATACT |
|
| Reverse:
GTGCAGGGTCCGAGGTATTC |
Western blot analysis
Total proteins of midbrain tissues and SH-SY5Y cells
were isolated using RIPA buffer (Beyotime Institute of
Biotechnology), and the concentration was estimated using a
Bradford Protein Assay kit (Beyotime Institute of Biotechnology).
Proteins (30 µg/lane) were loaded into and separated by 10%
SDS-PAGE, and then transferred onto PVDF membranes (EMD Millipore).
After blocking in 5% non-fat milk for 2 h at room temperature, the
membrane was incubated with primary antibodies at 4°C overnight.
The membrane was then incubated with a corresponding HRP-labeled
donkey anti-rabbit secondary antibody (1:3,000; cat. no. ab6802;
Abcam) for 1 h at room temperature. Finally, the blots were
visualized with an ECL kit (cat. no. ab133406; Abcam). GAPH was
used as an internal control. The primary antibodies included:
rabbit anti-caspase-3 (1:1,000, cat. no. ab49822; Abcam), rabbit
anti-Bcl2 (1:2,000; cat. no. ab196495; Abcam), rabbit anti-Bax
(1:3,000, cat. no. 2772; Cell Signaling Technology, Inc.), rabbit
anti-GAPDH (1:5,000; cat. no. ab125247; Abcam), LC3II (rabbit;
1:3,000; cat. no. ab229327; Abcam), Beclin (rabbit; 1:5,000; cat.
no. ab62557; Abcam), phosphorylated (p)-5′AMP-activated protein
kinase (AMPK; rabbit; 1:1,000; cat. no. ab23875; Abcam), AMPK
(rabbit; 1;5,000; cat. no. ab3760; Abcam), p-mTOR (rabbit;
1:10,000; cat. no. ab137133; Abcam) and mTOR (rabbit; 1:10,000;
cat. no. ab109268; Abcam). Subsequently, the membranes were
incubated with an anti-rabbit IgG secondary antibody (1:1,000, cat.
no. sc-2357; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. Protein were visualized using ChemiDoc XRS System
(Bio-Rad Laboratories, Inc.) equipped with a 12-bit digital camera
coupled to the Quantity One software (version 4.6.3; Bio-Rad
Laboratories, Inc.).
TUNEL assay
The midbrain tissue and treated SH-SY5Y cells were
fixed using 4% paraformaldehyde for 30 min at 25°C, then
dehydrated, paraffin-embedded and sectioned (5 µm). Slides were
covered with VECTASHIELD® mounting medium (Vector
Laboratories, Inc.) with DAPI. Subsequently, the slices were
incubated with proteinase K (10 µg/ml; Beyotime Institute of
Biotechnology) for 10 min at 37°C. The detection of
apoptosis-specific nuclear DNA fragmentation was conducted using a
Colorimetric TUNEL Apoptosis Assay kit (Beyotime Institute of
Biotechnology) for 1 h at 37 °C in the dark. The TUNEL-positive
cell nucleus was detected using an Axiovert 200 fluorescence
microscope (magnification, ×200; Olympus Corporation), and
TUNEL-positive cells were observed under fluorescent microcopy
(Olympus Corporation) and counted in six random fields.
Flow cytometry analysis
After being transfected with sh-circ, shNC,
miR-29c-3p NC or miR-29c-3p inhibitor, SH-SY5Y cells were collected
and washed three times with PBS. Cells were then resuspended in
annexin V binding buffer, and cell apoptosis was analyzed using an
annexin V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology). The cells were incubated with 5 µl Annexin V-FITC
and 10 µl PI for 20 min at 25°C in the dark. Cell apoptosis was
analyzed using a flow cytometer FACSCanto II (version 6.13; BD
Biosciences). The apoptotic rate (%) was calculated as the
percentage of the early + late apoptotic cells.
Luciferase reporter assay
The fragment of circSAMD4A containing the wild-type
(WT) or mutant (MUT) of the miR-29c-3p binding site was amplified
and inserted into a PGL3 luciferase vector (Promega Corporation),
to construct the luciferase reporter vectors, PGL3-circSAMD4A-WT
and PGL3-circSAMD4A-MUT. 293 cells were co-transfected with 100 ng
constructed luciferase reporter vector and 50 nM miR-29c-3p mimic
using Lipofectamine 3000 at 37°C for 48 h. After 48 h of
incubation, 293 cells were harvested to determine the luciferase
activity using a Dual-Glo Luciferase assay kit (Promega
Corporation). Relative luciferase activity of the 3′UTR reporter
constructs was normalized by Renilla activity.
Statistical analysis
All the experiments were performed independently
three times, and data are presented as the mean ± SD. The data were
analyzed using GraphPad Prism, version 7.0 software (GraphPad
Software, Inc.). Comparisons between two groups were analyzed using
paired Student's t-test. Comparisons between different groups were
analyzed using one-way ANOVA with Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
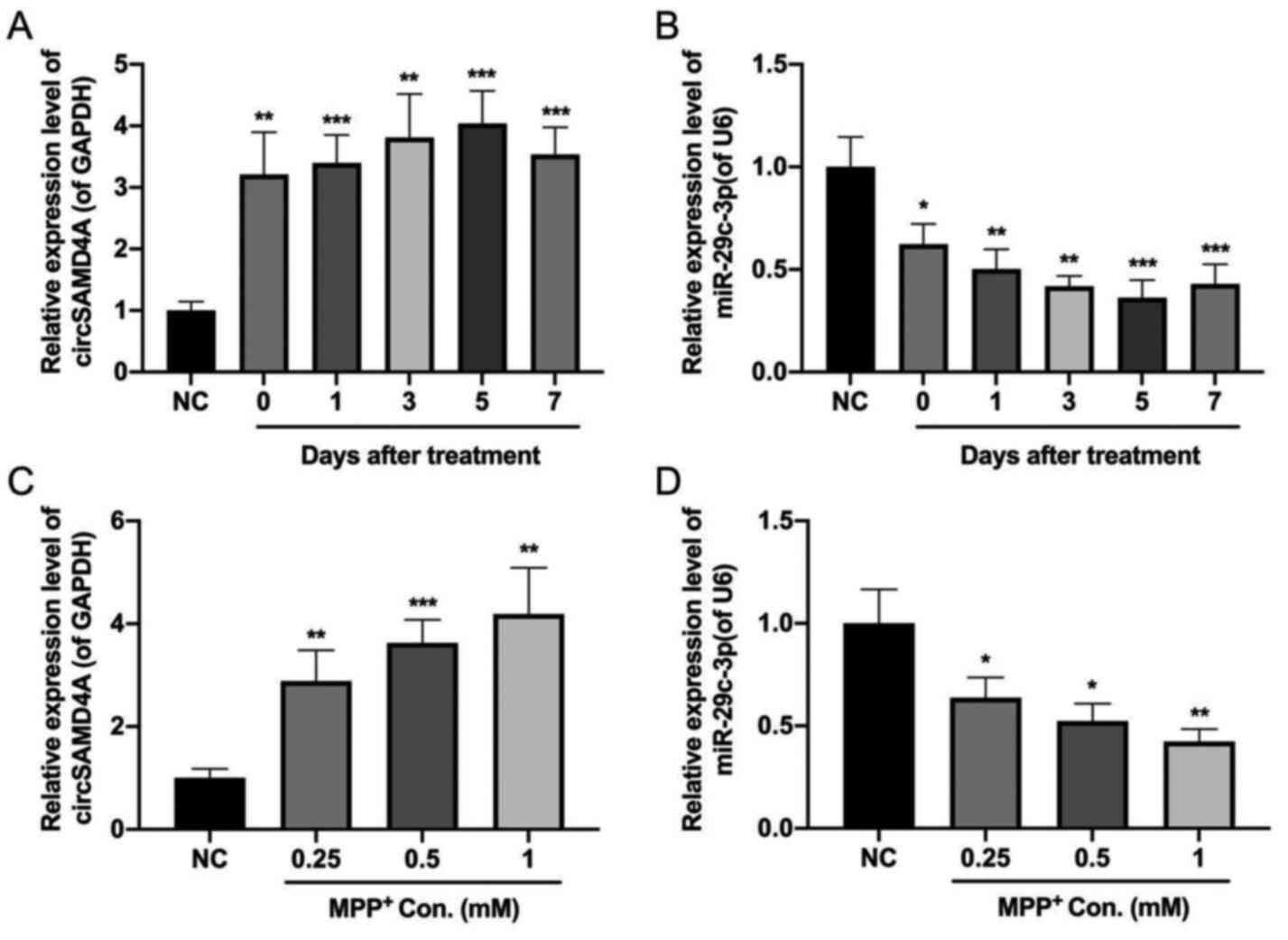
circSAMD4A and miR-29c-3p are
dysregulated in PD
To investigate whether circSAMD4A and miR-29c-3p
served a role during the pathogenesis of PD, their expression
levels were measured in both PD animals and cellular models using
RT-qPCR. The results indicated that the expression level of
circSAMD4A was upregulated, while miR-29c-3p was downregulated in
the PD animal model (Fig. 1A and
B). Consistently, MPP+ treatment increased
circSAMD4A expression but decreased miR-29c-3p expression in
SH-SY5Y cell lines (Fig. 1C and D).
Taken together, these results suggested that circSAMD4A and
miR-29c-3p served a role in the pathogenesis of PD.
circSAMD4A targets and negatively
regulates miR-29c-3p
To further study the relationship of circSAMD4A and
miR-29c-3p in the pathogenesis of PD, it was predicted whether
there was a complementary sequence between circSAMD4A and
miR-29c-3p using TargetScan software (release 7.2, http://www.targetscan.org). Fig. 2A (upper panel) shows the predicted
recognition of the miR-29c-3p sequence in circSAMD4A transcripts.
To verify the interaction between circSAMD4A and miR-29c-3p, the
transfection efficiency of miR-29c-3p mimics in 293 cells was
determined. The results demonstrated that miR-29c-3p expression was
significantly increased in miR-29c-3p mimics group compared with
that in miR-control group, suggesting the successful transfection
of miR-29c-3p mimics in 293 cells (Fig. S1A). Next, a dual-luciferase
reporter driven by WT-circSAMD4A or MUT-circSAMD4A was established
and co-transfected into 293 cells with miR-29c-3p mimic or the
miR-control. The results indicated that the luciferase activity of
WT-circSAMD4A was decreased in miR-29c-3p-overexpressing 293 cells,
while there was no effect of miR-29c-3p transfection on the
luciferase activities of MUT-circSAMD4A (Fig. 2A; lower panel).
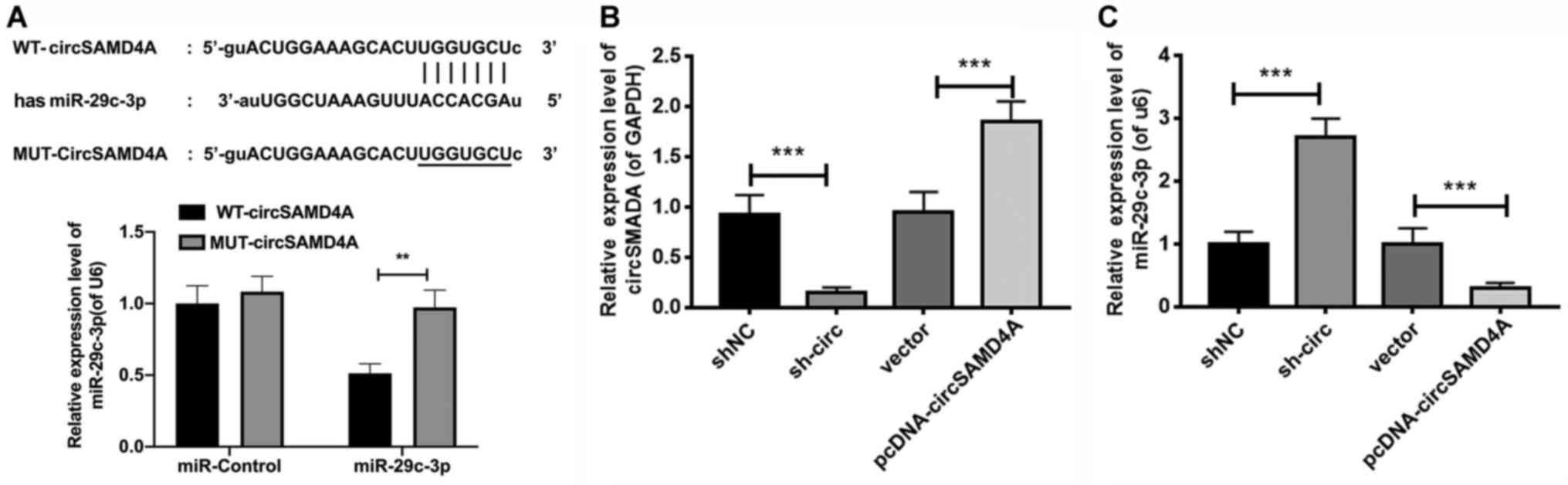 | Figure 2.circSAMD4A targets and negatively
regulates miR-29c-3p. (A) Upper panel, TargetScan analysis showing
the putative recognition sequence of miR-29c-3p in circSAMD4A.
Lower panel, a dual-luciferase reporter was used to verify the
interaction between circSAMD4A and miR-29c-3p in 293 cells. The
relative expression levels of (B) circSAMD4A and (C) miR-29c-3p
were detected in SH-SY5Y cells transfected with sh-circ,
pcDNA-circSAMD4A or matched controls via reverse
transcription-quantitative PCR. **P<0.01, ***P<0.001.
sh-circ, sh-circSAMD4A; miR, microRNA; circSAMD4A, circular RNA
sterile α motif domain containing 4A; WT, wild-type; MUT, mutant;
sh, short hairpin RNA; NC, negative control. |
Next, circSAMD4A was knocked down and overexpressed
in SH-SY5Y cells via transfection, and the results demonstrated
that circSAMD4A was significantly downregulated in the sh-circ
group compared with the shNC group; while it was upregulated in
pcDNA-circSAMD4A group compared with the vector group, suggesting
the successful transfection of circSAMD4A shRNAs and
circSAMD4A-overexpressed plasmid (Fig.
2B). Moreover, miR-29c-3p expression was measured using RT-qPCR
in sh-circ or pcDNA-circSAMD4A transfected cells, and it was found
that the expression level of miR-29c-3p was significantly increased
by sh-circ, while circSAMD4A overexpression inhibited miR-29c-3p
expression in SH-SY5Y cells (Fig.
2C). Together, the results demonstrated that circSAMD4A
directly targeted miR-29c-3p to inhibit its expression.
A miR-29c-3p inhibitor abrogates the
protective effects of circSAMD4A knockdown against MPTP, and causes
cell apoptosis in the PD animal model
After the interaction between circSAMD4A and
miR-29c-3p was confirmed, the effect of circSAMD4A and miR-29c-3p
on MPTP-induced apoptosis of dopaminergic neurons in PD mice was
examined. The sh-circ, shNC, miR-29c-3p NC or miR-29c-3p inhibitor
was intraperitoneally injected into the midbrain of mice. The
RT-qPCR results indicated that sh-circ transfection resulted in
downregulation of circSAMD4A but in the upregulation of miR-29c-3p
(Fig. 3A). Moreover, it was found
that co-transfection of sh-circ and miR-29c-3p inhibitor reversed
the sh-circSAMD4A-induced upregulation of miR-29c-3p (Fig. 3A). In addition, the level of
circSAMD4A was inhibited after co-transfection (Fig. 3A). The transfection effect of
miR-29c-3p inhibitor was verified in PD mice (Fig. S1B).
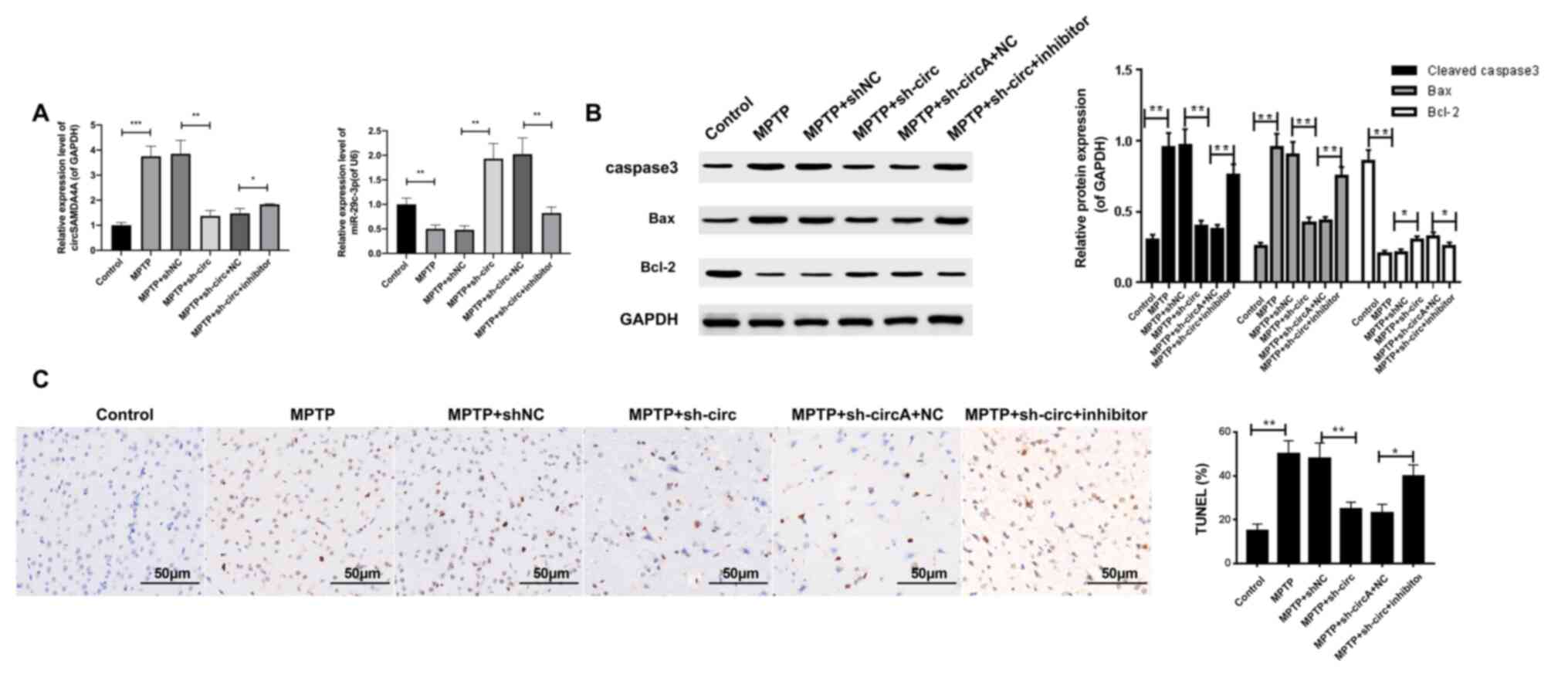 | Figure 3.A miR-29c-3p inhibitor abrogates the
protective effects of sh-circ against MPTP-induced cell apoptosis.
(A) Expression levels of circSAMD4A and miR-29c-3p in sh-circ,
shNC, miR-29c-3p NC or miR-29c-3p inhibitor-injected MPTP-induced
mice were measured via reverse transcription-quantitative PCR. (B)
Western blotting was used to determine protein expression levels of
caspase 3, Bcl2 and Bax in transfected mice. GAPDH or U6 were used
as an internal control. (C) Apoptotic cells in treated mice were
analyzed using the TUNEL assay. *P<0.05, **P<0.01,
***P<0.001. sh-circ, sh-circSAMD4A; miR, microRNA; circSAMD4A,
circular RNA sterile α motif domain containing 4A; sh, short
hairpin RNA; NC, negative control; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. |
Subsequently, the protein expression levels of
caspase 3, Bcl2 and Bax were determined using western blotting.
Compared with the control group, MPTP treatment significantly
increased the expression levels of caspase-3 and Bcl2, and
decreased the expression level of Bax, while sh-circ transfection
reversed the dysregulation of caspase-3, Bcl2 and Bax expression
induced by MPTP (Fig. 3B),
suggesting that sh-circ protected cells from MPTP-induced
apoptosis. However, co-transfection of sh-circ and miR-29c-3p
inhibitor abolished the protective effects of sh-circ against
MPTP-induced cell apoptosis (Fig.
3B). In a similar manner, the TUNEL assay demonstrated that
circSAMD4A knockdown significantly reduced MPTP-induced apoptosis
of DA neurons, and the miR-29c-3p inhibitor abrogated the
protective effect of circSAMD4A knockdown in the PD mice model
(Fig. 3C). Collectively, these
results indicated that the miR-29c-3p inhibitor abrogated the
protective effect on cell apoptosis caused by circSAMD4A knockdown
in the MPTP-induced PD animal model.
Knockdown of circSAMD4A exerts a
protective effect on MPP+-induced SH-SY5Y cell apoptosis
by regulating miR-29c-3p
After characterizing the role of circSAMD4A and
miR-29c-3p in the PD animal model, the effects of the transfection
of sh-circ or miR-29c-3p inhibitor on apoptosis were examined in
the PD cell model. Moreover, the expression levels of circSAMD4A
and miR-29c-3p in sh-circ- or miR-29c-3p-transfected SH-SY5Y cells
in the presence of MPP+ were determined. The results
showed that sh-circ transfection abolished the upregulation of
circSAMD4A and downregulation of miR-29c-3p induced by
MPP+ in SH-SY5Y cells. Moreover, this phenomenon was
abrogated by the co-transfection of sh-circ and miR-29c-3p
inhibitor (Fig. 4A).
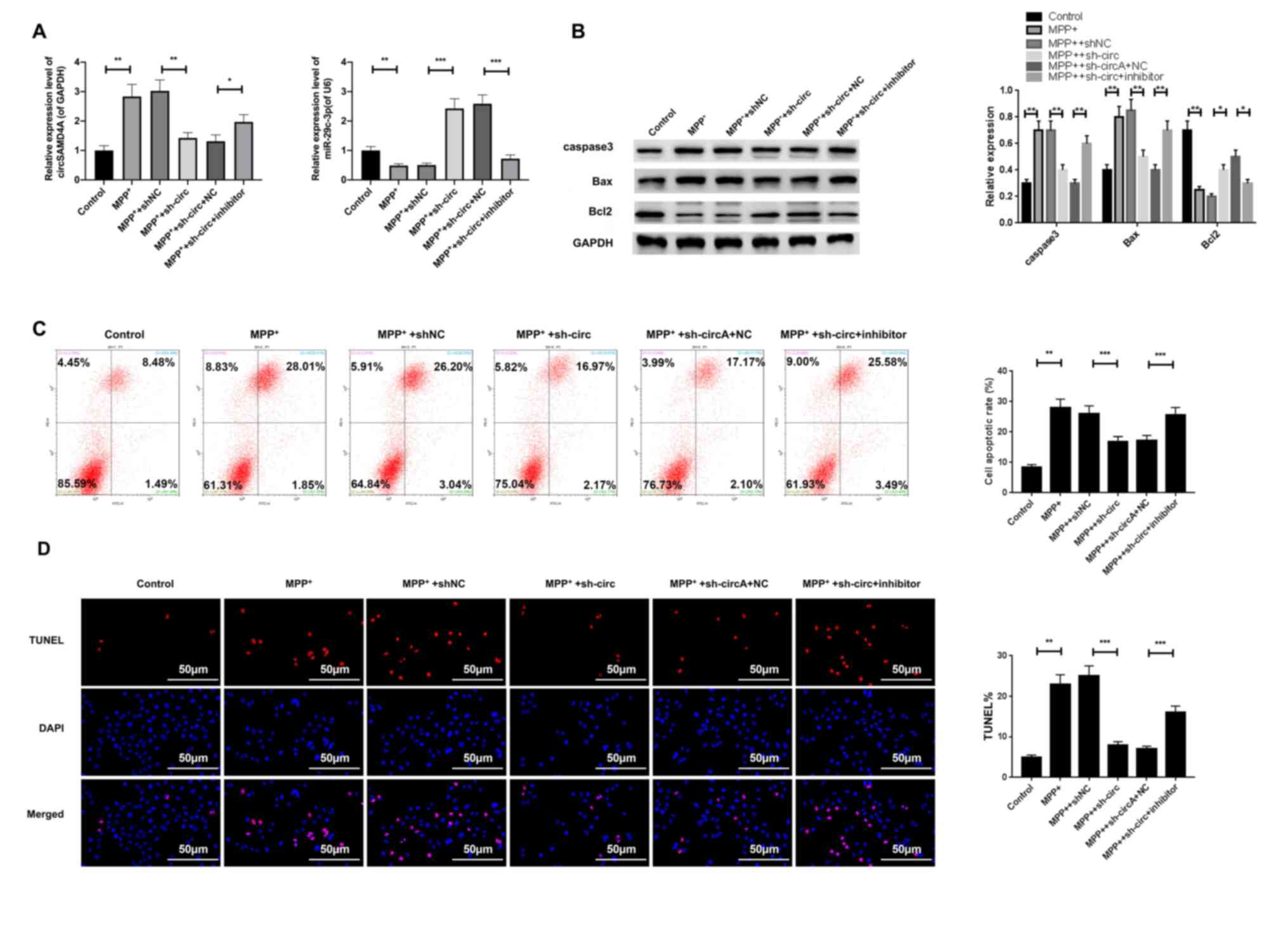 | Figure 4.A miR-29c-3p inhibitor abrogates the
protective effects of sh-circ against MPP+-induced
SH-SY5Y cell apoptosis. (A) Expression levels of circSAMD4A and
miR-29c-3p were measured via reverse transcription-quantitative PCR
SH-SY5Y cells treated with sh-circ, shNC, miR-29c-3p NC or
miR-29c-3p inhibitor. (B) Western blotting was used to determine
protein expression levels of caspase-3, Bcl2 and Bax in
MPP+-induced SH-SY5Y cells treated with sh-circ, shNC,
miR-29c-3p NC or miR-29c-3p inhibitor. GAPDH or U6 were used as an
internal control. (C) Flow cytometry analysis and (D) TUNEL
staining were performed to assess the effects of circSAMD4A
knockdown and miR-29c-3p inhibitor on MPP+-induced
apoptosis in SH-SY5Y cells. The apoptotic rate (%) was calculated
as the percentage of the early and late apoptotic cells.
*P<0.05, **P<0.01, ***P<0.001. sh-circ, sh-circSAMD4A;
miR, microRNA; circSAMD4A, circular RNA sterile α motif domain
containing 4A; sh, short hairpin RNA; NC, negative control;
MPP+, 1-methyl-4-phenylpyridinium. |
The western blot analysis of apoptosis-related
proteins demonstrated that sh-circ transfection reversed the
upregulation of caspase-3 and Bcl2 and downregulation of Bax
induced by MPP+ in SH-SY5Y cells; however, these effects
of sh-circ were abolished by co-transfection of sh-circ and the
miR-29c-3p inhibitor (Fig. 4B).
Furthermore, flow cytometry analysis identified that circSAMD4A
knockdown significantly repressed MPP+-induced SH-SY5Y
cell apoptosis; however, co-transfection with the miR-29c-3p
inhibitor abrogated this effect (Fig.
4C). Consistent results were obtained from TUNEL staining in
SH-SY5Y cells transfected with sh-circ or the miR-29c-3p inhibitor
(Fig. 4D). Thus, it was suggested
that the miR-29c-3p inhibitor abrogated the protective effect of
circSAMD4A knockdown against apoptosis in the
MPP+-induced PD cell model.
The inhibitory effect of knockdown of
circSAMD4A on autophagy is repressed by miR-29c-3p in both PD
animals and cellular models
To determine whether the dysregulation of circSAMD4A
and miR-29c-3p was associated with cell autophagy, the
autophagy-related proteins were assessed in both the PD model
animal and cells using western blotting. The results indicated that
MPTP treatment activated in vivo autophagy as shown by the
upregulation of LC3II and Beclin in the midbrain tissues of mice
(Fig. 5A). It was found that
knockdown of circSAMD4A inhibited the expression levels of LC3II
and Beclin in the presence of MPTP compared with the shNC group;
however, the miR-29c-3p inhibitor abolished this effect (Fig. 5A). Consistently, MPP+
also activated in vitro autophagy as shown by the
upregulation of LC3II and Beclin in SH-SY5Y cells (Fig. 5B). Knockdown of circSAMD4A
significantly decreased the expression levels of LC3II and Beclin
in the presence of MPP+ in SH-SY5Y cells compared with
the shNC group; however, the miR-29c-3p inhibitor abolished this
effect (Fig. 5B). Taken together,
these results indicated that the miR-29c-3p inhibitor abrogated the
repressive effects of sh-circ on autophagy in both PD animal and
cellular models.
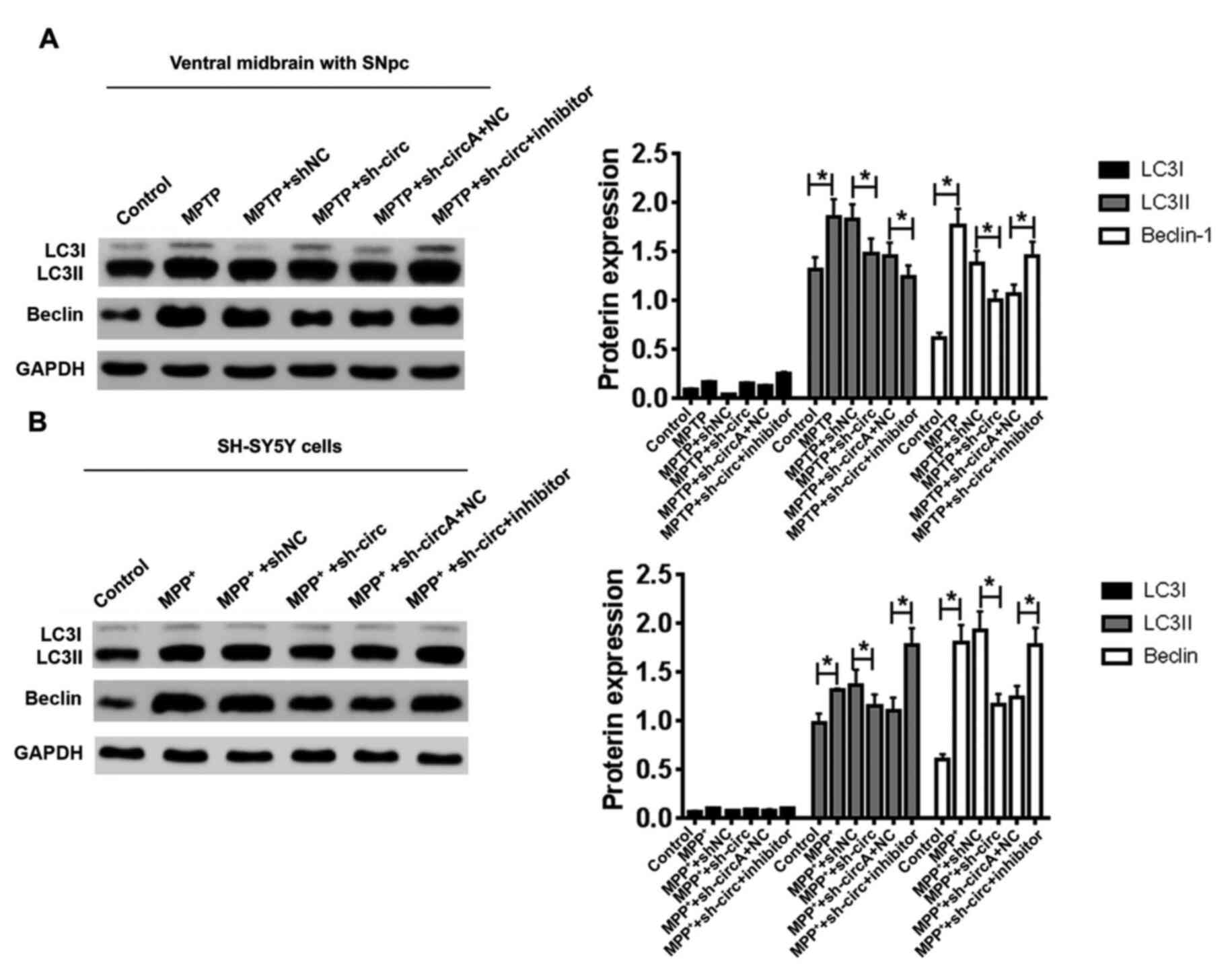 | Figure 5.A miR-29c-3p inhibitor abrogates the
repressive effects of sh-circ on autophagy. Western blotting was
used to determine protein expressions of LC3II and Beclin in
transfected (A) mice and (B) SH-SY5Y cells. GAPDH was used as an
internal control. *P<0.05. sh-circ, sh-circSAMD4A; miR,
microRNA; circSAMD4A, circular RNA sterile α motif domain
containing 4A; sh, short hairpin RNA; NC, negative control;
MPP+, 1-methyl-4-phenylpyridinium; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc, substantia
nigra pars compacta. |
A miR-29c-3p inhibitor abrogates the
regulatory effects of circSAMD4A knockdown on the AMPK/mTOR pathway
in vitro and in vivo
To determine the downstream molecular mechanism of
the circSAMD4A/miR-29c-3p axis, the AMPK/mTOR signaling cascade,
which has been reported to be engaged in autophagy (23), was investigated. Western blotting
results demonstrated that MPTP treatment resulted in increased
expression level of p-AMPK and decreased expression level of p-mTOR
in the midbrain tissues of mice (Fig.
6A). Knockdown of circSAMD4A reversed the upregulation of
p-AMPK and downregulation of p-mTOR induced by MPTP in vivo,
and the miR-29c-3p inhibitor abolished the effect of circSAMD4A
knockdown (Fig. 6A). Consistently,
MPP+ treatment significantly increased the expression
level of p-AMPK and decreased the expression level of p-mTOR in
SH-SY5Y cells (Fig. 6B). Knockdown
of circSAMD4A reversed the upregulation of p-AMPK and
downregulation of p-mTOR induced by MPP+ in
vitro, whereas the miR-29c-3p inhibitor abolished this effect
(Fig. 6B). Overall, these results
indicated that the miR-29c-3p inhibitor abrogated the regulatory
effects of sh-circ on the AMPK/mTOR pathway in vitro and
in vivo.
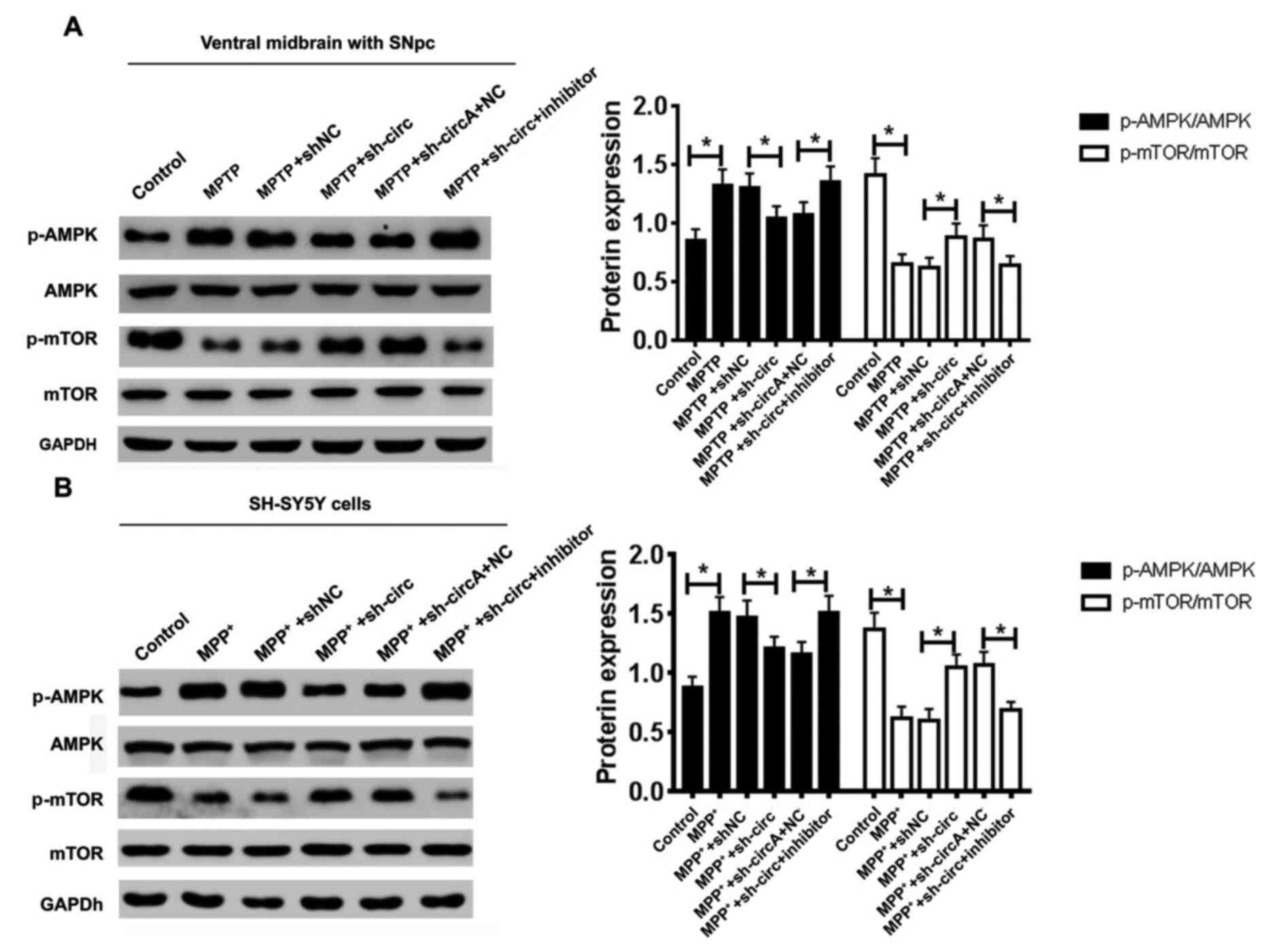 | Figure 6.A miR-29c-3p inhibitor abrogates the
regulatory effects of sh-circ on the AMPK/mTOR pathway. Effects of
circSAMD4A knockdown and miR-29c-3p inhibitor on the protein
expression levels of p-AMPK, AMPK, p-mTOR and mTOR were determined
via western blotting in both a (A) PD animal model and (B) PD
cellular model. *P<0.05. sh-circ, sh-circSAMD4A; miR, microRNA;
circSAMD4A, circular RNA sterile α motif domain containing 4A; sh,
short hairpin RNA; NC, negative control; MPP+,
1-methyl-4-phenylpyridinium; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc, substantia
nigra pars compacta; p-, phosphorylated; AMPK, 5′AMP-activated
protein kinase; PD, Parkinson's' disease. |
Discussion
As the most common type of chronic neurodegenerative
disorder, PD currently affects ~3% of the global population that
are >60 years of age (24,25).
Despite decades of extensive studies, the pathogenesis of PD
remains largely unknown. Reduced cell viability, increased cell
apoptosis and deficient autophagy flux of neurocytes have been
considered to play critical roles in PD pathogenesis (26–28).
To further investigate the underlying pathogenesis of PD, the
present study first constructed PD mouse and cellular models. By
comparing the application range of C57BL6J and C57BL6 mice, it was
discovered that C57BL6 mice are mainly used in tumor, immune,
genetic and physiological research, while C57BL6J mice have been
used in cardiovascular and cerebrovascular diseases, nervous system
diseases, metabolic and developmental diseases, and immune and
genetic diseases (29). PD is a
nervous system disease. Moreover, the present study consulted the
relevant data on the preparation of PD mouse models, and found that
C57BL6J mice were one of the main mouse varieties used for PD model
establishment (30–35). Therefore, in the current study, the
PD mouse model was constructed using C57BL/6J mice. Furthermore,
the PD cell model was created using SH-SY5Y cells, based on
previous studies (36,37).
Previous studies have shown that circRNAs can
regulate synaptic gene expression, cognition ability and memory
storage (38,39). Recently, circ-DLG associated protein
4 has been reported to exhibit neuroprotective effects on PD by
modulating cAMP responsive element binding protein 1 via miR-134-5p
(40). Moreover, in a transgenic
C. elegans PD model, Kumar et al (41) revealed that knockdown of circular
ZIP-2 decreased the aggregation of α-synuclein protein and
increased the lifespan by regulating the Daf-16 cascade via miR-60.
In the present study, it was found that circSAMD4A expression was
increased in the MPTP-induced PD animal model and the
MPP+-induced SH-SY5Y cell model. In vitro and
in vivo experiments also revealed that knockdown of
circSAMD4A repressed apoptosis and autophagy, and thereby
attenuated the cellular toxicity of MPTP or MPP+ toward
dopamine neurons or SH-SY5Y cells. Overall, these findings
suggested that circSAMD4A may be a useful diagnostic biomarker and
therapeutic target for PD.
Previous studies have reported that circRNAs can
serve as miRNA sponges to regulate the expression of specific genes
(42). In addition, miRNAs have
been shown to play a role during PD pathogenesis (43). For example, miR-7 can directly
inhibit the expression of α-synuclein, whose aggregation is a
specific PD marker (44). ciRS-7,
one of the most well-studied circRNAs in the central nervous
system, has been found to be closely associated with the
pathogenesis of AD (45). Recently,
miR-7 transfection was used to induce more efficient α-synuclein
inhibition in a HeLa cell line without ciRS-7 expression,
suggesting that ciRS-7 may regulate α-synuclein expression in a
miR-7-dependent manner, which correlated with the pathogenesis of
PD (46). Additionally, circSNCA
RNA has been shown to suppress autophagy and promote apoptosis in
SH-SY5Y cells by facilitating the expression of α-synuclein mRNA
via sponging miR-7 (47). In the
present study, miR-29c-3p was found to be targeted and regulated by
circSAMD4A. Although miR-29c-3p has been reported to participate in
the pathogenesis of multiple human diseases, such as colorectal
cancer, ovarian cancer and AD (48–50),
to the best of our knowledge, its role in PD has not been
previously reported. In the current study, miR-29c-3p was
identified to abolish the protective effects of circSAMD4A
knockdown against MPTP- or MPP+-induced apoptosis and
autophagy.
AMPK is a key cell receptor that is responsible for
monitoring alterations in energy regulation, while mTOR is a vital
signaling complex involved in numerous cellular processes (23). Both AMPK and mTOR have been reported
to be associated with the regulation of apoptosis and autophagy
during tumor progression (51,52).
Moreover, miR-124 was found to protect dopaminergic neurons by
modulating apoptosis and autophagy via the regulation of the
AMPK/mTOR cascade in PD (53).
Subsequently, the long non-coding RNA metastasis associated lung
adenocarcinoma transcript 1 was revealed to contribute to cellular
apoptosis in PD by targeting miR-124 (19). The present study demonstrated that
knockdown of circSAMD4A significantly reversed the upregulation of
p-AMPK and downregulation of p-mTOR in an MPTP-induced animal PD
model and MPP+-induced PD cell PD, which was abolished
by transfection with a miR-29c-3p inhibitor.
There were some limitations to the present study.
First, the positive results assessing the function of circRNAs in
PD were limited, Second, there was a lack of clinical studies to
verify the results. Therefore, further studies will be needed.
In conclusion, the present study indicated that
circSAMD4A participated in apoptosis and autophagy of dopaminergic
neurons by regulating the AMPK/mTOR pathway via miR-29c-3p in PD.
The current findings may nonetheless contribute to the development
of effective candidate drugs for PD treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WSW participated in the study design and manuscript
writing. RXL performed experiments, analyzed data and participated
in manuscript writing. JJZ participated in statistical analysis and
manuscript writing. YL performed experiments and analyzed data. WSW
and YL confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments followed stated protocols
from the Research Committee of Ningbo No. 6 Hospital and permission
was obtained from the Animal Experiment Center of the Institute of
Radiation Medicine of the Chinese Academy of Medical Sciences
[permit no. SCXK(JING)2019-0010].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
circRNAs/circs
|
circular RNAs
|
|
PD
|
Parkinson's disease
|
|
MPTP-HCl
|
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
AMPK
|
5′AMP-activated protein kinase
|
|
miR
|
microRNA
|
References
|
1
|
Beitz JM: Parkinson's disease: A review.
Front Biosci (Schol Ed). 6:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tysnes OB and Storstein A: Epidemiology of
Parkinson's disease. J Neural Transm (Vienna). 124:901–905. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schrag A, Hovris A, Morley D, Quinn N and
Jahanshahi M: Young-vs. older-onset Parkinson's disease: Impact of
disease and psychosocial consequences. Mov Disord. 18:1250–1256.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stern M, Dulaney E, Gruber SB, Golbe L,
Bergen M, Hurtig H, Gollomp S and Stolley P: The epidemiology of
Parkinson's disease: A case-control study of young-onset and
old-onset patients. Arch Neurol. 48:903–907. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elbaz A, Carcaillon L, Kab S and Moisan F:
Epidemiology of Parkinson's disease. Rev Neurol (Paris). 172:14–26.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider RB, Iourinets J and Richard IH:
Parkinson's disease psychosis: Presentation, diagnosis and
management. Neurodegener Dis Manag. 7:365–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orimo S: New development of diagnosis and
treatment for Parkinson's disease. Rinsho Shinkeigaku. 57:259–273.
2017.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salzman J: Circular RNA Expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-New member of noncoding RNA with novel functions. Exp Biol Med
(Maywood). 242:1136–1341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jahani S, Nazeri E, Majidzadeh AK, Jahani
M and Esmaeili R: Circular RNA; a new biomarker for breast cancer:
A systematic review. J Cell Physiol. 235:5501–5510. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: CircRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu F, Han B, Wu S, Yang L, Leng S, Li M,
Liao J, Wang G, Ye Q, Zhang Y, et al: Circular RNA TLK1
aggravates neuronal injury and neurological deficits after ischemic
stroke via miR-335-3p/TIPARP. J Neurosci. 39:7369–7393. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai Y, Zhang Y, Han B, Yang L, Chen X,
Huang R, Wu F, Chao J, Liu P, Hu G, et al: Circular RNA DLGAP4
ameliorates ischemic stroke outcomes by targeting mir-143 to
regulate endothelial-mesenchymal transition associated with
blood-brain barrier integrity. J Neurosci. 38:32–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong GH, An FM, Wang Y, Bian M, Wang D and
Wei CX: Comprehensive Circular RNA profiling reveals the regulatory
role of the CircRNA-0067835/miR-155 pathway in temporal lobe
epilepsy. Cell Physiol Biochem. 51:1399–1409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akhter R: Circular RNA and Alzheimer's
disease. Adv Exp Med Biol. 1087:239–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Wang H, Shang H, Chen X, Yang S,
Qu Y, Ding J and Li X: Circular RNA circ_0000950 promotes neuron
apoptosis, suppresses neurite outgrowth and elevates inflammatory
cytokines levels via directly sponging miR-103 in Alzheimer's
disease. Cell Cycle. 18:2197–2214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y and Jing Z: CircSAMD4A accelerates
cell proliferation of osteosarcoma by sponging miR-1244 and
regulating MDM2 mRNA expression. Biochem Biophys Res Commun.
516:102–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Zhang Q, Zhang J, Pan W, Zhao J and
Xu Y: Long non-coding RNA MALAT1 contributes to cell apoptosis by
sponging miR-124 in Parkinson disease. Cell Biosci. 7:192017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paschall AV and Liu K: An orthotopic mouse
model of spontaneous breast cancer metastasis. J Vis Exp.
540402016.PubMed/NCBI
|
|
21
|
Creamer-Hente MA, Lao FK, Dragos ZP and
Waterman LL: Sex- and Strain-related differences in the stress
response of Mice to CO2 euthanasia. J Am Assoc Lab Anim
Sci. 57:513–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirsch L, Jette N, Frolkis A, Steeves T
and Pringsheim T: The incidence of Parkinson's disease: A
systematic review and meta-analysis. Neuroepidemiology. 46:292–300.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shalash AS, Hamid E, Elrassas HH, Bedair
AS, Abushouk AI, Khamis M, Hashim M, Ahmed NS, Ashour S and
Elbalkimy M: Non-motor symptoms as predictors of quality of life in
egyptian patients with parkinson's disease: A cross-sectional study
using a culturally adapted 39-item parkinson's disease
questionnaire. Front Neurol. 9:3572018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui B, Guo X, You Y and Fu R: Farrerol
attenuates MPP+ -induced inflammatory response by TLR4 signaling in
a microglia cell line. Phytother Res. 33:1134–1141. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malley KR, Koroleva O, Miller I,
Sanishvili R, Jenkins CM, Gross RW and Korolev S: The structure of
iPLA2 β reveals dimeric active sites and suggests
mechanisms of regulation and localization. Nat Commun. 9:7652018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suresh SN, Chavalmane AK, Dj V,
Yarreiphang H, Rai S, Paul A, Clement JP, Alladi PA and Manjithaya
R: A novel autophagy modulator 6-Bio ameliorates SNCA/α-synuclein
toxicity. Autophagy. 13:1221–1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanduzzi Zamparelli M, Compare D, Coccoli
P, Rocco A, Nardone OM, Marrone G, Gasbarrini A, Grieco A, Nardone
G and Miele L: The metabolic role of gut microbiota in the
development of nonalcoholic fatty liver disease and cardiovascular
disease. Int J Mol Sci. 17:12252016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramalingam M, Huh YJ and Lee YI: The
impairments of α-synuclein and mechanistic target of rapamycin in
rotenone-induced SH-SY5Y cells and mice model of Parkinson's
disease. Front Neurosci. 13:10282019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pupyshev AB, Tikhonova MA, Akopyan AA,
Tenditnik MV, Dubrovina NI and Korolenko TA: Therapeutic activation
of autophagy by combined treatment with rapamycin and trehalose in
a mouse MPTP-induced model of Parkinson's disease. Pharmacol
Biochem Behav. 177:1–11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ge G, Chen C, Guderyon MJ, Liu J, He Z, Yu
Y, Clark RA and Li S: Regulatable lentiviral hematopoietic stem
cell gene therapy in a mouse model of Parkinson's disease. Stem
Cells Dev. 27:995–1005. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Campos PS, Kawamura L, Hasegawa K,
Kumei Y and Zeredo JL: Analysis of respiratory movements in a mouse
model of late Parkinson's disease submitted to stress. Respir
Physiol Neurobiol. 251:50–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugumar M, Sevanan M and Sekar S:
Neuroprotective effect of naringenin against MPTP-induced oxidative
stress. Int J Neurosci. 129:534–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Zhang Y, Li R, Zhu L, Fu B and
Yan T: Salidroside ameliorates Parkinson's disease by inhibiting
NLRP3-dependent pyroptosis. Aging (Albany NY). 12:9405–9426. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang
W, Wang W, Sun Y and Zhang H: Curcumin protects an SH-SY5Y cell
model of parkinson's disease against toxic injury by regulating
HSP90. Cell Physiol Biochem. 51:681–691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xicoy H, Wieringa B and Martens GJ: The
SH-SY5Y cell line in Parkinson's disease research: A systematic
review. Mol Neurodegener. 12:102017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zimmerman AJ, Hafez AK, Amoah SK,
Rodriguez BA, Dell'Orco M, Lozano E, Hartley BJ, Alural B, Lalonde
J, Chander P, et al: A psychiatric disease-related circular RNA
controls synaptic gene expression and cognition. Mol Psychiatry.
25:2712–2727. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ayers D and Scerri C: Non-coding RNA
influences in dementia. Noncoding RNA Res. 3:188–194. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng Z, Zhang L, Wang S and Hong Q:
Circular RNA circDLGAP4 exerts neuroprotective effects via
modulating miR-134-5p/CREB pathway in Parkinson's disease. Biochem
Biophys Res Commun. 522:388–394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar L, Shamsuzzama, Jadiya P, Haque R,
Shukla S and Nazir A: Functional characterization of novel circular
RNA molecule, circzip-2 and its synthesizing gene zip-2 in C.
Elegans model of parkinson's disease. Mol Neurobiol.
55:6914–6926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao MS, Ai Y and Wilusz JE: Biogenesis
and functions of circular RNAs come into focus. Trends Cell Biol.
30:226–240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leggio L, Vivarelli S, L'Episcopo F,
Tirolo C, Caniglia S, Testa N, Marchetti B and Iraci N: microRNAs
in Parkinson's disease: From pathogenesis to novel diagnostic and
therapeutic approaches. Int J Mol Sci. 18:26982017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Junn E, Lee KW, Jeong BS, Chan TW, Im JY
and Mouradian MM: Repression of alpha-synuclein expression and
toxicity by microRNA-7. Proc Natl Acad Sci USA. 106:13052–13057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lukiw WJ: Circular RNA (circRNA) in
Alzheimer's disease (AD). Front Genet. 4:3072013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang
W, Sun Y and Zhang H: CircSNCA downregulation by pramipexole
treatment mediates cell apoptosis and autophagy in Parkinson's
disease by targeting miR-7. Aging (Albany NY). 10:1281–1293. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu Y, Xu J, Xu J, Cheng J, Jiao D, Zhou C,
Dai Y and Chen Q: Lower serum levels of miR-29c-3p and miR-19b-3p
as biomarkers for alzheimer's disease. Tohoku J Exp Med.
242:129–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang S, Jin J, Tian X and Wu L:
hsa-miR-29c-3p regulates biological function of colorectal cancer
by targeting SPARC. Oncotarget. 8:104508–104524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luo H, Zhu W, Mo W and Liang M:
High-glucose concentration aggravates TNF-alpha-induced cell
viability reduction in human CD146-positive periodontal ligament
cells via TNFR-1 gene demethylation. Cell Biol Int. 44:2383–2394.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu C, Wang W, Jia Y, Liu X, Tong Z and Li
B: Inhibition of AMPK/autophagy potentiates parthenolide-induced
apoptosis in human breast cancer cells. J Cell Biochem.
115:1458–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X,
Lu G and Zhang S: miR-124 regulates cell apoptosis and autophagy in
dopaminergic neurons and protects them by regulating AMPK/mTOR
pathway in Parkinson's disease. Am J Transl Res. 8:2127–2137.
2016.PubMed/NCBI
|