Introduction
Diabetic encephalopathy (DE) is a serious chronic
complication of diabetes (1,2) and
its clinical manifestations include loss of learning and memory
abilities, and cognitive dysfunction (3). At present, the pathogenesis of DE is
unclear, but substantial data have shown that neuronal apoptosis is
one of the key pathogenic mechanisms of DE (2,4).
Studies have found that neurons are regulated by the mitochondrial
apoptosis pathway, death receptor apoptosis pathway and endoplasmic
reticulum apoptosis pathway and these pathways ultimately affect
cognitive function (4,5). A recent study revealed that
Ganoderma lucidum triterpenoids improve cognitive
impairment, alleviate neuronal damage and inhibit apoptosis in
hippocampal tissues and cells in Alzheimer's disease by inhibiting
the Rho-associated protein kinase 1 signaling pathway (6). Although research has shown that the
hippocampus is the main lesion site in DE and that hippocampal
neuronal apoptosis is closely associated with the occurrence of
cognitive dysfunction, the mechanism of action remains to be
elucidated (7).
Myosin light chain kinase (MLCK), a key molecule in
the calcium signaling pathway (8),
is expressed in hippocampal neurons, where it can accelerate the
mobilization of synaptic vesicles, promote the release of various
neurotransmitters and regulate the elongation of growth cones
(9,10). A previous study found that the
increase in nerve cell apoptosis observed in a rat model of
ischemia-reperfusion brain injury might be associated with
upregulation of MLCK expression and that the addition of a specific
MLCK inhibitor (ML-7) significantly reduces neuronal apoptosis
(11). In addition, increased MLCK
expression affects the cytoskeletal structure and this effect leads
to the occurrence of apoptosis (12). Our previous studies found that MLCK
serves a role in hippocampal neuronal apoptosis, but the regulatory
mechanism needs further examination (13,14).
Previous research has shown that hippocampal neuronal apoptosis is
regulated by multiple pathways, including the MAPK/JNK signaling
pathway and the Wnt/β-catenin signaling pathway (15). The JNK signaling pathway not only
serves an important role in regulating apoptosis, but is also
associated with neurodegenerative diseases, such as Alzheimer's
disease (16). A number of factors,
including inflammatory cytokines, oxidative damage and a
high-glucose (HG) environment, can activate the JNK signaling
pathway (17) and thereby the
mitochondrial apoptotic pathway, which affects expression of the
apoptosis-related proteins Bcl-2 and Bax, induces the release of
cytochrome c from mitochondria and activates the downstream caspase
signaling pathway (18). However,
JNK does not act universally as a pro-apoptotic signaling pathway
because JNK activation can also exert antiapoptotic activity in
certain situations (19). As the
role of the JNK signaling pathway in DE has not been fully
elucidated, the present study aimed to clarify the relationship
between the JNK signaling pathway and MLCK in the apoptosis of
hippocampal neurons in DE.
Green tea, a non-fermented tea, contains a number of
tea polyphenols, alkaloids, amino acids, polysaccharides and other
components. A typical brewed green tea contains 30–45% green tea
catechins, including epicatechin (EC), epicatechin-3-gallate (ECG),
epigallocatechin and epigallocatechin-3-gallate (EGCG) (20). A previous study suggested that green
tea has anti-diabetic and anti-ageing activity and lowers the blood
cholesterol leve (20), which
results in reduced incidence of neurodegenerative diseases and
protects the nervous system (21).
Epidemiological research has begun to show that green tea
suppresses cognitive decline (22).
In diabetes development, whether green tea can
protect against the cognitive dysfunction caused by diabetes and
the potential pharmacological mechanism remains to be elucidated.
Therefore, the purpose of the present study was to elucidate the
mechanism of action of green tea in hippocampal neuronal apoptosis
in DE. It is hoped that the present study will contribute to a
deeper understanding of the pharmacological mechanism of green
tea.
Materials and methods
Experimental animals
A total of 300 healthy newborn (24-h old)
Sprague-Dawley (SD) rats (6–12 g) and 66 healthy adult SD rats
(180–220 g, male) were purchased from the Experimental Animal
Center of Guizhou Medical University [animal license no.
SYXK(Qian)2018-0001, Guiyang, China]. The 300 newborn SD rats were
used to cultivate primary hippocampal neurons; generally, 10
newborn rats provided 2×105 hippocampal neurons and
primary hippocampal neurons do not divide in culture. In addition,
60 adult SD rats were used to establish a type 1 diabetes rat model
and six adult rats were used to obtain serum. Prior to the
experiments, the animals were maintained under a 12-h light/dark
cycle in an indoor environment with a temperature of 22±2°C and
50±10% humidity, and provided with sterile water and food ad
libitum. The experiments were performed in strict accordance with
the guidelines established by the National Institutes of Health for
the Use of Laboratory Animals (National Institutes of Health
publication no. 85-23, 1985) and the experiments were approved by
the Guizhou Medical University Animal Care and Use Committee
(approval no. 1800954; Guiyang, China).
Preparation of green tea serum and
blank serum
Blank serum and green tea serum were prepared
according to a previous study (23). Green tea powder (20.0 g) was weighed
and 100 ml sterile distilled water was added to the powder. The
solution was mixed in a water bath shaker at 70°C for 1 h. The
mixture was filtered through a sieve and green tea extract was then
obtained for intragastric use. A total of six healthy SD rats were
randomly selected and divided into one of two groups: The rats in
the control group were intragastrically administered normal saline
at a dosage of 20 ml/kg/day and those in the model group were
intragastrically administered green tea concentrate at a dosage of
20 ml/kg twice per day for 3 consecutive days. After 1.5 h of
gavage on the last day of treatment, the SD rats were anaesthetized
via intraperitoneal injection of sodium pentobarbital (30 mg/kg)
until they lost consciousness. Subsequently, 5 ml of blood was
drawn from the femoral artery (peripheral blood) and centrifuged at
1,006.2 × g for 10 min at 4 °C and the upper layer containing serum
was aspirated. The rats were sacrificed via cervical dislocation.
The green tea serum and blank serum were then inactivated at 56°C
for 30 min and diluted in maintenance medium to obtain low-dose
blank serum (25% blank serum), medium-dose blank serum (50% blank
serum), high-dose blank serum (100% blank serum), low-dose green
tea serum (25% green tea serum), medium-dose green tea serum (50%
green tea serum) and high-dose green tea serum (100% green tea
serum). All sera were stored in an ultra-low temperature
refrigerator at −80°C.
High-performance liquid chromatography
(HPLC)
The components in the blank serum and green tea
serum were detected by HPLC using an Agilent 1100 system (Agilent
Technologies, Inc.) according to a previous study (24). First, the serum samples were
pretreated and the HPLC test conditions confirmed: Diamonsil C18
column (250×4.6 mm, 5 µm), mobile phase consisting of acetonitrile
(A) and 0.03% phosphoric acid aqueous solution (B), gradient
elution (0 min, 8% A; 18 min, 19% A; 25 min, 23% A; 45 min, 26% A;
70 min, 37% A; 80 min, 63% A; and 95 min, 70% A), volume flow rate
of 0.9 ml/min, column temperature of 30°C, detection wavelength of
278 nm and injection volume of 10 µl.
Hippocampal neuronal cell culture and
identification
The primary hippocampal neurons were cultured
according to a previously published method (14). Newborn SD rat were sacrificed by
cervical dislocation and the mortality of the animals confirmed by
cessation of heartbeat. Primary hippocampal neurons were extracted
from newborn rat hippocampal tissue. The basal medium glucose
concentration was 25 mmol/l glucose (Neurobasal-A medium, without
FBS; Gibco; Thermo Fisher Scientific, Inc.). After 7 days of
culture, the hippocampal neurons were incubated with anti-neuron
specific enolase (NSE) antibody (1:100; cat. no. M02930; Wuhan
Boster Biological Technology, Ltd.) and their purity was determined
by immunocytochemistry. The extracted cells were plated at
2×105/ml and rinsed in PBS three times for 2 min each
time. Then, 4% paraformaldehyde was used to fix the cells at room
temperature for 30 min, followed by rinsing with PBS three times
for 4 min each time, and placed in 0.3% Triton X-100 and soaked for
10 min. Slides were washed again with PBS three times for 4 min
each time, and put into 3% H2O2 for 15 min to
eliminate the influence of endogenous peroxidase. Then, 3% goat
serum was used for blocking at room temperature for 20 min to block
the effect of non-specific binding sites. After blocking, the
slides were rinsed with PBS three times for 2 min each time. Then,
the slides were incubated with rabbit anti-rabbit NSE primary
antibody (1:100; cat. no. ab180943; Abcam) and incubated overnight
at 4°C on a shaker. The next day, cells were incubated with goat
anti-rabbit IgG H&L (HRP-conjugated) (1:500; cat. no. ab6721;
Abcam) and incubated at room temperature for 1.5 h. The sides were
washed with PBS three times for 5 min each time, and then put into
the enzyme-labeled avidin for 20 min. The cells were incubated with
DAB in the dark for 20 min. After washing, the cells were stained
with hematoxylin at room temperature for 10 sec. The slides were
observed under an optical microscope, the field of view was
selected at random and cell morphology was observed under the
microscope, and the brown-stained cells were considered positive
cells. The hippocampal neuron growth status was assessed via
inverted microscopy (magnification, ×200). The purity of the
neurons was estimated using ImageJ v1.45s (National Institutes of
Health).
Cellular activity assay
The hippocampal neurons were seeded into a 96-well
plate at a density of 1,000/well, grown for 5 days and divided into
the following groups: i) Control group (25 mmol/l glucose); ii) HG
group (45 mmol/l glucose); iii) HG + low-dose blank serum group (45
mmol/l glucose + 10% total volume of low-dose blank serum); iv) HG
+ medium-dose blank serum group (45 mmol/l glucose + 10% total
volume of medium-dose blank serum); v) HG + high-dose blank serum
group (45 mmol/l glucose + 10% total volume of high-dose blank
serum); vi) HG + low-dose green tea serum group (45 mmol/l glucose
+ 10% total volume of low-dose green tea serum); vii) HG +
middle-dose green tea serum group (45 mmol/l glucose + 10% total
volume of medium-dose green tea serum); viii) HG + high-dose green
tea serum group (45 mmol/l glucose + 10% total volume of high-dose
green tea serum); ix) HG + SP group [45 mmol/l glucose + 10 µmol/l
SP600125 (JNK inhibitor; Sigma-Aldrich; Merck KGaA)]; and x) HG +
ML-7 group [45 mmol/l glucose + 10 µmol/l ML-7 (MLCK inhibitor;
Sigma-Aldrich; Merck KGaA)]. Cellular activity was assessed using
Cell Counting Kit-8 (CCK-8) assays according to a previous study
(25). Briefly, 10 µl CCK-8
solution (cat. no. CK04; Dojindo Molecular Technologies, Inc.) was
added to each well and the cultures were incubated in a water bath
at 37°C for 2 h. The optical density at 450 nm was then measured
using a microplate reader and the activities of the cells in the
different groups were calculated. All CCK-8 assays were performed
in triplicate and repeated three times.
Cell apoptosis analysis
The cell culture medium used was the same as in
Hippocampal neuronal cell culture and identification.
Differentially treated hippocampal neuronal cells were cultured for
48 h and apoptosis was then assessed via incubation in the dark
with an Annexin V-FITC apoptosis detection kit (Nanjing KeyGen
Biotech Co., Ltd.) according to the manufacturer's protocol. The
apoptosis rate was determined by calculating the percentage of
early and late apoptotic cells. The cells were analyzed via
fluorescence-activated cell sorting using a flow cytometer (Navios;
Beckman Coulter, Inc.) and the results were analyzed using FlowJo
v10 (FlowJo LLC) and GraphPad Prism 5 (GraphPad software,
Inc.).
Diabetes induction
The present study established the type 1 diabetes
rat model by the administration of an intraperitoneal injection of
streptozotocin (STZ; Sigma-Aldrich; Merck KGaA). A total of 60 male
SD rats were randomly divided into two groups: The negative control
(NC) group and the model group. The rats in the NC group (n=12)
were fasted for 12 h, intraperitoneally injected with citric
acid-sodium citrate buffer (0.1 mol/l, pH 4.2) and given daily
administrations of an equal volume of sterile distilled water. The
rats in the model group (n=48) were fasted for 12 h and
administered an STZ solution (STZ dissolved in citric acid-sodium
citrate buffer, 50 mg/kg) via intraperitoneal injection to induce
diabetes. As in our previous study (13), successful replication of this
diabetes model was verified if the fasting blood glucose level at
72 h after STZ injection was ≥16.7 mmol/l and remained >16.7
mmol/l for 3 consecutive weeks. The model group was then divided
into four groups (n=12): i) The rats in the diabetes model (DM)
group received an equal volume of distilled water; ii) the rats in
the low-dose green tea extract group received 5 ml/kg/day green tea
extract + 15 ml/kg/day sterile distilled water; iii) the rats in
the medium-dose green tea extract group received 10 ml/kg/day green
tea extract + 10 ml/kg/day sterile distilled water; and iv) the
rats in the high-dose green tea extract group received 20 ml/kg/day
green tea extract. Timed gavage was performed every day for 8 weeks
to process the rats. The fasting blood glucose level and weight of
the rats was measured every 3 weeks and the effect of green tea on
the rat blood glucose levels was observed in the different
groups.
Morris water maze test
In the 12th week after the diabetes model was
established, the Morris water maze test was performed to assess the
learning and memory abilities of the rats. According to a previous
study (26), the basal swimming
speed of the rats in each group was first tested. The DM group
developed cognitive dysfunction at 12 weeks, as shown by the Morris
water maze test, which included positioning navigation experiments
and space exploration experiments. The water maze circular constant
temperature pool had a diameter of 160 cm and a height of 50 cm,
while the circular platform had a diameter of 12 cm and its
position was adjustable. In this experiment, the height was set to
30 cm, and the water temperature was kept at 23±1°C. The circular
platform was placed in the center of the southeast quadrant of the
pool ~1 cm below the water surface. In the beginning, the rats were
placed in the water from a position different from the edge of the
northeast quadrant. The DigBehv animal behavior video tracking
system (DigBehv-LM4; Shanghai Jiliang Software Technology Co.,
Ltd.) was used to track and record the time that the rats reached
the platform, which was measured as the escape latency period. The
rats that found the platform within 60 sec were treated as a
success. If the rat did not find the platform during this time,
they were manually guided to the platform and kept there for 30
sec, and the escape latency of this rat was deemed to be 60 sec.
The experiment was performed continuously for 5 days in total, four
times a day. The escape latency was recorded on the last day of the
experiment and the mean was calculated. During the space
exploration experiment on the 6th day of the experiment, most
conditions were left unchanged, but the platform in the pool was
removed and the rats were allowed to enter the pool at any one of
the marked points. The program recorded the rats' swimming
trajectory within 60 sec and recorded their swimming tracks in 60
sec. The number of crossings over the original platform during 60
sec was considered to evaluate the space exploration ability of the
rat.
Hematoxylin and eosin (H&E)
staining
Morphological changes in the hippocampus of the rats
in the different groups were observed via H&E staining. The
hippocampal tissue was dissected and paraffin-embedded sections
were prepared (slice thickness, 4 µm). The slices were dehydrated
by ethanol gradient (80, 95 and 100% ethanol), soaked in xylene two
times for 5 min each time to make the slices transparent, stained
with hematoxylin for 1 min and stained with eosin solution for 1
min at 25°C, and finally mounted with neutral gum. Slices were
observed with a Nikon Eclipse E100 microscope (Nikon
Corporation).
Transmission electron microscopy (TEM)
of the hippocampal tissue ultrastructure
Rats were anaesthetized via intraperitoneal
injection of sodium pentobarbital (100 mg/kg) and subjected to
intracardiac perfusion-fixation using a solution of 0.9% sodium
chloride (VWR International, LLC), then all rats were sacrificed
via cervical dislocation. and 4% paraformaldehyde (Sigma-Aldrich;
Merck KGaA) in 0.1 M PBS (pH 7.4). The hippocampal tissue was
obtained and placed in fixative. Shanghai Fucheng Biotechnology
Co., Ltd., processed the samples and the samples were processed
according to the following steps. The samples (slice thickness, 70
nm) were fixed at room temperature in 2.5% glutaraldehyde (pH 7.4)
for 2 h and embedded in agarose with low melting point. After
washing three times with 0.1 M PBS (pH 7.2), samples were fixed in
1% osmic acid at 4°C for 2 h. Then, the samples were dehydrated in
a graded series of ethanol. Subsequently, the samples were embedded
in Epon-Araldite resin for penetration and placed in a model for
polymerization. Ultrathin sections were collected for
microstructure analysis. Following counterstaining with 3% uranyl
acetate and 2.7% lead citrate, slices were observed with a HT7800
transmission electron microscope (Hitachi, Ltd.).
Analysis of apoptosis in hippocampal
tissue
The degree of apoptosis in the hippocampal tissues
from each group was detected through terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labelling (TUNEL) assays using a
TUNEL Apoptosis kit (Nanjing KeyGen Biotech Co., Ltd.) according to
the manufacturer's recommended protocol. The positive cells in each
group were counted under a microscope (a brown color indicated
positive apoptotic cells) and the degree of apoptosis in the
hippocampus was assessed using ImageJ software v1.6.0 (National
Institutes of Health).
Western blot analysis
Following collection of hippocampal tissue and
hippocampal neurons, protein was extracted using a protein
extraction kit (Nanjing KeyGen Biotech Co., Ltd.). Protein
concentration was determined using a BCA assay (Pierce; Thermo
Fisher Scientific, Inc.). The protein samples (20 µg) were
separated via SDS-polyacrylamide on 10% gels. The target protein
was then transferred to a 0.45 µm Immobilon-P PVDF membrane (cat.
no. IPVH00010; Merck KGaA) via the wet transfer method and the
membrane was blocked with 5% skimmed milk for 2 h at room
temperature and incubated overnight at 4°C on a shaker with the
following antibodies: Rabbit anti-β-actin (1:5,000; cat. no.
ab8227; Abcam), rabbit anti-JNK (1:2,000; cat. no. 9252S; Cell
Signaling Technology, Inc.), rabbit anti-p-JNK (1:2,000; cat. no.
4668; Cell Signaling Technology, Inc.), rabbit anti-MLCK (1:5,000;
cat. no. ab76092; Abcam), rabbit anti-Bcl-2 (1:1,000; cat. no.
ab182858; Abcam) and rabbit anti-Bax (1:1,000; cat. no. ab32503;
Abcam). The membrane was then subjected to three 10-min rinses in
TBS with 0.1% Tween-20 buffer (TBST), incubated with HRP-conjugated
sheep anti-rabbit IgG (1:20,000; cat. no. 7074; Cell Signaling
Technology, Inc.) secondary antibody for 2 h at room temperature
and rinsed three times for 10 min in TBST buffer. The ECL method
(Amersham; Cytiva) was used to display the signals from the target
proteins and a gel imaging system was used for image collection.
The image grey values were analyzed and compared using ImageJ
software v1.6.0 (National Institutes of Health) to calculate the
relative expression levels. Western blot analyses were repeated at
least three times for each protein.
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS v19.0 software (IBM Corp.) was used for data
analysis. Inter-group variation was measured using a one-way ANOVA,
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HG leads to increased apoptosis in
hippocampal neurons
The present study first prepared primary cultured
hippocampal neurons and detected the purity of the cells by
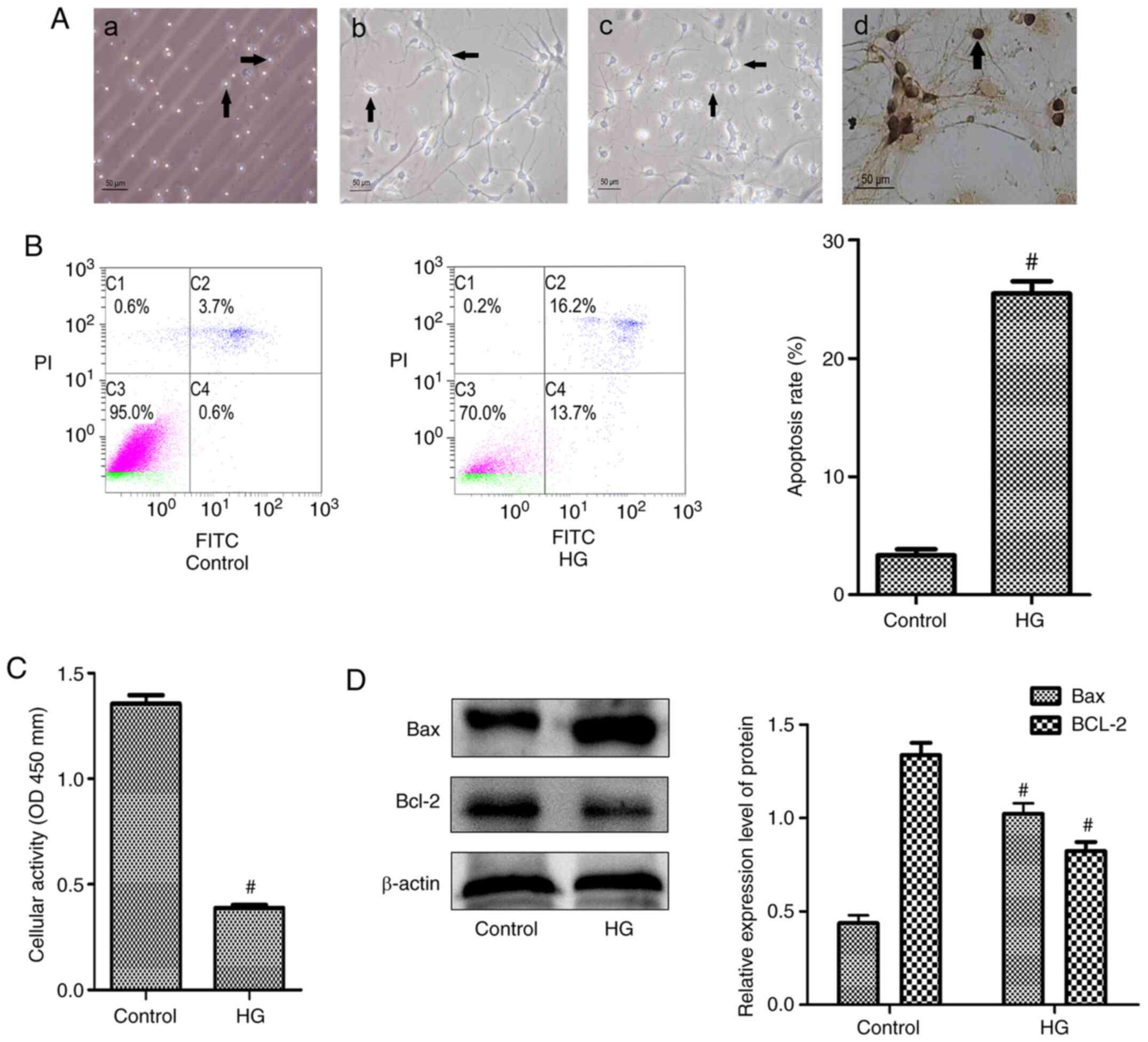
staining for NSE (Fig. 1A).
Hippocampal neuronal apoptosis was detected in the HG group
(Fig. 1B). The HG group
demonstrated a significantly increased apoptosis rate compared with
the control group. Cellular activity was also detected using a
CCK-8 assay and the results demonstrated that the cellular activity
in the HG group was significantly decreased (Fig. 1C). In addition, the expression of
the apoptosis-related proteins Bcl-2 and Bax in the two groups was
determined and the HG group demonstrated decreased Bcl-2 levels and
increased Bax levels compared with the control group (Fig. 1D).
Increased apoptosis of hippocampal
neurons in diabetic rats with cognitive dysfunction
The present study successfully replicated diabetes,
as shown by the fact that blood glucose levels ≥16.7 mmol/l were
maintained for >3 weeks (Fig.
2A) and the diabetes model was maintained for 2 months. Prior
to cognitive function tests, the basal swimming speed of the rats
in each group was tested and no differences were found between
groups (Table SI). The Morris
water maze test demonstrated that the rats in the diabetic group
exhibited significant declines in both learning and memory
(Fig. 2B and C; Table SII), as illustrated by the lines in
Fig. S1C, which showed the
movement of the rats and the decreased cognitive function of the
diabetic rats. Rat hippocampal tissues were then collected for
observation and H&E staining (Fig.
2D). Specifically, the morphology of the neurons in the CA1
subfield of the hippocampi of rats was detected by H&E
staining. Analysis of the NC group demonstrated that the nerve
cells in each structure were complete, but staining of the
hippocampal tissues of the DM group revealed that the neurons
adopted a disordered arrangement and that the structure of the
vertebral body and nucleus of nerve cells was destroyed.
Furthermore, the number of hippocampal neurons was reduced and
vacuoles were observed in the cytoplasm. The hippocampal neuron
ultrastructure in the NC group was also observed via TEM (Fig. 2E) and it was noted that the
morphological structure of the hippocampus was intact, the nuclei
were large, the cytoplasm was rich in content and intact, the
mitochondria were numerous and well-arranged and the endoplasmic
reticulum was regular. By contrast, analysis of the diabetic rats
revealed that the neurons were damaged, the mitochondria number was
reduced and the structure was destroyed. TUNEL assays were used to
detect apoptosis of hippocampal neurons and the CA1 region of the
hippocampus selected for observation and evaluation of neuronal
apoptosis; a brown-black color indicated apoptotic cells.
Assessment of the NC group revealed intact cells in the hippocampal
tissue, few apoptotic cells and a regular arrangement of normal
cells; however, more brown-black cells, which indicated apoptosis,
were observed in the DM group (Figs.
2F and S2A; Table SIII). To further verify these
changes, western blot analysis was performed and the results
confirmed increased expression of the apoptosis-related protein Bax
and reduced expression of the apoptosis-related protein Bcl-2 in
the DM group (Fig. 2G). Taken
together, these results showed that increased apoptosis of
hippocampal neurons led to decreased cognitive function in diabetic
rats.
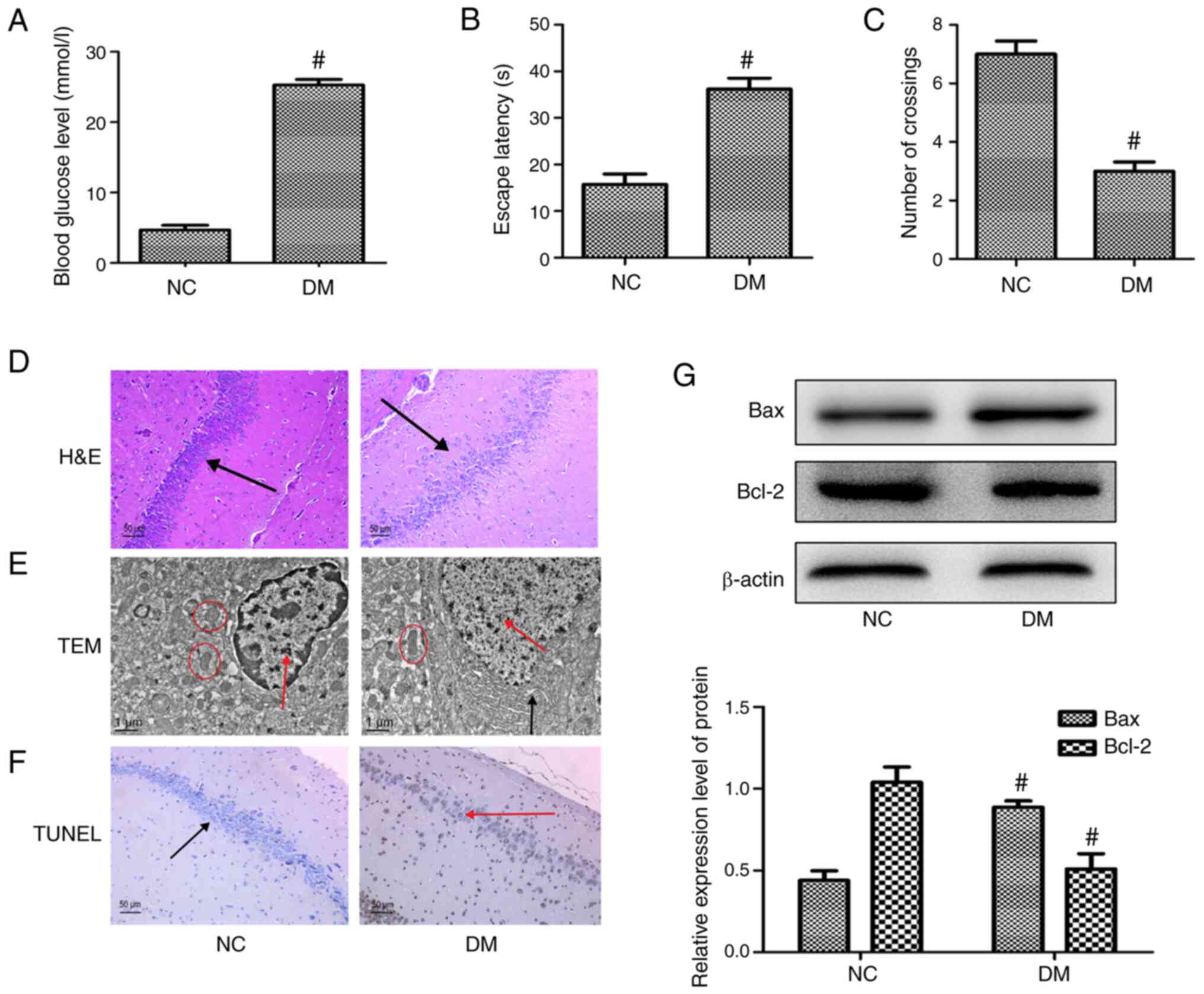 | Figure 2.Increased apoptosis of hippocampal
neurons in rat models of diabetic cognitive dysfunction. (A) Blood
glucose levels in different groups in the 12th week. (B) Escape
latency in the Morris water maze test in the 12th week. (C) Number
of rat crossings in the Morris water maze test in the 12th week.
(D) H&E staining showing the morphology of neurons in the CA1
subfield of the hippocampus (magnification, ×200), black arrow
indicates hippocampal neurons. (E) TEM observation of hippocampus
ultrastructure (magnification, ×10,000), red arrows indicate
nuclei; black arrows indicate endoplasmic reticulum; red circles
indicate mitochondria. (F) TUNEL was used to detect apoptosis in
the hippocampus (magnification, ×200), red arrow indicates
apoptotic hippocampal neurons; black arrow indicates normal
hippocampal neurons. (G) Expression of apoptosis-associated
proteins Bcl-2 and Bax in hippocampal neurons examined by western
blot analysis. Each data point represents the mean ± standard
deviation (n=3). #P<0.05 vs. the NC group. H&E,
hematoxylin and eosin; TEM, transmission electron microscopy; NC,
negative control; DM, diabetes model. |
Identification of six components in
green tea serum
To further study the components in green tea serum,
blank serum (Fig. 3A), mixed
standards (Fig. 3B) and green tea
serum (Fig. 3C) were assessed via
HPLC. A total of six components were detected in green tea serum:
Gallic acid, catechin, caffeine, EGCG, EC and ECG at mass
concentrations of 3.6, 4.2, 54.6, 22.8, 3.8 and 4.8 µg/ml,
respectively.
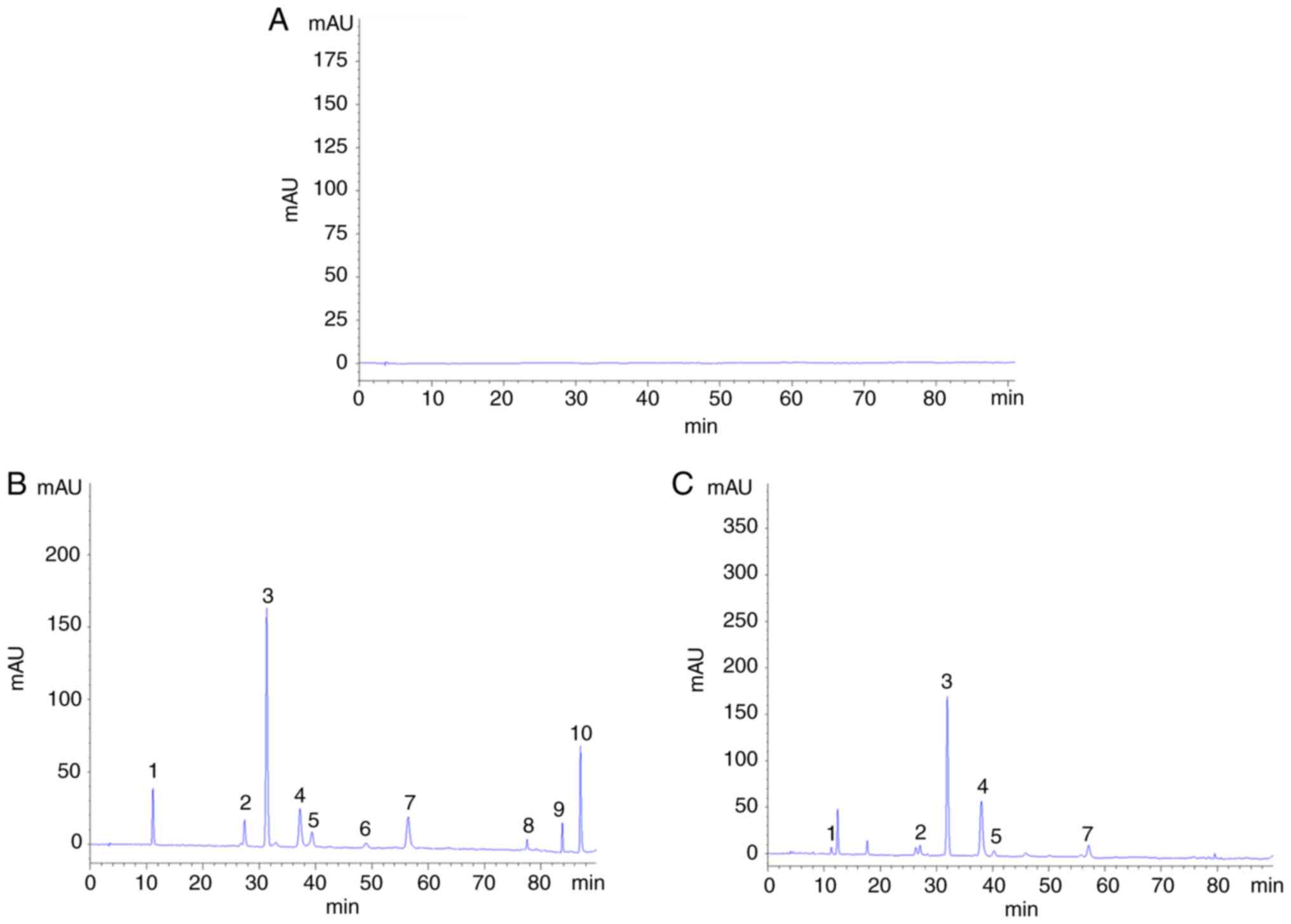 | Figure 3.Green tea components in green tea
serum. (A) The 10 tea components in blank serum. (B) The 10 green
tea ingredients in the mixed standard. (C) The 10 green tea
ingredients in tea serum. 1, gallic acid; 2, catechin; 3, caffeine;
4, epigallocatechin catechin gallate; 5, epicatechin; 6, gallic
acid catechin gallate; 7, epicatechin gallate; 8, rutin; 9,
quercetin; 10, kaempferol. |
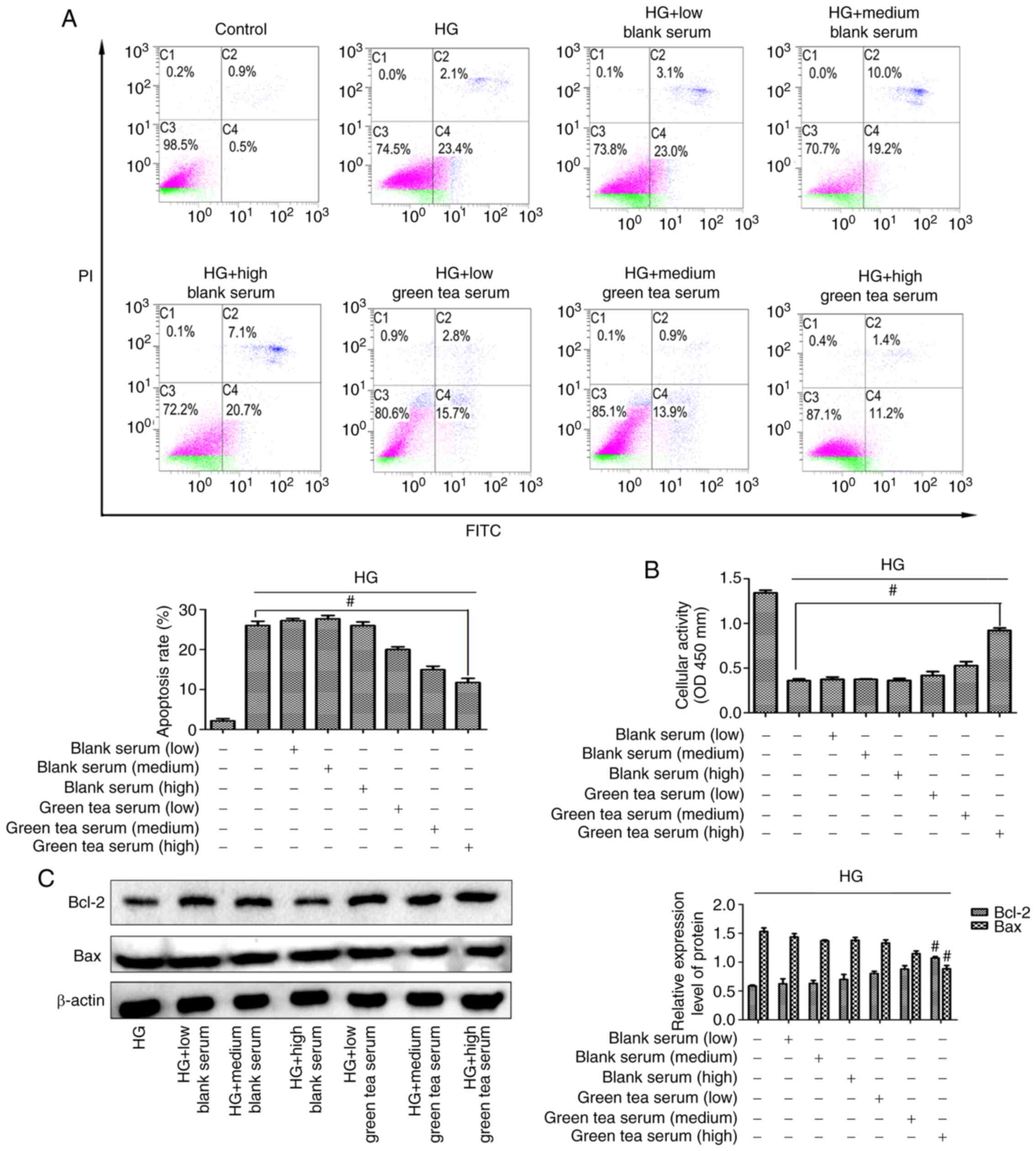
Green tea serum inhibits Bax to
protect hippocampal neurons from HG-induced apoptosis
First, data were obtained from an in vitro
cell culture study in which no difference was found in apoptotic
rate among the low-, medium- and high-dose blank serum groups and
the HG group. In the low-, medium- and high-dose green tea serum
groups, the apoptotic rate demonstrated a downward trend and the
highest dose of green tea serum exerted the most significant effect
(Fig. 4A). In addition, no
difference in cellular activity was found among the low-, medium-
and high-dose blank serum groups and the HG group and the cellular
activity was increased in the low-, medium- and high-dose green tea
serum groups (Fig. 4B). It was also
found that the expression of the apoptotic proteins Bcl-2 and Bax
was not significantly different between the low-, medium- and
high-dose blank serum groups and the HG group, but the high-dose
green tea serum group exhibited decreased Bax levels and increased
Bcl-2 levels compared with the HG group (Fig. 4C). Overall, these results confirmed
that green tea serum could inhibit Bax to protect hippocampal
neurons from HG-induced apoptosis.
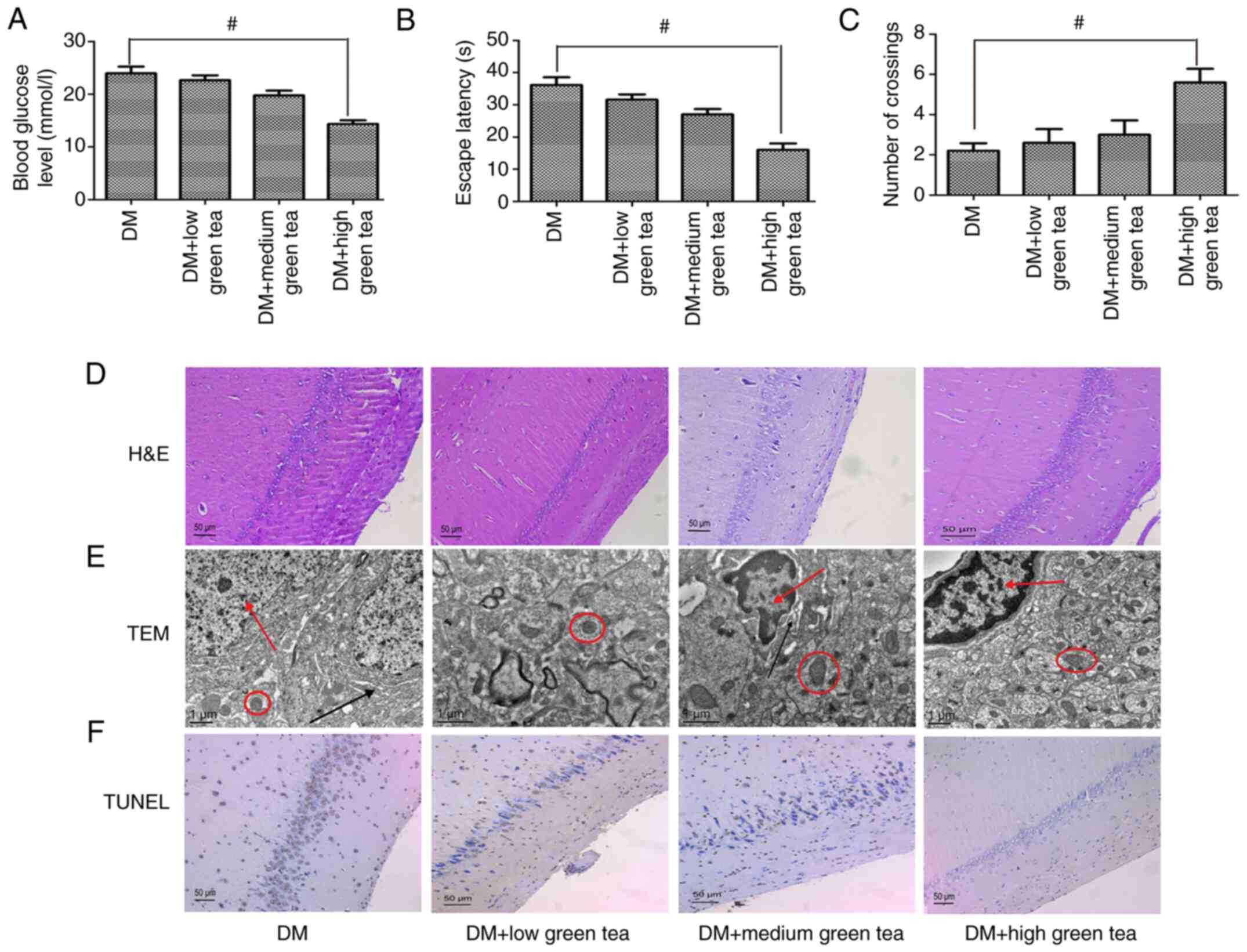
Green tea improves cognitive
dysfunction in diabetic rats by inhibiting hippocampal neuronal
apoptosis
To further probe the effects of green tea in
vivo, green tea extract was administered to diabetic rats and
found that green tea reduced blood glucose levels (Figs. 5A and S1A) and slowed the weight loss in these
rats (Fig. S1B). The results of
the Morris water maze test demonstrated that green tea improved the
learning and memory abilities of the diabetic rats (Fig. 5B and C), as illustrated by the lines
in Fig. S1C, which show the
movement of the rats. H&E staining of hippocampal tissues from
the green tea-treated rats demonstrated that the hippocampal neuron
cell structure was more complete, the nucleolus was clearer and the
number of cells was higher than observed in the other groups
(Fig. 5D). Similar results were
obtained by TEM, which demonstrated that green tea protected
against changes to the ultrastructure of hippocampal neurons
(Fig. 5E). The CA1 region of the
hippocampus was subsequently selected for observation and
evaluation of neuronal apoptosis in the various groups. The low-,
medium- and high-dose green tea groups exhibited decreased
hippocampal neuronal apoptosis and the high-dose green tea group
had the lowest apoptosis rate and presented regularly arranged
cells (Figs. 5F and S2). Taken together, these results
indicated that green tea can reduce blood sugar levels and improve
cognitive function in diabetic rats.
Green tea protects against hippocampal
neuronal apoptosis via the JNK/MLCK pathway
The JNK signaling pathway serves an important role
in apoptosis and our previous study found that MLCK serves a role
in hippocampal neuronal apoptosis in DE (14). Therefore, the present study further
examined the relationship between the JNK pathway and MLCK. First,
it was found that the JNK signaling pathway regulates MLCK
expression and that activation of the JNK pathway with HG promoted
MLCK expression in hippocampal neurons (Fig. 6A). The mechanism through which green
tea protects hippocampal neurons from apoptosis was further
clarified by flow cytometry and the results demonstrated that the
HG + SP and HG + green tea serum groups exhibited significantly
reduced apoptotic rates (Fig. 6B).
CCK-8 assays demonstrated that cellular activity was increased in
the HG + SP and HG + green tea serum groups (Fig. 6C). Western blot analysis
demonstrated that the green tea serum groups exhibited decreased
p-JNK, MLCK and Bax expression and increased Bcl-2 expression
(Fig. 6D) and similar results were
detected in model rats treated with green tea. The most significant
decreases in p-JNK, MLCK and Bax expression and the most
significant increases in Bcl-2 expression among the green
tea-treated groups were found in the high-dose green tea group
(Fig. 6E). Taken together, these
results suggested that green tea protects against hippocampal
neuronal apoptosis by inhibiting activation of the JNK/MLCK pathway
in DE.
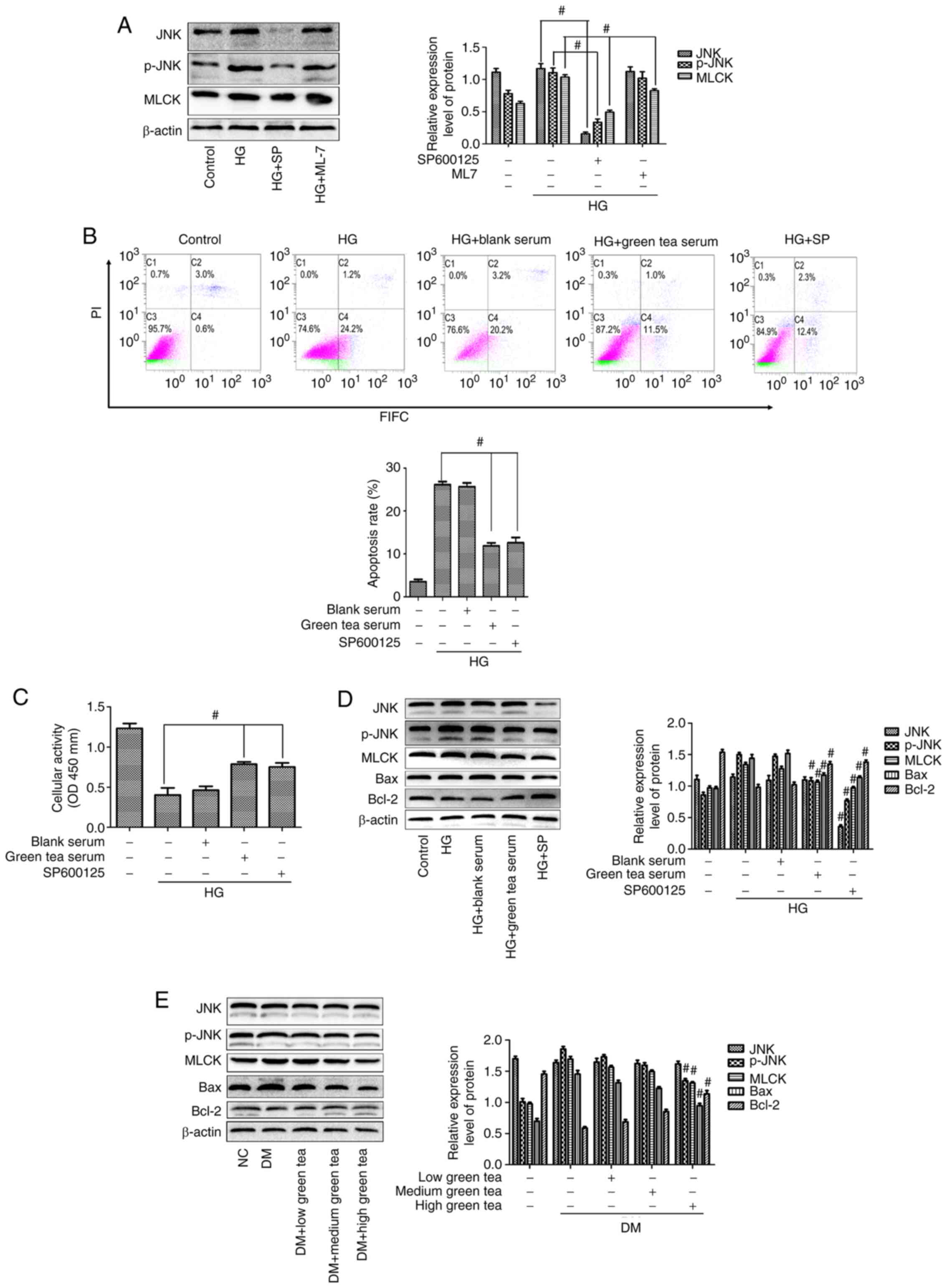 | Figure 6.Green tea protects against
hippocampal neuronal apoptosis via the JNK/MLCK pathway. (A)
Relationship between the JNK pathway and MLCK was explored via
western blotting. (B) Flow cytometry was used to detect apoptosis
in each group. (C) Cell activity in each group was tested using
Cell Counting Kit-8 assays. (D) JNK, p-JNK, MLCK, Bcl-2 and Bax
expression was detected in vitro. Each data point represents
the mean ± standard deviation. #P<0.05 vs. the HG
group. (E) JNK, p-JNK, MLCK, Bcl-2 and Bax expression detected
in vivo via western blotting. Each data point represents the
mean ± standard deviation. #P<0.05 vs. the DM group.
MLCK, myosin light chain kinase; HG, high-glucose; p-,
phosphorylated; DM, diabetes model; SP, SP600125; OD, optical
density; NC, negative control. |
Discussion
The pathogenesis of DE is complicated and it is
currently difficult to identify effective therapeutic targets for
DE (27). It is becoming
epidemiologically clear that the intake of green tea suppresses
cognitive decline. A previous study indicated that green tea intake
might reduce the risk of dementia, Alzheimer's disease, mild
cognitive impairment and severe cognitive impairment (28), but the exact mechanism of action of
green tea remains unclear. Previous studies have found that the
occurrence of diabetic cognitive dysfunction is associated with
apoptosis, oxidative stress, perturbed calcium homeostasis, altered
energy metabolism and microvascular disease (29–31).
Our previous study found that hippocampal neuronal apoptosis is an
important mechanism leading to DE development (14). Hyperglycemia can lead to the
occurrence of downstream apoptosis through activation of the JNK
signaling pathway and activation of this pathway can induce the
mitochondrial apoptotic pathway, which is involved in the
pathogenesis of diabetic cognitive dysfunction. As mentioned in a
literature review, the JNK signaling pathway serves not only an
important role in apoptosis, but also a substantial role in the
pathogenesis of neurological disorders (32). JNK, an important member of the MAPK
family, has three isoforms, JNK1, JNK2 and JNK3 (16). The JNK pathway can activate a
variety of signaling factors, including TNF-α, epidermal growth
factor, G protein-coupled receptors and stress, which results in
cytochrome c release and subsequent activation of the mitochondrial
apoptosis pathway and the occurrence of apoptosis (33). Several studies have shown that the
JNK signal transduction pathway is involved in the neurotoxic
damage induced by amyloid β and closely associated with the
apoptosis of hippocampal neurons (34,35).
Dendropanax morbifera has been reported to improve diabetic
cognitive dysfunction by reducing the level of oxidative stress and
inhibiting the JNK signaling pathway (36). A recent study found that Nuanxin
Capsules, which are a Traditional Chinese Medicine that have a
protective effect against heart failure, can reduce Bcl-2
expression and increase Bax expression by inhibiting activation of
the JNK pathway, ultimately inhibiting apoptosis induced via the
mitochondrial pathway (37). The
present study demonstrated that hyperglycemia promoted hippocampal
neuron apoptosis by activating the JNK signaling pathway, which
serves a central role in DE onset and development. The JNK
signaling pathway regulates neuronal apoptosis during the
development of diabetic encephalopathy, but a previous study found
that activation of the PI3K/Akt/GSK3β pathway can improve tau
hyperphosphorylation and axon damage and thereby exert cognitive
protection (38). In short,
cognitive dysfunction caused by DE is the result of multiple
pathways. The present study only studied apoptosis of hippocampal
neurons and further evidence is needed.
MLCK is an important functional protein that
regulates smooth muscle contraction. Activated MLCK phosphorylates
the myosin light chain and phosphorylated myosin light chain
induces smooth muscle contraction (8). MLCK was the first CaM-dependent kinase
to be discovered and free Ca2+ obtained after the
binding of MLCK to calmodulin activates MLCK, which in turn
activates contractile proteins (39). MLCK expression is increased in the
hippocampi of rats with diabetic cognitive dysfunction. Inhibition
of MLCK expression has also been found to decrease the hippocampal
neuronal apoptosis rate, but the underlying mechanism remains
unknown (10). At present, few
studies have investigated the MLCK and JNK pathways, but a previous
study found that loss of MLCK leads to disruption of cell-cell
adhesion and invasive behavior in breast epithelial cells via
increased expression of JNK signaling (40). Thus, the present study further
investigated the relationship between MLCK and the JNK pathway. It
used specific protein inhibitors (SP600125 and ML-7) that act on
hippocampal neurons and found that activation of the JNK pathway
promoted MLCK expression, leading to changes in the expression of
the apoptosis-related proteins Bax and Bcl-2 and ultimately
affecting the occurrence of apoptosis. Although the results
indicated that MLCK is regulated by the JNK pathway, which is
inconsistent with previous studies, the present study might have
some limitations. For example, it did not directly elucidate the
relationship between JNK and MLCK at the gene level. Despite its
best attempts, no method to address the low transfection efficiency
of hippocampal neurons was found, which is an important issue to
address in future studies and it is intended to further verify the
regulatory relationship between JNK and MLCK through
immunoprecipitation experiments. HT-22 cells were selected as a
model cell line to optimize the in vitro experiments to
solve the problem of low transfection efficiency. The authors are
currently establishing hippocampus-specific MLCK-knockout mice to
explore the precise mechanisms underlying these effects.
Current pharmacological therapies for diabetes have
failed to prevent DE development and there is an urgent need for
alternative treatment strategies. Green tea is one of the most
frequently and heavily consumed beverages worldwide and retains
more of the natural ingredients in tea than other types of tea
(20) Compared with other teas,
green tea contains more tea polyphenols, alkaloids, theanine and
other biologically active substances, the most abundant of which
are EGCG and caffeine (41). For
the present study, a seropharmacological method was selected to
study the effects of green tea on neurons. Compared with other
methods, seropharmacology, a novel method for pharmacological study
of Traditional Chinese Medicine (42), has improved effects on assessing the
actual effects of a drug on the body and the pharmacological
effects of the drug itself and its metabolites (43). Therefore, green tea serum was
obtained from green tea-gavaged rats via seropharmacological
methods according to the literature and six active components in
green tea serum were detected using HPLC. Among these components,
EGCG and caffeine were present at the highest concentrations, which
is similar to the levels of the components found in green tea.
Dietary factors might serve a role in prevention of
cognitive dysfunction and among these factors, beverages are
considered useful because their intake does not substantially
affect other dietary habits and is more acceptable (1). Green tea is one of the most common
beverages consumed worldwide and serves a role in neurodegenerative
diseases and improving cognitive function (21). Tea polyphenols, alkaloids and
theanine, which are the main ingredients of green tea, penetrate
the blood-brain barrier and enter the brain tissue (44). A previous study found that green tea
catechins have antioxidant activity, which can improve cognitive
function in patients with Alzheimer's disease by scavenging free
radicals (45). Green tea
polyphenols reduce the inflammation associated with encephalopathy
and improve cognitive ability by inhibiting NFK-β activation
(46). Treatment with EGCG in
vitro (10 nmol/l) and in vivo (2.5 mg/kg; equivalent
human dose of 0.27 mg/kg) significantly improves the survival rate
of hippocampal neurons and the cognitive function of the organism,
potentially by influencing the activity of nerve cells by
activating the PI3K/AKT signaling pathway (47). The present study found that
intervention with green tea decreased blood glucose levels in
diabetic rats and improved their memory and learning abilities. In
addition, the cell experiments demonstrated that green tea serum
protected against HG-induced apoptosis of hippocampal neurons by
reducing the expression of the pro-apoptotic gene Bax and
increasing cell viability. Therefore, HG-induced hippocampal
neurons were treated with a JNK inhibitor and green tea serum to
explore the mechanism through which green tea protects neurons. The
experimental results of the present study demonstrated that green
tea serum significantly inhibited p-JNK expression and JNK
signaling pathway activation, which resulted in protection of cells
from apoptosis. Similar results were obtained using animal models.
In summary, the findings of the present study suggested that the
beneficial effects through which green tea consumption improves
cognitive function in diabetic rats might be associated with
decreased hippocampal neuronal apoptosis. Afzal et al
(48) reported that EGCG, the main
component of green tea catechins, exerts neuroprotective effects
because EGCG inhibits amyloid-β aggregation. Τhe present study
provided the first demonstration of the effect of green tea in
improving cognitive function in diabetes and clarifies the
mechanism of action through which green tea protects cognitive
function through anti-apoptotic activity. However, the present
study has a number of limitations. First, it is not known whether
the improvement in cognitive function occurred due to a combination
of multiple ingredients in green tea or a single ingredient and
thus it is planned to study the individual components of green tea
to observe their effects on DE and provide further evidence to
elucidate the mechanism through which green tea protects against
hippocampal neuron apoptosis.
In conclusion, the present study found that
hyperglycemia promoted hippocampal neuronal apoptosis by activating
the JNK/MLCK signaling pathway, reducing Bcl-2 expression and
increasing Bax expression in diabetic rats with cognitive
impairment. Green tea reduced the occurrence of hippocampal
neuronal apoptosis by inhibiting activation of the JNK/MLCK
signaling pathway, increasing Bcl-2 expression and reducing Bax
expression and these effects ultimately protected against diabetic
cognitive dysfunction.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81960151 and
81960822), the Guizhou Provincial Department of Education
Innovation Group Major Research Project [grant no. (2018)021] and
the Guizhou Provincial Science and Technology Department Support
Program [grant no. (2019)2802].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WP and XL designed the study, YX and SL and LZ
conducted all the experiments. LD, WQ and JZ interpreted and
analyzed the data, drafted the manuscript and revised it critically
for important intellectual content. All authors read and approved
the final manuscript. WP, XL and YX were responsible for confirming
the authenticity of the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Guizhou
Medical University Animal Care and Use Committee (approval no.
1800954; Guiyang, China) and were conducted in accordance with the
National Institutes of Health guidelines for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dong M, Ren M, Li C, Zhang X, Yang C, Zhao
L and Gao H: Analysis of metabolic alterations related to
pathogenic process of diabetic encephalopathy rats. Front Cell
Neurosci. 12:5272019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Díaz-Gerevini GT, Daín A, Pasqualini ME,
López CB, Eynard AR and Repossi G: Diabetic encephalopathy:
Beneficial effects of supplementation with fatty acids ω3 and
nordihydroguaiaretic acid in a spontaneous diabetes rat model.
Lipids Health Dis. 18:432019. View Article : Google Scholar
|
|
3
|
Liu LJ, Lu XJ, Gao JM, Wang RJ and Cheng
GX: Effect of the APP17 peptide on diabetic encephalopathy. J Biol
Regul Homeost Agents. 33:251–257. 2019.PubMed/NCBI
|
|
4
|
Kim TW and Park HS: Physical exercise
improves cognitive function by enhancing hippocampal neurogenesis
and inhibiting apoptosis in male offspring born to obese mother.
Behav Brain Res. 347:360–367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu S, Min D, Zeng J, Ju Y, Liu Y and Chen
X: Transplantation of stem cells from human exfoliated deciduous
teeth decreases cognitive impairment from chronic cerebral ischemia
by reducing neuronal apoptosis in rats. Stem Cells Int.
2020:63930752020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu N, Huang Y, Jiang Y, Zou L, Liu X, Liu
S, Chen F, Luo J and Zhu Y: Ganoderma lucidum triterpenoids
(GLTs) reduce neuronal apoptosis via inhibition of ROCK signal
pathway in APP/PS1 transgenic Alzheimer's disease mice. Oxid Med
Cell Longev. 2020:98940372020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhusal A, Rahman MH, Lee IK and Suk K:
Role of hippocampal lipocalin-2 in experimental diabetic
encephalopathy. Front Endocrinol (Lausanne). 10:252019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Y, Liu P, Li Z, Shi Y, Huang J, Li S,
Liu Y, Zhang Z, Wang Y, Zhu W, et al: The effect of myosin light
chain kinase on the occurrence and development of intracranial
aneurysm. Front Cell Neurosci. 12:4162018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizui T, Sekino Y, Yamazaki H, Ishizuka Y,
Takahashi H, Kojima N, Kojima M and Shirao T: Myosin II ATPase
activity mediates the long-term potentiation-induced exodus of
stable F-actin bound by drebrin A from dendritic spines. PLoS One.
9:e853672014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Wu X, Yue HY, Zhu YC and Xu J:
Myosin light chain kinase facilitates endocytosis of synaptic
vesicles at hippocampal boutons. J Neurochem. 138:60–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang HF, Li TB, Liu B, Lou Z, Zhang JJ,
Peng JJ, Zhang XJ, Ma QL, Peng J and Luo XJ: Inhibition of myosin
light chain kinase reduces NADPH oxidase-mediated oxidative injury
in rat brain following cerebral ischemia/reperfusion. Naunyn
Schmiedebergs Arch Pharmacol. 388:953–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuo CY, Chou TY, Chen CM, Tsai YF, Hwang
GY and Hwang TL: Hepatitis B virus X protein disrupts stress fiber
formation and triggers apoptosis. Virus Res. 175:20–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y, Wang F, Chen S, Liu M, Pan W and Li
X: The protective effect of radix polygoni multiflori on diabetic
encephalopathy via regulating myosin light chain kinase expression.
J Diabetes Res. 2015:4847212015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Li C, Du G, Pan M, Liu G, Pan W and
Li X: High glucose upregulates myosin light chain kinase to induce
microfilament cytoskeleton rearrangement in hippocampal neurons.
Mol Med Rep. 18:216–222. 2018.PubMed/NCBI
|
|
15
|
Akchiche N, Bossenmeyer-Pourié C, Pourié
G, Koziel V, Nédélec E, Guéant JL and Daval JL: Differentiation and
neural integration of hippocampal neuronal progenitors: Signaling
pathways sequentially involved. Hippocampus. 20:949–961.
2010.PubMed/NCBI
|
|
16
|
Zhao Y, Xin Y and Chu H: MC4R is involved
in neuropathic pain by regulating JNK signaling pathway after
chronic constriction injury. Front Neurosci. 13:9192019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Kannan A and Gangwani L:
ZPR1-dependent neurodegeneration is mediated by the JNK signaling
pathway. J Exp Neurosci. 13:11790695198679152019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Xue AY, Li ZL and Yin Z: Liraglutide
promotes apoptosis of HepG2 cells by activating JNK signaling
pathway. Eur Rev Med Pharmacol Sci. 23:3520–3526. 2019.PubMed/NCBI
|
|
19
|
Wei H, Ren Z, Tang L, Yao H, Li X, Wang C,
Mu C, Shi C and Wang H: JNK signaling pathway regulates the
development of ovaries and synthesis of vitellogenin (Vg) in the
swimming crab Portunus trituberculatus. Cell Stress Chaperones.
25:441–453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki T, Pervin M, Goto S, Isemura M and
Nakamura Y: Beneficial effects of tea and the green tea catechin
epigallocatechin-3-gallate on obesity. Molecules. 21:E13052016.
View Article : Google Scholar
|
|
21
|
Di Lorenzo A, Nabavi SF, Sureda A,
Moghaddam AH, Khanjani S, Arcidiaco P, Nabavi SM and Daglia M:
Antidepressive-like effects and antioxidant activity of green tea
and GABA green tea in a mouse model of post-stroke depression. Mol
Nutr Food Res. 60:566–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pervin M, Unno K, Takagaki A, Isemura M
and Nakamura Y: Function of green tea catechins in the Brain:
Epigallocatechin gallate and its metabolites. Int J Mol Sci.
20:E36302019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Liu G, Xu Y, Gao C, Pan W and Li X:
Protective effect of green tea on hippocampal neurons in rats under
high glucose. Shandong Medicin. 58:35–39. 2018.(In Chinese).
PubMed/NCBI
|
|
24
|
Huang Y, Shi T, Luo X, Xiong H, Min F,
Chen Y, Nie S and Xie M: Determination of multi-pesticide residues
in green tea with a modified QuEChERS protocol coupled to
HPLC-MS/MS. Food Chem. 275:255–264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu H and Li S: Long non-coding RNA MT1JP
exerts anti-cancer effects in breast cancer cells by regulating
miR-92-3p. Gen Physiol Biophys. 39:59–67. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu MH, Sheen JM, Chen YC, Yu HR, Tain YL
and Huang LT: Rats with prenatal dexamethasone exposure and
postnatal high-fat diet exhibited insulin resistance, and spatial
learning and memory impairment: Effects of enriched environment.
Neuroreport. 31:265–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang B, Huang J, Li J and Zhong Y: Control
of macrophage autophagy by miR-384-5p in the development of
diabetic encephalopathy. Am J Transl Res. 10:511–518.
2018.PubMed/NCBI
|
|
28
|
Kakutani S, Watanabe H and Murayama N:
Green tea intake and risks for Dementia, Alzheimer's disease, mild
cognitive impairment, and cognitive impairment: A systematic
review. Nutrients. 11:E11652019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai Y, Meng X, Ye T, Xie W, Sun G and Sun
X: Inhibiting the NLRP3 inflammasome activation with MCC950
ameliorates diabetic encephalopathy in db/db mice. Molecules.
23:E5222018. View Article : Google Scholar
|
|
30
|
Jing YH, Zhang L, Gao LP, Qi CC, Lv DD,
Song YF, Yin J and Wang DG: Autophagy plays beneficial effect on
diabetic encephalopathy in type 2 diabetes: Studies in vivo and in
vitro. Neuroendocrinol Lett. 38:27–37. 2017.PubMed/NCBI
|
|
31
|
Wang Z, Huang Y, Cheng Y, Tan Y, Wu F, Wu
J, Shi H, Zhang H, Yu X, Gao H, et al: Endoplasmic reticulum
stress-induced neuronal inflammatory response and apoptosis likely
plays a key role in the development of diabetic encephalopathy.
Oncotarget. 7:78455–78472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Ren X, Hu X, Zhou L, Zhang C and
Zhang M: Cadmium-induced apoptosis through reactive oxygen
species-mediated mitochondrial oxidative stress and the JNK
signaling pathway in TM3 cells, a model of mouse Leydig cells.
Toxicol Appl Pharmacol. 368:37–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Li X, Fan L, Chen B, Liu J, Tao Y
and Wang X: Negative pressure wound therapy promoted wound healing
by suppressing inflammation via down-regulating MAPK-JNK signaling
pathway in diabetic foot patients. Diabetes Res Clin Pract.
150:81–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Zhou S, Qian X, Zhang Y and Zhao J:
Over-expressed Bax inhibitor 1 (BI-1) inhibits apoptosis of
hippocampal neurons via endoplasmic reticulum IRE1-JNK pathway in
rats with subarachnoid hemorrhage. Xibao Yu Fenzi Mianyixue Zazhi.
33:1316–1322. 2017.(In Chinese). PubMed/NCBI
|
|
35
|
Cai Y, Jiang W, Zhou AL, Zhou M and Xu L:
Effect of oxymatrine on apoptosis of hippocampal neurons by p38/JNK
signaling pathway. Zhongguo Zhongyao Zazhi. 42:731–738. 2017.(In
Chinese). PubMed/NCBI
|
|
36
|
Kim JM, Park SK, Guo TJ, Kang JY, Ha JS,
Lee S, Lee U and Heo HJ: Anti-amnesic effect of Dendropanax
morbifera via JNK signaling pathway on cognitive dysfunction in
high-fat diet-induced diabetic mice. Behav Brain Res. 312:39–54.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lou T, Ma J, Xie Y, Yao G, Fan Y, Ma S and
Zou X: Nuanxin capsule enhances cardiac function by inhibiting
oxidative stress-induced mitochondrial dependent apoptosis through
AMPK/JNK signaling pathway. Biomed Pharmacother. 135:1111882021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang S, He B, Hang W, Wu N, Xia L, Wang X,
Zhang Q, Zhou X, Feng Z, Chen Q, et al: Berberine alleviates tau
hyperphosphorylation and axonopathy-associated with diabetic
encephalopathy via restoring PI3K/Akt/GSK3β pathway. J Alzheimers
Dis. 65:1385–1400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei S, Czerwinska E, Czerwinski W, Walsh
MP and MacDonald JF: Regulation of NMDA receptor activity by
F-actin and myosin light chain kinase. J Neurosci. 21:8464–8472.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim DY and Helfman DM: Loss of MLCK leads
to disruption of cell-cell adhesion and invasive behavior of breast
epithelial cells via increased expression of EGFR and ERK/JNK
signaling. Oncogene. 35:4495–4508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Q, Bao L, Zimering J, Zan K, Zhang Z,
Shi H, Zu J, Yang X, Hua F, Ye X, et al: The protective role of
(−)-epigallocatechin-3-gallate in thrombin-induced neuronal cell
apoptosis and JNK-MAPK activation. Neuroreport. 26:416–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu H, Wu Q, Peng C and Zhou L: Study on
the antiviral activity of San Huang Yi Gan Capsule against
hepatitis B virus with seropharmacological method. BMC Complement
Altern Med. 13:2392013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siu WS, Ko CH, Wong HL, Gao S, Shum WT,
Lau CB, Hung LK and Leung PC: Seropharmacological study on
osteogenic effects of post-absorption ingredients of an
osteoprotective herbal formula. Chin J Integr Med. 23:25–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chu KO, Wang CC, Chu CY, Choy KW, Pang CP
and Rogers MS: Uptake and distribution of catechins in fetal organs
following in utero exposure in rats. Hum Reprod. 22:280–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Molino S, Dossena M, Buonocore D, Ferrari
F, Venturini L, Ricevuti G and Verri M: Polyphenols in dementia:
From molecular basis to clinical trials. Life Sci. 161:69–77. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spagnuolo C, Moccia S and Russo GL:
Anti-inflammatory effects of flavonoids in neurodegenerative
disorders. Eur J Med Chem. 153:105–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ortiz-López L, Márquez-Valadez B,
Gómez-Sánchez A, Silva-Lucero MD, Torres-Pérez M,
Téllez-Ballesteros RI, Ichwan M, Meraz-Ríos MA, Kempermann G and
Ramírez-Rodríguez GB: Green tea compound
epigallo-catechin-3-gallate (EGCG) increases neuronal survival in
adult hippocampal neurogenesis in vivo and in vitro. Neuroscience.
322:208–220. 2016. View Article : Google Scholar
|
|
48
|
Afzal M, Safer AM and Menon M: Green tea
polyphenols and their potential role in health and disease.
Inflammopharmacology. 23:151–161. 2015. View Article : Google Scholar : PubMed/NCBI
|