Introduction
Cerebral ischemic stroke is a common clinical
condition that is considered a major cause of morbidity and
mortality in adults worldwide, which caused ~5.5 million deaths in
2016 alone (1–4). Ischemic stroke is essentially brain
damage-induced by the restoration of blood flow to an ischemic
area, which promotes further ischemic organ damage (5,6).
Although significant advances have been made in the diagnosis and
therapy of ischemic stroke, several factors, such as smoking and
delayed hospitalization, limit the recovery of patients with this
condition (7,8). Inflammation and apoptosis are crucial
biological events involved in the progression of ischemic stroke,
and the inhibition of inflammation has been reported to decrease
nerve tissue damage (9,10). Therefore, inhibiting inflammation
and apoptosis may be an efficient strategy for the treatment of
cerebral ischemia/reperfusion injury (IRI) (11).
Long non-coding RNAs (lncRNAs) have been shown to
interact with specific microRNAs (miR) to regulate gene expression
(12). Previous studies have also
demonstrated that certain lncRNAs play crucial roles in cerebral
ischemia reperfusion. For example, Ren et al (13) reported that lncRNA KCNQ1 opposite
strand/antisense transcript 1 promoted OGD/R injury by regulating
the miR-9/matrix metalloproteinase (MMP)8 signaling axis in
cultured primary cortical neurons. In addition, the lncRNA small
nucleolar RNA host gene (SNHG)12 was found to protect primary
hippocampal neuronal cells and N2a cells from OGD/R injury by
downregulating miR-199a expression and upregulating sirtuin 1
expression via the activation of the 5′AMP-activated protein kinase
signaling pathway (14). Zhong
et al (15) revealed that
lncRNA SNHG14 promoted cerebral IRI via modulating the
miR-136-5p/Rho associated coiled-coil containing protein kinase 1
axis. In addition, previous studies have reported that the lncRNA
AK139328 was associated with IRI in various types of organs. For
example, the expression levels of AK139328 were found to be
upregulated in the following in mouse plasma after liver IRI using
microarray technology, and the knockdown of AK139328 expression
exerted a protective role over the liver injury (16). AK139328 expression levels were also
reported to be upregulated in diabetic mice following myocardial
ischemia/reperfusion using microarray analysis (17). Furthermore, AK139328 was also
discovered to be involved in the pathogenesis of acute kidney
injury and pathological cardiac remodeling (18,19).
However, the current understanding of the functions of AK139328 are
limited and the associations between AK139328 and other disease
types remain unclear.
As AK139328 has been reported to play an important
role in IRI, the present study aimed to determine whether AKI39328
was also associated with cerebral IRI. An in vitro cerebral
OGD/R model was established in PC12 cells. The expression levels of
AK139328 were analyzed in patients with cerebral ischemic stroke
and in OGD/R-induced PC12 cells. The effects of AK139328 knockdown
on inflammation, oxidative stress, apoptosis and PC12 cell neurite
outgrowth were also evaluated. The present study may provide
valuable insight into novel potential approaches for the treatment
of cerebral ischemic stroke.
Materials and methods
Clinical specimens
Between January 2019 and October 2019, blood samples
were collected from 30 patients who had suffered a cerebral
ischemic stroke (female to male patient ratio, 17:13; aged 25–65
years) and 30 healthy individuals who were recruited to The
Affiliated Hospital of Yangzhou University (Yangzhou, China). The
study was approved by the Ethics Committee of The Affiliated
Hospital of Yangzhou University (approval no. 2018-004-01) and all
patients provided written informed consent prior to participation
in the study. The inclusion criteria were as follows: Patients were
admitted within 48 h from the onset of stroke, and diagnosed by
cerebral imaging and a neurologist. The exclusion criteria were as
follows: Intracranial hemorrhage, hematological diseases,
pregnancy, cancer, severe renal failure, severe liver failure,
recent myocardial infarction and ongoing treatment with
anti-inflammatory drugs (20). To
obtain the plasma samples, 2 ml of blood was collected in
S-Monovette® EDTA-KE tubes (Sarstedt, Inc.), which was
subsequently centrifuged at 2,000 × g for 1 min at 4°C. The
supernatant was transferred into a new tube and further centrifuged
at 4,000 × g for 5 min at 4°C. The plasma was then acquired and
stored at −80°C until required for further analysis.
Cell culture and treatment
PC12 cells cultured under OGD/R conditions have been
extensively studied as an in vitro model system for the
identification of mechanisms of neuronal death following ischemic
insult and for potential neuroprotective targets (21–23).
In the present study, PC12 cells were purchased from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA), 100 µg/ml
streptomycin and 100 U/ml penicillin. Cells were maintained under
humidified conditions with 5% CO2 and 95% air at
37°C.
To establish the OGD/R model, PC12 cells were
maintained in glucose-free DMEM supplemented with 10% FBS and
streptomycin/penicillin mixture in an oxygen-free incubator with 5%
CO2 and 95% N2 at 37°C for 2 h. Following
hypoxic exposure, the cells were incubated in normal medium
containing FBS and streptomycin/penicillin in an atmosphere
containing 95% air and 5% CO2 at 37°C for 12 h. PC12
cells in the control group were incubated under normoxic conditions
containing 95% air and 5% CO2 at 37°C for 24 h.
Cell transfection
Knockdown of AK139328 expression was performed using
short hairpin RNA (shRNA) targeting AK139328 (shRNA-AK139328-1;
5′-CACCGGAAACTCAGCTATCACATGCCGAAGCATGTGATAGCTGAGTTTCC-3′),
shRNA-AK139328-2
(5′-CACCGCAGCAGAAAGACATGTTTGGCGAACCAAACATGTCTTTCTGCTGC-3′) and
corresponding scrambled negative control (shRNA;
5′-CACCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG-3′),
which were both synthesized by Shanghai GenePharma Co., Ltd. PC12
cells were transfected with 500 ng/µl shRNA-AK139328-1/2 or shRNA
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The cells were harvested for use in subsequent experiments at 48 h
post-transfection.
MTT assay
MTT assay was performed to analyze cell viability.
PC12 cells were plated into 96-well plates (5×103
cells/well). Following 24 h of incubation at 37°C, cells received
OGD/R treatment as described above. Then, 20 µl MTT solution (5
mg/ml) was added into each well and incubated with the cells for 4
h at 37°C. MTT solution was subsequently discarded and 150 µl DMSO
was added to each well for 10 min at room temperature to dissolve
the purple formazan crystals. The optical density of each well was
measured using a microplate reader at a wavelength of 570 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from PC12 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The concentration and quality of RNA were assessed using a
NanoDrop™ 3000 spectrophotometer (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using a PrimeScript RT Master mix (Takara
Bio, Inc.) according to the manufacturer's protocol. qPCR was
subsequently performed using a SYBR Premix ExTaq kit (Takara Bio,
Inc.) with the following reaction conditions: 95°C for 2 min,
followed by 40 cycles of 95°C for 20 sec and 65°C for 40 sec. The
primer sequences were as follows: lncRNA-AK139328 forward,
5′-CCAGTTCTTGGTCCTGGTGT-3′ and reverse, 5′-GTGTCTGCAACCCGATAGGT-3′;
GAPDH forward, 5′-AGCCACATCGCTCAGACAC-3′ and reverse,
5′-GCCCAATACGACCAAATCC-3′. The relative expression levels of the
target genes were calculated using the 2−∆∆Cq method
(24). GAPDH was used as the
internal reference gene, and the relative expression level was
normalized to GAPDH.
Detection of inflammatory cytokines
and reactive oxygen species (ROS) levels
PC12 cells were plated in the 96-well plates
(5×103 cells/well) for incubation for 24 h, followed by
exposure to OGD/R. Then, the culture medium was collected and
centrifugated for 5 min at 12,000 × g and 4°C to obtain the
supernatant of PC12 cells. The levels of TNF-α, IL-1β and IL-6 in
the supernatant of PC12 cells were measured using the corresponding
ELISA kits (cat. no. 210-TA-005 for TNF-α; cat. no. 201-LB-005 for
IL-1β; cat. no. S6050 for IL-6; R&D Systems, Inc.) according to
the manufacturers' protocols. A ROS assay kit (cat. no. JL13783;
Shanghai Jianglai Biological Technology Co., Ltd.) was used to
evaluate the intracellular ROS levels in OGD/R-treated PC12 cells.
Each experimental condition was plated five times and all assays
were independently repeated three times.
Flow cytometric analysis of
apoptosis
Flow cytometry was performed to determine the
induction of cell apoptosis using an Annexin V-FITC/PI Apoptosis
Detection kit (Sigma-Aldrich; Merck KGaA). After the transfected
PC12 cells were treated with OGD/R as described above, the cells
were harvested, resuspended in 0.5 ml PBS and incubated with 5 µl
Annexin V-FITC for 15 min and 10 µl PI (10 mg/ml) in the dark for 5
min. Apoptotic cells were analyzed using a FACScan flow cytometer
(BD Biosciences). The data were analyzed using flow cytometry
software (iSort™ Automated Cell Sorter; version A.0; Thermo Fisher
Scientific, Inc.). The cell apoptosis rate calculated as the
percentage of early and late apoptotic cells. The experiments were
performed in triplicate.
Immunofluorescence staining
Transfected PC12 cell slides were fixed with 4%
paraformaldehyde at room temperature for 20 min and blocked with 5%
skimmed milk at room temperature for 1 h. Subsequently, the cells
were incubated with an anti-microtubule associated protein-2
(MAP-2; 1:1,000; cat. no. ab75713; Abcam) or anti-growth associated
protein (GAP)-43 primary antibody (1:500; cat. no. ab75810; Abcam)
overnight at 4°C. Following the primary antibody incubation, slides
were incubated with Alexa Fluor® 594-conjugated
secondary antibody (1:1,000; cat. no. A-11012; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at 37°C. Cell nuclei were
counterstained with DAPI for 5 min at room temperature. Stained
cells were observed under a laser confocal microscope (Olympus
Corporation; magnification, ×200).
Western blotting
Total protein was extracted from transfected
OGD/R-treated cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a BCA assay kit
(Beyotime Institute of Biotechnology) and protein samples (20
µg/lane) were separated via 10% SDS-PAGE. The separated proteins
were subsequently transferred onto PVDF membranes and blocked with
5% non-fat milk at room temperature for 1 h. The membranes were
then incubated with the following primary antibodies overnight at
4°C: Anti-endothelial nitric oxide synthase (eNOS; 1:1,000; cat.
no. ab5589; Abcam), anti-MAP-2 (1:1,000; cat. no. ab32454; Abcam),
anti-GAP-43 (1:1,000; cat. no. ab75810; Abcam), anti-Bcl-2
(1:1,000; cat. no. ab196495; Abcam), anti-Bax (1:1,000; cat. no.
ab182733; Abcam), anti-cleaved caspase-3 (1:5,000; cat. no.
ab214430; Abcam), anti-caspase-3 (1:2,000; cat. no. ab184787;
Abcam) and anti-GAPDH (1:2,500; cat. no. ab9485; Abcam). Following
the primary antibody incubation, the membranes were washed twice
with TBS with 0.05% Tween-20 (TBST) and incubated with goat
anti-rabbit IgG H&L secondary antibody (1:2,000; cat. no.
ab6721; Abcam) for 1 h at room temperature. GAPDH was used as the
internal loading control. Protein bands were visualized using ECL
substrate (Pierce; Thermo Fisher Scientific, Inc.) and analyzed
with ImageJ software (version 3.0; National Institutes of
Health).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.) and GraphPad Prism 6.0 software (GraphPad
Software, Inc.). Statistical differences between two groups were
determined using an unpaired Student's t-test, while a one-way
ANOVA followed by a Tukey's post hoc test was used to determine
statistical differences among multiple groups. Data are presented
as the mean ± SD. All experiments were repeated at least three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
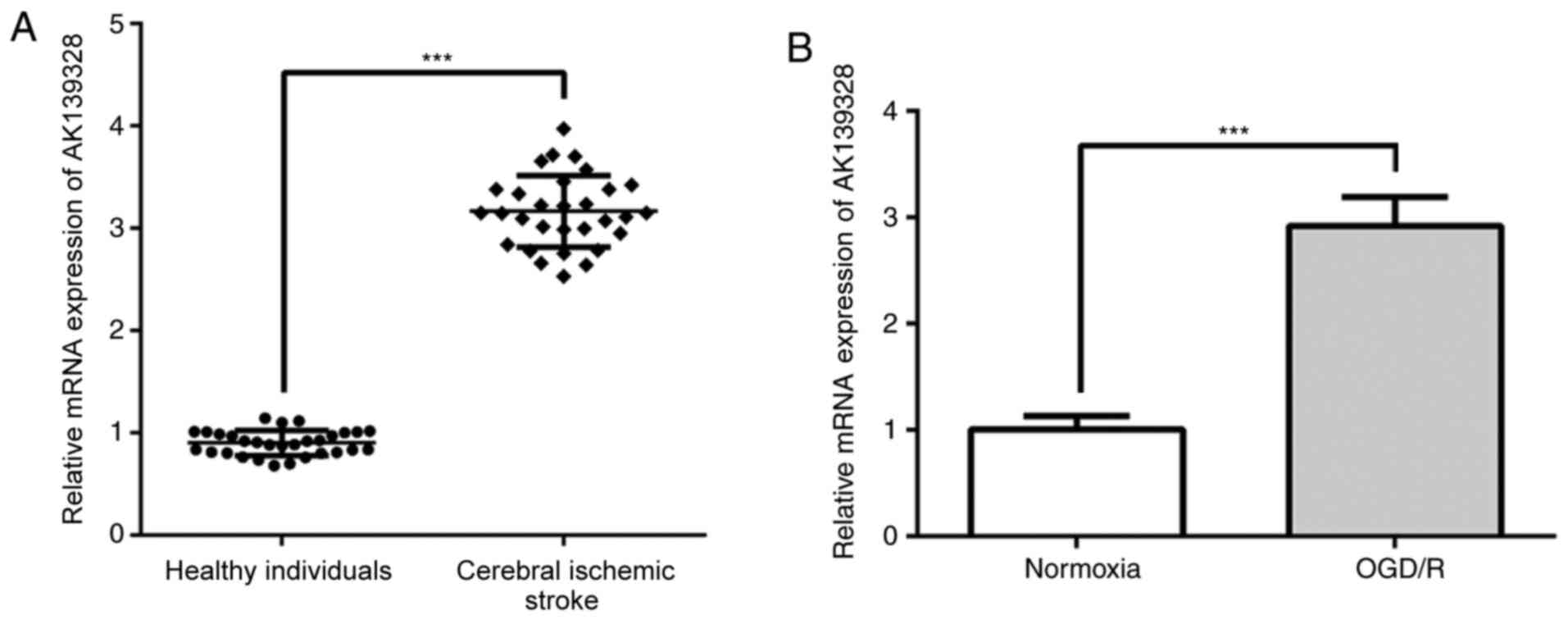
AK139328 expression levels are
upregulated in patients who had suffered a cerebral ischemic stroke
and in OGD/R-treated PC12 cells
To investigate the role of lncRNA AK139328 in
cerebral ischemic stroke, the expression levels of AK139328 were
first analyzed in clinical specimens. AK139328 expression levels
were significantly upregulated in the plasma of patients who had
experienced a cerebral ischemic stroke compared with those noted in
healthy individuals (Fig. 1A). In
addition, AK139328 expression levels were determined in an in
vitro OGD/R model. The RT-qPCR results revealed a significant
upregulation in AK139328 expression levels in OGD/R-induced PC12
cells compared with cells cultured under normoxic conditions
(Fig. 1B). These data indicated
that AK139328 may participate in the pathophysiology of cerebral
IRI.
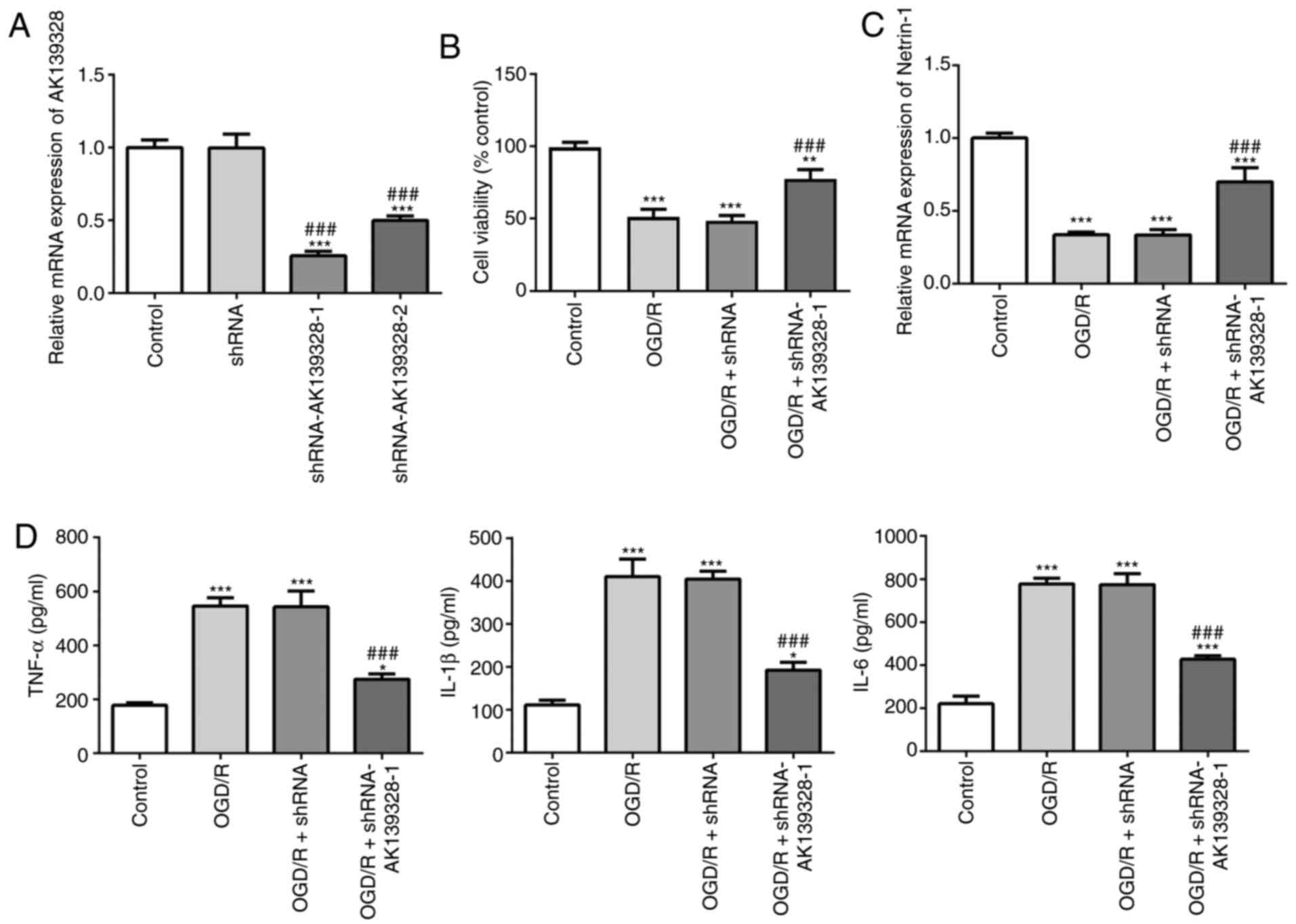
Knockdown of AK139328 expression
promotes cell viability and upregulates Netrin-1 expression
levels
To determine the effects of AK139328 on PC12 cells
induced with OGD/R, AKI39328 expression was knocked down in PC12
cells and the transfection efficiency was evaluated using RT-qPCR
(Fig. 2A). The results indicated
that AK139328 expression levels were significantly downregulated in
cells transfected with shRNA-AK139328-1 or shRNA-AK139328-2
compared with the control and shRNA-transfected cells. Moreover,
the expression levels of AK139328 were lowest in
shRNA-AK139328-1-transfected cells; therefore, shRNA-AK139328-1 was
selected for use in subsequent experiments. The results of the MTT
assay revealed that, after OGD/R induction, cell viability was
significantly decreased compared with the control group, while
silencing of AK139328 partially alleviated the OGD/R-induced loss
in cell viability (Fig. 2B). In
addition, the expression levels of Netrin-1 were significantly
downregulated by OGD/R treatment compared with the control group;
however, this downregulation was partially reversed following the
knockdown of AK139328 (Fig.
2C).
Knockdown of AK139328 expression
alleviates OGD/R-induced inflammatory injury in PC12 cells
The effects of AK13928 on inflammatory injury were
subsequently determined. The results obtained from the ELISAs
revealed that the protein levels of TNF-α, IL-1β and IL-6 in PC12
cells following exposure to OGD/R were significantly elevated
compared with the control cells, while the production of these
inflammatory factors was inhibited following transfection of the
cells with shRNA-AK139328-1 in the presence of OGD/R (Fig. 2D). These results suggested that the
knockdown of AK139328 may play an inhibitory role over the
inflammatory responses induced by OGD/R.
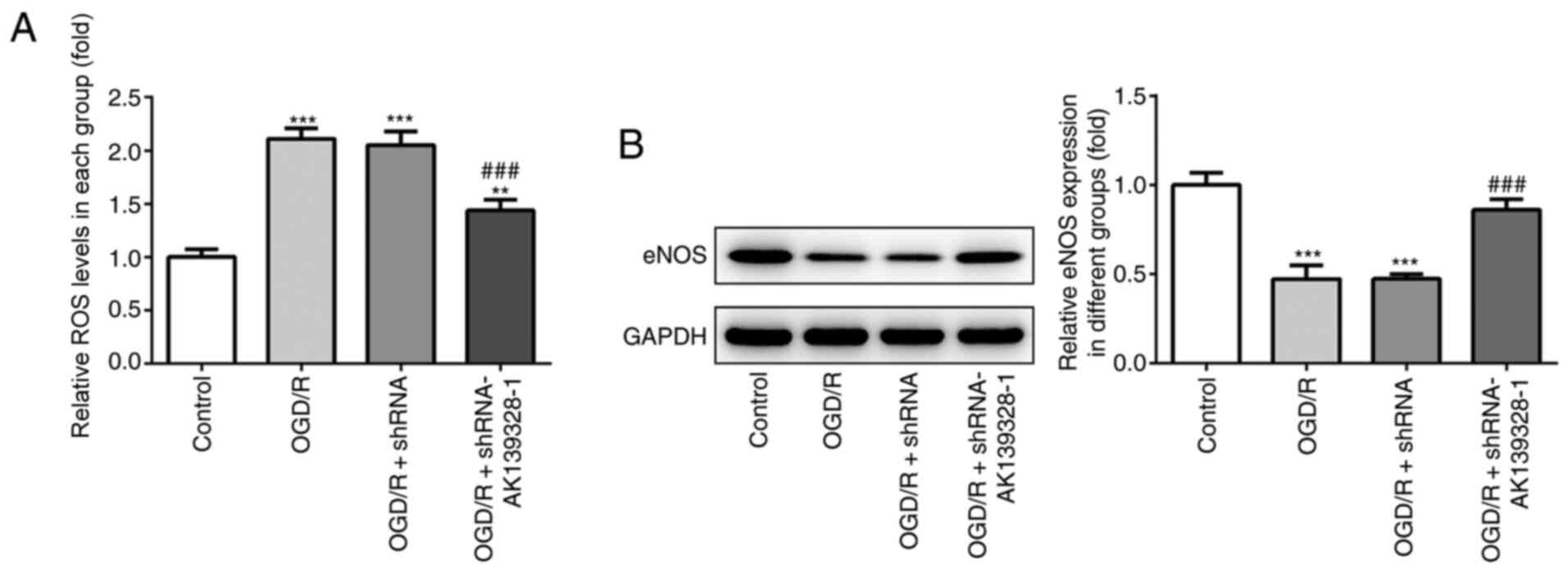
Knockdown of AK139328 reduces
oxidative stress in PC12 cells induced by OGD/R
Subsequently, the effects of AK139328 silencing on
the induction of oxidative stress were determined in OGD/R-treated
PC12 cells. OGD/R treatment significantly increased the production
of ROS compared with control cells, whereas the concurrent
transfection with shRNA-AK139328-1 partially reduced the increased
levels of ROS in PC12 cells (Fig.
3A). In addition, the protein expression levels of eNOS were
analyzed by western blotting. OGD/R injury significantly
downregulated the protein expression levels of eNOS compared with
the control group, while AK139328 silencing upregulated eNOS
protein expression levels in OGD/R-injured PC12 cells (Fig. 3B). These findings suggested that the
knockdown of AK139328 expression may alleviate OGD/R-induced
oxidative stress levels in PC12 cells.
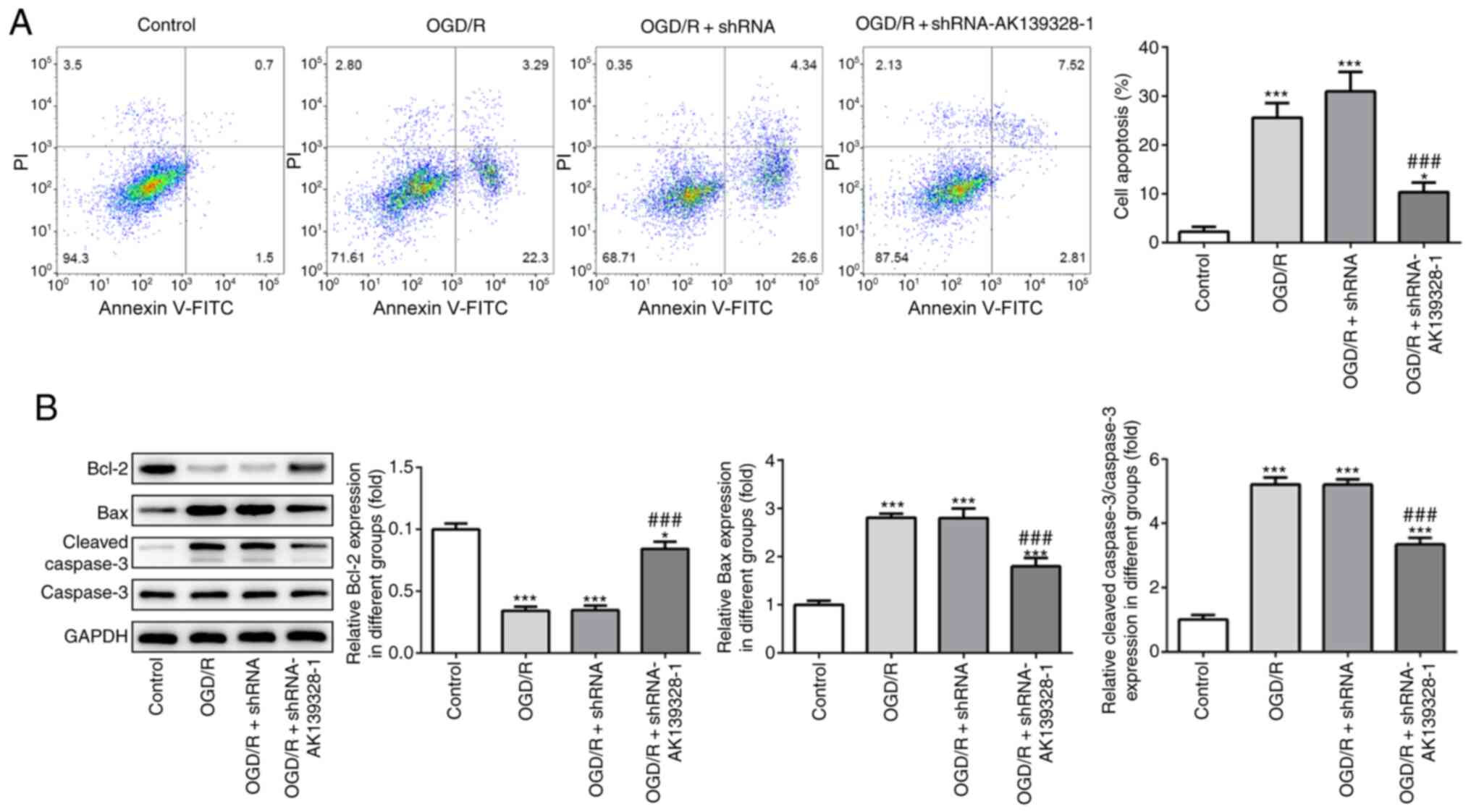
Induction of cell apoptosis by OGD/R
is suppressed by AK139328 knockdown
The effects of AK139328 silencing on the induction
of OGD/R-injured PC12 cell apoptosis were determined to investigate
the role of AK139328 in cerebral IRI. Flow cytometry data
demonstrated that OGD/R significantly increased PC12 cell apoptosis
compared with control cells, while AK139328 silencing decreased the
apoptotic rate in OGD/R-treated PC12 cells (Fig. 4A). Moreover, western blotting
analysis revealed that Bcl-2 expression levels were significantly
downregulated, while the expression levels of Bax and cleaved
caspase-3/caspase-3 were significantly upregulated following the
induction of OGD/R compared with control cells. However,
transfection with shRNA-AK139328-1 exerted inhibitory effects on
cell apoptosis by partially reversing the trends in the expression
levels of Bcl-2, Bax and cleaved caspase-3 observed in
OGD/R-treated PC12 cells (Fig. 4B).
These data indicated that the knockdown of AK139328 may exert
suppressive effects in OGD/R-mediated PC12 cell apoptosis.
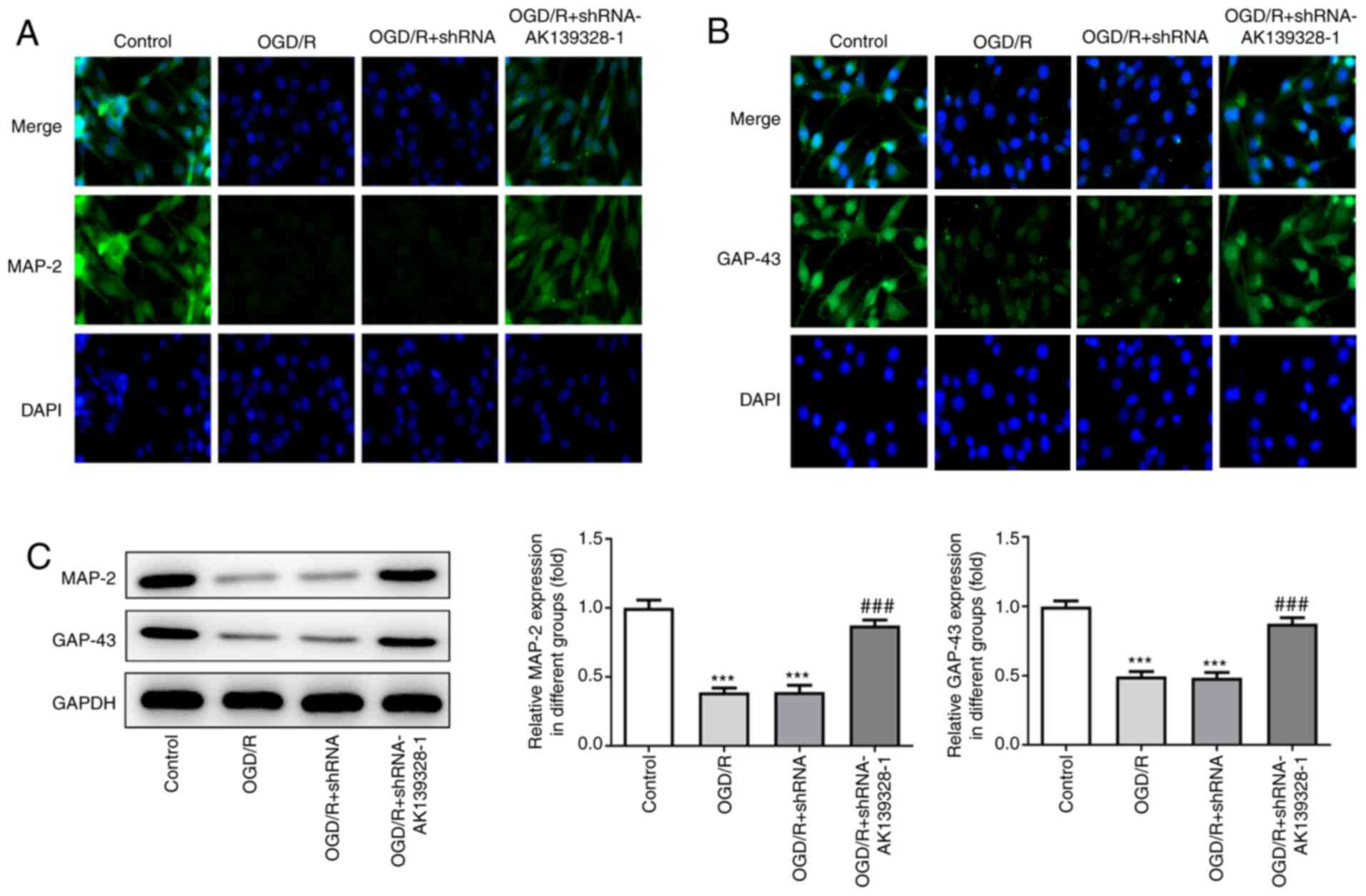
Silencing of AK139328 promotes neurite
outgrowth in OGD/R-injured PC12 cells
The effects of AK139328 on the neurite outgrowth of
PC12 cells injured by OGD/R were analyzed. The results from the
immunofluorescence staining demonstrated that MAP-2 and GAP-43
expression levels were increased in cells cultured under normal
conditions, while the expression levels were slightly decreased by
OGD/R treatment (Fig. 5A and B).
Notably, the transfection with shRNA-AK139328-1 rescued
OGD/R-induced decreased expression levels of MAP-2 and GAP-43 in
OGD/R-induced PC12 cells. In addition, the protein expression
levels of MAP-2 and GAP-43 were significantly downregulated in
OGD/R-treated PC12 cells, which were subsequently reversed by
AK139328 silencing (Fig. 5C). These
data suggested that AK139328 silencing may protect neurite
outgrowth in PC12 cells following the induction of OGD/R.
Discussion
The present study investigated the expression levels
of lncRNA AK139328 in OGD/R-stimulated cells. The data demonstrated
that AK139328 expression levels were upregulated in patients with
cerebral ischemic stroke and in OGD/R-stimulated PC12 cells.
Furthermore, the knockdown of AK139328 expression exerted
inhibitory roles over the inflammatory response, oxidative stress
and induction of apoptosis in OGD/R-stimulated PC12 cells. In
addition, knockdown of AK139328 accelerated the process of neurite
outgrowth following exposure of PC12 cells to OGD/R treatment.
Alterations in the expression levels of specific
lncRNAs have been used as biomarkers for the diagnosis and
treatment of several human diseases, including cancer and
cardiovascular diseases (25–27).
It has been well documented that lncRNA AK139328 plays a crucial
role in multiple diseases. For example, Pei et al (28) revealed that AK139328 expression
levels were upregulated in OGD/R-induced vascular endothelial
cells, whereas lncRNA AK139328 knockdown reduced inflammation,
oxidative stress and apoptosis in a rat hindlimb
ischemia/reperfusion model by regulating the PI3K/AKT/eNOS
signaling pathway. In addition, another previous study found that
upregulated expression levels of AK139328 promoted cell viability,
invasion and cell cycle progression in thyroid cancer, while
AK139328 knockdown exerted the opposite results (29). AK139328 was also reported to be
abnormally expressed and involved in the pathogenic mechanism of
myocardial IRI, pathological cardiac remodeling, hepatic IRI and
acute kidney injury (16–19,30).
Yu et al (17) reported that
the knockdown of AK139328 expression suppressed cardiomyocyte
autophagy and apoptosis in diabetic mice, resulting in the
amelioration of myocardial IRI by modulating miR-204-3p expression.
In addition, it has been shown that AK139328 expression was
upregulated in ischemia/reperfusion-treated mouse livers, whereas
the knockdown of AK139328 expression alleviated IRI in the liver by
activating the AKT signaling pathway and inhibiting the activity of
NF-κB (30). Thus, due to the
observed important role of AK139328 in IRI, the present study aimed
to investigate whether AKI39328 was also associated with cerebral
IRI. To the best of our knowledge, the current study was the first
to investigate the role of AK139328 in cerebral IRI. Plasma samples
were collected from patients who had experienced a cerebral
ischemic stroke and the expression levels of AK139328 were
detected. The results demonstrated a significant upregulation in
AK139328 expression levels in clinical samples of patients who had
suffered from cerebral ischemic stroke. Moreover, in the OGD/R cell
model, AK139328 expression levels were upregulated compared with
the cells that were not stimulated with OGD/R, which is consistent
with previous reports (16,17).
Although glucose and oxygen deprivation can be
attenuated by reperfusion of cerebral blood, the process of
reperfusion exacerbates the inflammatory response, oxidative stress
and apoptosis, which further aggravates the progression of cerebral
damage caused by reperfusion (31–33).
Previous studies have demonstrated that the inhibition of the
inflammatory response, oxidative stress and apoptosis rescued cells
from OGD/R injury (34,35). Gaire et al (36) reported that Terminalia
chebula extract prevented OGD/R injury in PC12 cells and
suppressed lipopolysaccharide-induced activation of microglia
through inhibition of the oxidative and inflammatory processes. A
previous study also indicated that inhibition of inducible NOS
reduced cell apoptosis induced by OGD to protect PC12 cells by
regulating lactate dehydrogenase and cytochrome c release
and caspase-3 activity (37). In
the current study, the knockdown of AK139328 expression in
OGD/R-induced PC12 cells resulted in a considerable reduction in
the inflammatory response, which was evidenced by decreased levels
of TNF-α, IL-1β and IL-6 and by upregulated expression levels of
Netrin-1. Of note, increased Netrin-1 expression has been found to
be associated with improved prognosis of ischemic stroke, and
Netrin-1 is considered to be a potential prognostic biomarker for
ischemic stroke (38). AK139328
knockdown further reduced the induction of oxidative stress by
decreasing ROS levels and upregulating eNOS protein expression
levels. In addition, decreased cell apoptosis was observed
following the knockdown of AK139328 expression, which was evidenced
by a decreased apoptotic rate, accompanied by downregulated Bax and
cleaved caspase-3 expression levels and upregulated Bcl-2 levels.
These data suggested a regulatory role for AK139328 in the
inflammatory response, oxidative stress and apoptosis of
OGD/R-induced PC12 cells.
Neurite outgrowth is a key step and indicator for
functional recovery following cerebral ischemic stroke (39). MAPs play important roles in
neuritogenesis and growth via regulating microtubule stability and
altering microtubule dynamics (40). MAP-2 is a member of the MAP family
of enzymes, which are essential for neurite initiation in cultured
cerebral neurons (41). GAP-43 is a
crucial indicator for evaluating axon injury and the regenerative
response in the mature central nervous system. The upregulation of
GAP-43 expression was found to be an important mechanism for
functional recovery after cerebral ischemia (42,43). A
previous study reported that GAP-43 and MAP-2 could be regarded as
neuronal growth markers, and downregulated expression levels of
GAP-43 and MAP-2 reflected inhibited neurite outgrowth (44,45).
In the present study, MAP-2 and GAP-43 expression levels were
downregulated in OGD/R-induced cells, indicating that OGD/R
induction resulted in an inhibited neurite outgrowth. AK139328
silencing reversed the effects of OGD/R induction on MAP-2 and
GAP-43 expression levels, thus promoting neurite outgrowth.
In conclusion, the findings of the current study
suggested that the knockdown of AK139328 may protect against OGD/R
induction in PC12 cells via the inhibition of the inflammatory
response, inhibiting the induction of oxidative stress and the
concomitant apoptosis, and accelerating neurite outgrowth. The
present study may expand the current knowledge of cerebral ischemic
stroke and provide further insight into the identification of novel
therapeutic approaches for cerebral ischemic stroke.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW conceived and designed the study, and LL and BZ
performed the experiments and analyzed the data. ZW and LL
interpreted the data. LL wrote the manuscript and ZW revised the
manuscript. All authors read and approved the final version of the
manuscript and agree to be accountable for all aspects of the
research. ZW and LL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Yangzhou University (Yangzhou, China)
and all patients provided written informed consent for their
participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li C, Liu Y, Tang P, Liu P, Hou C, Zhang
X, Chen L, Zhang L and Gu C: Hydrogen sulfide prevents
OGD/R-induced apoptosis by suppressing the phosphorylation of p38
and secretion of IL-6 in PC12 cells. Neuroreport. 27:230–234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Z, Weian C, Susu H and Hanmin W:
Protective effects of mangiferin on cerebral ischemia-reperfusion
injury and its mechanisms. Eur J Pharmacol. 771:145–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Xu Z, Chen X, Li Y, Chen C, Wang
C, Zhu J, Wang Z, Chen W, Xiao Z, et al: MicroRNA-182-5p attenuates
cerebral ischemia-reperfusion injury by targeting Toll-like
receptor 4. Biochem Biophys Res Commun. 505:677–684. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collaborators GBDS; GBD 2016 Stroke
Collaborators, : Global, regional, and national burden of stroke,
1990–2016: A systematic analysis for the Global Burden of Disease
Study 2016. Lancet Neurol. 18:439–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Li S, Wang S, Yu N and Liu J: The
effect of mitochondrial calcium uniporter on mitochondrial fission
in hippocampus cells ischemia/reperfusion injury. Biochem Biophys
Res Commun. 461:537–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oda E and Kawai R: A possible
cross-sectional association of serum total bilirubin with coronary
heart disease and stroke in a Japanese health screening population.
Heart Vessels. 27:29–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prabhakaran S, Ruff I and Bernstein RA:
Acute stroke intervention: A systematic review. JAMA.
313:1451–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meschia JF and Brott T: Ischaemic stroke.
Eur J Neurol. 25:35–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin R, Liu L, Zhang S, Nanda A and Li G:
Role of inflammation and its mediators in acute ischemic stroke. J
Cardiovasc Transl Res. 6:834–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong L, Tang Y, An R, Lin M, Chen L and Du
J: RTN1-C mediates cerebral ischemia/reperfusion injury via ER
stress and mitochondria-associated apoptosis pathways. Cell Death
Dis. 8:e30802017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu SY, Tang L and Zhou SH: Long noncoding
RNAs: New players in ischaemia-reperfusion injury. Heart Lung Circ.
27:322–332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren Y, Gao XP, Liang H, Zhang H and Hu CY:
LncRNA KCNQ1OT1 contributes to
oxygen-glucose-deprivation/reoxygenation-induced injury via
sponging miR-9 in cultured neurons to regulate MMP8. Exp Mol
Pathol. 112:1043562020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin WL, Yin WG, Huang BS and Wu LX: LncRNA
SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral
ischemia/reperfusion injury through activating AMPK signaling
pathway. Neurosci Lett. 690:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p /ROCK1.
Cancer Gene Ther. 26:234–247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Luo Y, Yang W, Ding L, Wang J, Tu
J, Geng B, Cui Q and Yang J: Comparison analysis of dysregulated
lncrna profile in mouse plasma and liver after hepatic
ischemia/reperfusion injury. PLoS One. 10:e01334622015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lncRNA AK139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou P, Chen Z, Zou Y and Wan X: Roles of
non-coding RNAs in acute kidney injury. Kidney Blood Press Res.
41:757–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Wang B, Yang YX, Jia QJ, Zhang A,
Qi ZW and Zhang JP: Long noncoding RNAs in pathological cardiac
remodeling: A review of the update literature. BioMed Res Int.
2019:71595922019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Wang L, Han Z, Dong J, Pang D, Fu
Y and Li L: KLF4 alleviates cerebral vascular injury by
ameliorating vascular endothelial inflammation and regulating tight
junction protein expression following ischemic stroke. J
Neuroinflammation. 17:1072020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abu Raya S, Trembovler V, Shohami E and
Lazarovici P: A tissue culture ischemic device to study eicosanoid
release by pheochromocytoma PC12 cultures. J Neurosci Methods.
50:197–203. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H,
Liang F, Li HB, Zhao Y, Xu X, Yang K, et al: Parthenolide
attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β
pathway in PC12 cells. Biomed Pharmacother. 89:1159–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y and Zhang Y: lncRNA ZFAS1 improves
neuronal injury and inhibits inflammation, oxidative stress, and
apoptosis by sponging miR-582 and upregulating NOS3 expression in
cerebral ischemia/reperfusion injury. Inflammation. 43:1337–1350.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X
and Tai S: LncRNA HOXA-AS2 and its molecular mechanisms in human
cancer. Clin Chim Acta. 485:229–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sallam T, Sandhu J and Tontonoz P: Long
noncoding RNA discovery in cardiovascular disease: Decoding form to
function. Circ Res. 122:155–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pei Z, Wang S, Mao Y, Li Q, Wu Z and Zheng
X: Silencing LncRNA AK139328 protects vascular endothelial cells
from ischemia-reperfusion injury by increasing PI3K/Akt signaling.
Oncotarget. 5:224532015.Retrieved from. https://www.oncotarget.com/article/22453/text/
|
|
29
|
Li S, Zhao Z, Li Y, Zhang Y, Dong L, Liang
Y, Mao Y and Ma J: The long noncoding RNA AK 139328 promotes the
oncogenesis in thyroid cancer. Int J Clin Exp Med. 10:11894–11902.
2017.
|
|
30
|
Chen Z, Jia S, Li D, Cai J, Tu J, Geng B,
Guan Y, Cui Q and Yang J: Silencing of long noncoding RNA AK139328
attenuates ischemia/reperfusion injury in mouse livers. PLoS One.
8:e808172013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thompson JW, Narayanan SV and Perez-Pinzon
MA: Redox signaling pathways involved in neuronal ischemic
preconditioning. Curr Neuropharmacol. 10:354–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mo ZT, Li WN, Zhai YR and Gong QH: Icariin
attenuates OGD/R-induced autophagy via Bcl-2-dependent cross talk
between apoptosis and autophagy in PC12 cells. Evid Based
Complement Alternat Med. 2016:43430842016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Y and Xu J: Sanggenon C ameliorates
cerebral ischemia-reperfusion injury by inhibiting inflammation and
oxidative stress through regulating RhoA-ROCK signaling.
Inflammation. 43:1476–1487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Ma Y, Wei X and Fan T:
Neuroprotective effect of licochalcone A against oxygen-glucose
deprivation/reperfusion in rat primary cortical neurons by
attenuating oxidative stress injury and inflammatory response via
the SIRT1/Nrf2 pathway. J Cell Biochem. 119:3210–3219. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rao G, Zhang W and Song S: MicroRNA-217
inhibition relieves cerebral ischemia/reperfusion injury by
targeting SIRT1. Mol Med Rep. 20:1221–1229. 2019.PubMed/NCBI
|
|
36
|
Gaire BP, Jamarkattel-Pandit N, Lee D,
Song J, Kim JY, Park J, Jung S, Choi HY and Kim H: Terminalia
chebula extract protects OGD-R induced PC12 cell death and inhibits
lps induced microglia activation. Molecules. 18:3529–3542. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang H, Koubi D, Zhang L, Kuo J,
Rodriguez AI, Hunter TJ, Gautam SC and Levine RA: Inhibitors of
iNOS protects PC12 cells against the apoptosis induced by oxygen
and glucose deprivation. Neurosci Lett. 375:59–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo D, Zhu Z, Zhong C, Peng H, Wang A, Xu
T, Peng Y, Xu T, Chen CS, Li Q, et al: Increased Serum Netrin-1 Is
Associated With Improved Prognosis of Ischemic Stroke. Stroke.
50:845–852. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang F, Guo S, Liao H, Yu P, Wang L, Song
X, Chen J and Yang Q: Resveratrol enhances neurite outgrowth and
synaptogenesis via sonic hedgehog signaling following
oxygen-glucose deprivation/reoxygenation injury. Cell Physiol
Biochem. 43:852–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riederer BM: Microtubule-associated
protein 1B, a growth-associated and phosphorylated scaffold
protein. Brain Res Bull. 71:541–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shalavadi MH, Chandrashekhar VM and
Muchchandi IS: Neuroprotective effect of Convolvulus
pluricaulis Choisy in oxidative stress model of cerebral
ischemia reperfusion injury and assessment of MAP2 in rats. J
Ethnopharmacol. 249:1123932020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Gao X, Wang Q, Yang Y, Liu H, Zhang
B and Li L: Retinoic acid protects from experimental cerebral
infarction by upregulating GAP-43 expression. Braz J Med Biol Res.
50:e55612017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li S and Carmichael ST: Growth-associated
gene and protein expression in the region of axonal sprouting in
the aged brain after stroke. Neurobiol Dis. 23:362–373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen MK, Peng CC, Maner RS, Zulkefli ND,
Huang SM and Hsieh CL: Geniposide ameliorated fluoxetine-suppressed
neurite outgrowth in Neuro2a neuroblastoma cells. Life Sci.
226:1–11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui W, Ren Y, Wang S, Zeng M, Han S, Li J
and Han R: The role of caveolin-1 in morphine-induced structural
plasticity in primary cultured mouse cerebral cortical neurons.
Neurosci Lett. 665:38–42. 2018. View Article : Google Scholar : PubMed/NCBI
|